Abstract
Angiogenesis is frequent in non-small-cell lung cancer (nsclc) and is associated with more aggressive disease. Many clinical trials have evaluated the addition of antiangiogenic therapy to standard therapies for patients with nsclc. Bevacizumab, a monoclonal antibody directed against serum vascular endothelial growth factor, in combination with carboplatin–paclitaxel chemotherapy, has been shown to improve survival for patients with nsclc. However, bevacizumab-based therapy is not suitable for many nsclc patients, including those with squamous histology, poor performance status, brain metastases, and the presence of bleeding or thrombotic disorders. Similar efficacy has also been seen with carboplatin–pemetrexed followed by maintenance pemetrexed chemotherapy. In the second-line setting, the addition of ramucirumab to docetaxel—or the addition of bevacizumab to paclitaxel—has resulted in a modest improvement in efficacy, although the clinical importance of those findings is questionable. Many trials in nsclc have also evaluated oral antiangiogenic compounds, both in the first line in combination with chemotherapy and upon disease progression either as combination or single-agent therapy. No clear improvements in overall survival have been observed, although a subgroup analysis of a trial evaluating the addition of nintedanib to docetaxel showed improved survival that was limited to patients with adenocarcinoma. Those findings require validation, however.
All of the oral antiangiogenic agents result in added toxicities. Some agents have resulted in an increased risk of death, limiting their development. Available evidence supports a limited number of antiangiogenic therapies for patients with nsclc, but no biomarkers to help in patient selection are currently available, and additional translational research is needed to identify predictive biomarkers for antiangiogenic therapy.
Keywords: Non-small-cell lung cancer, antiangiogenic therapy, monoclonal antibodies, tyrosine kinase inhibitors, overall survival, progression-free survival
INTRODUCTION
Angiogenesis—the formation of new blood vessels from pre-existing vessels1—is observed in many different cancers, including lung cancer2. The process depends on many activating and inhibiting factors that regulate angiogenesis and potentially influence the aggressiveness of cancer3. The most important factors associated with angiogenesis include vascular endothelial growth factor (vegf)4,5, platelet-derived growth factor6, and fibroblast-derived growth factor7. Vascular endothelial cells depend for their survival on serum vegf, which stimulates proliferation and migration, inhibits apoptosis, and modulates endothelial permeability8. Antiangiogenic therapy aims to disrupt those processes by normalizing the abnormal vasculature in cancer, improving delivery of chemotherapy, enhancing its anti-vascular effect, and preventing rapid repopulation after systemic treatment.
APPROVED ANTIANGIOGENIC AGENTS IN FIRST-LINE THERAPY OF NON-SMALL-CELL LUNG CANCER
Bevacizumab
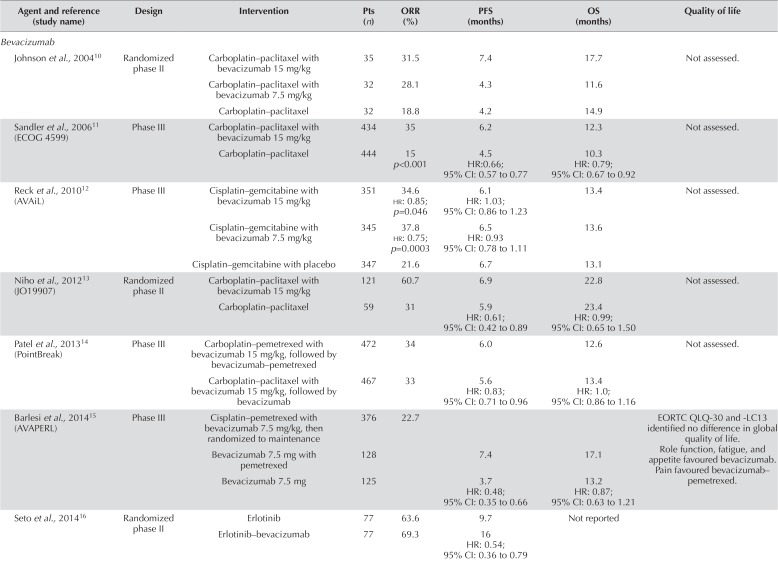
Bevacizumab is a humanized monoclonal antibody that binds serum vegf, preventing its binding to and activation of vegf receptor 29. Multiple trials (Table i) have evaluated bevacizumab in combination with platinum-based chemotherapy as initial therapy for advanced and metastatic non-small-cell lung cancer (nsclc).
TABLE I.
Summary of trials evaluating intravenous antiangiogenic therapies in first-line systemic therapy for non-small-cell lung cancer
| Agent and reference (study name) | Design | Intervention | Pts (n) | ORR (%) | PFS (months) | OS (months) | Quality of life |
|---|---|---|---|---|---|---|---|
| Bevacizumab | |||||||
| Johnson et al., 200410 | Randomized phase II |
Carboplatin–paclitaxel with bevacizumab 15 mg/kg | 35 | 31.5 | 7.4 | 17.7 | Not assessed. |
| Carboplatin–paclitaxel with bevacizumab 7.5 mg/kg | 32 | 28.1 | 4.3 | 11.6 | |||
| Carboplatin–paclitaxel | 32 | 18.8 | 4.2 | 14.9 | |||
| Sandler et al., 200611 (ECOG 4599) | Phase III | Carboplatin–paclitaxel with bevacizumab 15 mg/kg | 434 | 35 | 6.2 | 12.3 | Not assessed. |
| Carboplatin–paclitaxel | 444 | 15 p<0.001 | 4.5 HR:0.66; 95% CI: 0.57 to 0.77 |
10.3 HR: 0.79; 95% CI: 0.67 to 0.92 |
|||
| Reck et al., 201012 (AVAiL) | Phase III | Cisplatin–gemcitabine with bevacizumab 15 mg/kg | 351 | 34.6 hr: 0.85; p=0.046 |
6.1 HR: 1.03; 95% CI: 0.86 to 1.23 |
13.4 | Not assessed. |
| Cisplatin–gemcitabine with bevacizumab 7.5 mg/kg | 345 | 37.8 hr: 0.75; p=0.0003 |
6.5 HR: 0.93 95% CI: 0.78 to 1.11 |
13.6 | |||
| Cisplatin–gemcitabine with placebo | 347 | 21.6 | 6.7 | 13.1 | |||
| Niho et al., 201213 (JO19907) | Randomized phase II |
Carboplatin–paclitaxel with bevacizumab 15 mg/kg | 121 | 60.7 | 6.9 | 22.8 | Not assessed. |
| Carboplatin–paclitaxel | 59 | 31 | 5.9 HR: 0.61; 95% CI: 0.42 to 0.89 |
23.4 HR: 0.99; 95% CI: 0.65 to 1.50 |
|||
| Patel et al., 201314 (PointBreak) | Phase III | Carboplatin–pemetrexed with bevacizumab 15 mg/kg, followed by bevacizumab–pemetrexed | 472 | 34 | 6.0 | 12.6 | Not assessed. |
| Carboplatin–paclitaxel with bevacizumab 15 mg/kg, followed by bevacizumab | 467 | 33 | 5.6 HR: 0.83; 95% CI: 0.71 to 0.96 |
13.4 HR: 1.0; 95% CI: 0.86 to 1.16 |
|||
| Barlesi et al., 201415 (AVAPERL) | Phase III | Cisplatin–pemetrexed with bevacizumab 7.5 mg/kg, then randomized to maintenance | 376 | 22.7 | EORTC QLQ-30 and -LC13 identified no difference in global quality of life. Role function, fatigue, and appetite favoured bevacizumab. Pain favoured bevacizumab–pemetrexed. |
||
| Bevacizumab 7.5 mg with pemetrexed | 128 | 7.4 | 17.1 | ||||
| Bevacizumab 7.5 mg | 125 | 3.7 HR: 0.48; 95% CI: 0.35 to 0.66 |
13.2 HR: 0.87; 95% CI: 0.63 to 1.21 |
||||
| Seto et al., 201416 | Randomized phase II |
Erlotinib | 77 | 63.6 | 9.7 | Not reported | |
| Erlotinib–bevacizumab | 77 | 69.3 | 16 HR: 0.54; 95% CI: 0.36 to 0.79 |
||||
| Zhou et al., 201517 (BEYOND) | Phase III | Carboplatin–paclitaxel with bevacizumab 15 mg/kg | 138 | 54 | 9.2 | 24.3 | Not assessed. |
| Carboplatin–paclitaxel with placebo | 138 | 26 | 6.5 HR: 0.40; 95% CI: 0.29 to 0.54 |
17.7 HR: 0.68; 95% CI: 0.50 to 0.93 |
|||
| Zinner et al., 201518 (PRONOUNCE) | Phase III | Carboplatin–pemetrexed, followed by pemetrexed | 182 | 23.6 | 3.9a | 10.5 | Not assessed. |
| Carboplatin–paclitaxel with bevacizumab 15 mg/kg, followed by bevacizumab | 179 | 27.4 | 2.9a HR: 0.85; 90% CI: 0.70 to 1.04 | 11.7 HR: 1.07; 95% CI: 0.83 to 1.36 |
|||
| Ramucirumab | |||||||
| Camidge et al., 201419 | Phase II | Carboplatin–paclitaxel with ramucirumab | 41 | 55 | 7.8 95% CI: 5.5 to 9.86 |
16.8 95% CI: 14.8 to 28.6 |
Not assessed. |
| Doebele et al., 201520 | Randomized phase II |
Cisplatin– or carboplatin–pemetrexed with ramucirumab 10 mg/kg, followed by pemetrexed–ramucirumab | 69 | 49.3 | 7.2 HR: 0.75; 90% CI: 0.55 to 1.03 |
13.9 HR: 0.83; 95% CI: 0.56 to 1.22 |
Not assessed. |
| Cisplatin– or carboplatin–pemetrexed followed by pemetrexed | 71 | 38 | 5.6 | 10.4 | |||
| Aflibercept | |||||||
| Chen et al., 201421 | Phase II | Cisplatin–pemetrexed–aflibercept | 42 | 26.3 | 5 95% CI: 4.3 to 7.1 |
NR | Trial stopped early because of 3 cases of reversible posterior leucoencephalopathy syndrome. |
Progression-free survival with no grade 4 toxicity.
Pts = patients; ORR = objective response rate; PFS = progression-free survival; OS = overall survival; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio; CI = confidence interval; EORTC = European Organisation for Research and Treatment of Cancer; QLQ-30 = Quality of Life Questionnaire; LC13 = Lung Cancer module.
The tolerability of bevacizumab in combination with chemotherapy in all subtypes of nsclc was established in a phase i trial22. However, a subsequent randomized phase ii trial of carboplatin–paclitaxel, with or without bevacizumab, in advanced and untreated nsclc, demonstrated significant toxicities associated with the use of bevacizumab10. Eight patients treated with chemotherapy and bevacizumab died because of adverse effects, including hemorrhage of unknown origin, hemoptysis, liver failure, aspiration pneumonia, Aspergillus lung abscess, and chronic obstructive pulmonary disease. Six patients experienced major bleeding, with four fatalities. Major hemoptysis was associated with squamous-cell histology, and subsequent clinical trials of bevacizumab in nsclc included only patients with nonsquamous histology, good performance status, and no history of thrombosis, bleeding, gross hemoptysis, or brain metastasis.
The efficacy of bevacizumab in combination with chemotherapy in nsclc was first demonstrated in the Eastern Cooperative Oncology Group (ecog) 4599 trial11. Patients were randomized to carboplatin–paclitaxel either alone or with bevacizumab 15 mg/kg every 21 days. Overall survival (os) was improved for patients randomized to carboplatin–paclitaxel with bevacizumab [12.3 months vs. 10.3 months; hazard ratio (hr): 0.79; 95% confidence interval (ci): 0.67 to 0.92]. Significant improvements were also observed in the overall response rate (orr) and progression-free survival (pfs).
However, conflicting information about os has been observed in three other first-line trials evaluating the addition of bevacizumab to platinum-based chemotherapy in advanced nsclc12,13,17. Longer pfs was seen in the avail trial comparing cisplatin–gemcitabine with or without bevacizumab 7.5 mg/kg or 15 mg/kg. The final analysis failed to demonstrate any significant improvement in os for either dose of bevacizumab in combination with cisplatin–gemcitabine. The adverse effect profile in the avail trial was similar to that in ecog 4599. The addition of bevacizumab to chemotherapy is associated with significant, increased risks of neutropenia, febrile neutropenia, hyponatremia, hypertension, proteinuria, headache, rash, and bleeding events.
Additional trials have evaluated platinum-based chemotherapy plus bevacizumab compared with other contemporary first-line treatments for advanced nsclc14,15. The randomized phase iii PointBreak trial compared two chemotherapy backbones: carboplatin–pemetrexed followed by maintenance pemetrexed, and carboplatin–paclitaxel, both combined with bevacizumab 15 mg/kg14. In the pemetrexed arm, pfs was significantly prolonged (6.0 months vs. 5.6 months; hr: 0.83; 95% ci: 0.71 to 0.96), but that difference did not translate into any improvement in os. Related grades 3 and 4 neutropenia, febrile neutropenia, sensory neuropathy, and alopecia were significantly lower in the pemetrexed arm.
The strategy of maintenance pemetrexed was also evaluated in the avaperl trial15. Patients with nonsquamous nsclc were randomized to bevacizumab maintenance, with or without pemetrexed, after first-line bevacizumab–cisplatin–pemetrexed. Prolonged pfs was observed with maintenance pemetrexed and bevacizumab (7.4 months vs. 3.7 months; hr: 0.48; 95% ci: 0.35 to 0.66), but no significant difference in os was seen (17.1 months vs. 13.2 months; hr: 0.87; 95% ci: 0.63 to 1.21). Currently, there is no clear value to the addition of maintenance pemetrexed to bevacizumab-based therapy.
The safety and efficacy of carboplatin–paclitaxel plus bevacizumab was also evaluated against a non-bevacizumab regimen of carboplatin–pemetrexed followed by maintenance pemetrexed (pronounce trial)18. No superiority was achieved in the primary endpoint of pfs without grade 4 toxicity or in the secondary outcomes of pfs, os, orr, or disease control rate.
One randomized phase ii trial evaluated the addition of bevacizumab to erlotinib in Japanese patients with EGFR mutation–positive nsclc16. A significant improvement in pfs was seen, but os data have not been reported. Randomized trials to validate those findings are ongoing, and at present, bevacizumab should not routinely be added to an egfr tyrosine kinase inhibitor (tki) in this population.
Examining the body of evidence, the addition of bevacizumab to carboplatin–paclitaxel chemotherapy improves os23. However, the benefits are modest, and the incremental toxicities are significant. Convincing evidence to support the addition of bevacizumab to other platinum-based chemotherapy is lacking. Decisions about treatment should reflect not only efficacy and toxicity, but also competing treatment strategies. Based on data from the pronounce trial, treatment with carboplatin–pemetrexed followed by maintenance pemetrexed represents an alternative to carboplatin–paclitaxel and bevacizumab, with similar efficacy and likely improved toxicity18.
OTHER ANTIANGIOGENIC AGENTS EVALUATED IN FIRST-LINE THERAPY FOR NSCLC
Monoclonal Antibodies
Other monoclonal antibodies have been evaluated in combination with platinum-based chemotherapy as first-line therapy for advanced nsclc (Table i).
Ramucirumab is a human immunoglobulin G monoclonal antibody that specifically binds to the extracellular domain of vegf receptor 2, blocking binding of the vegf ligand to that receptor24. Promising data were observed in a single-arm phase ii trial of ramucirumab in combination with carboplatin–paclitaxel19. A randomized phase ii trial compared the addition of ramucirumab to pemetrexed–cisplatin or pemetrexed–carboplatin, followed by maintenance pemetrexed, with chemotherapy alone20. The trial did not meet its primary endpoint of improved pfs. However, the observed pfs (7.2 months vs. 5.6 months) and orr (49% vs. 38%) both favoured the addition of ramucirumab. The most common adverse events were thrombocytopenia, neutropenia, fatigue, anemia, nausea, back pain, and hypertension.
Aflibercept is a recombinant fusion protein, consisting of the extracellular domain of the human vegf receptor (vegfr), fused to the hinge region of the human immunoglobulin G1 Fc domain25. Commonly called “vegf trap,” it binds all isoforms of serum vegf, vegf-b, and human placental growth factor. The addition of aflibercept to cisplatin–pemetrexed did not appear to improve efficacy in a trial of untreated patients with nsclc21. The trial was closed early because of 3 confirmed cases of reversible posterior leucoencephalopathy syndrome.
Neither ramucirumab or aflibercept are used in the first-line management of nsclc.
TKIs
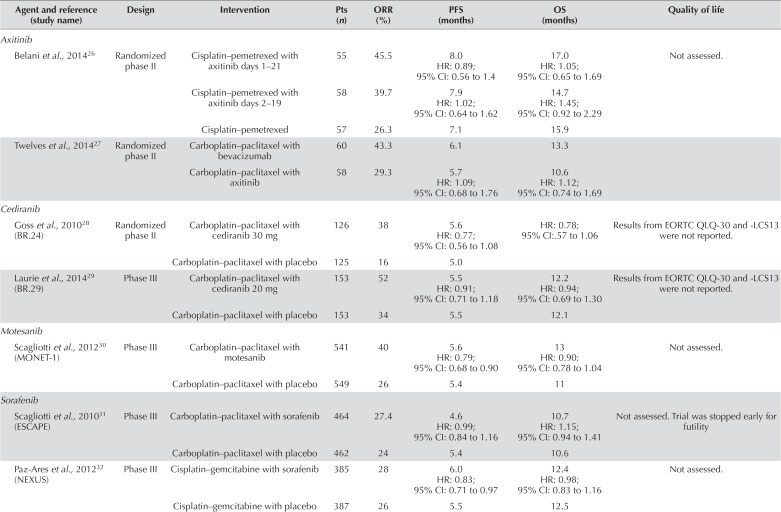
Multiple trials in patients with advanced nsclc have evaluated the addition of oral tkis with antiangiogenic activity to platinum-based chemotherapy (Table ii). However, no such tki has demonstrated a clear improvement in efficacy, and many have added considerable toxicity to standard platinum-based chemotherapy regimens. Axitinib, an oral potent and selective vegfr tki showed preclinical antitumour efficacy in combination with chemotherapy agents in multiple tumour models34. A randomized phase ii trial evaluated cisplatin–pemetrexed alone or in combination with two dose schedules of axitinib26. Patients in both axitinib arms experienced a higher response rate, but no significant difference in pfs or os. A second randomized phase ii trial compared carboplatin–paclitaxel plus bevacizumab with carboplatin–paclitaxel plus axitinib27. There was no statistically significant difference in efficacy between the treatments, and axitinib was less well-tolerated, resulting in more temporary discontinuations and dose reductions because of side effects. Axitinib remains investigational as a treatment for nsclc.
TABLE II.
Summary of trials evaluating oral antiangiogenic agents in first-line systemic therapy for non-small-cell lung cancer
| Agent and reference (study name) | Design | Intervention | Pts (n) | ORR (%) | PFS (months) | OS (months) | Quality of life |
|---|---|---|---|---|---|---|---|
| Axitinib | |||||||
| Belani et al.,201426 | Randomized phase II |
Cisplatin–pemetrexed with axitinib days 1–21 | 55 | 45.5 | 8.0 HR: 0.89; 95% CI: 0.56 to 1.4 |
17.0 HR: 1.05; 95% CI: 0.65 to 1.69 |
Not assessed. |
| Cisplatin–pemetrexed with axitinib days 2–19 | 58 | 39.7 | 7.9 HR: 1.02; 95% CI: 0.64 to 1.62 |
14.7 HR: 1.45; 95% CI: 0.92 to 2.29 |
|||
| Cisplatin–pemetrexed | 57 | 26.3 | 7.1 | 15.9 | |||
| Twelves et al.,201427 | Randomized phase II |
Carboplatin–paclitaxel with bevacizumab | 60 | 43.3 | 6.1 | 13.3 | |
| Carboplatin–paclitaxel with axitinib | 58 | 29.3 | 5.7 HR: 1.09; 95% CI: 0.68 to 1.76 |
10.6 HR: 1.12; 95% CI: 0.74 to 1.69 |
|||
| Cediranib | |||||||
| Goss et al.,201028 (BR.24) | Randomized phase II |
Carboplatin–paclitaxel with cediranib 30 mg | 126 | 38 | 5.6 HR: 0.77; 95% CI: 0.56 to 1.08 |
HR: 0.78; 95% CI:.57 to 1.06 | Results from EORTC QLQ-30 and -LCS13 were not reported. |
| Carboplatin–paclitaxel with placebo | 125 | 16 | 5.0 | ||||
| Laurie et al.,201429 (BR.29) | Phase III | Carboplatin–paclitaxel with cediranib 20 mg | 153 | 52 | 5.5 HR: 0.91; 95% CI: 0.71 to 1.18 |
12.2 HR: 0.94; 95% CI: 0.69 to 1.30 |
Results from EORTC QLQ-30 and -LCS13 were not reported. |
| Carboplatin–paclitaxel with placebo | 153 | 34 | 5.5 | 12.1 | |||
| Motesanib | |||||||
| Scagliotti et al.,201230 (MONET-1) | Phase III | Carboplatin–paclitaxel with motesanib | 541 | 40 | 5.6 HR: 0.79; 95% CI: 0.68 to 0.90 |
13 HR: 0.90; 95% CI: 0.78 to 1.04 |
Not assessed. |
| Carboplatin–paclitaxel with placebo | 549 | 26 | 5.4 | 11 | |||
| Sorafenib | |||||||
| Scagliotti et al.,201031 (ESCAPE) | Phase III | Carboplatin–paclitaxel with sorafenib | 464 | 27.4 | 4.6 HR: 0.99; 95% CI: 0.84 to 1.16 |
10.7 HR: 1.15; 95% CI: 0.94 to 1.41 |
Not assessed. Trial was stopped early for futility |
| Carboplatin–paclitaxel with placebo | 462 | 24 | 5.4 | 10.6 | |||
| Paz-Ares et al.,201232 (NEXUS) | Phase III | Cisplatin–gemcitabine with sorafenib | 385 | 28 | 6.0 HR: 0.83; 95% CI: 0.71 to 0.97 |
12.4 HR: 0.98; 95% CI: 0.83 to 1.16 |
Not assessed. |
| Cisplatin–gemcitabine with placebo | 387 | 26 | 5.5 | 12.5 | |||
| Vandetanib | |||||||
| Heymach et al.,200833 | Randomized phase II |
Carboplatin–paclitaxel with vandetanib | 56 | 32 | 24 Weeks HR: 0.76; 95% CI: 0.51 to 1.14 |
10.2 HR: 1.15; 95% CI: 0.75 to 1.77 |
Not assessed. |
| Vandetanib | 73 | 7 | 11.5 Weeks HR: 1.26; 95% CI: 0.83 to 1.91 |
10.2 HR: 1.09; 95% CI: 0.70 to 1.72 |
|||
| Carboplatin–paclitaxel | 52 | 25 | 23 Weeks | 12.6 | |||
Pts = patients; ORR = objective response rate; PFS = progression-free survival; OS = overall survival; HR = hazard ratio; CI = confidence interval; EORTC = European Organisation for Research and Treatment of Cancer; QLQ-30 = Quality of Life Questionnaire; LC13 = Lung Cancer module.
Similar findings have been observed with other oral vegf inhibitors such as cediranib and motesanib. Despite promising data from phase i trials, the addition of cediranib (30–45 mg daily) to carboplatin–paclitaxel resulted in considerably increased toxicity necessitating dose reductions28,29. Some increased activity was observed at higher doses, but cediranib 20 mg daily failed to improve pfs or os29. The addition of motesanib to carboplatin–paclitaxel resulted in an increased risk of death and serious hemoptysis for patients with squamous histology30. The final analysis conducted in patients with nonsquamous histology showed improvements in pfs and orr for patients randomized to chemotherapy plus motesanib. However, those improvements did not translate to improvement in os. In the trial, increased toxicity was associated with motesanib, including higher incidences of neutropenia, gastrointestinal events, hypertension, pneumonia, cholecystitis, and gallbladder-related disorders.
Several multi-targeted tkis have been evaluated in combination with first-line platinum-based chemotherapy. Sorafenib, a potent inhibitor of several receptor tyrosine kinases—including braf, vegfr-2, vegfr-3, pdgfr, c-Kit, and Flt-3—has been evaluated in two large randomized trials. The escape trial evaluated the addition of sorafenib to carboplatin–paclitaxel31. Patients with disease of all histologic subtypes were included. The trial, which was stopped early for futility, failed to show any improvement in os (10.7 months vs. 10.6 months; hr: 1.15; 95% ci: 0.94 to 1.41). A subgroup analysis showed a shorter median os in patients with squamous-cell carcinoma randomized to sorafenib plus chemotherapy compared with chemotherapy alone (8.9 months vs. 13.9 months; hr: 1.85; 95% ci: 1.22 to 2.81). The incidence of drug-related adverse events was higher in patients receiving sorafenib. The most common adverse events were thrombocytopenia, rash, desquamation, hand–foot syndrome, pruritus, and hypertension. Similarly, in the nexus trial (limited to patients with nonsquamous nsclc), there was no improvement in the primary endpoint of os32. The toxicity profile was similar to that seen in the escape trial.
Trials evaluating vandetanib, a dual vegfr and egfr tki, have also failed to improve treatment outcomes for patients with advanced nsclc33. A randomized phase ii trial of vandetanib alone or carboplatin–paclitaxel with or without vandetanib did not show an improvement in os. The pfs for carboplatin–paclitaxel alone or in combination with vandetanib was similar, and vandetanib monotherapy was inferior to carboplatin–paclitaxel.
It is unclear why, in comparison with bevacizumab, which has resulted in modest improvements in os, oral antiangiogenic agents have not improved treatment efficacy in advanced nsclc. Improvements in some measures of efficacy, such as orr and pfs, have been observed with some agents. However, increased toxicities have often precluded the administration of those agents at full dose. Currently, no approved oral antiangiogenic agents have been recommended as first-line therapy in advanced nsclc.
APPROVED ANTIANGIOGENIC AGENTS AFTER PROGRESSION ON PLATINUM-BASED THERAPY
Antiangiogenic therapies have been extensively evaluated, either alone or in combination with other systemic therapies, in patients with nsclc progressing after initial platinum-based therapy. Monoclonal antibodies including bevacizumab and ramucirumab, as well as receptor tkis, have demonstrated some activity in this setting, although clinical practice has not been extensively modified at this time.
Bevacizumab
Multiple trials have evaluated bevacizumab in the second-line setting (Table iii). The phase iii ultimate trial randomized 166 patients with advanced nsclc progressing after first-line or second-line therapy 2:1 to weekly paclitaxel (90 kg/m2) on days 1, 8, and 15 in combination with bevacizumab 10 mg/kg on days 1 and 15 every 4 weeks or to intravenous docetaxel monotherapy 75 mg/m2 on a 3-week cycle37. Median follow-up was 28.9 months. The primary outcome, pfs, was significantly improved for patients randomized to paclitaxel–bevacizumab compared with those randomized to docetaxel (5.4 months vs. 3.9 months; hr: 0.62; 95% ci: 0.44 to 0.87). Furthermore, a significant improvement in the orr in favour of the combination therapy was noted (22.5% vs. 5.5%, p = 0.006). However, no differences in os were observed between the two groups (9.9 months vs. 11.4 months; hr: 1.18; 95% ci: 0.81 to 1.72). Weekly paclitaxel–bevacizumab was associated with less neutropenia, but more neuropathy and hypertension. Although the primary outcome was significantly improved in this study, the lack of improvement in os limits the clinical importance of these data.
TABLE III.
Intravenous antiangiogenic therapy in non-small-cell lung cancer after disease progression
| Agent and reference (study) | Design | Intervention | Pts (n) | ORR (%) | PFS (months) | OS (months) | Quality of life |
|---|---|---|---|---|---|---|---|
| Bevacizumab | |||||||
| Herbst et al.,200735 | Randomized phase II |
Docetaxel–pemetrexed with bevacizumab 15 mg/kg | 40 | 12.5 | 4.8 HR: 0.66; 95% CI: 0.38 to 1.16 |
12.6 HR: 0.71; 95% CI: 0.41 to 1.21 |
Not assessed. |
| Erlotinib with bevacizumab 15 mg/kg | 39 | 17.9 | 4.4 HR: 0.72; 95% CI: 0.42 to 1.23 |
13.7 HR: 0.78; 95% CI: 0.46 to 1.31 |
|||
| Docetaxel–pemetrexed with placebo | 41 | 12.2 | 3.0 | 8.6 | |||
| Herbst et al.,201136 (BeTa) | Phase III | Erlotinib with bevacizumab 15 mg/kg | 319 | 13 | 3.4 | 9.3 | Not assessed. |
| Erlotinib with placebo | 317 | 6 | 1.7 HR: 0.62; 95% CI: 0.52 to 0.75 |
9.2 HR: 0.97; 95% CI: 0.80 to 1.18 |
|||
| Cortot et al.,201637 (ULTIMATE) | Randomized phase III |
Paclitaxel (weekly) with bevacizumab 10 mg/kg | 111 | 22.5 | 5.4 | 9.9 | Not assessed. |
| Docetaxel | 55 | 5.5 p=0.006 |
3.9 HR: 0.62; 95% CI: 0.44 to 0.87 |
11.4 HR: 1.15; p=0.49 |
|||
| Bennouna et al.,201740 (AVaALL) | Phase III | Chemotherapy with bevacizumab | 485 in total | 9.7 | 4.9 HR: 0.85; 90% CI: 0.72 to 1.0 |
11.9 HR: 0.84; 90% CI: 0.71 to 1.0 |
|
| Chemotherapy alone | 6.7 | 3.8 | 10.2 | ||||
| Garon et al.,201438 (REVEL) | Phase III | Docetaxel with ramucirumab | 628 | 23 | 4.5 | 10.5 | |
| Docetaxel with placebo | 625 | 14 | 3.0 HR: 0.76; 95% CI: 0.68 to 0.86 |
9.1 HR: 0.86; 95% CI: 0.75 to 0.98 |
|||
| Aflibercept | |||||||
| Ramlau et al.,201239 (VITAL) | Phase III | Docetaxel with aflibercept | 456 | 23.3 | 5.2 HR: 0.82; 95% CI: 0.72 to 0.94 |
10.1 HR: 1.01; 95% CI: 0.87 to 1.17 |
No differences observed using the Lung Cancer Symptom Scale. |
| Docetaxel with placebo | 457 | 8.9 | 4.1 | 10.4 |
Pts = patients; ORR = objective response rate; PFS = progression-free survival; OS = overall survival; HR = hazard ratio; CI = confidence interval.
Additional trials examined bevacizumab in combination with erlotinib or with chemotherapy. Herbst et al. randomized patients to chemotherapy alone or to chemotherapy or erlotinib in combination with bevacizumab35. The addition of bevacizumab resulted in numerically higher orr, pfs, and os—findings that were not confirmed in a phase iii trial. The beta trial compared erlotinib and bevacizumab 15 mg/kg with erlotinib and placebo after progression on first-line therapy36. The primary endpoint in the study, os, was not significantly improved (9.3 months vs. 9.2 months; hr: 0.97; 95% ci: 0.80 to 1.18). However, significant improvements were observed in the secondary endpoints of pfs (3.4 months vs. 1.7 months) and orr (13% vs. 6%). Based on that evidence, the data are insufficient to recommend the combination of erlotinib and bevacizumab.
The role of bevacizumab in second-line therapy for patients who received bevacizumab in the first-line setting is also unclear. The avaall trial randomized patients with nsclc progressing on first-line platinum doublet and bevacizumab therapy to continue bevacizumab with chemotherapy or to receive chemotherapy alone40. The results of the trial were presented at the 2017 American Society of Clinical Oncology annual meeting. Patients randomized to continue bevacizumab experienced longer os; however, the difference was not statistically significant (11.9 months vs. 10.2 months; hr: 0.84; 90% ci: 0.71 to 1.0). Similarly, no significant increase in pfs was observed.
Much as in first-line therapy, the addition of bevacizumab in second-line therapy was associated with an improvement in the intermediate outcomes of pfs and orr. Why those improvements fail to translate into an improvement in os is unclear. One argument is that significant crossover to bevacizumab occurred in the ultimate trial. Although the reported gains in efficacy could be important to some physicians and patients, they are generally not favourable from a cost-effectiveness standpoint. Second-line therapy with bevacizumab has not affected practice in the Canadian environment.
Ramucirumab
The revel trial randomized 1253 patients (all nsclc histologies) to docetaxel 75 mg/m2 plus ramucirumab 10 mg/kg or to docetaxel 75 mg/m2 plus placebo38. The addition of ramucirumab to docetaxel was associated with a significant improvement in pfs (4.5 months vs. 3.0 months; hr: 0.76; 95% ci: 0.68 to 0.86) and in os (10.5 months vs. 9.1 months; hr: 0.86; 95% ci: 0.75 to 0.98). The improvement in os was observed for all histologic subtypes. Compared with patients receiving docetaxel alone, those receiving the combination therapy experienced more grade 3 or greater adverse events (49% vs. 40%). The increase in toxicities included febrile neutropenia (16% vs. 10%), neutropenia (49% vs. 40%), leucopenia (14% vs. 12%), and hypertension (6% vs. 2%). However, death from adverse events did not differ between the groups (5% vs. 6%). Although ramucirumab has been approved by the U.S. Food and Drug Administration, the improvement in os is modest, such that a consideration of cost-effectiveness is important in the decision to give it. Alternative treatment strategies with immune checkpoint inhibitors likely offer greater benefit and should also be considered for this population41.
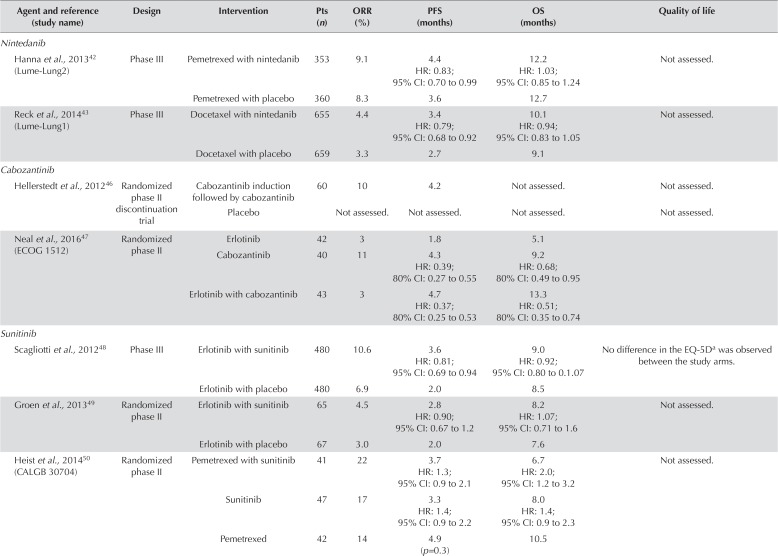
Nintedanib
Nintedanib is a multi-targeted antiangiogenic agent with sites of inhibition including vegfr, platelet-derived growth factor receptor, and fibroblast-derived growth factor receptor. It has been evaluated in combination with docetaxel or pemetrexed in two large phase iii randomized trials42,43. The first of those trials, lume-Lung 2, was conducted exclusively in patients with nsclc of nonsquamous histology42. Patients were randomized to pemetrexed plus nintedanib or to pemetrexed plus placebo after failure of first-line therapy. The study was discontinued early because an interim analysis suggested futility. No improvement was seen in os (12.2 months vs. 12.7 months; hr: 1.03; 95% ci: 0.85 to 1.24) or response rate (9.1% vs. 8.3%). However, pfs showed a significant improvement (4.4 months vs. 3.6 months; hr: 0.83; 95% ci: 0.7 to 0.99).
In the lume-Lung 1 trial, docetaxel plus nintedanib was compared with docetaxel plus placebo in all nsclc histologic subtypes43. Compared with patients randomized to docetaxel alone, those randomized to docetaxel plus nintedanib experienced a significantly improved pfs (3.4 months vs. 2.7 months; hr: 0.85; 95% ci: 0.75 to 0.96), which was the primary outcome. A modified hierarchical analysis for os was pre-specified to examine os in patients with adenocarcinoma who progressed within 9 months of commencing first-line therapy, then in all patients with adenocarcinoma, and finally in the overall intention-to-treat population. Significant improvements in os were seen in patients randomized to docetaxel plus nintedanib, in those with adenocarcinoma progressing within 9 months (10.9 months vs. 7.9 months), and in the entire adenocarcinoma subset (12.6 months vs. 10.3 months). No improvement in os was seen in the analysis of the overall study population. Nevertheless, the first two os analyses represent nonrandomized comparisons.
Patient-reported outcomes were also assessed in lume-Lung 1. No differences were observed in time to deterioration in global health status or in common lung cancer symptoms of cough, dyspnea, or pain44. Multiple post-hoc analyses of the data have been conducted to try to better define the patient population who might benefit from the addition of nintedanib45, but those analyses are all just hypothesis-generating.
The lume-Columbus trial was designed to verify the findings from lume-Lung 1 in patients with adenocarcinoma progressing after first-line therapy; however, that trial was discontinued. The findings in lume-Lung 1 and 2 were inconsistent for patients with adenocarcinoma. Nintedanib was approved for use in nsclc by the European Medicines Agency, but not by the U.S. Food and Drug Administration or Health Canada. The benefit of nintedanib in the second-line therapy of advanced nsclc therefore remains unclear.
OTHER ANTIANGIOGENIC AGENTS NOT APPROVED IN NSCLC
Multiple additional antiangiogenic agents have been tested in the second-line therapy of nsclc. Those agents include drugs that specifically target the vegf pathway (aflibercept) and multi-targeted tyrosine kinase inhibitors that could target a combination of vegfr, egfr, mesenchymal-to-epithelial transition, platelet-derived growth factor receptor, and other receptors (cabozantinib, sunitinib, sorafenib). Table iv presents findings from the relevant clinical trials for those drugs.
TABLE IV.
Summary of trials of oral antiangiogenic agents after failure of first-line therapy in non-small-cell lung cancer
| Agent and reference (study name) | Design | Intervention | Pts (n) | ORR (%) | PFS (months) | OS (months) | Quality of life |
|---|---|---|---|---|---|---|---|
| Nintedanib | |||||||
| Hanna et al.,201342 (Lume-Lung2) | Phase III | Pemetrexed with nintedanib | 353 | 9.1 | 4.4 HR: 0.83; 95% CI: 0.70 to 0.99 |
12.2 HR: 1.03; 95% CI: 0.85 to 1.24 |
Not assessed. |
| Pemetrexed with placebo | 360 | 8.3 | 3.6 | 12.7 | |||
| Reck et al.,201443 (Lume-Lung1) | Phase III | Docetaxel with nintedanib | 655 | 4.4 | 3.4 HR: 0.79; 95% CI: 0.68 to 0.92 |
10.1 HR: 0.94; 95% CI: 0.83 to 1.05 |
Not assessed. |
| Docetaxel with placebo | 659 | 3.3 | 2.7 | 9.1 | |||
| Cabozantinib | |||||||
| Hellerstedt et al.,201246 | Randomized phase II discontinuation trial | Cabozantinib induction followed by cabozantinib | 60 | 10 | 4.2 | Not assessed. | Not assessed. |
| Placebo | Not assessed. | Not assessed. | Not assessed. | Not assessed. | |||
| Neal et al.,201647 (ECOG 1512) | Randomized phase II | Erlotinib | 42 | 3 | 1.8 | 5.1 | |
| Cabozantinib | 40 | 11 | 4.3 HR: 0.39; 80% CI: 0.27 to 0.55 |
9.2 HR: 0.68; 80% CI: 0.49 to 0.95 |
|||
| Erlotinib with cabozantinib | 43 | 3 | 4.7 HR: 0.37; 80% CI: 0.25 to 0.53 |
13.3 HR: 0.51; 80% CI: 0.35 to 0.74 |
|||
| Sunitinib | |||||||
| Scagliotti et al.,201248 | Phase III | Erlotinib with sunitinib | 480 | 10.6 | 3.6 | 9.0 | No difference in the EQ-5Da was observed between the study arms. |
| HR: 0.81; | HR: 0.92; | ||||||
| 95% CI: 0.69 to 0.94 | 95% CI: 0.80 to 0.1.07 | ||||||
| Erlotinib with placebo | 480 | 6.9 | 2.0 | 8.5 | |||
| Groen et al.,201349 | Randomized phase II | Erlotinib with sunitinib | 65 | 4.5 | 2.8 HR: 0.90; 95% CI: 0.67 to 1.2 |
8.2 HR: 1.07; 95% CI: 0.71 to 1.6 |
Not assessed. |
| Erlotinib with placebo | 67 | 3.0 | 2.0 | 7.6 | |||
| Heist et al.,201450 (CALGB 30704) | Randomized phase II | Pemetrexed with sunitinib | 41 | 22 | 3.7 HR: 1.3; 95% CI: 0.9 to 2.1 |
6.7 HR: 2.0; 95% CI: 1.2 to 3.2 |
Not assessed. |
| Sunitinib | 47 | 17 | 3.3 HR: 1.4; 95% CI: 0.9 to 2.2 |
8.0 HR: 1.4; 95% CI: 0.9 to 2.3 |
|||
| Pemetrexed | 42 | 14 | 4.9 (p=0.3) | 10.5 | |||
| Sorafenib | |||||||
| Molina et al.,201151 (NCCTG626) | Randomized phase II |
Pemetrexed with sorafenib | 49 | 3.4 (p=0.22) | 9.4 (p=0.49) | Not assessed. | |
| Pemetrexed | 51 | 4.1 | 9.7 | ||||
| Spigel et al.,201152 (Lun160) | Randomized phase II |
Pemetrexed with sorafenib | 112 | 8 | 3.38 HR: 0.86; 95% CI: 0.60 to 1.22 |
7.62 HR: 0.89; 95% CI: 0.59 to 1.34 |
Not assessed. |
| Erlotinib with placebo | 56 | 11 | 1.94 | 7.23 | |||
| Wakelee et al.,201253 (E2501) | Randomized phase II | Sorafenib induction followed by sorafenib | 53b | 3 | 3.3 HR: 0.51; 95% CI: 0.30 to 0.87 |
13.7 HR: 0.67; 95% CI: 0.40 to 1.11 |
Not assessed. |
| Placebo | 52b | 2.0 | 9.0 | ||||
| Paz-Ares et al.,201554 (MISSION) | Phase III | Placebo with best supportive care | 353 | 0.9 | 1.4 | 8.3 | |
| Sorafenib with best supportive care | 350 | 4.9 | 2.8 HR: 0.54; 95% CI: 0.45 to 0.65 |
8.2 HR: 0.99; 95% CI: 0.84 to 1.17 |
|||
| Vandetanib | |||||||
| Herbst et al.,201055 (ZODIAC) | Phase III | Docetaxel with vandetanib | 694 | 17 | 4.0 HR: 0.79; 95% CI: 0.70 to 0.90 |
10.6 HR: 0.91; 95% CI: 0.78 to 1.07 |
Based on the lung cancer subscale of the FACT-Lc, time to symptom progression was delayed in the vandetanib arm. |
| Docetaxel with placebo | 697 | 10 | 3.2 | 10.0 | |||
| De Boer et al.,201156 (ZEAL) | Phase III | Pemetrexed with vandetanib | 256 | 19 | 17.6 Weeks HR: 0.86; 95% CI: 0.69 to 1.06 |
10.5 HR: 0.86; 95% CI: 0.65 to 1.13 |
Time to deterioration was longer based on the total score on the Lung Cancer Symptom Scale for the vandetanib arm. |
| Pemetrexed with placebo | 278 | 8 | 11.9 Weeks | 9.2 | |||
| Natale et al.,201157 (ZEST) | Phase III | Vandetanib | 623 | 12 | 2.6 HR: 0.98; 95% CI: 0.87 to 1.10 |
6.9 HR: 1.01; 95% CI: 0.89 to 1.16 |
Based on the EORTC QLQ-30, no difference in time to deterioration in symptoms was observed. |
| Erlotinib | 617 | 12 | 2.0 | 7.8 | |||
| Lee et al.,201258 (ZEPHYR) | Phase III | Vandetanib | 617 | 2.6 | 1.9 HR: 0.63; 95% CI: 0.54 to 0.74 |
8.5 HR: 0.95; 95% CI: 0.81 to 1.11 |
Based on the lung cancer subscale of the FACT-Lc, no significant difference between the groups was observed. |
| Placebo | 307 | 0.7 | 1.8 | 7.8 | |||
EuroQol Research Foundation, Rotterdam, Netherlands.
Enrolled 299 patients.
FACIT.org, Elmhurst, IL, U.S.A.
Pts = patients; ORR = objective response rate; PFS = progression-free survival; OS = overall survival; HR = hazard ratio; CI = confidence interval; FACT-L = Functional Assessment of Cancer Therapy–Lung; EORTC = European Organisation for Research and Treatment of Cancer.
Resistance to agents that block only vegfr can be mediated through pathways such as mesenchymal-to-epithelial transition because its ligand, hepatocyte growth factor, can synergistically activate vegfr. Cabozantinib, a multi-targeted tyrosine kinase inhibitor, might overcome a potential mechanism of resistance to vegfr therapy through blockade of both mesenchymal-to-epithelial transition and vegfr. Some efficacy of cabozantinib was observed in a phase ii randomized discontinuation trial46. All the patients, who had previously been heavily treated, were given cabozantinib for 12 weeks. At that point, they were randomized to continue on the drug or to switch to placebo. At 12 weeks, the response rate was 10%, and the pfs was 4.2 months. Data for the full randomized cohort were not available.
Recently, data from the ecog 1512 randomized phase ii trial comparing erlotinib alone, cabozantinib alone, and the combination erlotinib–cabozantinib in patients with previously treated egfr wild-type nsclc were published47. Greater efficacy was seen in both groups of patients treated with cabozantinib. Longer pfs was observed in patients receiving cabozantinib alone (4.3 months; hr: 0.39; 80% ci: 0.27 to 0.55) and erlotinib–cabozantinib (4.7 months; hr: 0.37; 80% ci: 0.25 to 0.53) than in those receiving erlotinib alone (1.8 months). Similarly, os was longer in patients randomized to cabozantinib alone (9.2 months; hr: 0.68; 80% ci: 0.49 to 0.95) or to cabozantinib-erlotinib (13.3 months; hr: 0.51; 80% ci: 0.35 to 0.74) than in those randomized to erlotinib alone (5.1 months). Those results appear promising, but require confirmation in a phase iii trial.
Multiple studies have assessed the utility of sunitinib in the second-line setting, observing no clear improvement in efficacy. A worse os appeared to be associated with sunitinib–pemetrexed than with pemetrexed alone (hr: 2.0; 95% ci: 1.2 to 3.2)50. Two additional trials investigated the combination sunitinib–erlotinib in patients with previously treated nsclc48,49. The pfs findings in those trials were discordant, and no improvement in os was observed in either trial. Notably, quality of life as measured by the EQ-5D (EuroQol Research Foundation, Rotterdam, Netherlands) showed no improvement. Sunitinib does not appear to have a role as an antiangiogenic therapy in nsclc.
Multiple trials have also investigated the role of sorafenib in the second line and beyond. Sorafenib was studied in a randomized discontinuation trial in 299 patients who had progressed on at least 2 prior lines of chemotherapy and an egfr-targeted therapy53. Patients received 2 months of oral sorafenib 400 mg twice daily. If a response was observed, patients would continue on treatment; those experiencing progression discontinued therapy. The remaining patients, those with stable disease (n = 105), were randomized to continue or discontinue therapy. An improvement in pfs was observed for the patients randomized to sorafenib (3.3 months vs. 2.0 months; hr: 0.51; 95% ci: 0.30 to 0.87). However, that improvement did not translate into an improvement in os (13.7 months vs. 9.0 months; hr: 0.67; 95% ci: 0.40 to 1.11). Interpreting the findings of the study requires caution, because an error in patient randomization occurred. Initially, 8 patients randomized to the sorafenib arm received a placebo, and 12 patients on the placebo arm received sorafenib.
Sorafenib has also been studied in combination with pemetrexed and erlotinib. The Lun 160 trial used a 2:1 randomization protocol in comparing erlotinib–sorafenib with erlotinib–placebo in patients after failure of 1 or 2 lines of prior chemotherapy52. Analysis revealed a trend toward a pfs benefit (hr: 0.86; 95% ci: 0.6 to 1.22) and no difference in response rate (8% vs. 11%). However, the data did suggest a possible benefit in the combination arm for patients with wild-type EGFR or tumours negative for egfr by fluorescence in situ hybridization. Furthermore, the North Central Cancer Treatment Group N0626 trial compared pemetrexed with pemetrexed–sorafenib after failure of first-line therapy51. No difference in pfs (3.4 months vs. 4.1 months, p = 0.22) or os (9.7 months vs. 9.4 months, p = 0.49) was observed.
Lastly, the Mission Trial compared sorafenib with placebo in heavily pretreated patients, finding a small difference in pfs (2.8 months vs. 1.4 months; hr: 0.54; 95% ci: 0.45 to 0.65), but no corresponding improvement in os (8.2 months vs. 8.3 months; hr: 0.99; 95% ci: 0.84 to 1.17)54. Despite some evidence of activity in the second-line setting, sorafenib alone or in combination with other agents has no clear role in nsclc.
Four trials—zeal, zodiac, zest, zephyr—have investigated the role of vandetanib in the second-line setting (Table iv). None of the trials showed any improvement in os. In zeal, 534 patients with nsclc were randomized to pemetrexed plus placebo or pemetrexed plus oral vandetanib 100 mg once daily56. Despite an improved orr (19% vs. 8%, p < 0.001), the pfs (17.6 weeks vs. 11.9 weeks; hr: 0.86; 95% ci: 0.69 to 1.06) and os (10.5 months vs. 9.2 months; hr: 0.86; 95% ci: 0.65 to 1.13) were not significantly improved. However, a delayed time to decline of lung cancer symptoms as assessed by the Lung Cancer Symptom Scale was demonstrated in the combination arm.
In zodiac, 1391 patients were randomized to docetaxel alone or to docetaxel plus vandetanib55. An improvement in pfs was observed (4.0 months vs. 3.2 months; hr: 0.79; 95% ci: 0.70 to 0.90), but did not translate into an improved os (10.6 months vs. 10 months; hr: 0.91; 95% ci: 0.78 to 1.07). As in zeal, the time to decline of lung cancer–related symptoms favoured the combination arm.
In zest, a study designed to assess superiority, 1240 patients who had received at least 1 or 2 lines of prior chemotherapy were randomized to oral vandetanib 300 mg daily or to oral erlotinib 150 mg once daily57. The overall results of the trial were negative, with no improvement seen in pfs (2.6 months vs. 2.0 months; hr: 0.98; 95% ci: 0.87 to 1.10), os (6.9 months vs. 7.8 months; hr: 1.01; 95% ci: 0.89 to 1.16), or the time to decline of lung cancer–related symptoms per the 30-question core Quality of Life Questionnaire from the European Organisation for Research and Treatment of Cancer and the associated LCS13 module for patients with lung cancer.
Lastly, in zephyr, 924 patients who had received chemotherapy as well as egfr-targeted therapy were randomized 2:1 to oral vandetanib 300 mg once daily or to placebo58. As in many of the trials in this setting, a small benefit in pfs was observed (1.9 months vs. 1.8 months; hr: 0.63; 95% ci: 0.54 to 0.74), but no improvement in os was demonstrated (8.5 months vs. 7.8 months; hr: 0.95; 95% ci: 0.8 to 1.11). Furthermore, no difference was noted in lung cancer–related symptoms, as identified by the Functional Assessment of Cancer Therapy–Lung (FACIT.org, Elmhurst, IL, U.S.A.). The body of evidence evaluating vandetanib fails to show any clear benefit for its use in previously treated patients with advanced nsclc.
SUMMARY
Targeting angiogenesis remains an important therapeutic strategy in the management of some solid-organ malignancies such as colon cancer. However, the utility of the approach in nsclc has not been as clearly established. The addition of bevacizumab to platinum-based chemotherapy in the first-line setting carries a modest benefit, although competing non-bevacizumab strategies are available. Some antiangiogenic therapies have been approved in the second line by the U.S. Food and Drug Administration (bevacizumab, ramucirumab) and the European Medicines Agency (nintedanib), but the extent of the improvements in pfs and os are modest and must be balanced against the expected toxicities and the costs associated with those agents. Furthermore, with the emergence of randomized data showing the efficacy of immunotherapy in both the first and second lines, and improvements in treatment options for molecularly driven nsclc (EGFR, ROS1, ALK, and so on), the utility of antiangiogenic therapies in the second-line setting in the management of patients with nsclc might be limited.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: PME has received speaker’s honoraria from Boehringer Ingelheim and Pfizer in the last 2 years. AA and GC have no disclosures to make. There are no funding sources for the present work.
REFERENCES
- 1.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–63. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/S0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–19. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkman J, Klagsbrun M. Vascular physiology. A family of angiogenic peptides. Nature. 1987;329:671–2. doi: 10.1038/329671a0. [DOI] [PubMed] [Google Scholar]
- 5.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006;81:1241–57. doi: 10.4065/81.9.1241. [DOI] [PubMed] [Google Scholar]
- 7.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Gerber HP, LeCouter J. The biology of vegf and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 9.Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti–vascular endothelial growth factor monoclonal anti-body for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 10.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase ii trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [Erratum in: N Engl J Med 2007;356:318] [DOI] [PubMed] [Google Scholar]
- 12.Reck M, von Pawel J, Zatloukal P, et al. on behalf of the BO17704 Study Group Overall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase iii trial (avail) Ann Oncol. 2010;21:1804–9. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niho S, Kunitoh H, Nokihara H, et al. on behalf of the JO19907 Study Group Randomized phase ii study of first-line carboplatin–paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer. 2012;76:362–7. doi: 10.1016/j.lungcan.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase iii study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage iiib or iv nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–57. doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab–pemetrexed after first-line cisplatin–pemetrexed–bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the avaperl (MO22089) randomized phase iii trial. Ann Oncol. 2014;25:1044–52. doi: 10.1093/annonc/mdu098. [DOI] [PubMed] [Google Scholar]
- 16.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–44. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Wu YL, Chen G, et al. beyond: a randomized, double-blind, placebo-controlled, multicenter, phase iii study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non–small-cell lung cancer. J Clin Oncol. 2015;33:2197–204. doi: 10.1200/JCO.2014.59.4424. [DOI] [PubMed] [Google Scholar]
- 18.Zinner RG, Obasaju CK, Spigel DR, et al. pronounce: randomized, open-label, phase iii study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients with advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2015;10:134–42. doi: 10.1097/JTO.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camidge DR, Berge EM, Doebele RC, et al. A phase ii, open-label study of ramucirumab in combination with paclitaxel and carboplatin as first-line therapy in patients with stage iiib/iv non-small-cell lung cancer. J Thorac Oncol. 2014;9:1532–9. doi: 10.1097/JTO.0000000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doebele RC, Spigel D, Tehfe M, et al. Phase 2, randomized, open-label study of ramucirumab in combination with first-line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/metastatic non–small cell lung cancer. Cancer. 2015;121:883–92. doi: 10.1002/cncr.29132. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Modiano MR, Neal JW, et al. A phase ii multicentre study of ziv-aflibercept in combination with cisplatin and pemetrexed in patients with previously untreated advanced/metastatic non-squamous non–small cell lung cancer. Br J Cancer. 2014;110:602–8. doi: 10.1038/bjc.2013.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margolin K, Gordon MS, Holmgren E, et al. Phase ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851–6. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 23.Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase ii/iii trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:20–30. doi: 10.1093/annonc/mds590. [DOI] [PubMed] [Google Scholar]
- 24.Youssoufian H, Hicklin DJ, Rowinsky EK. Review: monoclonal antibodies to the vascular endothelial growth factor receptor-2 in cancer therapy. Clin Cancer Res. 2007;13:5544s–8s. doi: 10.1158/1078-0432.CCR-07-1107. [DOI] [PubMed] [Google Scholar]
- 25.Lassoued W, Murphy D, Tsai J, Oueslati R, Thurston G, Lee WMF. Effect of vegf and vegf Trap on vascular endothelial cell signaling in tumors. Cancer Biol Ther. 2010;10:1326–33. doi: 10.4161/cbt.10.12.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belani CP, Yamamoto N, Bondarenko IM, et al. Randomized phase ii study of pemetrexed/cisplatin with or without axitinib for non-squamous non-small-cell lung cancer. BMC Cancer. 2014;14:290. doi: 10.1186/1471-2407-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twelves C, Chmielowska E, Havel L, et al. Randomised phase ii study of axitinib or bevacizumab combined with paclitaxel/carboplatin as first-line therapy for patients with advanced non-small-cell lung cancer. Ann Oncol. 2014;25:132–8. doi: 10.1093/annonc/mdt489. [DOI] [PubMed] [Google Scholar]
- 28.Goss GD, Arnold A, Shepherd FA, et al. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: ncic Clinical Trials Group BR24 study. J Clin Oncol. 2010;28:49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]
- 29.Laurie SA, Solomon BJ, Seymour L, et al. Randomized, double-blind trial of carboplatin and paclitaxel with daily cediranib or placebo in patients with advanced non–small cell lung cancer: ncic Clinical Trials Group study br29. Eur J Cancer. 2014;50:706–12. doi: 10.1016/j.ejca.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind phase iii study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: monet1. J Clin Oncol. 2012;30:2829–36. doi: 10.1200/JCO.2011.41.4987. [DOI] [PubMed] [Google Scholar]
- 31.Scagliotti G, Novello S, von Pawel J, et al. Phase iii study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–42. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 32.Paz-Ares LG, Biesma B, Heigener D, et al. on behalf of the NSCLC Research Experience Utilizing Sorafenib (nexus) investigators study group Phase iii, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol. 2012;30:3084–92. doi: 10.1200/JCO.2011.39.7646. [DOI] [PubMed] [Google Scholar]
- 33.Heymach J, Paz-Ares L, De Braud F, et al. Randomized phase ii study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5407–15. doi: 10.1200/JCO.2008.17.3138. [DOI] [PubMed] [Google Scholar]
- 34.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumour activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–83. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 35.Herbst RS, O’Neill VJ, Fehrenbacher L, et al. Phase ii study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25:4743–50. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 36.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (beta): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–54. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortot AB, Audigier-Valette C, Molinier O, et al. Weekly paclitaxel plus bevacizumab versus docetaxel as second or third-line treatment in advanced non-squamous non-small cell lung cancer (nsclc): results from the phase iii study ifct-1103 ultimate [abstract 9005] J Clin Oncol. 2016;34 doi: 10.1016/j.ejca.2020.02.022. [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.9005; cited 17 December 2017] [DOI] [PubMed] [Google Scholar]
- 38.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage iv non-small-cell lung cancer after disease progression on platinum-based therapy (revel): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 39.Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase iii trial. J Clin Oncol. 2012;30:3640–7. doi: 10.1200/JCO.2012.42.6932. [DOI] [PubMed] [Google Scholar]
- 40.Bennouna J, De Castro J, Dingemans AMC, et al. Efficacy and safety results from avaall: an open-label, randomized phase iii trial of standard of care (soc) with or without continuous bevacizumab (Bev) treatment beyond progression (pd) in patients (pts) with advanced non–small cell lung cancer (nsclc) progressing after first-line Bev and chemotherapy (chemo) [abstract 9004] J Clin Oncol. 2017;35 [Available online at: https://meetinglibrary.asco.org/record/145471/abstract; cited 17 December 2017] [Google Scholar]
- 41.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna NH, Kaiser R, Sullivan RN, et al. lume-Lung 2: a multicenter, randomized, double-blind, phase iii study of nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with advanced nonsquamous non-small cell lung cancer (nsclc) after failure of first-line chemotherapy [abstract 8034] J Clin Oncol. 2013;31 [Available online at: https://meetinglibrary.asco.org/record/81165/abstract; cited 17 December 2017] [Google Scholar]
- 43.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (lume-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–55. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 44.Novello S, Kaiser R, Mellemgaard A, et al. Analysis of patient-reported outcomes from the lume-Lung 1 trial: a randomised, double-blind, placebo-controlled, phase iii study of second-line nintedanib in patients with advanced non–small cell lung cancer. Eur J Cancer. 2015;51:317–26. doi: 10.1016/j.ejca.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Gottfried M, Bennouna J, Bondarenko I, et al. Efficacy and safety of nintedanib plus docetaxel in patients with advanced lung adenocarcinoma: complementary and exploratory analyses of the phase iii lume-Lung 1 study. Target Oncol. 2017;12:475–85. doi: 10.1007/s11523-017-0517-2. [DOI] [PubMed] [Google Scholar]
- 46.Hellerstedt BA, Edelman G, Vogelzang NJ, et al. Activity of cabozantinib (XL184) in metastatic nsclc: results from a phase ii randomized discontinuation trial (rdt) [abstract 7514] J Clin Oncol. 2012;30 [Available online at: https://meetinglibrary.asco.org/record/69424/abstract; cited 17 December 2017] [Google Scholar]
- 47.Neal JW, Dahlberg SE, Wakelee HA, et al. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ecog-acrin 1512): a randomised, controlled, open-label, multicentre, phase 2 trial. Lancet Oncol. 2016;17:1661–71. doi: 10.1016/S1470-2045(16)30561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase iii trial. J Clin Oncol. 2012;30:2070–8. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- 49.Groen HJ, Socinski MA, Grossi F, et al. A randomized, double-blind, phase ii study of erlotinib with or without sunitinib for the second-line treatment of metastatic non-small-cell lung cancer (nsclc) Ann Oncol. 2013;24:2382–9. doi: 10.1093/annonc/mdt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heist RS, Wang X, Hodgson L, et al. A randomized phase ii study to assess the efficacy of pemetrexed or sunitinib or pemetrexed plus sunitinib in the second-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9:214–21. doi: 10.1097/JTO.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molina JR, Dy GK, Foster NR, et al. A randomized phase ii study of pemetrexed (pem) with or without sorafenib (s) as second-line therapy in advanced non-small cell lung cancer (nsclc) of nonsquamous histology: ncctg N0626 study [abstract 7513] J Clin Oncol. 2011;29 [Available online at: http://ascopubs.org/doi/abs/10.1200/jco.2011.29.15_suppl.7513; cited 17 December 2017] [Google Scholar]
- 52.Spigel DR, Burris HA, 3rd, Greco FA, et al. Randomized, double-blind, placebo-controlled, phase ii trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2582–9. doi: 10.1200/JCO.2010.30.7678. [DOI] [PubMed] [Google Scholar]
- 53.Wakelee HA, Lee JW, Hanna NH, Traynor AM, Carbone DP, Schiller JH. A double-blind randomized discontinuation phase-ii study of sorafenib (BAY 43-9006) in previously treated non-small-cell lung cancer patients: Eastern Cooperative Oncology Group study E2501. J Thorac Oncol. 2012;7:1574–82. doi: 10.1097/JTO.0b013e31826149ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paz-Ares L, Hirsh V, Zhang L, et al. Monotherapy administration of sorafenib in patients with non–small cell lung cancer (mission) trial. J Thorac Oncol. 2015;10:1745–53. doi: 10.1097/JTO.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 55.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (zodiac): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–26. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase iii trial. J Clin Oncol. 2011;29:1067–74. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 57.Natale RB, Thongprasert S, Greco FA, et al. Phase iii trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:1059–66. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 58.Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase iii trial (zephyr) J Clin Oncol. 2012;30:1114–21. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]