Abstract
Endocrine therapy, a major modality in the treatment of hormone receptor (hr)–positive breast cancer (bca), has improved outcomes in metastatic and nonmetastatic disease. However, a limiting factor to the use of endocrine therapy in bca is resistance resulting from the development of escape pathways that promote the survival of cancer cells despite estrogen receptor (er)–targeted therapy. The resistance pathways involve extensive cross-talk between er and receptor tyrosine kinase growth factors [epidermal growth factor receptor, human epidermal growth factor receptor 2 (her2), and insulin-like growth factor 1 receptor] and their downstream signalling pathways—most notably pi3k/akt/mtor and mapk. In some cases, resistance develops as a result of genetic or epigenetic alterations in various components of the signalling pathways, such as overexpression of her2 and erα co-activators, aberrant expression of cell-cycle regulators, and PIK3CA mutations. By combining endocrine therapy with various molecularly targeted agents and signal transduction inhibitors, some success has been achieved in overcoming and modulating endocrine resistance in hr-positive bca. Established strategies include selective er downregulators, anti-her2 agents, mtor (mechanistic target of rapamycin) inhibitors, and inhibitors of cyclin-dependent kinases 4 and 6. Inhibitors of pi3ka are not currently a treatment option for women with hr-positive bca outside the context of clinical trial. Ongoing clinical trials are exploring more agents that could be combined with endocrine therapy, and biomarkers that would help to guide decision-making and maximize clinical efficacy. In this review article, we address current treatment strategies for endocrine resistance, and we highlight future therapeutic targets in the endocrine pathway of bca.
Keywords: Breast cancer, hormone-positive disease, endocrine resistance, targeted therapy, metastatic breast cancer
INTRODUCTION
Breast cancer (bca) is a heterogeneous disease encompassing several biologic subtypes that have different clinical behaviours and responses to treatment. The most common subtypes are hormone receptor (hr)–positive [estrogen (er) or progesterone (pgr) receptor–positive, or both], which together constitute the luminal subtype and account for about 75% of all cases1,2.
The first evidence for the estrogen-dependent nature of bca was obtained more than 100 years ago by observation of the regression of bca after oophorectomy3. With more advances in cancer treatment, several anti-hormonal approaches were developed, making endocrine therapy one of the earliest targeted treatments in bca. Established targeted endocrine strategies in bca include selective er modulators such as tamoxifen; aromatase inhibitors (ais), which block conversion of androgens to estrogens in peripheral tissues; selective er downregulators (for example, fulvestrant); and ovarian suppression or ablation, which prevents endogenous production of estrogen by the ovaries. Those endocrine treatments have provided clinical benefit and tumour regression with favourable toxicity profiles for patients with metastatic and nonmetastatic hr-positive disease4. In contrast, targeting pgr has demonstrated less impressive clinical results, with increased toxicity and side effects5,6. Currently, pgr is considered a marker for endocrine sensitivity, with higher pgr expression suggesting better sensitivity to endocrine blockade.
Resistance to endocrine therapies is a major factor limiting the use of those agents in er-positive bca. Approximately 50% of patients with metastatic er-positive disease achieve a complete or partial response or stabilization of their tumour with endocrine therapy; for the remaining patients, the benefit is limited because of intrinsic or de novo resistance7–9. The experience of the latter patients underpins the hypothesis that er is not the only survival pathway for these tumours and that escape pathways could have already developed that drive cancer cell survival despite the targeting of er with endocrine therapy7.
Overcoming endocrine resistance has been a major focus of recent clinical research, and a number of clinical trials have combined endocrine therapy with signal transduction inhibitors and molecularly targeted agents with the aim of modulating and overcoming potential resistance pathways. In this review, we highlight the available data on endocrine resistance in bca and discuss the most recent updates from major clinical trials that have targeted various molecular and signalling pathways involved in the development of endocrine resistance.
Definition of Endocrine Therapy Resistance
No standardized definition for endocrine therapy resistance in er-positive bca has been established. The complexity of endocrine resistance makes it difficult to clearly define the various types of resistance (intrinsic vs. acquired). Furthermore, the clinical data are limited; most of the information has come from preclinical studies, which have to be further investigated in well-designed studies. Generally, resistance can take either the de novo form (present before starting any treatment) or the acquired form (develops during therapy after an initial period of response).
These are the common clinical scenarios of endocrine resistance:
■ De novo resistance of metastatic disease to all hormonal therapies, or recurrence soon after the start of adjuvant hormonal therapy, with no response to further endocrine therapy
■ De novo resistance to some hormonal therapy, but sensitive to others
■ Acquired resistance after initial response to endocrine therapy, followed by shorter periods of response to serial endocrine therapies until the cancer becomes refractory to all endocrine agents
Clinical observation of cancers that respond to endocrine therapy after progression on another agent supports the existence of agent-specific and class-specific types of endocrine resistance10. In contrast to patients having bca that recurs or progresses shortly after cessation of endocrine therapy, bca in patients who experience a prolonged treatment-free interval (more than 12 months) might not be endocrine-resistant, and those patients might benefit from continued endocrine therapy11. Patients with high-burden or rapidly progressing metastatic disease that is life-threatening should be treated with systemic chemotherapy to work toward faster control of their disease. Endocrine therapy could then be offered to those experiencing a clinical response to chemotherapy.
Complexity of ER Signalling Pathways
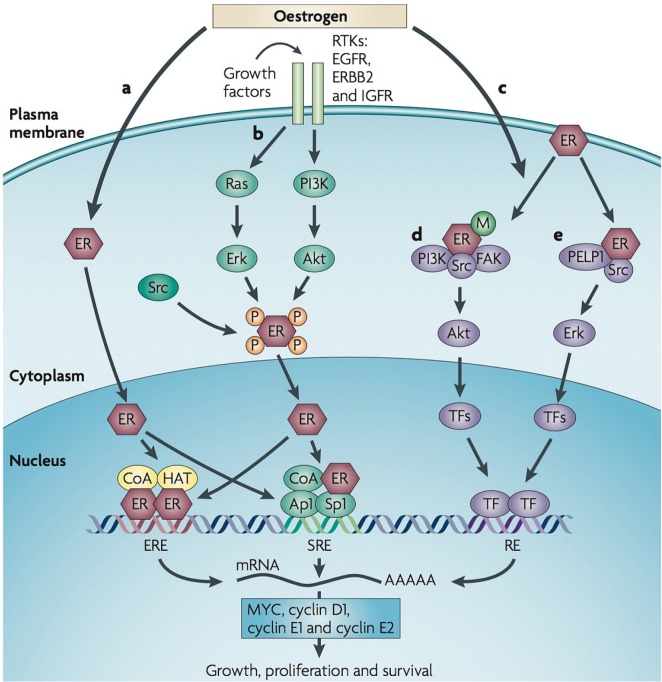
Estrogen has important effects on cellular processes, including cell proliferation and survival. Those actions are mediated through estrogen binding to its receptors (erα and erβ). Activation of erα is responsible for most estrogen effects on normal breast tissue (making an essential contribution to mammary development) and on cancerous breast tissue (leading to hormone-dependent tumour growth)12. The er signalling pathway (Figure 1) consists of a complex biologic network that involves several regulators and “cross-talk” between er and membrane receptor tyrosine kinases such as epidermal growth factor receptor (egfr), human epidermal growth factor receptor 2 (her2), and insulin-like growth factor 1 receptor (igf1r). Those signalling pathways regulate gene expression and control a variety of functions such as cell growth, proliferation, and survival14.
FIGURE 1.
Estrogen receptor (ER) signalling pathway. (a) Classic ER signalling leads to genomic function through estrogen ligand–receptor binding, leading to dimerization of ERs that complex with co-activators (CoA) and co-repressors and that then bind to specific DNA sites called estrogen response elements (EREs), which in turn regulate gene expression. The ER can also bind to other transcription factors, such as activation protein 1 (Ap1) and specificity protein 1 (Sp1), and functions as co-regulator to facilitate binding to serum response elements (SREs) and activate transcription. (b) The ER can also be activated through downstream events of receptor tyrosine kinases (RTKs) [epidermal growth factor receptor (EGRF), human epidermal growth factor receptor 2 (HER2, ERBB2), insulin-like growth factor receptor (IGFR)], which is sometimes called ligand-independent ER receptor activation. (c) Nongenomic functions can be mediated by ER activation inducing the assembly of protein complexes that activate signalling cascades that ultimately lead to transcription factor activation independent of ER binding to DNA. This pathway can rapidly regulate cellular processes through interaction with various signalling pathways including (d) ER–phosphoinositide 3-kinase (PI3K)–Src–focal adhesion kinase (FAK) complex that activates AKT, and (e) ER–Src–proline-, glutamate-, and leucine-rich protein 1 (PELP1) complexes that activate ERK. (Adapted with permission from Macmillan Publishers Ltd.: Musgrove and Sutherland, 200913.)
Activation of er has nuclear (genomic) and nongenomic functions. Classical er signalling leads to genomic functions through estrogen ligand–receptor binding, which leads to dimerization of ers that complex with co-activators and co-repressors. The complexes then bind to specific dna sites called estrogen response elements that in turn regulate expression of estrogen-responsive genes that are important in physiologic and pathologic processes, including breast tumour proliferation and progression15. Estrogen receptor–ligand complexes can also bind to other transcription factors such as activation protein 1, specificity protein 1, and nuclear factor κB, functioning as a coregulator to facilitate their binding to serum response elements, which in turn regulate the transcriptional activity of those factors and their responsive genes, triggering the co-activators into a higher state of activity16,17. That non-classical er transcriptional regulation mechanism was shown to be augmented, even in the absence of estrogen, under the stimulation of growth factors in bca cells that are resistant to endocrine therapy18,19.
Activation of er can also occur through downstream events of receptor tyrosine kinases (egfr, her2, igf1r)— sometimes called “ligand-independent er activation.” Bidirectional cross-talk between those pathways at multiple levels has been described14. For instance, estrogen can increase the expression of ligands such as transforming growth factor α and insulin-like growth factor 1 (igf1) that activate growth factor receptor pathways. On the other hand, er signalling can downregulate egfr and her2 while increasing the expression of igf1r, thus promoting the igf1r–igf1 axis, which in turn enhances the tyrosine kinase signalling and mediates resistance to antiestrogen through downstream signalling of the mapk (mitogenactivated protein kinase) and pi3k (phosphoinositide 3-kinase) pathways20. The expression of PAX2 (a transcription factor) leads to her2 repression, while activation of AIB1 (src-3) upregulates her2 transcription. Preclinical study suggested that the balance between PAX2 and AIB1 influences tamoxifen resistance through the regulation of her221. Expression of er and pgr can be further down-regulated by activation of the pi3k/akt (protein kinase B)/mtor (mechanistic target of rapamycin) and mapk pathways through growth factor receptors, which might reduce cellular dependency on estrogen, thereby bypassing the therapeutic approach of lowering estrogen levels by using either selective er modulators or ais22,23.
The nongenomic function can be mediated through er activation, inducing assembly of protein complexes that activate signalling cascades ultimately leading to transcription factor activation independent of er binding to dna. That function can rapidly regulate cellular processes by interaction with various signalling pathways such as the er–pi3k–Src–fak complex that activates akt and er–Src–pelp1 complexes, which in turn activate erk. The activated akt or erk stimulates cellular growth and at the same time protects cancer cells from apoptosis by phosphorylating and inactivating several key apoptotic molecules; those molecules in turn promote er-independent tumour growth13,14.
FACTORS ASSOCIATED WITH ENDOCRINE RESISTANCE
The mechanisms of endocrine resistance in hr-positive bca are complex and diverse; multiple molecules and pathways have been implicated. Much information has come from preclinical studies with tamoxifen. Many of the broad concepts that emerged from those studies will probably apply to resistance to other endocrine therapies, and more recent investigations have collected data about other alternative pathways that better correlate with specific types of resistance13,24.
ER and Co-regulators
The primary mechanism for de novo resistance to endocrine therapy is lack of erα expression. Another intrinsic mechanism was described in patients with inactive alleles of cytochrome P450 2D6 (CYP2D6), which leads to impaired enzymatic activity for converting tamoxifen to its active metabolite, endoxifen, thus decreasing the response to tamoxifen therapy25. A deficiency of the CYP2D6 enzyme is inherited as an autosomal recessive trait and presents in 7% of the white population and about 1% of the Asian population26.
A small proportion of patients develop acquired resistance because of erα expression loss (15%–20%) or er mutations (fewer than 1% in the primary tumour)27,28. On the other hand, loss of pgr expression occurs more frequently and has also been associated with increased growth factor signalling and endocrine resistance, making tumours more clinically aggressive and leading to worse patient outcomes. Additionally, her2 status can change with disease progression (14% of patients). Those findings have led to the recommendation for rebiopsy on progression in relapsed and metastatic disease to assess for changes in hormonal and her2 receptors, which can affect treatment choices23,29,30. As previously noted, the increased activity of specificity protein 1 and the dysregulation and over expression of er co-activators, most notably aib1, have also been implicated in tamoxifen resistance18,19.
The recent discovery of acquired mutations in the gene encoding for erα, ESR1, has implicated those mutations in the mechanism of resistance to estrogen deprivation therapy such as ais or oophorectomy in metastatic hr-positive bca. Advances in deep-sequencing technologies has shown the feasibility of detecting ESR1 mutations in liquid biopsies, which are thought to represent the most important metastatic tumour sites. Liquid biopsies can be obtained through noninvasive measurement of circulating tumour cells or cell-free dna in peripheral blood31. The most frequent ESR1 mutations, which occur in the ligand-binding domain of er, include alterations in the amino acids clustering between sequence numbers 534 and 538. Those activating mutations in the ligand-binding domain lead to strong constitutive activation of er, with subsequent tumour-cell proliferation in the presence or absence of estrogen.
The reported incidence of ESR1 mutations in metastatic breast sites ranges from 12% to 20%; it reaches up to 39% in women who have experienced progression while taking ais, and it is less than 1% in primary breast tumours in treatment-naïve women31. The presence of ESR1 mutations has been associated with poorer prognosis and limited response to ais. Interestingly, patients with ESR1 mutations who develop resistance to ais still respond to antiestrogen therapies using fulvestrant or tamoxifen, with fulvestrant having been associated with greater tumour inhibition in preclinical studies. Recent data have also shown the effectiveness of combined treatments with mtor inhibitors (bolero-2) and inhibitors of cyclin-dependent kinases (cdks) 4 and 6 (paloma 3) in patients with or without ESR1 mutations who have metastatic hr-positive bca progressing on prior ai treatment31,32.
Receptor Tyrosine Kinase (Growth Factor) Signalling Pathways
Ongoing endocrine therapy in bca can cause adaptive changes that result in activation of alternative signalling pathways such that cancer cells are no longer dependent on er stimulation for survival and proliferation25. Those signalling pathways involve bidirectional cross-talk between growth factors, cellular kinases, and er pathways at multiple levels, which can lead to endocrine resistance14. Pathway activation can also occur through other mechanisms such as overexpression of growth factors or their receptors (egfr, her2, and igf1r) or activation of downstream signalling (pi3k/akt/mtor and ras/mek/mapk), or both13,33,34. In some cases, deregulation and activation of those signalling pathways occur as a result of genetic or epigenetic alterations, such as HER2 gene amplification, activating mutations in the pi3k catalytic subunit or loss of expression of the pten tumour suppressor of the pi3k pathway28,35. Notably, activation of egfr and her2 signalling has been recognized as one of the factors most prominently contributing to endocrine resistance36,37. An important limiting factor for the activity of anti-egfr treatments is the lack of a biomarker, with exception of her2, which has been shown to be predictive for response to anti-her2 therapy in both hr-positive and hr-negative disease.
Cell-Cycle Regulators
Endocrine therapy in bca has both cytostatic and cytotoxic effects, as supported by clinical data demonstrating reduced cellular proliferation, induction of apoptosis, and reduction in growth rate as a result of cell-cycle arrest in the G1 phase38,39. Molecules that control the cell cycle include positive and negative regulators that have an important role in the estrogen effect to control cell-cycle progression from G1 to the S phase. Aberrant expression of those molecules has been associated with endocrine resistance13,40. Overexpression of the positive regulators Myc and cyclins E1 and D1 can lead to endocrine resistance either by activating cdks, which are important in the G1 phase, or by relieving the inhibitory effects of the negative regulators p21 and p27 on the cdks41,42. In addition, decreased expression of the cdk inhibitor (p21 or p27) and inactivation of the retinoblastoma tumour suppressor are also associated with resistance to endocrine therapy7,43,44.
CLINICAL TRIALS WITH STRATEGIES TO OVERCOME ENDOCRINE RESISTANCE
Selective ER Degrader or Downregulator
Fulvestrant is a steroidal selective er downregulator that binds er, blocks its function, and increases er degradation. Unlike other endocrine therapies, fulvestrant is administered as an intramuscular injection in postmenopausal women with hr-positive bca. The first approved dose (250 mg every 28 days) was shown in two randomized phase iii studies (0020 and 0021) to be as effective as anastrozole, and joint analysis of the studies showed no difference in median time to progression (mttp) between fulvestrant and anastrozole for postmenopausal women who had progressed on prior endocrine therapy (5.5 months vs. 4.1 months, p = 0.48)45–47. In the 0025 clinical trial, which compared fulvestrant (250 mg) with tamoxifen in the first-line setting in advanced bca, the primary endpoint of time to progression (ttp) was not different between the two arms (mttp for fulvestrant vs. tamoxifen: 6.8 months vs. 8.3 months; p = 0.088). The secondary endpoints, including clinical benefit rate, time to treatment failure, and overall survival (os), favoured of tamoxifen therapy. Unexpectedly, the study showed that fulvestrant did not meet the criteria for noninferiority to tamoxifen in the intention-to-treat population48.
Initial and subsequent data showed that, compared with a loading dose approach (500 mg on day 0, 250 mg on day 14 of month 1, and then 250 mg monthly), which reaches steady state within 28 days, fulvestrant at a 250 mg dose takes 3–6 months to achieve steady state49, which might allow for the use of higher doses to achieve quicker response and to limit the possibility of early relapses50. In the effect trial, loading-dose fulvestrant was compared with exemestane in women who had progressed while taking a nonsteroidal ai. The results showed similar efficacy, and the mttp was 3.7 months in both groups50.
Three studies have investigated the combination of loading-dose fulvestrant and ais; the contrasting results in those trials were likely related to the patient groups and their prior treatments. In the sofea and fact trials, no additional benefit was seen for the combination compared with the single agent. The study population in the sofea trial consisted of patients with acquired resistance to nonsteroidal ais, and the result showed similar median progression-free survival (mpfs) for fulvestrant alone and fulvestrant–anastrozole (4.8 months vs. 4.4 months, p = 0.98), and for fulvestrant alone and exemestane alone (4.8 months vs. 3.4 months, p = 0.56)51. In the fact study, more than two thirds of enrolees had received prior antiestrogen therapy, and the mttp was not different between loading-dose fulvestrant plus anastrozole and anastrozole alone (10.8 months vs. 10.2 months, p = 0.91)52. However, the swog 0226 study, whose enrolees included approximately 40% with de novo metastatic disease and 60% without prior adjuvant tamoxifen therapy, found superior outcomes with the fulvestrant–anastrozole combination compared with anastrozole alone or sequential anastrozole and fulvestrant [mpfs: 15 months vs. 13.5 months, p = 0.007; median os (mos): 47.7 months vs. 41.3 months, p = 0.05]53.
More data have shown that er downregulation using fulvestrant is a dose-dependent process. Approximately 70% er downregulation is observed with a single 250 mg dose; nearly 100% can be achieved with high-dose (hd) fulvestrant (500 mg monthly), which might offer greater antitumour activity with superior efficacy54. The phase iii confirm trial compared hd fulvestrant (500 mg on days 0, 14, and 28, and then every 28 days) with the approved dose (250 mg every 28 days) in postmenopausal women with recurrent or metastatic bca in whom prior endocrine therapy had failed. Compared with the lower 250 mg dose, hd fulvestrant was found to be associated with increased pfs: 6.5 months compared with 5.5 months (p = 0.006)55. The updated analysis of final os showed that, compared with fulvestrant 250 mg, hd fulvestrant was associated with an improved 4.1-month difference in mos (26.4 months vs. 22.3 months, p = 0.02)56.
More recently, hd fulvestrant was compared with anastrozole in the falcon trial, which included endocrine therapy–naïve postmenopausal women with locally advanced or metastatic hr-positive bca, finding a significantly improved mpfs in favour of hd fulvestrant compared with anastrozole (16.6 months vs. 13.8 months; hazard ratio: 0.797; p = 0.0486). A significantly enhanced treatment effect was seen in the subgroup analysis of patients having non-visceral disease (defined as skeletal, lymph-node, or soft-tissue metastasis) compared with those having visceral disease (mpfs: 22.3 months vs. 13.8 months). In contrast, for patients with visceral disease, no difference was observed between the two treatment groups (mpfs: 13.8 months vs. 15.9 months). A post hoc interaction test to assess for the consistency of treatment effects across the visceral and non-visceral subgroups resulted in a p value of 0.0092. The os data for the study have not yet been reported57.
The current data support the use of hd fulvestrant as initial therapy in postmenopausal women with advanced bca who are hr-positive and who have low-burden disease and no prior exposure to endocrine therapy. They also provide support for the use of hd fulvestrant as a single agent or in combination with other agents such as nonsteroidal ais or cdk 4/6 inhibitors in those who have progressed while taking prior endocrine therapy (see the discussion of the paloma-3 trial in the next subsection).
CDK 4/6 Inhibitors
The ckd 4/6 inhibitors are small molecules that interact with the cell-cycle machinery and interfere with the G1-to-S phase transition, thus leading to cell-cycle arrest and inhibiting cancer-cell growth. The efficacy of combining cdk 4/6 inhibitor with the ai letrozole was demonstrated in the paloma-1, paloma-2, and monaleesa-2 studies in postmenopausal women who had not previously received systemic treatment for hr-positive advanced bca58–60. Significant improvements in mpfs were observed for palbociclib and letrozole compared with letrozole alone in paloma-1 (20.2 months vs. 10.2 months; hazard ratio: 0.488; p = 0.0004) and paloma-2 (24.8 months vs. 14.5 months; hazard ratio: 0.58; p < 0.0001)58,59, and for ribociclib and letrozole compared with letrozole alone in monaleesa-2 (at the preplanned interim analysis, the mpfs was not reached vs. 14.7 months respectively; hazard ratio: 0.56; p < 0.0001)60. Subgroup analyses of the paloma-2 study confirmed a consistent benefit of combined palbociclib–letrozole in all subgroups, including in patients with visceral and non-visceral disease and in patients who had and had not received prior endocrine therapy. Both studies showed that cdk 4/6 inhibitor combined with letrozole improves efficacy, but with some additional toxicities, including neutropenia, leucopenia, and fatigue, and with hepatotoxicity and QTc prolongation being identified with ribociclib. However, the side effects can be successfully managed with appropriate supportive care, monitoring, and dose reductions.
The randomized phase iii paloma-3 study compared the combination of palbociclib and fulvestrant with fulvestrant alone. That study demonstrated a doubling of pfs in women who had progressed while taking an ai or within 1 month of completion of ai for advanced disease, or within 12 months of completing adjuvant hormonal therapy (mpfs: 9.2 months for palbociclib–fulvestrant vs. 3.8 months for fulvestrant alone; hazard ratio: 0.42; p < 0.001). The side effects were consistent with the toxicity profiles reported in earlier studies. Notably, the study population included pre- and perimenopausal women, who also received the luteinizing hormone–releasing hormone agonist goserelin, and study benefits were consistent in all subgroups regardless of menopausal status. Data for os were immature at the time of the interim analysis61.
A number of clinical trials continue to assess cdk 4/6 inhibitors as treatment for bca. The phase iii monaleesa-3 trial is exploring ribociclib–fulvestrant for patients with advanced bca who are treatment-naïve or who have progressed on only 1 prior line of endocrine therapy. The phase iii monaleesa-7 trial is exploring first-line endocrine therapy with ribociclib in combination with tamoxifen or a nonsteroidal ai and goserelin for premenopausal women with advanced hr-positive bca. Abemaciclib is another cdk 4/6 inhibitor that has shown single-agent activity in patients who have been heavily pretreated for metastatic hr-positive bca62, and ongoing phase iii trials are exploring its efficacy in combination with fulvestrant (monarch-2) or with a nonsteroidal ai (monarch-3) for patients with metastatic hr-positive bca.
Inhibitors of Growth Factor Signalling Pathways
As previously described, the egfr/her family has been implicated in endocrine resistance. Blockade of both the er and growth factor receptor signalling pathways has been investigated in many clinical trials, with the aim to reverse the resistance, restore endocrine sensitivity, and delay the need for chemotherapy. Results of clinical studies targeting egfr in bca, including a small-molecule tyrosine kinase inhibitor of egfr (gefitinib) and the dual tyrosine kinase inhibitor of egfr and her2 (lapatinib), have shown limited benefit when combined with endocrine therapy63,64. On the other hand, adding her2-targeted therapies to endocrine therapy has been associated with better outcomes than those achieved with endocrine therapy alone in patients with hr-positive advanced bca with her2 overexpression.
The combination of the monoclonal antibody to her2 (trastuzumab) and anastrozole, without chemotherapy, was explored in the tandem phase iii clinical trial in postmenopausal women with her2-positive, hr-positive metastatic bca. Compared with patients receiving anastrozole alone, those receiving combination treatment experienced better mpfs (4.8 months vs. 2.4 months; hazard ratio: 0.63; p = 0.0016). A numeric but nonsignificant improvement in os was observed in the combination arm (mos: 28.5 months vs. 23.9 months; p = 0.325); however, 70% of patients in the anastrozole-alone arm crossed over to receive trastuzumab after progression65.
With the evolution in the therapy for her2-positive bca, treatments combining chemotherapy and anti-her2 agents have significantly improved survival outcomes and should be considered the initial therapy for all patients with advanced her2-positive disease, including those with hr-positive bca. Patients who experience good control of their disease could be offered endocrine therapy in addition to maintenance anti-her2 therapy after chemotherapy66.
Pertuzumab is a humanized monoclonal antibody that binds to subdomain ii in the extracellular domain of her2, inhibiting her2/her3 heterodimerization, which then blocks downstream signalling pathways of her2 (mapk and pi3k). Dual her2 blockade with trastuzumab–pertuzumab plus docetaxel was shown (in the cleopatra trial) to be associated with significantly improved pfs and os in metastatic her2-positive bca66. The potential benefit of adding pertuzumab to ai and trastuzumab (with or without induction taxane chemotherapy) for first-line treatment of postmenopausal women with her2-positive, hr-positive advanced bca was investigated in the phase ii pertain clinical trial. The primary analysis in that study showed a significantly improved mpfs (by 3 months) in the added pertuzumab arm compared with the trastuzumab and ai arm (18.9 months vs. 15.8 months; hazard ratio: 0.65; p = 0.007). Notably, more than 50% of the patients in both arms had received induction taxane chemotherapy (docetaxel or paclitaxel for 18–24 weeks) combined with anti-her2 therapy before starting ais. Results in most subgroups favoured the pertuzumab arm, including the groups of patients who did (hazard ratio: 0.75; 95% confidence interval: 0.50 to 1.13) and did not receive induction chemotherapy (hazard ratio: 0.55; 95% confidence interval: 0.34 to 0.88)67. The ongoing phase iii detect v/chevendo study is comparing chemotherapy with endocrine therapy in combination with dual her2-targeted therapy (trastuzumab and pertuzumab) in patients with her2-positive, hr-positive metastatic bca.
Inhibitors of the PI3K/AKT/mTOR Pathway
The pi3k/akt/mtor signalling pathway is critical in many cellular processes controlling cell growth, proliferation, survival, and metabolism. Aberrations in this intracellular pathway have been implicated in development of many cancers and resistance to cancer therapy68. Multiple therapeutic strategies have been developed to target various components of the pathway in combination with endocrine therapy for the treatment of bca with endocrine therapy resistance.
Rapamycin—and its analogs temsirolimus, everolimus, and deforolimus—inhibit mtor activation, which is often involved in cancer-cell resistance to treatment69. The addition of everolimus to the steroidal ai (exemestane) was evaluated in comparison with exemestane alone in the phase iii bolero-2 trial, which included postmenopausal women with hr-positive, her2-negative advanced bca who had progressed on prior nonsteroidal ais. The primary endpoint, pfs, was significantly better in the combination treatment arm (mpfs: 6.9 months vs. 2.8 months; hazard ratio: 0.43; p < 0.001). However, the mos, which was the secondary endpoint and which was improved by 4.4 months with everolimus–exemestane, was not statistically significant (31 months vs. 26.6 months; hazard ratio: 0.89; p = 0.14). Notably, more than 85% of patients in both arms received post-study therapies70,71.
Furthermore, tamoxifen–everolimus was assessed in the phase ii tamrad study, in which postmenopausal women with hr-positive, her2-negative advanced bca who had received prior treatment with ais were randomized to everolimus–tamoxifen or to tamoxifen alone. The clinical benefit rate at 6 months (primary endpoint) was significantly better in the combination arm than in the tamoxifen-alone arm (61% vs. 42%, exploratory p = 0.045). The ttp and os were also improved in the everolimus–tamoxifen arm [mttp: 8.6 months vs. 4.5 months with tamoxifen alone; p = 0.002; mos: not reached vs. 32.9 months; hazard ratio: 0.45; p = 0.007 (from the last data update in September 2011)]72. Notably, the benefit of everolimus was greater for patients with secondary hormone resistance (defined as relapse >6 months after stopping adjuvant ais, or response for 6 months or more to ais in the metastatic setting)72.
In the most recent phase ii study (precog 0102), the addition of everolimus to hd fulvestrant, compared with fulvestrant–placebo, doubled the mpfs in postmenopausal women with hr-positive, her2-negative metastatic bca resistant to ai therapy (10.4 months vs. 5.1 months; hazard ratio: 0.6; p = 0.02)73.
The foregoing studies—bolero-2, tamrad, and precog 0102—show that the combination of everolimus with endocrine therapy was associated with improved efficacy for the treatment of postmenopausal women with advanced hr-positive bca that had progressed during prior endocrine therapy. However, the combined treatment has been associated with increased toxicities, including fatigue, stomatitis, pneumonitis, anemia, and metabolic abnormalities such as hyperglycemia70–73.
Another mtor inhibitor, temsirolimus, was evaluated in the horizon trial. That phase iii study compared temsirolimus–letrozole with letrozole–placebo as first-line treatment in ai-naïve postmenopausal women with hr-positive advanced bca. Adding temsirolimus to letrozole did not improve the primary endpoint (mpfs: 8.9 months vs. 9 months; hazard ratio: 0.90; p = 0.25), and the combination was associated with higher rates of grades 3 and 4 adverse events. The contrasting results of horizon compared with bolero-2 and tamrad are likely related to differences in the study population: where bolero-2 and tamrad included patients who had progressed on prior ai treatment, horizon enrolled ai-naïve patients for first-line treatment74.
A recent meta-analysis considered four phase ii and iii randomized trials that investigated mtor inhibitors (everolimus, temsirolimus, and sirolimus) in combination with endocrine therapy, comparing those combinations with endocrine therapy alone in metastatic luminal (hr-positive) bca. The pooled analyses (2147 patients) showed improved outcomes, including ttp or pfs, os, and overall response rate, for the combination therapy. Adverse events—and, in particular, those graded 3 and 4—were mostly increased with combination therapy (asthenia, fatigue, stomatitis, diarrhea, pneumonitis, rash, and dyspnea). The benefit of using mtor inhibitors should therefore be balanced with the patient’s quality of life, which could be degraded by the side effects and increased toxicity of these treatments75.
Alterations in the pi3k/akt pathway are frequently associated with resistance to endocrine therapy in bca. Somatic mutations in the pi3k catalytic subunit p110α (pik3ca) are the most common genetic alterations in that pathway76. Many pi3k inhibitors—including pan-pi3k agents, isoform-specific agents, and dual pi3k/mtor agents—have been developed and are being tested in combination with endocrine therapy in various-phase clinical trials. The phase iii belle-2 clinical trial evaluated the addition of the pan-pi3k inhibitor (buparlisib) with fulvestrant in postmenopausal women with hr-positive, her2-negative advanced bca who had progressed on prior ais. In contrast to belle-3, belle-2 excluded patients who had already received mtor inhibitor therapies. The study met its primary endpoint, demonstrating a modest mpfs improvement for buparlisib–fulvestrant compared with fulvestrant alone (6.9 months vs. 5 months; hazard ratio: 0.78; p < 0.001). However, patients with tumours harbouring PIK3CA mutations detected in circulating tumour dna achieved a clinically meaningful pfs improvement with the combination, but responded poorly to fulvestrant monotherapy77. The phase iii belle-3 trial used the same combination of buparlisib and fulvestrant in ai-treated patients with advanced bca who had progressed on or after mtor inhibitor–based treatment. The mpfs was improved with buparlisib–fulvestrant (3.9 months vs. 1.8 months with fulvestrant alone; hazard ratio: 0.67; p < 0.001). Exploratory subgroup analyses found that the benefit with combined treatment was confined to patients with PIK3CA-mutant tumours and to those with visceral disease. Approximately 90% of enrolled patients had progressed on or after treatment with an mtor inhibitor78. Both belle-2 and belle-3 observed higher rates of toxicity in the combination arm, including elevation of transaminases, mood disorders, and hyperglycemia, suggesting that a pan-pi3k inhibitor in this clinical setting might be too toxic. Data for os are still immature77,78.
In contrast to buparlisib, alpelisib is an α-specific pi3k inhibitor that is being assessed in the phase iii solar-1 trial in combination with fulvestrant in patients with hr-positive, her2-negative advanced bca after disease progression on prior ai therapy. The patients are being assigned to two cohorts (PIK3CA-mutant vs. non-mutant status in tumour tissue) before randomization. Another ongoing phase iii trial, sandpiper, is combining taselisib, another α-specific pi3k inhibitor, with fulvestrant in postmenopausal women with hr-positive, her2-negative, PIK3CA-mutant advanced bca who progressed on prior ais. The results of those studies will determine efficacy and toxicity and whether only tumours harbouring PIK3CA mutations should be treated with these specific targeted compounds.
Histone Deacetylase Inhibitors
Histone deacetylase (hdac) can interact with a variety of non-histones such as transcription factors and co-regulators, with varying functional effects. The repression of er at a transcriptional level by hdac is a potential mechanism of resistance. Preclinical and clinical data have shown that hdac inhibitors lower the levels of er suppression transcription, induce degradation of cyclin D1, and enhance the antiproliferative effects of endocrine therapy. Inhibitors of hdac therefore potentially offer a way to modulate the effects of hdac and thus reverse endocrine resistance.
The efficacy of adding a hdac inhibitor (entinostat) to continued ai therapy in women whose bca has progressed is therefore being tested, and results are awaited (see NCT00828854 at http://ClinicalTrials.gov). The benefit of combining another hdac inhibitor (vorinostat) with an ai is also being tested in women with metastatic disease who have previously derived clinical benefit from endocrine therapy (NCT01720602)13.
SUMMARY
Combining endocrine therapy with various molecularly targeted agents and signal transduction inhibitors has led to successes in overcoming and modulating endocrine resistance in hr-positive bca. Established strategies based on the available scientific data include hd fulvestrant as monotherapy in the first-line treatment of postmenopausal women with low-burden disease; hd fulvestrant as monotherapy or in combination with other agents such as nonsteroidal ais or cdk 4/6 inhibitors (for example, palbociclib, ribociclib) in patients who have progressed on prior endocrine therapy; a combination of cdk 4/6 inhibitors (palbociclib, ribociclib) with letrozole or other endocrine therapy as first-line treatment in patients with higher-risk disease, such as visceral metastasis; a combination of maintenance anti-her2 therapies with ais or other endocrine therapy; and a combination of mtor inhibition (everolimus) with exemestane, tamoxifen, or fulvestrant in patients who have progressed on nonsteroidal ais. Inhibitors of pi3k are not currently approved outside the context of clinical trials. The results of the ongoing sandpiper and solar-1 clinical trials are needed before pi3k inhibitors can be considered in treatment strategies. Finally, participation in clinical trials should always be encouraged to explore more options that can be combined with endocrine therapy to modulate endocrine resistance and to find biomarkers that can help in decision-making and in maximizing the chances of future success.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: CBM has received grants from Eli Lilly, AstraZeneca, Pfizer, and Novartis, and has received honoraria or consulting fees from Eli Lilly, AstraZeneca, Pfizer, and Novartis. AAF has no disclosures to make.
REFERENCES
- 1.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and her2 status. J Natl Cancer Inst. 2014;28:106–5. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (er), progesterone receptor (pr), and the human epidermal growth factor receptor 2 (her2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;156:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 3.Stockwell S. Classics in oncology. George Thomas Beatson, M.D. (1848–1933) CA Cancer J Clin. 1983;33:105–21. doi: 10.3322/canjclin.33.2.105. [DOI] [PubMed] [Google Scholar]
- 4.Gaughan EM, Come SE. Optimizing endocrine therapy for metastatic breast cancer. Curr Breast Cancer Rep. 2012;4:30–8. doi: 10.1007/s12609-011-0063-3. [DOI] [Google Scholar]
- 5.Robertson JF, Willsher PC, Winterbottom L, Blamey RW, Thorpe S. Onapristone, a progesterone receptor antagonist, as first-line therapy in primary breast cancer. Eur J Cancer. 1999;35:214–18. doi: 10.1016/S0959-8049(98)00388-8. [DOI] [PubMed] [Google Scholar]
- 6.Jonat W, Bachelot T, Ruhstaller T, Kuss I, Reimann U, Robertson JF. Randomized phase ii study of lonaprisan as second-line therapy for progesterone receptor–positive breast cancer. Ann Oncol. 2013;24:2543–8. doi: 10.1093/annonc/mdt216. [DOI] [PubMed] [Google Scholar]
- 7.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN. Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol. 1995;13:513–29. doi: 10.1200/JCO.1995.13.2.513. [DOI] [PubMed] [Google Scholar]
- 9.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–18. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 10.Miller TW. Endocrine resistance: what do we know? Am Soc Clin Oncol Educ Book. 2013. [Available online at: https://meetinglibrary.asco.org/record/78737/edbook#fulltext; cited 4 June 2017] [DOI] [PubMed]
- 11.Palmieri C, Patten DK, Januszewski A, Zucchini G, Howell SJ. Breast cancer: current and future endocrine therapies. Mol Cell Endocrinol. 2014;382:695–723. doi: 10.1016/j.mce.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5:271–81. doi: 10.1023/A:1009594727358. [DOI] [PubMed] [Google Scholar]
- 13.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–43. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 14.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331S–6S. doi: 10.1158/1078-0432.CCR-031212. [DOI] [PubMed] [Google Scholar]
- 15.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–19. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushner PJ, Agard DA, Greene GL, et al. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–17. doi: 10.1016/S0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 17.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 18.Johnston SR, Lu B, Scott GK, et al. Increased activator protein-1 dna binding and c-Jun NH2-terminal kinase activity in human breast tumors with acquired tamoxifen resistance. Clin Cancer Res. 1999;5:251–6. [PubMed] [Google Scholar]
- 19.Zhou Y, Yau C, Gray JW, et al. Enhanced NFκB and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer [abstract 12] Clin Cancer Res. 2001;7:4429s–35s. [Available online at: http://clincancerres.aacrjournals.org/content/7/12/4429s; cited 4 June 2017] [PubMed] [Google Scholar]
- 21.Hurtado A, Holmes KA, Geistlinger TR, et al. Regulation of ErbB2 by oestrogen receptor-pax2 determines response to tamoxifen. Nature. 2008;456:663–6. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creighton CJ, Fu X, Hennessy BT, et al. Proteomic and transcriptomic profiling reveals a link between the pi3k pathway and lower estrogen receptor (er) levels and activity in er+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui X, Zhang P, Deng W, et al. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003;17:575–88. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]
- 24.Sabnis G, Brodie A. Adaptive changes results in activation of alternate signaling pathways and resistance to aromatase inhibitor resistance. Clin Cancer Res. 2011;17:4208–13. doi: 10.1158/1078-0432.CCR-10-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: dna matters in breast cancer. Nat Rev Cancer. 2009;9:576–86. doi: 10.1038/nrc2683. [DOI] [PubMed] [Google Scholar]
- 26.Bertilsson L, Dahl ML, Dalén P, Al-Shurbaji A. Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol. 2002;53:111–22. doi: 10.1046/j.0306-5251.2001.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roodi N, Bailey LR, Kao WY, et al. Estrogen receptor gene analysis in estrogen receptor–positive and receptor–negative primary breast cancer. J Natl Cancer Inst. 1995;87:446–51. doi: 10.1093/jnci/87.6.446. [DOI] [PubMed] [Google Scholar]
- 28.Riggins RB, Schrecengost RS, Guerrero MS, Bouton AH. Pathways to tamoxifen resistance. Cancer Lett. 2007;256:1–24. doi: 10.1016/j.canlet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brankovic-Magic M, Janković R, Nesković-Konstantinović Z, Nikolić-Vukosavljević D. Progesterone receptor status of breast cancer metastases. J Cancer Res Clin Oncol. 2002;128:55–60. doi: 10.1007/s00432-001-0299-9. [DOI] [PubMed] [Google Scholar]
- 30.Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–8. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 31.Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in er-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7:11579. doi: 10.1038/ncomms11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–51. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faridi J, Wang L, Endemann G, Roth RA. Expression of constitutively active akt-3 in MCF-7 breast cancer cells reverses the estrogen and tamoxifen responsivity of these cells in vivo. Clin Cancer Res. 2003;9:2933–9. [PubMed] [Google Scholar]
- 34.deGraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mtor activity restores tamoxifen response in breast cancer cells with aberrant akt activity. Clin Cancer Res. 2004;10:8059–67. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 35.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the her tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–33. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knowlden JM, Hutcheson IR, Jones HE, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen resistant MCF-7 cells. Endocrinology. 2003;144:1032–44. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 37.Hutcheson IR, Knowlden JM, Madden TA, et al. Oestrogen receptor–mediated modulation of the egfr/mapk pathway in tamoxifen resistant MCF-7 cells. Breast Cancer Res Treat. 2003;81:81–93. doi: 10.1023/A:1025484908380. [DOI] [PubMed] [Google Scholar]
- 38.Dowsett M, Smith IE, Ebbs SR, et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin Cancer Res. 2006;12:1024s–30s. doi: 10.1158/1078-0432.CCR-05-2127. [DOI] [PubMed] [Google Scholar]
- 39.Doisneau-Sixou SF, Sergio CM, Carroll JS, Hui R, Musgrove EA, Sutherland RL. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr Relat Cancer. 2003;10:179–86. doi: 10.1677/erc.0.0100179. [DOI] [PubMed] [Google Scholar]
- 40.Jansen MP, Foekens JA, van Staveren IL, et al. Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling. J Clin Oncol. 2005;23:732–40. doi: 10.1200/JCO.2005.05.145. [DOI] [PubMed] [Google Scholar]
- 41.Span PN, Tjan-Heijnen VC, Manders P, Beex LV, Sweep CG. Cyclin-E is a strong predictor of endocrine therapy failure in human breast cancer. Oncogene. 2003;22:4898–904. doi: 10.1038/sj.onc.1206818. [DOI] [PubMed] [Google Scholar]
- 42.Butt AJ, McNeil CM, Musgrove EA, Sutherland RL. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr Relat Cancer. 2005;12(suppl 1):S47–59. doi: 10.1677/erc.1.00993. [DOI] [PubMed] [Google Scholar]
- 43.Chu IM, Hengst L, Slingerland JM. The cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Tenorio G, Berglund F, Esguerra Merca A, et al. Cytoplasmic p21WAF1/CIP1 correlates with akt activation and poor response to tamoxifen in breast cancer. Int J Oncol. 2006;28:1031–42. [PubMed] [Google Scholar]
- 45.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 46.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 47.Vergote I, Robertson JF. Fulvestrant is an effective and well-tolerated endocrine therapy for postmenopausal women with advanced breast cancer: results from clinical trials. Br J Cancer. 2004;90(suppl 1):S11–14. doi: 10.1038/sj.bjc.6601631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22:1605–13. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 49.Robertson JF, Erikstein B, Osborne KC, et al. Pharmacokinetic profile of intramuscular fulvestrant in advanced breast cancer. Clin Pharmacokinet. 2004;43:529–38. doi: 10.2165/00003088-200443080-00003. [DOI] [PubMed] [Google Scholar]
- 50.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor–positive, advanced breast cancer: results from efect. J Clin Oncol. 2008;26:1664–70. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 51.Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (sofea): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14:989–98. doi: 10.1016/S1470-2045(13)70322-X. [DOI] [PubMed] [Google Scholar]
- 52.Bergh J, Jönsson PE, Lidbrink EK, et al. fact: an open-label randomized phase iii study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30:1919–25. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 53.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–44. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robertson JF. Fulvestrant (Faslodex)—how to make a good drug better. Oncologist. 2007;12:774–84. doi: 10.1634/theoncologist.12-7-774. [DOI] [PubMed] [Google Scholar]
- 55.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the confirm phase iii trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor–positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 56.Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized confirm trial. J Natl Cancer Inst. 2014;106:djt337. doi: 10.1093/jnci/djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robertson JF, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor–positive advanced breast cancer (falcon): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 58.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, her2-negative, advanced breast cancer (paloma-1/trio-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 59.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–36. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 60.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 61.Turner NC, Ro J, André F, et al. on behalf of the paloma3 study group Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–19. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 62.Dickler MN, Tolaney SM, Rugo HS, et al. monarch 1, a phase ii study of abemaciclib, a cdk4 and cdk6 inhibitor, as a single agent, in patients with refractory hr+/her2–metastatic breast cancer. Clin Cancer Res. 2017;23:5218–24. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osborne CK, Neven P, Dirix LY, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor–positive metastatic breast cancer: a randomized phase ii study. Clin Cancer Res. 2011;17:1147–59. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–46. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 65.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2–positive, hormone receptor–positive metastatic breast cancer: results from the randomized phase iii tandem study. J Clin Oncol. 2009;27:5529–37. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 66.Baselga J, Cortés J, Kim SB, et al. on behalf of the cleopatra Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arpino G, Ferrero JM, de la Haba-Rodriguez J, et al. Primary analysis of pertain: a randomized, two-arm, open-label, multicenter phase ii trial assessing the efficacy and safety of pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in first-line patients with her2-positive and hormone receptor–positive metastatic or locally advanced breast cancer [abstract S3-04]. Presented at: 39th Annual San Antonio Breast Cancer Symposium; San Antonio, TX, U.S.A.. 6–10 December 2016; [Available online at: https://www.abstracts2view.com/sabcs16/view.php?nu=SABCS16L_973&terms=; cited 4 June 2017] [Google Scholar]
- 68.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase–akt pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 69.Jiang BH, Liu LZ. Role of mtor in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11:63–76. doi: 10.1016/j.drup.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [Updated in: Ann Oncol 2014;25:2357–62] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: overall survival results from bolero-2†. Ann Oncol. 2014;25:2357–62. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase ii trial of everolimus in combination with tamoxifen in patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer with prior exposure to aromatase inhibitors: a gineco study. J Clin Oncol. 2012;30:2718–24. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 73.Kornblum NS, Manola J, Klein P, et al. precog 0102: a randomized, double-blind, phase ii trial of fulvestrant plus everolimus or placebo in post-menopausal women with hormone receptor–positive, her2-negative metastatic breast cancer resistant to aromatase inhibitor therapy [abstract S1-02]. Presented at: 39th Annual San Antonio Breast Cancer Symposium; San Antonio, TX, U.S.A.. 6–10 December 2016; [Available online at: https://www.abstracts2view.com/sabcs16/view.php?nu=SABCS16L_952&terms=; cited 4 June 2017] [Google Scholar]
- 74.Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase iii placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202. doi: 10.1200/JCO.2011.38.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rotundo MS, Galeano T, Tassone P, Tagliaferri P. mtor inhibitors, a new era for metastatic luminal her2-negative breast cancer? A systematic review and a meta-analysis of randomized trials. Oncotarget. 2016;10(7):27055–66. doi: 10.18632/oncotarget.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.The Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baselga J, Im SA, Iwata H, et al. PIK3C A status in circulating tumor dna predicts efficacy of buparlisib plus fulvestrant in postmenopausal women with endocrine-resistant her+/her2–advanced breast cancer: first results from the randomized, phase iii belle-2 trial [abstract S6-01]. Presented at: 38th Annual San Antonio Breast Cancer Symposium; San Antonio, TX, U.S.A.. 8–12 December 2015; [Available online at: https://www.abstracts2view.com/sabcs15/view.php?nu=SABCS15L_826&terms=; cited 4 June 2017] [Google Scholar]
- 78.Di Leo A, Seok Lee K, Ciruelos E. belle-3: a phase iii study of buparlisib and fulvestrant in postmenopausal women with hr+, her2–, ai-treated, locally advanced or metastatic breast cancer, who progressed on or after mtor inhibitor–based treatment [abstract S4-07 ]. Presented at: 39th Annual San Antonio Breast Cancer Symposium; San Antonio, TX, U.S.A.. 6–10 December 2016; [Available online at: https://www.abstracts2view.com/sabcs16/view.php?nu=SABCS16L_1106&terms=; cited 4 June 2017] [Google Scholar]