Abstract
Although screening mammography has delivered many benefits since its introduction in Canada in 1988, questions about perceived harms warrant an up-to-date review. To help oncologists and physicians provide optimal patient recommendations, the literature was reviewed to find the latest guidelines for screening mammography, including benefits and perceived harms of overdiagnosis, false positives, false negatives, and technologic advances.
For women 40–74 years of age who actually participate in screening every 1–2 years, breast cancer mortality is reduced by 40%. With appropriate corrections, overdiagnosis accounts for 10% or fewer breast cancers. False positives occur in about 10% of screened women, 80% of which are resolved with additional imaging, and 10%, with breast biopsy. An important limitation of screening is the false negatives (15%–20%). The technologic advances of digital breast tomosynthesis, breast ultrasonography, and magnetic resonance imaging counter the false negatives of screening mammography, particularly in women with dense breast tissue.
Keywords: Breast cancer, screening mammography, digital breast tomosynthesis, overdiagnosis
INTRODUCTION
Breast cancer (bca) is the leading cause of cancer death in women worldwide. It is the main cause of cancer-related death in women in developing countries (where many have advanced disease at presentation), and it is the second-leading cause in women in developed countries1–3. In Canada, cancer is also the leading cause of premature mortality, as measured by potential years of life lost. Breast cancer has one of the highest potential years of life lost: almost 137,000 years, reflecting the burden of bca in younger women4. Since the 1988 peak in the bca mortality rate, estimates suggest that 32,000 bca deaths have been avoided in Canada for a variety of reasons, including early detection with screening and advances in bca treatment4. Screening mammography is the method most commonly used worldwide for the detection of early bca in asymptomatic women, and it is the only imaging modality proven to significantly lower bca mortality5.
In the present review, we cover screening for average-risk women, who represent 80% of those diagnosed with bca. It has been well established that women at high risk of bca, including carriers of gene mutations (for example, BRCA1 and BRCA2) or those with a lifetime risk of 25% or greater calculated using the ibis or boadicea risk assessment tools, benefit from annual screening with breast magnetic resonance imaging in addition to mammography6.
BENEFITS OF SCREENING MAMMOGRAPHY
In 2014, because of concerns about overdiagnosis with mammography, 29 experts in epidemiology, surgical oncology, oncology, radiology, pathology, physics, and genetics from 16 countries met at the International Agency for Research on Cancer as a Working Group to reassess the cancer-preventive and adverse effects of various methods of screening for bca7. All available high-quality observational cohort and case–control studies from 1989–2014 (approximately 40) were assessed and debated until a consensus was reached. A meta-analysis was not performed, but the greatest weight was given to cohort studies with the longest follow-up period and more robust designs. A distinction was made between women invited to screen, which results in only 60% participation in screening, and those who actually participate and undergo mammography. Results showed that women 50–69 years of age who were invited to attend mammographic screening experienced a 23% reduction in the risk of death from bca and that women who attended mammographic screening had a higher reduction in risk of 40%. Fewer studies have assessed the effectiveness of screening in women 40–44 or 45–49 years of age, and the risk reduction in those studies was less pronounced7. In addition to randomized controlled trials (rcts), many observational studies from modern service-based screening (that is, organized population-based screening) show pooled mortality reductions of 25% [relative risk (rr): 0.75; 95% confidence interval (ci): 0.69 to 0.81] among women invited to screening and 38% (rr: 0.62; 95% ci: 0.56 to 0.69) among those attending screening8.
The 2014 Pan-Canadian observational study examined the effect of mammographic screening on bca mortality given the variability of findings from observational studies in different countries where screening was implemented9. Of 12 Canadian breast screening programs, 7 programs representing 85% of the Canadian population participated in the study. Data about screens and bca diagnoses and deaths from 1990 to 2009 were obtained for 2.8 million participants in the screening programs and from the corresponding cancer registries (20.2 million person–years of observation in total). The average bca mortality among participants was 40% (95% ci: 33% to 48%), which is lower than the mortality for women who did not participate in screening as determined by provincial cancer registry data linked to screening program databases. The bca mortality reduction observed in the participating provinces was in the 27%–59% range. Age at entry into screening (40 years vs. 50 years) did not affect the magnitude of the average reduction in mortality (between 35% and 44%). The population’s awareness of bca and trends in treatment efficacy did not influence the results. The study concluded that participation in population-based mammography screening programs in Canada was associated with substantially reduced bca mortality for women 40–74 years of age.
Benefits: Number Needed to Invite Compared With Number Needed to Screen
Absolute benefit can be measured as the number needed to invite to screening (nni) or the number needed to screen (nns) to prevent 1 death10. The magnitude of the absolute benefit is influenced by the rr, the duration of follow-up, the underlying mortality risks in the population from which the estimate is derived, and whether the estimate is the nni or the nns.
The nni is based on rcts and is not a measure of who is actually screened, only who is invited to screening. Only 50%–70% participate when invited to screen11. The nni can be estimated from observational studies or rcts, but should not be used because the numbers will be inflated by deaths among women invited to screening who never attended screening12. That distinction was not made by the Canadian Task Force on Preventive Health Care13.
The nns is equivalent to the number needed to participate and indicates the actual number needed to be screened or to participate to see a benefit. It is the more accurate assessment of the benefit of screening and is increasingly being used in the literature.
Variable estimates of absolute benefit have been noted in the literature depending on whether the nni, nns, or other model inputs were used. As Table i shows, the nns estimates from the U.K. Independent Review and the Cochrane systematic review differed by a factor of almost 10: 180 compared with 20005,19. That difference is attributed to the Cochrane systematic review having used the nni rather than the nns and being based on a less-favourable mortality reduction (rr: 0.85 vs. 0.80) over a shorter screening program duration (10 years vs. 20 years), with follow-up limited to the period of the screening program. It is important to use long-term follow-up to estimate the nns. That factor is most evident in the Swedish Two-County Trial, in which it was observed that 922 women had to be screened 2–3 times during a 7-year period to prevent 1 bca death at 10 years of follow-up; that number declined to 414 women at 29 years of follow-up20. The latter estimate is similar to the American Cancer Society (acs) nns estimate of 462 for women 50–59 years of age at 15 years of follow-up, with a 40% mortality reduction10.
TABLE I.
Screening recommendations, by organization
| Organization | Target age (years) | Screening interval (years) | Period (years) | Number needed | Relative risk reduction | |
|---|---|---|---|---|---|---|
|
| ||||||
| To screen | To invite | |||||
| Canadian Task Force on Preventive Health Care13 | 50–74 | 2–3 | 11 | NA | Age 40–49: 2108 | 0.82 |
| Age 50–69: 721 | ||||||
| Canadian Association of Radiologists14 | 40–74+a | Age 40–49: 1 | 40+ | NA | NA | NA |
| Age 50–74+: 1–2 | ||||||
| U.S. Preventive Services Task Force15 | 50–74 | 2 | 10 | NA | Age 40–49: 3333 | Age 39–49: 0.88 |
| Age 50–59: 1250 | Age 50–59: 0.86 | |||||
| Age 60–69: 476 | Age 60–69: 0.67 | |||||
| Age 70–74: 769 | Age 70–74: 0.80 | |||||
| American Cancer Society10 | 45+b | Age 45–54: 1 | 15 | Age 40–49: 753 | 1770 | 0.75 Invited to screening |
| Age 55: 2 | Age 50–59: 462 | 835 | 0.62 Attending screening | |||
| Age 60–69: 355 | 835 | |||||
| American College of Radiology16 | 40c | 1 | 40+ | NA | NA | NA |
| American College of Obstetricians and Gynecologists17 | 40–75 | 1–2 | 40+ | NA | NA | NA |
| U.S. National Comprehensive Cancer Network18 | 40d | 1 | 40+ | NA | NA | NA |
| Cochrane Systematic Review19 | NA | NA | 10 | NA | 2000 | 0.81 |
| U.K. Independent Review5 | 50–70e | 3 | 20 | 50–79: 180 | NA | 0.80 |
Continue as long as life expectancy is 7–10 years.
Continue as long as life expectancy is ≥10 years.
Continue until life expectancy is <5–7 years.
Continue until life expectancy is ≤10 years.
The U.K. National Health Service is in the process of extending breast cancer screening to include mammography in women 47–73 years of age.
NA = not available.
Other benefits to screening include the reduction in costs associated with treatment. Treatment for individuals diagnosed at an earlier stage is less invasive and costly, which might reduce patient anxiety and improve prognosis21. From the patient’s perspective, breast-conservation surgery instead of mastectomy, a decreased need for chemotherapy, and less time off work are all huge benefits associated with earlier detection. A decreased likelihood of axillary lymph node metastases with screening can also result in fewer axillary lymph node dissections and reduced risk of lymphedema. A study from 1996 demonstrated that the cumulative costs of treatment for late-stage bca were US$50,000 to US$60,000 per patient, compared with US$18,000 to US$25,000 for treating early-stage bca22. Montero and colleagues23 estimated the costs of treating metastatic bca to be much higher at US$250,000, likely because of increased drug-related costs 20 years later and the increased costs of the medical delivery system. A Canadian study showed that the average undiscounted lifetime cost per case of treating women diagnosed with bca varied by stage, from $36,340 for stage iv or metastatic disease to $23,275 for stage i disease24.
Guidelines for Screening to Maximize Benefit
Most national screening guidelines suggest that there is value in mammography screening for women in their 40s10,15,17,18. An informed, personal choice for women in their early 40s is widely supported by the U.S. Preventive Services Task Force, the acs, and the Canadian Task Force on Preventive Health Care25,26. Several other North American medical societies recommend screening for women starting at age 40 (Table i). The acs recommends annual screening for women 45–54 years of age; women 55 years of age and older should then transition to biennial screening10. Because the bca growth rate is faster in premenopausal women, the optimal recommended screening interval for those women is annual27. In postmenopausal women, although the maximal benefit is achieved with annual screening, the incremental benefit of that approach compared with biennial screening is less marked, and in the relevant age group, most programs recommend biennial screening for maximal cost-effectiveness28.
Breast Cancer Screening in Young Women
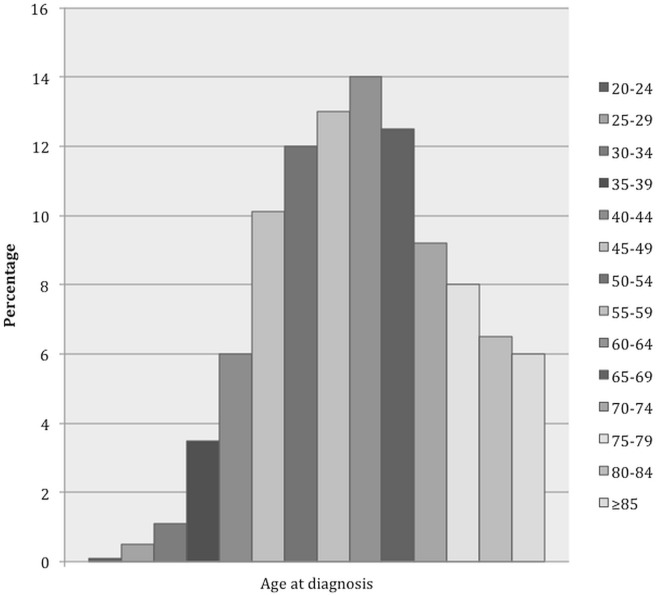
An often-touted reason not to screen women 40–49 years of age is that most bcas occur in women more than 50 years of age. However, 17% of bcas are diagnosed in women less than 50 years of age4, with fewer than 5% occurring in those less than 40 years of age10. It is more informative to express the incidence per decade, with 18% of bcas occurring in women 40–49, 23% in those 50–59, 26% in those 60–69, and 28% in those 70 and older according to U.S. Surveillance, Epidemiology, and End Results data29. No abrupt increase occurs at the age of 50. The incidence of bca can be further subdivided into 5-year age categories, as the acs has done10, with the most marked increase in bca incidence being seen in the 45–49 age category. Hence, the strong recommendation of the acs to begin screening at 45 years of age (Figure 1, Table i).
FIGURE 1.
Breast cancer (BCa) burden by age at diagnosis, 2007–2011. (A) Distribution of invasive female BCa cases (n = 292,369) by age at diagnosis. (B) Distribution of BCa deaths (n = 16,789, patients followed for up to 20 years) by age at diagnosis. (C) Distribution of person–years of life lost to BCa (n = 326,560, patients followed for up to 20 years) by age at diagnosis. Source: Oeffinger et al., 201510.
Limited studies have evaluated screening mammography for women 40–49 years of age. Many of the rcts were designed to include women 50–69 years of age. Although the Canadian National Breast Screening Study evaluated women 40–59 years of age30, it has been challenged because of poor-quality mammography and because the rct allocations were not blinded, with an excess of advanced bcas allocated to the screening arm31. The Canadian National Breast Screening Study is an outlier among the 8 rcts for screening mammography; it was the only study to show no bca mortality reduction from screening mammography.
In the Pan-Canadian study, which used data from the 3 provinces that perform screening in women 40–49 years of age, the relative bca mortality reduction with screening was 44%9. The U.K. Age rct reported the effect on bca mortality of mammographic screening for women 40–49 years of age at 17.7 years of follow-up32. From 1990 to 1997, 160,921 women 39–41 years of age in the Breast Screening Programme of the National Health Service were randomly assigned to either an intervention group that was offered annual screening by mammography or to a control group (1:2 allocation) that received usual medical care (screening starting at age 50). Results showed a 25% reduction in bca mortality in the intervention group compared with the control group in the first 10 years after diagnosis (rr: 0.75; 95% ci: 0.58 to 0.97), but not thereafter, once they started regular screening at age 50 (rr: 1.02; 95% ci: 0.80 to 1.30). The overall bca incidence during the 17-year follow-up was similar in the intervention and control groups. The authors concluded that their results supported an early reduction in bca mortality with annual screening in women 40–49 years of age.
HARMS OF BREAST CANCER SCREENING
False Positives
A false positive is defined as recall for additional testing after an abnormal mammogram, in which further evaluation determines that the initial abnormal finding is not cancer. False-positive results are one of the most common adverse effects of screening. Most will be resolved with further noninvasive imaging work-up, but a percentage will require further tissue diagnosis (for example, a core biopsy), with the findings being mostly benign. False-positive results invariably lead to some level of anxiety for screening participants. The variability in the recall rate is a result of many factors, including use of postmenopausal hormone therapy, greater mammographic density, first mammogram, longer intervals between screens, lack of previous mammograms for comparison33, and differences in performance and training of the interpreting radiologists34.
In Canada, data about abnormal recalls from screening programs are publicly available from the Canadian Partnership Against Cancer11. These quality indicators help to demonstrate the performance and effectiveness of provincial organized screening programs, summarized in Table ii. Most women who receive an abnormal screening result do not go on to be diagnosed with bca; however, additional assessment is required to reach a definitive diagnosis. The assessment process can include additional imaging with diagnostic mammographic views, breast ultrasonography, or core or fine-needle aspiration biopsy. Approximately 80% of women with an abnormal screen require only additional imaging; the remaining 20% require a biopsy for diagnosis11. Among women who require a breast biopsy, the expected rate of a malignant finding is less than 50% (30%–50%)11.
TABLE II.
Summary of quality indicators for women 50–69 years of age in organized breast cancer screening programs across Canada, 2011–2012 screen yearsa
| Quality indicator | Screening target | Performance |
|---|---|---|
| Rate (%) of ... | ||
| Participation | ≥70 | 54 |
| Retention within 30 months of subsequent screen | ≥90 | 82.6 |
| Annual screening within 18 months of subsequent screen | NA | 31.8 |
| Abnormal call subsequent screens | <5 | 7.2 |
| Invasive cancers (n) detected on subsequent screen (per 1000 screens) | >3 | 3.7 |
| In situ cancers (n) detected on subsequent screen (per 1000 screens) | NA | 0.8 |
| Sensitivity (%) | NA | 84.3 |
| Screen-detected invasive tumour size ≤15 mm (%) | >50 | 59.2 |
| Node-negative screen-detected invasive cancers (%) | >70 | 76.4 |
| Diagnostic interval (%) | ||
| First diagnostic assessment within 3 weeks | ≥90 | 66.1 |
| Final diagnosis (with no tissue biopsy) within 5 weeks | ≥90 | 79.1 |
| Final diagnosis (biopsy) within 7 weeks | ≥90 | 54.9 |
| Post-screen invasive cancers (n per 10,000 person–years), 12–24 months | <12 | 12.7 |
| Positive predictive value (%), subsequent screen | ≥6 | 6.5 |
Adapted from Canadian Partnership Against Cancer, 201211.
Overdiagnosis
“Overdiagnosis” is the diagnosis, as a result of screening, of a cancer (either invasive or in situ) that would never have been identified clinically or caused a problem in the individual’s lifetime. Several autopsy studies have demonstrated the frequent presence of breast malignancy in women with no diagnosis before death. Overdiagnosis can result in unnecessary worry, additional imaging or diagnostic work-up, and overtreatment. Reports of overdiagnosis in the literature range widely, from 0% to 57%35–38, which should call into question their scientific validity.
To obtain an accurate estimate for overdiagnosis, it is important that the screened and unscreened populations studied have similar risk factors for bca and that adjustments be made for any confounders. Lead-time bias—the time between detection of the disease as a result of screening and the time at which the diagnosis would normally have been made when the patient presented with symptoms—must be accounted for. Because of lead time, an excess incidence of bca is expected when screening starts. After the end of screening, a reduction in the incidence of bca should occur because of the earlier diagnosis of cancers during screening. If no overdiagnosis occurs, then the initial increase in bca in screened women should be fully compensated by a similar decline in bca in older women who no longer screen, called the “compensatory drop.” An interval of at least 5 years of follow-up is required to observe that drop. If follow-up is insufficient, then the compensatory drop will overestimate any overdiagnosis. If no adjustment is made for the compensatory drop, then estimates of overdiagnosis are much higher, on the order of 57% for in situ and invasive cancers39.
The estimation of overdiagnosis requires accurate correction for changes in the baseline incidence of bca. The problem is that the incidence of bca has changed over time40. Use of an incorrect assumption about the incidence of bca could inflate the estimate of the magnitude of overdiagnosis. For example, Bleyer and Welch41 reported that the incidence of bca increased by 0.25% per year between 1975 and 2008, and they estimated overdiagnosis to be 31%. But, 4 years later, Welch et al.42 reported that the incidence of bca was stable during the same time period. Those authors argued that the flat incidence line for metastatic bca was evidence for massive overdiagnosis from screening mammography. However, if the incidence of bca had risen steadily, then the flat incidence rate for metastatic bcas was, in reality, evidence of the benefit of screening and a low rate of overdiagnosis. In fact, the Connecticut registry documented a steady increase in the incidence of bca, by 1% per year, between 1940 and 1980, before screening mammography43. Then, between 1980 and 1987, an increase of 32% was reported by the U.S. Surveillance, Epidemiology, and End Results program, attributed to the advent of widespread screening mammography43. A recent study that appropriately adjusted for pre-screening trends found a 37% reduction in late-stage disease, with a reciprocal increase in early-stage disease, approximating the bca mortality reduction seen among women from 1990 through 200944.
Puliti and colleagues undertook a literature review of observational studies to estimate a range for overdiagnosis of bca, including carcinoma in situ, in 7 mammographic screening programs in Western Europe39. Studies were critically reviewed for the methods used to estimate counterfactual rates (what would have happened without screening) and to adjust for lead-time bias. The studies were then categorized as having “adequate” or “not adequate” adjustment for those two factors. The thirteen studies that satisfied the eligibility criteria reported 16 estimates of overdiagnosis. The literature review showed that the unadjusted overdiagnosis estimates ranged widely (from 0% to 54%), but concluded that the most plausible estimates of overdiagnosis ranged from 1% to 10%, the higher estimates being attributed to lack of correction for lead time bias or bca risk, or both. Data from long-term studies such as the Malmo rct after 15 years of follow-up confirm a similar rate of overdiagnosis of 10%45.
Overdetection and Ductal Carcinoma In Situ
It has been argued that the term “overdiagnosis” is not correct, with the correct term being “overdetection,” because the actual diagnosis of bca is performed by a pathologist after a lesion is detected, usually after an imaging work-up46. The overtreatment that accompanies overdetection is what causes the harm. Most overdetection is driven by the diagnosis of ductal carcinoma in situ (dcis). The literature contains much debate about the value of screen detection of dcis and subsequent treatment of the disease.
Before the widespread use of screening mammography in the United States, 6 cases of dcis were detected annually per 100,000 women; after the introduction of screening, 37 cases of dcis were detected per 100,000 women47. According to the acs, carcinoma in situ accounts for 20% of all new bca cases, the vast majority (83%) being dcis, a true (non-obligatory) cancer precursor48.
On mammography, dcis is most often detected as new microcalcifications (Figure 2), although it can present as a palpable mass. It can also be both mammographically and clinically occult. Breast magnetic resonance imaging (mri) has been shown to be more sensitive than mammography for detecting high nuclear grade dcis49. The main goal of bca screening is to detect bca early and thus to lower the incidence of locally advanced bca.
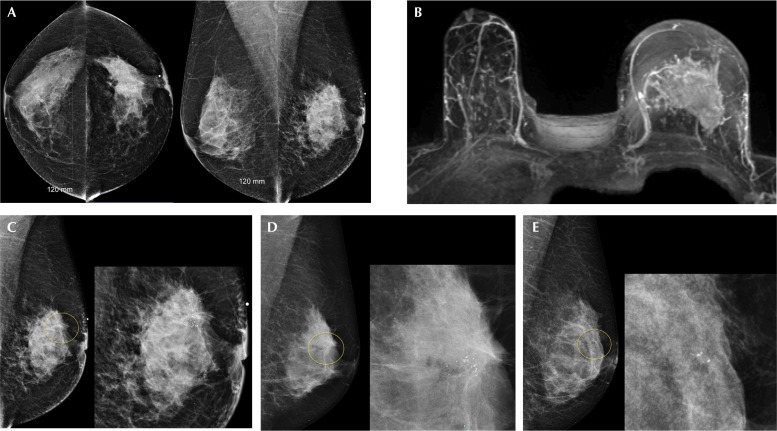
FIGURE 2.
Locally advanced breast cancer in a 56-year-old woman, with calcifications seen at the same site 5 years earlier, likely an evolution from ductal carcinoma in situ (DCIS). (A) Bilateral digital mammograms demonstrate heterogeneously dense breasts (American College of Radiology, BI-RADS C), with a large spiculated mass in the central left breast causing left nipple retraction corresponding to the palpable mass. An ultrasound-guided breast biopsy (not shown) confirmed invasive ductal carcinoma, with axillary node metastases. (B) Maximal-intensity projection image from magnetic resonance imaging shows tumour occupying most of the left breast, measuring more than 5 cm. (C) Photographic enlargement of the left breast mass shows fine pleomorphic calcifications within the mass, characteristic for DCIS. (D) Photographic enlargement of the left breast from a screening mammogram 2 years earlier shows a smaller cluster of calcifications within the same area, not detected at screening. (E) Photographic enlargement of the left breast from a screening mammogram 5 years earlier shows a very small group of fine pleomorphic calcifications, likely DCIS, identified only in retrospect.
Does detecting dcis reduce the rate of invasive cancer? Currently, no tools are available to predict which dcis will progress and which will not. In the United Kingdom, Duffy et al.50 conducted a retrospective population-based study that set out to estimate the association between detection of dcis at screening and the incidence of subsequent invasive interval bcas. Data were obtained for 5.2 million women 50–64 years of age who attended mammographic breast screening through the National Health Service during 2003–2007. Interval cancers diagnosed symptomatically within 36 months after the relevant screen were recorded. The average detection frequency of dcis was 1.6 per 1000 women screened. A significant negative association was observed for screen-detected dcis and the rate of invasive interval cancers; for every 3 screen-detected cases of dcis, 1 fewer invasive interval cancer occurred in the subsequent 3 years. The study concluded that detection and treatment of dcis was worthwhile for the prevention of future invasive disease. To mitigate the harm of overdiagnosis, women should be involved in the decision-making for dcis treatment, based on information about the risks of treatment compared with watchful waiting.
False Negatives
The overall sensitivity of mammography is 80%. Of bcas, 20% are not detected by mammography, but are detected by clinical symptoms such as a palpable mass or suspicious nipple discharge. False negatives are more likely with certain bcas—in particular, lobular carcinomas that tend to grow along the normal breast architecture in a lepidic pattern, making them more difficult to detect. False negatives are also more likely in patients with dense breast tissue, which masks bca. Breast tissue density is most commonly reported using the American College of Radiology’s 4-category Breast Imaging—Reporting and Data System. Sensitivity is highest in the lowest density category and lowest in the highest density category, with one study showing sensitivity decreased from 87% in fatty breasts to 63% in women with the densest breasts51.
TECHNOLOGIC ADVANCES AND DIGITAL BREAST TOMOSYNTHESIS
One technologic advance in screening mammography was the transition from film screen to digital mammography. The dmist trial showed that, in women with dense breasts, the sensitivity of digital mammography was significantly increased52. Another recent major technologic advance is digital breast tomosynthesis (dbt), a pseudo “three-dimensional” mammography technique in which multiple low-dose mammographic images are acquired of compressed breast from multiple angles and are then reconstructed into overlapping thin slices that can be displayed either individually or in a cine loop. Increasingly, dbt is being used as an adjunct screening tool for the detection of bca. Two-dimensional (2D) mammography and tomosynthesis can be obtained in a single compression, and synthesized 2D projection images can also be reconstructed from the dbt data53. The radiation dose received when dbt is combined with conventional 2D mammography is nearly double that of digital mammography alone, but within the established and acceptable safe dose limits53–56.
When combined with digital mammography, dbt helps to improve bca screening and diagnosis. Multiple studies have demonstrated that bca detection rates are improved by 33%–53% (sensitivity) and that false-positive recall rates are simultaneously reduced by 30%–40% (specificity)57–66. Several screening studies have shown incremental invasive cancer detection rates of 1.2–2 per 1000 screened women, with no increase in the detection of dcis59,62,63.
The main advantage of tomosynthesis is its ability to diminish the masking effect of tissue overlap and structure noise usually encountered with 2D mammography. That feature is particularly useful in the setting of dense breasts60,67 and helps to improve the radiologist’s reading confidence, with better characterization of masses68–70. If dbt is used in the screening setting, the marginal definition is equal to that of spot magnification, and so women with masses detected at screening can forego additional mammographic views and attend just for ultrasonography.
Few studies have investigated the long-term sustainability of the improved screening outcomes with dbt. A retrospective analysis looked at outcomes data from 3 years of dbt screening of an entire population at an academic centre. The results showed that dbt screening outcomes were sustainable, with a significant recall reduction, an increase in the cancer cases identified in recalled patients, and a decline in interval cancers71. The tmist trial is the first large randomized multicentric study to assess whether, compared with conventional mammography alone, dbt combined with digital mammography is more effective at lowering the incidence of advanced bcas (see NCT03233191 at http://ClinicalTrials.gov). In the United States and Canada, 165,000 asymptomatic women between the ages of 45 and 74 years will be enrolled. The study aims to provide a modern basis for implementation of the combination technology for bca screening. The Canadian Lead-in Study began recruitment in 2014, and the full study opened in 2017.
Currently, no widely accepted view for the supplemental screening of women with dense breasts has been reached, even though the sensitivity of screening mammography is recognized to be reduced in such women. No rcts have determined any mortality benefit from supplemental screening. Multiple studies have shown increased detection (3–4 per 1000) of small, invasive, node-negative cancers when supplementary screening is performed for women with dense breasts72,73. The j-start prospective rct of ultrasonography has shown favourable preliminary results for detecting early-stage cancers, with fewer interval cancers74. Currently, 32 U.S. states report on breast tissue density, and many recommend supplemental screening. Personalized screening could become more of a reality in the future, whereby, depending on risk and density, supplemental screening might be offered. That approach has been proposed in Quebec with the international Perspective Project75. Recently, studies of contrast-enhanced mammography have shown promise in improving the detection of bca by relying on its enhanced vascularity76,77. Although still experimental and currently used only in the diagnostic setting, that type of screening could have future applications. Breast mri has also recently been proposed as a method of screening for average-risk women: a recent study showed a high supplemental cancer detection rate of 15.5 per 1000 in 2120 average-risk women screened with mri78. In the latter study, more biologically active tumours were found with mri. However, given the higher cost, the requirement for intravenous contrast, and the lower specificity, breast mri has not become a part of routine screening.
SUMMARY
Attending screening mammography has the benefit of reducing bca mortality by 40% in average-risk women 40–74 years of age. Of the 10% false positives that occur in mammography, 8 of 10 are resolved by taking additional views or obtaining ultrasound images, with the remaining 2 being resolved by biopsy. For women who undergo biopsy, only 1 in 3 will be diagnosed with a malignancy. Overdiagnosis occurs in about 10% of screened women, represented mostly by the detection of dcis. False negatives with mammography are an important limitation, often being related to bcas hidden by dense breast tissue. Digital breast tomosynthesis has the potential to simultaneously increase cancer detection and lower the rate of false positives. In addition, supplemental screening with breast ultrasonography, breast mri, and contrast-enhanced mammography shows promise for further increasing the detection of biologically significant bcas in women at higher risk of bca. In 2018, based on the best available current evidence, screening mammography should be recommended every 1–2 years for women 40–74 years of age at average risk. In future, as assessment of risk and breast tissue density becomes a reality, more personalized screening will likely be added to that screening mammography regimen.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ren JS, Masuyer E, Ferlay J. Estimates of global cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 3.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(SICI)1097-0215(19990924)83:1<18::AID-IJC5>3.0.CO;2-M. [Erratum in: Int J Cancer 1999;83:870–3] [DOI] [PubMed] [Google Scholar]
- 4.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2016. Toronto, ON: Canadian Cancer Society; 2016. [Google Scholar]
- 5.Independent UK Panel on Breast Cancer Screening The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–86. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 6.Chiarelli AM, Prummel MV, Muradali D, et al. Effectiveness of screening with annual magnetic resonance imaging and mammography: results of the initial screen from the Ontario high risk breast screening program. J Clin Oncol. 2014;32:2224–30. doi: 10.1200/JCO.2013.52.8331. [DOI] [PubMed] [Google Scholar]
- 7.Lauby-Secretan B, Scoccianti C, Loomis D, et al. on behalf of the International Agency for Research on Cancer Handbook Working Group Breast-cancer screening—viewpoint of the iarc Working Group. N Engl J Med. 2015;372:2353–8. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 8.Broeders M, Moss S, Nystrom L, et al. on behalf of the euroscreen Working Group The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14–25. doi: 10.1258/jms.2012.012078. [DOI] [PubMed] [Google Scholar]
- 9.Coldman A, Phillips N, Wilson C, et al. Pan-Canadian study of mammography screening and mortality from breast cancer. J Natl Cancer Inst. 2014;106:dju261. doi: 10.1093/jnci/dju261. [Erratum in: J Natl Cancer Inst 2015;107:dju404] [DOI] [PubMed] [Google Scholar]
- 10.Oeffinger KC, Fontham ET, Etzioni R, et al. on behalf of the American Cancer Society Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599–614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canadian Partnership Against Cancer (cpac) Breast Cancer Screening in Canada: Monitoring and Evaluation of Quality Indicators—Results Report, January 2011 to December 2012. Toronto, ON: CPAC; 2017. [Google Scholar]
- 12.Tabar L, Vitak B, Yen MF, Chen HH, Smith RA, Duffy SW. Number needed to screen: lives saved over 20 years of follow-up in mammographic screening. J Med Screen. 2004;11:126–9. doi: 10.1258/0969141041732175. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M, Connor Gorber S, Joffres M, et al. on behalf of the Canadian Task Force on Preventive Health Care Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ. 2011;183:1991–2001. doi: 10.1503/cmaj.110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canadian Association of Radiologists (car) CAR Practice Guidelines and Technical Standards for Breast Imaging and Intervention. Ottawa, ON: CAR; 2016. [Available online at: https://car.ca/wp-content/uploads/Breast-Imaging-and-Intervention-2016.pdf cited 28 January 2018] [Google Scholar]
- 15.Siu AL, on behalf of the U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279–96. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the acr on the use of mammography, breast mri, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Practice Bulletin No. 179 Summary: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:241–3. doi: 10.1097/AOG.0000000000002151. [DOI] [PubMed] [Google Scholar]
- 18.Gradishar WJ, Anderson BO, Balassanian R, et al. nccn guidelines insights: breast cancer, version 1.2017. J Natl Compr Canc Netw. 2017;15:433–51. doi: 10.6004/jnccn.2017.0044. [DOI] [PubMed] [Google Scholar]
- 19.Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013:CD001877. doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabar L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260:658–63. doi: 10.1148/radiol.11110469. [DOI] [PubMed] [Google Scholar]
- 21.Webb ML, Kopans DB, Cady B. Reply to A failure analysis of invasive breast cancer: most deaths from disease occur in women not regularly screened. Cancer. 2014;120:2937–8. doi: 10.1002/cncr.28525. [DOI] [PubMed] [Google Scholar]
- 22.Legorreta AP, Brooks RJ, Leibowitz AN, Solin LJ. Cost of breast cancer treatment. A 4-year longitudinal study. Arch Intern Med. 1996;156:2197–201. doi: 10.1001/archinte.1996.00440180055007. [DOI] [PubMed] [Google Scholar]
- 23.Montero AJ, Eapen S, Gorin B, Adler P. The economic burden of metastatic breast cancer: a U.S. managed care perspective. Breast Cancer Res Treat. 2012;134:815–22. doi: 10.1007/s10549-012-2097-2. [DOI] [PubMed] [Google Scholar]
- 24.Will BP, Berthelot JM, Le Petit C, Tomiak EM, Verma S, Evans WK. Estimates of the lifetime costs of breast cancer treatment in Canada. Eur J Cancer. 2000;36:724–35. doi: 10.1016/S0959-8049(99)00340-8. [DOI] [PubMed] [Google Scholar]
- 25.Siu AL, Bibbins-Domingo K, Grossman DC, LeFevre ML, on behalf of the U.S. Preventive Services Task Force Convergence and divergence around breast cancer screening. Ann Intern Med. 2016;164:301–2. doi: 10.7326/M15-3065. [DOI] [PubMed] [Google Scholar]
- 26.Siu AL, on behalf of the U.S. Preventive Services Task Force Screening for depression in children and adolescents: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:360–6. doi: 10.7326/M15-2957. [DOI] [PubMed] [Google Scholar]
- 27.Eby PR. Evidence to support screening women annually. Radiol Clin North Am. 2017;55:441–56. doi: 10.1016/j.rcl.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Breast-cancer screening with mammography in women aged 40–49 years Swedish Cancer Society and the Swedish National Board of Health and Welfare. Int J Cancer. 1996;68:693–9. doi: 10.1002/(SICI)1097-0215(19961211)68:6<693::AID-IJC1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975–2014. Bethesda, MD: United States, National Cancer Institute; 2017. [Google Scholar]
- 30.Miller AB, Howe GR, Wall C. The national study of breast cancer screening protocol for a Canadian randomized controlled trial of screening for breast cancer in women. Clin Invest Med. 1981;4:227–58. [PubMed] [Google Scholar]
- 31.Kopans DB. The Canadian National Breast Screening Studies are compromised and their results are unreliable. They should not factor into decisions about breast cancer screening. Breast Cancer Res Treat. 2017;165:9–15. doi: 10.1007/s10549-017-4302-9. [DOI] [PubMed] [Google Scholar]
- 32.Moss SM, Wale C, Smith R, Evans A, Cuckle H, Duffy SW. Effect of mammographic screening from age 40 years on breast cancer mortality in the U.K. Age trial at 17 years’ follow-up: a randomised controlled trial. Lancet Oncol. 2015;16:1123–32. doi: 10.1016/S1470-2045(15)00128-X. [DOI] [PubMed] [Google Scholar]
- 33.Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481–92. doi: 10.7326/0003-4819-155-8-201110180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miglioretti DL, Gard CC, Carney PA, et al. When radiologists perform best: the learning curve in screening mammogram interpretation. Radiology. 2009;253:632–40. doi: 10.1148/radiol.2533090070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paci E, Duffy S. Overdiagnosis and overtreatment of breast cancer: overdiagnosis and overtreatment in service screening. Breast Cancer Res. 2005;7:266–70. doi: 10.1186/bcr1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopans DB, Smith RA, Duffy SW. Mammographic screening and “overdiagnosis”. Radiology. 2011;260:616–20. doi: 10.1148/radiol.11110716. [DOI] [PubMed] [Google Scholar]
- 37.Gotzsche PC, Hartling OJ, Nielsen M, Brodersen J, Jorgensen KJ. Breast screening: the facts—or maybe not. BMJ. 2009;338:b86. doi: 10.1136/bmj.b86. [DOI] [PubMed] [Google Scholar]
- 38.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 39.Puliti D, Duffy SW, Miccinesi G, et al. on behalf of the euroscreen working group Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen. 2012;19(suppl 1):42–56. doi: 10.1258/jms.2012.012082. [DOI] [PubMed] [Google Scholar]
- 40.Xie L, Semenciw R, Mery L. Cancer incidence in Canada: trends and projections (1983–2032) Health Promot Chronic Dis Prev Can. 2015;35(suppl 1):2–186. doi: 10.24095/hpcdp.35.S1.02. [DOI] [PubMed] [Google Scholar]
- 41.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 42.Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375:1438–47. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 43.Garfinkel L, Boring CC, Heath CW., Jr Changing trends. An overview of breast cancer incidence and mortality. Cancer. 1994;74(suppl):222–7. doi: 10.1002/cncr.2820741304. [DOI] [PubMed] [Google Scholar]
- 44.Helvie MA, Chang JT, Hendrick RE, Banerjee M. Reduction in late-stage breast cancer incidence in the mammography era: implications for overdiagnosis of invasive cancer. Cancer. 2014;120:2649–56. doi: 10.1002/cncr.28784. [DOI] [PubMed] [Google Scholar]
- 45.Zackrisson S, Andersson I, Janzon L, Manjer J, Garne JP. Rate of over-diagnosis of breast cancer 15 years after end of Malmo mammographic screening trial: follow-up study. BMJ. 2006;332:689–92. doi: 10.1136/bmj.38764.572569.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaffe MJ, Pritchard KI. Overdiagnosing overdiagnosis. Oncologist. 2014;19:103–6. doi: 10.1634/theoncologist.2014-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute (nci), Surveillance, Epidemiology, and End Results Program (seer) Cancer Statistics Branch, SEER Program Public Use Data Tapes 1973–1998, November 2000 submission. Bethesda, MD: NCI; 2017. Cancer Stat Facts: Female Breast Cancer. [Google Scholar]
- 48.Ward EM, DeSantis CE, Lin CC, et al. Cancer statistics: breast cancer in situ. CA Cancer J Clin. 2015;65:481–95. doi: 10.3322/caac.21321. [DOI] [PubMed] [Google Scholar]
- 49.Kuhl CK, Schrading S, Bieling HB, et al. mri for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–92. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 50.Duffy SW, Dibden A, Michalopoulos D, et al. Screen detection of ductal carcinoma in situ and subsequent incidence of invasive interval breast cancers: a retrospective population-based study. Lancet Oncol. 2016;17:109–14. doi: 10.1016/S1470-2045(15)00446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–75. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 52.Pisano ED, Gatsonis C, Hendrick E, et al. on behalf of the Digital Mammographic Imaging Screening Trial (dmist) investigators group Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–83. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 53.Nelson JS, Wells JR, Baker JA, Samei E. How does C-view image quality compare with conventional 2D ffdm? Med Phys. 2016;43:2538. doi: 10.1118/1.4947293. [DOI] [PubMed] [Google Scholar]
- 54.Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast. 2015;24:93–9. doi: 10.1016/j.breast.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng SS, Sechopoulos I. Clinical digital breast tomosynthesis system: dosimetric characterization. Radiology. 2012;263:35–42. doi: 10.1148/radiol.11111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavagnetto F, Taccini G, Rosasco R, Bampi R, Calabrese M, Tagliafico A. “In vivo” average glandular dose evaluation: one-to-one comparison between digital breast tomosynthesis and full-field digital mammography. Radiat Prot Dosimetry. 2013;157:53–61. doi: 10.1093/rpd/nct120. [DOI] [PubMed] [Google Scholar]
- 57.Poplack SP, Tosteson TD, Kogel CA, Nagy HM. Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography. AJR Am J Roentgenol. 2007;189:616–23. doi: 10.2214/AJR.07.2231. [DOI] [PubMed] [Google Scholar]
- 58.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (storm): a prospective comparison study. Lancet Oncol. 2013;14:583–9. doi: 10.1016/S1470-2045(13)70134-7. [DOI] [PubMed] [Google Scholar]
- 59.Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R., Jr Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am J Roentgenol. 2013;200:1401–8. doi: 10.2214/AJR.12.9672. [DOI] [PubMed] [Google Scholar]
- 60.Gilbert FJ, Tucker L, Gillan MG, et al. Accuracy of digital breast tomosynthesis for depicting breast cancer subgroups in a UK retrospective reading study (tommy trial) Radiology. 2015;277:697–706. doi: 10.1148/radiol.2015142566. [DOI] [PubMed] [Google Scholar]
- 61.Rafferty EA, Park JM, Philpotts LE, et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology. 2013;266:104–13. doi: 10.1148/radiol.12120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311:2499–507. doi: 10.1001/jama.2014.6095. [DOI] [PubMed] [Google Scholar]
- 63.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267:47–56. doi: 10.1148/radiol.12121373. [DOI] [PubMed] [Google Scholar]
- 64.Michell MJ, Iqbal A, Wasan RK, et al. A comparison of the accuracy of film-screen mammography, full-field digital mammography, and digital breast tomosynthesis. Clin Radiol. 2012;67:976–81. doi: 10.1016/j.crad.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Bernardi D, Ciatto S, Pellegrini M, et al. Prospective study of breast tomosynthesis as a triage to assessment in screening. Breast Cancer Res Treat. 2012;133:267–71. doi: 10.1007/s10549-012-1959-y. [DOI] [PubMed] [Google Scholar]
- 66.Svahn T, Andersson I, Chakraborty D, et al. The diagnostic accuracy of dual-view digital mammography, single-view breast tomosynthesis and a dual-view combination of breast tomosynthesis and digital mammography in a free-response observer performance study. Radiat Prot Dosimetry. 2010;139:113–17. doi: 10.1093/rpd/ncq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial. J Clin Oncol. 2016. [Epub ahead of print]. [DOI] [PubMed]
- 68.Noroozian M, Hadjiiski L, Rahnama-Moghadam S, et al. Digital breast tomosynthesis is comparable to mammographic spot views for mass characterization. Radiology. 2012;262:61–8. doi: 10.1148/radiol.11101763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elmaadawy M, Seely JM, Doherty GP, Lad SV. Digital breast tomosynthesis in the evaluation of focal mammographic asymmetry, do you still need coned compression views? [abstract]. Presented at: Radiological Society of North America 2012 Annual Meeting; Chicago, IL, U.S.A.. 25–30 November 2012; [Available online at: http://archive.rsna.org/2012/12026336.html; cited 9 April 2018] [Google Scholar]
- 70.Tagliafico A, Astengo D, Cavagnetto F, et al. One-to-one comparison between digital spot compression view and digital breast tomosynthesis. Eur Radiol. 2012;22:539–44. doi: 10.1007/s00330-011-2305-1. [DOI] [PubMed] [Google Scholar]
- 71.McDonald ES, Oustimov A, Weinstein SP, Synnestvedt MB, Schnall M, Conant EF. Effectiveness of digital breast tomosynthesis compared with digital mammography: outcomes analysis from 3 years of breast cancer screening. JAMA Oncol. 2016;2:737–43. doi: 10.1001/jamaoncol.2015.5536. [DOI] [PubMed] [Google Scholar]
- 72.Berg WA, Zhang Z, Lehrer D, et al. on behalf of the acrin 6666 investigators Detection of breast cancer with addition of annual screening ultrasound or a single screening mri to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening us in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265:59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
- 74.Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (j-start): a randomised controlled trial. Lancet. 2016;387:341–8. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 75.Gagnon J, Levesque E, Borduas F, et al. on behalf of the Clinical Advisory Committee on Breast Cancer Screening and Prevention Recommendations on breast cancer screening and prevention in the context of implementing risk stratification: impending changes to current policies. Curr Oncol. 2016;23:e615–25. doi: 10.3747/co.23.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewin JM, Isaacs PK, Vance V, Larke FJ. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology. 2003;229:261–8. doi: 10.1148/radiol.2291021276. [DOI] [PubMed] [Google Scholar]
- 77.Covington MF, Pizzitola VJ, Lorans R, et al. The future of contrast-enhanced mammography. AJR Am J Roentgenol. 2018;210:292–30. doi: 10.2214/AJR.17.18749. [DOI] [PubMed] [Google Scholar]
- 78.Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361–70. doi: 10.1148/radiol.2016161444. [DOI] [PubMed] [Google Scholar]