Abstract
Calcineurin inhibitors (CNIs, eg, tacrolimus) reduce short‐term kidney transplant failure, but chronic nephrotoxicity may contribute to late transplant loss. Elective conversion to inhibitors of the mammalian target of rapamycin (mTOR, eg, sirolimus) pathway might avoid long‐term CNI renal damage and improve outcomes. The 3C Study was a pragmatic randomized controlled trial of sequential randomizations between alemtuzumab and basiliximab induction therapy (at the time of surgery) and between tacrolimus and sirolimus maintenance therapy at 6 months posttransplantation. The primary outcome of this analysis was estimated glomerular filtration rate (eGFR) at 18 months after maintenance therapy randomization; 197 patients were assigned sirolimus‐based and 197 to tacrolimus‐based therapy. Allocation to sirolimus had no significant effect on eGFR at 18 months: baseline‐adjusted mean (SEM) eGFR was 53.7 (0.9) mL/min/1.73 m2 in the sirolimus group versus 54.6 (0.9) mL/min/1.73 m2 in the tacrolimus group (P = .50). Biopsy‐proven acute rejection (29 [14.7%]) vs 6 [3.0%]; P < .001) and serious infections (defined as opportunistic infections or those requiring hospitalization; 95 [48.2%] vs 70 [35.5%]; P = .008) were more common among participants allocated sirolimus. Compared with tacrolimus‐based therapy, sirolimus‐based maintenance therapy did not improve transplant function at 18 months after conversion and was associated with significant hazards of rejection and infection. ClinicalTrials.gov identifier NCT01120028 and ISRCTN88894088.
Keywords: clinical research/practice, immunosuppressant ‐ calcineurin inhibitor (CNI), immunosuppressant ‐ fusion proteins and monoclonal antibodies: alemtuzumab, immunosuppressant ‐ fusion proteins and monoclonal antibodies: basiliximab/daclizumab, immunosuppressant ‐ mechanistic target of rapamycin (mTOR), immunosuppression/immune modulation, kidney transplantation/nephrology
Short abstract
The 3C Study tested whether elective conversion to sirolimus‐based maintenance therapy was better than continued tacrolimus‐based maintenance therapy at 6 months after kidney transplantation and finds no improvement in transplant function, but increased risks of transplant rejection and infection with sirolimus.
Abbreviations
- ANCOVA
analysis of covariance
- BPAR
biopsy‐proven acute rejection
- CI
confidence interval
- CNI
calcineurin inhibitor
- CTSU
Clinical Trial Service Unit
- DBD
donation after brain death
- DCD
donation after circulatory death
- DSA
donor‐specific antibody
- eGFR
estimated glomerular filtration rate
- mTORi
mammalian target of rapamycin inhibitor
- mTOR
mammalian target of rapamycin
- RR
rate ratio
1. INTRODUCTION
Kidney transplantation is the best treatment for most patients with end‐stage kidney disease. However, despite improvements in short‐term transplant survival, long‐term rates have not improved in recent decades.1 Calcineurin inhibitors (CNIs, eg, tacrolimus) are central to modern immunosuppression but are associated with graft fibrosis and atrophy, worsening transplant function, and long‐term transplant failure.2, 3 Immunosuppression strategies that minimize CNI exposure may be expected, therefore, to reduce the rate of late transplant failure.4
Inhibitors of the mammalian target of rapamycin pathway (mTORi, eg, sirolimus) were first tested in various strategies to replace CNI; however, de novo use (ie, from the time of transplantation) is associated with complications,5, 6 and a large trial of late (ie, >6 months posttransplantation) conversion from CNI‐based therapy found no improvement in subsequent function.7 In contrast, early (ie, within 6 months of transplantation) conversion to mTORi has been shown in some (but not all) trials to improve transplant function compared with remaining on cyclosporine‐based immunosuppression.8, 9, 10, 11, 12 Another potential benefit of conversion to mTORi is a reduction in the risk of malignancy posttransplantation (especially skin cancer).13, 14, 15 However, a recent meta‐analysis suggested that sirolimus may be associated with an increased risk of all‐cause mortality.15
mTORi have more favorable effects than CNIs on tolerogenic T regulatory cells, and this effect may be most notable after alemtuzumab‐based induction therapy.16 A series of kidney transplant recipients treated with alemtuzumab, and elective conversion to sirolimus 6 months later reported good results,17 and it was hypothesized that the combination with alemtuzumab induction treatment enabled patients to become established on maintenance sirolimus without the problems that had limited previous studies (including increased rejection). No previous randomized controlled trials have compared mTORi with CNIs in combination with either alemtuzumab‐ or basiliximab‐based induction therapy: this is the overall issue addressed by the 3C Study. The first planned analysis of the 3C study, comparing the immediate effects of the 2 induction treatments, has been reported previously and showed a highly significant halving of rejection in patients treated with alemtuzumab.18 In the current report, the results of the sirolimus‐ versus tacrolimus‐based maintenance therapy randomization are presented.
2. METHODS
2.1. Trial design and participants
Details of the 3C Study methods have been reported previously,18, 19 and the trial design is summarized in Figure 1. The data analysis plan was finalized and published online at http://www.3cstudy.org in advance of any unblinded analyses being conducted and is available in the Supplementary Appendix. Approval from a national research ethics committee was obtained before enrolment. The study is registered at ClinicalTrials.gov, NCT01120028 and ISRCTN88894088.
Figure 1.
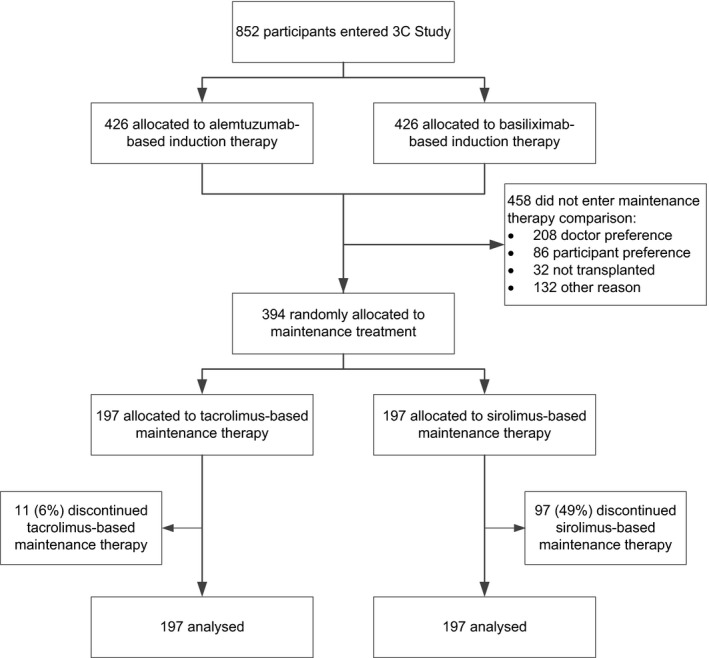
Trial profile
Patients aged 18 years or older were eligible to participate if scheduled to receive a kidney transplant within 24 hours. Eligibility was assessed and written informed consent was obtained. Induction therapy was randomly allocated (either alemtuzumab or basiliximab based), and all participants received tacrolimus and mycophenolate (and prednisolone if assigned basiliximab). Participants with a functioning transplant between 5 and 7 months after transplantation were eligible to participate in this comparison of tacrolimus‐ versus sirolimus‐based maintenance therapy with 2 exclusion criteria: (i) a proven rejection episode in the previous month and (ii) proteinuria in excess of 800 mg daily (estimated by spot urine protein:creatinine ratio) (Figure 1).
2.2. Randomization and blinding
Eligible and consenting participants were allocated the study treatment through minimized randomization (see Supplementary Appendix).20 Treatment allocation was concealed; investigators, clinicians, and patients had no foreknowledge of the upcoming treatment allocation.21 Patients were randomized equally to either (i) sirolimus‐based maintenance therapy (immediate cessation of tacrolimus and sirolimus started on next day: 3 mg daily [or 2 mg if weight <50 kg]; target trough concentration 6‐12 ng/mL for the first 6 months, thereafter 5‐10 ng/mL) or (ii) tacrolimus‐based maintenance therapy (target trough concentration 5‐7 ng/mL). All participants also received mycophenolate and prednisolone if considered necessary by the managing clinician. After randomized treatment allocation, local clinicians and participants were not blinded to treatment allocation.
2.3. Procedures
Participants were to be reviewed at 3 and 6 months after maintenance therapy randomization. At each follow‐up visit, blood pressure and weight were to be measured, and blood and urine samples were collected for local analysis of creatinine, full blood count, tacrolimus and sirolimus concentrations, and urine protein:creatinine ratio (or albumin:creatinine ratio, depending on local practice).
The primary outcome of estimated glomerular filtration rate (eGFR) at 18 months after maintenance therapy randomization was calculated by using the Modification of Diet in Renal Disease equation from creatinine measurements, which were measured locally, sent electronically to the UK Renal Registry (per routine practice), and provided to the study coordinating center (all blinded to treatment allocation). Information on all serious adverse events (including possible rejection episodes and opportunistic infections) and nonserious adverse events believed to be related to study treatment were recorded at each follow‐up visit. In addition, all participants were flagged with the UK Transplant Registry (for information about any transplant rejection and failure episodes), the UK Renal Registry (for information on transplant function and transplant failure), the Office for National Statistics (for site‐specific cancer and cause‐specific mortality), and the UK Health and Social Care Information Centre, the Information Services Division in Scotland and the Secure Anonymised Information Linkage databank in Wales (for information about any hospitalizations). All information from these registries was provided blinded to treatment allocation.
2.4. Statistical analysis
Analysis of covariance (ANCOVA) was used to compare mean levels of eGFR at 18 months between sirolimus‐ and tacrolimus‐allocated patients, with adjustment for each individual's eGFR at randomization into the maintenance phase of the trial. While it was hoped that about 500 of the 800 patients originally randomized in the induction phase of the trial would be willing and eligible to be re‐randomized in the maintenance phase, the randomization of 400 patients in the maintenance phase, together with chosen ANCOVA analysis, still provided >90% power at 2‐sided P = .05 to detect a 5 mL/min/1.73 m2 difference in eGFR at 18 months. The few participants with eGFR missing at 18 months had their value imputed using multiple imputation, with the results across imputations combined using the methods of Rubin.22 Time‐to‐event analyses prespecified log‐rank methods to calculate the average event rate ratio (RR), 95% confidence intervals (CIs), and its associated 2‐sided P value. However, for biopsy‐proven acute rejection (BPAR), the RR was subsequently estimated instead by the hazard ratio from a Cox proportional hazards regression model (because it was large and the log‐rank “1‐step” estimate of the RR increasingly underestimates the true RR as it becomes more extreme).23 All analyses were done according to the intention‐to‐treat principle among all randomized participants, and all P values are 2‐sided.23, 24 To investigate the possibility that sirolimus may have been of benefit among participants who could tolerate it, 2 types of exploratory analyses were conducted. First, we conducted a nonrandomized comparison of eGFR among those participants who remained compliant with their allocated maintenance therapy at 18 months after randomization, before and after adjustment for baseline characteristics. Second, an exploratory analysis preserving the randomized comparison used a score to predict noncompliance with sirolimus from baseline characteristics that was constructed by using standard logistic regression among the participants assigned sirolimus. This was then applied to all participants (including those assigned tacrolimus) to allow stratification by tertiles of risk of noncompliance. A standard test for trend was performed to explore whether the effect of sirolimus varied by risk of noncompliance and, in particular if there was any evidence to suggest that sirolimus was more beneficial among participants at the lowest risk of noncompliance. Unless stated otherwise, all analyses were prespecified. Analyses were done with SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.11.1 (http://www.R-Project.org).
2.5. Role of the funding source
The 3C Study was designed, conducted, and analyzed by the Clinical Trial Service Unit (CTSU) at Oxford University, which is the independent regulatory sponsor for the study, in collaboration with the Oxford Transplant Centre. The study was funded by grants from the NHS Blood and Transplant Research and Development program, Pfizer, and Novartis UK. The funding sources participated in discussions about the trial design and had a right to comment on (but not to require changes to) study reports. They had no involvement in data collection, analysis, interpretation, report writing, or the decision to submit for publication. The writing committee had full access to all data and accepts full responsibility for the content of this report.
3. RESULTS
Of the 852 participants who were randomly assigned to either alemtuzumab‐ or basiliximab‐based induction therapy, 820 were transplanted. Between April 2011 and July 2013, 394 of these participants were randomly assigned to sirolimus‐based therapy versus tacrolimus‐based therapy (Figure 1). The most common reasons for participants not entering the maintenance therapy comparison were managing physician decision and patient preference (Table S1). Mean age at this randomization was 52 years (SD 13), 264 (67%) were male, and 347 (88%) were white (Table 1). Two‐thirds of participants were receiving at least 720 mg daily of mycophenolic acid and one‐third were on prednisolone at the time of randomization (Table S2).
Table 1.
Baseline by treatment allocation at maintenance randomization
| Sirolimus (n = 197) | Tacrolimus (n = 197) | |
|---|---|---|
| Age (years) | ||
| ≤30 | 16 (8%) | 13 (7%) |
| >30 to ≤60 | 124 (63%) | 130 (66%) |
| >60 | 57 (29%) | 54 (27%) |
| Mean (SD) | 52 (13) | 52 (13) |
| Sex | ||
| Male | 132 (67%) | 132 (67%) |
| Female | 65 (33%) | 65 (33%) |
| Ethnic origin | ||
| White | 174 (88%) | 173 (88%) |
| Black | 5 (3%) | 7 (4%) |
| Asian | 13 (7%) | 15 (8%) |
| Other | 5 (3%) | 2 (1%) |
| Primary renal disease | ||
| Diabetes | 15 (8%) | 21 (11%) |
| Glomerulonephritis | 50 (25%) | 40 (20%) |
| Cystic kidney disease | 47 (24%) | 34 (17%) |
| Chronic pyelonephritis | 5 (3%) | 9 (5%) |
| Hypertension | 14 (7%) | 24 (12%) |
| Renovascular disease | 3 (2%) | 4 (2%) |
| Other | 63 (32%) | 65 (33%) |
| HLA mismatcha | ||
| Level 1 | 23 (12%) | 22 (11%) |
| Level 2 | 42 (21%) | 42 (21%) |
| Level 3 | 89 (45%) | 90 (46%) |
| Level 4 | 43 (22%) | 43 (22%) |
| Previous transplant | ||
| None | 181 (92%) | 181 (92%) |
| 1 | 13 (7%) | 13 (7%) |
| >1 | 3 (2%) | 3 (2%) |
| Highly sensitized | ||
| Yes | 4 (2%) | 7 (4%) |
| No | 193 (98%) | 190 (96%) |
| Type of donor | ||
| DBD | 66 (34%) | 65 (33%) |
| DCD | 65 (33%) | 69 (35%) |
| Living | 66 (34%) | 63 (32%) |
| eGFR (mL/min/1.73 m²) | ||
| <40 | 43 (22%) | 44 (22%) |
| ≥40 to <60 | 89 (45%) | 95 (48%) |
| ≥60 | 65 (33%) | 58 (29%) |
| Mean (SD) | 53.5 (16.8) | 52.6 (16.6) |
| Proteinuria (mg/g)b | ||
| <300 | 165 (84%) | 170 (86%) |
| ≥300 to <500 | 24 (12%) | 19 (10%) |
| ≥500 | 8 (4%) | 8 (4%) |
| Median (IQR) | 133 (80‐235) | 136 (82‐228) |
| Induction therapy strategy | ||
| Alemtuzumab‐based | 95 (48%) | 97 (49%) |
| Basiliximab‐based | 102 (52%) | 100 (51%) |
Data are n (%), mean (SD), or median (IQR).
DBD, donation after brain death; DCD, donation after circulatory death; eGFR, estimated glomerular filtration rate.
Level 1: 0‐0‐0; level 2: 0 DR + 0/1 B mismatches; level 3: [0 DR + 2B] or [1 DR + 0/1 B]; level 4: [1 DR + 2B] or [2 DR].
Measured using spot urine protein:creatinine ratio. The characteristics were well balanced between the trial groups at baseline; nominal P > .05 for between‐group differences in all the characteristics listed in the table.
At 3 months after maintenance therapy randomization, 148 (76%) of participants assigned sirolimus‐based therapy remained adherent to their randomized allocation compared with 195 (99%) of those assigned tacrolimus‐based therapy (Table S3). By 18 months after transplantation, these had reduced to 91 (48%) and 180 (94%), respectively. Reasons for discontinuing study treatment were collected during the first 6 months after randomization, at which point 56 (28%) of those assigned sirolimus had reported a reason for stopping allocated therapy (with the majority restarting tacrolimus) compared with 2 (1%) assigned tacrolimus. The most common medical reasons for stopping sirolimus reported during the first 6 months were rejection (6%) and infection (4%) (Table S4). Among participants assigned sirolimus‐based therapy and still receiving it, mean (SD) concentration was 8.7 (4.0) ng/mL and 6.9 (2.1) ng/mL at 3 and 18 months, respectively (Table S5A). Among participants assigned tacrolimus‐based therapy and still receiving it, the mean concentration was 7.4 (4.2) ng/mL and 7.0 (2.2) ng/mL at 3 and 18 months, respectively. Exploratory analyses showed that other immunosuppression did not differ during the period of follow‐up when such data were collected (up to 6 months postrandomization; Table S5B).
At 18 months after randomization, the mean (SE) eGFR among participants assigned sirolimus‐based therapy was 53.7 (0.9) compared with 54.6 (0.9) mL/min/1.73 m2 among those assigned tacrolimus‐based therapy (P = .50; Figure 2). Sensitivity analyses (including one which excluded the 10 [2.5%] patients who had their 18‐month eGFR input because it was not available) had no material effect on this result. There was no evidence that the effect on 18‐month eGFR differed by induction therapy allocation (P for heterogeneity = .80); among participants who received alemtuzumab‐based induction therapy, the mean eGFR among participants assigned sirolimus‐based therapy was 54.2 (1.5) compared with 55.5 (1.5) mL/min/1.73 m2 among those assigned tacrolimus‐based therapy. Similarly, among participants who received basiliximab‐based induction therapy, the mean eGFR among participants assigned sirolimus‐based therapy was 53.3 (1.2) compared with 53.9 (1.2) mL/min/1.73 m2 among those assigned tacrolimus‐based therapy (Figure S1). Neither baseline eGFR (P = .55) nor baseline proteinuria (P = .91) modified this effect. An apparent heterogeneity in effect depending on a history of a previous transplant was not supported by any parallel differences for other markers of immunologic risk (eg, HLA mismatch or sensitization status) after adjustment for multiple comparisons.
Figure 2.
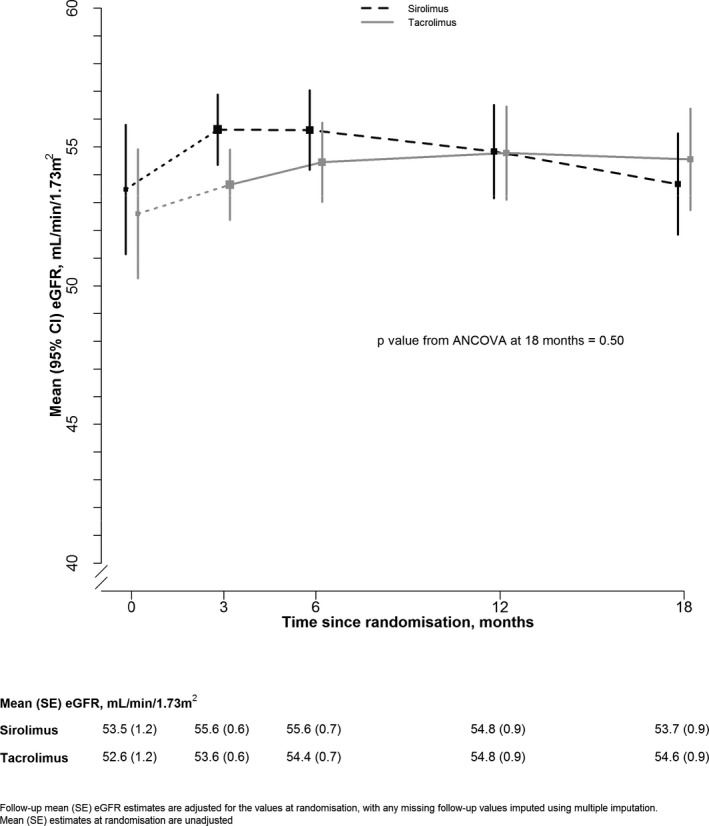
Effect of allocation to sirolimus‐based maintenance therapy on transplant function within the first 18 months
Exploratory analyses that compared the 100 participants who remained compliant with sirolimus with the 186 participants who remained compliant with tacrolimus yielded an apparent difference in transplant function among those participants allocated and adherent to sirolimus (difference in mean values 2.9 mL/min/1.73 m2, SE 1.4 mL/min/1.73 m2; adjusted P = .05; Table S6A). The 18‐month mean eGFR of participants who discontinued allocated treatment was 8.9 (4.2) mL/min/1.73 m2 lower among those assigned sirolimus compared with those assigned tacrolimus, whereas it was 2.9 (1.4) mL/min/1.73 m2 higher among those who did not discontinue allocated treatment. When participants were divided in a further exploratory analysis into 3 groups according to their risk of noncompliance with sirolimus, there was evidence that the effect of allocation to sirolimus varied with risk of noncompliance (P for trend = .05; Table S6B) such that allocation to sirolimus was associated with a 2.9 (SE 2.2) mL/min/1.73 m2 decrement among participants at high risk of noncompliance and a 3.2 (SE 2.2) mL/min/1.73 m2 improvement among participants with lowest risk of noncompliance.
During the 18 months after randomization, 29 (14.7%) of participants assigned sirolimus‐based therapy had at least 1 episode of BPAR compared with 6 (3.0%) of participants allocated tacrolimus‐based therapy, corresponding to a relative risk of 5.15 (95% CI 2.14‐12.41; P < .0001) (Table 2 and Figure 3). Post‐hoc analyses show this relative risk was similar among participants assigned alemtuzumab‐based and basiliximab‐based induction therapy (P for heterogeneity = .47) and was similar regardless of baseline dose of MPA (P for trend = .94) or prednisolone (P for heterogeneity = .65). Exploratory analyses indicated that the mean 18‐month eGFR of participants who experienced rejection was 7.4 (7.1) mL/min/1.73 m2 lower among those assigned sirolimus compared with those assigned tacrolimus, whereas it was 0.5 (1.3) mL/min/1.73 m2 higher among those who did not experience rejection. We saw no significant difference in transplant failure: 8 (4.1%) versus 4 (2.0%) (Table 2).
Table 2.
Effect of allocation to sirolimus‐based maintenance therapy on graft rejection, graft survival, and safety outcomes
| Sirolimus (n = 197) | Tacrolimus (n = 197) | Rate ratio (95% CI) | P value | |
|---|---|---|---|---|
| Graft rejection | ||||
| Cellular | ||||
| Banff 1 | 23 (11.7%) | 5 (2.5%) | ||
| Banff 2 | 1 (0.5%) | 0 (0.0%) | ||
| Banff 3 | 1 (0.5%) | 0 (0.0%) | ||
| Unknown | 0 (0.0%) | 0 (0.0%) | ||
| Any | 25 (12.7%) | 5 (2.5%) | ||
| Humoral | ||||
| Banff 1 | 1 (0.5%) | 0 (0.0%) | ||
| Banff 2 | 4 (2.0%) | 2 (1.0%) | ||
| Banff 3 | 0 (0.0%) | 0 (0.0%) | ||
| Unknown | 1 (0.5%) | 0 (0.0%) | ||
| Any | 6 (3.0%) | 2 (1.0%) | ||
| Unknown | 1 (0.5%) | 0 (0.0%) | ||
| All biopsy‐proven acute rejectiona | 29 (14.7%) | 6 (3.0%) | 5.15 (2.14‐12.41) | <.001 |
| Reasons for transplant failure | ||||
| Glomerular disease | 1 (0.5%) | 0 (0.0%) | ||
| Fibrosis/atrophy | 0 (0.0%) | 1 (0.5%) | ||
| Medical/surgical condition | 1 (0.5%) | 0 (0.0%) | ||
| Rejection | 2 (1.0%) | 0 (0.0%) | ||
| Unknown | 4 (2.0%) | 3 (1.5%) | ||
| Any graft failure | 8 (4.1%) | 4 (2.0%) | 1.99 (0.64‐6.18) | .23 |
| Serious infections | ||||
| Opportunistic infections | ||||
| Cytomegalovirus infection | 8 (4.1%) | 8 (4.1%) | 1.00 (0.38‐2.68) | .99 |
| BK virus infection | 1 (0.5%) | 3 (1.5%) | ||
| Fungal infection | ||||
| Noninvasive | 3 (1.5%) | 2 (1.0%) | ||
| Invasive | 0 (0.0%) | 1 (0.5%) | ||
| Any | 3 (1.5%) | 3 (1.5%) | 1.00 (0.20‐4.97) | 1.00 |
| Other opportunistic infection | ||||
| PCJ | 5 (2.5%) | 1 (0.5%) | ||
| Mycobaterial | 0 (0.0%) | 0 (0.0%) | ||
| Other | 8 (4.1%) | 7 (3.6%) | ||
| Any other opportunistic infection | 12 (6.1%) | 8 (4.1%) | ||
| Any opportunistic infection | 22 (11.2%) | 22 (11.2%) | 1.00 (0.56‐1.81) | .99 |
| Nonopportunistic infections | ||||
| Urinary tract | 30 (15.2%) | 29 (14.7%) | ||
| Respiratory tract | 32 (16.2%) | 19 (9.6%) | ||
| Gastrointestinal | 26 (13.2%) | 9 (4.6%) | ||
| Central nervous system | 1 (0.5%) | 1 (0.5%) | ||
| Other | 33 (16.8%) | 25 (12.7%) | ||
| Any nonopportunistic infection | 83 (42.1%) | 60 (30.5%) | 1.54 (1.11‐2.15) | .010 |
| Any serious infection | 95 (48.2%) | 70 (35.5%) | 1.51 (1.11‐2.06) | .008 |
| Cancer | ||||
| Hematologic cancer | ||||
| Posttransplantation lymphoproliferative disorder | 1 (0.5%) | 4 (2.0%) | ||
| Other hematologic cancer | 0 (0.0%) | 2 (1.0%) | ||
| Any hematologic cancer | 1 (0.5%) | 5 (2.5%) | 0.26 (0.05‐1.30) | .10 |
| Skin cancer | ||||
| Nonmelanoma skin cancer | 4 (2.0%) | 6 (3.0%) | 0.67 (0.19‐2.30) | .52 |
| Melanoma | 0 (0.0%) | 1 (0.5%) | ||
| Any skin cancer | 10 (5.1%) | 9 (4.6%) | 1.11 (0.45‐2.74) | .81 |
| Other cancerb | 7 (3.6%) | 6 (3.0%) | 1.16 (0.39‐3.45) | .78 |
| Any cancer | 17 (8.6%) | 17 (8.6%) | 1.00 (0.51‐1.97) | .99 |
| Mortality | ||||
| Cause of death | ||||
| Vascular | 7 (3.6%) | 2 (1.0%) | ||
| Infection | 1 (0.5%) | 1 (0.5%) | ||
| Cancer | 2 (1.0%) | 3 (1.5%) | ||
| Other | 1 (0.5%) | 3 (1.5%) | ||
| Any death | 11 (5.6%) | 9 (4.6%) | 1.23 (0.51‐2.95) | .64 |
Presumed rejection occurred in 5 vs 1 participants, making the total of any acute rejection (biopsy proven or presumed) 31 vs 7. PCJ, Pneumocystis jirovecii.
Rate ratio for any biopsy‐proven acute rejection was calculated using Cox proportional hazards model.
Other cancer consists of lung (1 vs 2), gastrointestinal (1 vs 0), hepatobiliary (1 vs 0), breast (1 vs 0), and other (3 vs 3).
Figure 3.
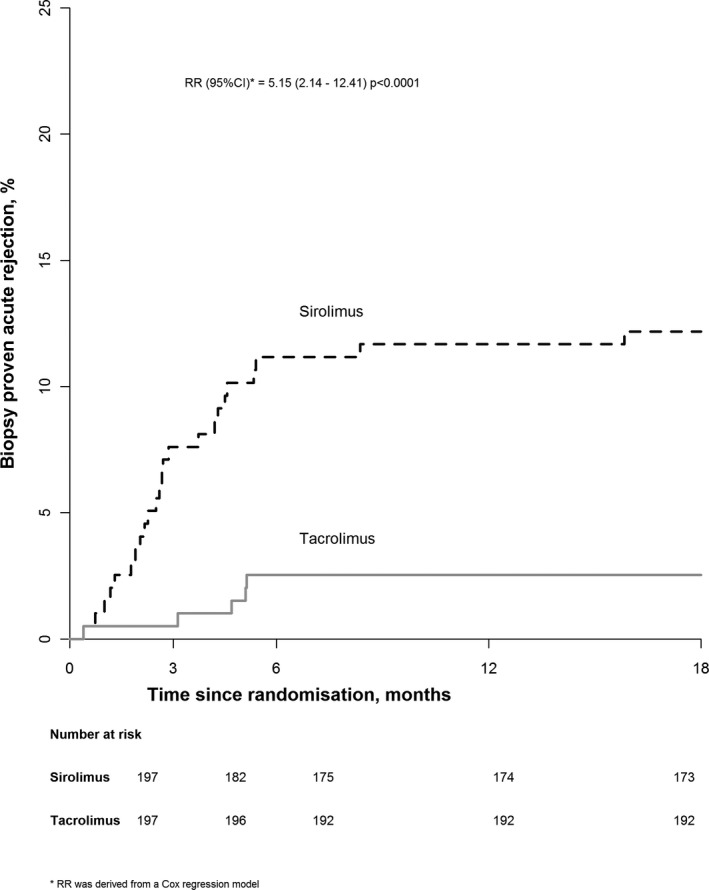
Life‐table plot of the effect of allocation to sirolimus‐based maintenance therapy on any biopsy‐proven rejection
More participants assigned sirolimus‐based therapy experienced a serious infection (defined as an opportunistic infection or one requiring hospitalization): 95 (48.2%) vs 70 (35.5%) (Table 2). This reflected an excess in nonopportunistic infections (83 [42.1%] vs 60 [30.5%], in particular, respiratory and gastrointestinal infections) but no excess in opportunistic infections (22 [11.2%] vs 22 [11.2%]). Allocation to sirolimus‐based therapy had no significant effect on cancer, either overall (17 [8.6%] vs 17 [8.6%]) or in specific sites, nor was there any significant effect on mortality (11 [5.6%] vs 9 [4.6%]; Table 2). There was also no significant effect on the composite outcome of death or transplant failure (18 [9.1%] vs 13 [6.6%]).
There was no significant effect on new‐onset diabetes (14 [7.1%] vs 11 [5.6%]) or on major vascular events (10 [5.1%] vs 13 [6.6%]) (Table S7). Anemia was significantly more common among participants assigned sirolimus‐based therapy (127 [64%] vs 96 [49%]; P = .002) (Table S8). Participants assigned sirolimus‐based therapy also had significantly more proteinuria (difference in geometric means at 6 months 201% [75% to 418%]; P < .001) and higher cholesterol and triglyceride concentrations at 6 months (Table S9).
4. DISCUSSION
The 3C Study was set up to investigate whether it is feasible to establish patients on long‐term CNI‐free immunosuppression and whether this is associated with reduced graft attrition (using kidney function [eGFR] as a surrogate in this analysis). Based on previous work, we hypothesized that the use of alemtuzumab‐based induction therapy might enable patients to be established on sirolimus‐based therapy without the complications (wound healing, rejection, mouth ulceration, pneumonitis) that have limited such strategies previously.17
Our findings suggest that elective conversion to sirolimus at about 6 months after kidney transplantation does not improve subsequent transplant function and carries significant risks of rejection and infection and that this detriment applies even in the context of the (previously demonstrated) benefits of alemtuzumab induction therapy. We included a broad range of different types of participant and recruited about one‐eighth of all kidney transplant recipients transplanted in the participating centers during the recruitment period. These results are likely therefore to be relevant to a broad population of patients.
Our findings are discrepant with previous published trials that have explored CNI avoidance strategies. However, with 394 participants in this second randomization, the 3C Study is the largest such trial (almost twice as large as the next largest trial using sirolimus). It is notable that most previous studies have used cyclosporine‐based therapy as the comparator (in part because this was previously the regimen approved by the US Food and Drug Administration). However, tacrolimus is now by far the most widely used CNI in the United States and worldwide and – in conjunction with mycophenolate – is the clinically most relevant comparator. The only previous trial comparing a sirolimus‐based strategy with a tacrolimus‐based strategy also found no benefit.25 It is possible that cyclosporine is more nephrotoxic than tacrolimus or that, historically, cyclosporine was used at higher equivalent concentrations than tacrolimus is used in current practice. In the Elite‐SYMPHONY study, low‐dose tacrolimus (target trough concentration 3‐7 ng/mL, similar to that used in the 3C Study) provided better transplant function at 1 year compared with standard‐ or low‐dose cyclosporine.5 Consistent with this is a recent report from the Westmead Hospital, Sydney, which found less evidence of nephrotoxicity in serial protocol kidney biopsy samples taken from kidney–pancreas transplant recipients between 1999 and 2012 (an era when tacrolimus was the predominant CNI) compared with those taken between 1987 and 2000 (when cyclosporine was used).3 This has significant implications for the interpretation of data from clinical trials that have compared novel immunosuppressants with cyclosporine rather than tacrolimus.9, 26 Whether this is drug specific or dose specific, the way in which tacrolimus is currently used appears to be substantially less nephrotoxic than older CNI regimens.
Another possible reason for the apparent discrepancy was that adherence to sirolimus‐based therapy was low in the 3C Study, with only half of all participants allocated sirolimus still taking it 18 months after randomization. The adherence was somewhat worse than we had anticipated but nonetheless in keeping with previous studies.8, 25, 27, 28, 29, 30 The low adherence level in the 3C Study reduces the sensitivity of the trial to detect a true difference in transplant function but also, importantly, means that estimates of the hazards may be less than would be observed with full adherence. It is possible that the development of donor‐specific antibodies (DSAs) may be an important determinant of long‐term transplant function,31 but we did not measure DSAs in this trial.
One of the underlying hypotheses for the 3C Study was that the use of lymphocyte‐depleting induction therapy might facilitate the conversion to sirolimus at 6 months, as suggested by a small pilot study.17 However, we found no evidence that sirolimus‐based maintenance therapy was more effective after alemtuzumab‐based than nondepleting basiliximab‐based induction therapy. Alemtuzumab has been suggested to induce a state of “prope” (near) tolerance32 and, in combination with sirolimus, induces more tolerogenic regulatory T cells.16 Despite favorable results of the pilot study,17 we found no effect on transplant function: this is consistent with the findings of small trials using histologic outcomes.16 Our finding that allocation to sirolimus‐based maintenance therapy substantially increased the risk of BPAR is also consistent with findings in previous studies.8, 12
We investigated the relevance of compliance to these results with 2 exploratory analyses. The first of these yielded an apparent difference in eGFR between the 100 participants who remained compliant with sirolimus and the 180 participants who remained compliant with tacrolimus. This result is, of course, of uncertain relevance because those who discontinued allocated sirolimus (the nature of which was known because the trial was not blinded) may have done so for adverse effects whose risk of occurrence may be correlated with renal function. Such an analysis may not be a reliable indication, therefore, of the differential effects of sirolimus on renal function (as compared with tacrolimus). We explored this further and found weak evidence that participants more likely to remain adherent to sirolimus might derive greater benefit from allocation to the sirolimus arm. Taken together, the results from these 2 analyses should perhaps still be considered only as hypothesis generating and require independent confirmation.
Infections were also more common with sirolimus‐based therapy. There was no overall effect on opportunistic infections: other studies have suggested that sirolimus may interfere with viral replication and therefore reduce the incidence of cytomegalovirus infections.33, 34 We observed no benefit (or hazard), but there were too few such infections in the 3C Study to make robust inferences. The excess in all serious infections was due to a higher rate of nonopportunistic infections (notably respiratory and gastrointestinal infections) among participants assigned sirolimus‐based therapy. Given the overlap with known symptomatic side effects of sirolimus (eg, pneumonitis) it is possible that misclassification (ie, attribution of symptoms to an infective cause rather than to a direct drug effect) explains some of this excess. However, such symptoms would not typically resolve without cessation of sirolimus.
We did not observe any effect on cancer incidence. Sirolimus has been shown to inhibit the development of nonmelanoma skin cancer and other cancers.13, 14, 15, 35 However, most posttransplantation malignancies take several years to develop, and the time horizon for these analyses was likely to be too early to observe any such benefit. Furthermore, this trial alone is too small to detect plausible effects on cancer incidence.
We observed the known effects of sirolimus on proteinuria.36 The mechanism of sirolimus‐induced proteinuria is unclear,37 but proteinuria is a recognized risk factor for subsequent transplant failure.38 With only 12 transplant failures during the period of observation, our power to detect an effect on this is extremely limited, but 2 meta‐analyses found no effect of sirolimus on transplant survival.11, 15
The increase in non–high‐density lipoprotein cholesterol of 0.5 mmol/L would be consistent with a 10% increased risk of major vascular events.39 Posttransplantation diabetes mellitus was nonsignificantly more common among participants assigned sirolimus‐based therapy, so overall the impact of sirolimus on cardiovascular risk was adverse. We observed no significant effect on major vascular events or all‐cause mortality, but with only 23 and 20 such events, respectively, the study's power was negligible. A recent meta‐analysis has suggested that sirolimus is associated with an increased risk of death.15 However, this effect was only marginally statistically significant in unadjusted analyses (P = .04), and most of the data were from trials in which sirolimus was used de novo and not following elective conversion as we did in our trial.
A limitation of the 3C Study was that just under half of all participants recruited at the time of transplantation entered this comparison. However, the baseline characteristics of these participants were similar to those of the overall trial cohort, suggesting that this result is likely to be generalizable. Information on concomitant immunosuppression and reasons for stopping were collected for only 6 months after randomization. A further limitation of the 3C Study was the requirement for it to be open label. However, the primary outcome (eGFR at 18 months after randomization) was collected by linkage with the UK Renal Registry, which, in turn, collects its data through routine extraction from hospital databases, making it highly unlikely that the values entered would be biased with respect to treatment allocation. Although these analyses were based only on 18‐month follow‐up, the 3C Study has established linkage with appropriate national registries, so longer‐term follow‐up will be conducted in a cost‐effective manner and may yield informative results.
Our findings suggest that compared with continuation of a tacrolimus‐based regimen, elective conversion to sirolimus‐based maintenance therapy does not improve transplant function 18 months later (regardless of induction therapy) and is associated with significant hazards of rejection and infection.
5. CONTRIBUTORS
All authors contributed to the collection and analysis of the data and to the preparation of the report.
6. WRITING COMMITTEE
R. Haynes, L. Blackwell, N. Staplin, W. G. Herrington, J. R. Emberson, P. K. Judge, B. C. Storey, M. J. Landray, P. N. Harden, C. Baigent, P. J. Friend.
7. STEERING COMMITTEE
Chair P. J. Friend*; members R. Haynes (clinical coordinator)*, C. Baigent*, P. Harden*, M. J. Landray*, M. Akyol, A. Asderakis, A. Baxter, S. Bhandari, P. Chowdhury, M. Clancy, P. Gibbs, A. Hammad, W. G. Herrington, K. Jayne, G. Jones, N. Krishnan, M. Lay, D. Lewis, I. Macdougall, C. Nathan, J. Neuberger, C. Newstead, R. Pararajasingam, C. Puliatti, K. Rigg, P. Rowe, A. Sharif, N. Sheerin, S. Sinha, N. Torpey, C. J. E. Watson; *co‐principal investigators.
8. DATA MONITORING COMMITTEE
Chair P. Morris; members K. Wheatley, D. Abramowicz; nonvoting statisticians J. R. Emberson, L. Blackwell.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. The 3C Study was initiated, conducted, and interpreted independently of the study funders (NHS Blood and Transplant, Novartis UK, and Pfizer UK). In addition, CTSU receives core support from the UK Medical Research Council, British Heart Foundation and Cancer Research UK. The Clinical Trial Service Unit, and Epidemiological Studies Unit, which is part of the University of Oxford, has a staff policy of not accepting honoraria or consultancy fees (http://www.ctsu.ox.ac.uk).
Supporting information
ACKNOWLEDGMENTS
The most important acknowledgment is to the participants in the 3C Study and the local clinical center staff, steering committee, and data monitoring committee. The study was funded by Pfizer Ltd, Novartis Pharmaceuticals UK Ltd, and NHS Blood and Transplant (project reference 09‐15‐01‐03). We acknowledge the support of the UK Transplant Registry, UK Renal Registry, Health and Social Care Information Centre, Office for National Statistics, Secure Anonymised Information Linkage Databank, and NHS National Services Scotland.
The 3C Study Collaborative Group . Campath, calcineurin inhibitor reduction, and chronic allograft nephropathy (the 3C Study) – results of a randomized controlled clinical trial. Am J Transplant. 2018;18:1424–1434. https://doi.org/10.1111/ajt.14619
REFERENCES
- 1. Meier‐Kriesche HU, Schold JD, Kaplan B. Long‐term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4(8):1289‐1295. [DOI] [PubMed] [Google Scholar]
- 2. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326‐2333. [DOI] [PubMed] [Google Scholar]
- 3. Nankivell BJ, P'Ng CH, O'Connell PJ, Chapman JR. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: comparison of cyclosporine and tacrolimus eras. Transplantation. 2016;100(8):1723‐1731. [DOI] [PubMed] [Google Scholar]
- 4. Ekberg H. Calcineurin inhibitor sparing in renal transplantation. Transplantation. 2008;86(6):761‐767. [DOI] [PubMed] [Google Scholar]
- 5. Ekberg H, Tedesco‐Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562‐2575. [DOI] [PubMed] [Google Scholar]
- 6. Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94(6):547‐561. [DOI] [PubMed] [Google Scholar]
- 7. Schena FP, Pascoe MD, Alberu J, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24‐month efficacy and safety results from the CONVERT trial. Transplantation. 2009;87(2):233‐242. [DOI] [PubMed] [Google Scholar]
- 8. Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant. 2009;9(5):1115‐1123. [DOI] [PubMed] [Google Scholar]
- 9. Budde K, Becker T, Arns W, et al. Everolimus‐based, calcineurin‐inhibitor‐free regimen in recipients of de‐novo kidney transplants: an open‐label, randomised, controlled trial. Lancet. 2011;377(9768):837‐847. [DOI] [PubMed] [Google Scholar]
- 10. Mulay AV, Cockfield S, Stryker R, Fergusson D, Knoll GA. Conversion from calcineurin inhibitors to sirolimus for chronic renal allograft dysfunction: a systematic review of the evidence. Transplantation. 2006;82(9):1153‐1162. [DOI] [PubMed] [Google Scholar]
- 11. Lim WH, Eris J, Kanellis J, et al. A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Transplant. 2014;14(9):2106‐2119. [DOI] [PubMed] [Google Scholar]
- 12. de Fijter JW, Holdaas H, Oyen O, Sanders JS, Sundar S, Bemelman FJ, et al. Early Conversion From Calcineurin Inhibitor‐ to Everolimus‐Based Therapy Following Kidney Transplantation: Results of the Randomized ELEVATE Trial. Am J Transplant. 2017;17:1853‐1867. [DOI] [PubMed] [Google Scholar]
- 13. Euvrard S, Morelon E, Rostaing L, et al. Sirolimus and secondary skin‐cancer prevention in kidney transplantation. N Engl J Med. 2012;367(4):329‐339. [DOI] [PubMed] [Google Scholar]
- 14. Hoogendijk‐van den Akker JM, Harden PN, Hoitsma AJ, et al. Two‐year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31(10):1317‐1323. [DOI] [PubMed] [Google Scholar]
- 15. Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta‐analysis of individual patient data. BMJ. 2014;349:g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruggenenti P, Perico N, Gotti E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation. 2007;84(8):956‐964. [DOI] [PubMed] [Google Scholar]
- 17. Sutherland AI, Akhtar MZ, Zilvetti M, et al. Alemtuzumab and sirolimus in renal transplantation: six‐year results of a single‐arm prospective pilot study. Am J Transplant. 2014;14(3):677‐684. [DOI] [PubMed] [Google Scholar]
- 18. 3C Study Collaborative Group . Alemtuzumab‐based induction treatment versus basiliximab‐based induction treatment in kidney transplantation (the 3C Study): a randomised trial. Lancet. 2014;384(9955):1684‐1690 [DOI] [PubMed] [Google Scholar]
- 19. Haynes R, Baigent C, Harden P, et al. Campath, calcineurin inhibitor reduction and chronic allograft nephropathy (3C) study: background, rationale, and study protocol. Transplantation Res. 2013;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103‐115. [PubMed] [Google Scholar]
- 21. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. J Am Med Assoc. 1995;273(5):408‐412. [DOI] [PubMed] [Google Scholar]
- 22. Rubin D. Multiple imputation for non‐response in surveys. New York: John Wiley; 1987. [Google Scholar]
- 23. Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Brit. J Cancer. 1977;35(1):1‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Brit. J Cancer. 1976;34(6):585‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silva HT Jr, Felipe CR, Garcia VD, et al. Planned randomized conversion from tacrolimus to sirolimus‐based immunosuppressive regimen in de novo kidney transplant recipients. Am J Transplant. 2013;13(12):3155‐3163. [DOI] [PubMed] [Google Scholar]
- 26. Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long‐term outcomes in kidney transplantation. N Engl J Med. 2016;374(4):333‐343. [DOI] [PubMed] [Google Scholar]
- 27. Guba M, Pratschke J, Hugo C, et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short‐term calcineurin inhibitor‐based quadruple therapy in de novo renal transplant patients: one‐year analysis of a randomized multicenter trial. Transplantation. 2010;90(2):175‐183. [DOI] [PubMed] [Google Scholar]
- 28. Heilman RL, Younan K, Wadei HM, et al. Results of a prospective randomized trial of sirolimus conversion in kidney transplant recipients on early corticosteroid withdrawal. Transplantation. 2011;92(7):767‐773. [DOI] [PubMed] [Google Scholar]
- 29. Pankewycz O, Leca N, Kohli R, et al. Conversion to low‐dose tacrolimus or rapamycin 3 months after kidney transplantation: a prospective, protocol biopsy‐guided study. Transplantation Proc. 2011;43(2):519‐523. [DOI] [PubMed] [Google Scholar]
- 30. Smith MP, Newstead CG, Ahmad N, et al. Poor tolerance of sirolimus in a steroid avoidance regimen for renal transplantation. Transplantation. 2008;85(4):636‐639. [DOI] [PubMed] [Google Scholar]
- 31. Matas AJ. Chronic progressive calcineurin nephrotoxicity: an overstated concept. Am J Transplant. 2011;11(4):687‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative campath 1H, and low‐dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351(9117):1701‐1702. [DOI] [PubMed] [Google Scholar]
- 33. Nashan B, Gaston R, Emery V, et al. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor‐based immunosuppressive therapy in de novo renal transplant recipients. Transplantation. 2012;93(11):1075‐1085. [DOI] [PubMed] [Google Scholar]
- 34. Mallat SG, Tanios BY, Itani HS, et al. CMV and BKPyV infections in renal transplant recipients receiving an mTOR inhibitor‐based regimen versus a CNI‐based regimen: a systematic review and meta‐analysis of randomized, controlled trials. Clin J Am Soc Nephrol. 2017;12:1321‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gutierrez‐Dalmau A, Campistol JM. Kaposi's sarcoma after renal transplantation. N Engl J Med. 2005;353(8):846‐847. [PubMed] [Google Scholar]
- 36. Bumbea V, Kamar N, Ribes D, et al. Long‐term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transplant. 2005;20(11):2517‐2523. [DOI] [PubMed] [Google Scholar]
- 37. Diekmann F, Andres A, Oppenheimer F. mTOR inhibitor‐associated proteinuria in kidney transplant recipients. Transplantation Rev. 2012;26(1):27‐29. [DOI] [PubMed] [Google Scholar]
- 38. Fernandez‐Fresnedo G, Plaza JJ, Sanchez‐Plumed J, Sanz‐Guajardo A, Palomar‐Fontanet R, Arias M. Proteinuria: a new marker of long‐term graft and patient survival in kidney transplantation. Nephrol Dial Transplant. 2004;19(Suppl 3):iii47‐iii51 [DOI] [PubMed] [Google Scholar]
- 39. Cholesterol Treatment Trialists C, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


