Abstract
We investigated whether high‐quality in vitro matured (IVM) oocytes can be distinguished from poor ones based on the morphological changes after treatment with hyperosmotic medium containing 0.2 mol/L sucrose in pigs. We hypothesize that IVM oocytes maintaining round shape have higher quality than mis‐shapened oocytes following dehydration. Oocyte quality was verified by determining embryonic developmental competence using in vitro fertilization, nuclear transfer and parthenogenetic activation. In all cases, the round oocytes had greater (p < .05) developmental competence than that of mis‐shapened oocytes in terms of blastocyst rate and total cell number in blastocysts obtained after 6 days of in vitro culture. We also confirm that round aged oocytes are higher in quality than mis‐shapened aged oocytes. In an attempt to find out why high‐quality oocytes maintain a round shape whereas poorer oocytes become mis‐shapened following sucrose treatment, we examined the arrangement of actin microfilaments and microtubules. Abnormal organization of these cytoskeletal components was higher (p < .05) in mis‐shapened oocytes compared to round oocytes after 52 hr of IVM. In conclusion, sucrose treatment helps selection of high‐quality oocytes, including aged oocytes, in pigs. Abnormal cytoskeleton arrangements partly explain for low developmental competence of mis‐shapened oocytes.
Keywords: embryonic development competence, oocyte quality, pig, sucrose
1. INTRODUCTION
The in vitro production (IVP) of embryos in porcine has increasingly gained relevance for biomedical applications as pigs and humans share similarities in physiology, anatomy, genome and organ size (reviewed in Abeydeera, 2002; Gil et al., 2010; Muenthaisong, Dinnyes, & Nedambale, 2011). Genetically modified pigs could be used as research models for human genetic disease, for xenotransplantation, as well as for stem cell research (Gil et al., 2010; Hou et al., 2016; Okere & Nelson, 2002). Among the widely used techniques in porcine IVP, in vitro fertilization (IVF), somatic cell nuclear transfer (SCNT) and parthenogenetic activation (PA) are essential for agricultural and biomedical applications (Gil et al., 2010; Muenthaisong et al., 2011). Oocyte quality is a determinant in the success of these techniques (El‐Baby et al., 2017; Gil et al., 2010; Marteil, Parpaillon, & Kubiak, 2009; Wu et al., 2007) because of its effects on the subsequent development competence of the embryo (Bakri et al., 2016; Goeseels & Panich, 2002; Krisher, 2003) and implantation (Danasouri et al., 2016). In addition, since SCNT and intracytoplasmic sperm injection (ICSI) are technically demanding and time‐consuming procedures, elimination of poor oocytes would increase efficiency. Moreover, as culturing good embryos together with poor‐quality embryos could reduce the developmental potential of the good embryos (Tao, Robichaud, Mercier, & Ouellette, 2013), elimination of poor embryos before culture would help to avoid this negative effect. Since numerous porcine oocytes can be collected from ovaries obtained from slaughterhouses, it would be advantageous to select only the good ones for experimentation following in vitro maturation (IVM). Although the colorimetric brilliant cresyl blue (BCB) assay has been widely used to classify the oocyte quality in many species (Alm et al., 2005; Ericsson, Boice, Funahashi, & Day, 1993; Heleil, Kuzmina, Novikova, Torner, & Alm, 2010; Ishizaki, Watanabe, Bhuiyan, & Fukui, 2009; Rodriguez‐Gonzalez, Lopez‐Bejar, Izquierdo, & Paramio, 2003; Wang et al., 2012; Wu et al., 2007), its use may be not recommended as it causes DNA damage, inducing apoptosis and compromising embryo development (Opiela et al., 2008; Scholkamy, Darwish, & Mahmoud, 2015; Wongsrikeao et al., 2006).
Sucrose treatment was initially used for diffusing cryoprotectants out of the embryos during the dehydration step in oocyte/embryo cryopreservation (Leibo, 1984). Sucrose was then used as a method to aid enucleation in SCNT in mice (Wang, Liu, Li, Lian, & Chen, 2001), but it also enabled the classification of oocyte quality. The poorer quality oocytes shrunk in an irregular manner, whereas good‐quality oocytes shrank spherically and showed a swelling around the meiotic spindle after treatment with the medium containing sucrose at slightly different concentrations dependent on the species (Lee et al., 2014; Liu, Sung, Barber, & Yang, 2002; Wang et al., 2001). However, the mechanism influencing oocyte quality based on the response in hyperosmotic medium is not fully understood.
In this study, we investigated whether high‐quality porcine IVM oocytes, including in vitro aged oocytes, can be distinguished from the poorer ones based on the morphological changes after being treated with 0.2 mol/L sucrose. This was determined by comparing the developmental ability of the two morphological types of oocytes in vitro. We then examined their cytoskeleton which is known to determine cell shape.
2. MATERIALS AND METHODS
2.1. Oocyte collection and IVM
Porcine ovaries were collected from pre‐pubertal cross‐bred gilts (Landrace × Large White × Duroc) at a local abattoir and transported to the laboratory in Dulbecco's phosphate buffered saline (PBS; Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) at 35°C within 1 hr. Cumulus‐oocyte complexes (COCs) were aspirated from 3–6 mm follicles, and cultured in groups of 40–50 in 500 μL of modified North Carolina State University (NCSU)‐37 medium (Petters & Wells, 1993) without oil overlay according to Kikuchi et al. (2002) in four‐well dishes (Nunclon Multidishes, Nalge Nunc International, Roskilde, Denmark) for 22 hr. The IVM medium was modified by adding 10% (v/v) porcine follicular fluid, 0.6 mmol/L cysteine (Sigma, St. Louis, MO, USA), 50 mmol/L β‐mercaptoethanol (Axon Medchem, Groningen, Netherlands), 1 mmol/L dibutyryl cyclic adenosine 3ʹ,5ʹ‐monophosphate (dbcAMP; Sigma), 10 IU/mL equine chorionic gonadotropin (Serotropin; ASKA Pharmaceutical Co. Ltd., Tokyo, Japan), and 10 IU/mL human chorionic gonadotropin (Puberogen; Novartis Animal Health, Tokyo, Japan). The COCs were then transferred to IVM medium without dbcAMP and hormones and cultured for another 22 hr. Aged oocytes were cultured in the second IVM medium for 30 hr. IVM was performed in 5% CO2 and 20% O2 at 38.5°C. Aged oocytes are oocytes that matured in vitro for a longer period of time compared with control oocytes. In this study, aged oocytes were matured for 52 hr whereas control oocytes were matured for 44 hr.
2.2. Sucrose treatment
After IVM, cumulus cells were removed from the COCs by gentle pipetting after treatment with 0.1% (w/v) hyaluronidase (Sigma) for 2–5 min, and the denuded oocytes were then washed three times in Medium 199 (with Hanks’ balanced salts; Sigma) supplemented with 10% (v/v) fetal calf serum (FCS) (Gibco, Life Technologies Inc., Grand Island, NE, USA), 20 mmol/L HEPES (Dojindo Laboratories, Kumamoto, Japan), antibiotics (100 units/mL penicillin G potassium [Sigma] and 0.1 mg/mL streptomycin sulfate [Sigma]), pH adjusted to 7.4. After washing, oocytes selected for the presence of the polar body were treated with 0.2 mol/L sucrose in Medium 199 supplemented with HEPES and 5% FCS (524 mOsm/L) for 5 min. Based on the changes of morphology after sucrose treatment, the oocytes were divided into two groups, termed round and mis‐shapened (Figure 1a,b). After treatment with sucrose, the oocytes were washed three times in Medium 199 with HEPES plus 5% FCS to completely remove sucrose. Oocytes were then used for either IVF, SCNT or PA.
Figure 1.
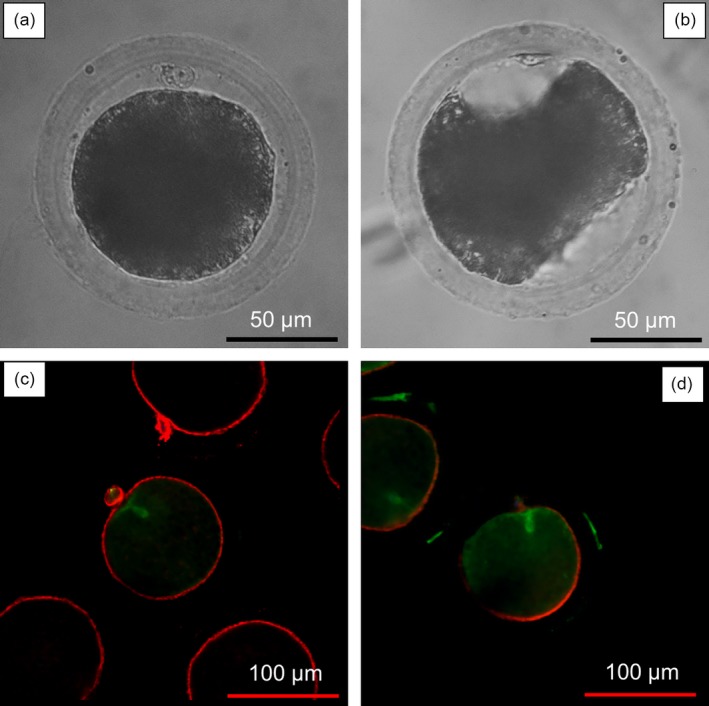
Morphological changes and arrangement of actin microfilaments and microtubules in round (a and c) and mis‐shapened oocytes (b and d) after treatment with sucrose at 52 hr of in vitro maturation
2.3. IVF
IVF was conducted according to Kikuchi et al. (2002). The medium for IVF was a modified Pig‐FM (Suzuki et al., 2002), comprising 90 mmol/L NaCl, 12 mmol/L KCl, 25 mmol/L NaHCO3, 0.5 mmol/L NaH2PO4, 0.5 mmol/L MgSO4, 10 mmol/L sodium lactate, 10 mmol/L HEPES, 8 mmol/L CaCl2, 2 mmol/L sodium pyruvate, 2 mmol/L caffeine, and 5 mg/mL bovine serum albumin (BSA; Fraction V, Sigma). Groups of 15–20 oocytes were transferred into 100 μL IVF droplets covered with paraffin oil. Frozen‐thawed epididymal spermatozoa were pre‐incubated in Medium 199 (with Earle's salts and HEPES, pH adjusted to 7.8) for 15 min. The sperm were then diluted to give a final concentration of 1 × 105 sperm/mL in the IVF medium droplet with oocytes. Sperm and oocytes were co‐incubated for 30 min at 38.5°C in 5% CO2, 5% O2, and 90% N2. The oocytes and bound sperm were then transferred into fresh IVF droplets without sperm for another 2.5 hr under the same conditions.
2.4. SCNT
The cumulus cells were removed from COCs following IVM and then collected, washed with PBS and used as donor cells for SCNT on the same day. SCNT was performed according to Akagi et al. (2008) with modifications. In brief, sucrose‐treated oocytes with a first polar body were enucleated in HEPES‐buffered Medium 199 supplemented with 20% FCS and 5 μg/mL Cytochalasin D (Sigma). Successful enucleation was confirmed by staining individual karyoplasts in 20 μg/mL Hoechst 33342 (Sigma) with visualization in a fluorescence microscope. Donor cells were transferred to the perivitelline space of enucleated oocytes. The oocyte‐cell complex was orientated between a pair of parallel electrodes, 1 mm apart, in fusion medium comprising 0.3 mol/L mannitol, 1 mmol/L CaCl2, 0.1 mmol/L MgCl2, and 0.5 mmol/L HEPES and 0.1 mg/mL BSA. A single direct current (DC) pulse of 3 kV/cm for 10 μs was applied for oocyte‐cell fusion using electro cell fusion generator LF101 (Nepa Gene Co., Ltd. Chiba, Japan). Fused embryos were activated 3–4 hr after fusion in the same manner as for PA oocytes and cultured as described below.
2.5. Parthenogenetic activation
Sucrose‐treated oocytes were parthenogenetically activated by a single DC pulse of 1.5 kV/cm for 100 μs using the same mannitol buffer as for fusion in SCNT. Electrically stimulated oocytes were then cultured 3 hr in IVC‐PyrLac supplemented with 5 μg/mL cytochalasin D. The oocytes were then transferred to IVC‐PyrLac as described below.
2.6. IVC
Presumptive zygotes produced by IVF, PA and SCNT were then transferred into IVC media. Two types of IVC media were prepared (Kikuchi et al., 2002). The basic IVC medium was NCSU‐37 modified by the addition of 0.4% (w/v) BSA and 50 μmol/L β‐mercaptoethanol. Embryos were cultured at 38.5°C under 5% CO2, 5% O2, and 90% N2 in IVC‐PyrLac (basic IVC medium with the addition of 0.17 mmol/L sodium pyruvate and 2.73 mmol/L sodium lactate) from Day 0 to Day 2, and then in IVC‐Glu (basic medium supplemented instead with 5.55 mmol/L glucose) until Day 6. Day 0 was designated the day of IVF, PA or SCNT.
2.7. Actin and tubulin staining
Staining of actin microfilaments and microtubules was performed according to Somfai et al. (2011). Briefly, the oocytes were denuded using a fine glass pipette in HEPES‐buffered Medium 199 (Hanks’ salt) supplemented with 5% FCS and 0.1% (w/v) hyaluronidase. After sucrose treatment, the round or mis‐shapened oocytes were then fixed with 3.7% (w/v) paraformaldehyde for 1 hr at room temperature (RT). After washing in PBS with 0.2% (w/v) Triton X‐100 (PBS‐T) at RT for 30 min, the oocytes were kept in a blocking solution, comprising PBS with 20 mg/ml BSA and 150 mmol/L glycine overnight at 4°C. After washing three times in PBS‐T, the oocytes were incubated with anti‐α‐tubulin primary antibody (Sigma T‐5168) at a dilution of 1:100 for 2 hr at 37°C in PBS‐T. The oocytes were then washed twice in PBS‐T. Following that, they were incubated with Alexa Fluor 488‐conjugated secondary antibody (A21121; Invitrogen, Tokyo, Japan) (1:1500) for 1 hr at 37°C. After two consecutive washings in PBS‐T, the oocytes were incubated with 1 IU/ml rhodamine phalloidin (R415; Invitrogen) in PBS‐T for 30 min at RT to stain F‐actin and washed twice again. The oocytes were then mounted on glass slides with anti‐fade solution (Component A; A2828; Invitrogen) supplemented with 10 μg/ml Hoechst 33342 (Calbiochem, San Diego, CA, USA). The mounted samples were sealed with clear nail polish and kept in the dark at 4°C until observation.
Fluorescent signals revealing the distribution of actin microfilaments and the position of meiotic spindles were visualized with a confocal laser scanning microscope (D‐eclipse C1; Nikon, Tokyo, Japan) equipped with an argon‐krypton‐helium/neon ion laser for simultaneous observation of Alexa Fluor 488 and Rhodamine using 488/543 nm excitation barrier filter combinations, respectively. Fluorescent images of microtubules and actin microfilaments were taken and simultaneously merged.
In metaphase II oocytes, normal actin microfilaments were considered to be evenly spaced beneath the plasma membrane, whereas abnormal microfilaments were discontinuously arranged or diffused (Sun et al., 2001). Normal microtubules in mature oocytes were classified as having the polar body cap integrated, whereas abnormal microtubules did not or were discontinuously arranged (Figure 1c,d) (Sun et al., 2001).
2.8. Evaluation of total cell number in blastocysts
To evaluate the total cell number in embryos, Day 6 blastocysts were stained with Hoechst 33342. Blastocysts were washed in PBS and treated for 5 min with 99.5% ethanol supplemented with 50 μg/mL Hoechst 33342. The blastocysts were then washed in PBS supplemented with 0.3% polyvinylpyrrolidone (Sigma) and mounted in glycerol on a glass slide and covered with a cover slip. The blastocysts were gently flattened under the coverslip, so that the cells appeared in the same focal plane. Total cell numbers were counted using an epi‐fluorescent microscope (Nikon Diaphot 200; Nikon, Tokyo, Japan).
2.9. Experimental design
2.9.1. Effect of sucrose treatment on further embryonic development
Oocytes after 44 hr of IVM were denuded and treated either with or without 0.2 mol/L sucrose (as control group). All oocytes with a first polar body from each group were then fertilized with frozen‐thawed epididymal spermatozoa. The blastocyst rate and total cell number in blastocysts on Day 6 were compared between groups.
2.9.2. Development of round and mis‐shapened oocytes following IVF, SCNT, and PA
After 44 hr of IVM, the oocytes were freed of cumulus and treated with 0.2 mol/L sucrose. Based on the changes in morphology after sucrose treatment, the oocytes were divided into two groups, round or mis‐shapened. The oocytes were separately then used for IVF, SCNT, and PA. The blastocyst rates and total cell number in blastocysts on Day 6 were determined. In addition, the sperm penetration, monospermy, and polyspermy rates were evaluated 10 hr post‐IVF and were compared between the two groups.
2.9.3. Actin microfilament, microtubule structure, and developmental competence of round and mis‐shapened oocytes after 44 and 52 hr of maturation
After 44 and 52 hr of IVM, oocytes were denuded and treated with hyperosmotic medium containing 0.2 mol/L sucrose. The oocytes were then divided into either round or mis‐shapened oocyte groups. A number of oocytes were subjected to immuno‐fluorescence staining for actin microfilaments and microtubules, whereas the remains were used for IVF, followed by IVC.
2.10. Statistical analysis
The data on sperm penetration, monospermy, polyspermy (for IVF), blastocyst rates (expressed as percentage development of total embryos cultured), and mean cell number in blastocysts, as well as percentages of oocytes with abnormal actin microfilament and microtubules, were analyzed by one‐way analysis of variance followed by Tukey test using the Statview 5 software package (SAS Institute Inc., Cary, NC, USA). Percentage data were arcsine transformed before analysis. Data are expressed as mean ± SEM. p < .05 was considered statistically significant.
3. RESULTS
3.1. Effect of sucrose treatment on further embryonic development
There was no significant difference (p > .05) in the blastocyst formation rates, or the total cell number in blastocysts on Day 6, after IVF between the sucrose treatments (34.0% ± 5.7% and 40.9% ± 3.8%, respectively) compared to the control group (34.6% ± 6.0% and 44.7% ± 2.8%).
3.2. Development of round and mis‐shapened oocytes after IVF, SCNT, and PA
The results showed that after IVF there was no significant difference (p > .05) in penetration, monospermy, and polyspermy rates between the round group (55.5% ± 4.9%, 78.0% ± 2.9%, and 22.0% ± 2.9%, respectively) and the mis‐shapened group (45.0% ± 2.9%, 76.9% ± 7.7%, and 23.1% ± 7.7%, respectively) following IVF. However, the percentage of oocytes developing to the blastocyst stage and total cell numbers in blastocysts on Day 6 from the round group (33.0% ± 4.4% and 49.2% ± 2.1%, respectively) were significantly greater (p < .05 and p < .01) than those from mis‐shapened oocytes (10.5% ± 3.8% and 33.8% ± 4.7%, respectively) (Figure 2a).
Figure 2.
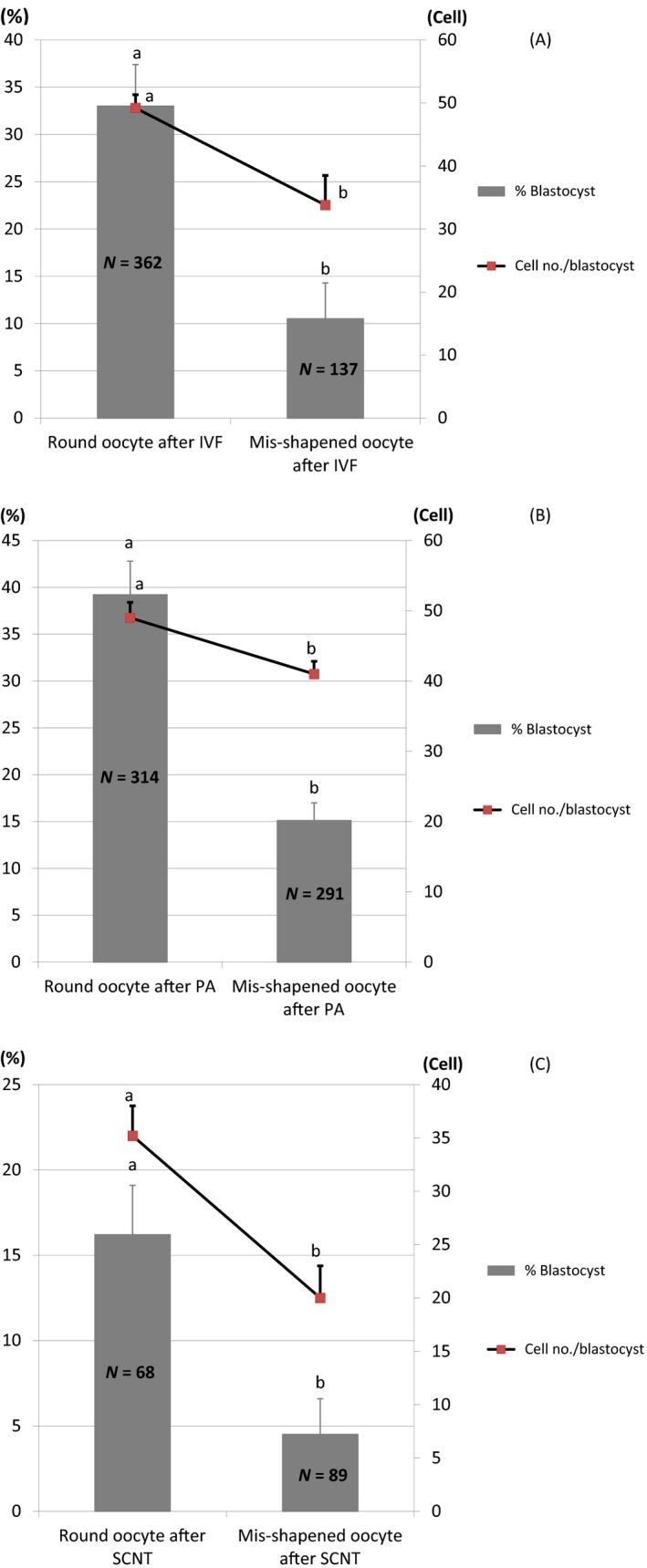
Developmental competence of round and mis‐shapened oocytes after in vitro fertilization (IVF) (A), somatic cell nuclear transfer (SCNT) (B), and parthenogenetic activation (PA) (C). Data are expressed as mean ± SEM. abValues with different superscripts are significantly different (p < .05)
Three to five replications of each IVP method were carried out
Similar to the results with IVF, the percentage of oocytes developing to the blastocyst stage and the total cell numbers in blastocysts on Day 6 after SCNT in the round group (16.2% ± 2.9% and 35.2% ± 2.8%, respectively) were significantly greater (p < .05) than those in the mis‐shapened group (4.5% ± 2.1% and 20.0% ± 3.0%, respectively) (Figure 2b).
Consistent with the results from IVF and SCNT, both the proportion of embryos that developed into blastocysts and their total cell number on Day 6 in PA derived from the round group (39.2% ± 3.6% and 49.0% ± 2.2%, respectively) were significantly greater (p < .01 and p < .05, respectively) than those from the mis‐shapened group (15.1% ± 1.9% and 41.0% ± 1.8%, respectively) (Figure 2c).
3.3. Actin microfilament, microtubule structure, and developmental competence of round and mis‐shapened oocytes after 44 and 52 hr of maturation
There was no significant difference (p > .05) in the proportion of abnormal actin microfilaments and microtubules in mis‐shapened oocytes (2.8% ± 1.7% and 4.7% ± 2.0%, respectively) compared to round oocytes (0.6% ± 0.6% and 1.3% ± 0.6%, respectively) after 44 hr of IVM (Figure 3a). However, after 52 hr of IVM, the proportion of mis‐shapened oocytes with abnormal actin microfilaments and microtubules increased dramatically (27.5% ± 2.3% and 18.8% ± 4.2%, respectively) and was significantly higher (p < .05) than those in the round oocytes (3.4% ± 0.5% and 0.7% ± 0.7%, respectively) (Figure 3b).
Figure 3.
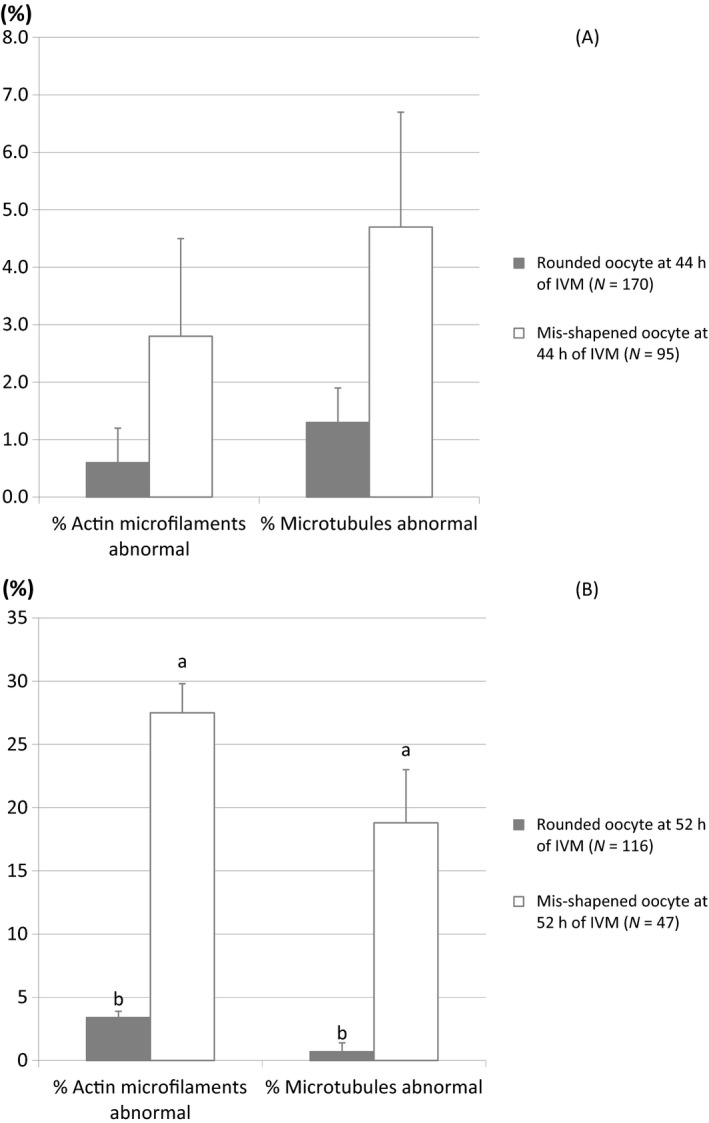
Actin microfilaments and microtubules in round and mis‐shapened oocytes at 44 hr (a) and 52 hr (b) of in vitro maturation.
Data are expressed as mean ± SEM.
abValues with different superscripts are significantly different (p < .05). Three replications were carried out
In agreement with the results shown previously in Figure 2a, we found that the blastocyst rate and cell number in blastocysts derived from round oocytes fertilized after 44 hr of IVM (40.6% ± 3.5% and 56.6 ± 3.0, respectively) were significantly higher (p < .05) than those derived from mis‐shapened oocytes fertilized after 44 hr of IVM (16.4% ± 2.2% and 37.6% ± 4.3%, respectively) (Table 1). Although the proportion of blastocyst derived from round oocytes fertilized after 52 hr of IVM (28.6% ± 3.5%) were significantly higher (p < .05) than that derived from mis‐shapened oocytes fertilized after 52 hr of IVM (3.6% ± 1.4%), cell numbers in blastocysts derived from these two groups were not significantly different (Table 1). The percentage of round oocytes drastically dropped from 60% (160/276) after 44 hr of IVM to 28.4% (77/271) after 52 hr of IVM (Table 1).
Table 1.
Development of porcine oocytes matured for 44 and 52 hr to blastocysts
| Maturation duration (hr) | Oocyte shape after sucrose treatment | No. of embryos | No. of blastocysts (%) | Cell no. in blastocysts |
|---|---|---|---|---|
| 44 | Round | 160 | 65 (40.6 ± 3.5)a | 56.6 ± 3.0a |
| Mis‐shapened | 116 | 19 (16.4 ± 2.2)b | 37.6 ± 4.3b | |
| 52 | Round | 77 | 21 (28.6 ± 3.5)ab | 47.7 ± 4.2ab |
| Mis‐shapened | 194 | 7 (3.6 ± 1.4)c | 28.0 ± 7.2b |
Three replications were performed.
abcValues with different superscripts are significantly different (p < .05).
Data are expressed as mean ± SEM.
4. DISCUSSION
In the present study, we examined whether good‐quality oocytes can be distinguished from low‐quality ones based on simply incubating oocytes in hyperosmotic medium supplemented with 0.2 mol/L sucrose for 5 min and assessing the resulting morphology. After short incubation in hyperosmotic medium containing 0.2 mol/L sucrose, all the oocytes dehydrated and shrank in size. However, a number of oocytes still maintain a round shape, whereas others become mis‐shapened or “deformed”. Based on previous reports, we hypothesize that oocytes that maintain a round shape are of higher quality than oocytes having “irregular” shape after treatment with hyperosmotic medium containing 0.2 mol/L sucrose. The suitable sucrose concentration depended on species such as 0.09 mol/L in mouse (Wang et al., 2001), 0.3 mol/L in bovine (Liu et al., 2002) or 0.345 mol/L in pigs (Lee et al., 2014). In this study, we have examined with several sucrose concentrations (data not shown) and used hyperosmotic medium containing 0.2 mol/L sucrose. According to the morphological changes, we observed a similar effect as previous studies in determining oocyte quality.
We first examined whether treating oocytes with sucrose would compromise developmental competence of porcine oocytes. There was no significant difference in the blastocyst formation rate and the total cell number in blastocysts on Day 6 after IVF between sucrose treatment and control groups. Therefore, sucrose treatment per se did not have a negative effect on embryonic developmental competence. This observation was similar to previous reports in mouse (Wang et al., 2001), bovine (Liu et al., 2002) and pigs (Lee et al., 2014).
We next examined our hypothesis that oocytes that maintain a round shape in 0.2 mol/L sucrose medium are of higher quality, whereas those oocytes that become mis‐shapened are of poorer quality. The results showed that oocytes that maintained a round shape had significantly greater developmental competence than that of mis‐shapened oocytes after IVF, SCNT or PA in terms of the blastocyst formation rates and the total cell number in blastocysts on Day 6. These results were similar to previous reports in mouse (Wang et al., 2001), bovine (Liu et al., 2002) and pigs (Lee et al., 2014) and confirmed our hypothesis. This study shows that sucrose treatment could help to distinguish good‐quality oocytes from poorer quality, and the treatment did not interfere with further developmental competence. Therefore, this suggests that a treatment with 0.2 mol/L sucrose followed by morphological evaluation is a simple and efficient method for selection of high‐quality porcine IVM oocytes.
Microfilaments and microtubules are major components of the cytoskeletal network that determine the shape and intra‐cellular movement of organelles in mammalian cells and oocytes (Kim, Funahashi, Prather, Schatten, & Day, 1996; Sun & Schatten, 2006; Sun et al., 2001; Wang, Abeydeera, Prather, & Day, 2000). Moreover, both microtubule and microfilament dynamics are integrated and interact with chromosomal changes during oocyte maturation, providing a framework for chromosomal movement and cell division (Kim et al., 1996). The cytoskeleton is also an important modulator of many fertilization and post‐fertilization events (Kim, Chung, & Day, 1997; Sun et al., 2001). Actin microfilaments in particular have an important role in cell division (Cao & Wang, 1990; Kim et al., 1996) and abnormal microfilament distribution has been observed in in vitro matured and fertilized oocytes (Wang, Abeydeera, Han, Prather, & Day, 1999). Based on these findings, we hypothesized higher frequencies of microfilament and microtubule abnormalities in mis‐shapened oocytes compared to round oocytes following hyperosmotic treatment. To confirm this, we examined the arrangement of actin microfilaments and microtubules in mis‐shapened and round oocytes at 44 and 52 hr of IVM. Porcine oocytes are typically matured for 44 hr before fertilization, SCNT or PA. Although the incidence of abnormal actin microfilaments and microtubules in mis‐shapened oocytes was similar to that in round oocytes after 44 hr of IVM, this proportion increased dramatically and was significantly greater than that in round oocytes after 52 hr of IVM (Figure 3). Previous studies reported that both microtubules and microfilaments might not be required for pronuclear formation (Kim et al., 1997). This may explain why there were no differences in sperm penetration, monospermy, and polyspermy rates between round and mis‐shapened oocytes after IVF. However, normal actin filaments are required for normal cell division (Cao & Wang, 1990; Sun et al., 2001). Thus, abnormal actin filament distribution is a possible reason for the abnormal cleavage, fragmentation and low cell numbers commonly associated with in vitro produced porcine embryos (Wang et al., 1999, 2000). In addition, microtubule assembly affects pronuclear migration, syngamy and cytokinesis in zygotes (Sun et al., 2001), and is required for contractile ring formation during cleavage (Sun & Schatten, 2006). Taken together, this suggests that the abnormalities of actin microfilaments and microtubules that occur more frequently in mis‐shapened oocytes compared to round oocytes at later stages of IVM may be one reason for the lower developmental competence of mis‐shapened oocytes.
The remarkable increase in proportion of abnormal actin microfilaments and microtubules in mis‐shapened oocytes after 52 hr of IVM led us to perform IVF and follow development of those aged oocytes. It has been widely known that in vitro aging causes various functional disorders in oocytes and in derived zygotes and embryos, including chromosomal anomalies, apoptosis, decreased fertilization rate, increased incidence of polyspermy, reduction in maturation promoting factor (reviewed in Miao, Kikuchi, Sun, & Schatten, 2009), and cytoskeletal abnormalities (Somfai et al., 2011). However, we found that, in our study, round aged oocytes have similar developmental competence compared with round oocytes matured in vitro for 44 hr in terms of development to blastocysts and total cell number in blastocysts. Unlike round aged oocytes, mis‐shapened aged oocytes had lower potential to reach blastocyst stage compared with mis‐shapened oocytes matured in vitro for 44 hr. This suggested that sucrose treatment might also help to select good‐quality oocytes among aged oocytes. This gives another option for researchers working with aged oocytes besides reversing the aging process by controlling the activity of maturation promoting factor (Kikuchi et al., 2000) or by transferring nuclear material of an aged oocyte into the cytoplast of a fresh oocyte (reviewed in Miao et al., 2009). Nevertheless, the percentage of round oocytes = drastically dropped from 60% (160/276) after 44 hr of IVM to 28.4% (77/271) after 52 hr of IVM, suggesting fewer and fewer good‐quality oocytes can be selected among the aged oocytes. Moreover, a good blastocyst rate alone does not guarantee normal development to term.
In conclusion, a hyperosmotic medium containing 0.2 mol/L sucrose assists the selection of high‐quality oocytes, including aged oocytes, in pigs. The maintenance of a round shape after treatment with sucrose and the integrity of actin microfilaments and microtubules are criteria for high‐quality porcine oocytes. However, further research on the arrangement of the cytoskeleton at later developmental time points in IVF, SCNT and PA embryos comparing round and mis‐shapened oocytes is required.
ACKNOWLEDGMENTS
We would like to thank Ms. Ando, Ms. Suzuki and Ms. Nagai for technical assistance. This study was partly supported by a JSPS – MBIE/RSNZ joint bilateral research project (David Wells, Kazuhiro Kikuchi, Takashi Nagai and Thanh Quang Dang‐Nguyen).
Dang‐Nguyen TQ, Nguyen HT, Somfai T, et al. Sucrose assists selection of high‐quality oocytes in pigs. Anim Sci J. 2018;89:880–887. https://doi.org/10.1111/asj.13015
REFERENCES
- Abeydeera, L. R. (2002). In vitro production of embryos in swine. Theriogenology, 57, 257–273. [DOI] [PubMed] [Google Scholar]
- Akagi, S. , Kaneyama, K. , Adachi, N. , Tsuneishi, B. , Matsukawa, K. , Watanabe, S. , … Takahashi, S. (2008). Bovine nuclear transfer using fresh cumulus cell nuclei and in vivo‐ or in vitro‐matured cytoplasts. Cloning and Stem Cells, 10, 173–180. [DOI] [PubMed] [Google Scholar]
- Alm, H. , Torner, H. , Lohrke, B. , Viergutz, T. , Choneim, I. M. , & Kanitz, W. (2005). Bovine blastocyst development rate in vitro is influenced by selection of oocytes by brilliant cresyl blue staining before IVM as indicator for glucose‐6‐phosphate dehydrogenase activity. Theriogenology, 63, 2194–2205. [DOI] [PubMed] [Google Scholar]
- Bakri, N. M. , Ibrahim, S. F. , Osman, N. A. , Hasan, N. , Jaffar, F. H. F. , Rahman, Z. A. , & Osman, K. (2016). Embryo apoptosis identification: Oocyte grade or cleavage stage? Saudi Journal of Biological Sciences, 23, S50–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. G. , & Wang, Y. L. (1990). Mechanism of the formation of contractile ring in dividing cultured animal cells. II. Cortical movement of microinjected actin filaments. Journal of Cell Biology, 111, 1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danasouri, E. L. , Sterzik, K. , Rinaldi, L. , Pacchiarotti, A. , Desanto, M. , & Selman, H. (2016). Effect of transferring a morphologically impaired embryo on the pregnancy and implantation rates. European Review for Medical and Pharmacological Sciences, 20, 394–398. [PubMed] [Google Scholar]
- El‐Baby, A. A. H. , Mahmoud, K. G. M. , El‐Raey, M. , Ahmed, Y. F. , Abouel‐Roos, M. E. A. , & Sosa, G. A. M. (2017). Impact of using brilliant cresyl blue stain on oocyte and embryo selection. Egyptian Journal of Veterinary Science, 48, 43–51. [Google Scholar]
- Ericsson, S. A. , Boice, M. L. , Funahashi, H. , & Day, B. N. (1993). Assessment of porcine oocytes using brilliant cresyl blue. Theriogenology, 39, 214. [Google Scholar]
- Gil, M. A. , Cuello, C. , Parrilla, I. , Vazquez, J. M. , Roca, J. , & Martinez, E. A. (2010). Advances in swine in vitro embryo production technologies. Reproduction Domestic Animal, 45, 40–48. [DOI] [PubMed] [Google Scholar]
- Goeseels, S. B. , & Panich, P. (2002). Effects of oocyte quality on development and transcriptional activity in early bovine embryos. Animal Reproduction Science, 71, 143–155. [DOI] [PubMed] [Google Scholar]
- Heleil, B. , Kuzmina, T. , Novikova, N. , Torner, H. , & Alm, H. (2010). Effect of prolactin on development competence of bovine oocytes selected by brilliant cresyl blue staining. Journal of Reproduction and Infertility, 1, 1–7. [Google Scholar]
- Hou, D. R. , Jin, Y. , Nie, X. W. , Zhang, M. L. , Ta, N. , Zhao, L. H. , … Li, R. F. (2016). Derivation of porcine embryonic stem‐like cells from in vitro‐produced blastocyst‐stage embryos. Scientific Reports, 6, 25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki, C. , Watanabe, H. , Bhuiyan, M. M. U. , & Fukui, Y. (2009). Developmental competence of porcine oocytes selected by brilliant cresyl blue and matured individually in a chemically defined culture medium. Theriogenology, 72, 72–80. [DOI] [PubMed] [Google Scholar]
- Kikuchi, K. , Naito, K. , Noguchi, J. , Shimada, A. , Kaneko, H. , Yamashita, M. , … Toyoda, Y. (2000). Maturation/M‐phase promoting factor: A regulator of aging in porcine oocytes. Biology of Reproduction, 63, 715–722. [DOI] [PubMed] [Google Scholar]
- Kikuchi, K. , Onishi, A. , Kashiwazaki, N. , Iwamoto, M. , Noguchi, J. , Kaneko, H. , … Nagai, T. (2002). Successful piglet production after transfer of blastocysts produced by a modified in vitro system. Biology of Reproduction, 66, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Kim, N. H. , Chung, K. S. , & Day, B. N. (1997). The distribution and requirements of microtubules and microfilaments during fertilization and parthenogenesis in pig oocytes. Journal of Reproduction and Fertility, 111, 143–149. [DOI] [PubMed] [Google Scholar]
- Kim, N. H. , Funahashi, H. , Prather, R. S. , Schatten, G. , & Day, B. N. (1996). Microtubule and Microfilament dynamic in porcine oocytes during meiotic maturation. Molecular Reproduction and Development, 43, 248–255. [DOI] [PubMed] [Google Scholar]
- Krisher, R. L. (2003). The effect of oocyte quality on development. Journal of Animal Science, 82, E14–E23. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Lee, Y. , Park, B. , Elahi, F. , Jeon, Y. , Hyun, S. H. , & Lee, E. (2014). Development competence of IVM pig oocytes after SCNT in relation to the shrinkage pattern induced by hyperosmotic treatment. Theriogenology, 81, 974–981. [DOI] [PubMed] [Google Scholar]
- Leibo, S. P. (1984). A one‐step method for direct nonsurgical transfer of frozen‐thawed bovine embryos. Theriogenology, 21, 767–790. [DOI] [PubMed] [Google Scholar]
- Liu, J. L. , Sung, L. Y. , Barber, M. , & Yang, X. (2002). Hypertonic medium treatment for localization of nuclear material in bovine metaphase II oocytes. Biology of Reproduction, 66, 1342–1349. [DOI] [PubMed] [Google Scholar]
- Marteil, G. , Parpaillon, L. R. , & Kubiak, J. Z. (2009). Role of oocyte quality in meiotic maturation and embryonic development. Reproductive Biology, 9, 203–224. [DOI] [PubMed] [Google Scholar]
- Miao, Y. L. , Kikuchi, K. , Sun, Q. Y. , & Schatten, H. (2009). Oocyte aging: Cellular and molecular changes, developmental potential and reversal possibility. Human Reproduction Update, 15, 573–585. [DOI] [PubMed] [Google Scholar]
- Muenthaisong, S. , Dinnyes, A. , & Nedambale, T. L. (2011). Review of somatic cell nuclear transfer in pig. African Journal of Biotechnology, 10, 17384–17390. [Google Scholar]
- Okere, C. , & Nelson, L. (2002). Novel Reproductive Techniques in Swine Production – A Review. Asian Australasian Journal of Animal Science, 15, 445–452. [Google Scholar]
- Opiela, J. , Katska‐Ksiazkiewicz, L. , Lipinski, D. , Slomski, R. , Bzowska, M. , & Rynska, B. (2008). Interactions among activity of glucose‐6‐phosphate dedydrogenase in immature oocytes, expression of apoptosis‐related genes Bcl‐2 and Bax, and developmental competence following IVP in cattle. Theriogenology, 69, 546–555. [DOI] [PubMed] [Google Scholar]
- Petters, R. M. , & Wells, K. D. (1993). Culture of pig embryos. Journal of Reproduction and Fertility, 48, 61–73. [PubMed] [Google Scholar]
- Rodriguez‐Gonzalez, E. , Lopez‐Bejar, M. , Izquierdo, D. , & Paramio, M. T. (2003). Developmental competence of prepubertal goat oocytes selected with brilliant cresyl blue and matured with cysteamine supplementation. Reproduction Nutrition Development, 43, 179–197. [DOI] [PubMed] [Google Scholar]
- Scholkamy, T. H. , Darwish, S. F. , & Mahmoud, K. G. M. (2015). Effect of vitrification by straw and cryotop on DNA integrity using comet assay with reference to brilliant cresyl blue exposure in buffalo oocytes. Alexandria Journal of Veterinary Sciences, 46, 117–123. [Google Scholar]
- Somfai, T. , Kikuchi, K. , Kaneda, M. , Watanabe, S. , Mizutani, E. , Haraguchi, S. , … Nagai, T. (2011). Cytoskeletal abnormal in relation with meiotic competence and ageing in porcine and bovine oocytes during in vitro maturation. Anatomia Histologia Embryologia, 40, 335–344. [DOI] [PubMed] [Google Scholar]
- Sun, Q. Y. , Lai, L. , Park, K. W. , Kuhholzer, B. , Prather, R. S. , & Schatten, H. (2001). Dynamic events are differently mediated by microfilaments, microtubules, and mitogen‐activated protein kinase during porcine oocyte maturation and fertilization in vitro. Biology of Reproduction, 64, 879–889. [DOI] [PubMed] [Google Scholar]
- Sun, Q. Y. , & Schatten, H. (2006). Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction, 131, 193–205. [DOI] [PubMed] [Google Scholar]
- Suzuki, K. , Asano, A. , Eriksson, B. , Niwa, K. , Nagai, T. , & Rodriguez‐Martinez, H. (2002). Capacitation status and in vitro fertility of boar spermatozoa: Effects of seminal plasma, cumulus‐oocyte‐complexes‐conditioned medium and hyaluronan. International Journal of Andrology, 25, 84–93. [DOI] [PubMed] [Google Scholar]
- Tao, T. , Robichaud, A. , Mercier, J. , & Ouellette, R. (2013). Influence of group embryo culture strategies on the blastocyst development and pregnancy outcome. Journal of Assisted Reproduction and Genetics, 30, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. H. , Abeydeera, L. R. , Han, Y. M. , Prather, R. S. , & Day, B. N. (1999). Morphologic evaluation and actin filament distribution in porcine embryos. Biology of Reproduction, 60, 1020–1028. [DOI] [PubMed] [Google Scholar]
- Wang, W. H. , Abeydeera, L. R. , Prather, R. S. , & Day, B. N. (2000). Polymerization of nonfilamentous actin into microfilaments is an important process for porcine oocyte maturation and early embryo development. Biology of Reproduction, 62, 1177–1183. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Lin, J. , Huang, J. , Wang, J. , Zhao, Y. , & Chen, T. (2012). Selection of ovine oocytes by brilliant cresyl blue staining. Journal of Biomedicine and Biotechnology, 2012, 161372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. K. , Liu, J. L. , Li, G. P. , Lian, L. , & Chen, D. Y. (2001). Sucrose pretreatment for enucleation: An efficient and non‐damage method for removing the spindle of the mouse MII oocyte. Molecular Reproduction and Development, 58, 432–436. [DOI] [PubMed] [Google Scholar]
- Wongsrikeao, P. , Otoi, T. , Yamasaki, H. , Agung, B. , Taniguchi, M. , Naoi, H. , … Nagai, T. (2006). Effects of single and double exposure to brilliant cresyl blue on the selection of porcine oocytes for in vitro production of embryos. Theriogenology, 66, 366–372. [DOI] [PubMed] [Google Scholar]
- Wu, Y. G. , Liu, Y. , Zhou, P. , Lan, G. C. , Han, D. , Miao, D. Q. , & Tan, J. H. (2007). Selection of oocytes for in vitro maturation by brilliant cresyl blue staining: A study using the mouse model. Cell research, 17, 722–731. [DOI] [PubMed] [Google Scholar]


