Abstract
The treatment of advanced non-small-cell lung cancer (nsclc) has undergone a paradigm shift since the early 2000s. The identification of molecular subtypes of the disease, based on oncogenic drivers, has led to the development of personalized medicine and the ability to deliver molecularly targeted therapies to patients. In the 10 years that have elapsed since the discovery of the ALK gene in a patient with nsclc, several active drugs have moved rapidly from bench to bedside, and multiple others are currently in clinical trials. Those developments have led to important improvements in patient outcomes, while simultaneously raising key questions about the optimal treatment for ALK-positive nsclc. The inevitable emergence of resistance to alk-directed therapy is central to ongoing research and daily clinical practice for affected patients. In the present review, we highlight the current treatment landscape, the available and emerging clinical trials, and the evolving clinical decision-making in ALK-positive nsclc, with a focus on Canadian practice.
Keywords: alk inhibitors, nsclc, resistance, cns metastases, crizotinib, ceritinib, alectinib, brigatinib
INTRODUCTION
In 2007, Soda et al.1 first identified the EML4–ALK (echinoderm microtubule–associated protein 4) fusion oncogene in a patient with non-small-cell lung cancer (nsclc). That fusion is the result of an inversion in the short arm of chromosome 2, juxtaposing the N terminal of the EML4 gene’s promoter and the kinase domain of the ALK gene. After formation of the EML4-ALK fusion, the EML4 fusion partner mediates ligand-independent activation of alk and constitutive kinase activity, leading to proliferation and survival of the cancer cells responsible for 3%–7% of nsclcs2.
Nature, it turns out, is not without a sense of irony. The same unruly behaviour that gives the fusion its proliferative power is also its Achilles’ heel, rendering it susceptible to targeted inhibition3. The prognostic significance of ALK rearrangements in nsclc is unclear, but the identification of the fusion protein opens the door to targeted therapies, changing the natural history of the disease4.
TESTING FOR ALK IN ADVANCED NSCLC
Patients with ALK-positive nsclc have unique clinical and pathologic characteristics. Those characteristics include young age, never or light smoking history, adenocarcinoma histology, and the presence of signet-ring cells5. Rearrangements of ALK are typically mutually exclusive of other oncogenic drivers6. Although it might be more cost-effective to try to enrich the population of nsclc patients likely to test positive, a significant number of patients will be missed, denying them the benefits of ALK-directed therapies. Current international guidelines recommend ALK testing for all patients with nsclc having an adenocarcinoma component7.
The optimal situation for biomarker testing in advanced nsclc is reflex testing at initial diagnosis. That approach has been demonstrated to be feasible in Canadian practice, improving the time to the start of first-line therapy in patients with advanced nsclc8. In the initial studies of ALK-positive nsclc, fish (fluorescence in situ hybridization) testing was the standard method for detecting ALK rearrangements9. Although initially considered the “gold standard,” fish is a cumbersome and expensive test; other screening methods such as immunohistochemistry and next-generation sequencing have thus been explored10. The calk study was pivotal in implementing ALK testing in Canada through the optimization and standardization of laboratory-developed ALK immunohistochemistry and fish tests in 14 hospitals, enabling testing for ALK on a national scale with immunohistochemistry as a screen11.
CURRENTLY APPROVED THERAPIES IN CANADA
The urgency for devising a national approach to ALK testing and its funding was a direct response to the rapid advancement of personalized cancer therapy across the world. As alk inhibitors were approved by international bodies, Health Canada approvals followed. Drug availability necessitated a standardized approach to patient identification. Currently, 3 alk inhibitors—crizotinib, ceritinib, and alectinib—have been approved by both Health Canada and the U.S. Food and Drug Administration (fda). A 4th, brigatinib, recently received fda approval and is currently under review by Health Canada.
Obtaining government funding for new treatments in the era of biomarker-directed therapy poses several challenges. Given the relatively low frequency of many oncogenic drivers, including ALK, large phase iii studies targeting those smaller subgroups of nsclc patients are more difficult to conduct. Those difficulties challenge clinicians and payers alike, in terms of identifying appropriate clinical trial endpoints and defining the level of evidence required to prove superiority to current standards of care, especially in comparisons with chemotherapy. The other obvious barrier to funding is the high cost of these effective, yet expensive, medications. For years, even with Health Canada approvals for next-generation alk inhibitors, crizotinib was the only funded line of alk-directed therapy for Canadian patients. Despite the lower frequency of ALK-positive nsclc, the enormousness of the global lung cancer epidemic means that this patient population is still larger than that for many other diseases. Clinicians, patients, and their families are all acutely aware of the need for and clinical benefit of next-generation alk inhibitors. Currently, those agents are being accessed in Canada through compassionate programs, private insurance, or clinical trials.
Crizotinib
The first-in-class small-molecule oral tyrosine kinase inhibitor (tki) crizotinib was developed initially as a c-Met inhibitor, but it also targets alk, ros1, and met12. The fda granted accelerated approval to this first-generation alk inhibitor in 2011 based on promising data from phase i (profile 10019) and ii (profile 100513) trials that demonstrated significant (approximately 60%) objective response rates (orrs) and improvements (approximately 8 months) in median progression-free survival (mpfs) in often heavily pretreated ALK-positive patients. Based on those data, crizotinib received a conditional notice of compliance from Health Canada in April 2012.
Two subsequent randomized phase iii studies compared crizotinib with standard chemotherapy, leading to full approval for crizotinib and, until recently, positioning it as the “gold standard” for the first-line treatment of ALK-positive nsclc. The profile 100714 study of ALK-positive patients who had progressed on standard first-line platinum-based doublets randomized participants to crizotinib or to the standard-of-care chemotherapy with pemetrexed or docetaxel. Consistent with the phase ii study data, crizotinib demonstrated impressive results in the comparison with chemotherapy, showing improvements in orr (65% vs. 20%) and pfs [hazard ratio (hr): 0.49; 7.7 months vs. 3 months]. Then, in profile 101415, crizotinib was compared with platinum–pemetrexed chemotherapy in ALK-positive treatment-naïve patients. Once again, compared with chemotherapy, crizotinib was associated with improvements in orr (74% vs. 45%), mpfs (hr: 0.45; 10.9 months vs. 7 months), and importantly, quality of life. No differences in overall survival (os) were seen, presumably because of crossover16.
NEXT GENERATION ALK INHIBITORS
With alk-directed therapy becoming a new standard of care, the challenge of overcoming the inevitable development of resistance arose. Next-generation alk inhibitors were designed to be more potent against alk, to overcome crizotinib-associated resistance mutations, and to improve activity in the central nervous system (cns). In series of patients treated sequentially with first-line crizotinib followed by a second-generation alk inhibitor, durable benefits were evident, with a mpfs of approximately 18 months and a median os exceeding 4 years17,18.
Ceritinib
Ceritinib was the first next-generation inhibitor to show benefit in treating crizotinib-resistant ALK-positive nsclc. Compared with crizotinib, ceritinib has 20 times the potency for inhibiting alk, and cell-line studies of biopsies from patients who had developed crizotinib resistance indicate that ceritinib is a potent inhibitor of some of the resistance mutations found19. Ceritinib was granted accelerated fda approval in April 2014 and Health Canada approval in March 2015.
Updated results from the phase i ascend-1 trial reported orrs of 56% in patients pretreated with an alk inhibitor20. Interestingly, responses were seen both in patients with identified resistance mutations to crizotinib and in patients in whom no mechanism of resistance was identified (discussed in more detail later in this review). The single-arm phase ii ascend-2 study enrolled patients who had progressed on both crizotinib and chemotherapy, showing robust responses in patients with and without brain metastasis21. In the phase iii ascend-5 trial22, patients who had progressed on both crizotinib and platinum-based chemotherapy were randomized to ceritinib or to chemotherapy, with ceritinib treatment being associated with improvements in orr (39% vs. 7%) and mpfs (hr: 0.49; 5.4 months vs. 1.6 months). The foregoing results highlight the superiority of alk-directed therapy compared with chemotherapy in crizotinib-resistant patients who have already received platinum-based chemotherapy; they also raise the question of the optimal role and timing for chemotherapy in those patients.
Ceritinib has also demonstrated activity in alk-inhibitor-naïve patients. In ascend-1, such patients experienced an orr of 72% and a pfs of 18.4 months19. In ascend-3, a single-arm phase ii study in this population, the orr was 64%, and the pfs was also 18.4 months. Importantly, patients who had brain lesions at study enrolment and who had received no prior brain radiotherapy (18.5% of patients), showed intracranial responses that matched or exceeded the whole-body response23.
Observed ceritinib activity in both crizotinib-resistant and -naïve patients led to the ascend-4 study looking at a potential role for ceritinib in first-line therapy24. That phase iii study compared ceritinib with chemotherapy in untreated ALK-rearranged nsclc and was positive for improvements favouring ceritinib in orr (72% vs. 27%) and mpfs (hr: 0.55; 16.6 months vs. 8.1 months). In a relatively small number of patients with measurable brain metastases, the intracranial response rate was 72% in ceritinib-treated patients. Yet despite that activity, the mpfs for patients with brain metastases was shorter than that for patients without brain metastases (10.7 months vs. 26.3 months), highlighting the challenge in managing such patients.
Alectinib
Alectinib is another highly selective alk inhibitor that has activity against crizotinib-resistant ALK mutations25. One unique feature of alectinib is that it is not a substrate for P-glycoprotein, which is implicated as a mechanism of cns resistance in patients taking crizotinib. Alectinib was granted accelerated fda approval in December 2015 and Health Canada approval in September 2016.
The first global approval for alectinib was granted in Japan, based on results of the phase i/ii AF-001JP study, which examined ALK-positive crizotinib-naïve patients. In 46 patients treated with alectinib, the orr was 94%, with an updated 4-year pfs of 52% and an os of 70%26,27.
In crizotinib-resistant patients, alectinib was assessed in two single-arm phase ii studies: a global phase ii study (NP28673)28 enrolled patients from 16 countries, and a North American phase ii study (NP28761)29 enrolled patients from the United States and Canada. A pooled analysis confirmed robust activity, with a systemic orr of 51% and a mpfs of 8.3 months30. The cns efficacy of alectinib was demonstrated in another pooled analysis of the same two studies, in which 60% of the patients had brain metastases. For patients with baseline measureable disease, the cns orr was 64%, and the median duration of response was 10.8 months31. Although those results are impressive, one of the challenges in cross-trial comparisons and interpretation of cns outcomes is the heterogeneity of patients with brain metastases (measurable versus non-measurable disease, and whether patients had previously received radiotherapy treatment).
Alectinib activity in treatment-naïve and -resistant patients and impressive cns responses provide the rationale for testing alectinib in the first-line setting for ALK-positive patients. Two randomized phase iii studies compared alectinib with crizotinib: j-alex, conducted in Japan, used a dose of 300 mg twice daily; and alex, conducted globally, used a dose of 600 mg twice daily. The initial data from j-alex demonstrated superiority for alectinib compared with crizotinib in terms of pfs [hr: 0.34; 99.7% confidence interval (ci): 0.17 to 0.71; p < 0.001]. The mpfs was not reached (95% ci: 20.3 months to not estimable) in the alectinib arm; it was 10.2 months (95% ci: 8.2 months to 12.0 months) in the crizotinib arm32. The alex study confirmed the superior efficacy and lower toxicity of alectinib compared with crizotinib in the primary treatment of ALK-positive nsclc33. Investigator-assessed pfs favoured alectinib over crizotinib, with a hr for disease progression or death of 0.47 (95% ci: 0.34 to 0.65); the independent review committee–assessed pfs also favoured alectinib, at 25.7 months (95% ci: 19.9 months to not estimable) compared with 10.4 months for crizotinib treatment (95% ci: 7.7 months to 14.6 months).
Alectinib was especially active in the cns: a cns progression event was observed in only 12% of patients in the alectinib group compared with 45% of patients in the crizotinib group (cause-specific hr: 0.16; 95% ci: 0.10 to 0.28; p < 0.001). In patients with measureable and non-measurable cns disease at baseline, the cns response rate favoured alectinib (59%; 95% ci: 46% to 71%) over crizotinib (26%; 95% ci: 15% to 39%). In terms of systemic response, the response rate was 82.9% for alectinib compared with 75.5% for crizotinib (p = 0.09), and the duration of response was longer with alectinib than with crizotinib, with the median duration of response being not estimable (95% ci: not estimable) compared with 11.1 months (95% ci: 7.9 months to 13 months). Grades 3–5 adverse events were less frequent with alectinib (41%) than with crizotinib (50%), as were rates of dose reduction, interruption, and discontinuation.
PRACTICAL MANAGEMENT OF SIDE EFFECTS
Given the lower frequency of ALK rearrangements in the broader population of patients with nsclc, the numbers of such patients might be limited in many clinical practices. Management of patients taking crizotinib and alectinib has been reviewed elsewhere34 and is beyond the scope of our review. Managing the adverse events arising from alk inhibition in these patients is key, especially considering that patients will be taking these agents for many months or even years. That management landscape thus highlights the need for ongoing education of the multidisciplinary team and of the patients themselves.
Treatment with ceritinib provides an important example of those education needs. At the starting dose of 750 mg, patients in the ascend-4 trial frequently required dose interruptions or reductions because of gastrointestinal toxicity or elevated liver enzymes. Nevertheless, the discontinuation rate in the study was quite low at 5%, reflecting the ability of practitioners to manage toxicities and modify dosing. The ascend-8 trial demonstrated that ceritinib 450 mg taken with food resulted in similar systemic exposure and a more favourable gastrointestinal safety profile than ceritinib 750 mg taken by fasting patients. Efficacy and long-term safety analyses are ongoing35. The resulting information will help clinicians to optimally manage patients taking ceritinib. Similarly, brigatinib, which recently received fda approval, has unique early pulmonary toxicity with a median time to onset of 2 days. Because grades 3 and 4 reactions occurred in roughly 3% of patients, clinicians must monitor carefully for any new or worsening respiratory symptoms36.
OPTIMAL FIRST-LINE THERAPY
The treatment of ALK-positive nsclc is a perfect example of rapid changes in data outpacing guidelines and regulatory approvals. The dramatic benefit seen in the alex trial for the first-line use of alectinib in ALK-positive patients highlights that situation. Although crizotinib currently remains the standard first-line option in many guidelines and is the alk inhibitor that is funded in that setting in Canadian practice, a rapid shift to alectinib is expected, with uptake determined by access to and funding of the drug.
Until recently, the algorithm for treating ALK-positive nsclc called for sequential treatment with first-line crizotinib, followed by a next-generation alk inhibitor. That approach has been associated with long-term disease control and impressive survival16,17.
The efficacy of the next-generation alk inhibitors and their improved cns penetration make them attractive options to consider in the first-line setting. Moreover, given that a significant proportion of patients who progress on first-line therapy do not go on to receive a subsequent line of therapy, the idea of giving “the best drug first” resonates in that setting, given the development of the more potent next-generation inhibitors.
The ascend-4 trial of ceritinib compared with chemotherapy in the first-line setting was initiated shortly before crizotinib became the standard of care. The positive results from ascend-4 reinforce the importance of treating ALK-positive patients with alk-directed therapy and provide another potential option in the first-line setting23. Although ceritinib proved to be better than chemotherapy, the ascend-4 study does not reveal anything about the relative efficacies of ceritinib and crizotinib.
A subsequent group of studies—including the afore-mentioned j-alex and alex trials testing alectinib, and ongoing studies of brigatinib [alta-1l (see NCT02737501 at http://ClinicalTrials.gov)], ensartinib [exalt3 (NCT02767804)], and lorlatinib [crown (NCT03052608)] as discussed in the next section—are comparing next-generation alk inhibitors with crizotinib. The question of the optimal sequence of therapies might not be fully answered by those studies. When looking at the “sprint” (that is, first-line therapy), the next-generation alk inhibitors are likely to prove superior to crizotinib, much as was demonstrated in alex. In the absence of head-to-head studies, reliance on cross-trial comparisons will be required to try to answer the question about the “best” first-line alk inhibitor. Another important question addresses the “marathon” (that is, the patient journey through sequential lines of therapy in nsclc), in which understanding how the selection of first-line therapy influences subsequent treatment options and outcomes. An additional issue to be addressed is whether certain patient subgroups are better suited for different approaches. For example, there is a need for a further understanding of how various ALK variants respond to treatment. The proposed (and long awaited) “ALK Master Protocol” would be valuable in answering some of those questions.
THERAPIES IN DEVELOPMENT
The goal of enhancing inhibitory activity against alk and overcoming the inevitable development of drug resistance continues to fuel the emergence of newer alk inhibitors. Many alk inhibitors are currently in development, the most noteworthy being brigatinib, lorlatinib, entrectinib, and ensartinib.
Brigatinib
Brigatinib is a highly selective and potent alk inhibitor that demonstrates substantial preclinical activity in overcoming ALK resistance mutations37. The randomized phase ii alta study evaluated two doses of brigatinib (90 mg or 180 mg daily after a 90 mg lead-in for 7 days) in patients experiencing progressive disease on crizotinib. In the most recent update, a trend toward better outcomes was observed for the 180 mg arm compared with the 90 mg arm. At the (recommended) 180 mg dose, the orr was 55%, with a median pfs of 16.7 months. In patients with brain metastases receiving that dose, the intracranial objective response was 67%, with a median duration of response of 16.6 months. Based on those data, the fda approved brigatinib in April 2017 at a recommended dose of 90 mg for 7 days, followed by 180 mg daily. The first-line phase iii alta-1l study started enrolment in April 2016 and is ongoing.
Lorlatinib
Lorlatinib is a selective brain-penetrant alk/ros1 tki, active against most known resistance mutations. In preclinical studies, lorlatinib demonstrated, compared with crizotinib, higher inhibition of wild-type EML4-ALK by a factor of 10, and higher inhibition of the L1196M gatekeeper resistance mutation by a factor of 40. Its activity against ALK kinase resistance mutations includes the highly resistant G1202R mutation38 (further discussed later in this section). The recommended phase ii dose was 100 mg daily. The phase ii study has enrolled 227 patients into 6 experimental cohorts and is demonstrating clinically meaningful activity, including substantial intracranial efficacy, in ALK-positive patients who were either treatment-naïve or for whom 1 or more prior alk tkis had failed39. That clinical benefit, seen even in heavily pretreated patients, makes lorlatinib a potential future option for overcoming resistance in patients who progress on next-generation alk inhibitors. The phase iii crown study—a comparison of crizotinib with lorlatinib in first-line therapy—is currently enrolling patients.
Entrectinib
Entrectinib is a potent inhibitor of ntrk, ros1, and alk fusions. In a phase i basket study involving treatment-naïve patients, it demonstrated significant responses in NTRK-, ROS1-, and ALK-positive patients. The orr for the ALK-positive group was 57%, comparable to that with crizotinib40. Entrectinib is being further investigated in the startrk-2 study, a phase ii basket trial (see NCT02568267 at http://ClinicalTrials.gov).
Ensartinib
Preclinical data for ensartinib demonstrated the potential to overcome resistance to crizotinib with good cns penetration. In a phase i/ii study, ensartinib showed activity in crizotinib-naïve patients, crizotinib-resistant patients, and patients with brain metastases41. The exalt3 study is an ongoing randomized phase iii study comparing ensartinib with crizotinib in first-line therapy36.
OVERCOMING ACQUIRED RESISTANCE TO ALK TKIS
First-line alk-targeted therapy often yields impressive responses, but acquired resistance invariably develops. Currently, therapy is at a crossroads, where we find two groups of ALK-positive patients who progress on first-line therapy: those who were treated with first-line crizotinib, and those who received first-line alectinib. Although those groups share some commonalities, the challenges and the approach to overcoming acquired resistance will differ.
Overcoming Specific Clinical Patterns of Resistance
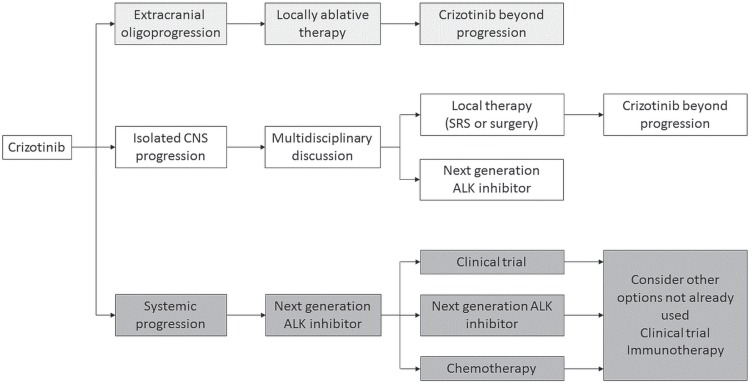
For many patients, disease progression will involve multiple sites. In others, distinct patterns of progression have been identified, including isolated cns progression in the setting of controlled extracranial disease, and extracranial oligoprogression. Tailoring treatment to the specific patient will help to optimize long-term outcomes (Figure 1).
FIGURE 1.
Current approach to managing ALK-positive non-small-cell lung cancer (NSCLC) in Canada. Crizotinib is still (for now) the standard first-line therapy for ALK-positive NSCLC. Distinct clinical patterns of resistance have been identified, highlighting the importance of multidisciplinary collaboration, particularly for patients with isolated central nervous system (CNS) progression. For patients with systemic progression, treatment with a next-generation ALK inhibitor is the standard of care. On subsequent progression, biopsy to determine molecular mechanisms of resistance is not routinely available and treatment is empirically selected. SRS = stereotactic radiosurgery.
The patterns of resistance dictate the need for new collaboration and education within the multidisciplinary team. In the setting of generalized progression, an under-standing of the underlying molecular mechanism of resistance is evolving as a key to decision-making and clinical management, highlighting the role of the pathologist. The important role for the radiotherapy team in treating cns progression and extracranial oligoprogression affirms the need for strong partnerships and multidisciplinary discussion in the clinic.
Isolated CNS progression
At roughly 20%–30%, the incidence of synchronous brain metastasis is similar in ALK-positive patients and in others with advanced nsclc42. Survival improvements resulting from ALK-directed therapies mean that patients are at greater risk of developing brain metastasis later in their disease course43. In the crizotinib era, poor cns penetration and drug efflux by P-glycoprotein meant that the brain was seen as a sanctuary site. Not surprisingly, for patients with untreated brain metastases, a pooled analysis of crizotinib in profile 1005 and profile 1007 indicated that systemic efficacy was greater than intracranial efficacy in terms both of orr (53% vs. 18%) and median time to progression (12.5 months vs. 7 months)44. In profile 1014, which included patients with treated brain metastases, a nonsignificant trend toward improved intracranial time to progression was observed for crizotinib compared with chemotherapy45. In the alex study, the cumulative incidence of cns progression at 12 months was 9.4% for alectinib compared with 41.4% for crizotinib. Those data highlight the challenge of overcoming cns progression in crizotinib-treated patients. In patients treated with first-line alectinib, an assessment of the patterns of cns progression requires longer follow-up from alex. Treating this group of patients who experience progression in the brain while on a next-generation alk inhibitor will be a new challenge.
When cns progression occurs with ongoing extracranial disease control, an approach of locally ablative therapy with radiotherapy to control cns disease might be possible, allowing for continuation of targeted therapy beyond progression. In a retrospective single-institution study of EGFR- and ALK-positive patients treated with local therapy for cns progression, the latter strategy was associated with a median time to next progression exceeding 7 months46. In profile 1014, the median duration of treatment with crizotinib beyond cns progression was 20.4 weeks15.
Local treatment strategies for brain metastases include whole-brain radiotherapy (wbrt), stereotactic radiosurgery, and surgical resection—either alone or in combination. In a series of ALK-positive patients with brain metastases, median os was approximately 4 years47. Given the long-term sequelae of wbrt, stereotactic radiosurgery is the preferred treatment strategy for many patients, with-holding wbrt until absolutely necessary.
The development of next-generation alk inhibitors associated with cns penetration and an ability to achieve meaningful cns responses changes the paradigm for the treatment of brain metastases. In the past, strategies for managing brain metastases relied heavily on radiotherapy. Next-generation alk inhibitors provide a new option for care in patients with cns progression, especially those who might otherwise be candidates for wbrt. In patients treated with first-line crizotinib, high intracranial response rates to next-generation alk inhibitors make those agents an important consideration. For patients in whom first-line alectinib fails, the data for intracranial response to currently available next-generation alk inhibitors is less robust. In the phase ii study of lorlatinib 39, an intracranial response rate of 48% was seen even in heavily pretreated patients (also see NCT02568267 at http://ClinicalTrials.gov).
An approach to treating brain metastases in the ALK-positive patient is discussed elsewhere, with an examination of scenarios ideal for tkis beyond progression, next-generation alk inhibitors, and integration with radiation48. Further data from clinical trials is needed to help guide decision-making for these patients. Ultimately, the treatment for cns metastases in ALK-positive nsclc will be individualized based on the specifics of the clinical picture, with a requirement for multidisciplinary collaboration and discussion.
Extracranial Oligoprogression
Most patients who experience systemic progression will do so at multiple sites, but in a select group of patients, sites of systemic progression are limited. In those patients, local ablative therapy can be considered to treat the resistant clones and to allow for continued benefit from tki suppression of sensitive areas of disease. In a retrospective series, the benefit of that strategy was reported as an improvement in mpfs of 5.5 months49. Importantly, the strategy was born in an era in which no next-generation alk inhibitors were readily available for use at progression. Careful selection procedures are therefore required to try to identify appropriate patients and the associated tumour biology that would benefit from locally ablative therapies compared with moving to the next line of therapy with a next-generation alk inhibitor.
Understanding Molecular Mechanisms of Resistance
Despite initial impressive responses, patients treated with alk inhibitors invariably progress because acquired resistance develops. An understanding of the underlying mechanisms of resistance will help to uncover the optimal strategies in the clinic for overcoming resistance.
Several series have analyzed post-progression biopsies in patients treated with alk inhibitors, contributing to an understanding of the molecular mechanisms of resistance50,51. Those mechanisms have been classified as alk-dependent “on-target” mechanisms in which the tumour-cell dependency on alk signaling persists, and alk-independent “off-target” mechanisms in which the tumour cells are no longer reliant on alk52.
The most common mechanism of “on target” resistance is the development of secondary mutations in the ALK tyrosine kinase domain. In crizotinib-resistant patients, such secondary mutations occur in 20%–30% of patients. The most common mutations in that scenario are the L1196M gatekeeper mutation and G1269A. Unlike the situation with EGFR-mutant nsclc, in which the T790M gatekeeper mutation is the dominant resistance mutation, the secondary mutations that have been identified in ALK-positive disease—including C1156Y, G1202R, I1171T, S1206Y, and E1210K, among others— constitute a much larger group. In patients treated with more potent and structurally different second-generation alk inhibitors, the frequency of ALK resistance mutations increases to more than 50%, and the spectrum of mutations changes, with the common emergence of the highly resistant G1202R mutation. Preclinical work has shown that, depending on the ALK resistance mutation, sensitivity to second-generation alk inhibitors differs. Lorlatinib, a third-generation alk inhibitor was shown to be active against all single ALK resistance mutations, including G1202R53.
An important mechanism of “off target” resistance is the activation of bypass signalling tracks. The first bypass mechanism identified was the epidermal growth factor receptor (egfr), but many others including her2 (human epidermal growth factor receptor 2), met, kit, and insulin-like growth factor 1 receptor have been identified, and future studies of paired pre- and post-tki biopsies will further validate bypass pathways that could be actionable as therapeutic targets50,54. Other mechanisms such as epithelial–mesenchymal transition—a cellular reprogramming resulting in a morphology change from an epithelial shape to a more spindled appearance that is associated with more invasive behaviour—have been implicated51.
Ultimately, further research and a better understanding of both mechanism types and their importance in alk inhibitor resistance is required.
Managing Patients with Acquired Resistance
How can the current understanding of alk-inhibitor-associated resistance translate into the clinic? The therapeutic approach to the development of resistance to crizotinib will differ from the approach to the development of resistance to the second-generation alk inhibitors.
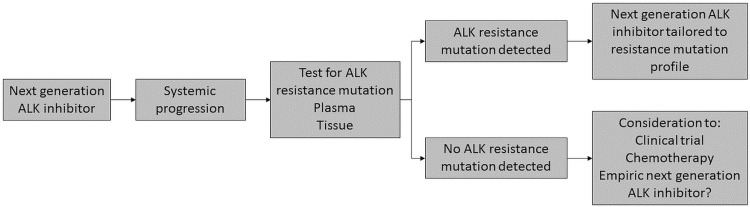
Most patients who develop disease progression while taking crizotinib will still respond to treatment with a next-generation alk inhibitor, even in the absence of a detectable resistance mutation. Therefore, the most appropriate treatment in the setting of crizotinib resistance is a second-generation alk inhibitor (Figure 1). The role of biopsy in understanding mechanisms of resistance after crizotinib would inform treatment for only a very small group of patients—for instance, the rare patients with G1202R mutations for whom a third-generation inhibitor clinical trial might be more appropriate. In patients progressing on a second-generation alk inhibitor, given a frequency of approximately 50% ALK resistance mutations, biopsy could be of value to inform the likelihood of response and the selection of the next line of alk-directed therapy (Figure 2). That approach requires a better understanding of how to translate the available in vitro data to patients in the clinic; further study is necessary. In the absence of molecular characterization, lorlatinib could become a promising option to use empirically, given its preclinical activity against ALK resistance mutations and the clinical benefit seen in pretreated patients.
FIGURE 2.
Potential future management algorithm for ALK-positive non-small-cell lung cancer (NSCLC). Especially as next-generation ALK inhibitors become standard in first-line therapy, the need to understand the underlying mechanisms of resistance so as to direct subsequent therapy is accelerated. Further data from clinical studies, lower testing costs, and collaboration with pathologists and payers will potentially lead to molecular testing to characterize patients and optimize management.
In routine clinical practice, performing a biopsy upon the development of resistance is both promising and problematic. Although the situation in EGFR-positive nsclc is proof-of-concept for routine biopsy on progression to look for T790M mutations, the situation with ALK is more complex. With EGFR, T790M is dominant in more than 60% of patients and leads to treatment with a single drug (osimertinib); in contrast, tumours in ALK-positive patients develop multiple different mutations, and no clear algorithm exists for selecting the optimal next treatment. The rapid development and implementation of plasma testing for the EGFR T790M mutation highlights the clinical ease, patient preference, and overall benefit of a noninvasive approach. Testing for ALK mutations in plasma has been evaluated in small studies55,56; further study is required before plasma testing for molecular mechanisms of resistance becomes a standard tool for monitoring patients on treatment and selecting the next therapeutic options. In Canada, as in many other jurisdictions, subsequent validation of the test in reference laboratories and government funding for the test will be essential for adopting it into routine clinical practice.
Enrolment on a clinical trial is an ideal option for ALK-positive patients who have developed resistance to therapy. Studies of third-generation alk inhibitors such as lorlatinib are attractive options, especially for patients with ALK resistance mutations. A number of interesting approaches are being taken in trials, including combining alk inhibitors with other targeted therapies in the hope of overcoming resistance arising from bypass pathways. Unfortunately, the difficulty of enrolling patients with nsclc to trials, especially when smaller cohorts are involved, is well documented57. The proposed “ALK Master Protocol” sponsored by the U.S. National Cancer Institute is a provocative study that hopes to answer some of the questions concerning the sequencing of tkis in affected patients and the use of routine molecular testing to understand the underlying mechanisms of resistance.
From a practical perspective, the options that are readily available in the clinic after progression on a next-generation alk inhibitor include further alk-directed therapy, chemotherapy, and immunotherapy. Especially in the absence of a biopsy to determine mechanisms of resistance, the current situation in Canada is a question of whether empiric selection of “the next available alk inhibitor” is more efficacious than cytotoxic chemotherapy. Although continuation of alk-targeted therapy is associated with responses in this scenario, brigatinib is being evaluated in a phase ii study in patients who have already been treated with a second-generation alk inhibitor (see NCT02706626 at http://ClinicalTrials.gov).
The benefits of pemetrexed-based chemotherapy in ALK-positive patients is well characterized58. What is unclear is the optimal timing of chemotherapy in this sequence of treatment. The use of immunotherapy in oncogene-driven cancers is associated with many unknowns. Patients who are ALK-positive are typically never-smokers with cancers possessing low tumour mutational burden, characteristics that both seem to predict for lower responsiveness to immunotherapy59,60. Notably, none of the EGFR- or ALK-positive patients in the recent update of keynote 010, which is comparing second-line pembrolizumab with docetaxel in PD-L1–expressing nsclc, were in the group of long-term responders61. The relative uncommonness of those patients and the fact that they are now being excluded from many immunotherapy trials further complicates the effort to answer the outstanding questions.
SUMMARY
The development of crizotinib for ALK-positive nsclc marked a change in the treatment paradigm that highlights the power of personalized medicine and targeted therapy. The rapid development of next-generation alk inhibitors to overcome resistance to crizotinib and now for use in the first-line setting has been associated with significant benefit and improvements in outcomes for patients— especially in the ability of the newer agents to penetrate the cns. The treatment of patients with ALK-positive disease requires a strong multidisciplinary approach to optimize outcomes. The multiple next-generation alk inhibitors that are available have raised new questions about optimal use and sequencing. A furthered understanding of the molecular mechanisms of resistance and the ability to conduct plasma testing in the future will enhance the tailoring of treatment to best fit individual patients. That understanding, in conjunction with data from ongoing clinical trials, brings continued hope for patients, challenges for both clinicians and payers, and the promise of improved outcomes for ALK-positive patients.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: JMR has received speaker and advisory board fees and participated in clinical trials funded by AstraZeneca, Bristol–Myers Squibb, Merck, Pfizer, and Roche. NC has no conflicts to disclose.
REFERENCES
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALK–positive lung cancer. Proc Natl Acad Sci U S A. 2008;105:19893–7. doi: 10.1073/pnas.0805381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumour activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4–ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS : an analysis of 1,683 patients with non–small cell lung cancer. Clin Cancer Res. 2013;19:4273–81. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32:3673–9. doi: 10.1200/JCO.2014.57.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menjak IB, Winterton-Perks Z, Raphael S, et al. Successful completion of EGFR/ALK testing in non-squamous non–small cell lung cancer (non-sq nsclc) with the implementation of reflex testing (rt) by pathologists [abstract 93] J Clin Oncol. 2016;34 doi: 10.1200/jco.2016.34.7_suppl.93. [Available online at: http://ascopubs.org/doi/abs/10.1200/jco.2016.34.7_suppl.93; cited 28 January 2018] [DOI] [Google Scholar]
- 9.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conklin CM, Craddock KJ, Have C, Laskin J, Couture C, Ionescu DN. Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol. 2013;8:45–51. doi: 10.1097/JTO.0b013e318274a83e. [DOI] [PubMed] [Google Scholar]
- 11.Cutz JC, Craddock KJ, Torlakovic E, et al. Canadian Anaplastic Lymphoma Kinase study: a model for multicenter standardization and optimization of ALK testing in lung cancer. J Thorac Oncol. 2014;9:1255–63. doi: 10.1097/JTO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 12.Nwizu T, Kanteti R, Kawada I, Rolle C, Vokes EE, Salgia R. Crizotinib (PF02341066) as a alk/met inhibitor—special emphasis as a therapeutic drug against lung cancer. Drugs Future. 2011;36:91–9. doi: 10.1358/dof.2011.036.02.1584112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crinò L, Kim D, Riely G, et al. Initial phase ii results with crizotinib in advanced ALK- positive non–small cell lung cancer (nsclc): profile 1005 [abstract 7514] J Clin Oncol. 2011;29 doi: 10.1200/jco.2011.29.15_suppl.7514. [Available online at: http://ascopubs.org/doi/abs/10.1200/jco.2011.29.15_suppl.7514; cited 29 January 2018] [DOI] [Google Scholar]
- 14.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 15.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [Erratum in: N Engl J Med 2015; 373:1582] [DOI] [PubMed] [Google Scholar]
- 16.Mok TS, Kim D, Wu Y, et al. Overall survival (os) for first-line crizotinib versus chemotherapy in ALK+ lung cancer: updated results from profile 1014 [abstract LBA50] Ann Oncol. 2017;28(suppl 5):v605–49. [Google Scholar]
- 17.Watanabe S, Hayashi H, Okamoto K, et al. Progression-free and overall survival of patients with ALK rearrangement–positive non–small cell lung cancer treated sequentially with crizotinib and alectinib. Clin Lung Cancer. 2016;17:528–34. doi: 10.1016/j.cllc.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Gainor JF, Tan DS, De Pas T, et al. Progression-free and overall survival in ALK-positive nsclc patients treated with sequential crizotinib and ceritinib. Clin Cancer Res. 2015;21:2745–52. doi: 10.1158/1078-0432.CCR-14-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friboulet L, Li N, Katayama R, et al. The alk inhibitor ceritinib overcomes crizotinib resistance in non–small cell lung cancer. Cancer Discov. 2014;4:662–73. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ascend-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–63. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crino L, Ahn MJ, De Marinis F, et al. Multicenter phase ii study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ascend-2. J Clin Oncol. 2016;34:2866–73. doi: 10.1200/JCO.2015.65.5936. [DOI] [PubMed] [Google Scholar]
- 22.Scagliotti G, Kim TM, Crino L, et al. Ceritinib vs chemotherapy (ct) in patients (pts) with advanced anaplastic lymphoma kinase (ALK)–rearranged (ALK+) non–small cell lung cancer (nsclc) previously treated with ct and crizotinib (crz): results from the confirmatory phase 3 ascend-5 study [abstract LBA42_PR] Ann Oncol. 2016;27(suppl 6) doi: 10.1093/annonc/mdw435.41. [DOI] [Google Scholar]
- 23.Felip E, Orlov S, Park K, et al. Phase 2 study of ceritinib in previously treated alki-naïve patients (pts) with ALK- rearranged (ALK+) non–small cell lung cancer (nsclc): whole body efficacy in all pts and in pts with baseline brain metastases (bm) [abstract 12080] Ann Oncol. 2016;27 doi: 10.1093/annonc/mdw383.03. [DOI] [Google Scholar]
- 24.Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ascend-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–29. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita K, Asoh K, Furuichi N, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802) Bioorg Med Chem. 2012;20:1271–80. doi: 10.1016/j.bmc.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (af-001jp study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–8. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 27.Nishio M, Kiura K, Seto T, et al. Final result of phase i/ii study (AF-001JP) of alectinib, a selective cns-active alk inhibitor, in ALK+ nsclc patients (pts) [abstract OA05.08] J Thorac Oncol. 2017;12(suppl 2):S1757. doi: 10.1016/j.jtho.2017.09.353. [DOI] [Google Scholar]
- 28.Barlesi F, Dingemans AC, Yang JC, et al. Updated efficacy and safety from the global phase ii NP28673 study of alectinib in patients (pts) with previously treated ALK+ non-small-cell lung cancer (nsclc) [abstract 1263P] Ann Oncol. 2016;27 [Google Scholar]
- 29.Gandhi L, Shaw A, Gadgeel S, et al. A phase ii, open-label, multicenter study of the alk inhibitor alectinib in an ALK+ non-small-cell lung cancer (nsclc) U.S./Canadian population who had progressed on crizotinib (NP28761) [abstract 8019] J Clin Oncol. 2015;33 doi: 10.1200/jco.2015.33.7_suppl.336. [Available online at: http://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.8019; cited 29 January 2018] [DOI] [Google Scholar]
- 30.Yang J, Ou SH, De Petris L, et al. Efficacy and safety of alectinib in ALK+ non-small-cell lung cancer (nsclc): pooled data from two pivotal phase ii studies (NP28673 and NP28761) [abstract e20507] J Clin Oncol. 2016;34 [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.e20507; cited 29 January 2018] [Google Scholar]
- 31.Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of cns response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:4079–85. doi: 10.1200/JCO.2016.68.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nokihara H, Hida T, Kondo M, et al. Alectinib (alc) versus crizotinib (crz) in alk-inhibitor naïve ALK- positive non–small cell lung cancer (ALK+ nsclc): primary results from the j-alex study [abstract 9008] J Clin Oncol. 2016;34 [Available online at: https://meetinglibrary.asco.org/record/125294/abstract; cited 29 January 2018] [Google Scholar]
- 33.Peters S, Camidge DR, Shaw AT, et al. on behalf of the alex trial investigators Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 34.Rothenstein JM, Letarte N. Managing treatment-related adverse events associated with alk inhibitors. Curr Oncol. 2014;21:19–26. doi: 10.3747/co.21.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho BC, Kim D, Bearz A, et al. ascend-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)–rearranged metastatic non–small cell lung cancer (nsclc) J Thorac Oncol. 2017;12:1357–67. doi: 10.1016/j.jtho.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Ahn M, Camidge DR, Tiseo M, et al. Brigatinib in crizotinib-refractory ALK+ nsclc: updated efficacy and safety results from alta, a randomized phase 2 trial [abstract OA05.05] J Thorac Oncol. 2017;12(suppl 2):S1755–6. doi: 10.1016/j.jtho.2017.09.350. [DOI] [Google Scholar]
- 37.Huang WS, Liu S, Zou D, et al. Discovery of brigatinib (AP26113), a phosphine oxide–containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem. 2016;59:4948–64. doi: 10.1021/acs.jmedchem.6b00306. [DOI] [PubMed] [Google Scholar]
- 38.Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an alk/ros1 inhibitor, overcomes resistance to first and second generation alk inhibitors in preclinical models. Cancer Cell. 2015;28:70–81. doi: 10.1016/j.ccell.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon B, Shaw A, Ou S, et al. Phase 2 study of lorlatinib in patients with advanced ALK+/ROS1+ non-small-cell lung cancer [abstract OA05.06] J Thorac Oncol. 2017;12(suppl 2) doi: 10.1016/j.jtho.2017.09.351. [DOI] [Google Scholar]
- 40.Drilon A, Siena S, Ou SHI, et al. Safety and antitumor activity of the multitargeted pan-trk, ros1, and alk inhibitor entrectinib: combined results from two phase i trials (alka-372-001 and startrk-1) Cancer Discov. 2017;7:400–9. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reckamp KL, Infante JR, Blumenschein GR, et al. Phase i/ii trial of X-396, a novel anaplastic lymphoma kinase (alk) inhibitor, in patients with ALK+ non–small cell lung cancer (nsclc) [abstract] J Thorac Oncol. 2016;11(suppl):S36–7. doi: 10.1016/j.jtho.2015.12.062. [DOI] [Google Scholar]
- 42.Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, Davis FG. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 2014;17:122–8. doi: 10.1093/neuonc/nou099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108–11. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–8. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from profile 1014. J Clin Oncol. 2016;34:2858–65. doi: 10.1200/JCO.2015.63.5888. [DOI] [PubMed] [Google Scholar]
- 46.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene addicted non–small cell lung cancer. J Thorac Oncol. 2012;7:1807–14. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johung KL, Yeh N, Desai NB, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. 2016;34:123–9. doi: 10.1200/JCO.2015.62.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rusthoven CG, Doebele RC. Management of brain metastases in ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:2814–19. doi: 10.1200/JCO.2016.67.2410. [DOI] [PubMed] [Google Scholar]
- 49.Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiotherapy can safely and durably control sites of extra-cns oligoprogressive disease in ALK- positive lung cancer patients on crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892–8. doi: 10.1016/j.ijrobp.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non–small cell lung cancer. Clin Cancer Res. 2012;18:1472–82. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin JJ, Riely GJ, Shaw AT. Targeting alk: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–55. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation alk inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–33. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27(suppl 3):iii42–50. doi: 10.1093/annonc/mdw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gettinger SN, Zhang S, Hodgson JG, et al. Activity of brigatinib (brg) in crizotinib (crz) resistant patients (pts) according to ALK mutation status [abstract 9060] J Clin Oncol. 2016;34 doi: 10.1200/JCO.2016.66.9929. [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.9060; cited 29 January 2018] [DOI] [Google Scholar]
- 56.Horn L, Wakelee HA, Reckamp KL, et al. Plasma genotyping of patients enrolled on the expansion phase i/ii trial of W-396 in patients (pts) with ALK+ non–small cell lung cancer (nsclc) [abstract 9056] J Clin Oncol. 2016;34 [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.9056; cited 29 January 2018] [Google Scholar]
- 57.Baggstrom MQ, Waqar SN, Sezhiyan AK, et al. Barriers to enrollment in non–small cell lung cancer therapeutic clinical trials. J Thorac Oncol. 2011;6:98–102. doi: 10.1097/JTO.0b013e3181fb50d8. [DOI] [PubMed] [Google Scholar]
- 58.Park S, Park TS, Choi CM, et al. Survival benefit of pemetrexed in lung adenocarcinoma patients with anaplastic lymphoma kinase gene rearrangements. Clin Lung Cancer. 2015;16:e83–9. doi: 10.1016/j.cllc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (tmb) in lung cancer (lc) and relationship with response to PD-1/PD-L1 targeted therapies [abstract 9017] J Clin Oncol. 2016;34 [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.9017; cited 29 January 2018] [Google Scholar]
- 60.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herbst R, Garon E, Kim D, et al. keynote-010: durable clinical benefit in patients with previously treated, PD-L1–expressing nsclc who completed pembrolizumab [abstract OA03.07] J Thorac Oncol. 2017;12(suppl):S254–5. doi: 10.1016/j.jtho.2016.11.243. [DOI] [Google Scholar]