Abstract
Oligodendrocyte‐formed myelin sheaths play important roles in the neuronal functions in the central nervous system. In demyelinating diseases, such as Multiple Sclerosis, the myelin sheaths are damaged and the remyelinating process is somehow hindered. Restoration of the myelin sheaths requires the differentiation of the oligodendrocyte precursor cells (OPCs) into mature oligodendrocytes (OLs). To discover small molecule compounds that might promote the OPC to OL differentiation, a high‐throughput screening system is established and L‐ascorbyl‐2‐phosphate (As‐2P), a stable form of Vitamin C (Vc), is found to greatly enhance the OPC to OL differentiation. As‐2P promotes gradual expression of OL lineage markers, including O4, CNPase and MBP, in a dose‐ and time‐dependent manner. It also facilitates the formation of myelin sheaths in OPC‐neuron co‐culture. As‐2P also promotes the repair of the myelin sheaths in vivo and provides significant therapeutic effect in a cuprizone‐mediated demyelination animal model. Interestingly, As‐2P's function in promoting OPC differentiation is not related to its antioxidant activity. And an intracellular rather than an extracellular mechanism might be involved. Considering the safe use of Vc as a dietary supplement for many years, it might also be used as an alternative medicine for CNS demyelinating diseases.
Keywords: differentiation, myelin, oligodendrocyte, oligodendrocyte progenitor cell, remyelination, vitamin C
1. INTRODUCTION
Oligodendrocytes (OLs) form myelin sheaths by wrapping around the axons in the central nervous system (CNS). Damage to the myelin sheath will disrupt axonal conduction and cause severe neurological dysfunctions (Baumann & Pham‐Dinh, 2001; Popescu & Lucchinetti, 2012). In adult CNS, a natural regenerative process of the myelin will kick in after injury, that is, oligodendrocyte precursor cells (OPCs) will migrate to the area of injury and differentiate into OLs and restore myelin sheaths (Gensert & Goldman, 1997). However, in a number of demyelinating diseases, exemplified by multiple sclerosis (MS), this regeneration process is hindered (Franklin, 2002). Currently available treatments for MS all aimed to modulate the immune responses (Haghikia, Hohlfeld, Gold, & Fugger, 2013). Although effective in reducing the relapse rate and the formation of new lesions, these drugs have very limited effects in preventing the progression of disability (Trapp & Nave, 2008). Thus, promoting OPC to OL differentiation and recovery of myelin sheaths and neuronal functions have been proposed to be the new goal of MS therapy (Cole, Early, & Lyons, 2017; Zhang, Guo, & Lu, 2013).
During development, OPCs are generated from neural progenitor cells (NPCs) of the ventricular zones throughout the brain and spinal cord (van Tilborg et al., 2018). The OPCs expressing platelet‐derived growth factor receptor alpha (PDGFRα) and proteoglycan glial antigen 2 (NG2) then proliferate and migrate throughout the CNS (Bergles & Richardson, 2015; Goldman & Kuypers, 2015; Zhang, 2001). OPCs give rise to immature premyelinating OLs that express surface antigen O1, O4, and 2′,3′‐cyclic nucleotide‐3′‐phosphohydrolase (CNPase) before differentiating into mature myelin basic protein (MBP)‐positive and myelin oligodendrocyte glycoprotein (MOG)‐positive myelinating OLs in white matter (Pfeiffer, Warrington, & Bansal, 1993; Zhang, 2001). Unlike many progenitors, OPCs remain abundant in the adult CNS (Crawford, Stockley, Tripathi, Richardson, & Franklin, 2014; Nunes et al., 2003), which makes it possible to develop remyelinating drugs by modulating the differentiation process of these cells.
A number of extrinsic and intrinsic factors have been discovered to control the OPC to OL differentiation and myelination processes. Transcription factors such as Olig1, Olig2, Sox‐10, Nkx2.2, and Ying Yang 1 are required for the generation of mature OLs, while Id2, Id4, Hes5, and Sox6 are critical in maintaining OPCs in their undifferentiated state (Emery, 2010; Zhang et al., 2013). Several pathways, including neuregulin/ErbB2, NGF/TrkA, Notch and Wnt/β‐catenin pathways, have also been found to mediate OPC to OL differentiation (Chan et al., 2004; Fancy et al., 2009; Guo et al., 2015; Park, Miller, Krane, & Vartanian, 2001; Zhang et al., 2009). A number of G protein‐coupled receptors, such as GPR17, GPR37, M1/M3 (muscarinic receptors), and KOR (kappa opioid receptor), also participate in the differentiation and maturation of OLs and the formation of myelin sheaths (Chen et al., 2009; Deshmukh et al., 2013; Du et al., 2016; Mei et al., 2016; Yang, Vainshtein, Maik‐Rachline, & Peles, 2016). Several pharmacological compounds, including XAV939, benztropine, clemastine, miconazole, and clobetasol, have been found to promote OPC to OL differentiation in vitro and remyelination in vivo (Deshmukh et al., 2013; Fancy et al., 2011; Mei et al., 2014; Najm et al., 2015).
In the present study, we established an in vitro OPC to OL differentiation system to screen compounds that might facilitate the generation of mature OLs. And L‐ascorbyl‐2‐phosphate (As‐2P), a stable form of Vitamin C (Vc), was found to greatly promotes OPC differentiation, OL maturation and myelin formation. In a cuprizone‐mediated demyelination animal model, As‐2P also showed significant therapeutic effects.
2. MATERIALS AND METHODS
2.1. Reagents
Laminin, poly‐ornithine, thyroid hormone (T3), sodium l‐ascorbyl‐2‐phosphate (As‐2P), paraformaldehyde (PFA), Bis(cyclohexanone)oxaldihydrazone (Cuprizone), Hoechst 33342, ascorbic acid, n‐acetylcysteine, resveratrol, poly‐d‐lysine, papain, l‐cysteine, insulin, transferrin, progesterone, putrescine, BSA, and 5‐Fluoro‐2′‐deoxyuridine were purchased from Sigma–Aldrich. Phloretin (PT), Vitamin E (α‐tocopherol) and Glutathione reduced form (GSH) were purchased from Tokyo Chemical Industry. l‐carnitine hydrochloride and Vitamin B1 (Thiamine hydrochloride) were purchased from TargetMol. Rat tail collagen, collagenase II and dispase II were purchased from Roche. EGF, bFGF, and PDGF‐AA were purchased from Peprotech.
2.2. OPC differentiation
Neural progenitor cells (NPCs) were purified from the dissected cerebral cortex of E14.5 mouse embryos by culturing (Chen et al., 2007). NPCs were expanded as neurospheres in the NPC medium (DMEM/F12 (Gibco), 20 ng mL−1 EGF, 20 ng mL−1 bFGF, 2% B27 (Invitrogen), 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin). Neurospheres were passaged every other day and never allowed to reach confluence. Neurospheres from passages 3–5 were used for the differentiation assay. To generate OPCs, neurospheres were dissociated into single cells with accutase (Millipore, SF006) and seeded onto poly‐ornithine (5 μg mL−1) plus laminin (1 μg mL−1)‐coated plates in OPC medium (DMEM/F12, 10 ng mL−1 bFGF, 10 ng mL−1 PDGF‐AA, 2% B27, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin) for 2 days. To induce OPC differentiation, the OPC medium were changed into basal medium (DMEM/F12, 2% B27, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin) and cultured for another 4–5 days.
2.3. High‐throughput screening and imaging
For the primary screen, NPCs were seeded at a density of 8,000/well onto poly‐ornithine (5 μg mL−1) plus laminin (1 μg mL−1)‐coated 96‐well plates in OPC medium for 2 days and then in basal medium containing various compounds at a concentration of 20 μM for another 4–5 days. Thyroid‐hormone T3 (100 nM) and DMSO (0.2%, v/v) were included in each assay plate as positive and vehicle controls. The cells were then fixed with 4% paraformaldehyde (PFA) and stained with anti‐MBP antibody (Covance, SMI‐94R, 1:500) and secondary antibody conjugated to Alexa Fluor 488 (Thermo Fisher, A‐11001, 1:1,000). Hoechst 33342 was used to identify cell nuclei. Eleven images per well (representing different locations in a single well) were captured and the nuclei and MBP‐positive cells were quantified using the Operetta high content analysis system (PerkinElmer).
2.4. Immunofluorescence staining
Cells were fixed with 4% PFA in phosphate buffered saline (PBS) for 15 min at room temperature. After washed with PBS three times, the cells were blocked and permeated with PBS containing 1% BSA and 0.1% Triton (without Triton for O4 staining) for 30 min at room temperature. Then cells were incubated with the relevant primary antibody at 4°C overnight. After PBS washing for three times, cells were incubated with the appropriate fluorescence‐conjugated secondary antibody for 1 hr at room temperature. Nuclei were stained with Hoechst 33,342 (10 mg mL−1). Antibodies used in this assay are as follows: anti‐Nestin (Millipore, MAB353, 1:400), anti‐A2B5 (Sigma, A8229, 1:500), anti‐Olig2 (Millipore, AB9610, 1:200), anti‐MBP (Covance, SMI‐94R, 1:500), anti‐Ki67 (Cell signaling, 9129S, 1:300), anti‐MBP (Abcam, ab7349, 1:750), anti‐O4 (R&D Systems, MAB1326, 1:1,000), anti‐CNPase (Millipore, MAB326, 1:500), anti‐NF‐200 (Sigma, N4142 and N5389, 1:1,000), anti‐Caspr (Abcam, ab34151, 1:500). TUNEL staining was carried out according to the manufacturer's instructions (Roche, 11684795910).
2.5. OPC‐DRG neuron coculture
Dorsal root ganglions (DRGs) isolated from postnatal (P5‐P10) mice were incubated in Hank's balanced salt solution (HBSS) containing papain (3 U mL−1) and l‐cysteine (0.36 mg mL−1) for 10 min at 37°C. After removal of papain solution, DRGs were further incubated in HBSS containing collagenase II (100 U mL−1) and dispase II (2 U mL−1) for 10 min at 37°C. After thorough washing, the dissociated DRG neurons were seeded at a density of 20,000 cells/well onto poly‐d‐lysine (80 μg mL−1) and rat tail collagen (25 μg mL−1) coated 48‐well plate and maintained in OL‐medium (DMEM (Gibco), 2% B27, Glutamax (Gibco), insulin (5 μg mL−1), transferrin (50 μg mL−1), 0.5% FBS, progesterone (0.2 μM), putrescine (100 μM), BSA (0.1 mg mL−1) 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin) for 9 days, and 5‐Fluoro‐2'‐deoxyuridine (10 μM) was added to remove contaminating glia cells for the first 7 days. After 9 days, 3 × 104 OPCs freshly isolated from P0‐2 postnatal mice cortices with anti‐AN2 microbeads (Miltenyi) were added per well to DRG neurons, and the co‐cultures were maintained for another 6 or 14 days in OL‐medium. Drugs were added after the addition of OPCs. Cultures were fixed and stained with anti‐NF‐200 (Sigma), anti‐MBP (Covance and Abcam) and anti‐Caspr (Abcam) antibodies. Images (11 pictures/well) were taken and analyzed using the Operetta high content analysis system, and myelination was identified as neuritis double positive for MBP and NF‐200 staining.
2.6. Cuprizone‐induced demyelination mouse model
Female C57BL/6 mice (9 weeks) were fed with 0.2% (w:w) cuprizone (Sigma) mixed into a ground standard rodent chow. Cuprizone diet was maintained for 5 weeks, thereafter cuprizone‐infused food was removed and the animals were given standard chow. The mice receive daily intraperitoneal injection of As‐2P (200 mg kg−1) after cuprizone withdrawal. The treatment lasted for 1, 2, or 3 weeks. Mice were anesthetized and perfused with PBS followed by 4% PFA. Brains were removed and fixed in 4% PFA overnight, and then sectioned and stained for histopathological analysis. All the mice were maintained in pathogen‐free conditions, and all experimental procedures were approved and conducted in accordance with international guidelines for the care and use of laboratory animals and were approved by the Animal Ethics Committee of Shanghai Institute of Materia Medica.
2.7. Histology and immunofluorescent analysis
Paraffin‐embedded coronal sections of brains were stained with Luxol fast blue (LFB, Sigma, S3382) for analysis of demyelination. Images were taken and quantitative image analysis was performed using ImagePro software. Region of interest corresponding to corpus callosum was initially drawn using the “irregular AOI” tool, nonblue areas were then counted within the lesion using the “count and measure objects” tool. Percent of the demyelination area was calculated by the ratio of the nonblue area and total corpus callosum area. For immunofluorescent analysis, frozen coronal sections of brains were blocked and permeated with PBS containing 2% BSA and 0.3% Triton for 30 min at room temperature, then incubated with mouse anti‐MBP antibody (Covance, SMI‐94R, 1:500), mouse polyclonal anti‐MOG antibody (Millipore, AB5320, 1:200) and Rabbit polyclonal anti‐GST‐pi antibody (Millipore, AB5320, 1:200), Rabbit anti‐PDGFRα (Cell signaling, 3164S, 1:200) and Rabbit anti‐Olig2 (Millipore, AB9610, 1:200) at 4°C overnight. After thorough washing, the sections were stained with secondary antibody conjugated to Alexa Fluor 488 or Alexa Fluor 555 (Thermo Fisher, 1:1,000), and nuclei were stained with Hoechst 33342. Images were taken using an Olympus IX71 inverted fluorescent microscope, and quantitative image analysis was performed using ImagePro.
2.8. Electron microscopy
Brains were isolated from 4% PFA perfused mice, corpus callosum were isolated and fixed in PBS containing 2.5% glutaraldehyde for 2 hr. Then the corpus callosum were washed, fixed in 1% osmium tetroxide, dehydrated in acetone, and embedded in EPON. A 70‐nm thin sagittal sections were cut with a diamond knife and mounted on copper slot grids coated with Formvar and stained with uranyl acetate and lead citrate for examination on JEM‐1230 transmission electron microscope. G‐ratios were measured in ImagePro, ∼200 remyelinated axons were measure for each group.
2.9. Reverse transcription and PCR
Total RNA was extracted with Trizol (Invitrogen). About 1 μg RNA was used to synthesize cDNA using the PrimeScript RT Reagent Kit (Takara Bio) according to the manufacturer's protocol. To detect SVCTs, PCR was performed using diluted reverse transcription products, 30 cycles were used for SVCT1, SVCT2, and GAPDH transcripts amplification. All products of RT‐PCR were analyzed by 1.5% agarose gel electrophoresis. The primer pairs used are as follows: SVCT1 sense, 5′‐AAAGCAGCATGAGGTCGTGG‐3′, antisense, 5′‐ACTGAAGCACGTCAGGTAATG‐3′; SVCT2 sense, 5′‐TGGACGGCATACAAGTTCCAG‐3′, antisense, 5′‐GAAGACATCAGTCACCGTGAAG‐3′; GAPDH sense, 5′‐AGGTCGGTGTGAACGGATTTG‐3′, antisense, 5′‐TGTAGACCATGTAGTTGAGGTCA‐3′.
2.10. Western blot
NPC‐derived OPCs and differentiated OLs were lysed and boiled at 95–100°C for 10 min in sample buffer [50 mM Tris‐HCl, 2% w/v SDS, 10% glycerol, 1% β‐mercaptoethanol, 0.01% bromophenyl blue (pH 6.8)]. The samples were separated on SDS‐PAGE and transferred onto PVDF (polyvinylidene difluoride, Millipore) membranes. The membranes were first incubated with blocking buffer (TBS with 0.05% Tween 20, 5% nonfat milk) for 1 hr at room temperature and then incubated with rabbit anti‐GAPDH (CST, 1:10,000) and goat anti‐SVCT2 (Santa Cruz, sc‐9926) overnight at 4°C. After thorough washing, the membranes were incubated with anti‐rabbit IgG HRP (CST, 1:10,000) or anti‐goat IgG HRP (CST, 1:10,000) for 1 hr. After washing, immunostaining was visualized using Amersham ECL Plus Western Blotting detection reagents (GE Healthcare).
2.11. Intracellular Vc measurements
As‐2P (150 μM), Phloretin (50 μM) or the combination of both were added from the day of OPC differentiation and maintained for 48 hr. Cells were then dissociated with accutase and collected by centrifugation. After washing, 5 × 105 cells in 20 μL water were lysed by freezing and thawing for several times to ensure complete cell lysis. The intracellular Vc level was measured by UPLC/Q‐TOF MS (Waters UPLC system and Waters Synapt G2 Q‐TOF high‐resolution mass spectrometer). l‐ascorbic acid (Vc, Sigma) was used as standard sample.
2.12. Intracellular ROS measurements
ROS were detected with 2,7‐dichlorofluorescein diacetate (DCFH‐DA, Beyotime) according to the manufacturers’ manual of a Reactive Oxygen Species Assay Kit (Beyotime). As‐2P and the other antioxidants were purchased from Sigma. The working concentrations of the antioxidants were: As‐2P, 150 μM; Vitamin B1, 30 μM; gluthatione (GSH), 5 μM; n‐acetylcysteine, 1 mM; resveratrol, 10 μM; Vitamin E, 30 μM; l‐carnitine hydrochloride, 100 μM. Antioxidants including As‐2P were added from the day of OPC differentiation and maintained for 48 hr.
2.13. Statistical analysis
Data were analyzed with GraphPad Prism software. For comparison between two groups, statistical evaluation was done by two‐tailed Student's t test. For multiple comparisons, one‐way ANOVA test was used. For all statistical tests, the p values <.05 were considered statistically significant. All error bars show standard error of the mean (SEM).
3. RESULTS
3.1. High‐throughput screening of compounds that promote OPC to OL differentiation
Although much knowledge is available for the isolation of primary OPCs from the CNS of rodents, it is still difficult to obtain sufficient quantity of these cells to screen thousands of compounds (Chen et al., 2007). OPCs used for chemical screen were generated from cortical NPCs, which can be expended in vitro for several passages (Figure 1a). NPCs proliferate as clonal aggregates termed “neurospheres” in NPC medium supplemented with EGF and bFGF, and express nestin (Figure 1b). NPCs can be induced to differentiate into NG2+ OPCs by culturing in OPC medium containing bFGF and PDGF‐AA for 2 days (Figure 1b). These OPCs can be further differentiated into MBP+ mature OLs in OL medium with no growth factors after 4–5 days (Figure 1b).
Figure 1.
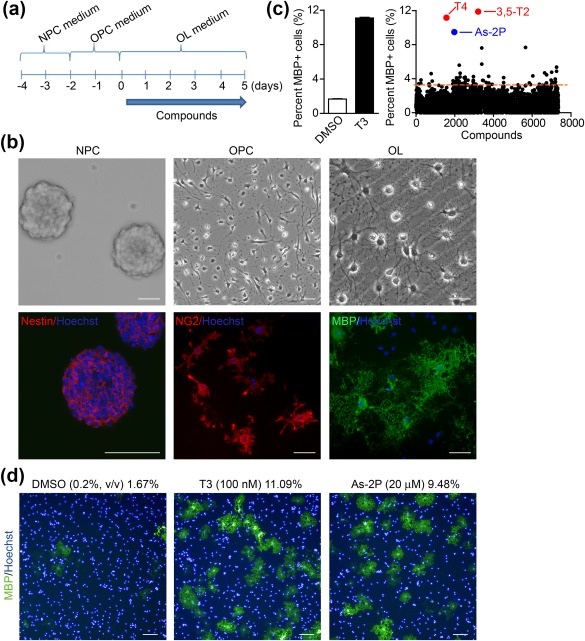
A NPC‐based phenotypic screening platform to discover inducers of OL differentiation and maturation. (a) Steps of OL differentiation from neurospheres generated from cortical NPCs of mouse E14.5 embryos: Neurosphere formation (NPC medium), OPC differentiation (OPC medium), and OL differentiation and maturation (OL medium). (b) Phase contrast (Top) and immunofluorescent staining of specific markers (Bottom) of NPCs (Nestin), OPCs (NG2), and OLs (MBP). Nuclei were stained with Hoechst. Scale bars, 40 μm. (c) The negative (DMSO) and positive (T3, 100 nM) controls of the high‐throughput screening system (Left). Scatter plot of the primary screen results of 7347 compounds (Right). The dotted line indicates two‐fold increase in the percentage of MBP+ cells comparing to DMSO. (d) Representative images of MBP+ cells in DMSO, T3, and As‐2P‐treated wells. Nuclei were stained with Hoechst. Scale bars, 100 μm [Color figure can be viewed at http://wileyonlinelibrary.com]
A 96‐well‐plate‐based chemical screening system was then established to identify inducers of OPC differentiation using mouse NPCs‐derived OPCs, and the percent of MBP+ cells were used as a readout. For in vitro screening, OPCs were treated with 0.2% (v/v) DMSO (negative control), thyroid hormone T3 (a known OPC differentiation inducer (Billon, Tokumoto, Forrest, & Raff, 2001), positive control), or compounds at a concentration of 20 μM. In DMSO‐treated group, 1.67% ± 0.03% of the cells became MBP+, and in T3‐treated group, 11.09% ± 0.07% of the cells turned MBP+ (Figure 1c). 7347 compounds from the Chinese National Compound Library were screened and 76 compounds were found to induce a two‐fold increase in the percentage of MBP+ cells comparing to DMSO (Figure 1c). Among them, a number of compounds or their targets have been previously reported to facilitate OPC to OL differentiation, including BRL‐52537 (selective κ agonist, 3.4%) (Du et al., 2016; Mei et al., 2016), 3,5‐diiodo‐l‐thyronine (thyroid hormone 3,5‐T2, 11.88%), thyroxine (thyroid hormone T4, 11.16%) (Billon et al., 2001), telenzepine hydrochloride (M1 muscarinic antagonist, 3.95%) (Deshmukh et al., 2013), clobetasol (glucocorticoid, 3.53%) (Najm et al., 2015) and donepezil (cholinesterase inhibitor, 3.82%) (Imamura et al., 2015), indicating that the assay is robust. It was very interesting to notice that a stable form of Vitamin C (Vc), sodium l‐ascorbyl‐2‐phosphate (As‐2P), also significantly increased the number of MBP+‐cells (Figure 1c,d).
3.2. As‐2P promotes OL differentiation and maturation in vitro
A careful dose‐response experiment was carried out. As‐2P's effect in inducing OPC to OL differentiation reached a plateau at a concentration of 150 μM (Figure 2a). The time‐dependent effect of As‐2P was also studied by adding 150 μM of As‐2P into the OL medium for various durations. The results indicate that the longer the treatment time, the better the effect (Figure 2b). In As‐2P (150 μM) treated OPCs, more than 20% of the cells became MBP+ at day 4. The induction efficiency was even higher than the known OL inducer T3 (100 nM) (Figure 2c,d). More interestingly, As‐2P and T3 displayed an additive effect in inducing the generation of MBP+‐OLs (Figure 2c,d), suggesting that they might act through different mechanisms. To evaluate whether As‐2P (150 μM) affect cell apoptosis or proliferation, TUNEL assay and Ki67 staining were carried out at day 4. As‐2P did not change the percentage of TUNEL+ cells while slightly increased the percentage of Ki67+ cells (Figure 2e–h). However, the slight (∼20%) increase in Ki67+ proliferating cells in the As‐2P group could not account for the robust appearance of OLs (∼10 times increase comparing to the control, Figure 2a), indicating As‐2P did promote the OPC to OL differentiation.
Figure 2.
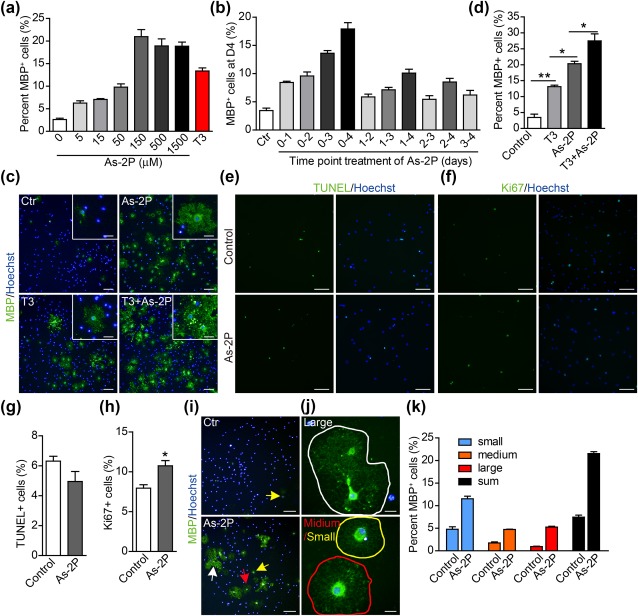
As‐2P promotes OL differentiation and maturation. (a) Dose‐dependent effect of As‐2P in inducing OPC differentiation into mature OLs. OPCs were treated with As‐2P for 4 days. (b) Time‐dependent effect of As‐2P (150 μM) in inducing OPC differentiation into mature OLs. (c) Mouse NPC‐derived OPCs were differentiated in the presence of As‐2P (150 μM), T3 (100 nM) or the combination of both for 4 days. OLs were stained with antibody against MBP (green). Nuclei were stained with Hoechst (blue). Scale bars, 100 μm; inset 25 μm. (d) Statistical analysis of the MBP+ cells in (c). Data are means ± SEM of three independent experiments. *p < .05, **p < .01 (one‐way ANOVA followed by Tukey's multiple comparison test). (e, f) Mouse NPC‐derived OPCs were differentiated in the presence of As‐2P (150 μM) or not for 4 days, cells were subjected to TUNEL (e) and Ki67 (f) staining. (g) Quantification of the TUNEL+ cells in (e). (h) Quantification of the Ki67+ cells in (f). Data are representative of three independent experiments, means ± SEM (n = 3). *p < .05 (Student's t test). (i, j) Typical morphology of large (>1,000 μm2, white arrow and circle), medium (500–1,000 μm2, red arrow and circle) and small (250–500 μm2, yellow arrow and circle) size OLs. The cells were stained with antibody against MBP (green). Nuclei were stained with Hoechst (blue). Scale bars in (i), 100 μm. Scale bars in J, 20 μm. (k) Statistical analysis of the MBP+ cells in (i) [Color figure can be viewed at http://wileyonlinelibrary.com]
The mature OLs typically possess complicated membrane processes and are larger in size than the immature OLs. As‐2P treatment not only increased the number of MBP+ cells, but also accelerated the maturation of OLs. At day 4, a significant part of the MBP+‐OLs showed membrane processes and were large in diameter in As‐2P treated wells, while the MBP+‐OLs in the control well are still very small (Figure 2i–k). As demonstrated in Figure 2b, As‐2P displayed beneficial effect at all stages of the differentiation process, and prolonged As‐2P treatment achieved its full potential. So the gradual expression of markers representing various maturating stages of OLs were studied in the presence of As‐2P. O4, a marker typically turned on at early stage of OPC to OL differentiation (Barateiro & Fernandes, 2014; Pfeiffer et al., 1993), were significantly increased at day 2 and 3 in As‐2P treatment groups (Figure 3a,d,e). And the membrane processes of O4+‐cells in the As‐2P‐treated groups were more abundant than the control groups (Figure 3a,e). Similarly, CNPase, a marker expressed slightly later than O4 during OL maturation (Zhang, 2001), was also significantly turned on by As‐2P treatment. CNPase+‐cells with clear membrane processes could be observed as early as day 2 in As‐2P‐treated cells, while in the control groups, such cells could only be observed at day 4 (Figure 3b,f). Again, the marker of mature OLs, MBP, was also turned on much earlier by the As‐2P treatment. Large diameter MBP+‐OLs could be observed as early as day 3, while in control groups, the mature OLs were typically seen in day 5 (Figure 3c,g). These data indicate that As‐2P promotes OL differentiation and maturation.
Figure 3.
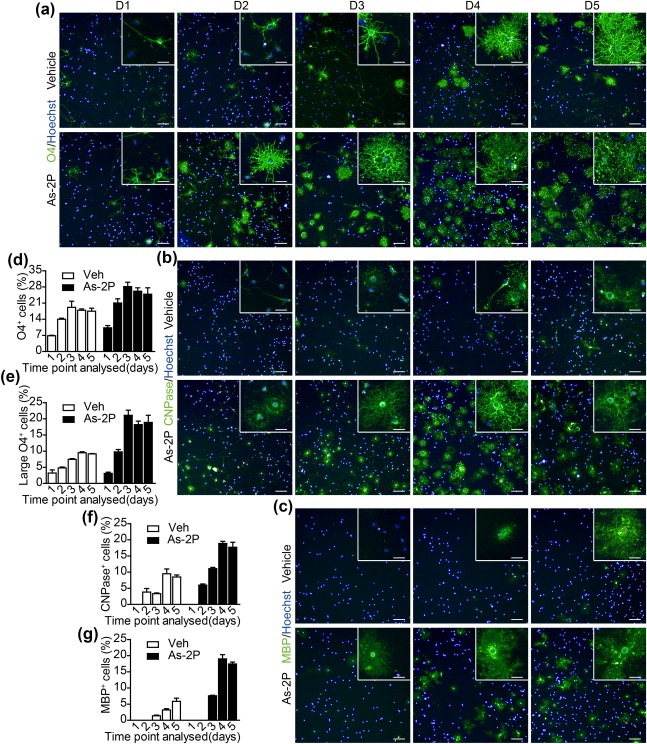
As‐2P promotes gradual expression of OL lineage markers. (a–c) NPC‐derived OPCs were differentiated in the presence of As‐2P (150 μM) or vehicle for 1–5 days. Cells were stained with antibodies against O4 (a), CNPase (b), and MBP (c). Typical morphologies of the cells were presented. Scale bars, 100 μm; inset 25 μm. (d, e) Statistical analysis of total (d) and large (>1000 μm2, e) O4+ cells presented in (a). (f, g) Statistical analysis of CNPase+ (f) and MBP+ (g) cells presented in (b and c) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. As‐2P enhances myelination in OPC‐neuron coculture in vitro
As‐2P's effect in promoting OPC to OL differentiation was also studied in primary OPCs. Mouse primary OPCs displayed a better differentiation potential comparing to the NPC‐derived OPCs without treatment (Figure 4a,4b vs. Figure 2c,d, vehicle control). However, As‐2P could still significantly increase the percentage of MBP+‐OLs generated from the primary OPCs (Figure 4a,b), and the best effect was also observed at 150 μM concentration. To evaluate the effect of As‐2P on myelin formation, an in vitro myelination system was set up by co‐culturing primary OPCs with dorsal root ganglion (DRG) neurons (O'Meara, Ryan, Colognato, & Kothary, 2011). The effect of AS‐2P in promoting myelination in the co‐culture was examined at day 6 and 14. Myelinated axons should be positive for both the OL marker MBP and the axon marker NF‐200 (200 kD neurofilament). The results showed a significant increase in the length of the myelinated axons in As‐2P‐treated co‐cultures at both day 6 and 14 (Figure 4c–f). Caspr (Contactin‐associated protein), which is clustered upon OL contact and ensheathment (Eisenbach et al., 2009; Pedraza, Huang, & Colman, 2009; Yang et al., 2016), was also studied at day 14 in the co‐culture. As demonstrated in Figure 4g,h, the axonal clustering of Caspr was significantly higher in As‐2P‐treated co‐cultures. These results suggest that As‐2P not only promote the generation of mature OLs from OPCs, but also promote the formation of myelin.
Figure 4.
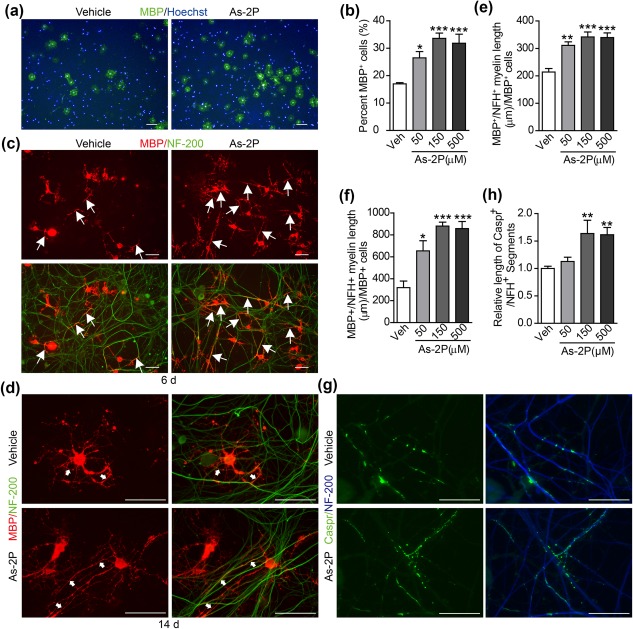
As‐2P enhances myelination in vitro. (a) Representative images of mouse primary NG2+‐OPCs treated with vehicle or As‐2P for 4 days and stained for MBP (green) and nuclei (Hoechst, blue). Scale bars, 100 μm. (b) Statistical analysis of the MBP+ cells after a 4‐day differentiation of the primary NG2+‐OPCs in the presence of various concentrations of As‐2P. Data are representative of three independent experiments, means ± SEM (n = 4). (c, d) Primary OPCs were co‐cultured with DRG‐neurons and treated with vehicle or As‐2P for 6 (c) or 14 (d) days. The cells were then immunostained for NF‐200 (neurofilament, green) and MBP (oligodendrocytes, red). Arrows indicate myelinated axons (double positive for NF‐200 and MBP). Scale bars, 40 μm. (e, f) Statistical analysis of myelinated axons in OPC‐neuron co‐cultures in the presence of various concentrations of As‐2P for 6 (c) or 14 (d) days. Data are representative of three independent experiments, means ± SEM (n=3). (g) Primary OPCs were co‐cultured with DRG‐neurons and treated with vehicle or As‐2P for 14 days and immunostained for Caspr (green) and NF‐200 (blue). Scale bars, 20 μm. (h) Statistical analysis of Caspr+ segments in OPC‐neuron cocultures in the presence of various concentrations of As‐2P for 14 days. Data are means ± SEM (n = 3). *p < .05, **p < .01, ***p < .001 versus vehicle control (one‐way ANOVA followed by Dunnett's multiple comparison test) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. As‐2P promotes remyelination in vivo
The ability of As‐2P to enhance remyelination in vivo was evaluated using a cuprizone‐induced demyelination model(Matsushima & Morell, 2001). The mice were given a diet containing 0.2% (w/w) cuprizone for 5 weeks to achieve complete demyelination at the corpus callosum region of the brain as demonstrated by Luxol fast blue staining (Figure 5a,b). Upon withdrawal of cuprizone, the mice were administered vehicle or As‐2P (200 mg kg−1) for 1–3 weeks (Figure 5a). Spontaneous remyelination could be observed in the vehicle‐treated group, and As‐2P treatment further enhanced the remyelination process (Figure 5b,c). The degree of remyelination was further evaluated by immunofluorescent staining of MBP, MOG and GST‐pi in the corpus callosum region after 3‐week treatment with As‐2P. Consistent with Luxol fast blue staining (Figure 5b), As‐2P treatment significantly increased the fluorescent intensity of MBP‐ and MOG‐staining, and the number of GST‐pi+ mature OLs (Figure 5d–g). The numbers of OPCs and OL‐lineage cells in the same region were also examined by immunofluorescent staining of PDGFRα and OLIG2, respectively. As‐2P treatment for 3 weeks led to a significant decrease of the PDGFRα+ OPCs and a significant increase of the Olig2+ OL‐lineage cells in corpus callosum of the cuprizone‐fed mice (Figure 5h–j).
Figure 5.
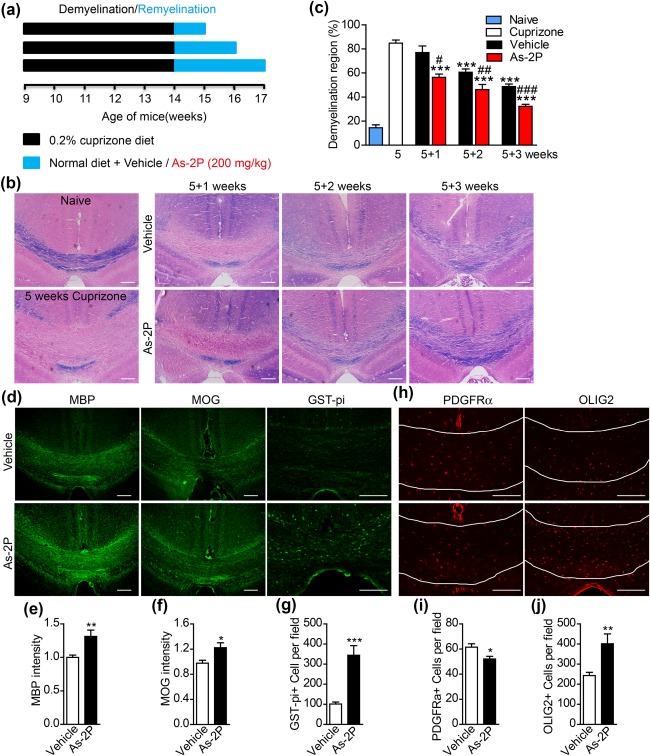
As‐2P promotes remyelination in vivo in cuprizone‐induced demyelination model. (a) A schematic drawing of the demyelination/remyelination mice model. C57BL/6 mice were given a diet containing 0.2% cuprizone 5 weeks. Following cuprizone withdrawal, the mice were treated with vehicle or As‐2P (200 mg kg−1) for 1, 2, or 3 weeks (wks). (b) Representative images of the corpus callosum region stained with Luxol fast blue after cuprizone and As‐2P treatment. Scale bars, 100 μm. (c) Statistical analysis of the demyelinating areas in (b). Data are means ± SEM (four mice in cuprizone group, three mice in 5 + 1 groups, seven mice in 5 + 2 groups, and ten mice in 5 + 3 groups; four sections from each mouse were analyzed). ***p < .001 versus cuprizone group, # p < .05, ## p < .01, ### p < .001 versus vehicle group (one‐way ANOVA followed by Tukey's multiple comparison test). (d) Immunofluorescent images of MBP, MOG, and GST‐pi staining in the corpus callosum region isolated from cuprizone‐fed mice treated with As‐2P or vehicle for 3 weeks. Scale bars, 100 μm. (e–g) Quantification of the fluorescent intensity of MBP, MOG and the number of GST‐pi+ cells in corpus callosum as presented in (d). Data are means ± SEM (three mice in each group; six sections from each mouse were analyzed). *p < .05, **p < .01, ***p < .001 versus vehicle group (Student's t test). (h) Immunofluorescent images of PDGFRα and OLIG2 staining in the corpus callosum region isolated from cuprizone‐fed mice treated with As‐2P or vehicle for 3 weeks. Scale bars, 100 μm. (i, j) Quantification of the number of PDGFRα+ and OLIG2+ cells in corpus callosum as presented in (h). Data are means ± SEM (three mice in each group; six sections from each mouse were analyzed). *p < .05, **p < .01 versus vehicle group (Student's t test) [Color figure can be viewed at http://wileyonlinelibrary.com]
The myelin status was further evaluated with electron microscopy. After 5 weeks of cuprizone treatment, most of the axons lost myelin sheath. Only very few axons still had loosely wrapped myelin (red asterisk, Figure 6a,b). Three weeks after cuprizone withdrawal, the vehicle group showed significant spontaneous remyelination, and the As‐2P treated animals had more myelinated exons (Figure 6a,b). Compared with the highly compact and tightly wrapped myelin in the naïve animals, the newly formed myelins were less tightly wrapped and typical structures such as the ends of the spiraling cytoplasmic processes of OLs could be observed (red arrows) (Deshmukh et al., 2013; Mi et al., 2007). G‐ratios of the remyelinated axons were evaluated. As‐2P not only increased the number of myelinated axons (Figure 6b), but also reduced the G‐ratio of the remyelinated axons (Figure 6c), indicating a better recovery.
Figure 6.
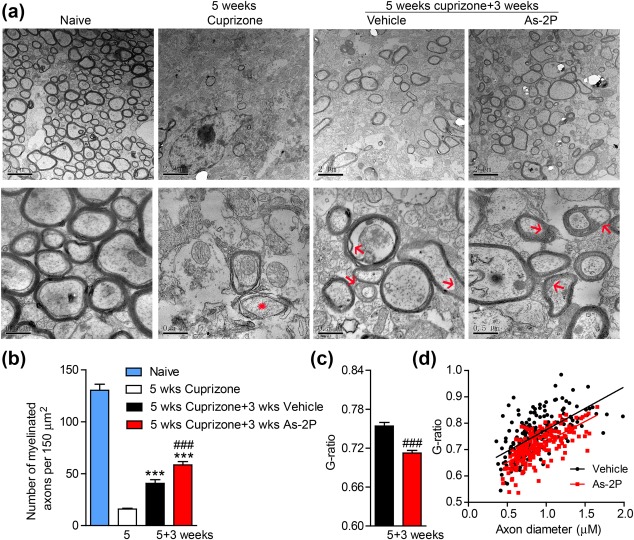
Evaluation of remyelination in cuprizone model with electron microscopy. (a) Representative electron microscopy images of the corpus callosum region isolated from cuprizone‐fed mice treated with As‐2P (200 mg kg−1) or vehicle for 3 weeks. Red asterisk indicates the demyelinating axon and the red arrows indicate the remyelinated axons. (b) Quantification of the myelinated axons from (a). Data are means ± SEM (three mice from each group, six sections from each mouse were analyzed). ***p < .001 versus cuprizone group, ### p < .001 versus vehicle group (one‐way ANOVA followed by Tukey's multiple comparison test). (c) Quantification of the G ratios of the remyelinated axons in (a). Data are means ± SEM (n = 200, ∼70 axons counted per mouse, three mice per group), ### p < .001 versus vehicle group (Student's t test). (d) The scatter plot displaying the individual G‐ratio values and axonal size distribution [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5. As‐2P enhances OPC differentiation independent of its antioxidant property
As‐2P (L‐ascorbyl‐2‐phosphate) is a stable form of Vc (l‐ascorbic acid, L‐AA), it does not oxidize in the media as does L‐AA (Du, Cullen, & Buettner, 2012). Thus, the use of As‐2P is preferred when studying the functions of Vc in cell culture. Both L‐AA and As‐2P were tested in the OPC differentiation assay, and both were found to significantly promote OPC to OL differentiation (Figure 7a,b). As‐2P displayed slightly better effect than L‐AA, which was consistent with its stability in solution. As‐2P may exert its effect within the cells or by modifying the extracellular environment (Fukui et al., 2017). As‐2P can be taken up by cells through sodium‐dependent Vc transporters (SVCTs) after modification by cell membrane esterases (Du et al., 2012). The SVCTs are crucial to maintain much higher intracellular concentrations of Vc than in the extracellular fluids in the brain (Sotiriou et al., 2002; Tsukaguchi et al., 1999). PCR analysis demonstrated the expression of Svct2, but not Svct1, in both OPCs and OLs (Figure 7c). Western blot analysis also confirmed the presence of SVCT2 protein in both OPCs and differentiated OLs (Figure 7d). SVCT2 inhibitor phloretin (PT, 50 μM) (Tsukaguchi et al., 1999) significantly reduced As‐2P's effect in inducing OPC to OL differentiation (Figure 7e,f). To confirm that PT actually inhibited the SVCT2 in OPCs, OPCs were cultured in the presence of As‐2P, PT or the combination of both for 48 h and the intracellular L‐AA were measured by UPLC/Q‐TOF MS. PT significantly reduced the intracellular concentration of L‐AA, indicating the effective blockage of SVCT2 (Figure 7g). These results indicate a possible intracellular action of As‐2P in inducing OPC differentiation. Vc is a well‐known antioxidant. Therefor a number of reported antioxidants, including Vitamin B1 (Vb1), glutathione (GSH), resveratrol, Vitamin E (Ve), n‐acetylcysteine (N‐ac) and L‐carnitine, were also tested in the OPC differentiation assay at commonly applied concentrations. Very interestingly, none of these antioxidants displayed significant effect in promoting OPC differentiation (Figure 7h,i), although many of them showed similar effect in reducing the steady‐state ROS level in OPCs (Figure 7j). These results indicate that As‐2P's effect in promoting OPC to OL differentiation might be independent of its antioxidant properties.
Figure 7.
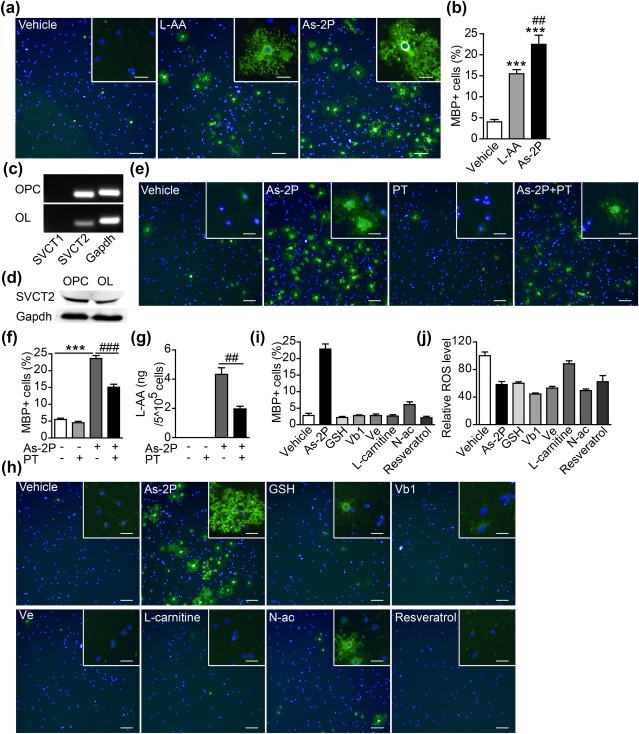
As‐2P's effect in inducing OL differentiation is independent of its antioxidant property. (a) Effects of two different forms of Vc, l‐ascorbic acid (L‐AA, 150 μM) and As‐2P (150 μM), in promoting the differentiation of OLs. Cells were stained with antibody against MBP (green). Nuclei were stained with Hoechst (blue). Scale bars, 100 μm; inset 25 μm. (b) Statistical analysis of MBP+ cells in (a); Data are representative of three independent experiments, means ± SEM (n = 4) ***p < .001 versus vehicle control, ## p < .01, versus L‐AA (one‐way ANOVA followed by Tukey's multiple comparison test). (c) PCR analysis of the mRNA level of Svct1 and Svct2 in NPC‐derived OPCs and differentiated OLs. (d) Western blot analysis showing the protein expression of Svct2 in NPC‐derived OPCs and differentiated OLs. (e) The effect of SVCT2 inhibitor phloretin (PT, 50 μM) on As‐2P‐stimulated generation of MBP+ OLs. Cells were stained with antibody against MBP (green). Nuclei were stained with Hoechst (blue). Scale bars, 100 μm; inset 25 μm. (f) Quantification of MBP+ cells in (e). Data are representative of three independent experiments, means ± SEM (n = 4) ***p < .001 versus control, ### p < .001, versus As‐2P (Student's t test). (g) OPCs were cultured in the presence of As‐2P (150 μM), PT (50 μM) or the combination of both for 2 days and the intracellular concentrations of L‐AA were measured by UPLC/Q‐TOF MS. Data are representative of three independent experiments, means ± SEM (n = 4), ## p < .01, versus As‐2P (Student's t test). (h) NPC‐derived OPCs were differentiated in the presence of various anti‐oxidants for 4 days. Cells were stained with antibodies against MBP (green). (i) Quantification of MBP+ cells in (h). (j) ROS measurement at day 2 post differentiation of OPCs treated as indicated [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
Vc is a common nutrient vital to human health. Its earliest medical use was to prevent and treat scurvy. Later studies have demonstrated that Vc is crucial for the development and maintenance of connective tissues, bones, healthy gums and also important for wound healing. Vc involves in a number of metabolic processes including the conversion of cholesterol to bile acids and the synthesis of catecholamine, tyrosine and peptide hormones (Chambial, Dwivedi, Shukla, John, & Sharma, 2013). It has been used as an alternative treatment in diverse diseases such as common cold, atherosclerosis, diabetes, metal intoxication, cancer, and neurodegenerative diseases (Chambial et al., 2013). Recent studies have showed that Vc could also promote somatic cell reprogramming and facilitate the generation of high quality induced pluripotent stem cells (Chen et al., 2013; Esteban et al., 2010). In this study, we demonstrated that Vc could also enhance the differentiation of OPCs to OLs and promote myelin formation both in vitro and in vivo.
Vc exerts its effects mostly as an antioxidant or as an enzyme cofactor (Du et al., 2012). Vc scavenges reactive oxygen species (ROS) to protect lipid membranes and proteins from free radical damage. However, our study showed that As‐2P's function in promoting OPC differentiation is not related to its antioxidant activity. Vc is a cofactor of the Fe2+‐and 2‐oxoglutarate‐dependent family of dioxygenases, including the well‐known collagen prolyl hydroxylases, which is important for proper assembly of collagens, and HIF (hypoxia‐inducible factor) prolyl hydroxylases, which plays important role in the hydroxylation and later ubiquitination and proteasomal degradation of HIF‐α (Du et al., 2012). Vc is thought to be important in Schwann cell‐based myelination of the DRG neurons because collagen‐ and laminin‐containing basal lamina of the Schwann cell is required in this process (Eldridge, Bunge, & Bunge, 1989; Eldridge, Bunge, Bunge, & Wood, 1987). Vc also plays a major role in peripheral nerve myelination in vivo, and has beneficial effects in the treatment of a common peripheral neuropathy model, the Charcot‐Marie‐Tooth neuropathy 1A mouse model (Passage et al., 2004). However, there is no clear evidence of the importance of Vc in OL‐mediated CNS myelination because OLs do not have a basal lamina (Colognato & Tzvetanova, 2011). Our study also demonstrated the Vc's effect in promoting OPC differentiation could be blocked by inhibition of the SVCT2 Vc transporter, indicating an intracellular mechanism rather than an extracellular one. Vc is also a cofactor for HIF‐prolyl hydroxylases (PHD1–3) and inhibits HIF signaling. There are studies demonstrating the existence of a crosstalk between the Wnt/β‐catenin and HIF pathways (Bogaerts, Heindryckx, Vandewynckel, Van Grunsven, & Van Vlierberghe, 2014). Canonical Wnt signaling functions as a potent inhibitor of OL maturation (Fancy et al., 2009, 2011). Activation of HIF pathway activates canonical Wnt signaling, which might in turn inhibits OPC differentiation and myelination. However, more careful investigation is needed to answer whether Vc promotes OPC to OL differentiation via HIF pathway inhibition.
Recently, Vc was discovered as a cofactor of the Jumonji family histone demethylases and Tet (ten‐eleven‐translocation, 1–3) family DNA demethylases, which also belong to the Fe2+‐ and 2‐oxoglutarate‐dependent dioxygenase family (Liu, Moyon, Hernandez, & Casaccia, 2016; Minor, Court, Young, & Wang, 2013; Tsukada et al., 2006). OPCs can be regulated by DNA‐ and histone‐modifying enzymes, allowing transcriptional activation or repression of genes regulating lineage specification(Liu et al., 2016). The Tet proteins catalyze the conversion of 5‐methylcytosine (5mC) into 5‐hydroxymethylcytosine (5hmC) and induce DNA demethylation (Ito et al., 2010). Tet proteins and 5hmC are abundant in mouse brain, and 5hmC level increases during neuronal differentiation in development (Kriaucionis & Heintz, 2009; Szulwach et al., 2011). Tet1 is involved in neural progenitor cell proliferation, learning and memory processes (Rudenko et al., 2013; Zhang et al., 2013). Tet proteins have been found to present a temporal expression during OL development and are necessary for OL differentiation in vitro (Zhao et al., 2014). The level of 5hmC also exhibits dynamic changes during OL maturation (Zhao et al., 2014). Another recent study combining transcriptomic analysis with genome‐wide RNA‐sequencing revealed genome‐wide differences in DNA methylation in pathology‐free regions of the brain derived from MS patients and control subjects. Genes regulating OL survival were hypermethylated and expressed in lower level, whereas genes related to proteolytic processing were hypomethylated and upregulated in expression (Huynh et al., 2014). It is very possible that Vc might influence OPC differentiation and myelination by regulating the activity of these epigenetic regulating enzymes.
In summary, we discovered that Vc greatly promotes OPC differentiation and maturation. It also promotes myelin formation both in vitro and in vivo, although the exact mechanism remains to be elucidated. Because Vc is a widely consumed dietary supplement and has also been approved by FDA for clinical uses, it might offer a therapeutic possibility for CNS demyelinating diseases.
AUTHOR CONTRIBUTION
Y.G. conducted most of the experiments, analyzed the results, and wrote the article. N.S. performed the ROS measurements and part of the animal studies. X.C. screened the compounds. Q.Y. provided technical assistance with animal studies. X.X. conceived the idea for the project, analyzed the results, and wrote the article. All authors reviewed the results and approved the manuscript.
ACKNOWLEDGMENT
This work was supported by grants from the Ministry of Science and Technology of China (2015CB964503, 2017YFA0104002), the Chinese Academy of Sciences (XDA16010202), and the National Natural Science Foundation of China (81425024, 81472862 and 31501189).
Guo Y‐, Suo N, Cui X, Yuan Q, Xie X. Vitamin C promotes oligodendrocytes generation and remyelination. Glia. 2018;66:1302–1316. https://doi.org/10.1002/glia.23306
Funding information Ministry of Science and Technology of China, Grant Number: 2015CB964503, 2017YFA0104002; the Chinese Academy of Sciences, Grant Number: XDA16010202; National Natural Science Foundation of China, Grant Number: 81425024, 81472862, 31501189
REFERENCES
- Barateiro, A. , & Fernandes, A. (2014). Temporal oligodendrocyte lineage progression: In vitro models of proliferation, differentiation and myelination. Biochimica Biophysica Acta, 1843(9), 1917–1929. https://doi.org/10.1016/j.bbamcr.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Baumann, N. , & Pham‐Dinh, D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological Reviews, 81(2), 871–927. [DOI] [PubMed] [Google Scholar]
- Bergles, D. E. , & Richardson, W. D. (2015). Oligodendrocyte development and plasticity. Cold Spring Harbour Perspective Biology, 8(2), a020453. https://doi.org/10.1101/cshperspect.a020453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon, N. , Tokumoto, Y. , Forrest, D. , & Raff, M. (2001). Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Developmental Biology, 235(1), 110–120. https://doi.org/10.1006/dbio.2001.0293 [DOI] [PubMed] [Google Scholar]
- Bogaerts, E. , Heindryckx, F. , Vandewynckel, Y. P. , Van Grunsven, L. A. , & Van Vlierberghe, H. (2014). The roles of transforming growth factor‐beta, Wnt, Notch and hypoxia on liver progenitor cells in primary liver tumours (Review). International Journal of Oncology, 44(4), 1015–1022. https://doi.org/10.3892/ijo.2014.2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambial, S. , Dwivedi, S. , Shukla, K. K. , John, P. J. , & Sharma, P. (2013). Vitamin C in disease prevention and cure: An overview. Indian Journal of Clinical Biochemistry, 28(4), 314–328. https://doi.org/10.1007/s12291-013-0375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. R. , Watkins, T. A. , Cosgaya, J. M. , Zhang, C. , Chen, L. , Reichardt, L. F. , … Barres, B. A. (2004). NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron, 43(2), 183–191. https://doi.org/10.1016/j.neuron.2004.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Guo, L. , Zhang, L. , Wu, H. , Yang, J. , Liu, H. , … Pei, D. (2013). Vitamin C modulates TET1 function during somatic cell reprogramming. Nature Genetics, 45(12), 1504–1509. https://doi.org/10.1038/ng.2807 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Balasubramaniyan, V. , Peng, J. , Hurlock, E. C. , Tallquist, M. , Li, J. , & Lu, Q. R. (2007). Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nature Protocols, 2(5), 1044–1051. https://doi.org/10.1038/nprot.2007.149 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Wu, H. , Wang, S. , Koito, H. , Li, J. , Ye, F. , … Lu, Q. R. (2009). The oligodendrocyte‐specific G protein‐coupled receptor GPR17 is a cell‐intrinsic timer of myelination. Nature Neuroscience, 12(11), 1398–1406. https://doi.org/10.1038/nn.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, K. L. H. , Early, J. J. , & Lyons, D. A. (2017). Drug discovery for remyelination and treatment of MS. Glia, 65(10), 1565–1589. https://doi.org/10.1002/glia.23166 [DOI] [PubMed] [Google Scholar]
- Colognato, H. , & Tzvetanova, I. D. (2011). Glia unglued: How signals from the extracellular matrix regulate the development of myelinating glia. Developmental Neurobiology, 71(11), 924–955. https://doi.org/10.1002/dneu.20966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, A. H. , Stockley, J. H. , Tripathi, R. B. , Richardson, W. D. , & Franklin, R. J. (2014). Oligodendrocyte progenitors: Adult stem cells of the central nervous system?. Experimental Neurology, 260, 50–55. https://doi.org/10.1016/j.expneurol.2014.04.027 [DOI] [PubMed] [Google Scholar]
- Deshmukh, V. A. , Tardif, V. , Lyssiotis, C. A. , Green, C. C. , Kerman, B. , Kim, H. J. , … Lairson, L. L. (2013). A regenerative approach to the treatment of multiple sclerosis. Nature, 502(7471), 327–332. https://doi.org/10.1038/nature12647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, C. , Duan, Y. , Wei, W. , Cai, Y. , Chai, H. , Lv, J. , … Xie, X. (2016). Kappa opioid receptor activation alleviates experimental autoimmune encephalomyelitis and promotes oligodendrocyte‐mediated remyelination. Nature Communications, 7, 11120 https://doi.org/10.1038/ncomms11120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, J. , Cullen, J. J. , & Buettner, G. R. (2012). Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochimica Biophysica Acta, 1826(2), 443–457. https://doi.org/10.1016/j.bbcan.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach, M. , Kartvelishvily, E. , Eshed‐Eisenbach, Y. , Watkins, T. , Sorensen, A. , Thomson, C. , … Peles, E. (2009). Differential clustering of Caspr by oligodendrocytes and Schwann cells. Journal of Neuroscience Research, 87(15), 3492–3501. https://doi.org/10.1002/jnr.22157 [DOI] [PubMed] [Google Scholar]
- Eldridge, C. F. , Bunge, M. B. , & Bunge, R. P. (1989). Differentiation of axon‐related Schwann cells in vitro: II. Control of myelin formation by basal lamina. Journal of Neuroscience, 9(2), 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge, C. F. , Bunge, M. B. , Bunge, R. P. , & Wood, P. M. (1987). Differentiation of axon‐related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. Journal of Cell Biology, 105(2), 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery, B. (2010). Regulation of oligodendrocyte differentiation and myelination. Science, 330(6005), 779–782. https://doi.org/10.1126/science.1190927 [DOI] [PubMed] [Google Scholar]
- Esteban, M. A. , Wang, T. , Qin, B. , Yang, J. , Qin, D. , Cai, J. , … Pei, D. (2010). Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell, 6(1), 71–79. https://doi.org/10.1016/j.stem.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Fancy, S. P. , Baranzini, S. E. , Zhao, C. , Yuk, D. I. , Irvine, K. A. , Kaing, S. , … Rowitch, D. H. (2009). Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes & Development, 23(13), 1571–1585. https://doi.org/10.1101/gad.1806309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy, S. P. , Harrington, E. P. , Yuen, T. J. , Silbereis, J. C. , Zhao, C. , Baranzini, S. E. , … Rowitch, D. H. (2011). Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nature Neuroscience, 14(8), 1009–1016. https://doi.org/10.1038/nn.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, R. J. (2002). Why does remyelination fail in multiple sclerosis?. Nature Reviews Neuroscience, 3(9), 705–714. https://doi.org/10.1038/nrn917 [DOI] [PubMed] [Google Scholar]
- Fukui, H. , Iwahashi, H. , Nishio, K. , Hagihara, Y. , Yoshida, Y. , & Horie, M. (2017). Ascorbic acid prevents zinc oxide nanoparticle‐induced intracellular oxidative stress and inflammatory responses. Toxicology & Industrial Health, 33(9), 687–695, https://doi.org/10.1177/0748233717707361 [DOI] [PubMed] [Google Scholar]
- Gensert, J. M. , & Goldman, J. E. (1997). Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron, 19(1), 197–203. [DOI] [PubMed] [Google Scholar]
- Goldman, S. A. , & Kuypers, N. J. (2015). How to make an oligodendrocyte. Development, 142(23), 3983–3995. https://doi.org/10.1242/dev.126409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, F. , Lang, J. , Sohn, J. , Hammond, E. , Chang, M. , & Pleasure, D. (2015). Canonical Wnt signaling in the oligodendroglial lineage–puzzles remain. Glia, 63(10), 1671–1693. https://doi.org/10.1002/glia.22813 [DOI] [PubMed] [Google Scholar]
- Haghikia, A. , Hohlfeld, R. , Gold, R. , & Fugger, L. (2013). Therapies for multiple sclerosis: Translational achievements and outstanding needs. Trends in Molecular Medicine, 19(5), 309–319. https://doi.org/10.1016/j.molmed.2013.03.004 [DOI] [PubMed] [Google Scholar]
- Huynh, J. L. , Garg, P. , Thin, T. H. , Yoo, S. , Dutta, R. , Trapp, B. D. , … Casaccia, P. (2014). Epigenome‐wide differences in pathology‐free regions of multiple sclerosis‐affected brains. Nature Neuroscience, 17(1), 121–130. https://doi.org/10.1038/nn.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, O. , Arai, M. , Dateki, M. , Ogata, T. , Uchida, R. , Tomoda, H. , & Takishima, K. (2015). Nicotinic acetylcholine receptors mediate donepezil‐induced oligodendrocyte differentiation. Journal of Neurochemistry, 135(6), 1086–1098. https://doi.org/10.1111/jnc.13294 [DOI] [PubMed] [Google Scholar]
- Ito, S. , D'Alessio, A. C. , Taranova, O. V. , Hong, K. , Sowers, L. C. , & Zhang, Y. (2010). Role of Tet proteins in 5mC to 5hmC conversion, ES‐cell self‐renewal and inner cell mass specification. Nature, 466(7310), 1129–1133. https://doi.org/10.1038/nature09303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaucionis, S. , & Heintz, N. (2009). The nuclear DNA base 5‐hydroxymethylcytosine is present in Purkinje neurons and the brain. Science, 324(5929), 929–930. https://doi.org/10.1126/science.1169786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Moyon, S. , Hernandez, M. , & Casaccia, P. (2016). Epigenetic control of oligodendrocyte development: Adding new players to old keepers. Current Opinions in Neurobiology, 39, 133–138. https://doi.org/10.1016/j.conb.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima, G. K. , & Morell, P. (2001). The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathology, 11(1), 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, F. , Fancy, S. P. J. , Shen, Y. A. , Niu, J. , Zhao, C. , Presley, B. , … Chan, J. R. (2014). Micropillar arrays as a high‐throughput screening platform for therapeutics in multiple sclerosis. Nature Medicine, 20(8), 954–960. https://doi.org/10.1038/nm.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, F. , Mayoral, S. R. , Nobuta, H. , Wang, F. , Desponts, C. , Lorrain, D. S. , … Chan, J. R. (2016). Identification of the kappa‐opioid receptor as a therapeutic target for oligodendrocyte remyelination. Journal of Neuroscience, 36(30), 7925–7935. https://doi.org/10.1523/JNEUROSCI.1493-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi, S. , Hu, B. , Hahm, K. , Luo, Y. , Kam Hui, E. S. , Yuan, Q. , … Wu, W. (2007). LINGO‐1 antagonist promotes spinal cord remyelination and axonal integrity in MOG‐induced experimental autoimmune encephalomyelitis. Nature Medicine, 13(10), 1228–1233. https://doi.org/10.1038/nm1664 [DOI] [PubMed] [Google Scholar]
- Minor, E. A. , Court, B. L. , Young, J. I. , & Wang, G. (2013). Ascorbate induces ten‐eleven translocation (Tet) methylcytosine dioxygenase‐mediated generation of 5‐hydroxymethylcytosine. Journal of Biological Chemistry, 288(19), 13669–13674. https://doi.org/10.1074/jbc.C113.464800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm, F. J. , Madhavan, M. , Zaremba, A. , Shick, E. , Karl, R. T. , Factor, D. C. , … Tesar, P. J. (2015). Drug‐based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature, 522(7555), 216–220. https://doi.org/10.1038/nature14335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, M. C. , Roy, N. S. , Keyoung, H. M. , Goodman, R. R. , McKhann, G., II , Jiang, L. , … Goldman, S. A. (2003). Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature Medicine, 9(4), 439–447. https://doi.org/10.1038/nm837 [DOI] [PubMed] [Google Scholar]
- O'Meara, R. W. , Ryan, S. D. , Colognato, H. , & Kothary, R. (2011). Derivation of enriched oligodendrocyte cultures and oligodendrocyte/neuron myelinating cocultures from post‐natal murine tissues. Journal of Visualized Experiments, 54(e3324), 1–9. https://doi.org/10.3791/3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. K. , Miller, R. , Krane, I. , & Vartanian, T. (2001). The erbB2 gene is required for the development of terminally differentiated spinal cord oligodendrocytes. Journal of Cell Biology, 154(6), 1245–1258. https://doi.org/10.1083/jcb.200104025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passage, E. , Norreel, J. C. , Noack‐Fraissignes, P. , Sanguedolce, V. , Pizant, J. , Thirion, X. , … Fontes, M. (2004). Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot‐Marie‐Tooth disease. Nature Medicine, 10(4), 396–401. https://doi.org/10.1038/nm1023 [DOI] [PubMed] [Google Scholar]
- Pedraza, L. , Huang, J. K. , & Colman, D. (2009). Disposition of axonal caspr with respect to glial cell membranes: Implications for the process of myelination. Journal of Neuroscience Research, 87(15), 3480–3491. https://doi.org/10.1002/jnr.22004 [DOI] [PubMed] [Google Scholar]
- Pfeiffer, S. E. , Warrington, A. E. , & Bansal, R. (1993). The oligodendrocyte and its many cellular processes. Trends in Cell Biology, 3(6), 191–197. https://doi.org/096289249390213K [pii] [DOI] [PubMed] [Google Scholar]
- Popescu, B. F. , & Lucchinetti, C. F. (2012). Pathology of demyelinating diseases. Annual Review of Pathology, 7, 185–217. https://doi.org/10.1146/annurev-pathol-011811-132443 [DOI] [PubMed] [Google Scholar]
- Rudenko, A. , Dawlaty, M. M. , Seo, J. , Cheng, A. W. , Meng, J. , Le, T. , … Tsai, L. H. (2013). Tet1 is critical for neuronal activity‐regulated gene expression and memory extinction. Neuron, 79(6), 1109–1122. https://doi.org/10.1016/j.neuron.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou, S. , Gispert, S. , Cheng, J. , Wang, Y. , Chen, A. , Hoogstraten‐Miller, S. , … Nussbaum, R. L. (2002). Ascorbic‐acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nature Medicine, 8(5), 514–517. https://doi.org/10.1038/nm0502-514 [DOI] [PubMed] [Google Scholar]
- Szulwach, K. E. , Li, X. , Li, Y. , Song, C. X. , Wu, H. , Dai, Q. , … Jin, P. (2011). 5‐hmC‐mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nature Neuroscience, 14(12), 1607–1616. https://doi.org/10.1038/nn.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp, B. D. , & Nave, K. A. (2008). Multiple sclerosis: An immune or neurodegenerative disorder? Annual Review of Neuroscience, 31, 247–269. https://doi.org/10.1146/annurev.neuro.30.051606.094313 [DOI] [PubMed] [Google Scholar]
- Tsukada, Y. , Fang, J. , Erdjument‐Bromage, H. , Warren, M. E. , Borchers, C. H. , Tempst, P. , & Zhang, Y. (2006). Histone demethylation by a family of JmjC domain‐containing proteins. Nature, 439(7078), 811–816. https://doi.org/10.1038/nature04433 [DOI] [PubMed] [Google Scholar]
- Tsukaguchi, H. , Tokui, T. , Mackenzie, B. , Berger, U. V. , Chen, X. Z. , Wang, Y. , … Hediger, M. A. (1999). A family of mammalian Na+‐dependent L‐ascorbic acid transporters. Nature, 399(6731), 70–75. https://doi.org/10.1038/19986 [DOI] [PubMed] [Google Scholar]
- van Tilborg, E. , de Theije, C. G. M. , van Hal, M. , Wagenaar, N. , de Vries, L. S. , Benders, M. J. , … Nijboer, C. H. (2018). Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia, 66(2), 221–238. https://doi.org/10.1002/glia.23256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. J. , Vainshtein, A. , Maik‐Rachline, G. , & Peles, E. (2016). G protein‐coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nature Communication, 7, 10884 https://doi.org/10.1038/ncomms10884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. R. , Cui, Q. Y. , Murai, K. , Lim, Y. C. , Smith, Z. D. , Jin, S. , … Xu, G. L. (2013). Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell, 13(2), 237–245. https://doi.org/10.1016/j.stem.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. C. (2001). Defining glial cells during CNS development. Nature Reviews Neuroscience, 2(11), 840–843. https://doi.org/10.1038/35097593 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Argaw, A. T. , Gurfein, B. T. , Zameer, A. , Snyder, B. J. , Ge, C. , … John, G. R. (2009). Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proceedings of the National Academy of Science of the United States of America, 106(45), 19162–19167. https://doi.org/10.1073/pnas.0902834106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Guo, T. B. , & Lu, H. (2013). Promoting remyelination for the treatment of multiple sclerosis: Opportunities and challenges. Neuroscience Bulletin, 29(2), 144–154. https://doi.org/10.1007/s12264-013-1317-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Dai, J. , Ma, Y. , Mi, Y. , Cui, D. , Ju, G. , … Jin, W. (2014). Dynamics of ten‐eleven translocation hydroxylase family proteins and 5‐hydroxymethylcytosine in oligodendrocyte differentiation. Glia, 62(6), 914–926. https://doi.org/10.1002/glia.22649 [DOI] [PubMed] [Google Scholar]


