Abstract
Background
To prospectively evaluate the role of procalcitonin (PCT) in screening of patients with thyroid nodules for medullary thyroid carcinoma (MTC).
Materials and methods
We measured PCT in 2705 patients with thyroid nodules referred to our centre between January 2011 and December 2017. Those with a positive PCT were operated after positive confirmatory tests such as fine‐needle aspiration, measurement of calcitonin (CT) in serum and fine‐needle aspiration washouts or CT stimulation testing. Patients with a negative PCT were operated based on the results of further diagnostics. The diagnostic performance of PCT was evaluated, and the best cut‐off level was selected by ROC curve analysis.
Results
Among 2705 patients, 9 with positive serum PCT (ie, above 0.1 μg/L) and 370 with negative PCT underwent thyroid surgery. MTC was histologically confirmed in all patients with positive PCT but not found in patients with negative PCT. Serum PCT levels were significantly higher in patients with MTC (median 0.64 μg/L, range 0.16‐12.9 μg/L) than in those without (median 0.075 μg/L, range 0.075‐0.16 μg/L; P < .0001). ROC curves were plotted to calculate the optimal PCT value separating patients with MTC from those without. The best cut‐off was 0.155 μg/L with sensitivity, specificity, positive and negative predictive values as well as accuracy of 100%, 99.7%, 91.7%, 100% and 99.7%, respectively. Positive and negative likelihood ratios were 329 and zero, respectively.
Conclusions
Measurement of PCT is a sensitive and accurate method for detecting MTC in patients with thyroid nodules and can thus be a reliable alternative to CT measurement.
Keywords: calcitonin, medullary thyroid carcinoma, procalcitonin, thyroid nodules
1. INTRODUCTION
Medullary thyroid cancer (MTC) harbours from the thyroid parafollicular C cells and accounts for ~3% to 5% of all thyroid carcinomas.1 The majority of MTCs are sporadic and generally present as a thyroid nodule. At the time of diagnosis, cervical lymph node and distant metastases are present in up to 50% and 10% of patients.1, 2 Calcitonin (CT) is a polypeptide hormone consisting of 32 amino acids mainly produced by the C cells. The measurement of CT concentrations in blood reflects the mass and activity of the C cells; it is generally performed using immunoassay‐based methods.3 There is a considerable body of evidence that the measurement of serum CT concentrations in patients with thyroid nodules can lead to an earlier diagnosis of MTC than the exclusive use of imaging procedures and/or fine‐needle aspiration cytology (FNAC).4, 5, 6, 7 However, there is some controversy regarding the screening of thyroid nodules with CT measurements as to whether this is a cost‐effective exercise in terms of reducing deaths.8, 9 In addition, different analytical, physiological, pharmacological and pathological factors may significantly reduce the specificity and overall accuracy of serum CT measurement.3 Due to the influence of these factors, there is a high variability in assay‐dependent and gender‐dependent cut‐offs used to discriminate between MTC, C‐cell hyperplasia (CCH) and healthy C cells.10 Additionally, CT is prone to in vitro decay at room temperature which makes rapid centrifugation and storage at −20°C of samples mandatory.11, 12
All in all, pros and cons of using CT to screen patients carrying thyroid nodules for MTC are differently balanced in currently available clinical guidelines: the ETA consensus report published in 2006 clearly states that routine measurement of CT should be applied in the management of thyroid nodule cases, as it is more sensitive than fine‐needle cytology.13 Besides, the more recent AACE, AME and ETA's common document declared that routine measurement of basal CT may be useful and strongly recommended its measurement in certain high‐risk groups.14 Finally, the ATA's guidelines panel decided to not recommend either for or against routine measurement of serum CT in patients with thyroid nodules8 but recommend that physicians decide whether the technique is useful in the management of patients in their clinic.9 If CT screening is adopted it should be noted that depending on the assay used, 56%‐88% of subjects without thyroid disease usually show a CT level below the functional sensitivity, while 3%‐10% have CT levels >10 pg/mL.15, 16 A CT stimulation test using pentagastrin or calcium should be performed to increase sensitivity and specificity of basal CT measurement and to avoid unnecessary thyroidectomies.9 Nevertheless, pentagastrin is not available any longer. An alternative method represents the calcium stimulation test, being described as sensitive as well, however, also having (potentially life threatening) side effects.17, 18 Additionally, cut‐offs for the calcium stimulation test are not yet well defined.19 All in all, a simple, safe and reliable complementary or alternative test to CT is warranted to increase the efficacy of a screening programme of thyroid nodules for MTC. Procalcitonin (PCT) is a CT precursor which is found at low serum concentrations in healthy individuals, but is produced in large amounts by extrathyroidal tissue during severe systemic inflammation, infection and sepsis.20 Notably, PCT assays are free from pre‐analytic drawbacks and produce consistent results across different analytical methods.21, 22 In recent years, serum PCT was also evaluated as a biomarker of MTC. Algeciras‐Schimnich et al23 collected data on a series of 133 MTC cases (42 cured and 91 actives), and 83 of 91 (91.2%) of the recurrent MTCs had PCT of over 0.15 μg/L, whereas none of the cured MTCs had positive PCT (specificity 100%). Furthermore, Kratsch et al measured serum CT and PCT in 437 patients with clinical conditions known to be associated with spurious hypercalcitoninemia such as chronic kidney diseases, Hashimoto's thyroiditis and proton‐pump inhibitor therapy. The number of spuriously increased basal PCT concentrations in those patients was lower than the number of increased CT concentrations as measured by 3 methods. Indeed, PCT concentrations <0.25 μg/L accurately excluded MTC in such patients.24 In a prospective study from our group,25 CT was measured in a consecutive series of 1236 patients carrying thyroid nodules, and 14 cases displayed increased CT levels (ie, >10 μg/L) and were selected for a pentagastrin stimulation test. Upon histology, 2 MTCs were recorded, and they had basal CT >100 ng/L and PCT of >0.1 μg/L. The main results of that study were that basal and pentagastrin‐stimulated CT had some false‐positive results, whereas all of the patients without MTC had undetectable levels of both basal and stimulated PCT with a 100% positive predictive value (PPV) and a 100% negative predictive value (NPV), respectively. Thus, a negative basal PCT excluded MTC without the need for additional stimulation tests. Such data were recently corroborated by Lim et al.26 They assessed 476 serum samples referred for CT measurements and evaluated the role of PCT in predicting negative CT results. In summary, the NPVs of PCT ranged from 95.4% to 100%. Finally, Machens et al27 compared preoperative PCT and CT in a series of 112 consecutive patients with previously untreated MTC. Receiver operating characteristics (ROC) analyses revealed similar diagnostic accuracy for PCT and CT for primary tumours, extrathyroid extension, number of lymph nodes involved and distant metastasis. Among 112 cases, 107 had PCT levels of more than 0.1 μg/L, with a sensitivity of 95.5%. The remaining 5 patients had a micro‐MTC with a mean diameter of 2 mm (range 0.4‐3.5 mm). Of interest, PCT levels were correlated with the number of lymph nodes involved and the extent of distant metastatic disease. As the PCT levels increased, the biochemical cure rates declined. On the whole, the above‐mentioned study results suggested that PCT could be a new standard of care in the management of MTC and, at minimum, is a useful complementary biomarker to exclude MTC in patients with suspicious thyroid nodules or suggestive symptoms.28 This study was undertaken to prospectively evaluate the diagnostic performance of PCT as the first‐line biomarker to screen patients with thyroid nodules for medullary thyroid carcinoma.
2. MATERIALS AND METHODS
2.1. Work‐up of thyroid nodules
As per our standard operative procedures patients with thyroid nodules undergo clinical examination, thyroid ultrasound and measurement of thyroid‐stimulating hormone (TSH; with additional measurement of free‐thyroxine [fT4] levels when abnormal). 99mTc‐pertechnetate scintigraphy was performed in patients with nodules larger than 10 mm and/or low TSH levels. Autonomously functioning nodules were treated accordingly or monitored at the discretion of the attending physician. Based on criteria for thyroid ultrasound proposed by the American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, European Thyroid Association (AACE/AME/ETA) guidelines,14 nodules were classified as low risk, intermediate risk or high risk of malignancy. Nodules at high risk and those at low‐to‐intermediate risk larger than 20 mm underwent cytological analysis of specimens gained by FNAC. Cytopathology results were reported according to the Società Italiana di Anatomia Patologica e Citopatologia/International Association of Pathology (SIAPEC/IAP) classification system (ie, TIR 1, nondiagnostic; TIR 2, benign; TIR 3, follicular lesion; TIR 4, suspicious for malignancy; TIR 5, malignant).29, 30 Patients with TIR 3, 4 and 5 cytological outcomes underwent surgery. Patients with benign cytology or unsuspicious US only underwent surgery in the presence of compressive symptoms. In the other cases, periodical clinical and US evaluation were performed every 12‐24 months according to the patients’ characteristics.
2.2. Study design and reference standard
For the purpose of this study, serum PCT was measured in patients with thyroid nodules referred to our centre between January 2011 and December 2016. Inclusion criteria were as follows: age >18 years and at least 1 nodule >10 mm in the largest diameter. Patients with personal or family history of MTC or multiple endocrine neoplasia, autonomously functioning nodules or severe systemic inflammation, infection and sepsis were excluded from the study. Patients with positive PCT results were evaluated by measuring serum CT and performing a confirmatory test consisting in either FNAC with CT measurement on fine‐needle aspiration washouts (FNA‐CT; ie, uninodular goitre) or CT stimulation test (ie, multinodular goitre), respectively. Patients with negative PCT but surgical indications (ie, compressive symptoms) also underwent preoperative CT measurement and, in case of positive results, a confirmatory test as described above.
2.3. Laboratory measurements
Thyroid‐stimulating hormone (TSH; with additional fT4 measurement if abnormal) was measured on the UniCell® DxI 800 fully automated random access analyser (Beckman Coulter, Nyon, Switzerland) according to the manufacturers’ specifications, as previously described (reference range: 0.40‐4.00 mUI/L). PCT was measured by fully automated homogenous time‐resolved amplified cryptate emission (TRACE) immunometric fluorescent assay on the Kryptor® system (Brahms Thermo Fischer, Hennigsdorf, Germany). The limits of detection and quantification are 0.02 and 0.075 μg/L, respectively, with an upper reference limit of 0.1 μg/L. CT was measured on the IMMULITE®2000 XPi platform (Siemens Healthcare Diagnostics, Erlangen, Germany) in strict adherence to the manufacturer's instructions. The CT assay is standardized against the 2nd International WHO calibrator 89/620. The limits of detection and quantification are 2.00 and 5.00 ng/L, respectively. Upper reference limits, as previously settled in our centre, are 7.7 ng/L for males and 5.5 ng/L for females, respectively.31
2.4. Fine‐needle aspiration with calcitonin measurement on needle washouts
Ultrasound‐guided FNAC was performed with a 23‐gauge needle attached to a 20‐mL plastic syringe. The aspirated fluid was in part expelled and directly smeared onto charged slides, fixed and stained with a May‐Grunwald‐Giemsa and/or Papanicolaou method. Cytologic specimen was evaluated by experienced cytopathologists. After aspiration, both needle and syringe were washed in 1 mL of normal saline solution, and the resulting samples were used for CT determination in FNAC washouts. Values of CT in FNAC washouts above 39.6 ng/L were considered positive for MTC.32, 33
2.5. Calcitonin stimulation test
The CT stimulation test was carried by intravenous administration of 25 mg/kg body weight of calcium gluconate at 5 mL/min, with continuous electrocardiographic monitoring. By the end of the infusion, blood samples were obtained at minute +2, +5, and +10, respectively. CT peak values above 544 ng/L (males) and 79 ng/L (females) were considered positive for MTC as previously reported.19
2.6. Surgery and surgical pathology
Patients demonstrating positive FNA‐CT or CT stimulation underwent total thyroidectomy and central neck dissection. Lateral compartment dissections were performed on indication of the attending physicians and surgeons based on preoperative US mapping and further complementary imaging procedures. Conventional pathology examination of surgical specimens with additional CT immunostaining was performed by an experienced endocrine pathologist in all cases.
2.7. Statistical analysis
For the convenience of data analyses, values of PCT and CT below the functional sensitivity were considered as equal to the functional sensitivity of assay methods (ie, CT and PCT values below 5 ng/L and 0.075 μg/L were included in the analysis as 5 ng/L and 0.075 μg/L, respectively). Categorical data were analysed by chi‐square testing or Z test for proportions while continuous data were analysed by parametric or nonparametric tests for differences in means or medians, respectively, depending on the normality of distribution. Continuous variables were dichotomized by receiver operating characteristics (ROC) curve analysis using the maximum value of Youden's index (J) as the most accurate cut‐off point. The predictivity tests, that is sensitivity, specificity, PPV, NPV and accuracy, were calculated according to Galen and Gambino.34 All statistical tests were performed by graphpad prism version 7 (GraphPad Software Inc., La Jolla, CA, USA).
3. RESULTS
The initial series included 3730 consecutive adult patients (2672 females and 1058 males, mean age 52 ± 18 years). After exclusion of 24 patients with a personal or family history of MTC or multiple endocrine neoplasia, 958 patients with autonomously functioning nodules, 43 patients with severe systemic inflammation (n = 34), infection (n = 7) and sepsis (n = 2), 2705 patients were included in the final series (1866 females [69%] and 839 males [31%], mean age 53 ± 17 years).
3.1. Patients with negative PCT measurement
A total of 2696 of 2705 (99.75%) patients had PCT values <0.1 μg/L. Among them, 322 underwent surgery and 16 (5%) had increased preoperative CT levels (9 males: 11.2‐78 ng/L; 7 females: 9.5‐47.6 ng/L) with neither a positive FNAC‐CT or a CT stimulation test. Histopathological analysis proved papillary thyroid carcinoma in 5 cases and benign goitre in 11 cases all without histopathological and immunohistochemical signs of MTC or C‐cell hyperplasia. The remaining 2374 patients had an unremarkable work‐up and were assigned to periodic follow‐up. Complete follow‐up (median 19, range 12‐55.2 months) data were available in 1892 patients. Among them, 47 (2.5%) underwent surgery with histological diagnosis of papillary thyroid carcinoma (n = 1) and benign goitre (n = 46), respectively, without histopathological and immunohistochemical signs of MTC or C‐cell hyperplasia. Three of 47 patients (6%) had increased preoperative CT levels (3 males: 16.2‐56 ng/L) with neither a positive FNAC‐CT or a CT stimulation test. Demographic, clinical and pathological data of these patients are summarized in Table 1. Overall, 369 patients with negative PCT underwent surgery, and none of them had histological and/or immunohistochemical evidences of MTC or C‐cell hyperplasia. All in all, negative PCT and CT values correctly identified 367 (99.5%) and 350 (95%) cases with no histopathological and immunohistochemical evidences of MTC, respectively.
Table 1.
Clinical and pathological characteristics of such patients with negative PCT measurement
| n = 369 | |
|---|---|
| Patients | |
| Age | 55 ± 21 y |
| Males/Females | 126 (34%)/243 (66%) |
| Surgery | |
| Lobectomy | 91 (25%) |
| Total thyroidectomy | 278 (75%) |
| Pathology | |
| Benign outcome | 298 |
| Malignant outcome | 71 |
| PTC | 61 |
| FTC | 9 |
| PDTC | 1 |
FTC, follicular thyroid carcinoma; PCT, procalcitonin; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma.
3.2. Patients with positive PCT measurement
As summarized in Table 2, positive PCT measurements (ie, ≥0.1 μg/L) were found in 9 of 2705 patients (0.33%). Of these, 7 (0.26%) showed concurrently positive serum CT (n = 7) with positive CT on FNAC washouts (n = 5) or stimulated CT measurements (n = 2), respectively. After surgery, MTC was histologically confirmed in all cases. None of them carried proto‐oncogene RET mutations. Among the other 2 patients 1 had a symptomatic multinodular goitre with slightly positive PCT and basal CT levels at 0.16 μg/L and 11.6 ng/L and a negative stimulation test (ie, peak of CT: 56.8 ng/L), respectively. He was operated due to compressive symptoms with a benign histological report and no evidence of MTC. The second patient had an asymptomatic uninodular goitre with a slightly increased PCT at 0.11 μg/L. CT levels in serum and FNAC washouts were normal, and a benign cytological diagnosis was rendered after FNAC. As PCT levels spontaneously normalized after 1 month he was only assigned to periodical monitoring with unremarkable results over 4.5 years.
Table 2.
Clinical, biochemical and pathological data of patients with positive PCT measurement
| Patient | Disease | Sex | Age (y) | TSH (mUI/L) | PCT (μg/L) | CT (ng/L) | FNAC | FNA‐CT (ng/L) | Ca‐CT (ng/L) | Pathology | TNM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | UNG | F | 41 | 1.25 | 0.11 | < 5 | Tir 2 | <5 | — | N.D. | N.A. |
| 2 | MNG | M | 55 | 0.97 | 0.16 | 11.6 | — | — | 56.8 | Benign | N.A. |
| 3 | UNG | F | 45 | 1.12 | 0.56 | 97.5 | Tir 1 | 1590.6 | — | MTC | T1a N1a M0 |
| 4 | UNG | M | 39 | 0.65 | 1.27 | 130.4 | Tir 4 | >2000 | — | MTC | T2 N0 M0 |
| 5 | MNG | M | 72 | 0.37 | 2.68 | 187.5 | — | — | 1890 | MTC | T2 N1b M0 |
| 6 | UNG | F | 44 | 3.56 | 0.21 | 28.5 | Tir 3 | 688.5 | — | MTC | T1a N0 M0 |
| 7 | MNG | F | 61 | 4.22 | 0.64 | 31.7 | — | — | 510.4 | MTC | T1b N0 M0 |
| 8 | UNG | M | 57 | 1.10 | 12.9 | 1350 | Tir 4 | >2000 | — | MTC | T3 N1b M1 |
| 9 | UNG | F | 64 | 0.98 | 0.16 | 52.6 | Tir 5 | 986.3 | — | MTC | T1a N0 M0 |
Ca‐CT, calcitonin after stimulation with intravenous calcium gluconate; FNAC‐CT, calcitonin on needle washouts, MTC, medullary thyroid carcinoma; N.A. not applicable; N.D., not done; PCT, procalcitonin; TSH, thyroid‐stimulating hormone.
3.3. Distribution of procalcitonin and calcitonin and ROC curve analysis
Procalcitonin and CT values obtained in patients operated with a definitive histological outcome of MTC (n = 7) were compared with those obtained in patients operated with a definitive histological exclusion of MTC (n = 371), respectively. Patients with MTC had significantly higher PCT levels (median 0.64 μg/L, range 0.16‐12.9 μg/L) and CT levels (median 455 ng/L, range 37.5‐3450 ng/L) than those with nonmedullary nodules (median 0.075 μg/L, range 0.075‐0.16 μg/L and median 5 ng/L, range 5‐73.2 ng/L, respectively; both P < .0001; Figure 1A,B). ROC curve analysis of PCT and CT diagnostic performance yielded statistically indistinguishable areas under the curve (AUCs) of 0.99 (95% CI: 0.99‐1.0) for PCT and 0.99 (95% CI: 0.97‐0.99) for PCT (Z test: ns; Figure 1C,D). The PCT and CT concentrations that provided the best diagnostic accuracy were 0.155 μg/L and 34.4 ng/L, respectively. Sensitivity, specificity, positive (PPV) and negative (NPV) predictive values and positive and negative likelihood ratios (and attached 95% CI) were 100% (58.9‐100) and 100% (58.9‐100), 99.7% (98.5‐100) and 98.3% (97.2‐99.7), 87.5% (47.4‐97.9) and 63.6% (30.9‐88.8), 100% (99‐100) and 100% (99‐100), 368 (52‐2605) and 92 (34.7‐343.8) and zero (both markers), respectively.
Figure 1.
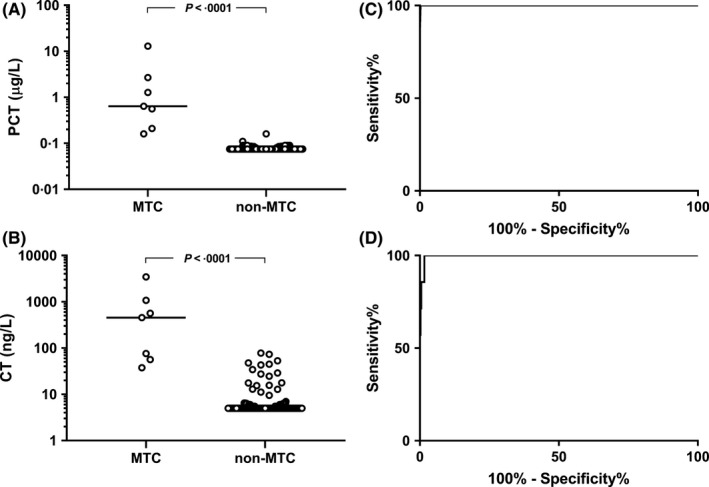
Procalcitonin (PCT) (A) and calcitonin (CT) (B) distribution in patients with histologically proved medullary thyroid carcinoma (MTC) and non‐MTC nodules (left side) and relative ROC curves (C, D)
4. DISCUSSION
Among 2705 patients with thyroid nodules a positive PCT detected 7 MTCs (0.26%). Basal serum CT levels were concordantly positive in these cases, and both markers were equally sensitive in detecting MTC among patients carrying thyroid nodules. In patients referred to surgery, thyroid tissues were intensively examined in macroscopical analysis with 0.5 cm serial sections and totally included in cassettes for histological and immunohistochemical examination when indicated. Although this intensive examination makes it unlikely, we cannot completely exclude the missing of small focal physiologic or preneoplastic C‐cell hyperplasia (ie, more than 50 cells) or micromedullary thyroid carcinoma, due to the depth of our sections (grossly at 5 mm and subsequently on 5‐7 μm histological slides). On the other hand, excepting multiple endocrine neoplasia syndromes and familial MTC (that were preliminarily excluded from the present study) the progression from C‐cell hyperplasia to MTC and the progression or microscopic MTC to clinically overt disease is under question.35 Indeed, the sensitivity and specificity of biomarkers testing should be based on the ability to diagnose clinically relevant MTC. In this setting, PCT and CT were equally sensitive in detecting MTC. However, among 378 patients with histologically confirmed MTC (n = 7) or nonmedullary outcome (n = 371), PCT and CT results were falsely positive in 2 (0.5%) and 19 (5%) patients, respectively. This is well in line with previous reports and confirms that PCT measurement could significantly reduce the need for confirmatory tests as CT measurement in FNAC washouts or stimulation test. In addition, PCT is less affected by analytical, physiological, pharmacological and pathological factors that can influence results of serum CT values and produce spurious hypercalcitoninemia. Finally, from the viewpoint of a routine clinical laboratory, it is more useful to implement the PCT assay which has a much wider range of indications than the CT assay. Notably, even if differently available PCT immunoassays produce consistent analytical result several studies have been carried out to evaluate the analytical performance and the possible implications on clinical classification of patients using different methods.36 Overall, the published studies showed a satisfactory analytical alignment between BRAHMS PCT Kryptor and the other methods (r ≥ .9) with good agreements (>80%) in patient classification at the 3 conventional diagnostic thresholds for diagnosis of bacterial infections 0.50, 2.0 and 10 ng/mL,37 but the unavoidable modest analytical bias between different IMAs suggests the use of the same method for longitudinal patient monitoring. In addition, no data on agreement between different methods are available in patients carrying thyroid nodules and MTC. As a consequence, the cut‐off selected in our study should not be directly transferred to different assays, and patients’ populations and proper evaluations by local laboratories are recommended.
In conclusion, our study proves that serum PCT measurement is a reliable and accurate alternative to CT as circulating tumour marker to screen patients carrying thyroid nodules for MTC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Giovanella L, Imperiali M, Piccardo A, et al. Procalcitonin measurement to screen medullary thyroid carcinoma: A prospective evaluation in a series of 2705 patients with thyroid nodules. Eur J Clin Invest. 2018;48:e12934 https://doi.org/10.1111/eci.12934
REFERENCES
- 1. Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88:1139‐1148. [DOI] [PubMed] [Google Scholar]
- 2. Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134‐2142. [DOI] [PubMed] [Google Scholar]
- 3. Bae YJ, Schaab M, Kratzsch J. Calcitonin as biomarker for the medullary thyroid carcinoma. Recent Results Cancer Res. 2015;204:117‐137. [DOI] [PubMed] [Google Scholar]
- 4. Trimboli P, Giovanella L, Valabrega S, et al. Ultrasound features of medullary thyroid carcinoma correlate with cancer aggressiveness: a retrospective multicenter study. J Exp Clin Cancer Res. 2014;33:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trimboli P, Treglia G, Guidobaldi L, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta‐analysis. Clin Endocrinol. 2015;82:280‐285. [DOI] [PubMed] [Google Scholar]
- 6. Trimboli P, Giovanella L, Crescenzi A, et al. Medullary thyroid cancer diagnosis: an appraisal. Head Neck. 2014;36:1216‐1223. [DOI] [PubMed] [Google Scholar]
- 7. Trimboli P, Giovanella L. Serum calcitonin negative medullary thyroid carcinoma: a systematic review of the literature. Clin Chem Lab Med. 2015;53:1507‐1514. [DOI] [PubMed] [Google Scholar]
- 8. Wells SA Jr, Asa SL, Dralle H, et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. d'Herbomez M, Caron P, Bauters C, et al. Reference range of serum calcitonin levels in humans: influence of calcitonin assays, sex, age, and cigarette smoking. Eur J Endocrinol. 2007;157:749‐755. [DOI] [PubMed] [Google Scholar]
- 11. Cavalier E, Carlisi A, Chapelle JP, Delanaye P. Analytical quality of calcitonin determination and its effect on the adequacy of screening for medullary carcinoma of the thyroid. Clin Chem. 2008;54:29‐30. [DOI] [PubMed] [Google Scholar]
- 12. Costante G, Durante C, Francis Z, Schlumberger M, Filetti S. Determination of calcitonin in C‐cell disease: clinical interest and potential pitfalls. Nat Clin Pract Endocrinol Metab. 2009;5:35‐44. [DOI] [PubMed] [Google Scholar]
- 13. Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787‐803. [DOI] [PubMed] [Google Scholar]
- 14. Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2016;22:622‐639. [DOI] [PubMed] [Google Scholar]
- 15. Pina G, Dubois S, Murat A, et al. Is basal ultrasensitive measurement of calcitonin capable of substituting for the pentagastrin‐stimulation test? Clin Endocrinol. 2013;78:358‐364. [DOI] [PubMed] [Google Scholar]
- 16. Daumerie C, Maiter D, Gruson D. Serum calcitonin estimation in medullary thyroid cancer: basal or stimulated levels? Thyroid Res. 2013;6(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doyle P, Duren C, Nerlich K, et al. Potency and tolerance of calcitonin stimulation with high‐dose calcium versus pentagastrin in normal adults. J Clin Endocrinol Metab. 2009;94:2970‐2974. [DOI] [PubMed] [Google Scholar]
- 18. Russo M, Scollo C, Padova G, Vigneri R, Pellegriti G. Cardiac arrest after intravenous calcium administration for calcitonin stimulation test. Thyroid. 2014;24:606‐607. [DOI] [PubMed] [Google Scholar]
- 19. Mian C, Perrino M, Colombo C, et al. Refining calcium test for the diagnosis of medullary thyroid cancer: cutoffs, procedures, and safety. J Clin Endocrinol Metab. 2014;99:1656‐1664. [DOI] [PubMed] [Google Scholar]
- 20. Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941‐952. [DOI] [PubMed] [Google Scholar]
- 21. Schuetz P, Christ‐Crain M, Huber AR, Mueller B. Long‐term stability of procalcitonin in frozen samples and comparison of Kryptor and VIDAS automated immunoassays. Clin Biochem. 2010;43:341‐344. [DOI] [PubMed] [Google Scholar]
- 22. Hausfater P, Brochet C, Freund Y, Charles V, Bernard M. Procalcitonin measurement in routine emergency medicine practice: comparison between two immunoassays. Clin Chem Lab Med. 2010;48:501‐504. [DOI] [PubMed] [Google Scholar]
- 23. Algeciras‐Schimnich A, Preissner CM, Theobald P, Finseth MS, Grebe SKG. Procalcitonin: a marker for the diagnosis and follow‐up of patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2009;94:861‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kratzsch J, Petzold A, Raue F, et al. Basal and stimulated calcitonin and procalcitonin by various assays in patients with and without medullary thyroid cancer. Clin Chem. 2011;57:467‐474. [DOI] [PubMed] [Google Scholar]
- 25. Giovanella L, Verburg FA, Imperiali M, Valabrega S, Trimboli P, Ceriani L. Comparison of serum calcitonin and procalcitonin in detecting medullary thyroid carcinoma among patients with thyroid nodules. Clin Chem Lab Med. 2013;51:1477‐1481. [DOI] [PubMed] [Google Scholar]
- 26. Lim SK, Guéchot J, Vaubourdolle M. Negative predictive value of procalcitonin in medullary thyroid carcinoma. Ann Biol Clin. 2016;74:213‐218. [DOI] [PubMed] [Google Scholar]
- 27. Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab. 2014;99:2986‐2994. [DOI] [PubMed] [Google Scholar]
- 28. Trimboli P, Seregni E, Treglia G, Alevizaki M, Giovanella L. Procalcitonin for detecting medullary thyroid carcinoma: a systematic review. Endocr Relat Cancer. 2015;22:157‐164. [DOI] [PubMed] [Google Scholar]
- 29. Fadda G, Basolo F, Bondi A, et al. Cytological classification of thyroid nodules. Proposal of the SIAPEC‐IAP Italian Consensus Working Group. Pathologica. 2010;102:405‐408. [PubMed] [Google Scholar]
- 30. Nardi F, Basolo F, Crescenzi A, et al. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest. 2014;37:593‐599. [DOI] [PubMed] [Google Scholar]
- 31. Giovanella L, Imperiali M, Ferrari A, et al. Thyroid volume influences serum calcitonin levels in a thyroid‐healthy population: results of a 3‐assay, 519 subjects study. Clin Chem Lab Med. 2012;50:895‐900. [DOI] [PubMed] [Google Scholar]
- 32. Trimboli P, Cremonini N, Ceriani L, et al. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity in detecting medullary thyroid cancer: a retrospective multicentre study. Clin Endocrinol. 2014;80:135‐140. [DOI] [PubMed] [Google Scholar]
- 33. Trimboli P, Guidobaldi L, Bongiovanni M, Crescenzi A, Alevizaki M, Giovanella L. Use of fine‐needle aspirate calcitonin to detect medullary thyroid carcinoma: a systematic review. Diagn Cytopathol. 2016;44:45‐51. [DOI] [PubMed] [Google Scholar]
- 34. Galen RS, Gambino SR. Beyond Normality: The Predictive Value and Efficiency of Medical Diagnosis. New York, NY: Wiley; 1975:10‐40. [Google Scholar]
- 35. Valle LA, Kloos RT. The prevalence of occult medullary thyroid carcinoma at autopsy. J Clin Endocrinol Metab. 2011;96:109‐113. [DOI] [PubMed] [Google Scholar]
- 36. Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34:263‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dipalo M, Guido L, Micca G, et al. Multicenter comparison of automated procalcitonin immunoassays. Pract Lab Med. 2015;2:22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]


