Abstract
Postpartum haemorrhage is a leading cause of maternal death worldwide. Oxytocin, currently the drug of choice for prevention of PPH, requires constant refrigeration. In pursuit of an alternative medicine, Ferring Pharmaceuticals have developed a heat‐stable formulation of carbetocin, an oxytocin analogue. This study aimed to define that formulation, and to investigate its stability under ICH climate zone IV conditions (30°C/75% relative humidity) for at least 3 years and at extreme temperatures, such as 60°C, for shorter periods of time. The development resulted in a heat‐stable carbetocin formulation consisting of 0.1 mg/mL carbetocin in sodium succinate buffer, mannitol, and methionine. The optimum pH was determined to be pH 5.45 (5.25–5.65). The generated stability data of this formulation show that ≥95% purity of the peptide was maintained for a minimum of 3 years at 30°C, 6 months at 40°C, 3 months at 50°C and 1 month at 60°C. In addition, the heat‐stable carbetocin formulation was not sensitive to freezing or light. The reported highly stable peptide formulation facilitates the distribution in low and middle‐income countries, where maintaining cold chain distribution is difficult.
Ferring Pharmaceuticals, the World Health Organization, and MSD# for Mothers have established a collaboration to develop this heat‐stable formulation of carbetocin for the prevention of post‐partum hemorrhage in women after vaginal childbirth, with the aim of making the medicine available in the public sector of developing countries that have a high burden of maternal mortality.
Keywords: carbetocin, heat stability, oxytocin, postpartum haemorrhage, uterotonic
Abbreviations
- HPLC
high performance liquid chromatography
- ICH
international conference of harmonization
- IU
international unit
- LMI
low and middle‐income
- MSD
Merck Sharp & Dohme
- PPH
postpartum haemorrhage
- RH
relative humidity
- UN
United Nations
- UNICEF
United Nations International Children's Emergency Fund
- WHO
World Health Organization
1. INTRODUCTION
Global efforts to improve health care for women during childbirth have contributed to a 44% decline (from 385 to 216 deaths per 100,000 live births) in maternal mortality between 1990 and 2015.1, 2 These gains, however, fell short of the United Nations 2015 Millennium Development Goals, which called for a 75% reduction. The proportion of mothers who die in childbirth remains 14 times higher in low and middle‐income (LMI) countries than in developed countries.3 A significant number of these maternal deaths are caused by postpartum haemorrhage (PPH).4 Effective prevention and treatment of PPH in LMI countries are therefore essential to achieve the new United Nations Sustainable Development Goal 3.1: to reduce the global maternal mortality ratio to less than 70 per 100,000 live births by 2030.5 Oxytocin is currently the uterotonic drug of choice for prevention and treatment of PPH.6 Oxytocin is widely available in LMI countries, but there are quality concerns. A recent systematic review of published studies on the quality of oxytocin concluded that there is a high prevalence of poor‐quality oxytocin in LMI countries.7 Studies have also demonstrated that oxytocin loses potency in field conditions, particularly in tropical climates.8, 9 Depending on the manufacturer, oxytocin products must be continually refrigerated (2°C to 8°C) or, for a limited amount of time, stored at room temperature (25°C or lower) to ensure stability.10 This makes it difficult to distribute oxytocin, under required conditions, particularly in many LMI countries, where climates are hot and humid and cold‐chain infrastructure is fragile.
It is highly desirable, therefore, to have an uterotonic that is stable in ICH climate zone IV (30°C and 75% relative humidity [RH]), and at even higher temperatures for shorter periods of time, to account for possible exposure to extreme conditions during shipment or storage.
Carbetocin, an oxytocin analogue, has been used for the prevention of PPH following Caesarean section births since 1997. While carbetocin and oxytocin molecules are similar, there are several molecular differences that enhance the stability of carbetocin relative to oxytocin, making it possible to formulate carbetocin into a highly stable liquid product.
The principal aim of this study was to define an optimized heat‐stable carbetocin formulation and to investigate its stability, comparing levels of degradation to that of oxytocin under the same conditions. Freeze‐thaw and photo‐stability studies were also conducted.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
Carbetocin and carbetocin impurities (Table 1) were purchased from PolypeptideLaboratories (Strasbourg, France). Succinic acid, D‐Mannitol, L‐methionine, and NaOH were purchased from Sigma‐Aldrich (St. Louis, Missouri, United States). Commercial product (PABAL®) of the heat‐stable carbetocin formulation in vials (0.1 mg/mL) was obtained from Ferring GmbH (Kiel, Germany). The clinical trial material of heat‐stable carbetocin formulation in ampoules (0.1 mg/mL) for the World Health Organization (WHO) Phase III trial (trial code A65870) was obtained from Patheon (Ferentino, Italy). Oxytocin SYNTOCINON® (10 IU/mL) was manufactured by Sigma‐Tau, Italy (Table 2).
Table 1.
Carbetocin and carbetocin impurities
| Material | Degradation Pathway |
|---|---|
| Carbetocin | N/A |
| [Gly9‐OH]carbetocin | Hydrolysis |
| [Asp5]carbetocin | Hydrolysis |
| [βAsp5]carbetocin | Hydrolysis |
| [Glu4]carbetocin | Hydrolysis |
| Carbetocin sulfoxide isomer 1 | Oxidation |
| Carbetocin sulfoxide isomer 2 | Oxidation |
| [D‐Cys6]carbetocin | N/A (synthesis related) |
| [des‐Gln4]carbetocin | N/A (synthesis related) |
| [D‐Asn5]carbetocin | Racemisation |
Table 2.
Batch details of products used in the investigation
| Product | Manufacturer | Batch Numbers | Comment |
|---|---|---|---|
| Heat‐stable carbetocin | Patheon, Ferentino, Italy | 13CAF1, 14CAF1, 14CAF2 | 1 mL in clear glass ampoules, clinical material for WHO trial |
| PABAL/ heat‐stable carbetocin | Ferring GmbH, Kiel, Germany | H14020, H14025, H14830, L13785 | 1 mL in clear glass vials, commercial product |
| SYNTOCINON 10 IU/mL | Sigma‐Tau, Italy | 1407120B | 1 mL in ampoules, commercial product |
2.2. Stability study of formulation screening samples
Carbetocin was dissolved to 0.1 mg/mL in an isotonic solution of 10 mM succinic acid, 47 mg/mL D‐mannitol, and 1 mg/ml L‐methionine. This solution was pH adjusted with 1 M NaOH to pH 4.0, 4.5, 5.2, 6.1, 6.5, and 7.0, and filled into vials. The vials were placed in a 40°C/75% RH cabinet and analysed for carbetocin content and impurities after 12 months.
2.3. Long‐term stability study of heat‐stable carbetocin formulation
Three batches of the final formulation of heat‐stable carbetocin in ampoules and vials were exposed to 30°C/75% RH for up to 3 years. The content and impurities of carbetocin were analysed after 0, 3, 6, 9, 12, 18, 24, and 36 months.
2.4. Accelerated stability study of heat‐stable carbetocin formulation
Three batches of the final formulation of heat‐stable carbetocin in ampoules and vials were exposed to 40°C/75% RH for up to 6 months. The content and impurities of carbetocin were analysed after 0, 1, 3, and 6 months.
2.5. Stability study at extreme heat conditions
Batches of heat‐stable carbetocin in vials and ampoules and 1 batch of SYNTOCINON 10 IU/mL (oxytocin) were monitored for 3 months at 50°C and for 1 month at 60°C. The batches of carbetocin and oxytocin were analysed after 0, 1, 2, and 3 months at 50°C and after 0, 1, 2, 3, and 4.3 weeks of storage at 60°C. The remaining amount of carbetocin and oxytocin at each time‐point was evaluated and compared with the same products stored in refrigerated conditions.
2.6. HPLC method
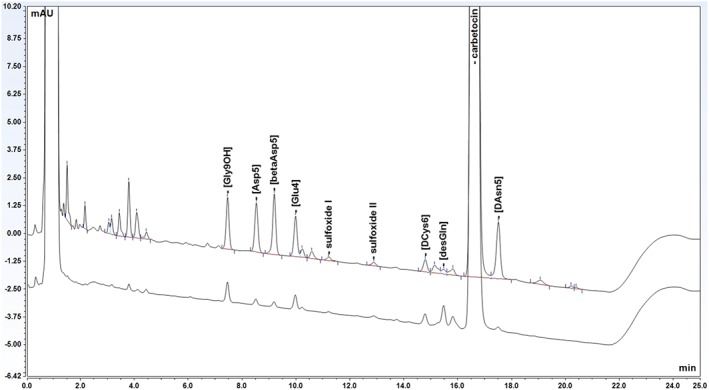
The content and impurities of carbetocin and the content of oxytocin were determined by gradient reversed‐phase LC using an Alliance HPLC (Waters, Milford, MA, USA) and a XBridge C18 column (3 × 150 mm, 3.5 μm) (Waters, Milford, MA, US). Mobile phase A was prepared by mixing 0.30 g of ammonium acetate (Sigma‐Aldrich, St. Louis, MO, USA) with 380 mL of acetonitrile (JT Baker, Deventer, The Netherlands) and approximately 1000 mL of milliQ purified water in a 2000‐mL volumetric flask. Eight milliliters of PIC B‐8 Low UV reagent (Waters, Milford, MA, USA) was added, and the solution was diluted to volume with Milli‐Q‐UF purified water (Millipore, Milford, MA, USA). Mobile phase B was prepared by mixing equal volumes of mobile phase A and acetonitrile. The flow rate was set to 0.8 mL/min, and the column temperature was 60°C. The injection volume was 40 μL and the detection wavelength 220 nm. A linear gradient was used from 0% to 25% of mobile phase B in 20 minutes. A chromatogram of carbetocin standard spiked with 9 related impurities is shown in Figure 1. All peaks were separated with baseline resolution (Rs ≥ 2.0).
Figure 1.
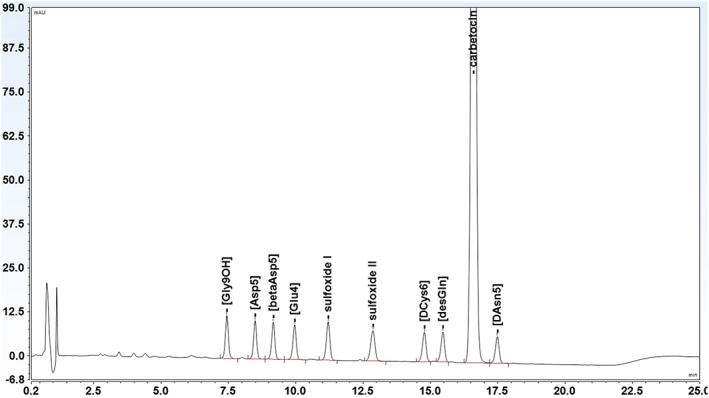
Chromatogram of carbetocin and 9 related impurities
3. RESULTS AND DISCUSSION
3.1. Stability study of carbetocin formulation screening samples
Figure 2 shows the amount of individual impurities and sum of impurities after 12 months at 40°C/75% RH as a function of pH.
Figure 2.
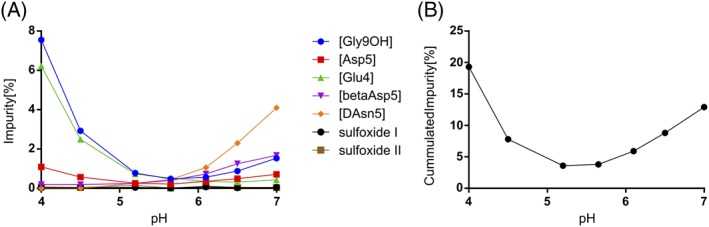
(A) Amount of individual impurities and (B) sum of carbetocin impurities, at different pH values after 12 months at 40°C/75% RH
The curve for sum of carbetocin impurities was found to form a U‐shape with an interpolated stability optimum at pH 5.45 (Figure 2) with low degradation (≤ 4%) after 12 months at 40°C/75% RH. Carbetocin was found to degrade mainly by deamidation of the glutamine residue and the amidated glycine C‐terminus at pH‐values below the optimum and by racemisation of the asparagine residue at pH values above the optimum (Figure 2). Due to the presence of an antioxidant (methionine), degradation by oxidation was negligible at all pH‐values. The final formulation of heat‐stable carbetocin was at this stage determined to be 0.1 mg/mL of carbetocin in 10 mM succinic acid, 47 mg/mL D‐mannitol, and 1 mg/ml of L‐methionine pH‐adjusted with NaOH to pH 5.25–5.65.
The found pH optimum of carbetocin at ≈ pH 5.5 is significantly higher than the pH range in which oxytocin is normally formulated (pH limits for oxytocin in European Pharmacopoeia is pH 3.5–4.0). The reason for this can be found in the structural differences between the 2 molecules.
There are 3 structural differences between carbetocin and oxytocin, highlighted by dash line circles in Figure 3: the N α of oxytocin is removed; sulphur atom on Cys1 is replaced by a methylene group, and Tyr2 is replaced by Tyr(Me).
Figure 3.
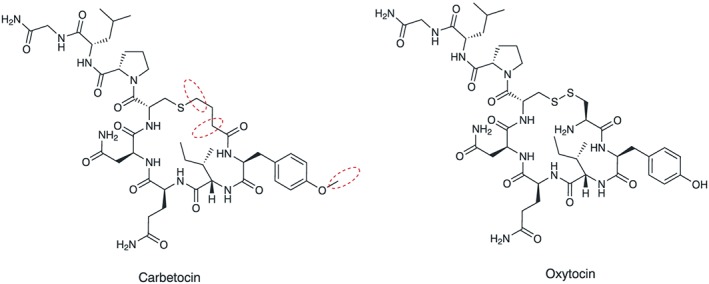
Structure of carbetocin and oxytocin. Structural differences marked with dashed line circles
These modifications reinforce the stability of carbetocin. The major degradation pathway of oxytocin at pH values ≥4 is initiated at the disulphide bond by beta‐elimination and formation of N‐terminal dehydroalanine, which is isomerized to enamine and hydrolyzed to pyruvoyl group. The latter triggers the dimerization by means of an aldol reaction.11 This process requires 2 features: a disulphide and a N α group. Carbetocin lacks both features; hence, the pathway of dimerization is blocked. Thus, the upper pH restriction to avoid dimerization is not relevant for carbetocin and allows for a broader pH interval to be explored, compared with oxytocin.11 The drawback of the low pH value required to reduce oxytocin dimerization is the increase of degradation by deamidation, which makes temperature control necessary.
The main degradation routes of carbetocin are found to be deamidation, oxidation, and racemisation. In analogy with oxytocin, deamidation of the amide side‐chains of asparagine and glutamine and the amidated glycine C‐terminus is favoured by low pH (acid‐catalyzed hydrolysis), and to some extent by high pH (direct base hydrolysis). The thioether linkage is sensitive to oxidation, which is accelerated by increasing pH. The racemisation of the asparagine residue from the L to the D‐form is an important degradation route at pH‐values above ≈ 6. Consequently, in order to develop a heat stable formulation of carbetocin, it is essential to identify an antioxidant that block the oxidation pathway throughout the investigated pH‐range and thereafter identify a solution pH where the sum of remaining degradation pathways is minimized.
3.2. Long term stability study—heat‐stable carbetocin
The long‐term stability of the final heat‐stable formulation in both vials and ampoules has been thoroughly studied in accordance with ICH guidelines at 30°C/75% RH for 36 months. The potency remained ≥95% at all time points, and the level of impurities did not exceed 2.5% (Figure 4).
Figure 4.
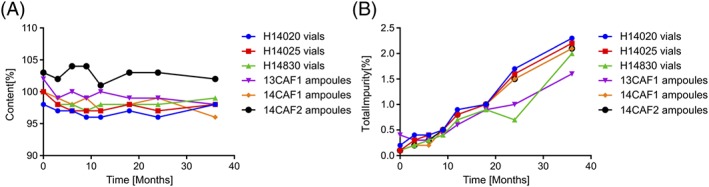
(A) Content of carbetocin and (B) sum of impurities, after storage at 30°C/75% RH for up to 36 months
3.3. Accelerated stability study—heat‐stable carbetocin
The accelerated stability of the final heat‐stable formulation in both vials and ampoules has been studied in accordance with ICH guidelines at 40°C/75% RH for 6 months. The potency remained ≥95% at all time points, and the level of impurities did not exceed 1.5% (Figure 5).
Figure 5.
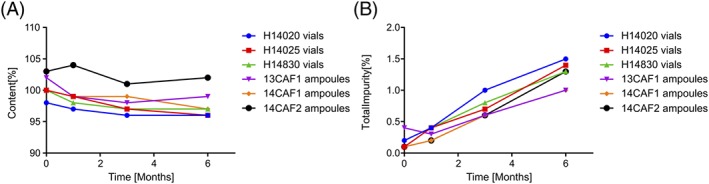
(A) Content of carbetocin and (B) sum of impurities, after storage at 40°C/75% RH for up to 6 months
3.4. Freeze–thaw stability study—heat‐stable carbetocin
Freeze‐thaw stability was examined by placing heat‐stable carbetocin batch 12CAR2 in vials at −20°C, 25°C, and 40°C sequentially for 24 hours each. The cycle was repeated 3 times, and then the product was stored at 30°C/75% RH for 36 months. No difference in content of carbetocin and level of impurities were observed between these samples and the vials from the same batch not exposed to the freeze‐thaw cycles (Table 3).
Table 3.
Results of freeze‐thaw study after 36 months at 30°C/75%
| Batch | Content of Carbetocin | Sum of Impurities |
|---|---|---|
| 12CAR2 | 0.098 mg/mL | 1.6% |
| Freeze‐thaw | ||
| 12CAR2 | 0.097 mg/mL | 1.6% |
| Normal storage |
3.5. Photostability study—heat‐stable carbetocin
Photostability was examined by irradiation of 1 batch of heat‐stable carbetocin vials in its primary container according to ICH Q1B. The samples were then analysed and compared with a dark control. No difference in content of carbetocin was observed between these samples and the dark control (Table 4). The observed level of sum of impurities of the irradiated samples were slightly increased compared with the dark control, but the overall level is negligible.
Table 4.
Results of photostability study
| Batch | Content of Carbetocin | Sum of Impurities |
|---|---|---|
| 12CAR2 irradiated | 0.101 mg/mL | 0.2% |
|
12CAR2 Dark control |
0.101 mg/mL | 0.1% |
3.6. Comparison of stability of oxytocin and carbetocin at extreme heat conditions
Figure 6 shows the remaining amounts of carbetocin and oxytocin after storage at 50°C and 60°C. See Figures 7 and 8 for overlay chromatograms of initial and last time point at 60°C.
Figure 6.
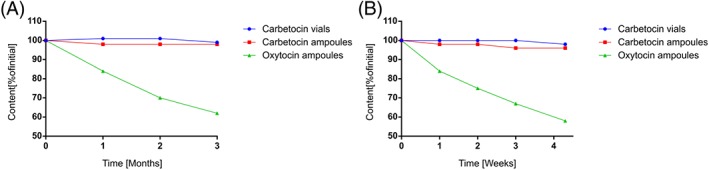
Remaining amount of oxytocin and carbetocin after storage at (A) 50°C and (B) 60°C
Figure 7.
Overlay chromatogram of heat‐stable carbetocin in ampoules before and after storage at 60°C for 1 month. Data in vials were similar (not shown)
Figure 8.
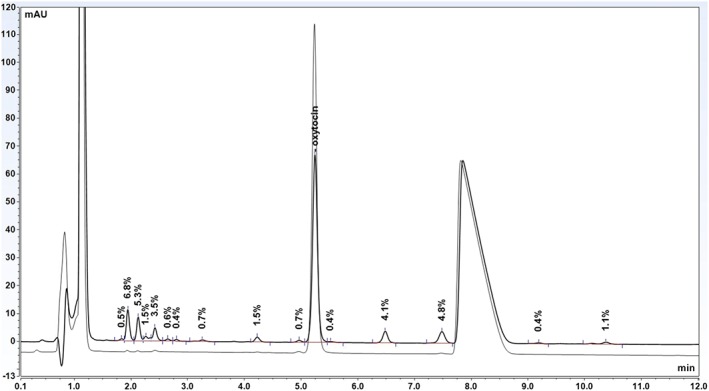
Overlay chromatogram of SYNTOCINON ampoules before and after storage at 60°C for 1 month. The large peak at approximately 8 minutes is a preservative
The decrease of oxytocin potency during storage at 50°C and 60°C was evaluated by curve fitting (Figure 6). The data indicated a nonlinear decrease of oxytocin over time, and a polynomial regression provided the best curve fit to the available data. By interpolation, the oxytocin potency was estimated to drop below 90% of initial value after approximately 17 days at 50°C and 4 days at 60°C. The carbetocin potency remained ≥95% of initial during the entire study at both 50°C and 60°C.
3.7. Discussion of stability studies
The heat‐stability data show that ≥95% potency of carbetocin was maintained for a minimum of 3 years at 30°C, 6 months at 40°C, 3 months at 50°C, and 1 month at 60°C. These results are in line with the findings of the initial formulation screening study, see Section 3.1. In addition, this new heat‐stable carbetocin formulation is not sensitive to freezing or light, in its primary container, making it a good uterotonic alternative in LMI countries where distribution and storage under controlled conditions can be difficult to achieve and/or maintain.
4. CONCLUSION
The molecular properties of carbetocin, an oxytocin analogue, has allowed for the development of an unusually stable peptide formulation under ICH climate zone IV (30°C and 75% RH) conditions for at least 3 years and at extreme temperatures, such as 60°C, for shorter periods of time.
Prior attempts to develop a heat‐stable oxytocin formulation for injection have been unsuccessful.12, 13, 14, 15 Peptides in solution are generally prone to undergo degradation via, e.g., deamidation, dimerization, and oxidation, making refrigeration in some cases necessary.
Minor modifications of the oxytocin peptide structure such as those introduced into the carbetocin structure can substantially change the sensitivity to degradation and allow for the development of a heat‐stable peptide liquid formulation. A uterotonic that can be stored at room temperature, in hot and humid environments, without compromising quality, and proven safe and effective, would be a significant breakthrough for maternal health in LMI countries.
Ferring Pharmaceuticals, the World Health Organization, and MSD for Mothers have established a collaboration to develop this heat‐stable formulation of carbetocin for the prevention of post‐partum hemorrhage in women after vaginal childbirth. The organizations are working together with the aim of making the medicine available in the public sector of developing countries that have a high burden of maternal mortality and where consistent refrigeration is burdensome. As part of that collaboration, the WHO is nearing completion of a randomized, double‐blind non‐inferiority trial comparing heat‐stable carbetocin to the standard intervention (oxytocin) for the prevention of PPH after vaginal birth.16
Malm M, Madsen I, Kjellström J. Development and stability of a heat‐stable formulation of carbetocin for the prevention of postpartum haemorrhage for use in low and middle‐income countries. J Pep Sci. 2018;24:e3082 https://doi.org/10.1002/psc.3082
MSD is known as Merck in the US and Canada
REFERENCES
- 1. WHO , 2016. Maternal Mortality fact sheet, http://www.who.int/mediacentre/factsheets/fs348/en/ (accessed: October, 2017).
- 2. UNICEF , 2017. UNICEF Data: Monitoring the Situation for Women and Children, Statistics by Topic, Maternal Health, Maternal Mortality, updated Feb. 2017, https://data.unicef.org/topic/maternal-health/maternal-mortality/ (accessed: October, 2017).
- 3. Filippi V, Chou D, Ronsmans C, Graham W, Saet L. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities. Third Edition (Volume 2). Levels and Causes of Maternal Mortality and Morbidity. Chapter 3 in Black RE, Laxminarayan R, Temmerman M, et al., editors. Washington (DC): The International Bank for Reconstruction and Development / The World Bank. 2016. https://doi.org/10.1596/978-1-4648-0348-2_ch3 [PubMed] [Google Scholar]
- 4. Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066‐1074. https://doi.org/10.1016/S0140-6736(06)68397-9 [DOI] [PubMed] [Google Scholar]
- 5. United Nations , 2015, Sustainable Development Goals, Goal 3, http://www.un.org/sustainabledevelopment/health/ (accessed: October, 2017)
- 6. WHO , 2012. WHO recommendations for the prevention and treatment of postpartum haemorrhage, http://apps.who.int/iris/bitstream/10665/75411/1/9789241548502_eng.pdf?ua=1 (accessed: October, 2017) [PubMed]
- 7. Torloni MR, Gomes Freitas C, Kartoglu UH, Metin Gulmezoglu A, Widmer M. Quality of oxytocin available in low‐ and middle‐income countries: a systematic review of the literature. BJOG. 2016;123(13):2076‐2086. https://doi.org/10.1111/1471-0528.13998 [DOI] [PubMed] [Google Scholar]
- 8. Hogerzeil HV, Walker GJA, de Goeje MJ, Stability of injectable oxytocics in tropical climates: results of field surveys and simulation studies on ergometrine, methylergometrine, and oxytocin. WHO. 1993. Available at: http://apps.who.int/medicinedocs/pdf/s2205e/s2205e.pdf (accessed: October, 2017)
- 9. De Groot, ANJA , Vree TB, Hogerzeil HV, Walker GJA, Stability of oral oxytocics in tropical climates—results of simulation studies on oral ergometrine, oral methylergometrine, buccal oxytocin, and buccal desamino‐oxytocin. WHO. 1994. Available at: http://apps.who.int/medicinedocs/pdf/s2231e/s2231e.pdf (accessed Oct. 31, 2017)
- 10. PATH . Rapid Assessment of Economic Value for Oxytocin in the Uniject™ Prefill Injection System. Seattle: PATH; 2011. [Google Scholar]
- 11. Wisniewski K, Finnman J, Flipo M, Galyean R, Schteingart CD. On the mechanism of degradation of oxytocin and its analogues in aqueous solution. Biopolymers. 2013;100(4):408‐421. https://doi.org/10.1002/bip.22260 [DOI] [PubMed] [Google Scholar]
- 12. Hawe A, Poole R, Romeijn S, Kasper P, van der Heijden R, Jiskoot W. Towards heat‐stable oxytocin formulations: analysis of degradation kinetics and identification of degradation products. Pharm Res. 2009;26(7):1679‐1688. https://doi.org/10.1007/s11095-009-9878-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avanti C, Permentier HP, Dam AV, et al. A new strategy to stabilize oxytocin in aqueous solutions: II. Suppression of cysteine‐mediated intermolecular reactions by a combination of divalent metal ions and citrate. Mol Pharm. 2012;9(3):554‐562. https://doi.org/10.1021/mp200622z [DOI] [PubMed] [Google Scholar]
- 14. Avanti C, Amorij JP, Setyaningsih D, et al. A new strategy to stabilize oxytocin in aqueous solutions: I. The effects of divalent metal ions and citrate buffer. AAPSJ. 2011;13(2):284‐290. https://doi.org/10.1208/s12248-011-9268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gard JW, Alexander JM, Bawdon RE, Albrecht JT. Oxytocin preparation stability in several common obstetric intravenous solutions. Am J Obstet Gynecol. 2002;186(3):496‐498. https://doi.org/10.1067/mob.2002.121104 [DOI] [PubMed] [Google Scholar]
- 16. Widmer M, Piaggio G, Abdel‐Aleem H, et al. Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally: study protocol for a randomized controlled trial. Trials. 2016;17(1):143 https://doi.org/10.1186/s13063-016-1271-y [DOI] [PMC free article] [PubMed] [Google Scholar]


