Abstract
The treatment of squamous non-small-cell lung cancer (nsclc) is evolving. In the past, the backbone of treatment was chemotherapy, with very few other options available. Fortunately, that situation is changing, especially with a better understanding of tumour biology. Various strategies have been tried to improve patient outcomes. The most notable advance must be immunotherapy, which has revolutionized the treatment paradigm for lung cancer in patients without a driver mutation. Immunotherapy is now the treatment of choice in patients who have progressed after chemotherapy and is replacing chemotherapy as upfront therapy in a selected population. Other strategies have also been tried, such as the addition of targeted therapy to chemotherapy. Targeted agents include ramucirumab, an inhibitor of vascular endothelial growth factor receptor 2, and necitumumab, a monoclonal antibody against epithelial growth factor receptor. Recently, advances in molecular profiling have also been applied to tumours of squamous histology, in which multiple genetic alterations, including mutations and amplifications, have been described. Research is actively seeking targetable mutations and testing various therapies in the hopes of further improving prognosis for patients with squamous nsclc. Here, we review the various advances in the treatment of squamous nsclc and present a proposed treatment algorithm based on current evidence.
Keywords: Non-small-cell carcinoma of the lung, squamous cell carcinoma, targeted therapy, immunotherapy
INTRODUCTION
Lung cancer is a common malignancy and remains the cancer with the highest mortality rate in Canada1. Unfortunately, it is often diagnosed at an advanced incurable stage. Non-small-cell lung cancer (nsclc) encompasses approximately 85% of lung cancers, with 20% of those cancers being squamous cell carcinoma (scc)2. Squamous cell carcinoma is strongly associated with smoking. Its incidence has been steadily declining over time, mostly because of a parallel reduction in smoking.
Historically, the systemic treatment of advanced scc was limited to palliative chemotherapy. Appropriate treatment delivery can be challenging because of the general demographics of the disease, with scc patients tending to be older and to have more comorbidities. In recent years, more therapeutic options have become available. The most noteworthy would be immunotherapy, which has changed the treatment paradigm in nsclc by improving outcomes. Nonetheless, we are still far from personalized medicine in scc of the lung—an approach that has been realized for adenocarcinomas, in which the discovery of driver mutations (such as those in ALK and EGFR) has allowed for the use of targeted therapy in selected patients. Recently, broader molecular genotyping has led to the description of multiple mutations that are potentially targetable in scc. Data relating to targeted agents are still limited, and ongoing research is trying to determine the efficacy of such agents in a clinical setting.
Here, we review approved therapies for advanced and metastatic scc of the lung. We also discuss various targeted therapies that are available or currently being investigated (Table i).
TABLE I.
Targeted therapy agents mentioned in this article
| Agent | Target | Side effects (not exhaustive) |
|---|---|---|
| Monoclonal antibodies | ||
| Bevacizumab | VEGF-A | Hypertension, neutropenia, bleeding, impaired wound healing, proteinuria, gastrointestinal perforation |
| Ramucirumab | VEGFR2 | Hypertension, neutropenia, bleeding, impaired wound healing, proteinuria, infusion reaction, gastrointestinal perforation |
| Cetuximab | EGFR | Fatigue, rash, diarrhea, infusion reaction, hypomagnesemia |
| Necitumumab | EGFR | Fatigue, rash, diarrhea, infusion reaction, hypomagnesemia |
| Tyrosine kinase inhibitors | ||
| Erlotinib | EGFR | Gastrointestinal symptoms (diarrhea), rash, elevated liver enzymes |
| Gefitinib | EGFR | Gastrointestinal symptoms (diarrhea), rash, elevated liver enzymes |
| Afatinib | EGFR, HER2 | Gastrointestinal symptoms (diarrhea), rash, elevated liver enzymes |
| Crizotinib | ALK, MET, ROS1 | Rash, diarrhea, elevated liver enzymes, vision disorder, QT prolongation |
| Multikinase inhibitor | ||
| Pazopanib | FGFR1, VEGFR1–3, PDGFR, Kit | Fatigue, cytopenia, increased liver enzymes, gastrointestinal symptoms (nausea, diarrhea), QT prolongation, hypertension, hair depigmentation |
| mTOR inhibitor | ||
| Everolimus | mTOR | Fatigue, cytopenia, diarrhea, stomatitis, hypersensitivity syndrome, pneumonitis |
| Immune checkpoint inhibitors | ||
| Nivolumab | PD-1 | Autoimmune side effects including fatigue, infusion reaction, rash, gastrointestinal symptoms (diarrhea, colitis), endocrinopathies (dysthyroidism, adrenal insufficiency), increased liver enzymes, pneumonitis |
| Pembrolizumab | PD-1 | |
VEGF = vascular endothelial growth factor; VEGFR2 = vascular endothelial growth factor receptor 2; EGFR = epidermal growth factor receptor (also known as HER1 or ErbB1); HER2 = human epidermal growth factor receptor 2 (also known as ErbB2/neu); ALK = anaplastic lymphoma kinase; MET = hepatocyte growth factor receptor; Flt-3 = fms-related tyrosine kinase; FGFR1 = fibroblast growth factor receptor 1; PDGFR = platelet-derived growth factor receptor; mTOR = mechanistic target of rapamycin.
CYTOTOXIC CHEMOTHERAPY IN SCC
Initial Chemotherapy
Doublet chemotherapy, which must include a platinum agent (cisplatin or carboplatin) and which is given for 4–6 cycles, is a standard of care in first-line treatment of scc of the lung. In a large meta-analysis, two-drug regimens (compared with single-agent chemotherapy) were associated with significantly increased rates of response and overall survival (os)3. Such regimens are generally offered to patients with a good performance status, and randomized studies comparing those regimens with best supportive care have shown a benefit in terms of survival and quality of life4. A variety of drugs can be paired with platinum, the most common being paclitaxel, gemcitabine, or vinorelbine. Prolonged administration of this initial chemotherapy is not recommended; although chemotherapy use can have an effect on progression-free survival (pfs), its effect on survival has not been formally demonstrated5.
No particular platinum-based doublet regimen has been proved to confer a clinically significant advantage over the others6. The choice is often based on safety profile and convenience of administration. Treatment intensification by adding a third chemotherapy agent has also not yielded positive outcomes. Response rates were increased, but did not translate into increased survival rates7. Histology offers insight into chemotherapy selection, but personalizing chemotherapy in scc of the lung is not yet achievable. Molecular biomarkers such as ERCC1 and RRM1 have not helped to identify potential responders in a clinical setting, despite encouraging preclinical data8,9.
Notably, pemetrexed is not indicated in scc of the lung, whether in the first or subsequent lines of treatment. In a large phase iii study comparing cisplatin–gemcitabine with cisplatin–pemetrexed in nsclc10, the survival benefit observed in the cisplatin–pemetrexed arm introduced pemetrexed as an attractive treatment choice for lung adenocarcinoma, especially given its better tolerability. In contrast, the cisplatin–gemcitabine doublet was associated with better survival in scc. A detrimental effect on survival of cisplatin–pemetrexed was also observed in patients who had received prior chemotherapy. A possible explanation for pemetrexed resistance could be higher expression of one of its targets, thymidylate synthase, in squamous tumours11.
Maintenance Therapy
Maintenance therapy aims to delay disease progression. Strategies include “switch maintenance” (in which initial chemotherapy is followed by a maintenance drug different from those used in the initial regimen) and “continuation maintenance” (in which a drug used in the initial regimen is continued alone, after the platinum agent has been dropped). Maintenance is offered when a patient experiences a favourable response or stable disease after first-line chemotherapy; patients who progress on their platinum doublet move on to second-line salvage therapy.
Various maintenance strategies using cytotoxic chemotherapy have been studied, including docetaxel12, gemcitabine, and pemetrexed. Those agents have not been adopted as effective maintenance strategies in scc. For example, with gemcitabine, a longer pfs was noted without an effect on os13. The most widely used maintenance chemotherapy in nsclc is pemetrexed14, but that approach does not apply in scc. In the jmen study, patients with non-squamous histology experienced a 3.2-month significant survival advantage. In contrast, patients with scc derived no benefit from maintenance pemetrexed [hazard ratio (hr): 1.03; p = 0.896]15.
Erlotinib was also studied in the maintenance setting. In the saturn trial, a modest improvement in os was observed for erlotinib maintenance compared with placebo (12 months vs. 11 months), but os was not statistically different between the scc (approximately 40% of the population) and the adenocarcinoma patients16. The iuno study compared maintenance erlotinib with erlotinib at disease progression in patients with advanced non-EGFR-mutated nsclc (36% scc). In contrast to the saturn trial, iuno found that maintenance erlotinib was not superior to second-line treatment (os: 9.7 months vs. 9.5 months; p = 0.82) in patients with EGFR wild-type lung cancer17. After the results of iuno were reported, the U.S. Food and Drug Administration (fda) modified the indication for erlotinib in nsclc, limiting it to EGFR mutation–positive tumours18.
Based on the foregoing studies, maintenance therapy has been restricted mostly to nonsquamous histologies.
Chemotherapy at Progression
Upon disease progression after platinum doublet chemotherapy, patients deemed fit for treatment would, until recently, be offered single-agent docetaxel19. This taxane derivative has been proved effective when compared with best supportive care. Treatment with docetaxel is associated with significant prolongation of survival (7.0 months vs. 4.6 months; p = 0.047), with the benefits of therapy outweighing toxicity20. Compared with best supportive care, docetaxel is also associated with less deterioration in quality of life21.
For many years, single-agent docetaxel was thus considered the standard second-line treatment at progression. That situation has changed with data now showing that immunooncology therapies are more effective than docetaxel in this setting. Docetaxel is now considered a possible treatment after progression on immunotherapy. Notably, docetaxel has not been compared with best supportive care in a third-line setting after immunotherapy failure.
IMMUNOTHERAPY
Immunotherapy has changed the treatment paradigm for nsclc, especially in patients with no driver mutations. It has introduced a new treatment modality for scc of the lung, for which proven and available treatments are not as diverse as they are for adenocarcinoma.
Immuno-oncology therapies include various immune checkpoint inhibitors such as antibodies directed against ctla-4, PD-1, and PD-L1. The fundamental mechanism of those drugs is to stimulate a patient’s T-cell immune response to recognize and destroy cancer cells22. Carrying a high mutational burden can increase the likelihood of responding to immuno-oncology agents, and scc— because it is related to smoking and exposure to a multitude of carcinogens—is a tumour that ranks among those with the highest mutational burden23. Immunotherapy has a toxicity profile different from that of cytotoxic chemotherapy, and it seems to be better tolerated overall.
Immunotherapy in First-Line Treatment
In the keynote-24 trial, pembrolizumab, a PD-1 inhibitor, was associated with improved pfs and os in treatment-naïve patients with 50% tumour-cell staining for PD-L1 (compared with standard doublet chemotherapy)24. Pembrolizumab was continued in those patients until progression or unacceptable toxicity. The median pfs of 10.3 months, compared with 6.0 months for those receiving chemotherapy, represented a significant improvement [hr: 0.50; 95% confidence interval (ci): 0.37 to 0.68; p < 0.001], with a more favourable toxicity profile. Median survival was not reached in either arm at the time of analysis. The pfs advantage was detected in all histology types, including scc (18% of the entire cohort; hr: 0.35; 95% ci: 0.17 to 0.71). Those positive outcomes were not seen in CheckMate 026, which compared first-line nivolumab, another PD-1 inhibitor, with chemotherapy25. That trial did not meet its primary endpoint of pfs (4.2 months vs. 5.9 months, p = 0.251). However, less toxicity was observed in the nivolumab arm (grades 3–4 adverse events: 17.6% vs. 50.6%). CheckMate 026 was less restrictive with respect to PD-L1 expression, with 5% being a cut-off for high expression.
The keynote-24 trial is pivotal and will change the first-line treatment of metastatic nsclc without a driver mutation. Health Canada granted approval for pembrolizumab in treatment-naïve patients who have high tumour PD-L1 expression26 and no contraindications to immunotherapy, the most common being active or recently treated autoimmune disease and interstitial lung disease. That approval has started changing practice, with some guidelines modifying recommendations for patients with advanced nsclc and no driver mutations. Pembrolizumab is now the preferred first-line treatment for patients with 50% or greater PD-L1 expression27.
Immunotherapy at Progression
Nivolumab was the first immuno-oncology agent approved for the treatment of lung cancer. More specifically with respect to scc, the phase iii CheckMate 017 trial randomly assigned 272 patients with stage iv scc progressing during or after platinum-based chemotherapy to nivolumab or docetaxel. Median os was improved in the nivolumab group (9.2 months vs. 6.0 months; p < 0.001; hr: 0.59; 1-year os: 42% vs. 24%).
The immunotherapy response rate is fairly low (<20%); however, it seems that a subset of patients can derive long-term benefit from immunotherapy. In CheckMate 017, efficacy was independent of PD-L1 tumour expression28, a result that differed from that in CheckMate 057, in which PD-L1 expression was predictive in a population having nonsquamous nsclc29. Additionally, in the keynote-010 study, which enrolled 1034 patients, participants experienced a longer os with pembrolizumab than with docetaxel. In that study, PD-L1 tumour expression had to be at least 1%. The median os was 10.4 months with pembrolizumab 2 mg/kg and 8.5 months with docetaxel (p = 0.0008). The survival advantage was demonstrated for all histology subtypes, including the scc subset (hr: 0.74; 95% ci: 0.50 to 1.09). The difference was more marked in patients with PD-L1 expression exceeding 50% (approximately 40% of patients), which further validated PD-L1 selection. In such patients, os was significantly longer with pembrolizumab (median: 14.9 months vs. 8.2 months; p = 0.0002)30.
Pembrolizumab has also been granted accelerated approval by the fda in advanced or metastatic high microsatellite instability or mismatch-repair-deficient solid tumours upon progression, regardless of tumour type31. That approval was based on efficacy data, the overall response rate being 39.6% in 149 patients. However, high microsatellite instability seems to be very infrequent in nsclc32.
Other anti–PD-L1 agents such as atezolizumab and durvalumab are also active in second- or subsequent-line treatment for lung cancer, including scc. In the phase iii randomized oak study comparing atezolizumab with second-line docetaxel, atezolizumab was associated with a survival benefit regardless of histology or PD-L1 expression. In the squamous subgroup (26%), median os was superior at 8.9 months compared with 7.7 months (hr: 0.73; 95% ci: 0.54 to 0.98)33.
Currently, nivolumab is approved for the treatment of advanced or metastatic nsclc with progression on or after platinum-based chemotherapy, regardless of PD-L1 expression. Pembrolizumab, in the second line or beyond, is approved in patients with metastatic nsclc and PD-L1 expression of 1% or greater. Government funding in Canada has been accepted in various provinces, making nivolumab and pembrolizumab more accessible.
Combination Immunotherapy With or Without Chemotherapy
Studies have demonstrated that specific chemotherapies commonly used in lung cancer can enhance the immunologic response to cancers34. That observation has led to research into combinations of immunotherapy and chemotherapy. Adding chemotherapy might add benefit for patients. It can be associated with higher response rates and more durable responses. It could also make combination therapy a treatment of choice for a broader population35.
Combination immunotherapy could include giving an anti–ctla-4 agent with a PD-1 or PD-L1 inhibitor. Such agents could be given together with or without standard chemotherapy. Early-phase trials are looking at various combination strategies. For example, in Canada, the Canadian Cancer Trials Group br.34 trial (NCT03057106 at http://ClinicalTrials.gov) is looking at durvalumab–tremelimumab given with or without chemotherapy in patients with metastatic nsclc. Another trial, keynote-021 (NCT02039674), is studying pembrolizumab in combination with chemotherapy or immunotherapy in participants with metastatic nsclc. Based on a cohort in keynote-021, pembrolizumab given with carboplatin–pemetrexed was recently approved by the fda for the first-line treatment of adenocarcinoma. Many combination trials are under way, and more details are expected in the future.
ANTIANGIOGENESIS AGENTS
Blocking neovascularization and tumour proliferation can theoretically reduce tumour growth. Bevacizumab is the most commonly studied antiangiogenesis agent in nsclc. It targets the vascular endothelial growth factor pathway by inhibiting vascular endothelial growth factor A.
Bevacizumab has shown efficacy in nsclc. It can also cause massive pulmonary hemorrhage, sometimes fatally so, as reported in earlier trials testing the combination of chemotherapy and bevacizumab36. Those events occurred more frequently in scc, which led to the early exclusion of patients with scc from the pivotal bevacizumab trials37,38, including the randomized trial showing an os improvement of 2 months for paclitaxel–carboplatin plus bevacizumab compared with chemotherapy alone. Squamous-cell histology and the presence of hemoptysis are therefore contraindications to the administration of bevacizumab in nsclc.
Other antiangiogenic agents have also been tested in nsclc39. Ramucirumab, which binds directly to vascular endothelial growth factor receptor 2, demonstrated modest activity in nsclc. Although patients with squamous histology could participate in the related trials, patients with major invasion of airway or blood vessels, a cavitating lesion, or a recent history of hemoptysis were notably excluded. The phase iii revel trial assigned 1253 patients to docetaxel with or without ramucirumab after progression on a platinum doublet40. A small but statistically significant difference favoured the combination. The os, pfs, and response rate were all improved. For the squamous-cell subgroup (25% of patients), outcomes were similar but nonsignificant (os: 9.5 months vs. 8.2 months; hr: 0.88; 95% ci: 0.69 to 1.12; p = 0.319). Ramucirumab is the only antiangiogenesis drug that has an indication in scc. It has been approved by the fda41, but not by Health Canada. An approach using ramucirumab has not been widely accepted because the cost–benefit ratio of the combination seems marginal42.
ANTI-EGFR AGENTS
Mutations of EGFR are very rare in scc and seem to be present only in mixed adenosquamous carcinomas43. The epidermal growth factor receptor (egfr) tyrosine kinase inhibitors are usually the treatment of choice for patients with an activating EGFR mutation, mostly seen in adenocarcinoma44. These targeted agents have revolutionized the care of EGFR-positive lung cancer and are conventionally used first45,46.
However, egfr tyrosine kinase inhibitors have also been used in patients who progress after chemotherapy, regardless of tumour histology. Chemotherapy is usually more effective than erlotinib for second-line treatment in previously treated patients with nsclc who have EGFR wild-type tumours. In the tailor trial, docetaxel was superior to erlotinib in EGFR wild-type nsclc. Median os was 8.2 months compared with 5.4 months (p = 0.05), with a similar outcome for the scc subgroup (os hr: 0.57; 95% ci: 0.32 to 1.03)47. Nonetheless, in the Canadian-led br.21 trial, a survival benefit was shown for egfr inhibitors compared with placebo in an unselected population that included a squamous-cell subset48. The response rate to erlotinib was low at 8.9%, and yet pfs (2.2 months vs. 1.8 months, p < 0.001) and os (6.7 months vs. 4.7 months, p < 0.001) were better with erlotinib than with placebo. The benefit was seen mostly in adenocarcinoma patients; in the other histology subtypes, the survival difference was not significant (hr: 0.8; 95% ci: 0.6 to 1.0; p = 0.7).
Recently, the lux-Lung 8 trial compared afatinib, an irreversible ErbB receptor family blocker, with erlotinib as second-line treatment in patients with advanced scc of the lung after a platinum doublet49. The pfs (median: 2.6 months vs. 1.9 months; hr: 0.81; p = 0.0103) and os (median: 7.9 months vs. 6.8 months; hr: 0.81; p = 0.0077) were both improved in the afatinib group. The ErbB family of receptors, including egfr, is overexpressed in scc, which could explain the outcome benefit seen with afatinib50. Afatinib could be an option for fit patients who have progressed on several lines of treatment and who are desirous of further treatment.
Inhibitors of the egfr pathway also include monoclonal antibodies, of which the most studied are cetuximab and necitumumab. Their effect does not seem to be associated with an activating EGFR mutation, and so their use could be warranted in scc. Cetuximab, a recombinant monoclonal antibody against egfr, is not currently of use in nsclc. The flex trial randomized 1125 previously untreated patients to cisplatin–vinorelbine with or without cetuximab. No change in outcome was reported. In the scc subset (more than one third of the population), a trend toward benefit was observed for the combination arm (9 months vs. 8.2 months), but the difference did not reach statistical significance51.
Investigation into anti-egfr monoclonal antibody activity continued with necitumumab, a second-generation agent. The squire trial enrolled 1093 patients with scc. Patients were randomized to treatment with cisplatin–gemcitabine given with or without necitumumab (days 1 and 8 of a 21-day cycle). Up to 6 cycles of combination therapy were administered, with patients having the possibility of continuing on maintenance necitumumab if they achieved clinical benefit (51% received maintenance for a median of 4 cycles). Compared with chemotherapy alone, the addition of necitumumab was associated with a survival advantage (11.5 months vs. 9.9 months; hr: 0.84; 95% ci: 0.74 to 0.96; p = 0.01) and an improvement in the disease control rate (82% vs. 77%, p = 0.043)52.
The squire study was one of the first trials demonstrating a survival advantage for a new agent combined with doublet chemotherapy in a scc-only population. The fda and Health Canada approved necitumumab in combination with cisplatin–gemcitabine for the treatment of patients with metastatic squamous nsclc who have not received prior therapy for advanced disease53,54. The clinical significance of necitumumab seems incremental and modest. The survival benefit of 1.6 months comes with a significantly increased risk of toxicities, which include thromboembolism, hypomagnesemia, and severe skin reactions (grades 3–4 adverse events: 7%, 9%, and 7% respectively with necitumumab vs. 2%, 1%, and 1% with chemotherapy alone).
PRECISION MEDICINE WITH TARGETED THERAPY
Next-generation technologies have provided invaluable information about cancer genomics, including those for lung cancer. The Cancer Genome Atlas project has published a comprehensive genomic characterization of scc. They sequenced lung samples from 178 patients with scc and identified a potentially targetable gene or pathway alteration in most of the samples studied55. Alterations in the fibroblast growth factor receptor (fgfr) kinase family were common. Mutations in PIK3CA, PTEN, TP53, CDKN2A, NOTCH1 were also described. That study has opened the door to personalized medicine in scc56. Describing the multitude of genetic aberrations in scc is beyond the scope of this review; however, the subsections that follow discuss the mutations most often found in scc—among them, PIK3CA mutations, fgfr amplification, MET mutations, and the potential targets currently studied in clinical trials.
FGFR1
Amplification of fgfr1 occurs in approximately 20% of patients with scc of the lung57. The fgfr pathway plays a key role in signal transduction in lung cancer. Activation is responsible for igniting the pi3k/akt and Ras/mapk pathways that stimulate growth and angiogenesis in several cancers, including scc58. Amplification of fgfr is associated with smoking and confers a worse prognosis59,60.
Inhibitors of fgfr include nintedanib, pazopanib, and ponatinib, which target multiple tyrosine kinases61. Originally, fgfr was thought to be a very promising target because some inhibitors showed activity in preclinical models. However, clinical trials in lung cancer have shown only minimal activity of such inhibitors at the cost of increased toxicities, which include hypertension, gastrointestinal side effects, and rash62. For example, results with pazopanib have been disappointing. A clinical trial comparing maintenance pazopanib with placebo after platinum doublet chemotherapy in patients with nsclc was terminated at the futility interim analysis63.
The phase iii lume-Lung 1 study, in which nintedanib was combined with docetaxel, assessed the efficacy and safety of the combination as second-line therapy for nsclc without any molecular selection. In the nintedanib arm, os was improved only for the adenocarcinoma histology sub-group, but not for the overall cohort (median: 10.1 months vs. 9.1 months; hr: 0.94; 95% ci: 0.83 to 1.05; p = 0.2720)64. Studies with nintedanib (NCT01346540 and NCT01948141 at http://ClinicalTrials.gov) are continuing in a molecularly selected population with fgfr1 amplification. Other fgfr inhibitors are also in development and remain in the investigational phase65,66.
PI3K Pathway
Genetic alterations in the PI3K family include mutations in PIK3CA, amplification of akt3, and inactivation of PTEN. The incidence varies depending on trial reports, but genetic alterations of PI3K seem to present in approximately 50% of patients with scc of the lung. The pi3k pathway is believed to be critical to the signal transduction system regulating essential cellular functions including cell growth and proliferation67,68. Various trials have previously tested everolimus, an inhibitor of the mechanistic target of rapamycin (mtor) complex 1, with disappointing results69,70.
Several pi3k inhibitors are being actively developed, including isoform-specific and pan-isoform pi3kca inhibitors and dual pi3kca–mtor inhibitors. None has yet shown impressive results. For example, buparlisib, an oral inhibitor of class i pi3k was deemed futile in a phase ii study71. Trials investigating other selective pi3k inhibitors such as taselisib are in progress either as single agents or combined with chemotherapy.
c-Met
In nsclc, multiple mechanisms of MET activation have been described. In the recently published phase iii met-lung study, patients with nsclc were randomized to onartuzumab, an anti-met antibody, plus erlotinib or to erlotinib alone. Increased expression of the met protein was not associated with improved pfs or os in patients who received both agents72.
Although those results were disappointing, there is now renewed interest in MET exon 14 skipping mutation as a potential oncogenic driver. The deletion of the juxtamembrane domain of met leads to enhanced signalling through the met receptor pathway. To date, the deletion has been described in adenocarcinoma, in which 3%–4% of tumours harbour the mutation. New data suggest that the mutation could be also detected in scc, possibly in the adenosquamous variant. Patients with the mutation might respond to c-Met inhibitors. Clinically significant tumour responses have been reported with off-label use of anti-met tyrosine kinase inhibitors such as crizotinib or cabozantinib73,74.
Multiple trials are under way evaluating the efficacy of targeted agents in scc. The Lung-map study (NCT02154490 at http://ClinicalTrials.gov) is a lung cancer master protocol for patients with advanced scc after progression on first-line treatment75. It is based on genomic testing of tumours through a next-generation sequencing platform. This collaborative clinical trial, led by swog, with Canadian Cancer Trials Group participation, aims to match predictive biomarkers with targeted drugs. Each biomarker is a single-arm phase ii study in itself, and the substudies function independently. The primary objective for the studies is overall response rate, which could lead to a phase iii trial randomizing a targeted treatment compared with standard-of-care therapy. This initiative is innovative in that it assesses compatibility with various novel treatments, increasing the likelihood of patients receiving a targeted agent. Further, patients without any mutation are offered immunotherapy, including nivolumab alone or a combination of nivolumab and ipilimumab.
SUMMARY
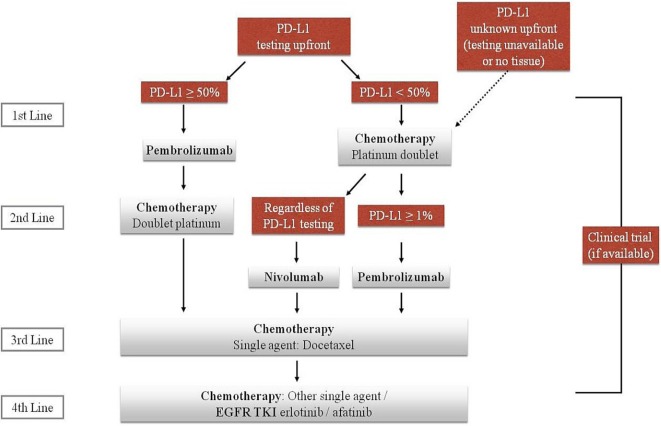
Squamous cell carcinoma is entering the era of precision medicine. Noteworthy developments during recent years are modifying the classical treatment algorithms that previously were based mostly on chemotherapy (Figure 1). Immunotherapy, in first or subsequent lines of treatment, has been a practice-changing development for patients without a driver mutation. Evidence from a randomized controlled trial now supports first-line treatment with pembrolizumab in patients with high PD-L1 expression. Nonetheless, it is accepted that patients who progress after doublet chemotherapy and who have not previously received immunotherapy are to be offered immunotherapy (for example, nivolumab) regardless of PD-L1 expression. Researchers are looking into the increased benefit such drugs bring and are broadening the spectrum of administration. Thus, multiple ongoing trials are testing combination immunotherapies with or without chemotherapy. Some improvement in outcome has also been seen with ramucirumab and necitumumab combined with chemotherapy, although clinical application of those agents has not yet been widely accepted in Canada because of modest efficacy, significant toxicity, and cost. Lastly, tumour molecular sequencing has described a multitude of new mutations in scc. Active research will probably provide more insight into the most targetable mutations and potentially effective agents. The discovery of newer treatments is definitely bringing hope to patients with advanced scc.
FIGURE 1.
The current algorithm for the treatment of small-cell lung cancer. EGFR TKI = epidermal growth factor receptor tyrosine kinase inhibitor.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: SAL reports honoraria from Boehringer Ingelheim, Roche, AstraZeneca, Pfizer, and UpToDate, and grant support from Pfizer in the form of an unrestricted educational grant. ND was the recipient of a Novartis Young Investigator Award in 2017. GN has no conflicts to disclose.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2016. Toronto, ON: Canadian Cancer Society; 2016. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014. Bethesda, MD: National Cancer Institute; 2017. [Available online at: https://seer.cancer.gov/csr/1975_2014; cited 30 April 2017] [Google Scholar]
- 3.Chemotherapy in non–small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials Non-Small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 4.NSCLC Meta-Analyses Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–25. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soon YY, Stockler MR, Askie MA, Boyer MJ. Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27:3277–83. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 6.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 7.Comella P, Filippelli G, De Cataldis G, et al. on behalf of the Southern Italy Cooperative Oncology Group Efficacy of the combination of cisplatin with either gemcitabine and vinorelbine or gemcitabine and paclitaxel in the treatment of locally advanced or metastatic non-small-cell lung cancer: a phase iii randomised trial of the Southern Italy Cooperative Oncology Group (sicog 0101) Ann Oncol. 2007;18:324–30. doi: 10.1093/annonc/mdl396. [DOI] [PubMed] [Google Scholar]
- 8.Bepler G, Williams C, Schell MJ, et al. Randomized international phase iii trial of ERCC1 and RRM1 expression–based chemotherapy versus gemcitabine/carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:2404–12. doi: 10.1200/JCO.2012.46.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SM, Falzon M, Blackhall F, et al. Randomized prospective biomarker trial of ercc1 for comparing platinum and non-platinum therapy in advanced non-small-cell lung cancer: ercc1 trial (et) J Clin Oncol. 2017;35:402–11. doi: 10.1200/JCO.2016.68.1841. [DOI] [PubMed] [Google Scholar]
- 10.Scagliotti GV, Parikh P, von Pawel J, et al. Phase iii study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 11.Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger rna and protein levels for thymidylate synthase. Cancer. 2006;107:1589–96. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 12.Fidias PM, Dakhil SR, Lyss AP, et al. Phase iii study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small cell lung cancer. J Clin Oncol. 2009;27:591–8. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 13.Brodowicz T, Krzakowski M, Zwitter M, et al. on behalf of the Central European Cooperative Oncology Group Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non–small cell lung cancer: a phase iii trial. Lung Cancer. 2006;52:155–63. doi: 10.1016/j.lungcan.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (paramount): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 15.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 16.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 17.Cicènas S, Geater SL, Petrov P, et al. Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (iuno study) Lung Cancer. 2016;102:30–7. doi: 10.1016/j.lungcan.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 18.United States, Department of Health and Human Services, Food and Drug Administration (fda) Erlotinib (Tarceva) [Web page] Silver Spring, MD: FDA; 2016. [Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm525739.htm; cited 30 April 2017] [Google Scholar]
- 19.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 21.Dancey J, Shepherd FA, Gralla RJ, Kim YS. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase iii trial. Lung Cancer. 2004;43:183–94. doi: 10.1016/j.lungcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Errico A. Immunotherapy: PD-1–PD-L1 axis: efficient checkpoint blockade against cancer. Nat Rev Clin Oncol. 2015;12:63. doi: 10.1038/nrclinonc.2014.221. [DOI] [PubMed] [Google Scholar]
- 23.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reck M, Rodriguez-Abreu D, Robinson AG, et al. on behalf of the keynote-024 investigators Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 25.Socinski M, Creelan B, Horn L, et al. CheckMate 026: a phase 3 trial of nivolumab vs investigator’s choice (ic) of platinum-based doublet chemotherapy (pt-dc) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1)–positive nsclc [abstract LBA7_PR] Ann Oncol. 2016;27(suppl 6) [Google Scholar]
- 26.Merck Canada . Keytruda: Pembrolizumab. Kirkland, QC: Merck Canada; 2017. [product monograph] [Available online at: https://pdf.hres.ca/dpd_pm/00040232.PDF; cited 1 December 2017] [Google Scholar]
- 27.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Non–Small Cell Lung Cancer. Fort Washington, PA: NCCN; 2017. Ver. 5.2017. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (free registration required); cited 30 April 2017] [Google Scholar]
- 28.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non-small-cell lung cancer (keynote-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 31.United States Department of Health and Human Services, Food and Drug Administration (fda) FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication [Web page] Silver Spring, MD: FDA; 2017. [Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm; cited 1 December 2017] [Google Scholar]
- 32.Scarpa A, Cataldo I, Salvatore L. Microsatellite Instability – Defective DNA Mismatch Repair: ESMO Biomarker Factsheet [Web page] Nice, France: European Society for Medical Oncology; 2017. [Available at: http://oncologypro.esmo.org/Education-Library/Factsheets-on-Biomarkers/Microsatellite-Instability-Defective-DNA-Mismatch-Repair#eztoc1701983_0_0_5_12; cited 1 December 2017] [Google Scholar]
- 33.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2016;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436–43. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris SJ, Brown J, Lopez J, Yap TA. Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med. 2016;13:171–93. doi: 10.20892/j.issn.2095-3941.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase ii trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 38.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase iii trial (avail) Ann Oncol. 2010;21:1804–9. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabchi S, Blais N. Antiangiogenesis for advanced non-small-cell lung cancer in the era of immunotherapy and personalized medicine. Front Oncol. 2017;7:52. doi: 10.3389/fonc.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage iv non-small-cell lung cancer after disease progression on platinum-based therapy (revel): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 41.United States, Department of Health and Human Services, Food and Drug Administration (fda) Cyramza (Ramucirumab) Silver Spring, MD: FDA; 2017. [prescribing information] [Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125477s011lbl.pdf; cited 30 April 2017] [Google Scholar]
- 42.U.K. National Institute for Health and Care Excellence (nice) Ramucirumab for Previously Treated Locally Advanced or Metastatic Non-Small-Cell Lung Cancer. London, UK: NICE; 2016. [appraisal consultation document] [Available online at: https://www.nice.org.uk/guidance/ta403/documents/appraisal-consultation-document; cited 1 December 2017] [Google Scholar]
- 43.Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res. 2012;18:1167–76. doi: 10.1158/1078-0432.CCR-11-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 45.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 46.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 47.Garassino MC, Martelli O, Broggini M, et al. on behalf of the tailor trialists Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (tailor): a randomised controlled trial. Lancet Oncol. 2013;14:981–8. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. on behalf of the National Cancer Institute of Canada Clinical Trials Group Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 49.Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (lux-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16:897–907. doi: 10.1016/S1470-2045(15)00006-6. [DOI] [PubMed] [Google Scholar]
- 50.Hirsh V. New developments in the treatment of advanced squamous cell lung cancer: focus on afatinib. Onco Targets Ther. 2017;10:2513–26. doi: 10.2147/OTT.S104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (flex): an open-label randomised phase iii trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 52.Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage iv squamous non-small-cell lung cancer (squire): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–74. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 53.United States, Department of Health and Human Services . FDA approves Portrazza to treat advanced squamous non-small cell lung cancer [news release] Silver Spring, MD: FDA; 2015. Food and Drug Administration (fda) [Available online at: https://wayback.archive-it.org/7993/20170111160748/ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm474131.htm; cited 8 February 2018] [Google Scholar]
- 54.Health Canada . Notice – Prescription Drug List (PDL): Multiple additions [Web page] Ottawa, ON: Health Canada; 2017. [Available at: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/pdl-ord/pdl-ldo-noa-ad-2017-04-21-eng.php; cited 30 April 2017] [Google Scholar]
- 55.Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. J Thorac Oncol. 2012;7:924–33. doi: 10.1097/JTO.0b013e31824cc334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heist RS, Mino-Kenudson M, Sequist LV, et al. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol. 2012;7:1775–80. doi: 10.1097/JTO.0b013e31826aed28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salgia R. Fibroblast growth factor signaling and inhibition in non–small cell lung cancer and their role in squamous cell tumors. Cancer Med. 2014;3:681–92. doi: 10.1002/cam4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Gao W, Xu J, et al. The role of FGFR1 gene amplification as a poor prognostic factor in squamous cell lung cancer: a meta-analysis of published data. Biomed Res Int. 2015;2015:763080. doi: 10.1155/2015/763080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang J, Liu X, Wang S, et al. Prognostic value of FGFR gene amplification in patients with different types of cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e105524. doi: 10.1371/journal.pone.0105524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soldera SV, Leighl NB. Update on the treatment of metastatic squamous non-small cell lung cancer in new era of personalized medicine. Front Oncol. 2017;7:50. doi: 10.3389/fonc.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent MD. Promising targets and current clinical trials in metastatic squamous cell lung cancer. Front Oncol. 2014;4:320. doi: 10.3389/fonc.2014.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Brien ME, Gaafar R, Hasan B, et al. on behalf of eortc-lcg Maintenance pazopanib versus placebo in non–small cell lung cancer patients non-progressive after first line chemotherapy: a double blind randomised phase iii study of the lung cancer group, eortc 08092 (eudract: 2010-018566-23, NCT01208064) Eur J Cancer. 2015;51:1511–28. doi: 10.1016/j.ejca.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 64.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (lume-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–55. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 65.Soria JC, DeBraud F, Bahleda R, et al. Phase i/iia study evaluating the safety, efficacy, pharmacokinetics, and pharmaco-dynamics of lucitanib in advanced solid tumors. Ann Oncol. 2014;25:2244–51. doi: 10.1093/annonc/mdu390. [DOI] [PubMed] [Google Scholar]
- 66.Lim SH, Sun JM, Choi YL, et al. Efficacy and safety of dovitinib in pretreated patients with advanced squamous non–small cell lung cancer with FGFR1 amplification: a single-arm, phase 2 study. Cancer. 2016;122:3024–31. doi: 10.1002/cncr.30135. [DOI] [PubMed] [Google Scholar]
- 67.Papadimitrakopoulou V. Development of pi3k/akt/mtor pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J Thorac Oncol. 2012;7:1315–26. doi: 10.1097/JTO.0b013e31825493eb. [DOI] [PubMed] [Google Scholar]
- 68.Spoerke JM, O’Brien C, Huw L, et al. Phosphoinositide 3-kinase (pi3k) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to pi3k inhibitors in lung cancer preclinical models. Clin Cancer Res. 2012;18:6771–83. doi: 10.1158/1078-0432.CCR-12-2347. [DOI] [PubMed] [Google Scholar]
- 69.Ramalingam SS, Owonikoko TK, Behera M, et al. Phase ii study of docetaxel in combination with everolimus for second-or third-line therapy of advanced non-small-cell lung cancer. J Thorac Oncol. 2013;8:369–72. doi: 10.1097/JTO.0b013e318282709c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Besse B, Leighl N, Bennouna J, et al. Phase ii study of everolimus–erlotinib in previously treated patients with advanced non-small-cell lung cancer. Ann Oncol. 2014;25:409–15. doi: 10.1093/annonc/mdt536. [DOI] [PubMed] [Google Scholar]
- 71.Vansteenkiste JF, Canon JL, De Braud F, et al. Safety and efficacy of buparlisib (BKM120) in patients with pi3k pathway-activated non–small cell lung cancer: results from the phase ii basalt-1 study. J Thorac Oncol. 2015;10:1319–27. doi: 10.1097/JTO.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spigel DR, Edelman MJ, O’Byrne K, et al. Results from the phase iii randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage iiib or iv non-small-cell lung cancer: metlung. J Clin Oncol. 2017;35:412–20. doi: 10.1200/JCO.2016.69.2160. [DOI] [PubMed] [Google Scholar]
- 73.Camidge RD, Ou SHI, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET–amplified non–small cell lung cancer [abstract 8001] J Clin Oncol. 2014;32 [Available online at: http://ascopubs.org/doi/abs/10.1200/jco.2014.32.15_suppl.8001; cited 28 January 2018] [Google Scholar]
- 74.Paik PK, Drilon A, Fan P, et al. Response to met inhibitors in patients with stage iv lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5:842–9. doi: 10.1158/2159-8290.CD-14-1467. [Erratum in: Cancer Discov 2016;6:330] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herbst RS, Gandara DR, Hirsch FR, et al. Lung master protocol (Lung-map)—a biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: swog S1400. Clin Cancer Res. 2015;21:1514–24. doi: 10.1158/1078-0432.CCR-13-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]