Abstract
Behavioural flexibility is crucial for adaptive behaviour, and recent evidence suggests that cholinergic interneurons of the striatum play a distinct role. Previous studies of cholinergic function have focused on strategy switching by the dorsomedial or ventral striatum. We here investigated whether cholinergic interneurons in the dorsolateral striatum play a similar role at the level of switching of habitual responses. Because the dorsolateral striatum is particularly involved in habitual responding, we developed a habit substitution task that involved switching habitual lever‐press responses to one side to another. We first measured the effect of cholinergic activation in the dorsolateral striatum on this task. Chemogenetic activation of cholinergic interneurons caused an increase in the response rate for the substituted response that was significantly greater than the increase normally seen in control animals. The increase was due to burst‐like responses with shorter inter‐press intervals. However, there was no effect on inhibiting the old habit, or on habitual responding that did not require a switch. There was also no effect on lever‐press performance and its reversal before lever‐press responses became habitual. Conversely, neurochemically specific ablation of cholinergic interneurons did not significantly change habitual responding or response substitution. Thus, activation –but not ablation –of cholinergic interneurons in the dorsolateral striatum modulates expression of a new habit when an old habit is replaced by a new one. Together with previous work, this suggests that striatal cholinergic interneurons facilitate behavioural flexibility in both dorsolateral striatum in addition to dorsomedial and ventral striatum.
Keywords: acetylcholine, basal ganglia, behavioural flexibility, chemogenetics
Introduction
Habits, as commonly understood, provide automatic and efficient control over action (Yin & Knowlton, 2006), which can be highly advantageous. More formally, Dickinson contrasted learning of habits with action–outcome learning, emphasizing that habitual action was not immediately sensitive to its consequences (Dickinson, 1985). Although habitual actions are efficient, if the environment changes then a habit may need to be modified to remain adaptive. Behavioural flexibility –the ability to break one habit and substitute a new response –is crucial to this end.
Acetylcholine is a neuromodulator in the striatum and mainly released from its intrinsic striatal cholinergic interneurons (CINs). The activity of CINs in awake animals is related to behavioural contexts such as reward probability (Morris et al., 2004), stimulus location (Ravel et al., 2006) or current state (Lee et al., 2006; Stalnaker et al., 2016). Many pieces of evidence also suggest that CINs play a causal role in behavioural flexibility underlying reversal learning (Ragozzino, 2003; Bradfield et al., 2013; Okada et al., 2014) and set‐shifting (Aoki et al., 2015). However, it has been difficult to selectively and temporarily manipulate their activity and previous studies have relied on selective lesions or pharmacology. Recently, new tools, optogenetics (Witten et al., 2011) and ‘Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)’ (Roth, 2016) have become available, which, when combined in transgenic animals, make selective manipulation possible.
We have demonstrated using a set‐shifting paradigm that a selective lesion of CINs in the dorsomedial striatum (DMS) leaves initial learning of a behavioural strategy intact, but impairs a subsequent switch to a new strategy due to an increase in perseverative responses and a decrease in exploration of new rules (Aoki et al., 2015). These findings suggest that CINs inhibit neurons representing an old strategy and enhance plasticity underlying exploration of new rules. This raises a question whether CINs play a similar role in other striatal areas. As the dorsolateral striatum (DLS) has been associated with habit learning (Yin et al., 2004; Yin & Knowlton, 2006; Gremel & Costa, 2013), we here studied the flexibility of habits in DLS.
We investigated the hypothesis that CINs in DLS play a role in switching one habitual response to another. In this study, animals formed a habit under a random‐interval (RI) schedule of reinforcement (Dickinson et al., 1983; Yin & Knowlton, 2006), and subsequently performed a habit substitution task in which reversal of habitual responses was required. Using this new paradigm, we sought to elucidate the functional role of CINs in switching habitual responses, using chemogenetic activation, or neurochemically specific ablation to manipulate CINs.
Materials and Methods
Ethical approval
All procedures involving animals were approved by the Committee for the Care and Use of Animals at the Okinawa Institute of Science and Technology.
Animals
Transgenic rats (ChAT‐Cre rats, male Long‐Evans) that express Cre‐recombinase in cholinergic cells were used in the experiments (Witten et al., 2011). A few, male wild‐type Long‐Evans rats (Charles River Laboratories Japan, Japan) were also used in a control group for clozapine‐N‐oxide (CNO) administration. All animals were provided with food and water ad libitum, and housed under standard conditions (12‐h/12‐h light/dark cycle, at 23 °C) until 5 days before behavioural experiments. Thereafter, animals were food‐restricted to approximately 85% of their average weight.
Surgical procedures
Viral injections or sham surgery were conducted on a stereotaxic frame under isoflurane anaesthesia (initial, 3.5%; maintenance, 2.5–3%). We bilaterally injected AAV8‐DIO‐hM3Dq‐mCherry (UNC Vector Core, USA) into the DLS, which resulted in expression of the receptor specifically on the cholinergic interneurons (Experiment 1, 3, 4 and 5). For ablation of CINs (Experiment 6), we injected AAV8‐mCherry‐flex‐diphtheria toxin (DTA, a kind gift by Dr. Naoshige Uchida, UNC Vector Core, USA). For sham operation, animals underwent the same surgical procedure but received no viral injection. Coordinates of injection sites were as follows: from bregma or dural surface, AP: +0.8 mm, ML: 3.7 mm, and depth: 4.6 and 3.3 mm. Injection volume was 0.5 μl in each location. After 10 days of recovery, behavioural experiments were commenced.
Behavioural experiment and analysis
Behavioural apparatus
We used an operant chamber (Med Associates. Inc., USA) equipped with two levers, food magazine and a house light. Throughout a session, a house light was tuned on and both left and right levers were presented.
Continuous reinforcement schedule (CRF)
After animals were habituated to a chamber on the first day, rats learned to lever‐press for a food reward (a sucrose pellet, 45 mg, TestDiet, USA) on a CRF schedule. Daily CRF sessions lasted either until 60 pellets were obtained (60 lever‐presses) or until 40 min passed. Both left and right levers were presented but only a left side was active, so that animals learnt to press the left lever almost exclusively (Fig. 2B). Animals moved to a next stage after completing at least four CRF sessions.
Figure 2.
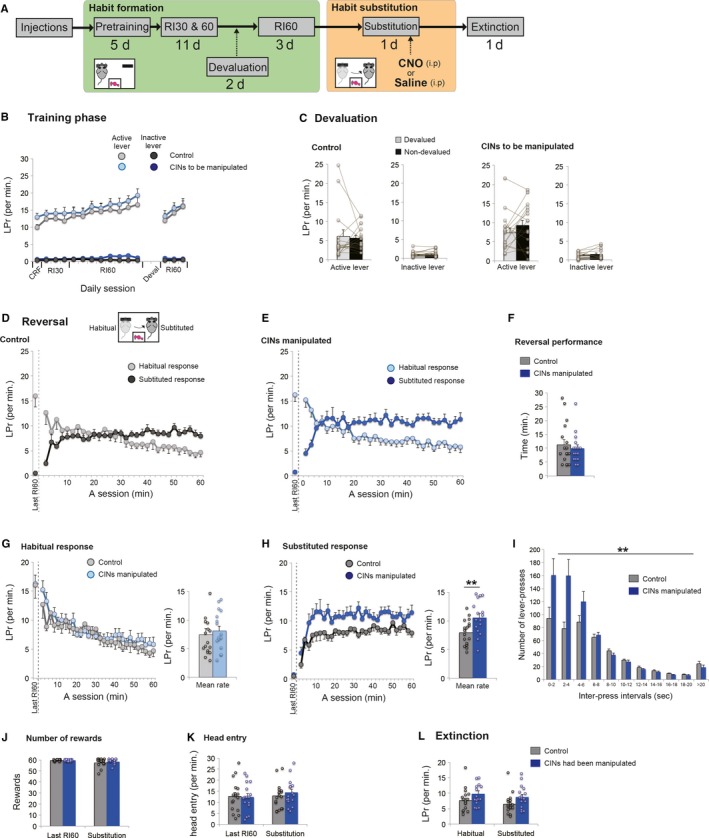
Chemogenetic activation of CINs in a habit substitution task. (A) A flow chart of experiment 1. Animals formed a habitual response under RI30 and RI60 (habit formation period). Next, a habit substitution task was commenced, during which CINs were manipulated. (B) Lever‐press rate (LPr) during habit formation. (C) Lever‐press rate during a 5‐min extinction test under the outcome devaluation procedure. Note that both groups showed insensitivity to the outcome devaluation, indicating that a habit has been formed successfully. (D and E) Lever‐press rate in a habit substitution task that involves reversal of responses. Animals needed to inhibit one habitual response and substitute a new response to an opposite lever. Both control rats (D) and rats with activation of CINs (E) show a successful reversal of lever‐press responses in the course of the session. Lever‐press rate is calculated by 2‐min bins. Lever‐press rate on the last day of RI60 is shown on the left‐hand of each panel (separated by a dashed line). (F) Evaluation of animal's reversal performance. Based on the number of lever‐presses in 2‐min bins (D and E), we compared the first time that animals scored the greater number of substituted responses than habitual ones. No statistical difference is seen in reversal performance. (G and H) Comparisons of habitual (G) and substituted (H) response rate between control rats and rats with activation of CINs. The neighbouring bar plots indicate mean response rate in the session. Note that in contrast to habitual responses, the number of substituted responses is significantly increased in rats with CIN activation than control rats. (I) Histogram of inter‐press intervals of substituted responses. Those responses are divided into 10 bins with 2‐s intervals (J and K) The number of rewards obtained (J) and head entry rate (K) during a habit substitution task. (L) Lever‐press rate in a following extinction test. Scatter plots indicate individual data points. Double asterisks indicate P < 0.01. Final group size is follows: control rats, n = 17 (virus‐control = 9; CNO‐control = 8); rats with CIN activation, n = 16.
Habit formation period (RI30 and RI60)
As established elsewhere (Dickinson et al., 1983; Dickinson, 1985; Yin et al., 2004; Yin & Knowlton, 2006; Gremel & Costa, 2013; O'Hare et al., 2015; Gremel et al., 2016), a random‐interval schedule of reinforcement (RI) was used for the habit formation. Daily sessions lasted 60 min. Both left and right levers were presented throughout the session, but only the left lever was active. During the initial 3 days, animals started on an RI30 schedule in which a reward was available at random times with an average interval of 30 s. For the next 8 days, animals performed the RI60 schedule (average interval of 60 s). These procedures resulted in a lever‐press habit (Figs 2C and 3C).
Figure 3.
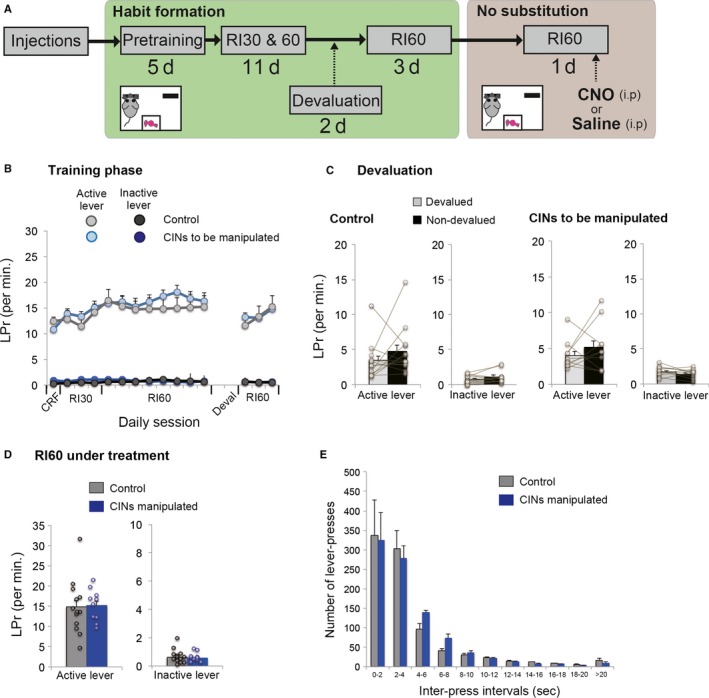
Chemogenetic activation of CINs in responses under a RI60 schedule without habit substitution. (A) A flow chart of experiment 3. Similar to the Experiment 1, animals formed a habit under the random‐interval schedule. In a test day, animals with or without CIN activation perform the same RI60 schedule without a switch of habitual responses. This procedure controls the effect of activating CINs on lever‐press behaviour and its reinforcement in general. (B) Lever‐press rate during a habit formation period under RI schedules. (C) Lever‐press rate during an outcome devaluation test. Note that both groups successfully formed a habit as confirmed by insensitivity to the outcome devaluation. (D) Lever‐press rate on both active and inactive levers in a test day under the same RI60 schedule. (E) Inter‐press intervals of habitual responses to an active side on the same testing day. Note that CIN activation had no effect on responses. Scatter plots indicate individual data points. Final group size is follows: control rats, n = 13 (virus‐control = 5; CNO‐control = 8); CINs manipulated rats, n = 10.
Outcome devaluation test
To confirm habit formation, we performed an outcome devaluation test on two successive days (Dickinson et al., 1983; Dickinson, 1985; Yin et al., 2004; Yin & Knowlton, 2006; Gremel & Costa, 2013; O'Hare et al., 2015; Gremel et al., 2016). Animals were given access to either sucrose pellets (devalued condition) or grain pellets (non‐devalued condition) freely, before a 5‐min extinction test. The sucrose–grain, grain–sucrose order was counterbalanced within an experimental group. Habitual response is by definition insensitive to outcome devaluation, so we compared the number of lever‐presses during extinction between devalued and non‐devalued conditions. After the devaluation procedure, animals were again trained on the same RI60 for additional 3 days prior to a testing session.
Experiment 1: Cholinergic activation in a habit substitution task
As described in Fig. 2A, once animals had formed a habit, they moved to a habit substitution task in which a contingency was reversed; a previously inactive lever now became active while the previously active lever was no longer rewarding. Thus, animals needed to suppress a habitual response and substitute a newly reinforced response on the opposite lever. The reinforcement schedule remained the same (RI60). For chemogenetic activation of CINs, animals that had been injected virus were administered clozapine‐N‐oxide (CNO, 3 mg/kg, dissolved by dimethyl sulfoxide (DMSO) in saline, i.p.) 40 min prior to a testing session. Control animals that underwent sham surgery were similarly treated by CNO. Virus‐injected control rats received saline containing DMSO. Importantly, CINs were activated only when CNO was administered to animals that had previously been injected with the virus for DREADDs (Roth, 2016). Final group size for this experiment is as follows: control rats, n = 17 (virus‐control = 9; CNO‐control = 8); rats with CIN activation, n = 16.
Experiment 2: Second devaluation test immediately after the habit substitution task
To examine whether the substituted responses in the habit substitution task were habitual or goal‐directed, another group of intact rats was used to perform the outcome devaluation test immediately after the habit substitution task. Rats performed exactly the same experimental schedule as experiment 1 (Fig. 2A), and subsequently were tested by the second devaluation procedure (Fig. S1A). A total of eight intact rats were used in this experiment.
Experiment 3: Cholinergic activation under continuation on RI60 without reversal
As in Fig. 3A, after animals had formed a habit, chemogenetic activation of CINs was applied while they continued responding under the same RI60 schedule without reversal of responses. Drug administration (CNO or saline) was made i.p. 40 min before the testing session. Final group size is follows: control rats, n = 13 (virus‐control = 5; CNO‐control = 8); CINs manipulated rats, n = 10.
Experiment 4: Cholinergic activation in an early phase of learning
As in Fig. 4A, chemogenetic activation of CINs was applied in an early phase of learning. Animals completed CRF and 2 days of RI30, and received either CNO or saline on third day of RI30. Drug administration (CNO or saline) was made i.p. 40 min before the testing session. Next day, we performed the outcome devaluation test to investigate a possibility of accelerated habit formation by the prior cholinergic activation. Final group size is as follows: control rats, n = 13 (virus‐control = 7; CNO‐control = 6); CINs manipulated rats, n = 10.
Figure 4.
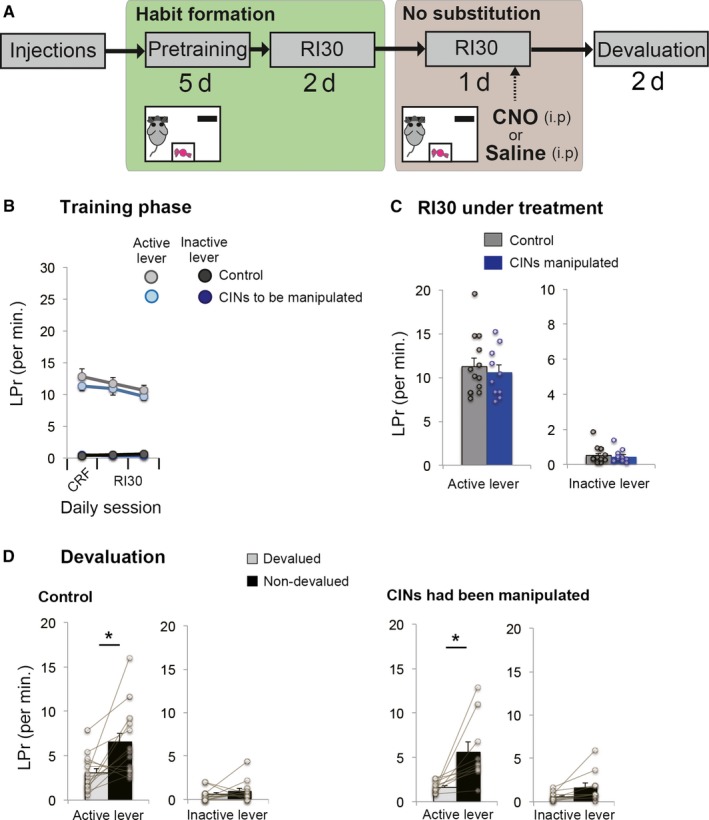
Chemogenetic activation of CINs in an early phase of learning. (A) A flow chart of experiment 4. Animals underwent a continuous reinforcement schedule followed by RI30. Unlike the other experiments, chemogenetic activation was performed 2 days after the initiation of RI30 schedule. After the one‐day activation, the devaluation test was performed to examine whether the prior treatment accelerated habit formation. This procedure controlled for the facilitative effect of activating CINs on new lever‐press learning or habit formation, regardless of switching habits. (B) Lever‐press rate before cholinergic manipulation. Experiment and control groups showed similar baseline lever‐press rates. (C) Performance of RI30 under cholinergic manipulation. Cholinergic activation does not affect lever‐press performance in the early phase of learning. (D) A devaluation test following cholinergic activation showed that both control and rats with cholinergic interneurons that had been activated are sensitive to outcome devaluation, indicating that there is no effect of the cholinergic manipulation on lever‐press rate or habit learning before the habit has been formed. Scatter plots indicate individual data points. Final group size is follows: control rats, n = 13 (virus‐control = 7; CNO‐control = 6); CINs manipulated rats, n = 10.
Experiment 5: Cholinergic activation in a substitution (reversal) task before a lever‐press becomes habitual
The experimental flow is indicated in Fig. 5A. Animals completed CRF and 2 days of RI30. Next, under treatment with either CNO or saline, animals were tested on a substitution (reversal) task under the same RI30 schedule. Importantly, at this point, their lever‐press responses have not yet become habitual, as our pilot experiment (data not shown) and Experiment 4 in this study (Fig. 4D) have determined that only 2 to 3 days of RI30 were not sufficient for animals to make a habit. On the last day, animals performed an extinction test. Final group size is follows: control rats, n = 12 (virus‐control = 6; CNO‐control = 6); rats with CIN activation, n = 10.
Figure 5.
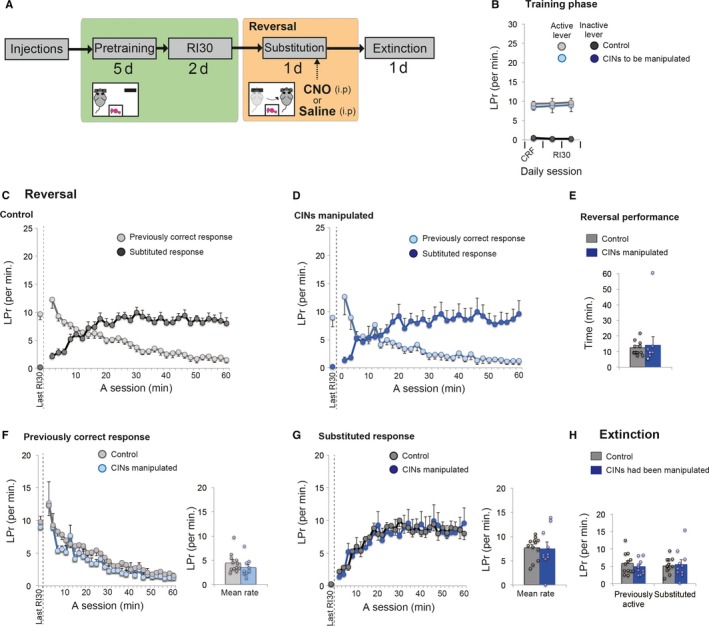
Chemogenetic activation of CINs in a substitution (reversal) task before a habit has been developed. (A) A flow chart of experiment 5. As in Experiment 4, animals underwent continuous reinforcement schedule followed by 2 days of RI30. On the third day of RI30, chemogenetic activation was applied when rats performed a substitution task in which response contingency was reversed. After the one‐day activation, an extinction test was conducted to evaluate learning of response contingency. (B) Lever‐press rate during training before cholinergic activation. Experiment and control groups showed similar baseline lever‐press rates on the last day. (C and D) Lever‐press rate during reversal under RI30. Both control rats (C) and rats with activation of CINs (D) showed a successful reversal of lever‐press responses in the course of the session. Lever‐press rate of the last day of RI30 is shown on the left‐hand of each panel (separated by a dashed line). (E) Animal's reversal performance. Based on the number of lever‐presses in 2‐min bins (C and D), we compared the first time that animals scored a greater number of substituted responses with presses on the previously correct lever. F and G, Comparison of previously correct (F) and substituted (G) response rates between control and experimental groups. The neighbouring bar plots indicate mean response rate in the session. (H) Lever‐press rate in the subsequent extinction test. Scatter plots indicate individual data points. Final group size is follows: control rats, n = 12 (virus‐control = 6; CNO‐control = 6); rats with CIN activation, n = 10.
Experiment 6: Effect of specific ablation of cholinergic interneurons on a substitution task
As in Fig. 6B, once animals had formed a habitual response as determined by the outcome devaluation, they received AAV8‐mCherry‐flex‐DTA or a control agent (saline). As our preliminary experiment has confirmed that the ablation occurred within 4 days after DTA injections, we resumed additional RI60 after recovery for 4 days. After completing RI60, both groups performed a habit substitution task in the same manner as Experiment 1. Final group size is follows: control rats, n = 18; lesioned rats, n = 18.
Figure 6.
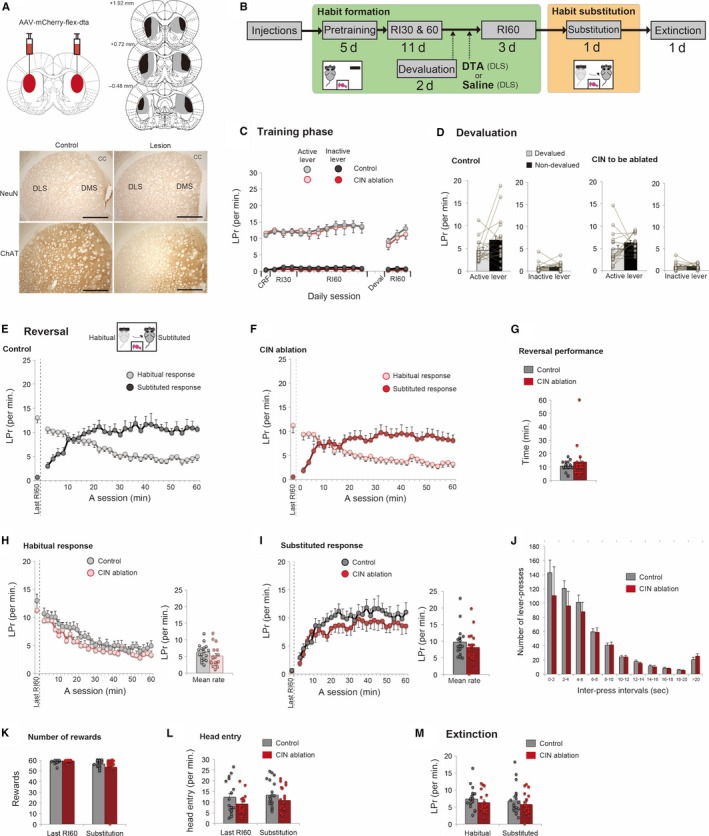
Effect of DTA‐mediated cholinergic ablation on a habit substitution task. (A) Injection of AAV‐mCherry‐flex‐DTA into DLS causes specific ablation of cholinergic interneurons. Scale = 1 mm. Absence of labelled neurons is only evident in ChAT staining after the lesion and it is selective to DLS. (B) A flow chart of experiment 6. Animals formed a habitual response under RI30 and RI60 (habit formation period). Next, those animals are divided into two groups based on their lever‐press performance (Lesion or Control). After the 4‐day recovery period, additional RI60 was continued and later a habit substitution task was commenced. An extinction test was conducted after the habit substitution. (C) Lever‐press rate (LPr) in a habit formation phase. (D) Lever‐press rate during a 5‐min extinction test under the outcome devaluation procedure. Note that both groups showed insensitivity to the outcome devaluation, indicating that a habit has been formed successfully. (E and F) Lever‐press rate in a habit substitution task that involves reversal of responses. Both control rats (E) and rats with ablation of CINs (F) successfully reversed their responses in the course of the session. Lever‐press rate of the last day of RI60 is shown on the left‐hand of each panel. (G) Animal's reversal performance. This comparison is based on the first time that animals scored a greater number of substituted responses than habitual ones in 2‐min bins. (H and I) Comparisons of habitual (H) and substituted (I) response rate between control and cholinergic ablated rats. The corresponding bar plots indicate the mean response rate across a session. (J) Histogram of inter‐press intervals of substituted responses. (K and L) The number of obtained rewards (K) and head entry rate (L) during a habit substitution task. (M) Lever‐press rate in the subsequent extinction test. Scatter plots indicate individual data points. Final group size is follows: control rats, n = 18; lesioned rats, n = 18.
Behavioural analyses
Lever‐press rate on active and inactive levers during a daily 60‐min session was calculated. For test sessions, the response rate on each lever throughout a session was calculated and binned into 2‐min intervals. Inter‐press intervals were binned into 2‐s intervals. To examine reversal performance during substitution of responses, we defined reversal as the first time that animals made more substituted responses than previously correct responses in a 2‐min bin. In case that an animal did not make a successful reversal in a 60 min session, its reversal performance (time) was measured as 60 min (Figs 5E and 6G). The head entry rate and the number of rewards obtained were also measured during testing sessions.
Electrophysiological recording
For electrophysiology experiments, a subset of animals was injected with the same virus: AAV8‐DIO‐hM3Dq‐mCherry at post‐natal day (P) 14 in the right hemisphere using the following stereotaxic coordinates: AP +0.7, ML +1.5 from bregma, and DV ‐1.7 from dura. To allow ample expression of the hM3Dq receptors on CINs, rats were used for electrophysiological recordings at ages between P50 and 60. Rats were anaesthetized with isoflurane, decapitated, and the brain was quickly removed and placed in oxygenated NMDG cutting solution containing in mM: 92 NMDG, 2.5 KCl, 1.25 NaH2 PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na‐ascorbate, 3 Na‐pyruvate, 0.5 CaCl2·, 10 MgCl2 and titrated to 7.2–7.4 pH with HCl. 300 μm striatal slices were obtained using a vibratome (VT1200S, Leica), incubated at 34 °C for at least 30 min and rested at room temperature for 1 h in oxygenated ACSF containing in mM: 126.0 NaCl, 2.5 KCl, 2.0 CaCl2, 2.0 MgCl2, 18.0 NaHCO3, 1.25 NaHPO4, 10.0 glucose. Whole‐cell and loose cell‐attached recordings were acquired with pClamp 10 software with Multiclamp 700B amplifier and Digidata 1440A (Molecular Devices, CA). CINs visualized by large somata under differential interference contrast (DIC) were identified as mCherry positive and negative with fluorescence camera (Olympus DP72). Glass pipettes with resistance between 4 and 6 MΩ were filled with a potassium‐based internal solution containing in mM: 119 K‐MeSO4, 12 KCl, 1 MgCl2, 0.1 CaCl2, 10 HEPES, 1 EGTA, 0.4 Na‐GTP, 2 Mg‐ATP, (280–300 mOsm, pH 7.3 adjusted with KOH). Whole‐cell or loose‐seal cell‐attached (seal of less than 100 MΩ) recordings were obtained to monitor spontaneous firing activity. Once a stable baseline was recorded, the synthetic ligand, CNO (10 uM), was bath applied for 5 min at 2 ml/min flow rate.
Histology
After the completion of behavioural experiments, the animals were deeply anaesthetized by isoflurane or sodium pentobarbital and subsequently perfused with 4% paraformaldehyde in 100 mM sodium phosphate buffer at pH 7.4. Brains were extracted and post‐fixed in the same fixative. After gelatin embedding, coronal sections (60 μm) were prepared using a vibratome (VT1000S, Leica) and were collected sequentially into four vials.
To visualize the expression of hM3Gq on CINs, we performed dual fluorescent staining against choline acetyltransferase (ChAT) and mCherry tagged with the injected virus (Fig. 1A). Sections were prepared by blocking with 5% goat donkey and 0.2% Triton‐×100 in PBS for one hour. Primary antibody staining was carried out in 2% goat serum and 0.2% Triton‐×100 in PBS for 48 h at 4 °C (1 : 100, goat anti‐ChAT, Millipore AB144P; 1 : 500, rabbit anti‐mCherry, Abcam ab167453). Secondary antibody staining for fluorescent images was incubated in 2% goat serum and 0.2% Triton‐×100 in PBS for 4 h at 25 °C (1 : 400, donkey anti‐goat: Alexa 488; 1 : 400, anti‐rabbit: Alexa 594, Life Technologies A11055 and A21206, respectively). Stained sections were mounted on slide grasses with a coverslip by Vectashield (Ventor Laboratories, USA).
Figure 1.
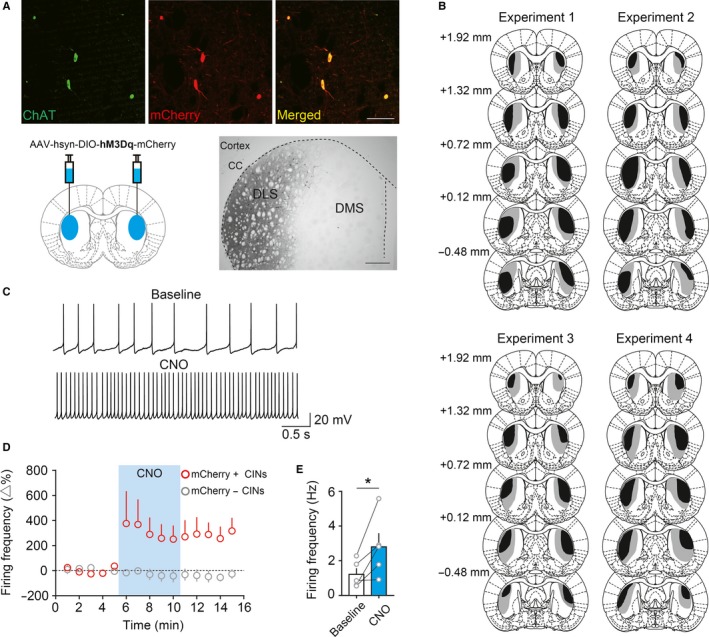
Specific DREADD (hM3Dq) activation of cholinergic interneurons in dorsolateral striatum. (A) Specific viral infection to CINs in DLS. AAV‐hsyn‐DIO‐hM3Dq‐mCherry was injected into bilateral DLS (Blue areas). This yields specific viral expression to CINs as ChAT and mCherry are co‐localized in fluorescent images. A representative DAB‐stained section shows the selective viral spread to DLS. Scale bars, Fluorescent images = 100 μm; Brightfield DAB = 500 μm. CC, corpus callosum. B, The extent of viral spread showing the largest (grey) and the smallest (black) viral spread in experimental groups. Distance from bregma is shown on the left‐hand. Note that the viral spread is restricted to DLS. (C) in vitro electrophysiological recordings from CINs showing an increase in firing rate under CNO application. (D) Time course of the change in firing rate of mCherry‐positive CINs (red, n = 5) and mCherry‐negative CINs (grey, n = 2). (E) A significant increase in firing rate of mCherry‐positive CINs under CNO. Scatter plots indicate individual data points. An asterisk indicates P < 0.05.
The extent of viral expression was determined by single immunohistochemical staining of mCherry using a metal enhanced 3,3′‐diaminobenzidine‐tetrahydrochloride method (DAB). Sections were pretreated with 3% H2O2 for 30 min and blocked in 5% goat serum. These sections were incubated in a primary antibody as mentioned earlier, and in a subsequent biotinylated secondary antibody (1 : 400, goat anti‐rabbit : biotin, Life Technologies, B2770). The DAB staining included an extra incubation step with streptavidin‐conjugated horseradish peroxidase in 0.2% Tween‐20 and PBS for one hour at 22 °C (Vectastain ABC Kit, PK‐4000) and finally resolved using DAB (ME DAB Substrate Kit, #34065, Thermo Scientific, USA). DAB‐stained sections were mounted and coverslipped by Entellan new (Merck, Germany).
The extent of DTA lesions was examined by ChAT staining using the same immunohistochemical procedures (primary, 1 : 100, goat anti‐ChAT, Millipore AB144P; secondary, 1 : 400, rabbit anti‐goat : biotin, Life Technologies, A10518) followed by DAB visualization. To confirm that lesions caused by DTA were specific to cholinergic cells and did not affect other cells, NeuN staining was performed using primary (1 : 1000, mouse anti‐NeuN, Abcam ab104224), secondary antibody staining (1 : 500, goat anti‐mouse : biotin, Life Technologies, B2763), and DAB.
Images of representative sections were obtained by confocal microscopy (LSM‐510, Leica) or digital light microscopy (BZ‐9000, Keyence). To determine the largest or smallest viral spread in each group of animals (Fig. 1B), we referred to the adjacent DAB‐stained sections and projected the extent of the viral spread on a standard rat brain atlas (Paxinos & Watson, 2004).
Experimental design and statistical analyses
A between‐subject comparison with treatment type as a factor was used for behavioural analysis. We made two controls for DREADDs: CNO administration (sham surgery followed by CNO administration) and viral injection (viral injection followed by saline administration). Neither intervention works alone (Ferguson et al., 2011; Roth, 2016). In fact, behavioural parameters examined in this study showed no statistical differences between two controls. Here, we combined them as a single control group for data presentation and statistical tests, but the number of animals used for each control is indicated above as well as in figure legends. In the DTA experiment, we compared two groups of rats with or without cholinergic ablation. In a slice electrophysiological experiment to test whether CNO increased firing of CINs, we compared the firing rate under CNO application with its baseline activity by a within‐subject comparison.
SPSS statistics version 21 (SPSS Japan, Inc., Japan) was used for all the statistical tests. A change in firing rate of CINs relative to its baseline was compared using a Student's paired t‐test. In an outcome devaluation test, lever‐press rates under devalued and non‐devalued conditions were analysed by a Student's paired t‐test. For a habit substitution task, we analysed the lever‐press rate using a two‐way repeated measures anova with treatment type as a between‐subject factor and time as a within‐subject factor. Inter‐press intervals were also analysed by a two‐way repeated measures anova with treatment type and inter‐press interval as between‐ and within‐subject factors, respectively. Reversal performance, the number of head entries and of obtained rewards, was examined by a Student's unpaired t‐test between groups. A difference of P < 0.05 was considered significant. Data are shown as mean and SEM.
Results
We injected AAV‐hsyn‐DIO‐hM3Dq‐mCherry bilaterally into DLS. This resulted in specific expression of the virus on CINs, identified by co‐localization of mCherry and ChAT (Fig. 1A). The viral spread was restricted to DLS (Fig. 1A, B). To confirm functionality of DREADDs, we performed electrophysiological recording from CINs in slice preparations (Fig. 1C–E). The spontaneous firing rate of CINs increased significantly relative to baseline during CNO application (Fig. 1C and E, Student's paired t‐test, P = 0.045). The CNO treatment increased the firing rate of mCherry‐positive CINs. The activity of mCherry‐negative CINs was not affected (Fig. 1D).
Figure 2A shows a flow chart of Experiment 1. Chemogenetic manipulation of CINs was restricted to a session in which animals performed a habit substitution task. Prior to habit substitution, during the habit formation period, rats in both control and experimental groups responded similarly and almost exclusively to an active lever (Fig. 2B). Continued RI schedules led animals to form a lever‐press habit, as confirmed by insensitivity to outcome devaluation (Fig. 2C); statistical comparisons between devalued and non‐devalued conditions were as follows: Student's paired t‐test, control group on an active lever, t 16 = 0.352, P = 0.729; control group on an inactive lever, t 16 = −1.249, P = 0.230; to be manipulated group on an active lever, t 15 = −1.190, P = 0.253; to be manipulated group on an inactive lever, t 15 = −1.014, P = 0.326. Following additional RI60 for 3 days, nearly identical response rates were observed between groups on the last day of RI60 (Fig. 2B): Student's unpaired t‐test, the response rate on an active lever, t 31 = −0.85, P = 0.932; the rate on an inactive lever, t 31 = −1.323, P = 0.195.
Next day, we tested animals on a habit substitution task involving a change in contingencies, in which animals had to respond on the opposite, previously inactive lever. Both control rats and rats with cholinergic activation made successful reversal of responses, as lever‐press rates between habitual and substituted responses crossed over mid‐session (Fig. 2D and E). There was no statistical difference in reversal performance between groups (Fig. 2F, Student's unpaired t‐test, t 31 = 0.616, P = 0.542). We then compared habitual and substituted responses separately. Between‐group comparison of the habitual response rate showed no difference (Fig. 2G); a two‐way repeated measures anova with treatment and time as factors indicated no significant main effect of treatment (F 1,31 = 0.219, P = 0.643), and no treatment by time interaction (F 1,31 = 1.403, P = 0.205). On the other hand, examining the rate of substituted responses revealed a clear difference (Fig. 2H); a two‐way repeated measures anova indicated a significant main effect of treatment (F 1,31 = 8.315, P = 0.007) without treatment by time interaction (F 1,31 = 1.009, P = 0.424), showing that activation of CINs significantly increased the rate of substituted responses. We analysed distribution of inter‐press intervals of substituted responses (Fig. 2I). A two‐way repeated measures anova with treatment and inter‐press interval as factors indicated a significant main effect of treatment (F 1,31 = 8.754, P = 0.006) and significant interaction between treatment and inter‐press intervals (F 1,31 = 5.413, P = 0.004), suggesting that a significant increase in the number of substituted responses was due to more frequent burst‐like responses with shorter inter‐press intervals (Fig. 2I). In the habit substitution task, there were no effects of CIN activation on the number of rewards obtained (Fig. 2J, Student's unpaired t‐test, t 31 = −0.958, P = 0.346) or head entry rate (Fig. 2K, Student's unpaired t‐test, t 31 = −0.713, P = 0.481), which indicates no change in reward‐seeking behaviour. A following extinction test resulted in no effect of prior treatment (Fig. 2L). A two‐way repeated measures anova with treatment and levers as factors revealed no statistical differences between groups (main effect of treatment, F 1,31 = 3.656, P = 0.065; interaction, F 1,31 = 0.000, P = 0.995). In sum, chemogenetic activation of CINs increased the performance of substituted responses when a previous, but now invalid, habit had to be modified.
It is important to determine whether the substituted response was habitual or not. In Experiment 2, intact rats went through all the same procedure as Experiment 1 until the habit substitution task, and performed second outcome devaluation test immediately after the substitution (Fig. S1). The results from this procedure indicated that the substituted responses were insensitive to outcome devaluation (Fig. S1E); Devaluation caused no significant difference in the number of responses on the previously active lever, t 7 = 1.424, P = 0.197; or on a substituted lever, t 7 = −0.117, P = 0.910 (Student's paired t‐test), suggesting that animals replaced an old habit with a new habitual response rather than with a new goal‐directed response.
Next, we sought to address whether chemogenetic activation of CINs amplifies the performance of rewarded responses in general, or it specifically affects a substituted response when a switch of habitual responses is required. In Experiment 3, animals similarly acquired a habit and were manipulated their CINs while responding under the same RI60 schedule without reversal (Fig. 3A). Rats in both control and experiment groups successfully formed a habit after extensive RI schedules (Fig. 3B), as confirmed by insensitivity to outcome devaluation (Fig. 3C): Student's paired t‐test, comparing non‐devalued and devalued condition showed no significant differences (control group active lever, t 12 = −1.236, P = 0.240; control group inactive lever, t 12 = −1.767, P = 0.103; experiment group active lever, t 9 = −0.944, P = 0.370; experiment group inactive lever, t 9 = 1.012, P = 0.338). On the last day of RI60, control and experiment groups showed nearly identical response rates (Fig. 3B): Student's unpaired t‐test, response rate on active lever, t 21 = 0.122, P = 0.904; rate on an inactive lever, t 21 = −0.576, P = 0.571. Subsequently, animals with or without cholinergic activation performed the same RI60 schedule without a change in contingency. Comparing lever‐press responses on either lever showed no differences (Fig. 3D): Student's unpaired t‐test between groups on the active lever, t 21 −0.186, P = 0.855; on the inactive lever, t 21 = 0.246, P = 0.808. Similarly, inter‐press intervals of responses to the continuingly active lever were not affected (Fig. 3E), as a two‐way repeated measures anova indicated no main effect of treatment (F 1,21 = 0.034, P = 0.855) and no interaction between treatment and inter‐press intervals (F 1,21 = 0.213, P = 0.723). These results demonstrate that chemogenetic activation of CINs has no general effect on habitual responses (Fig. 3), but it does affect the substituted responses when a switch of habitual responses is required (Fig. 2).
The result of Experiment 3 could have been due to a ceiling effect because animals had already acquired a fast response rate (Fig. 3B and D). To rule out this possibility, in Experiment 4, we activated CINs in an earlier phase of habit learning (Fig. 4A). Here, cholinergic interneurons were activated on the third day of RI30, and we found that the lever‐press performance on either side of levers was not affected by the activation (Fig. 4C); Student's unpaired t‐test between groups on the active lever, t 21 = −0.512, P = 0.614; on the inactive lever, t 21 = −0.335, P = 0.741. Thus, cholinergic activation does not simply facilitate the performance of currently rewarded responses.
Another question is whether activation of CINs during habit learning might facilitate the acquisition of a lever‐press habit. To test for this possibility, we performed an outcome devaluation test following the cholinergic activation (Fig. 4A and D). The results still indicated sensitivity to the devaluation (Fig. 4D, control group active lever, t 12 = −3.059, P = 0.01; control group inactive lever, t 12 = −0.899, P = 0.386; experiment group active lever, t 9 = −3.235, P = 0.01; experiment group inactive lever, t 9 = −2.309, P = 0.046), demonstrating that cholinergic activation does not accelerate habit acquisition.
To further confirm the specific involvement of CINs in habit substitution, in Experiment 5, the same cholinergic activation was applied on third day of RI30, but the contingency was reversed (Fig. 5A). Note that the previous experiment (Experiment 4) has revealed that at this point on third day of RI30 a lever‐press response has not yet become habitual. So, we here attempted to clarify whether cholinergic activation affects a switch of responses while a response is goal‐directed. We found that cholinergic activation resulted in no change in reversal performance (Fig. 5C–E, Student's unpaired t‐test for reversal performance between groups, t 20 = −0.348, P = 0.732). Likewise, separate analysis revealed that response rates were unchanged either on the previously correct lever (Fig. 5F, t 20 = 1.196, P = 0.246) or on the substituted lever (Fig 5G, t 20 = 0.109, P = 0.914). These results suggest that cholinergic activation in DLS was rather specific to switching of habitual responses without an effect on a reversal under goal‐directed responses.
Lastly, we sought to address whether selective ablation of cholinergic interneurons from dorsolateral striatum influences the habit substitution (Fig. 6). To mimic Experiment 1 (cholinergic activation after habit formation), in Experiment 6, cholinergic ablation was made after a habit had been formed (Fig. 6B). Once we confirmed in both groups of animals that lever‐press responses have become habitual (Fig. 6C and D), AAV‐mCherry‐flex‐DTA was injected into DLS of ChAT‐Cre rats (Fig. 6A). As the injected virus deletes CINs within 4 days, we commenced additional RI60 after a 4‐day recovery period. Comparing the response rate on the last RI60 of ablated rats with that of the control rats, there was no difference (Fig. 6C): Student's unpaired t‐test between groups on the active lever, t 34 = 0.952, P = 0.348; on the inactive lever, t 34 = 0.523, P = 0.604. During a habit substitution task, both groups showed a successful reversal (Figs 6E and F) and its performance was unaffected by cholinergic ablation (Fig. 6G, Student's unpaired t‐test between groups, t 34 = −0.893, P = 0.378). A separate analysis indicated that habitual responses were not affected by the ablation (Fig. 6H, Student's unpaired t‐test between groups, t 34 = 1.276, P = 0.211). Focusing on the performance of substituted responses, rats with cholinergic ablation showed a slight decrease in the number of the substituted response, but it was not significant (Fig. 6I, Student's unpaired t‐test between groups, t 34 = 1.078, P = 0.289). These results suggest that ablation of cholinergic interneurons has no clear effect on the habit substitution. Combined with a facilitative effect of CIN activation, we consider that the cholinergic activation is sufficient to modulate animal's performance to replace an old habit with a new response, but its necessity might be limited.
Discussion
We investigated a possible role for cholinergic interneurons (CINs) in the neuronal mechanism by which one habit is substituted for another. After a change in contingencies, we found that the lever‐press rate on the newly reinforced lever was increased by chemogenetic activation of CINs, relative to control animals. This effect was specific to the newly reinforced responses. Previously reinforced, but now unreinforced responses were decreased to a similar extent in both experimental and control animals, indicating this was not due to a general increase in motor activity. There were also no differences in reward‐seeking behaviour between experimental and control animals, indicating that activation of CINs had no impact on motivation in general. Various control experiments in the present study demonstrated that activation of CINs had no effect on any of: habitual responding without a shift; response performance during habit learning; or reversal before a response becomes habitual. Taken together, these findings suggest a specific involvement of CINs of the dorsolateral striatum in a switch of habitual responses. To the best of our knowledge, this is the first study to associate striatal cholinergic interneurons with habit substitution.
We have previously used reversal learning and set‐shifting paradigms to test the function of cholinergic interneurons in the dorsomedial striatum (Aoki et al., 2015). In that study, we found that immunotoxic lesions of the cholinergic interneurons had no effect on reversal learning, but impaired set‐shifting by increasing the number of perseverative responses after a change in contingencies. In the present study, we chemogenetically increased firing of cholinergic interneurons in the DLS, and measured the effect on reversal of habitual responses, the nature of which was confirmed by outcome devaluation. Consistent with the previous study, we saw no impairment of reversal performance. However, we found an increased rate of the substituted response in the present study, in contrast to the previous study in which there was increased perseveration on the previous, now unreinforced, response. This difference may be due to the opposite direction of the manipulations, namely chemogenetic activation of cholinergic interneurons in the present study, and their destruction by the immunotoxic lesion in the previous study.
In the present study, we used a free operant procedure in which animals were able to lever‐press as much as they wanted, and under these conditions, we measured an increased rate of responding on the substituted response during chemogenetic activation of CINs. This selective increase in rate suggests that the substituted responses were more strongly facilitated under cholinergic stimulation. This effect would not have been observable if we had used a discrete trial procedure. We suggest that future studies of cholinergic function would benefit from a free operant procedure.
The physiological and morphological properties of the CINs are similar throughout the striatum (Kawaguchi et al., 1995). However, there are regional differences in striatal function. For example, several studies have indicated involvement of the dorsomedial striatum in action–outcome learning and dorsolateral striatum in habit learning (Yin et al., 2004; Yin & Knowlton, 2006). It is plausible to suggest that CINs provide a common behavioural operation, ‘a switch of behaviour’, in these different regions. Previous studies have focused on the dorsomedial striatum and these have shown that CINs are important for behavioural flexibility (Ragozzino, 2003; Brown et al., 2010; Bradfield et al., 2013; Okada et al., 2014; Stalnaker et al., 2016). However, few studies have investigated the role of CINs in the DLS using behavioural paradigms addressing the currently understood functional specialization of that region. Our findings support the idea that CINs in the dorsolateral striatum are also involved in switching of behaviour, as evidenced by the specific effect of activating CINs on the newly reinforced habitual responding after a change in reinforcement contingencies. Thus, there appears to be a common operation expressed in different ways according to the functional specialization of the striatal region.
Cholinergic interneurons have a distinctive firing pattern of tonic activity interrupted by pauses associated with motivationally significant events (Aosaki et al., 1995; Morris et al., 2004; Apicella et al., 2009). The present chemogenetic activation increases tonic firing rates and also occludes pauses. Our electrophysiological confirmation of the effect of the CNO in brain slices showed a manifold increase in firing rate. Although the mechanism of the pause is unknown, the level of excitation would surely overwrite any naturally occurring pause responses in vivo. Therefore, it is not possible to say whether the effects we observed were due to the increased tonic activity and acetylcholine concentration in general, or to overwriting pauses with induced firing activity.
How can we explain the specificity of CIN activation to increased substituted response rate? At this stage, we can only speculate. Both learning and performance factors may be involved. In relation to learning, activity‐dependent synaptic plasticity at cortico‐striatal synapses can involve either potentiation or depression. Reduced activation of muscarinic type 1 receptors (M1Rs) on spiny projection neurons has been implicated in depression of cortico‐striatal synapses (Wang et al., 2006) and such depression has been associated with habit suppression (O'Hare et al., 2015). On the other hand, activation of M1Rs (Martella et al., 2009; Lv et al., 2017) has been associated with potentiation. These mechanisms of M1R‐dependent modulation of synaptic plasticity may be involved in switching to new substituted responses by redirecting synaptic transmission in an alternative circuit. Alternatively, over‐activation of CINs might facilitate response generalization, in the light of evidence that the DLS is necessary for generalization of habitual responses (Hilario et al., 2012). Optogenetic stimulation of D2R‐expressing spiny projection neurons (D2 SPNs) in the DLS leads to more generalization of habitual responses, whereas stimulation of D1R‐expressing neurons (D1 SPNs) does not (Vicente et al., 2016). As D2 SPNs express only excitatory M1Rs in contrast to D1 SPNs containing both M1Rs and inhibitory M4R, it is possible that increasing cholinergic activity biases the overall balance between those two types of SPNs towards preferential involvement of D2 SPNs. This mechanism might lead to an increase in substituted response rate by facilitating generalization of lever‐press habits.
Conflict of interest
The authors declare no conflict of interest.
Data accessibility
Data size provided in this study is large, so that they will be available upon a request to corresponding authors.
Author contributions
S.A. and J.R.W. designed a concept and experiments of this study. S.A. performed surgery and behavioural experiments. S.A., A.W.L and Y.A. conducted histological analysis. A.Z. and S.Z. made a slice recording. S.A. and J.R.W. drafted and wrote this manuscript. All authors joined discussion and approved the final version of the manuscript.
Supporting information
Fig. S1. 2nd devaluation test immediately after animals undergo the habit substitution task.
Acknowledgements
This study was supported by Human Frontier Science Program (J.W.) and JSPS Grant‐in‐Aid for Challenging Exploratory Research and Grant‐in‐Aid for Young Scientists (A) (S.A.).
Edited by: Paul Bolam
All peer review communications can be found with the online version of the article.
Contributor Information
Sho Aoki, Email: sho.aoki@oist.jp.
Jeffery R. Wickens, Email: wickens@oist.jp.
References
- Aoki, S. , Liu, A.W. , Zucca, A. , Zucca, S. & Wickens, J.R. (2015) Role of striatal cholinergic interneurons in set‐shifting in the rat. J. Neurosci., 35, 9424–9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki, T. , Kimura, M. & Graybiel, A.M. (1995) Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J. Neurophysiol., 73, 1234–1252. [DOI] [PubMed] [Google Scholar]
- Apicella, P. , Deffains, M. , Ravel, S. & Legallet, E. (2009) Tonically active neurons in the striatum differentiate between delivery and omission of expected reward in a probabilistic task context. Eur. J. Neurosci., 30, 515–526. [DOI] [PubMed] [Google Scholar]
- Bradfield, L. A. , Bertran‐Gonzalez, J. , Chieng, B. & Balleine, B. W. (2013) The thalamostriatal pathway and cholinergic control of goal‐directed action: interlacing new with existing learning in the striatum. Neuron, 79, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, H.D. , Baker, P.M. & Ragozzino, M.E. (2010) The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J. Neurosci., 30, 14390–14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, A. (1985) Actions and Habits: the development of behavioural autonomy. Philos. Trans. R. Soc. Lon. B Biol Sci., 308, 67–78. [Google Scholar]
- Dickinson, A. , Nicholas, D.J. & Adams, C.D. (1983) The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Q. J. Exp. Psychol. B, 35, 35–51. [Google Scholar]
- Ferguson, S.M. , Eskenazi, D. , Ishikawa, M. , Wanat, M.J. , Phillips, P.E.M. , Dong, Y. , Roth, B.L. & Neumaier, J.F. (2011) Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat. Neurosci., 14, 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel, C.M. & Costa, R.M. (2013) Orbitofrontal and striatal circuits dynamically encode the shift between goal‐directed and habitual actions. Nat. Commun., 4, 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel, C.M. , Chancey, J.H. , Atwood, B.K. , Luo, G. , Neve, R. , Ramakrishnan, C. , Deisseroth, K. , Lovinger, D.M. et al (2016) Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron, 90, 1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario, M. , Holloway, T. , Jin, X. & Costa, R.M. (2012) Different dorsal striatum circuits mediate action discrimination and action generalization. Eur. J. Neurosci., 35, 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, Y. , Wilson, C.J. , Augood, S.J. & Emson, P.C. (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci., 18, 527–535. [DOI] [PubMed] [Google Scholar]
- Lee, I.H. , Seitz, A.R. & Assad, J.A. (2006) Activity of tonically active neurons in the monkey putamen during initiation and withholding of movement. J. Neurophysiol., 95, 2391–2403. [DOI] [PubMed] [Google Scholar]
- Lv, X. , Dickerson, J.W. , Rook, J.M. , Lindsley, C.W. , Conn, P.J. & Xiang, Z. (2017) M1 muscarinic activation induces long‐lasting increase in intrinsic excitability of striatal projection neurons. Neuropharmacology, 118, 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella, G. , Tassone, A. , Sciamanna, G. , Platania, P. , Cuomo, D. , Viscomi, M.T. , Bonsi, P. , Cacci, E. et al (2009) Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain, 132, 2336–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, G. , Arkadir, D. , Nevet, A. , Vaadia, E. & Bergman, H. (2004) Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron, 43, 133–143. [DOI] [PubMed] [Google Scholar]
- O'Hare, J.K. , Ade, K.K. , Sukharnikova, T. , Van Hooser, S.D. , Palmeri, M.L. , Yin, H.H. & Calakos, N. (2015) Pathway‐specific striatal substrates for habitual behavior. Neuron, 89, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, K. , Nishizawa, K. , Fukabori, R. , Kai, N. , Shiota, A. , Ueda, M. , Tsutsui, Y. , Sakata, S. et al (2014) Enhanced flexibility of place discrimination learning by targeting striatal cholinergic interneurons. Nat. Commun., 5, 3778. [DOI] [PubMed] [Google Scholar]
- Paxinos, G. & Watson, C. (2004) The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Ragozzino, M.E. (2003) Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol. Learn. Mem., 80, 257–267. [DOI] [PubMed] [Google Scholar]
- Ravel, S. , Sardo, P. , Legallet, E. & Apicella, P. (2006) Influence of spatial information on responses of tonically active neurons in the monkey striatum. J. Neurophysiol., 95, 2975–2986. [DOI] [PubMed] [Google Scholar]
- Roth, Bryan L. (2016) DREADDs for neuroscientists. Neuron, 89, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker, T.A. , Berg, B. , Aujla, N. & Schoenbaum, G. (2016) Cholinergic interneurons use orbitofrontal input to track beliefs about current state. J. Neurosci., 36, 6242–6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente, A.M. , Galvão‐Ferreira, P. , Tecuapetla, F. & Costa, R.M. (2016) Direct and indirect dorsolateral striatum pathways reinforce different action strategies. Curr. Biol., 26, R267–R269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Kai, L. , Day, M. , Ronesi, J. , Yin, H.H. , Ding, J. , Tkatch, T. , Lovinger, D.M. et al (2006) Dopaminergic control of corticostriatal long‐term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron, 50, 443–452. [DOI] [PubMed] [Google Scholar]
- Witten, I.B. , Steinberg, E.E. , Lee, S.Y. , Davidson, T.J. , Zalocusky, K.A. , Brodsky, M. , Yizhar, O. , Cho, S.L. et al (2011) Recombinase‐driver rat lines: tools, techniques, and optogenetic application to dopamine‐mediated reinforcement. Neuron, 72, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H.H. & Knowlton, B.J. (2006) The role of the basal ganglia in habit formation. Nat. Rev. Neurosci., 7, 464–476. [DOI] [PubMed] [Google Scholar]
- Yin, H.H. , Knowlton, B.J. & Balleine, B.W. (2004) Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur. J. Neurosci., 19, 181–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. 2nd devaluation test immediately after animals undergo the habit substitution task.
Data Availability Statement
Data size provided in this study is large, so that they will be available upon a request to corresponding authors.


