Summary
Background
Despite a rising incidence of inflammatory bowel disease (IBD) in Hispanics in the United States, there are no studies examining the relationship between immigrant generation and IBD onset among Hispanics.
Aims
To determine whether age of IBD diagnosis, time from immigration to IBD diagnosis and IBD phenotype, differed across immigration periods in South Florida Cuban immigrants.
Methods
This was a cohort of consecutively identified Cuban‐born adults who developed IBD in the United States and were followed in gastroenterology (GI) clinic. We divided time cohorts of immigration by historical relevance: before 1980, 1980‐1994 and 1995‐to‐present. We examined differences across time cohorts in diagnosis age, time from immigration to IBD diagnosis, and IBD phenotype (ie, IBD type, disease location).
Results
A total of 130 Cuban patients with IBD were included. Age of IBD diagnosis was older in Cubans arriving before 1980 than in those arriving between 1980‐1994 or after 1995 (44.7 vs 33.79 and 33.71, respectively, P<.0001). Time between immigration and diagnosis was shorter in patients arriving to the US after 1980 (31.77 years, Standard deviation (SD) 12.83 (<1980) vs 17.13 years, SD 8.55 (1980‐1994) and 8.30 years, SD 4.72 (1995‐to‐present). IBD phenotype, including type of IBD, disease location and surgeries, did not differ significantly across time cohorts.
Conclusions
Our study describes changing patterns of IBD onset following immigration in Cubans, suggesting that environmental changes either in the United States, Cuba or both are resulting in faster IBD onset in younger immigrant generations. These studies can inform the search for environmental triggers that may result in IBD.
Short abstract
Linked Content
This article is linked to Kuenzig and Benchimol, and Damas et al, Actis and Pelicano, and Damas and Abreu, papers. To view these articles visit https://doi.org/10.1111/apt.14168, https://doi.org/10.1111/apt.14200, https://doi.org/10.1111/apt.14249 and https://doi.org/10.1111/apt.14278.
1. INTRODUCTION
Inflammatory bowel disease (IBD) affects approximately 1.5 million people residing in the United States, and IBD incidence is rising in many developing countries.1, 2 Despite recent characterisation of the genetic risk alleles that contribute to the onset of IBD, genetic predisposition does not explain the steadily rising incidence of IBD in countries such as Japan and India.3 The prevalence of IBD is also increased in immigrant populations arriving from previously low IBD incidence areas to countries where the disease is more prevalent.4, 5, 6 The study of immigrants arriving to regions with high IBD prevalence offers a unique opportunity to examine environmental risk factors for IBD.6 Because South Florida is a gateway for Latin American immigrants, researchers in this area are uniquely positioned to study the effect of immigration on development of IBD in Hispanic immigrants and to characterise the IBD phenotype within this population.
Studies examining the Hispanic population with respect to IBD are often limited by heterogeneity among Hispanics. Most US‐based studies of Hispanics classify all Hispanics into a single pan‐ethnic group. Not surprisingly, these studies often yield differing results. For instance, Mexican Hispanics differ from Argentinian Hispanics, not only because of their distinct genetic ancestry, but also because of cultural influences that can significantly affect health seeking behaviours and disease outcomes.7 Unlike other areas of the United States, where individuals of Mexican heritage are the most prevalent Hispanic group, the composition of our IBD Hispanic patient cohort in South Florida is primarily Cuban.8 Another unique aspect of the Cuban immigrant population is that Cuban immigrants have been coming to the United States since the 1950s—earlier than other immigrant Hispanic groups to South Florida—providing an opportunity to examine whether IBD phenotype is changing over time.
Cuban immigrants are also a unique subgroup of Hispanics to study given the relatively unchanged environment within Cuba compared to the rapid “westernization” of other Latin American countries. In an earlier study, composed largely of Cubans, we found that Hispanic immigrants were developing ulcerative colitis more often than Crohn's disease, and at a much older age compared to American‐born Hispanics or non‐Hispanic Whites. These changes in IBD presentation highlight the sizeable role of the environment in IBD expression among Hispanics.9
We chose to focus our attention on the Cuban population migrating to the United States because it is a natural experiment that allows us to evaluate the effect of timing, and therefore changing environmental exposures, in a group of individuals originating from the same country. Our study is the first to examine IBD phenotype in relation to immigration‐related variables among Hispanics. In this study, we examined1 whether age of IBD diagnosis, and the time between immigration and diagnosis, is changing in Cuban immigrants arriving in the US;2 and whether IBD phenotype (ie, type of IBD, location of disease, Crohn's disease behaviour, IBD‐related surgeries) differed across time cohorts.
2. METHODS
2.1. Study population
Our sample included adult IBD patients seen in our gastroenterology (GI) clinics between 2008 and 2015, who self‐identified as Hispanic, and were Cuban‐born but developed IBD symptoms after arrival in the United States. Our study was approved by the University of Miami IRB. We also included a cohort of non‐immigrant Hispanic IBD controls to account for barriers to access to health care among immigrant Cubans.
To examine the veracity of our results, and to investigate the possibility that any cohort differences might be due to immigration or to “historical” differences (eg, changes in access to care over time or improved IBD detection), we included a comparison cohort of non‐immigrant, self‐identified Cuban Americans. The Cuban‐born and US‐born cohorts were derived from the same “universe” of IBD patients followed in our clinic. In addition, to minimise lead‐time bias and to make sure that older people would not have a greater chance of having disease because of their age, we examined differences using people in the same age range. To do this, we selected people who were at least 40 years old and who were diagnosed with IBD before the age of 40. This method has been employed in prior studies to minimise lead‐time bias.11 If the cohort difference continued to emerge, that would mean that our findings could not be explained simply in terms of people living longer.
2.2. Study procedures and data collection
A bilingual research coordinator administered a detailed intake form to patients at the time of their GI clinic visit. All patients provided detailed demographic information on an intake form (provided in English and Spanish), including self‐identified race/ethnicity, country of birth and age of immigration to the United States. All self‐identified Hispanics who were born in Cuba were included in the present analyses. Patients who did not provide their ethnicity were not included in the present analyses. Medical information provided by patients included gender, IBD family history, smoking history, religious background, and medical and surgical histories.
The attending physician confirmed participants’ IBD diagnosis by reviewing participants’ medical records and their endoscopic, histological, radiological, and surgical findings. The attending physician determined IBD phenotype, including luminal location of disease and Crohn's disease behaviour (eg, perforating, stricturing), according to the Montreal Classification system, after reviewing patient records.
2.3. Exposure variables: immigration cohorts
We created “time in residence” immigrant cohort groups to reflect the different waves of immigration occurring from Cuba to South Florida. Initially, we created four cohort subgroups based on year of arrival: before 1960, 1960‐1979, 1980‐1994 and 1995 to present. These time cohorts were selected because they reflect the largest waves of immigrants from Cuba to the United States. However, only two patients had immigrated to the United States before 1960, so we combined them with participants who had immigrated between 1960 and 1979 (heretofore referred to as stated earlier 1980).
2.4. Covariates
We compared demographic variables and immigration‐related variables, including current age, gender, education level, Jewish family history, family history of IBD, smoking history (prior to diagnosis, at diagnosis and current), and age at immigration across time cohorts. We also compared duration of disease between time cohorts (Table 1).
Table 1.
Patient demographics in Cuban immigrants with inflammatory bowel disease. SD, standard deviation
| N | Periods of immigration | ||||
|---|---|---|---|---|---|
| Before 1980 | 1980‐1994 | 1995‐present | a P‐value | Total | |
| 44 | 42 | 44 | 130 | ||
| Mean age at immigration (SD) | 12.7 (10.6) | 16.5 (10.3) | 25.5 (13.1) | <.001 | 16.6 (18.8) |
| Mean duration of disease in years (SD) | 14.9 (11.7) | 7.6 (13.6) | 2.8 (8.4) | <.001 | 5.7 (13.2) |
| Mean current age (SD) | 58.50 (10.1) | 43.1 (12.5) | 37.2 (13.6) | <.001 | 45.8 (22.5) |
| Gender, % female | 59.1 | 35.7 | 31.8 | .020 | 42.3 |
| Self‐identified Jewish, (%) | 1 (2.3) | 0 (0) | 1 (2.3) | .608 | 2 (1.5) |
| Current smoker (%) | 2 (4.7) | 7 (16.7) | 4 (9.1) | .264 | 13 (10.1) |
| Former smoker at time of diagnosis (%) | 13 (30.2) | 11 (26.2) | 8 (18.2) | .343 | 32 (24.8) |
| Education level (%) | |||||
| Less than high school | 3 (10.0) | 1 (3.7) | 2 (6.5) | .364b | 6 (6.8) |
| High school | 10 (33.3) | 16 (59.3) | 16 (51.6) | 42 (47.7) | |
| College or higher | 17 (56.7) | 10 (37.0) | 13 (41.9) | 40 (45.5) | |
| Family history of IBD (%) | 5 (11.4) | 2 (4.7) | 6 (13.6) | .536 | 13 (10.0) |
P‐value is comparing differences across the three time cohorts.
Information was missing on a few participants.
Bold values correspond to P‐values <.05.
2.5. Outcomes and analyses
Our objectives were to determine whether age at IBD diagnosis, and time from immigration to IBD diagnosis, differs by immigration cohort. We also sought to evaluate whether IBD phenotype (type of IBD, location of luminal disease, Crohn's disease behaviour, IBD‐related surgeries and hospitalisations) differs across different time cohorts of Cuban immigrants.
We used ANOVAs and chi‐square tests to compare demographic factors across immigration cohorts (Table 1). We used a univariate general linear model for age of IBD diagnosis and a Cox proportional‐hazards model to examine differences in elapsed time from immigration to IBD diagnosis between time‐in‐residence cohorts. For IBD phenotype (separating CD and UC), we used multinomial logistic regression for our categorical variables (location of disease and CD behavioru), and binomial logistic regression for dichotomous variables (IBD type, upper GI tract disease, perianal disease. Binomial logistic regression was also used for history of IBD‐related surgeries and IBD‐related hospitalisations. SPSS 22 (IBM Corp, Armonk, NY, USA) was used to conduct analyses.
3. RESULTS
3.1. Demographic characteristics of Cuban immigrants
A total of 130 Cuban‐born individuals developed IBD in the United States and were included in our primary analyses. An additional 42 Cubans who developed IBD in Cuba were excluded from our analysis. Table 1 provides the demographic characteristics among immigrants who received an IBD diagnosis in the United States. Mean age at immigration was different among all three time cohorts, with age at immigration being oldest in the 1995‐to‐present arrival group (44.64 years (<1980), 41.12 years (1980‐1994) and 60.60 years. (1995‐to‐present); F (2, 127)=14.48 P<.001). There were more women in the earliest time‐in‐residence cohort than in the later cohorts (59% (<1980), 35.7% (1980‐1994), 31.8% (1995‐to‐present); X2 (2, N=130) P=.020). As a result of statistical differences observed across these variables (age of immigration and gender), we controlled for them in subsequent analyses. Duration of disease was longer in the cohort arriving prior to 1980 compared to later immigration waves and therefore was added as a covariate in the IBD phenotype analyses (M=14.93, SD=11.7 (<1980); M=7.6, SD=13.6 (1980‐1994); M=2.17, SD=8.4 (1995‐to‐present); F (2, 127)=13.88, P<.001). There were no differences between time‐in‐residence cohorts with respect to Jewish religion, smoking history (past or present), or education level (Table 1). Similarly, no differences were observed when examining demographics across time cohorts by ulcerative colitis or Crohn's disease separately (data not shown).
3.2. Age of diagnosis stratified by periods of immigration
Age at diagnosis among Cuban immigrants differed by time‐in‐residence cohort and was younger in patients arriving since 1980 (44.47 years. SD 16.4 (<1980), 33.71 years. SD 14.0 (1980‐1994), 33.79 years. SD 11.9 (1995‐to‐present)). Patients who immigrated after 1980 developed IBD approximately 10 years earlier compared to those arriving before 1980 (Beta values of −10.7 (CI=−16.85 to −4.67) (1980‐1994) and −10.6 (CI=−16.69 to −4.66) (1995‐to‐present), P=.0006 in both cases). In this linear regression model (univariate general linear model), we use a logarithmic transformation for age of IBD diagnosis because it was a nonlinear variable. To validate the assumptions of linear regression we examined the residuals within our final model. Looking at the results of the Shapiro‐Wilk statistic test we can see that the residuals come from a normal distribution (P=.1232).
3.3. More recent immigration from Cuba is a risk factor for earlier onset of IBD
Time between immigration and diagnosis was shorter in patients arriving in the United States after 1980 (mean 31.77 years for <1980, 17.13 years for 1980‐1994, 8.30 years for 1995 to present) (Figure 1). More specifically, using a Cox proportional hazard model, we observed that the 1980‐1994 cohort and the 1995 to present cohort had a significantly shorter time interval between immigration and age of diagnosis compared to the 1960‐1979 cohort (HR=4.74, CI: 2.64‐8.50, P<.001 and HR=18.45 (8.1‐41.5), P<.001 respectively). This effect emerged while adjusting for age at immigration and gender. Cumulative hazard ratios were generated to evaluate the risk of developing IBD following immigration. The proportional hazards assumption was satisfied (Figure S1). Time‐in‐residence cohort was used as a predictor of the hazard function. We observed similar findings when examining Crohn's and ulcerative colitis by cohorts (data not shown).
Figure 1.
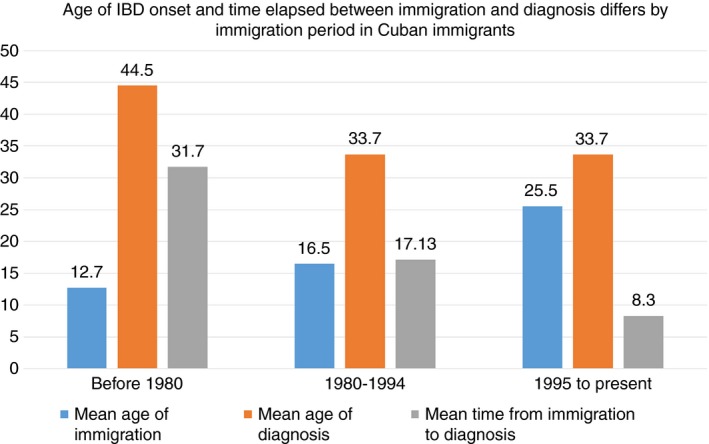
Cuban immigrants arriving to the US after 1980 are developing IBD at a younger age and within a shorter time from immigration, despite adjusting for differences in age of immigration
In our additional analyses, we examined differences using people in the same age range so that older people would not have a greater chance of having IBD because of their age. Within this subsample, the mean current age and age range in each time‐in‐residence cohort were similar: in those arriving before 1980, the mean age was 54.2 years (range: 40.5‐77.2), in participants arriving between 1980 and 1994, the mean age was 46.8 years (range: 40.3‐63.8), and in participants arriving between 1995‐present, the mean age was 51.4 years (range: 40.4‐70.7). In this subgroup of 43 individuals, we estimated a Cox proportional hazard model controlling for account gender and age at immigration. Similar to the results of the primary analyses, we found a shorter time interval between immigration and IBD diagnosis in later immigration cohorts. In the cohort arriving prior to 1980, the mean time to IBD diagnosis was 18.0 years (SD 9.1); in the cohort arriving between 1980 and 1994, this mean was 12.49 (SD 8.17); and in the cohort arriving after 1995, this mean was 7.15 (SD 7.1), P<.0001.
Last, to ensure that our results were not explained by differences in access to care across time, we examined whether age of diagnosis was also occurring at a younger age in recent generations of non‐immigrant Cuban Americans. To do this, we grouped 253 non‐immigrant Cuban Americans into the same three time cohorts examined, based on the date of their diagnosis. We then examined the mean age of diagnosis across the three time cohorts (using brackets based on date of diagnosis). We found that the mean age of diagnosis did not differ significantly across time cohorts in the non‐immigrant sample (before 1980 [13.0 years, SD 6.56] vs 1980‐1994 [19.75 years, SD 10.11] vs 1995‐to‐present [22.79 years, SD 11.11], P=.165).
3.4. IBD phenotype stratified by time‐in‐residence cohorts
There were no IBD phenotypic differences observed among Cubans across time‐in‐residence cohorts (Table 2). Specifically, there were no differences in development of Crohn's vs UC or location of disease (ie, upper GI tract Crohn's, pancolitis, proctitis, etc.) for either Crohn's disease or ulcerative colitis across time cohorts (Table 2). A greater proportion of Cuban immigrants developed perianal disease in the two later immigration cohorts compared to the cohort arriving prior to 1980, but this finding was no longer significant after taking into account disease duration (13.8% [<1980], 42.9% [1980‐1994], 37.5%[1995 to present]; OR=1.96 [95% CI: 0.98‐3.92], P=.055); Table 2. No differences between time cohorts emerged in Crohn's disease behaviour, abdominal surgeries, or perianal surgeries (Table 2). There was also no cohort difference in prevalence of colectomy in UC (6.6% [<1980], 5.5% [1980‐1994], 0% [1995‐to‐present]); OR=0.8 (95% CI: 0.95‐2.32), P=.68) (Table 2).
Table 2.
Inflammatory bowel disease phenotype using montreal classification in Cuban immigrants diagnosed in the United States, stratified by period of immigration
| Total N | Period of immigration | |||
|---|---|---|---|---|
| <1980 | 1980‐1994 | 1995‐present | P‐valuea | |
| 44 | 42 | 44 | ||
| Mean time from immigration to IBD diagnosis | 31.7 (12.8) | 17.13 (8.5) | 8.30 (4.7) | <.001 b |
| Mean age of IBD diagnosis (SD) | 44.5 (16.4) | 33.7 (14.0) | 33.7 (11.9) | <.001 b |
| Crohn's disease (%) | 29 (65.9) | 24 (57.1) | 31 (70.5) | .396 |
| Ulcerative colitis (%) | 15 (34.1) | 18 (42.9) | 13 (29.5) | |
| Crohn's disease (n) | 29 | 24 | 31 | |
| Upper GI Crohn's | 0 (0) | 1 (2.4) | 2 (4.5) | .399 |
| Ileal | 13 (29.5) | 8 (19.0) | 12 (27.3) | .996 |
| Colonic | 4 (9.0) | 3 (7.1) | 7 (15.9) | |
| Ileocolonic | 12 (27.3) | 11 (53.4) | 12 (27.3) | |
| Ulcerative colitis (n) | 15 | 18 | 13 | |
| Proctitis | 1 (6.7) | 1 (5.6) | 1 (7.7) | .275 |
| Left sided colitis | 7 (46.7) | 9 (50.0) | 1 (7.7) | |
| Pancolitis | 7 (46.7) | 8 (44.4) | 9 (69.2) | |
| Crohn's behaviour | ||||
| Fibrostenotic | 15 (34.1) | 13 (30.9) | 9 (31.0) | .187 |
| Inflammatory | 17 (38.6) | 9 (21.4) | 17 (38.6) | |
| Internal perforating | 6 (13.6) | 3 (7.1) | 5 (11.4) | |
| Perianal perforating | 4 (13.8) | 9 (42.9) | 12 (37.5) | .055c |
| IBD‐related surgeries | 20 (45.5) | 15 (35.7) | 14 (31.8) | .397 |
| Abdominal surgeries for Crohn's disease | 13 (44.8) | 11 (45.8) | 10 (32.3) | .501 |
| Total proctocolectomy ulcerative colitis | 1 (6.6) | 1 (5.5) | 0 (0) | .675 |
| Perianal surgeries | 1 (3.4) | 3 (12.5) | 5 (16.1) | .251 |
| Hospitalisations | 21 (47.7) | 26 (61.9) | 13 (29.5) | .112 |
Phenotypic information was missing on a few patients. Certain patients with Crohn's disease had upper gastroenterology tract disease and ileal or colonic disease and therefore the sum of n for disease location of Crohn's and for Crohn's disease behaviour is greater than the total number of Crohn's patients.
P‐value is comparing differences across the three time‐cohorts.
P‐value after taking into account covariates (gender and age of immigration) in univariate analysis of variance.
P‐value for perianal disease was attenuated to nonsignificance (.055) after taking into account duration of disease using logistic regression.
Bold values correspond to p‐values <.05.
4. DISCUSSION
We are witnessing a global rise in IBD incidence.2 Immigrants from low risk areas to high‐risk areas offer opportunities to examine what changes or exposures are taking place in the new country. The present study emerged from our observations in the clinic that we were seeing more Cuban immigrants developing IBD and that the time from immigration to disease manifestations was getting shorter. Our study is the first to examine onset of IBD following immigration among Hispanics living in the United States. To our knowledge, there are no other studies looking at the relationship between immigrant generation and IBD onset among Hispanics. It is also the first study to examine IBD in Cubans, one of the largest Hispanic groups in the United States and the largest Hispanic group in Florida.7 Because Cubans have immigrated to the United States in waves, comparing across these waves allows us the opportunity to study the evolution of IBD in this population.
In this study, we found that Cubans who arrived in the United States after 1980 are at significantly higher risk of developing IBD at younger ages, and within a shorter period of time following immigration, compared to Cubans arriving prior to 1980. This was true for both ulcerative colitis and Crohn's disease and remained true even after adjusting for differences in population demographics, including gender and age at immigration. Our results suggest that, once IBD onset occurs, the phenotype of IBD is similar, including IBD‐related surgeries, location of luminal disease, and IBD‐related hospitalisations. These findings offer unique insights for identifying key environmental factors that may accelerate the process of IBD development not only in Cubans, but in immigrants from various backgrounds moving from low to high IBD incidence areas such as the United States.
Our findings allow us to speculate that present‐day Cuban immigrants may have a greater risk of exposure to an undefined environmental risk factor that was perhaps not as prevalent in earlier waves of immigration, particularly prior to the 1980s. Although previous epidemiologic studies have demonstrated increasing IBD incidence in immigrants arriving from low to high incidence areas, particularly immigrants arriving as children and second‐generation immigrants (individuals born in the United States to immigrant parents), there are no studies suggesting an evolving United States receiving environment over different time periods and the impact that this changing environment exerts on health among immigrants.4, 5, 11, 12
We propose several hypotheses as to why Cuban immigrants may be developing IBD faster in the United States than before. First, we may be witnessing more rapid acculturation—especially dietary acculturation—among more recent Cuban immigrants having relatives and/or friends already in the United States who facilitate the process of assimilation into American culture. The question then is, how does this faster/greater dietary assimilation—if this is indeed responsible for the cohort differences in IBD incidence—influence IBD risk in more recent generations of Cuban immigrants? Previous studies suggest that higher levels of acculturation are associated with increased smoking, obesity and lower intake of fruits and vegetables.13, 14, 15, 16, 17 Although the prevalence of smoking was not different across time‐in‐residence cohorts, it may be that more recent Cuban immigrants adopt an American diet faster than their predecessors—a postulated risk factor for IBD for those with a susceptible genetic background.
Alternatively, it is possible that the difference in IBD onset observed across generations of immigrant Hispanics may not be related to changes that occurred in the United States, but rather to differences occurring in Cuba. Given the current political environment in Cuba, we do not have access to epidemiologic data from Cuba that would allow us to account for changing environmental and public health patterns, including early childhood factors such as breastfeeding, mode of birth delivery, antibiotic exposure and changing patterns in hygiene‐related risk factors (eg, access to clean water, parasitic infections, sharing of bedrooms). In addition, individuals in Cuba now are more likely to be exposed to US products earlier and more often as a result of Cuban immigrants travelling back to Cuba and/or sending American products to their relatives in Cuba.18 Nevertheless, although these changes may be taking place in Cuba, they are minimal compared to the rapid westernisation witnessed in other developing countries.19 Therefore, the study of IBD in Cubans offers a unique opportunity to “control” for westernisation given Cuba's geographic isolation as an island and its political climate, which have led to stable diet and lifestyle behaviours for the last 50 years. We hope to test these hypotheses over time in future work.
Strengths of our study include our complete phenotype data on a cohort of IBD subjects originating from the same country, as well as our detailed intake data on immigration and onset of IBD symptoms. In turn, this information allowed us to make comparisons of IBD phenotype occurring in the United States among immigrants arriving at different time intervals—something that has not been done before. Another strength of our study is our use of cautious statistical methods, and accounting for differences in demographics (ie, education level, gender, duration of disease) in our statistical models to reduce the likelihood of committing a Type I error. We also minimised lead‐time bias by accounting for calendar age and analysing a subgroup of patients similar in age.
The limitations of our study are inherent to the study of immigrants presenting at a clinic. Our study is subject to immigration bias and selection bias given that we only examined a subset of the Cuban population immigrating to the United States (particularly Miami)—specifically, those who presented at our clinic. However, Cuban immigrants have settled primarily in South Florida across waves of immigration, allowing for more direct comparisons of people staying in one US environment.20 Another limitation is that our patients were not followed prospectively over time from immigration to development of IBD, so our study is susceptible to recall bias. However, participants are likely to recall the year when they immigrated—and as a result, our primary outcome (age of diagnosis in relation to immigration) was likely not affected by recall bias. In addition, because we conducted an exploratory study to test a clinical observation, the findings of our study should be interpreted with caution because the study was not designed (and powered) specifically to examine the cohort differences observed here. Last, our study may be subject to differences in access to care or health care seeking behaviours across time‐in‐residence cohorts. With that said, Cuban immigrants are different than other immigrant groups because they are provided with healthcare insurance upon arrival as a result of federal healthcare programs for Cuban exiles. This law has been in place for decades and applies to all three time cohorts examined.21 Furthermore, when we conducted parallel analyses with non‐immigrant Hispanics to determine whether our findings could be attributed to overall improvement in IBD detection or access to health care in all patients (immigrants or not) in more recent years, we found no cohort differences among non‐immigrant Cuban Americans. This result suggests that our findings are likely not a result of global improvement of access to care among Hispanics or better detection/increased awareness of IBD in recent decades.
In conclusion, for the first time, we have documented a temporal relationship between time‐in‐residence and the development of IBD among immigrant Hispanics in the United States. We have demonstrated that IBD is occurring within a shorter period of time following immigration in more recent waves of Cuban immigrants. This finding may be a reflection of changing environmental exposures in the United States that increasingly predispose immigrants to develop IBD. Given the restarting of relations between the United States and Cuba, the present results suggest that such influence may produce dramatic changes in the Cuban microenvironment and may increase the prevalence of IBD there. Future studies should focus on identifying these changing environmental exposures, which may include dietary factors, pollutants, exposure to antibiotics and the resulting change in the microbiome. We hope that our study will inspire work in this direction.
AUTHORSHIP
Guarantor of the article: Oriana M. Damas, MD.
Author contributions: Oriana M. Damas: responsible for the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting of the article; Danny J. Avalos: acquisition of data; Ana Palacio: design of the study, revising it critically for important intellectual content; Lissette Gomez: analysis of the data; Maria A. Quintero: acquisition of the data; Amar R. Deshpande: revising it critically for important intellectual content; Daniel A. Sussman: revising it critically for important intellectual content; Jacob L. McCauley: data interpretation and analysis, revising it critically for important intellectual content; Johanna Lopez: analysis of the data; Seth J. Schwartz: design of the study, analysis and interpretation of the data, revising it critically for important intellectual content; Maria T. Abreu: interpretation of the data, revising it critically for important intellectual content.
All authors approved the final version of the manuscript.
Supporting information
ACKNOWLEDGEMENTS
Declaration of personal interest: The authors of this manuscript have reported no conflicts of interest.
Damas OM, Avalos DJ, Palacio A, et al. Inflammatory bowel disease is presenting sooner after immigration in more recent US immigrants from Cuba. Aliment Pharmacol Ther. 2017;46:303–309. https://doi.org/10.1111/apt.14145
Funding information
This study was funded in part by the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant (R01DK104844 to M.T.A. and J.L.M.). The Micky & Madeleine Arison Family Foundation Crohn's & Colitis Discovery Laboratory (to M.T.A.), The Martin Kalser Chair in Gastroenterology (to M.T.A), and grants AA023994 and AA021888 (to S.S.).
The Handling Editor for this article was Dr Ashwin Ananthakrishnan, and it was accepted for publication after full peer‐review.
The copyright line for this article was changed on 29 May 2018 after original online publication.
This article is linked to Kuenzig and Benchimol, and Damas et al, Actis and Pelicano, and Damas and Abreu, papers. To view these articles visit https://doi.org/10.1111/apt.14168, https://doi.org/10.1111/apt.14200, https://doi.org/10.1111/apt.14249 and https://doi.org/10.1111/apt.14278.
REFERENCES
- 1. Cosnes J, Gower‐Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785‐1794. [DOI] [PubMed] [Google Scholar]
- 2. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205‐217. [DOI] [PubMed] [Google Scholar]
- 3. Thia KT, Loftus EV Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167‐3182. [DOI] [PubMed] [Google Scholar]
- 4. Benchimol EI, Mack DR, Guttmann A, et al. Inflammatory bowel disease in immigrants to Canada and their children: a population‐based cohort study. Am J Gastroenterol. 2015;110:553‐563. [DOI] [PubMed] [Google Scholar]
- 5. Benchimol EI, Manuel DG, To T, et al. Asthma, type 1 and type 2 diabetes mellitus, and inflammatory bowel disease amongst South Asian immigrants to Canada and their children: a population‐based cohort study. PLoS ONE. 2015;10:e0123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ko Y, Kariyawasam V, Karnib M, et al. Inflammatory bowel disease environmental risk factors: a population‐based case–control study of Middle Eastern migration to Australia. Clin Gastroenterol Hepatol. 2015;13:1453‐1463. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz SJ, Unger JB, Zamboanga BL, Szapocznik J. Rethinking the concept of acculturation: implications for theory and research. Am Psychol. 2010;65:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennis SRR‐V, M.; Albert, N.G. The Hispanic Population: 2010. In: Commerce USDo, editor. U.S. Census Bureau; 2010.
- 9. Damas OM, Jahann DA, et al. Phenotypic manifestations of inflammatory bowel disease differ between Hispanics and non‐Hispanic whites: results of a large cohort study. Am J Gastroenterol. 2013;108:231‐239. [DOI] [PubMed] [Google Scholar]
- 10. Picco MF, Goodman S, Reed J, Bayless TM. Methodologic pitfalls in the determination of genetic anticipation: the case of Crohn disease. Ann Intern Med. 2001;134:1124‐1129. [DOI] [PubMed] [Google Scholar]
- 11. Bar‐Gil Shitrit A, Koslowsky B, Kori M, et al. IBD: an emergent disease among Ethiopian Jews migrating to Israel. Inflamm Bowel Dis. 2015;21:631‐635. [DOI] [PubMed] [Google Scholar]
- 12. Barreiro‐de Acosta M, Alvarez Castro A, Souto R, Iglesias M, Lorenzo A, Dominguez‐Muñoz JE. Emigration to western industrialized countries: a risk factor for developing inflammatory bowel disease. J Crohns Colitis 2011;5:566‐569. [DOI] [PubMed] [Google Scholar]
- 13. Abraido‐Lanza AF, Florez KR. Do healthy behaviors decline with greater acculturation? implications for the Latino mortality paradox. Soc Sci Med. 2005;61:1243‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dey AN, Lucas JW. Physical and mental health characteristics of U.S.‐and foreign‐born adults: United States, 1998–2003 Advance data from vital and health statistics; no 369. Hyattsville, MD: National Center for Health Statistics; 2006. [PubMed] [Google Scholar]
- 15. Eamranond PP, Wee CC, Legedza AT, Marcantonio ER, Leveille SG. Acculturation and cardiovascular risk factor control among Hispanic adults in the United States. Public Health Rep. 2009;124:818‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myers HF, Kagawa‐Singer M, Kumanyika SK, Lex BW, Markides KS. Panel III: Behavioral risk factors related to chronic diseases in ethnic minorities. Health Psychology. 1995;14:613. [DOI] [PubMed] [Google Scholar]
- 17. Perez‐Escamilla R, Putnik P. The role of acculturation in nutrition, lifestyle, and incidence of type 2 diabetes among Latinos. J Nutr. 2007;137:860‐870. [DOI] [PubMed] [Google Scholar]
- 18. Sesin C. Need a School Uniform In Cuba? These Miami Shops Have This. http://www.nbcnews.com Accessed March 5, 2015.
- 19. Jensen LA. Cultural‐developmental scholarship for a global world: An introduction In: Jensen LA, ed. The Oxford handbook of human development and culture: An interdisciplinary perspective. New York: Oxford University Press; 2015:3‐13. [Google Scholar]
- 20.Lopez, Gustavo. Pew Research Center. Hispanic Trends. http://www.pewhispanic.org/2015/09/15/hispanics-of-cuban-origin-in-the-united-states-2013/ Accessed August 8, 2016.
- 21. Overview of eligibility for non‐citizens in Medicaid and CHIP. https://www.medicaid.gov/medicaid/outreach-and-enrollment/downloads/overview-of-eligibility-for-non-citizens-in-medicaid-and-chip.pdf Accessed February 22, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


