Summary
Background
Venous leg ulcers (VLUs) are typically painful and heal slowly. Compression therapy offers high healing rates; however, improvements are not usually sustained. Exercise is a low‐cost, low‐risk and effective strategy for improving physical and mental health. Little is known about the feasibility and efficacy of supervised exercise training used in combination with compression therapy patients with VLUs.
Objectives
To assess the feasibility of a 12‐week supervised exercise programme as an adjunct therapy to compression in patients with VLUs.
Methods
This was a two‐centre, two‐arm, parallel‐group, randomized feasibility trial. Thirty‐nine patients with venous ulcers were recruited and randomized 1 : 1 either to exercise (three sessions weekly) plus compression therapy or compression only. Progress/success criteria included exercise attendance rate, loss to follow‐up and patient preference. Baseline assessments were repeated at 12 weeks, 6 months and 1 year, with healing rate and time, ulcer recurrence and infection incidents documented. Intervention and healthcare utilization costs were calculated. Qualitative data were collected to assess participants’ experiences.
Results
Seventy‐two per cent of the exercise group participants attended all scheduled exercise sessions. No serious adverse events and only two exercise‐related adverse events (both increased ulcer discharge) were reported. Loss to follow‐up was 5%. At 12 months, median ulcer healing time was lower in the exercise group (13 vs. 34·7 weeks). Mean National Health Service costs were £813·27 for the exercise and £2298·57 for the control group.
Conclusions
The feasibility and acceptability of both the supervised exercise programme in conjunction with compression therapy and the study procedures is supported.
Short abstract
What's already known about this topic?
Almost 70% of all leg ulcers have a venous component.
Up to 30% of venous leg ulcers (VLUs) do not respond to compression alone, remain open after 1 year of treatment and need an average of 51 treatment visits to heal.
Adjunct therapies to compression are needed.
Exercise can form part of the therapeutic pathway, but evidence to determine whether exercise training has an effect on ulcer healing and quality of life is limited.
What does this study add?
The findings support the feasibility and acceptability of supervised exercise training as an adjunct therapy for adults with VLUs.
The preliminary data also support the potential effectiveness of exercise training in improving ulcer healing.
An appropriately powered, multicentre trial is required to confirm the clinical and cost‐effectiveness of the intervention.
Linked Comment: https://doi.org/10.1111/bjd.16523.
https://doi.org/10.1111/bjd.16618 available online
Almost 70% of all leg ulcers have a venous component.1 Occurrence of venous leg ulcers (VLUs) increases with age, with the U.K. prevalence in those > 65 years of age being estimated at about 3%.2 VLUs arise from venous valve incompetence and calf muscle pump insufficiency, which leads to venous stasis and hypertension. This results in microcirculatory changes and localized tissue ischaemia.3, 4 The natural history of VLUs is of a continuous cycle of healing and breakdown over decades:5 VLUs are typically painful and heal slowly, resulting in an impaired quality of life (QoL), social isolation and reduced work productivity.6 Treatment of this major health problem results in a considerable cost to the National Health Service (NHS): each ulcer costs up to £1981 per year;7 estimated total healthcare costs are between £198 million and £400 million per year,8, 9 with 65% of these costs occurring in the community.10
Lower‐limb compression therapy is an established first‐line therapy for VLUs,11 with approximately 50% of VLUs closing within 24 weeks.10 Nevertheless, recurrence rates remain high (up to 56% within 4 years).12 Furthermore, up to 30% of VLUs do not respond to compression alone, remain open after 1 year of treatment and need an average of 51 treatment visits to heal.10, 11, 13 Therefore, it is important to develop adjunct therapies to compression, which would improve healing outcomes.
Lifestyle factors, including nutrition, exercise and smoking, are mentioned in guidelines on the management of VLUs but receive relatively little emphasis.14 Exercise training might enhance ulcer healing and other aspects of health, and is routinely prescribed for other cardiovascular diseases (e.g. peripheral arterial disease and coronary artery disease).15, 16 In patients with VLUs, supervised calf muscle exercise has been shown to increase calf muscle pump function and improve lower‐limb haemodynamics,17, 18 as well as mobility and QoL.19, 20, 21 A recent systematic review suggested further research to determine whether exercise training has an effect on ulcer healing and QoL.22
Our team recently completed ‘FISCU’ [Feasibility of Implementing Supervised exercise training alongside Compression therapy in people with venous Ulceration; a National Institute for Health Research‐funded study (PB‐PG‐0213‐30029)]5 to assess the feasibility of a 12‐week supervised exercise programme combining aerobic, resistance and flexibility exercises as an adjunct therapy to compression in patients with VLUs. We report on rates of screening, eligibility, recruitment, retention, outcome completion, exercise adherence and adverse events (AEs). We also report on reasons for exclusion and nonconsent, sample characteristics, the distribution and completeness of potential primary outcomes, and provide information on preliminary data on effectiveness and healthcare resource use.
Patients and methods
A full description of methods is available in our previously published protocol paper.5 The study was a two‐arm, parallel‐group, randomized controlled feasibility trial conducted in two U.K. sites (Lincoln and Sheffield). Ethics approval was granted by the NHS National Research Ethics Service, Yorkshire and the Humber (Sheffield) Committee (14/YH/0091), and all participants provided written informed consent prior to enrolment. The trial was prospectively registered (Current Controlled Trials ISRCTN10205425).
Participants
Participants were recruited from community nursing and tissue viability teams or services, community and outpatient leg ulcer clinics, and newspaper advertisement. Inclusion and exclusion criteria are given in Table 1.
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| At least 18 years old | Unsuitable for or unable to exercise (determined by clinician) |
| Have at least one VLU of primarily venous aetiology (determined by clinician) with maximum diameter of at least 1 cm | Unable or unwilling to tolerate lower‐limb compression |
| ABPI of at least 0·8 (recorded within the previous 3 months) | Insulin‐controlled diabetes mellitus |
| Able and willing to tolerate lower‐limb compression | Pregnancy |
| Coexisting skin conditions, vasculitis, deep venous occlusion or malignant/atypical ulceration | |
| Require major surgery | |
| Leg ulcer with maximum diameter < 1 cm | |
| Have had an ulcer at the same site within the previous 3 months | |
| Unable or do not wish to consent to participation in the trial |
VLU, venous leg ulcer; ABPI, ankle brachial pressure index.
Randomization, allocation concealment and blinding
Following baseline assessments, participants were randomly assigned 1 : 1 to an intervention group or a control group. Participants were stratified by ulcer size (maximum ulcer diameter 1–3 cm or > 3 cm in any direction). Outcome assessors were blinded to group allocation.
Interventions
All participants received standard compression therapy directed by experienced tissue‐viability nurses, following standard local practice. Patients were reviewed in clinics as considered clinically necessary, with no interference by the study team.
Participants randomized to the exercise group were invited to attend three sessions of supervised exercise each week for 12 weeks (total of 36 sessions) at one of the two study exercise training facilities (Sheffield Hallam University and University of Lincoln). For details on the exercise components see Table S1 (Supporting Information).
Study schedule and assessments
During visit 1, after written informed consent had been obtained and eligibility confirmed (which included a medical examination), the following baseline measurements were recorded at one of the two research centres (Sheffield Hallam University, University of Lincoln): (i) demographic data, including age, sex and socioeconomic status; (ii) clinical history, current medications, stature, body mass, ankle and calf circumference; (iii) ulcer size; (iv) ankle brachial pressure index (ABPI; a Doppler‐determined measurement of ABPI was performed according to the procedures of Aboyans et al.,23 unless a reading < 3 months old could be obtained from clinical records, following the patient's consent); (v) baseline exercise history; (vi) health‐related QoL (HRQoL) questionnaires (EQ‐5D‐5L and VEINES‐QOL);24, 25, 26 (vii) lower‐limb cutaneous microvascular function (methods and results reported elsewhere);27 (viii) physical fitness, using three items from the Senior Fitness Test (6‐min walking test, chair sit and reach, chair sit and stand)28 and ankle range of motion assessed using a bi‐plane ankle goniometer.
All participants were given a resource use diary to complete at home for the duration of the study (to conduct health economics analysis).
Participants were then randomized to one of the two groups, as described above.
At 12 weeks and 12 months, participants had the following measures and tests repeated: physical fitness, microvascular function, ulcer‐related clinical data (size, status and recurrence) and medications, body mass and HRQoL questionnaires. A copy of the resource use diary was also taken. A postal assessment involving the completion of HRQoL questionnaires was also undertaken at 6 months.
Feasibility and acceptability outcomes
Recruitment rates were measured as rate of invited participants who were eligible and consenting. Acceptability of allocation was assessed by examining reasons for dropout in discontinuing participants and comparing attrition rates between the two study groups. Suitability of measurement procedures was evaluated by outcome completion rates and reasons for missing data. Attrition rate was established as discontinuation of intervention and loss to follow‐up measurement for all conditions. The acceptability of the exercise programmes was assessed by using session attendance and compliance data and participant feedback via one‐to‐one semi‐structured interviews conducted with a subgroup of participants after the 3‐month follow‐up visit (detailed analysis will be presented elsewhere). The safety of exercise training was also assessed by exploring reasons for dropout from the exercise programme and the number and type of AEs that occurred in each group.
Sample size
Sample size calculation was based on willingness for randomization and aimed to recruit 80 participants within an 18‐month recruitment period (Table S1; see Supporting Information).
Data analysis
All analyses were conducted on an intention‐to‐treat basis, conducted in SPSS version 24 (IBM, Armonk, NY, U.S.A.). Missing data were reported by trial arm with description of underlying reasons.
Baseline
Summary tables report all baseline variables, and clinical, fitness and patient‐reported outcome variables. Continuous variables were summarized with descriptive statistics. Frequency counts and percentages were provided for categorical data.
Feasibility and acceptability
For success criteria, see Table 2. Outcomes used to assess the feasibility and acceptability of key trial parameters were rates of eligibility, recruitment, retention, outcome completion, exercise adherence and AEs.5 Group preference, reasons for exclusion and nonconsent, sample characteristics and the distribution of potential primary outcomes are presented.
Table 2.
Criteria for success/progression
| An appropriate primary outcome variable is defined |
| At least 67% of randomly assigned patients in the exercise group are compliant with the intervention (defined as at least 75% of the scheduled sessions completed as planned) |
| Loss to follow‐up at 12 months is < 20% |
| Patient preferences are not so strong that they result in the conclusion that a randomized controlled trial is not a feasible design |
Clinical, fitness and patient‐reported outcomes
Descriptive statistics are presented for clinical, fitness and patient‐reported outcomes at each time point.
Economic evaluation
A prospective economic evaluation was rehearsed to develop and refine the methods for a subsequent definitive trial (Appendix S1; see Supporting Information).
Results
Figure 1 shows the flow of participants through the trial. Recruitment took place between July 2014 and May 2016, with all follow‐up data collection completed by May 2017. The trial was extended for 3 months to allow extra recruitment time.
Figure 1.
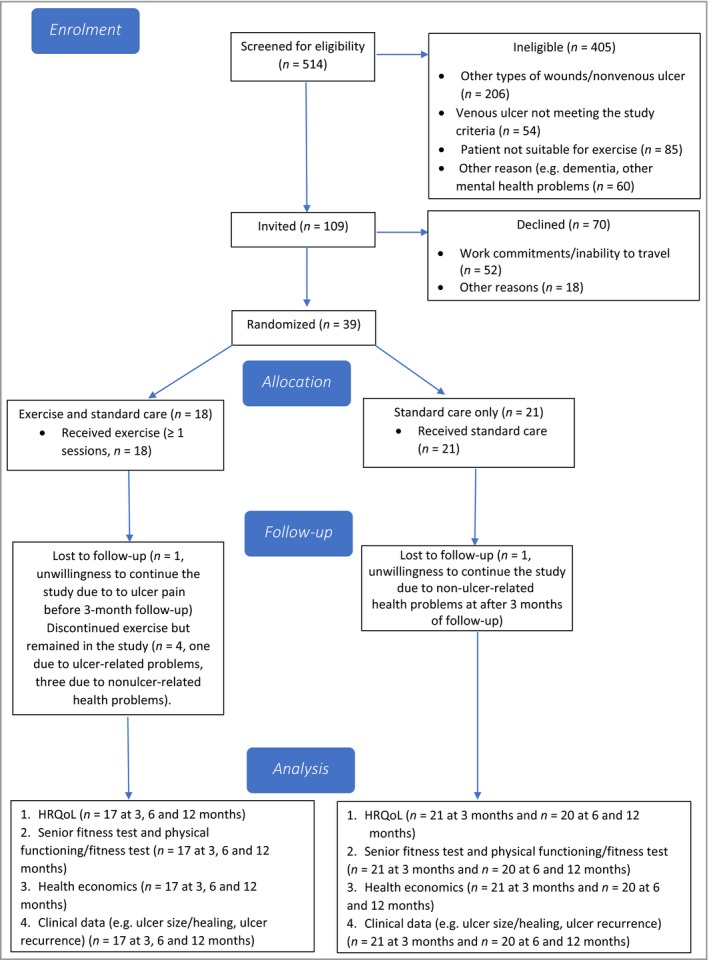
Flow of participants through the trial. HRQoL, health‐related quality of life.
Screening, eligibility and recruitment
A summary of feasibility and acceptability data is presented in Table 3. All success criteria were met (e.g. 72% of participants completed all exercise sessions, loss to follow‐up was 5%, patient preference to the exercise group was 44%, whereas median ulcer healing time was chosen as the primary outcome for the definitive trial). Of 514 patients screened for participation, 109 met the eligibility criteria and 39 (24 men, 15 women) were recruited, giving eligibility and recruitment rates of 21% and 36%, respectively. Sites 1 and 2 recruited 38 and one participants, respectively. Reasons for nonconsent and exclusion are shown in Figure 1.
Table 3.
Summary of trial feasibility and acceptability data
| Methodological issues | Findings | Evidence |
|---|---|---|
| What factors influenced eligibility and what proportion of those screened were eligible? | Tissue viability clinics see a variety of patient wounds, the majority of which are not ulcers or of venous origin | 109/514 screened were eligible; the most common reasons for noneligibility were ulcer not of venous origin or other type of wound present (n = 206); the main reasons for nonconsent were mainly of social origin (e.g. work commitments or difficulty travelling; n = 52) |
| Was recruitment successful? | Recruitment was slower than anticipated | 39 participants were recruited within a 21‐month period |
| Were eligible patients recruited? | Conversion rate to recruitment was within our primary targets | 39/109 (36%) eligible participants were recruited in the study |
| Were participants successfully randomized and did randomization yield equality in groups? | Randomization process worked well | Similar sized groups, well‐balanced on stratification and most other variables; however, QoL scores were higher at baseline in the exercise group |
| Were blinding procedures adequate? | Blinding of outcome assessors and ulcer healing assessments worked well | Two different assessors were used at follow‐up sessions. No discussions were reported between participants and assessors on their study experience during follow‐up sessions. Assessment of digital ulcer photographs was completed by a team member unaware of the association between study ID numbers and group allocation |
| Did participants adhere to the intervention? | We experienced a very high attendance rate | 13/18 (72%) of the exercise group participants attended 100% of the scheduled exercise sessions; 512/648 (79%) of the scheduled sessions were completed |
| Was the intervention acceptable to the participants? | Qualitative and quantitative data from exercise participants suggest that the intervention was acceptable | Of the 27 participants who expressed a preference for a specific group before allocation (12 of the study participants did not express a preference), 17 (69% among those expressing preference; 44% among all) preferred exercise. Patient interviews (reported elsewhere) have also suggested a high degree of satisfaction |
| Was the intervention safe? | Our preliminary safety data appear favourable | Two nonserious AEs (excess fluid discharge from ulcer) were noted during the study; no bandaging was affected during the exercise sessions |
| Were outcome assessments completed? | Outcome completion rates were very high | See ‘Results’ section |
| Was it possible to calculate intervention and healthcare utilization costs? | Yes | Cost of exercise programme: £610·22 per participant. Total costs per participant were £2412·2 (including out‐of‐pocket expenses) and £1537·10 for control and exercise group patients, respectively |
| Was retention to the study good? | Retention was very high | Retention rate 95% |
| Did all components of the protocol work together? | From the point that the recruitment procedures were modified, components had strong synergy | There were no major difficulties identified in the various processes and the researchers’ ability to implement them. For example, if participants were recruited, there was excellent collaboration between the care and the research team in regards to data capture (e.g. tracing, ulcer photography) |
| Was an appropriate outcome defined for the definitive trial? | Yes | Based on our study and previous research experience, a reduction in ulcer healing time appears to be the most appropriate outcome for the definitive trial |
QoL, quality of life; AE, adverse event.
Group allocation, group preference and participant characteristics
Eighteen participants were allocated to exercise and 21 to usual care. Seventeen (63%) of 27 participants expressed a preference for exercise (12 expressed no preference). Participant characteristics at baseline are shown in Table 4; the groups were well balanced for most variables except QoL.
Table 4.
Summary of baseline demographics
| Baseline characteristics | Intervention (n = 18) | Control (n = 21) | Combined (n = 39) |
|---|---|---|---|
| Male | 9 (50) | 14 (67) | 23 (59) |
| Mean ± SD age (y) | 65·4 ± 14·9 | 61·9 ± 10·9 | 63·5 ± 12·8 |
| Working | 8 (44) | 6 (29) | 14 (36) |
| White ethnicity | 17 (94) | 21 (100) | 38 (98) |
| Mean ± SD body mass (kg) | 102·1 ± 29·4 | 104·9 ± 24·3 | 103·6 ± 26·5 |
| Mean ± SD SBP (mmHg) | 143 ± 20 | 140 ± 18 | 141 ± 19 |
| Mean ± SD DBP (mmHg) | 79 ± 10 | 84 ± 13 | 81 ± 12 |
| Mean ± SD HR (bpm) | 72 ± 13 | 69 ± 11 | 70 ± 12 |
| Smoking status | 4 (22) | 5 (24) | 9 (23) |
| Mean ± SD alcohol consumption (units weekly) | 8 ± 13 | 9 ± 14 | 8 ± 13 |
| Key medications | |||
| Antiplatelet/anticoagulant | 7 (39) | 5 (24) | 12 (31) |
| Statin | 3 (17) | 5 (24) | 8 (21) |
| ACE inhibitor | 1 (6) | 1 (5) | 2 (5) |
| Beta blocker | 3 (17) | 6 (29) | 9 (23) |
| Calcium channel blocker | 1 (6) | 2 (10) | 3 (8) |
| Diuretic | 4 (22) | 3 (14) | 7 (18) |
| Comorbidities | 12 (67) | 16 (76) | 27 (69) |
| Hypertension | 7 (39) | 4 (19) | 11 (28) |
| History of other CVD | 1 (6) | 8 (38) | 9 (23) |
| Noninsulin‐dependent diabetes | 4 (22) | 4 (19) | 8 (21) |
| History of cancer | 2 (11) | 1 (5) | 3 (8) |
| Hypercholesterolaemia | 1 (6) | 2 (10) | 3 (8) |
| Ulcer‐related | |||
| Had ulcer before | 11 (61) | 14 (67) | 25 (64) |
| Mean ± SD duration of reference ulcer (months) | 12·7 ± 19·9 | 7·1 ± 8·1 | 7·9 ± 14·8 |
| Mean ± SD time since diagnosis of reference ulcer (months) | 8·9 ± 13·7 | 6·1 ± 8·0 | 7·4 ± 10·9 |
| Had ulcer at same site (> 3 months previously) | 3 (17) | 3 (14) | 6 (15) |
| Median (range) ulcer length (cm) | 2·6 (1·2 to 13·5) | 2·8 (1·2 to 11·8) | 2·7 (1·2 to 13·5) |
| Median (range) ulcer width (cm) | 1·9 (0·9–10·1) | 1·9 (1·1–6·5) | 1·9 (0·9–10·1) |
| Median (range) ulcer area (cm2) | 4·9 (1·9–136·4) | 5·7 (1·3–56·6) | 5·0 (1·3–136·4) |
| Mean ± SD ABPI | 1·0 ± 0·1 | 1·1 ± 0·2 | 1·1 ± 0·2 |
| Physical activity and fitness | |||
| Walking with difficulty | 8 (44) | 10 (48) | 18 (46) |
| No walking | 5 (28) | 7 (33) | 12 (31) |
| Walking < 1 h | 3 (17) | 5 (24) | 8 (21) |
| Walking 1–3 h | 6 (33) | 5 (24) | 11 (28) |
| Walking ≥ 3 h | 4 (22) | 4 (19) | 8 (21) |
| Exercise/physical activity other than walking | 14 (78) | 16 (76) | 30 (77) |
Data are n (%) unless otherwise indicated. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; bpm, beats per min; ABPI, ankle brachial pressure index.
Retention
Retention rate was 95%. Two of 39 participants formally left the study; one from the exercise group for ulcer pain before the 3‐month assessment and one from the control group for nonulcer‐related health reasons before the 6‐month assessment. All others completed all assessment sessions. Four participants withdrew from exercise training due to family commitments and nonulcer‐related health reasons.
Exercise attendance and safety data
Of the 18 exercise participants, 13 (72%) completed all sessions; overall session completion rate was 79% (n = 512/648). No bandage slippage/misplacement was detected during exercise sessions. We observed two exercise‐related AEs (both excessive discharge from the ulcer). Actions taken included the removal of resistance exercises and postponement of exercise sessions.
Physical function and body mass
Participants in the exercise group showed higher mean values at 3 months in all tests (Table 5). Results stabilized at 12 months for all tests except plantar flexion. Weight reduction was modest for the exercise group in relation to the baseline (mean ± SD 103·9 ± 24 kg at baseline vs. 99·8 ± 28·4 kg at 12 months). In contrast there was an increase in weight in the control group (mean ± SD 102·6 ± 25·6 kg vs. 105·7 ± 25·2 kg at 12 months).
Table 5.
Physical fitness/function indices
| Exercise group | Control group | |||||
|---|---|---|---|---|---|---|
| Test | Baseline | 3 months | 12 months | Baseline | 3 months | 12 months |
| 6‐min walk distance (m) | 276 ± 100 | 290 ± 123 | 291 ± 122 | 280 ± 141 | 284 ± 138 | 273 ± 146 |
| Chair sit‐to‐stand (repetitions) | 8 ± 4 | 10 ± 4 | 9 ± 4 | 9 ± 4 | 9 ± 4 | 8 ± 4 |
| Chair sit‐and‐reach (score) | −6·4 ± 11·4 | 2·6 ± 16·0 | 2·2 ± 11·8 | −2·8 ± 13·6 | −0·8 ± 11·3 | −1·7 ± 11·9 |
| Plantar flexion (°) | 18·7 ± 21·0 | 22 ± 15·9 | 17·6 ± 12·8 | 15·1 ± 9·1 | 19·0 ± 22·3 | 14·7 ± 9·4 |
| Dorsiflexion (°) | 20·5 ± 14 | 22·9 ± 14·8 | 18·9 ± 15·8 | 20·3 ± 16·5 | 18·7 ± 24·2 | 17·4 ± 15·3 |
| Ankle range of movement (°) | 39·2 ± 19·9 | 44·9 ± 21·3 | 36·6 ± 20·8 | 35·4 ± 19·7 | 37·7 ± 43·2 | 32·1 ± 18·9 |
Data are mean ± SD.
Ulcer‐related data
Median ulcer size was similar between groups at 12 months (Table 6), but healing rate was higher in the intervention group (83% vs. 60%), with shorter median (range) ulcer healing time [13 (3·9–52) vs. 34·7 (4·3–52) weeks]. Recurrence rates were low in both groups (two in the intervention group, one in the control group).
Table 6.
Ulcer‐related data
| Exercise | Control | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |
| Median (range) ulcer size | ||||||||
| Length (cm) | 2·6 (1·2–13·5) | 0 (0–5) | NA | 0 (0–5·5) | 2·8 (1·2–11·8) | 1·3 (0–10·2) | NA | 0 (0–14) |
| Width (cm) | 1·9 (0·9–10·1) | 0 (0–6·5) | NA | 0 (0–3·4) | 1·9 (1·1–6·5) | 1 (0–7·7) | NA | 0 (0–10·5) |
| Area (cm2) | 4·9 (1·9–136·4) | 0 (0–26) | NA | 0 (0–18·7) | 5·7 (1·3–56·6) | 1·5 (0–78·5) | NA | 0 (0–147) |
| Whether healed, n (%) | 9/17 (53) | 11/17 (65) | 14/17 (83) | 3/21 (14) | 8/20 (40) | 12/20 (60) | ||
| Median (range) time of ulcer healing (weeks) | 13 (3·9–52) | 34·7 (4·3–52) | ||||||
| Reoccurrence of ulcer, n (%) | 0/17 (0) | 1/17 (6) | 2/17 (12) | 0/20 (0) | 2/17 (12) | 1/19 (5) | ||
NA, not available.
Health‐related quality of life
Participants in the intervention group started the study with a higher EQ‐5D utility score than the control group (Table 7; mean ± SD 0·8022 ± 0·17 vs. 0·6010 ± 0·35). This difference was maintained throughout the study. A similar difference was observed with EQ visual analogue scale, VEINES‐QOL (overall score and symptom score) and pain score, although for VEINES‐QOL and pain score, the difference between groups was increased from 3 months onwards.
Table 7.
Summary of health status, disease‐specific quality of life and pain data
| Exercise | Control | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |
| EQ‐5D‐5L utility score | 0·8022 ± 0·17 | 0·8567 ± 0·15 | 0·8147 ± 0·21 | 0·7874 ± 0·28 | 0·6010 ± 0·35 | 0·5698 ± 0·42 | 0·5740 ± 0·40 | 0·5825 ± 0·41 |
| EQ‐VAS score | 69·03 ± 15·13 | 75·35 ± 15·38 | 71·47 ± 21·34 | 75·53 ± 20·37 | 57·43 ± 19·84 | 64·33 ± 22·74 | 58·70 ± 26·21 | 56·20 ± 27·58 |
| VEINES‐QoL: main | 53·68 ± 24·62 | 69·53 ± 26·13 | 67·49 ± 27·75 | 67·23 ± 29·86 | 42·65 ± 24·70 | 47·24 ± 29·57 | 51·79 ± 33·62 | 52·46 ± 34·81 |
| VEINES symptom subdomain | 62·03 ± 26·52 | 75·18 ± 24·76 | 73·24 ± 26·26 | 73·41 ± 31·73 | 53·17 ± 28·82 | 54·60 ± 32·11 | 58·13 ± 30·05 | 58·53 ± 33·58 |
| Pain score | 24·44 ± 27·3 | 15·9 ± 27·7 | 16·5 ± 28·4 | 7·9 ± 22·8 | 30·95 ± 31·6 | 22·1 ± 32·8 | 28·0 ± 36·3 | 30·5 ± 36·6 |
Data are mean ± SD.
Health economic data
There were no missing data for procedure costs. Mean exercise intervention costs per participant was £610·22, including staff time, room hire and patient reimbursement. Mean total NHS costs (based on NHS National Tariff Schedules and calculated based on visits and usage of NHS resources) were calculated as £813·27 for the exercise and £2298·57 for the control group.
Personal costs were calculated using a diary with ‘out‐of‐pocket’ expenses being estimated at £113·63 and £174·58 for the exercise and control groups, respectively.
The mean ‘per patient’ cost savings to the NHS from the exercise intervention was £875·08. Similarly, the ‘per patient’ less ‘out‐of‐pocket’ expenses to participants, as a result of participation in exercise intervention, was £60·95. The combined per ‘per patient’ mean total cost saving was £936·03 (Table 8 and Appendix S1; see Supporting Information).
Table 8.
Summary of annual costs to the National Health Service (NHS), out‐of‐pocket (OOP) expenses of treatment and intervention study cost by group
| Cost type | Total (£) | Mean Per patient (£) | ||||
|---|---|---|---|---|---|---|
| Exercise | Control | Combined | Exercise | Control | Combined | |
| NHS healthcare professional | 12 724·00 | 34 573·00 | 47 297·00 | 748·47 | 1646·33 | 1244·66 |
| A&E | 0 | 2110·00 | 2110·00 | 0 | 100·48 | 55·53 |
| Inpatient care | 0 | 9365·00 | 9365·00 | 0 | 445·95 | 246·45 |
| Diagnostic tests | 257·00 | 746·00 | 1003·00 | 15·12 | 35·52 | 26·39 |
| Medicine (free prescriptions) | 844·60 | 1476·00 | 2320·60 | 49·68 | 70·29 | 61·07 |
| Total cost to NHS | 13 825·60 | 48 270·00 | 62 095·60 | 813·27 | 2298·57 | 1634·09 |
| Cost to patients | ||||||
| Travel | 1081·66 | 2341·68 | 3423·34 | 63·63 | 111·51 | 90·09 |
| Medicine | 229·10 | 413·44 | 642·54 | 13·48 | 19·69 | 16·91 |
| Equipment | 621·00 | 911·00 | 1532·00 | 36·53 | 43·38 | 40·32 |
| Total OOP expenses | 1931·76 | 3666·12 | 5597·88 | 113·63 | 174·58 | 147·31 |
| Intervention study cost | ||||||
| Exercise intervention delivery | 10 984·00 | NA | 10 984·00 | 610·22 | 610·22 | |
| Including study outcome measures cost | 14 051·17 | 3255·17 | 17 306·33 | 780·62 | 155·01 | 455·43 |
A&E, Accident and Emergency; NA, not applicable.
Discussion
We successfully assessed several feasibility study aspects, including recruitment, baseline and follow‐up measurements, as well as the feasibility and preliminary effectiveness of a supervised exercise programme for people with VLUs. Our main finding was that the study procedures were feasible and acceptable.
The feasibility and acceptability of using a supervised exercise regime as an adjunct therapy to compression therapy had been an area of uncertainty prior to this study. Indeed, during the preliminary study stages, such a notion was met with scepticism by some clinicians and patients, who believed that exercise may be either inappropriate or harmful, and may delay rather than promote healing – an attitude that has also been documented in the literature.29, 30 Nevertheless, the majority of the eligible patients had a positive attitude towards undertaking exercise in addition to following a therapeutic pathway based on compression therapy. This was irrespective of whether they consented to take part and reasons for nonparticipation will help make the programme more accessible (i.e. by choosing appropriate venues). Our feasibility data show few AEs, with no bandage misplacement or slippage incidents, one of the biggest concerns of collaborating clinicians.
Exercise attendance was 79%, with 72% of participants completing all sessions. This is high considering that many participants were old, frail and had no previous exercise experience. This was achieved without employing any specific adherence‐enhancing components or provision of behavioural change support, which could have potentially improved attendance rates and the effect of the intervention even further.31 This suggests great interest and self‐motivation from our participants, which will be a decisive factor for the success of a definitive trial and any wider roll‐out of the intervention. However, despite our success, it is our plan to incorporate cognitive–behavioural strategies as part of any future trial to optimize exercise adherence and increase any potential, positive effect.
With regard to practicality our intervention was primarily delivered within a university setting, at some distance from the clinics in which our participants were treated. The high attendance rate suggests that this did not have a negative impact on the outcome, although it may have affected recruitment rate. Recruitment rate may be improved in the definitive trial, where 12‐week exercise referral schemes will be utilized for the intervention delivery.32 Delivery with an option of times in community‐based venues increases accessibility but comes with a number of challenges (as adherence and success varies);33 recent research suggests that these schemes can offer QoL and physical activity gains.34
Our findings support the feasibility of using diaries to collect economic data on patients’ usage of NHS resources, healthcare visits, prescriptions and out‐of‐pocket expenses. The findings suggest potential savings to both the NHS (£875·08 per patient) and patients (£60·95 reduction in out‐of‐pocket expenses). Nevertheless, as our analysis was purely descriptive, an appropriate health economics analysis in the definitive trial will provide greater certainty regarding cost‐effectiveness.
Overall, no major difficulties were identified in the design or implementation of trial procedures. For example, the blinding procedures ran as intended, the rates of retention and outcome completion (including the 6‐month postal assessment) was very good, and from a point onwards there was an excellent communication between clinical and research teams, which allowed smooth recruitment. There was an imbalance between groups in the EQ‐5D‐5L data; this did not affect the success of our study but may need to be considered in planning the definitive trial.
Designing, setting up and managing a definitive, multicentre study has other challenges besides recruitment rate, data collection and exercise delivery. One important issue is the recruitment of a sufficient number of sites which will: (i) deliver the required number of participants; (ii) have experienced clinicians to act as local Principal Investigators; (iii) have dedicated tissue viability services, which will support and promote the study; and (iv) have a good communication level between exercise deliverers, tissue viability clinics, local stakeholders (e.g. NHS Trusts) and the main research team. It is therefore advisable that a clinical trials unit is utilised to safeguard data quality and guarantee database management, in addition to costing dedicated personnel (e.g. a trial coordinator and a trial manager) for day‐to‐day study management and involving experienced research sites in previous, similar studies (which would safeguard a consistent delivery of the trial protocol).
Our findings support the feasibility and acceptability of both the supervised exercise programme in conjunction with compression therapy and the study procedures and all our success criteria were met.5 In addition, our results suggest that there may be significant potential benefit in healing rates and that, if this were confirmed in a full trial, the introduction of supervised exercise for VLU may well also save costs for the NHS. The next step will be the design and implementation of an appropriately powered, multicentre trial, which is required to provide answers to the questions of the clinical and cost‐effectiveness of the intervention.
Supporting information
Table S1 Further particulars of the supervised exercise programme.
Appendix S1 Health economics and quality of life analysis.
Video S1 Author video.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
We acknowledge the study participants for supporting this research. We would also like to acknowledge the following people: the tissue viability nurses who supported recruitment and data collection; Dr Mark Boon (Conisbrough Group Practice) for his role as co‐applicant on the original grant application and providing clinical assessments to Sheffield participants; Mrs Sue Kesterton and Mr Alexandros Mitropoulos (Sheffield Hallam University) for leading and supporting the assessments; Mr Trevor Simpson (University of Lincoln) for acting as the clinical assessor in Lincoln; Mr Nicholas Bell (Director, Research Development Unit) for representing the study sponsor (Sheffield Health and Social Care NHS Foundation Trust) in the Steering Group; our Patient and Public Involvement steering group members; Professor Ian Chetter (Chair of Surgery HYMS/University of Hull; Steering Group Chair), Professor Catherine Hewitt (University of York) and Dr Jude Watson (University of York) for being members of our steering group; Mrs Janice Wiseman (Research and Innovation Manager, Lincolnshire Community Health Services NHS Trust) for her support in setting up Lincoln as the second study site. This article presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0213‐30029). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding sources This paper presents independent research funded by the National Institute for Health Research under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0213‐30029).
Conflicts of interest None declared
https://doi.org/10.1111/bjd.16618 available online
The copyright line for this article was changed on 18th May 2018 after original online publication
References
- 1. Nelzén N, Bergqvist D, Lindhagen A. Leg ulcer etiology – a cross sectional population study. J Vasc Surg 1991; 14:557–64. [PubMed] [Google Scholar]
- 2. Donnelly R, London N. ABC of Arterial and Venous Disease, 2nd edn Chichester: Wiley Blackwell/BMJ, 2009. [Google Scholar]
- 3. Browse NL, Burnand KG. The cause of venous ulceration. Lancet 1982; 2:243–5. [DOI] [PubMed] [Google Scholar]
- 4. Coleridge Smith PD, Thomas P, Scurr JH, Dormandy JA. Causes of venous ulceration: a new hypothesis. BMJ 1988; 296:1726–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tew GA, Michaels J, Crank H et al Supervised exercise training as an adjunctive therapy for venous leg ulcers: study protocol for a randomised controlled trial. Trials 2015; 6:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. J Tissue Viability 2009; 18:13–19. [DOI] [PubMed] [Google Scholar]
- 7. Guest JF, Ayoub N, Greaves T. Clinical outcomes and cost‐effectiveness of an externally applied electroceutical device in managing venous leg ulcers in clinical practice in the UK. J Wound Care 2015; 24:572–4. [DOI] [PubMed] [Google Scholar]
- 8. Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008; 104:44–5. [PubMed] [Google Scholar]
- 9. Thurlby K, Griffiths P. Community leg ulcer clinics vs home visits: which is more effective? Br J Community Nurs 2002; 7:260–4. [DOI] [PubMed] [Google Scholar]
- 10. Guest JF, Vowden K, Vowden P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J Wound Care 2017; 26:292–303. [DOI] [PubMed] [Google Scholar]
- 11. O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012; 11:CD000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gohel MS, Barwell JR, Taylor M et al Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ 2007; 335:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drew P, Posnett J, Rusling L. Wound Care Audit Team. The cost of wound care for a local population in England. Int Wound J 2007; 4:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. NICE Clinical Knowledge Summaries. Leg ulcers – venous. Available at: https://cks.nice.org.uk/leg-ulcer-venous (last accessed 25 June 2017).
- 15. Layden J, Michaels J, Bermingham S et al Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012; 345:e4947. [DOI] [PubMed] [Google Scholar]
- 16. Balady GJ, Williams MA, Ades PA et al Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2007; 115:2675–82. [DOI] [PubMed] [Google Scholar]
- 17. Kan YM, Delis KT. Hemodynamic effects of supervised calf muscle exercise in patients with venous leg ulceration: a prospective controlled study. Arch Surg 2001; 136:1364–9. [DOI] [PubMed] [Google Scholar]
- 18. Jull A, Parag V, Walker N et al The prepare pilot RCT of home‐based progressive resistance exercises for venous leg ulcers. J Wound Care 2009; 18:497–503. [DOI] [PubMed] [Google Scholar]
- 19. O'Brien J, Finlayson K, Kerr G, Edwards H. Evaluating the effectiveness of a self‐management exercise intervention on wound healing, functional ability and health‐related quality of life outcomes in adults with venous leg ulcers: a randomised controlled trial. Int Wound J 2017; 14:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meagher H, Ryan D, Clarke‐Moloney M et al An experimental study of prescribed walking in the management of venous leg ulcers. J Wound Care 2012; 21:421–30. [DOI] [PubMed] [Google Scholar]
- 21. Heinen M, Borm G, van der Vleuten C et al The Lively Legs self‐management programme increased physical activity and reduced wound days in leg ulcer patients: results from a randomized controlled trial. Int J Nurs Stud 2012; 49:151–61. [DOI] [PubMed] [Google Scholar]
- 22. Yim E, Kirsner RS, Gailey RS et al Effect of physical therapy on wound healing and quality of life in patients with venous leg ulcers: a systematic review. JAMA Dermatol 2015; 151:320–7. [DOI] [PubMed] [Google Scholar]
- 23. Aboyans V, Criqui MH, Abraham P et al Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation 2012; 126:2890–909. [DOI] [PubMed] [Google Scholar]
- 24. The EuroQol Group . EuroQol– a new facility for the measurement of health related quality of life. Health Policy 1990; 16:199–208. [DOI] [PubMed] [Google Scholar]
- 25. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35:1095–108. [DOI] [PubMed] [Google Scholar]
- 26. Lamping DL, Schroter S, Kurz X et al Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient‐reported measure of symptoms and quality of life. J Vasc Surg 2003; 37:410–19. [DOI] [PubMed] [Google Scholar]
- 27. Tew GA, Gumber A, McIntosh E et al Effects of supervised exercise training on lower‐limb cutaneous microvascular reactivity in adults with venous ulcers. Eur J Appl Physiol 2018; 118:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rikli RE, Jones CJ. Senior Fitness Test Manual, 2nd edn Champaign, IL: Human Kinetics, 2013. [Google Scholar]
- 29. O'Brien J, Finlayson K, Kerr G, Edwards H. The perspectives of adults with venous leg ulcers on exercise: an exploratory study. J Wound Care 2014; 23:496–8. [DOI] [PubMed] [Google Scholar]
- 30. McCulloch J, Mahoney E, McCallon S. Enhancing the role of physical therapy in venous leg ulcer management. JAMA Dermatol 2015; 151:327. [DOI] [PubMed] [Google Scholar]
- 31. National Institute for Clinical Excellence . Behaviour change: general approaches. Available at: https://www.nice.org.uk/Guidance/PH6 (last accessed 17 July 2017).
- 32. National Institute for Clinical Excellence . Physical activity: exercise referral schemes. Available at: https://www.nice.org.uk/guidance/ph54 (last accessed 17 July 2017).
- 33. Williams NH, Hendry M, France B et al Effectiveness of exercise‐referral schemes to promote physical activity in adults. Br J Gen Pract 2007; 57:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duda JL, Williams GC, Ntoumanis N et al Effects of a standard provision versus an autonomy supportive exercise referral programme on physical activity, quality of life and well‐being indicators: a cluster randomised controlled trial. Int J Behav Nutr Phys Act 2014; 11:10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Further particulars of the supervised exercise programme.
Appendix S1 Health economics and quality of life analysis.
Video S1 Author video.
Powerpoint S1 Journal Club Slide Set.


