abstract
Objectives
Social anxiety disorder (SAD) is a serious and prevalent psychiatric condition, with a heritable component. However, little is known about the characteristics that are associated with the genetic component of SAD, the so‐called “endophenotypes”. These endophenotypes could advance our insight in the genetic susceptibility to SAD, as they are on the pathway from genotype to phenotype. The Leiden Family Lab study on Social Anxiety Disorder (LFLSAD) is the first multiplex, multigenerational study aimed to identify neurocognitive endophenotypes of social anxiety.
Methods
The LFLSAD is characterized by a multidisciplinary approach and encompasses a variety of measurements, including a clinical interview, functional and structural magnetic resonance imaging and an electroencephalography experiment. Participants are family members from 2 generations, from families genetically enriched for SAD.
Results
The sample (n = 132 participants, from 9 families) was characterized by a high prevalence of SAD, in both generations (prevalence (sub)clinical SAD: 38.3%). Furthermore, (sub)clinical SAD was positively related to self‐reported social anxiety, fear of negative evaluation, trait anxiety, behavioral inhibition, negative affect, and the level of depressive symptoms.
Conclusions
By the multidimensional character of the measurements and thorough characterization of the sample, the LFLSAD offers unique opportunities to investigate candidate neurocognitive endophenotypes of SAD.
Keywords: EEG, endophenotypes, family study, MRI, social anxiety disorder
1. INTRODUCTION
Social anxiety disorder (SAD) is a prevalent mental disorder, with an estimated lifetime prevalence around 13% (Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012). Patients with SAD have an extreme fear of being negatively evaluated by others in social situations (American Psychiatric Association, 2013). SAD has a considerable impact on the life of patients, as the disorder has a typical onset during late childhood or early adolescence, and is characterized by a chronic course (Beard, Moitra, Weisberg, & Keller, 2010; Beesdo‐Baum et al., 2012; Haller, Cohen Kadosh, Scerif, & Lau, 2015; Miers, Blöte, de Rooij, Bokhorst, & Westenberg, 2013; Miers, Blöte, Heyne, & Westenberg, 2014; Steinert, Hofmann, Leichsenring, & Kruse, 2013; Westenberg, Gullone, Bokhorst, Heyne, & King, 2007; Wittchen & Fehm, 2003). SAD patients experience impairments in multiple domains, including education, work, and social life; they report a lower quality of life and suffer often from comorbid psychopathology, such as other anxiety disorders, depression, and substance abuse (Acarturk, de Graaf, van Straten, Ten Have, & Cuijpers, 2008; Dingemans, van Vliet, Couvée, & Westenberg, 2001; Fehm, Pelissolo, Furmark, & Wittchen, 2005; Mack et al., 2015; Meier et al., 2015; Stein & Stein, 2008). Insight in the factors that play a role in the development of SAD is therefore of uttermost importance, in order to be able to reduce long‐term effects of SAD by developing effective preventive interventions and early treatment programs (Beauchaine, Neuhaus, Brenner, & Gatzke‐Kopp, 2008).
Several studies have indicated that genetic predispositions, as well as environmental, biological, and temperamental factors, interact in the pathogenesis of SAD, as reviewed by Wong and Rapee (2016), Spence and Rapee (2016), and Fox and Kalin (2014). Family and twin studies pointed to a heritability of SAD of around 50% (Bandelow et al., 2016; Gottschalk & Domschke, 2016; Isomura et al., 2015; Smoller, 2015); however, the search for specific genes underlying the susceptibility to SAD has been proven difficult. To start, SAD is a heterogeneous disorder, and the diagnosis is based on clinical assessments and not on biologically based measurements (Bearden, Reus, & Freimer, 2004; Glahn, Thompson, & Blangero, 2007; Gottesman & Gould, 2003). In addition, it is assumed that multiple interacting genetic variants, with relatively small individual effects, contribute to the vulnerability for SAD, complicating their detection (Binder, 2012; Munafò & Flint, 2014). Furthermore, epigenetic mechanisms, reflecting the interaction between genetic background and environmental influences, are of importance, requiring multilevel studies integrating data on psychopathology, (epi)genetics, and environment (Gottschalk & Domschke, 2016; Schiele & Domschke, 2017). Given these complexities, studies into the genes that contribute to the pathophysiology may be facilitated by defining endophenotypes related to SAD (Bas‐Hoogendam et al., 2016).
Endophenotypes are measurable characteristics on the pathway from genotype to phenotype (Gottesman & Gould, 2003; Lenzenweger, 2013b) and offer several possibilities to advance our understanding of the genetic susceptibility to SAD (Bas‐Hoogendam et al., 2016). Endophenotypes could shed light on the pathways leading to disorder phenotypes (Flint, Timpson, & Munafò, 2014; Miller & Rockstroh, 2013), can be used to identify individuals at risk (Puls & Gallinat, 2008), and could aid in the development of animal models for psychopathology (Gould & Gottesman, 2006). Furthermore, they offer starting points for therapeutic interventions (Garner, Möhler, Stein, Mueggler, & Baldwin, 2009) and can be useful in transdiagnostic research as proposed by the NIMH Research Domain Criteria (RDoC) (Sanislow et al., 2010). For a conceptual framework on neurobiological endophenotypes of SAD, we refer to Bas‐Hoogendam et al. (2016).
Endophenotypes are defined as meeting the following criteria (Glahn et al., 2007; Gottesman & Gould, 2003; Lenzenweger, 2013b; Puls & Gallinat, 2008): (1) They are associated with the disorder; (2) they are state‐independent traits, already present in a preclinical state; (3) they are heritable; and (4) they cosegregate with the disorder within families of probands, with nonaffected family members showing altered levels of the endophenotype in comparison with the general population. Furthermore, endophenotypes are ideally more strongly related to the disorder of interest in comparison with other psychiatric conditions (Lenzenweger, 2013a), but given the shared genetic influences between psychiatric disorders, certain endophenotypes are likely related to more than one disorder (Cannon & Keller, 2006).
1.1. objective of the Leiden Family Lab study on Social Anxiety Disorder
To determine which disease‐related characteristics may serve as endophenotypes, participants with SAD as well as their relatives need to be extensively phenotyped. Families are essential to allow investigating the heritability of the feature (criterion 3) and the cosegregation of the candidate endophenotype with the disorder within the family (criterion 4, first element), whereas case–control studies and longitudinal studies are needed to examine the other endophenotype criteria (criteria 1 and 2, respectively; Bas‐Hoogendam et al., 2016). In addition, adequately matched control families are needed to investigate the second element of criterion 4, namely, whether nonaffected family members show altered levels of the endophenotype when compared with the general population. To the best of our knowledge, the Leiden Family Lab study on Social Anxiety Disorder (LFLSAD) is the first multiplex (i.e., multiple cases of the disorder within one family), multigenerational family study aimed to determine neurocognitive endophenotypes of SAD, as measured with magnetic resonance imaging (MRI) and electroencephalography (EEG), investigating the heritability of candidate endophenotypes and the cosegregation of the candidate endophenotypes with the disorder within the family. Two important aspects of the study deserve to be highlighted.
First, the multiplex, multigenerational design was chosen to maximize statistical power to detect genetic and environmental influences on SAD‐related characteristics. Having multiple cases within a family instead of sporadic cases enriches the sample for a heritable basis of the disease and the detection of genetic factors. Furthermore, a sample consisting of large families, composed of several related nuclear families (parents with their children), is likely to share more heritable factors than a same‐sized sample of unrelated nuclear families, hence more statistical power to distinguish shared environmental effects from genetic effects (Williams & Blangero, 1999; cf. Gur et al., 2007).
Second, the LFLSAD focuses on neurocognitive SAD endophenotypes as measured with MRI and EEG, as these are noninvasive, widely applied, and safe methods to investigate structural and functional properties of the human brain. Importantly, these methods are complementary: EEG has good temporal precision to capture electrocortical activity associated with attentional SAD‐related biases and can be used to study candidate endophenotypes related to processing social judgments (Harrewijn, van der Molen, van Vliet, Tissier, & Westenberg, 2018; Van der Molen et al., 2014) and to performing a public speaking task (Harrewijn, van der Molen, van Vliet, Houwing‐Duistermaat, & Westenberg, 2017; Harrewijn, Van der Molen, & Westenberg, 2016). MRI enables precise spatial localization of the brain regions implicated in SAD and provides valuable insights in the structure and connectivity of the brain and the functioning of brain regions such as the amygdala and the prefrontal cortex during viewing neutral faces in a habituation and conditioning task (cf. Bas‐Hoogendam, van Steenbergen, Westenberg, & van der Wee, 2015; Blackford, Allen, Cowan, & Avery, 2013; Blackford, Avery, Cowan, Shelton, & Zald, 2011; Davis, Johnstone, Mazzulla, Oler, & Whalen, 2010) and processing social norm violations (Bas‐Hoogendam, van Steenbergen, Kreuk, van der Wee, & Westenberg, 2017; Bas‐Hoogendam, van Steenbergen, van der Wee, & Westenberg, 2018; Blair et al., 2010). Typically, neurocognitive endophenotypes are assumed to be closer to the genotype than, for example, psychological constructs (Cannon & Keller, 2006). However, data collection in the LFLSAD was not limited to these measures: in order to achieve comprehensive phenotyping of the participants, a variety of additional measurements was included, as described in detail below. To this aim, the LFLSAD was performed by a multidisciplinary team of clinicians, neuroscientists, and statisticians.
In the current paper, the design and methods of the LFLSAD are presented. Furthermore, characteristics of the LFLSAD sample are described, including analyses on its psychological features. Hypotheses with respect to the candidate neurocognitive endophenotypes have been preregistered in 2014 on the Open Science Framework website (osf.io) and are available online (links provided in the Supporting Information; Bas‐Hoogendam et al., 2014). Results of the analyses into candidate neurocognitive endophenotypes of SAD are reported in other papers and conference abstracts (Bas‐Hoogendam et al., 2015; Bas‐Hoogendam, van Steenbergen, van der Wee, & Westenberg, 2017a, 2017b; Harrewijn et al., 2018; Harrewijn, van der Molen, et al., 2017) and in preparation.
2. METHODS
2.1. study design and setting
The LFLSAD is a cross‐sectional, two‐generation multiplex family study on the neurocognitive characteristics that are genetically linked to SAD. The study is a collaboration between Leiden University (Institute of Psychology) and the Leiden University Medical Center (LUMC; Departments of (Child) Psychiatry and Department of Medical Statistics and Bioinformatics) and is embedded within the Leiden University research profile area “Health, prevention and the human life cycle.” Data collection took place at Leiden University and the LUMC between October 2013 and July 2015.
2.2. sample
Families were considered eligible for inclusion when they contained at least one adult, aged 25–55 years, with a primary diagnosis of SAD (from now on referred to as the “proband”), of whom at least one child, aged 8–21 years and living at home with the proband, showed SAD symptoms at a clinical or subclinical level (referred to as the “proband's SA‐child”). For these participants, comorbidity with other internalizing disorders was allowed; however, families were excluded when the proband or the proband's SA‐child suffered of other psychiatric diagnoses, especially developmental disorders (e.g., autism).
In addition to the proband and its SA‐child, the proband's spouse, other children (age ≥8 years) and the proband's sibling(s) and their spouse(s) with their child(ren) (age ≥8 years) were invited to participate. In Figure 1, we depict a pedigree starting with the grandparental generation (0) on which no data were collected for reasons of feasibility; probands and siblings belonging to generation 1; and proband's and siblings' offspring (generation 2). We aimed to include families with at least eight family members, to enable reliable estimations of the relation between endophenotype and SAD.
Figure 1.
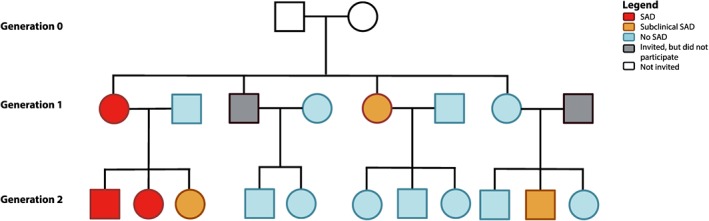
Example of a family within the Leiden Family Lab study on Social Anxiety Disorder. Families were included based on the combination of a parent with social anxiety disorder (SAD; “proband”: depicted in red) and a proband's child with SAD (red) or (sub)clinical SAD (orange). In addition, family members of two generations were invited, independent from the presence of SAD within these family members (no SAD: light blue; did not participate: gray). Grandparents (Generation 0; white) were not invited for participation. This family is slightly modified to guarantee anonymity; however, the number of family members and the frequency of (sub)clinical SAD are depicted truthfully. Squares and circles represent men and women, respectively
Family members of the proband and proband's SA‐child were included independent of the presence of psychopathology. All participants were required to have sufficient comprehension of the Dutch language.
2.3. sample size and power calculation
The aim of the LFLSAD was twofold. First, the study aimed to estimate the association between SAD and neurocognitive putative endophenotypes (Bas‐Hoogendam et al., 2014; Bas‐Hoogendam et al., 2016; Harrewijn et al., 2018; Harrewijn, Schmidt, Westenberg, Tang, & van der Molen, 2017; Harrewijn, van der Molen, et al., 2017); second, the significance of clustering of these endophenotypes within families (i.e., genetic effects) was addressed. To estimate this heritability, a joint mixed model taking the ascertainment process and familiar relationships into account will be used (Tissier, Tsonaka, Mooijaart, Slagboom, & Houwing‐Duistermaat, 2017). Power calculations, performed by co‐author J. H. D., revealed that 12 families with eight to 12 family members (average: 10 members per family) were required for sufficient power (i.e., minimally 80%) to investigate these two questions (details provided in the Supporting Information).
2.4. procedure
2.4.1. Recruitment
Families were recruited through media exposure, such as interviews in Dutch newspapers, on television and radio; furthermore, the study was brought to the attention of patient organizations such as the “Anxiety, Compulsion and Phobia association” (in Dutch: “Angst, Dwang en Fobie stichting”) and the “Association of Shy People” (“Vereniging van Verlegen Mensen”), to clinical psychologists, general practitioners, and mental health care organizations. In the media items, we asked families in which multiple family members experienced “extreme shyness” to contact us.
2.4.2. Screening procedure and inclusion of families
Potential probands were screened for eligibility by a telephone call or an email, depending on their preference. This screening contained questions with respect to the presence of social anxiety in the proband and the proband's SA‐child, the age of the proband and his or her child(ren), and the potential number of family members that could be invited for the study. In addition, probands were further informed about the study. When they passed the screening and showed interest in participation, an information letter was sent to the proband and his or her nuclear family members, containing detailed information about the study. Two weeks later, participants were contacted by telephone and any questions about the study were answered. Next, the proband, the proband's spouse, and the proband's SA‐child were invited to come to the LUMC for an introductory meeting and structured clinical interview, in order to confirm the presence of a primary diagnosis of SAD (proband) and (sub)clinical social anxiety (proband's SA‐child). Furthermore, a screening was performed to exclude the presence of autism in the proband and the proband's SA‐child.
When the inclusion criteria were met, the proband and his or her nuclear family were included in the study and invited for the remaining measurements (Table 1). In addition, we asked the proband to contact his or her sibling(s), in order to confirm that they were interested to be informed about participation in the study. Given a positive response, these siblings, together with their partner and/or children, were invited to participate by the investigators. Given the inherent characteristic of socially anxious people to avoid new situations and their tendency to stay out of the spotlights, we encouraged participants to visit the lab together with their family members, in order to make them feel more comfortable. Although we emphasized the importance of including as many family members as possible within the study, we also indicated that each individual was free to decide whether or not to participate (Figure 1).
Table 1.
Measurements included in the LFLSAD
| Measurement | Instrument | Age group (year) | |
|---|---|---|---|
| Clinical Interview | Diagnoses of mental (Axis 1) disorders according to DSM criteria | MINI‐Plus | 18+ |
| MINI‐Kid | 8–17 | ||
| Questionnaires | Social anxiety symptoms | LSAS‐SR | 18+ |
| SAS‐A | 8–17 | ||
| Fear of negative evaluation | BFNE‐II‐R | 8+ | |
| General anxiety | STAI trait | 8+ | |
| STAI state (before and after MRI scan) | 8+ | ||
| Depressive symptoms | BDI‐II | 18+ | |
| CDI | 8–17 | ||
| Affect | PANAS | 8+ | |
| Temperament | BIS/BAS | 13+ | |
| BIS/BAS‐C | 8–12 | ||
| Autism screening | AQ | 18+ | |
| SRS, completed by both parents about their child(ren) | 8–17 | ||
| Handedness | EHI | 8+ | |
| Estimation of intelligence | IQ | WAIS‐IV subtests (similarities and block design) | 17+ |
|
WISC subtests (similarities and block design) |
8–16 | ||
| MRI scan | Structural and functional MRI | 8+ | |
| EEG experiment | EEG measurement, including collection of saliva for cortisol measurements | 8+ | |
| Genotyping | Collection of saliva | Oragene•DNA OG‐500 kit | 8+ |
Note. MINI‐Plus = Mini‐Plus International Neuropsychiatric Interview (MINI Plus version 5.0.0; Sheehan et al., 1998; van Vliet & de Beurs, 2007); MINI‐Kid = MINI Kid interview version 6.0 (Bauhuis et al., 2013; Sheehan et al., 2010); LSAS‐SR = Liebowitz Social Anxiety Scale—self‐report (Fresco et al., 2001; Mennin et al., 2002); SAS‐A = Social Anxiety Scale—adolescents (La Greca & Lopez, 1998); BFNE‐II‐R = revised Brief Fear of Negative Evaluation II scale (Carleton et al., 2006; Leary, 1983); STAI = State–Trait Anxiety Inventory (Spielberger et al., 1970); BDI‐II = Beck Depression Inventory II (Beck et al., 1996; Van der Does, 2002); CDI = Children's Depression Inventory (Kovacs, 1983, 1985; Timbremont & Braet, 2002); PANAS = Positive and Negative Affect Schedule (Peeters et al., 1996; Watson et al., 1988); BIS/BAS = Behavioral Inhibition and Behavioral Activation Scales (Carver & White, 1994); BIS/BAS‐C = Behavioral Inhibition and Behavioral Activation Scales for children (Muris et al., 2005); AQ = Autism‐Spectrum Quotient questionnaire (Baron‐Cohen et al., 2001); SRS = Social Responsiveness Scale (Constantino et al., 2003; Roeyers et al., 2011); EHI = Edinburgh Handedness Inventory (Oldfield, 1971); WAIS = Wechsler Adult Intelligence Scale IV (Wechsler et al., 2008); WISC = Wechsler Intelligence Scale for Children III (Wechsler, 1991); MRI = magnetic resonance imaging; EEG = electroencephalography; LFLSAD = Leiden Family Lab study on Social Anxiety Disorder.
2.4.3. Ethics
The study (P12.061) was approved by the Medical Ethical Committee of the LUMC in June 2012. All participants received written and verbal information with respect to the objectives and procedure of the study; information letters were age adjusted, to make them understandable for children and adolescents as well. Participants provided informed consent prior to participation, according to the Declaration of Helsinki. Both parents signed the informed consent form for their children, whereas children between 12 and 18 years of age signed the form themselves as well. Every participant received €75 for participation (duration whole test procedure, including breaks: 8 hr), and travel expenses were reimbursed. Furthermore, participants were provided with lunch/diner, snacks, and drinks during their visit to the lab. Confidentiality of the research data was maintained by the use of a unique research ID number for each participant.
2.5. measurements
All participants took part in the same measurements; the order of the measurements differed between participants depending on their availability and lab resources. However, as described above, for the proband, the proband's spouse, and the proband's SA‐child, the clinical interview always preceded the other measurements. Age‐appropriate instruments were used to evaluate certain constructs. Measurements are listed in Table 1 and explained below.
2.5.1. Diagnosis of mental disorders
Structured clinical interviews using the Mini‐International Neuropsychiatric Interview‐Plus (version 5.0.0; Sheehan et al., 1998; van Vliet & de Beurs, 2007) or the Mini‐International Neuropsychiatric Interview‐Kid (version 6.0; Bauhuis, Jonker, Verdellen, Reynders, & Verbraak, 2013; Sheehan et al., 2010) were used to determine the presence of psychiatric diagnoses according to Diagnostic and Statistical Manual of Mental Disorders (DSM)‐IV‐R criteria (Axis 1). Interviews were conducted by trained clinicians and were recorded. These recordings were used to determine the presence of (sub)clinical SAD. Clinical SAD was diagnosed using the DSM‐IV‐R criteria for the generalized subtype of SAD, but the clinician verified whether the DSM‐5 criteria for SAD were also met in order to establish the diagnosis. Participants were classified as having subclinical SAD when they met the criteria for SAD as described in the DSM‐5, but without showing obvious impairments in social, occupational, or other important areas of functioning (Criterion G; American Psychiatric Association, 2013).
2.5.2. Self‐report assessments of anxiety and associated constructs
Social anxiety was assessed on a dimensional scale using the self‐report version of the Liebowitz Social Anxiety Scale (LSAS‐SR; Fresco et al., 2001; Mennin et al., 2002) or the Social Anxiety Scale for Adolescents (SAS‐A; La Greca & Lopez, 1998). The LSAS‐SR measures fear in and avoidance of situations that are likely to elicit social anxiety, with good internal consistency (Heimberg et al., 1999). The SAS‐A determines social anxiety in children and adolescents, with satisfactory levels of internal consistency (Miers et al., 2013).
Fear of negative evaluation was assessed with the revised Brief Fear of Negative Evaluation (BFNE)‐II‐R scale (Carleton, McCreary, Norton, & Asmundson, 2006), which is a revision of the BFNE questionnaire (Leary, 1983). The BFNE‐II‐R is a self‐report questionnaire with excellent internal consistency and good convergent validity (Carleton, Collimore, & Asmundson, 2007).
The State–Trait Anxiety Inventory (Spielberger, Gorsuch, & Lushene, 1970; see Spielberger & Vagg, 1984, for psychometric properties) was used to determine self‐reported trait anxiety, as well as state anxiety before and after the MRI scan.
Severity of self‐reported depressive symptoms was assessed using the Beck Depression Inventory II (Beck, Steer, & Brown, 1996; Van der Does, 2002) or the Children's Depression Inventory (Kovacs, 1983, 1985; Timbremont & Braet, 2002). Due to ethical reasons, an item asking about suicide was removed from the Children's Depression Inventory (cf. Miers, Blöte, & Westenberg, 2010).
The general mood of the participant, experienced in the last couple of weeks, was assessed by the self‐report Positive and Negative Affect Schedule (Peeters, Ponds, & Vermeer, 1996; Watson, Clark, & Tellegen, 1988), which is a reliable and valid instrument to measure affect (Crawford & Henry, 2004).
The sensitivity for the temperamental traits “behavioral inhibition” and “behavioral activation” was assessed using the self‐report BIS/BAS (Carver & White, 1994; Franken, Muris, & Rassin, 2005) or the BIS/BAS scales for children (BIS/BAS‐C; Muris, Meesters, de Kanter, & Timmerman, 2005).
2.5.3. Autism screening
Adult participants were screened for autism using the self‐report Autism‐Spectrum Quotient questionnaire (Baron‐Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001); parents completed the Dutch version of Social Responsiveness Scale about their child(ren) (Constantino et al., 2003; Roeyers, Thys, Druart, De Schryver, & Schittekatte, 2011).
2.5.4. Handedness
Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971).
2.5.5. Estimation of intelligence
Two subscales of the Wechsler Adult Intelligence Scale IV (Wechsler, Coalson, & Raiford, 2008) or Wechsler Intelligence Scale for Children III (Wechsler, 1991), the similarities (verbal comprehension) and block design (perceptual reasoning) subtests, were administered to obtain an estimate of cognitive functioning.
2.5.6. Structural and functional MRI measurements
A detailed description of the MRI session is included in the Supporting Information. The session consisted of a high‐resolution T1 scan, two diffusion tensor imaging scans, and a magnetization transfer ratio scan. In addition, a high‐resolution EPI scan and a B0 fieldmap were acquired. Functional MRI data were collected during resting‐state and during two functional paradigms: an amygdala paradigm investigating amygdala habituation (based on the work of Blackford et al., 2013; Blackford et al., 2011; Schwartz, Wright, Shin, Kagan, Whalen, et al., 2003; Schwartz, Wright, Shin, Kagan, & Rauch, 2003) and conditioning (Davis et al., 2010), and the revised Social Norm Processing Task (Bas‐Hoogendam, van Steenbergen, Kreuk, et al., 2017).
2.5.7. EEG measurements
A detailed description of the EEG session is included in the Supporting Information. The session consisted of multiple resting‐state measurements, as well as two task paradigms: a social judgment paradigm (Harrewijn et al., 2018; Van der Molen et al., 2014) and a social performance task (Harrewijn et al., 2016; Harrewijn, van der Molen, et al., 2017). At several time points before and during this task, task‐induced mood was measured and saliva samples were collected to measure cortisol.
2.5.8. Biosampling for DNA isolation
Saliva samples were collected for future genotyping, using the Oragene•DNA OG‐500 self‐collection kits (Genotek, Ottawa, Ontario, Canada).
2.6. data analysis for the current paper
2.6.1. Sample characterization
We investigated sociodemographic differences between the generations using chi‐square tests (male/female ratio, native country, and education level) and linear regression models (age and estimated IQ). These regression models were fitted in R (R Core Team, 2016), with generation as independent variable. Because of the relationships between the participants, genetic correlations between family members were modeled by including random effects (lmekin function).
Next, in order to verify that the LFLSAD sample is genetically enriched for SAD, several analyses were performed. First, the presence of clinical and subclinical SAD was determined. Furthermore, the heritability of (sub)clinical SAD within the sample was estimated using the software package SOLAR (Sequential Oligogenic Linkage Analysis Routines; Almasy & Blangero, 1998). Heritability indicates how strong genetic effects influence a certain trait and is defined as the proportion of the variation in a phenotype that can be attributed to additive genetic effects (Almasy & Blangero, 2010; Wray & Visscher, 2008). SOLAR uses maximum likelihood techniques to attribute variance in the phenotype to either genetic or environmental effects, based on a kinship matrix for the genetic component and an identity matrix for the unique environmental component. Here, we did not include a shared environmental component, to keep the model as simple as possible. We corrected for ascertainment (de Andrade & Amos, 2000) by indicating that families were selected based on the proband and the proband's SA‐child. Age and gender were included as covariates and were removed from the model when their effect was not significant (p > .05).
2.6.2. Characterization of participants with and without (sub)clinical SAD
To further characterize the sample, we investigated differences between participants with and without (sub)clinical SAD with respect to male/female ratio, generation, presence of (comorbid) psychopathology (chi‐square tests; Bonferroni‐corrected p value for psychopathology: p = .003 [15 tests]), age, and estimated IQ (regression models with genetic correlations as random effects). Furthermore, we examined the relationships between (sub)clinical SAD and self‐reported levels of anxiety and anxiety‐related constructs. When different questionnaires were used for adults and children/adolescents, z scores were used (see Supporting Information for reference values). The following constructs were investigated: level of social anxiety (z score), level of fear of negative evaluation, level of depressive symptoms (z score), level of negative affect, level of trait anxiety, and the level of inhibited temperament (z score). Regression models were fitted in R, with (sub)clinical SAD as the independent variable; the outcomes of the questionnaires were the dependent variables of interest. Age and gender were included as covariates, and the genetic correlations between family members were modeled by including random effects. A Bonferroni‐corrected p value of .008 was used (six tests).
3. RESULTS
3.1. recruitment and inclusion
Given the nature of SAD, recruitment of families meeting the inclusion criteria was a time‐consuming process, taking place between Summer 2013 and Summer 2015. Nine families were included in the LFLSAD, including 133 family members (Figure 2). All probands were recruited by media exposure and contacts with patient associations, and none of the probands had not been treated for SAD before entering the study. Due to insufficient proficiency of the Dutch language, data of one participant (partner of a proband's sibling) were excluded. Sociodemographic characteristics of the remaining sample (n = 132) are summarized in Table 2.
Figure 2.
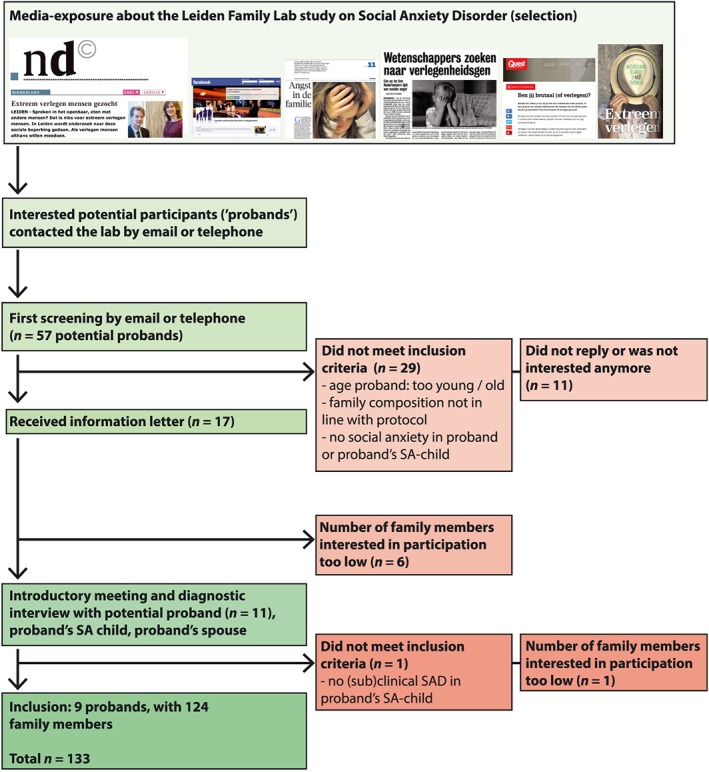
Flowchart of inclusion of the Leiden Family Lab study on Social Anxiety Disorder (LFLSAD)
Table 2.
Sociodemographic characteristics of the LFLSAD sample per generation
| Generation 1 (n = 62) | Generation 2 (n = 70) | Statistical analysis | |
|---|---|---|---|
| Gender (n) | χ2(1) = 1.05, p = .38 | ||
| Male/female | 29/33 | 39/31 | |
| Age in years (mean ± SD) | 46.2 ± 6.6 | 17.9 ± 6.2 | β = −30.4, p < .001 |
| Range | 31.0–61.5 | 8.2–32.2 | |
| Native country (n) | χ2(1) = 0.40, p = .84 | ||
| The Netherlands | 57 | 65 | |
| Other | 5 | 5 | |
| Education level (n)a | χ2(1) = 3.28, p = .19 | ||
| Low | 11 | 22 | |
| Intermediate | 25 | 26 | |
| High | 25 | 22 | |
| Estimated IQ (mean ± SD)b | 104.0 ± 11.8 | 107.2 ± 10.6 | β = 2.5, p = .13 |
Note. Education level was classified as follows: low = primary education (elementary school) and prevocational education; intermediate = higher secondary education (higher general continued education, preuniversity secondary education) and postsecondary education (intermediate vocational education); high = tertiary education (higher professional education, university); LFLSAD = Leiden Family Lab study on Social Anxiety Disorder.
Generation 1 (education completed): Data from 61 participants; Generation 2 (education completed or currently following): Data from 70 participants.
Generation 1: Data from 58 participants; Generation 2: Data from 66 participants.
On average, each family contained 14.7 participating family members (range: 4–35). The sample included 68 males and 64 females, who were equally divided over the generations. As expected based on the design, the generations differed significantly in age, but not in estimated IQ (Table 2). Availability of data is illustrated in Figure 3.
Figure 3.
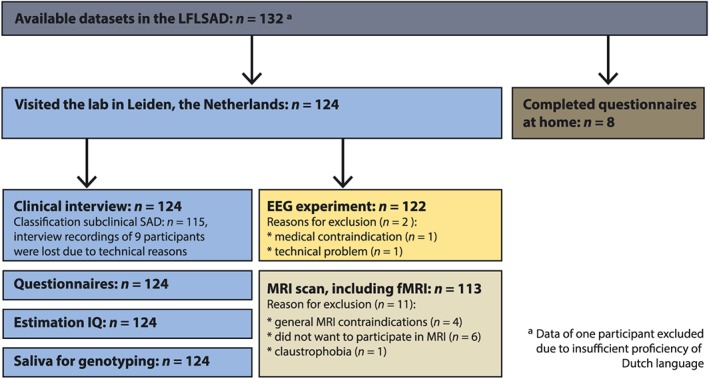
Overview of available data within the Leiden Family Lab study on Social Anxiety Disorder (LFLSAD)
3.2. characterization of the LFLSAD sample
An overview of clinical diagnoses within the sample is presented in Table 3, whereas scores on the dimensional self‐assessments of anxiety and anxiety‐related constructs are displayed in Table 4. Diagnostic interviews showed that social anxiety was highly prevalent within the sample, in both generations: In addition to the nine probands, who were selected based on a primary diagnosis of SAD, 10 of their family members (generation 1: n = 6; generation 2: n = 4, of whom three proband's SA‐children) met the criteria for clinical SAD. Furthermore, 25 family members (six of them proband's SA‐children) were classified as having subclinical SAD. Total percentage of (sub)clinical SAD cases within the sample was 38.3% (generation 1: 40.4%; generation 2: 36.5%). The validity of the diagnoses as established by the clinical interviews was confirmed by the self‐report questionnaires: participants meeting the DSM criteria for generalized SAD (n = 19) also met literature‐based cutoff scores for generalized social anxiety (a score ≥60 on LSAS; Mennin et al., 2002, or a score ≥50 on SAS‐A; Storch, Masia‐Warner, Dent, Roberti, & Fisher, 2004), with an average score (±SD) of 68.1 ± 24.2 on the LSAS (n = 17) and a score of 55.5 ± 0.7 (n = 2) on the SAS‐A, whereas participants with subclinical SAD reported scores of 38.2 ± 23.7 (LSAS; n = 12) and 37.5 ± 9.7 (SAS‐A; n = 13), respectively.
Table 3.
Clinical diagnoses of DSM Axis 1 diagnoses within the LFLSAD sample, per generation
| Generation 1 | Generation 2 | |
|---|---|---|
| SAD (number of cases; %)a | 15; 25.9% | 4; 6.1% |
| Subclinical SAD (number of cases; %)b | 6; 11.5% | 19; 30.2% |
| (Sub)clinical SAD—cumulative (number of cases; %)b | 21; 40.4% | 23; 36.5% |
| Other psychopathologyc | ||
| Depressive episode—Current | 1 | 1 |
| Depressive episode—Past | 16 | 9 |
| Dysthymia—Current | 1 | 2 |
| Dysthymia—Past | 1 | 1 |
| Panic disorder—Lifetime | 6 | 1 |
| Agoraphobia—Current | 5 | 2 |
| Agoraphobia—Lifetime | 1 | 1 |
| Separation anxiety—Present | n/a | 1 |
| Specific phobia—Present | 2 | 3 |
| Generalized anxiety disorder—Present | 3 | 0 |
| Obsessive–compulsive disorder—Present | 1 | 0 |
| Alcohol dependence—Present | 1 | 1 |
| Alcohol dependence—Lifetime | 1 | 3 |
| Drug dependence—Lifetime | 1 | 0 |
| Bulimia nervosa—Present | 1 | 0 |
Note. SAD = social anxiety disorder; DSM = Diagnostic and Statistical Manual of Mental Disorders; n/a = not assessed; LFLSAD = Leiden Family Lab study on Social Anxiety Disorder.
Generation 1: Data from 58 participants; Generation 2: Data from 66 participants (30 participants: MINI‐Plus; 36 participants: MINI‐Kid).
Generation 1: Data from 52 participants; Generation 2: Data from 63 participants.
Generation 1: Data from 58 participants; Generation 2: Data from 60 participants (30 participants: MINI‐Plus; 30 participants MINI‐Kid).
Table 4.
Self‐report assessments of anxiety and associated constructs within the LFLSAD sample, per generation
|
Generation 1 (n = 62) (mean ± SD; range) |
Generation 2 (n = 70) (mean ± SD; range) |
||
|---|---|---|---|
| LSAS‐SRa | Total | 31.4 ± 25.0 (2–95) | 33.7 ± 23.3 (7–98) |
| Fear | 16.1 ± 13.0 (0–52) | 17.0 ± 13.2 (0–58) | |
| Avoidance | 15.3 ± 12.8 (0–50) | 16.7 ± 11.1 (2–42) | |
| SAS‐Ab | Total | 35.8 ± 9.2 (20–56) | |
| Fear of negative evaluation | 14.9 ± 5.2 (8–26) | ||
| Social avoidance and distress—New | 13.9 ± 4.6 (6–26) | ||
| Social avoidance and distress—General | 6.9 ± 2.3 (4–14) | ||
| BFNE‐II‐Rc | Total | 16.3 ± 11.6 (0–48) | 15.0 ± 10.5 (0–47) |
| STAI traitd | Total | 36.0 ± 10.4 (20–64) | 35.0 ± 8.1 (21–57) |
| BDIa | Total | 7.3 ± 8.1 (0–32) | 7.6 ± 7.0 (1–30) |
| CDIb | Total | 6.6 ± 4.5 (0–23) | |
| Positive affectd | Total | 32.3 ± 7.3 (15–47) | 32.7 ± 5.7 (21–45) |
| Negative affectd | Total | 17.5 ± 6.9 (10–40) | 16.9 ± 5.0 (10–31) |
| BIS‐BASe | BIS—Total | 19.8 ± 4.5 (7–28) | 18.5 ± 3.9 (9–28) |
| BAS—Total | 37.2 ± 5.0 (26–50) | 39.1 ± 4.3 (31–48) | |
| BIS‐BAS‐Cf | BIS—Total | 7.2 ± 4.2 (1–17) | |
| BAS—Total | 17.6 ± 5.2 (9–27) | ||
Note. LSAS‐SR = Liebowitz Social Anxiety Scale—self‐report (Fresco et al., 2001; Mennin et al., 2002); SAS‐A = Social Anxiety Scale—adolescents (La Greca & Lopez, 1998); BFNE‐II‐R = revised Brief Fear of Negative Evaluation II scale (Carleton et al., 2006; Leary, 1983); STAI = State–Trait Anxiety Inventory (Spielberger et al., 1970); BDI‐II = Beck Depression Inventory II (Beck et al., 1996; Van der Does, 2002); CDI = Children's Depression Inventory (Kovacs, 1983, 1985; Timbremont & Braet, 2002); BIS/BAS = Behavioral Inhibition and Behavioral Activation Scales (Carver & White, 1994); BIS/BAS‐C = Behavioral Inhibition and Behavioral Activation Scales for children (Muris et al., 2005); LFLSAD = Leiden Family Lab study on Social Anxiety Disorder.
Generation 1: Data from 62 participants; Generation 2: Data from 33 participants.
Generation 2: Data from 37 participants.
Generation 1: Data from 60 participants; Generation 2: Data from 70 participants.
Generation 1: Data from 62 participants; Generation 2: Data from 70 participants.
Generation 1: Data from 62 participants; Generation 2: Data from 52 participants.
Generation 2: Data from 18 participants.
A heritability analysis using SOLAR indicated that (sub)clinical SAD had a moderately high heritability, which was significant at trend level (h 2 = 0.43, p = .09). Age and gender did not significantly influence the model and were therefore removed (age: p = .78; gender: p = .62).
Comorbid diagnoses in the nine probands included depression (past, n = 3), panic disorder (n = 2), agoraphobia (current, n = 2), specific phobia (n = 1), and obsessive–compulsive disorder (n = 1). Assessment of other psychopathology in their family members indicated that depression (past and current, n = 24), agoraphobia (past and current, n = 7), and panic disorder (n = 5) were most common diagnoses in the LFLSAD sample. Furthermore, several participants met criteria for alcohol dependence (current and lifetime, n = 6), dysthymia (current and past, n = 5), specific phobia (n = 4), generalized anxiety disorder (n = 3), separation anxiety (n = 1), drug dependence (n = 1), and bulimia nervosa (n = 1; Table 3).
3.3. characterization of participants with and without (sub)clinical SAD
A characterization of the participants with and without (sub)clinical SAD is presented in Table 5. There were no differences between family members with and without (sub)clinical SAD with respect to the presence of other DSM diagnoses (at Bonferroni‐corrected p value < .003). However, all self‐reported measures of interest were significantly related to (sub)clinical SAD (Table 6). Age was not a significant predictor in the models; gender was, however, significantly related to the level of the level of behavioral inhibition (at Bonferroni‐corrected p value < .008), the level of fear of negative evaluation, and the level of negative affect (at uncorrected p value < .05), with higher levels in females compared with males.
Table 5.
Characteristics of participants with and without (sub)clinical SAD
| (Sub)clinical SAD (n = 44) | No (sub)clinical SAD (n = 71) | Statistical analysis | |
|---|---|---|---|
| Demographics | |||
| Male/female | 22/22 | 35/36 | χ2(1) = 0.005, p = 1.00 |
| Generation 1/Generation 2 | 21/23 | 31/40 | χ2(1) = 0.18, p = 0.70 |
| Age in years (mean ± SD) | 30.0 ± 15.5 | 30.8 ± 15.8 | β = 0.82, p = 0.78 |
| Estimated IQ (mean ± SD) | 104.6 ± 11.8 | 105.7 ± 10.8 | β = 1.39, p = 0.50 |
| Other psychopathologya | |||
| Depressive episode—Current | 1 | 1 | χ2(1) = 0.16, p = 1.00 |
| Depressive episode—Past | 12 | 11 | χ2(1) = 3.00, p = 0.09 |
| Dysthymia—Current | 3 | 0 | χ2(1) = 5.32, p = 0.047 * |
| Dysthymia—Past | 1 | 1 | χ2(1) = 0.17, p = 1.00 |
| Panic disorder—Lifetime | 5 | 2 | χ2(1) = 3.88, p = 0.10 |
| Agoraphobia—Current | 5 | 2 | χ2(1) = 3.88, p = 0.10 |
| Agoraphobia—Lifetime | 0 | 2 | χ2(1) = 1.18, p = 0.53 |
| Separation anxiety—Present | 0 | 1 | χ2(1) = 0.63, p = 1.00 |
| Specific phobia—Present | 2 | 3 | χ2(1) = 0.02, p = 1.00 |
| Generalized anxiety disorder—Present | 2 | 1 | χ2(1) = 1.19, p = 0.55 |
| Obsessive–compulsive disorder—Present | 1 | 0 | χ2(1) = 1.74, p = 0.37 |
| Alcohol dependence—Present | 1 | 1 | χ2(1) = 0.16, p = 1.00 |
| Alcohol dependence—Lifetime | 1 | 3 | χ2(1) = 0.25, p = 1.00 |
| Drug dependence—Lifetime | 1 | 0 | χ2(1) = 1.78, p = 0.36 |
| Bulimia nervosa—Present | 1 | 0 | χ2(1) = 1.74, p = 0.37 |
| Self‐report measurements | |||
| Social anxiety symptoms (z score; mean ± SD) | 3.0 ± 3.3 | 0.2 ± 1.8 | See Table 6 |
| Fear of negative evaluation (mean ± SD) | 23.4 ± 12.5 | 12.5 ± 8.0 | See Table 6 |
| Trait anxiety (mean ± SD) | 39.1 ± 9.6 | 32.9 ± 8.5 | See Table 6 |
| Behavioral inhibition (z score; mean ± SD) | 0.4 ± 1.2 | −0.4 ± 1.0 | See Table 6 |
| Depressive symptoms (z score; mean ± SD) | 0.0 ± 0.8 | −0.5 ± 0.7 | See Table 6 |
| Negative affect (mean ± SD) | 20.6 ± 6.9 | 15.3 ± 4.7 | See Table 6 |
Note. SAD = social anxiety disorder.
Generation 1: Data from 52 participants; Generation 2: Data from 57 participants (28 participants: MINI‐Plus; 29 participants MINI‐Kid).
Significant at uncorrected p value of .05.
Table 6.
Associations with (sub)clinical SAD
| Constructs | Relation with (sub)clinical SAD | Relation with age | Relation with gender | ||||
|---|---|---|---|---|---|---|---|
| n | β (SE) | p | β (SE) | p | β (SE) | p | |
| Social anxiety (z score) | 115 | 2.76 (0.45) | 1.3 * 10−9 ** | 0.02 (0.01) | .10 | 0.40 (0.44) | .36 |
| Fear of negative evaluation | 113 | 10.83 (1.85) | 5.0 * 10−9 ** | 0.08 (0.06) | .18 | 4.10 (1.80) | .02* |
| Trait anxiety | 115 | 5.97 (1.67) | 3.5 * 10−4 ** | 0.02 (0.05) | .69 | 3.09 (1.63) | .06 |
| Behavioral inhibition (z score) | 115 | 0.82 (0.19) | 1.7 * 10−5 ** | 0.00 (0.01) | .49 | 0.71 (0.19) | 1.2 * 10−4 ** |
| Depressive symptoms (z score) | 115 | 0.53 (0.14) | 1.4 * 10−4 ** | 0.00 (0.00) | .37 | 0.17 (0.14) | .20 |
| Negative affect | 115 | 5.32 (1.04) | 3.1 * 10−7 ** | 0.02 (0.03) | .64 | 2.54 (1.02) | .01* |
Note. SAD = social anxiety disorder.
Significant at uncorrected p value of .05.
Significant at Bonferroni‐corrected p value of .008.
4. DISCUSSION
Here, we describe the background, objective, design, and methods of the LFLSAD and present data characterizing the sample. The study is unique in several aspects.
To start, the LFLSAD is the first multiplex, multigenerational family study on SAD, including 132 participants from nine families. The composition of the sample (families were selected based on at least two SAD cases within one nuclear family, multiplex, and multiple nuclear families involving two generations from the same family were included, multigenerational; see Figure 1) boosts statistical power to observe genetic and environmental effects on SAD‐related traits (Williams & Blangero, 1999).
In addition, families were recruited from the general population (Figure 2), and none of the participants with SAD within the sample (n = 19) was treated for the disorder before entering the study. This is in line with several reports on social anxiety, indicating that SAD is frequently underdiagnosed because of the low help‐seeking behavior of patients; furthermore, SAD is often not adequately recognized by clinicians (Alonso et al., 2018; Dingemans et al., 2001; Fehm et al., 2005; Ruscio et al., 2008). Thereby, the sample of the LFLSAD represents socially anxious families from the community (Dingemans et al., 2001), including participants who are on a daily basis limited by their SAD symptoms (following Criterion G of the DSM‐5 definition, stating that “the fear, anxiety, or avoidance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning”; American Psychiatric Association, 2013), but those SAD cases are not a selection of cases who have received treatment for SAD in the past.
Next, following our criteria that were aimed to include families who were enriched for genetic susceptibility to SAD, the disorder was highly prevalent within the sample: although the lifetime prevalence of SAD is estimated to be around 13% in the general population (Kessler et al., 2012), the prevalence of (sub)clinical SA in the sample was 38.3%, with a heritability of 0.43. In addition, the scores on the dimensional self‐assessments of social anxiety were also indicative of elevated levels of social anxiety. It is interesting to note that, although SAD is often comorbid with major depressive disorder (Meier et al., 2015), the prevalence of depressive episodes within the sample was in the range of the general population: the lifetime prevalence of past and/or present depressive episodes within the LFLSAD was 22.9% (27 cases in 118 participants), whereas population studies indicated that the lifetime prevalence of major depressive disorder within the community ranges between 17.1% (Jacobi et al., 2004) and 28.2% (Vandeleur et al., 2017). These results suggest that the sample is specifically enriched for SAD and not for depression.
Furthermore, as the majority of the participants (n = 124) visited the lab in Leiden and completed a variety of measurements including, among others, a structured clinical interview, self‐report questionnaires, and collection of saliva for future genotyping (Table 1; Figure 3), the LFLSAD sample is an extensively characterized sample. This enables detailed (future) analyses on the relationship between the social anxiety phenotype on the one hand and neurocognitive candidate endophenotypes of SAD on the other.
Here, we presented data on the relationship between (sub)clinical SAD and anxiety‐related constructs, showing that (sub)clinical SAD is positively related to increased levels of self‐reported social anxiety, fear of negative evaluation, and depressive symptoms, to higher trait anxiety, to the temperamental tendency to be behaviorally inhibited, and to higher levels of negative affect (Table 6). These findings are in line with previous reports indicating a relationship between (sub)clinical social anxiety and these self‐reported traits (Bas‐Hoogendam, van Steenbergen, Pannekoek, et al., 2017; Campbell et al., 2009; Carleton et al., 2007; Clauss & Blackford, 2012; Goldin, Manber, Hakimi, Canli, & Gross, 2009; Harrewijn et al., 2016; Rytwinski et al., 2009; Stein, Chartier, Lizak, & Jang, 2001) and underscore the validity of the LFLSAD sample.
Looking back at the power analyses performed before the start of the study, which showed that including 12 families of on average 10 family members would result in sufficient statistical power, the actual LFLSAD sample contains less families (i.e., nine), but with, on average, more family members per family (14.7 family members). In comparison with the original sample composition, this actual sample contains comparable statistical power to investigate candidate endophenotypes of SAD. The first results on neurocognitive endophenotypes emerging from the LFLSAD (Bas‐Hoogendam et al., 2015; Bas‐Hoogendam, van Steenbergen, van der Wee, et al., 2017a, 2017b; Harrewijn et al., 2018; Harrewijn, van der Molen, et al., 2017) underscore the potential of such a study design.
Some limitations of the LFLSAD design should be mentioned. First of all, the LFLSAD has a relatively small sample size, which is due to the novelty and complexity of performing a family study in this population. Furthermore, given the cross‐sectional nature of the study, the LFLSAD data do not allow for testing the state independency of the candidate neurocognitive endophenotypes (endophenotype criterion 2). In addition, as no control families were included, comparing the levels of the candidate endophenotypes between nonaffected family members and participants from the general population (second part of endophenotype criterion 4) is not possible. Finally, we did not acquire data with respect to potential environmental influences such as traumatic life events and aversive social experiences, which could play an important role in the etiology and maintenance of SAD (Brook & Schmidt, 2008; Norton & Abbott, 2017; Wong & Rapee, 2016).
5. CONCLUSION
To conclude, the LFLSAD provides a unique opportunity to examine candidate neurocognitive endophenotypes of this serious disorder. It is our hope that the results of this study will provide clues for future‐directed gene linkage studies, to gain more insight in the genetic vulnerability for SAD.
DECLARATION OF INTEREST STATEMENT
The authors report no conflicts of interest.
Supporting information
Data S1 Supporting Information
ACKNOWLEDGMENTS
The Leiden Family Lab study on Social Anxiety Disorder and Janna Marie Bas‐Hoogendam are funded by Leiden University Research Profile “Health, Prevention and the Human Life Cycle” and the Institute of Psychology of Leiden University. These funding sources had no involvement in writing this paper nor in the decision to submit this work for publication.
Furthermore, we are grateful to the Royal Dutch Academy of Sciences (KNAW), which enabled the organization of the Social Anxiety Conference, consisting of a 2‐day KNAW Academy Colloquium and a Master Class day (June 6–8, 2011, Leiden, the Netherlands), and we thank the Lorentz Center (Leiden, the Netherlands) for their financial and practical support in organizing the workshop “Endophenotypes of Social Anxiety Disorder: Can we detect them and are they useful in clinical practice?” which took place December 14–18, 2015 (https://www.lorentzcenter.nl/lc/web/2015/754/info.php3?wsid=754).
Bas‐Hoogendam JM, Harrewijn A, Tissier RLM, et al. The Leiden Family Lab study on Social Anxiety Disorder: A multiplex, multigenerational family study on neurocognitive endophenotypes. Int J Methods Psychiatr Res. 2018;27:e1616 10.1002/mpr.1616
Present address: Anita Harrewijn, Department of Human Development and Quantitative Methodology, University of Maryland, College Park, USA; or Emotion and Development Branch, National Institutes of Mental Health, Bethesda, USA.
REFERENCES
- Acarturk, C. , de Graaf, R. , van Straten, A. , Ten Have, M. , & Cuijpers, P. (2008). Social phobia and number of social fears, and their association with comorbidity, health‐related quality of life and help seeking: A population‐based study. Social Psychiatry and Psychiatric Epidemiology, 43, 273–279. 10.1007/s00127-008-0309-1 [DOI] [PubMed] [Google Scholar]
- Almasy, L. , & Blangero, J. (1998). Multipoint quantitative‐trait linkage analysis in general pedigrees. The American Journal of Human Genetics, 62, 1198–1211. 10.1086/301844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy, L. , & Blangero, J. (2010). Variance component methods for analysis of complex phenotypes. Cold Spring Harbor Protocols, 2010, pdb.top77 10.1101/pdb.top77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, J. , Liu, Z. , Evans‐Lacko, S. , Sadikova, E. , Sampson, N. , Chatterji, S. , … Thornicroft, G. (2018). Treatment gap for anxiety disorders is global: Results of the World Mental Health Surveys in 21 countries. Depression and Anxiety., 35, 195–208. 10.1002/da.22711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM‐5®) (Fifth ed.). Washington, DC: American Psychiatric Association Publishing. [Google Scholar]
- de Andrade, M. , & Amos, C. I. (2000). Ascertainment issues in variance components models. Genetic Epidemiology, 19, 333–344. [DOI] [PubMed] [Google Scholar]
- Bandelow, B. , Baldwin, D. , Abelli, M. , Altamura, C. , Dell'Osso, B. , Domschke, K. , … Riederer, P. (2016). Biological markers for anxiety disorders, OCD and PTSD—A consensus statement. Part I: Neuroimaging and genetics. The World Journal of Biological Psychiatry, 17, 321–365. 10.1080/15622975.2016.1181783 [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen, S. , Wheelwright, S. , Skinner, R. , Martin, J. , & Clubley, E. (2001). The Autism‐Spectrum Quotient (AQ): Evidence from Asperger syndrome/high‐functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31, 5–17. 10.1023/A:1005653411471 [DOI] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , Blackford, J. U. , Brühl, A. B. , Blair, K. S. , van der Wee, N. J. A. , & Westenberg, P. M. (2016). Neurobiological candidate endophenotypes of social anxiety disorder. Neuroscience & Biobehavioral Reviews, 71, 362–378. 10.1016/j.neubiorev.2016.08.040 [DOI] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , Harrewijn, A. , van der Molen, M. J. W. , van Steenbergen, H. , van Vliet, I. , Houwing‐Duistermaat, J. , … Westenberg, P. M. (2014). Profiling endophenotypes in social anxiety disorder: A neurocognitive approach. General Background and Key Question of Project. 10.17605/OSF.IO/E368H [DOI]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , Kreuk, T. , van der Wee, N. J. A. , & Westenberg, P. M. (2017). How embarrassing! The behavioral and neural correlates of processing social norm violations. PLoS One, 12, e0176326 10.1371/journal.pone.0176326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , Pannekoek, J. N. , Fouche, J.‐P. , Lochner, C. , Hattingh, C. J. , … van der Wee, N. J. A. (2017). Voxel‐based morphometry multi‐center mega‐analysis of brain structure in social anxiety disorder. NeuroImage: Clinical, 16, 678–688. 10.1016/j.nicl.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , van der Wee, N. J. A. , & Westenberg, P. M. (2017a). Social norm processing as an endophenotype of social anxiety disorder: A family study in two generations. European Neuropsychopharmacology, 27, S49–S50. 10.1016/S0924-977X(17)30120-7 [DOI] [Google Scholar]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , van der Wee, N. J. A. , & Westenberg, P. M. (2017b). Subcortical brain volumes as endophenotypes of social anxiety disorder—Preliminary findings from the Leiden Family Study on Social Anxiety Disorder. European Neuropsychopharmacology, 27, S1021 10.1016/S0924-977X(17)31789-3 [DOI] [Google Scholar]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , van der Wee, N. J. A. , & Westenberg, P. M. (2018). Not intended, still embarrassed: Social anxiety is related to increased levels of embarrassment in response to unintentional social norm violations. European Psychiatry, 52, 15–21. 10.1016/j.eurpsy.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Bas‐Hoogendam, J. M. , van Steenbergen, H. , Westenberg, P. M. , & van der Wee, N. J. A. (2015). Social conditioning of neutral faces: A pilot‐study on brain functioning in social anxiety patients and their unaffected first‐degree relatives. European Neuropsychopharmacology, 25, S573–S574. 10.1016/S0924-977X(15)30803-8 [DOI] [Google Scholar]
- Bauhuis, O. , Jonker, K. , Verdellen, C. , Reynders, J. , & Verbraak, M. (2013). De introductie van een Nederlandstalig instrument om DSM‐IV‐Tr‐diagnoses bij kinderen te stellen. Kind & Adolescent Praktijk, 12, 20–26. 10.1007/s12454-013-0005-5 [DOI] [Google Scholar]
- Beard, C. , Moitra, E. , Weisberg, R. B. , & Keller, M. B. (2010). Characteristics and predictors of social phobia course in a longitudinal study of primary‐care patients. Depression and Anxiety, 27, 839–845. 10.1002/da.20676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden, C. E. , Reus, V. I. , & Freimer, N. B. (2004). Why genetic investigation of psychiatric disorders is so difficult. Current Opinion in Genetics & Development, 14, 280–286. 10.1016/j.gde.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Beauchaine, T. P. , Neuhaus, E. , Brenner, S. L. , & Gatzke‐Kopp, L. (2008). Ten good reasons to consider biological processes in prevention and intervention research. Development and Psychopathology, 20, 745–774. 10.1017/S0954579408000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. , Steer, R. , & Brown, G. (1996). Manual for the Beck Depression Inventory‐II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beesdo‐Baum, K. , Knappe, S. , Fehm, L. , Höfler, M. , Lieb, R. , Hofmann, S. G. , & Wittchen, H.‐U. (2012). The natural course of social anxiety disorder among adolescents and young adults. Acta Psychiatrica Scandinavica, 126, 411–425. 10.1111/j.1600-0447.2012.01886.x [DOI] [PubMed] [Google Scholar]
- Binder, E. B. (2012). The genetic basis of mood and anxiety disorders—Changing paradigms. Biology of Mood & Anxiety Disorders, 2, 1–3. 10.1186/2045-5380-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford, J. U. , Allen, A. H. , Cowan, R. L. , & Avery, S. N. (2013). Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience, 8, 143–150. 10.1093/scan/nsr078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford, J. U. , Avery, S. N. , Cowan, R. L. , Shelton, R. C. , & Zald, D. H. (2011). Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience, 6, 621–629. 10.1093/scan/nsq073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, K. S. , Geraci, M. , Hollon, N. , Otero, M. , DeVido, J. , Majestic, C. , … Pine, D. S. (2010). Social norm processing in adult social phobia: atypically increased ventromedial frontal cortex responsiveness to unintentional (embarrassing) transgressions. The American Journal of Psychiatry, 167, 1526–1532. 10.1176/appi.ajp.2010.09121797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook, C. A. , & Schmidt, L. A. (2008). Social anxiety disorder: A review of environmental risk factors. Neuropsychiatric Disease and Treatment, 4, 123–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, D. W. , Sareen, J. , Stein, M. B. , Kravetsky, L. B. , Paulus, M. P. , Hassard, S. T. , & Reiss, J. P. (2009). Happy but not so approachable: The social judgments of individuals with generalized social phobia. Depression and Anxiety, 26, 419–424. 10.1002/da.20474 [DOI] [PubMed] [Google Scholar]
- Cannon, T. D. , & Keller, M. C. (2006). Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology, 2, 267–290. 10.1146/annurev.clinpsy.2.022305.095232 [DOI] [PubMed] [Google Scholar]
- Carleton, R. N. , Collimore, K. C. , & Asmundson, G. J. G. (2007). Social anxiety and fear of negative evaluation: Construct validity of the BFNE‐II. Journal of Anxiety Disorders, 21, 131–141. 10.1016/j.janxdis.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Carleton, R. N. , McCreary, D. R. , Norton, P. J. , & Asmundson, G. J. G. (2006). Brief fear of negative evaluation scale—Revised. Depression and Anxiety, 23, 297–303. 10.1002/da.20142 [DOI] [PubMed] [Google Scholar]
- Carver, C. S. , & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67, 319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Clauss, J. A. , & Blackford, J. U. (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta‐analytic study. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 1066–1075. 10.1016/j.jaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J. N. , Davis, S. A. , Todd, R. D. , Schindler, M. K. , Gross, M. M. , Brophy, S. L. , … Reich, W. (2003). Validation of a brief quantitative measure of autistic traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview‐Revised. Journal of Autism and Developmental Disorders, 33, 427–433. 10.1023/A:1025014929212 [DOI] [PubMed] [Google Scholar]
- Crawford, J. R. , & Henry, J. D. (2004). The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non‐clinical sample. British Journal of Clinical Psychology, 43, 245–265. 10.1348/0144665031752934 [DOI] [PubMed] [Google Scholar]
- Davis, F. C. , Johnstone, T. , Mazzulla, E. C. , Oler, J. A. , & Whalen, P. J. (2010). Regional response differences across the human amygdaloid complex during social conditioning. Cerebral Cortex, 20, 612–621. 10.1093/cercor/bhp126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans, A. E. , van Vliet, I. M. , Couvée, J. , & Westenberg, H. G. (2001). Characteristics of patients with social phobia and their treatment in specialized clinics for anxiety disorders in the Netherlands. Journal of Affective Disorders, 65, 123–129. 10.1016/S0165-0327(00)00238-X [DOI] [PubMed] [Google Scholar]
- Fehm, L. , Pelissolo, A. , Furmark, T. , & Wittchen, H.‐U. (2005). Size and burden of social phobia in Europe. European Neuropsychopharmacology : The Journal of the European College of Neuropsychopharmacology, 15, 453–462. 10.1016/j.euroneuro.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Flint, J. , Timpson, N. , & Munafò, M. (2014). Assessing the utility of intermediate phenotypes for genetic mapping of psychiatric disease. Trends in Neurosciences, 37, 733–741. 10.1016/j.tins.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. S. , & Kalin, N. H. (2014). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. The American Journal of Psychiatry, 171, 1162–1173. 10.1176/appi.ajp.2014.14040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken, I. H. A. , Muris, P. , & Rassin, E. (2005). Psychometric properties of the Dutch BIS/BAS scales. Journal of Psychopathology and Behavioral Assessment, 27, 25–30. 10.1007/s10862-005-3262-2 [DOI] [Google Scholar]
- Fresco, D. M. , Coles, M. E. , Heimberg, R. G. , Liebowitz, M. R. , Hami, S. , Stein, M. B. , & Goetz, D. (2001). The Liebowitz Social Anxiety Scale: A comparison of the psychometric properties of self‐report and clinician‐administered formats. Psychological Medicine, 31, 1025–1035. 10.1017/S0033291701004056 [DOI] [PubMed] [Google Scholar]
- Garner, M. , Möhler, H. , Stein, D. J. , Mueggler, T. , & Baldwin, D. S. (2009). Research in anxiety disorders: From the bench to the bedside. European Neuropsychopharmacology : The Journal of the European College of Neuropsychopharmacology, 19, 381–390. 10.1016/j.euroneuro.2009.01.011 [DOI] [PubMed] [Google Scholar]
- Glahn, D. C. , Thompson, P. M. , & Blangero, J. (2007). Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Human Brain Mapping, 28, 488–501. 10.1002/hbm.20401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin, P. R. , Manber, T. , Hakimi, S. , Canli, T. , & Gross, J. J. (2009). Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66, 170–180. 10.1001/archgenpsychiatry.2008.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman, I. I. , & Gould, T. D. (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. The American Journal of Psychiatry, 160, 636–645. 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- Gottschalk, M. G. , & Domschke, K. (2016). Novel developments in genetic and epigenetic mechanisms of anxiety. Current Opinion in Psychiatry, 29(1), 32–38. 10.1097/YCO.0000000000000219 [DOI] [PubMed] [Google Scholar]
- Gould, T. D. , & Gottesman, I. I. (2006). Psychiatric endophenotypes and the development of valid animal models. Genes, Brain, and Behavior, 5, 113–119. 10.1111/j.1601-183X.2005.00186.x [DOI] [PubMed] [Google Scholar]
- Gur, R. E. , Nimgaonkar, V. L. , Almasy, L. , Calkins, M. E. , Ragland, J. D. , Pogue‐Geile, M. F. , … Gur, R. C. (2007). Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. American Journal of Psychiatry, 164, 813–819. 10.1176/ajp.2007.164.5.813 [DOI] [PubMed] [Google Scholar]
- Haller, S. P. W. , Cohen Kadosh, K. , Scerif, G. , & Lau, J. Y. F. (2015). Social anxiety disorder in adolescence: How developmental cognitive neuroscience findings may shape understanding and interventions for psychopathology. Developmental Cognitive Neuroscience, 13, 11–20. 10.1016/j.dcn.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrewijn, A. , Schmidt, L. A. , Westenberg, P. M. , Tang, A. , & van der Molen, M. J. W. (2017). Electrocortical measures of information processing biases in social anxiety disorder: A review. Biological Psychology, 129, 324–348. 10.1016/J.BIOPSYCHO.2017.09.013 [DOI] [PubMed] [Google Scholar]
- Harrewijn, A. , van der Molen, M. J. W. , van Vliet, I. M. , Houwing‐Duistermaat, J. J. , & Westenberg, P. M. (2017). Delta‐beta correlation as a candidate endophenotype of social anxiety: A two‐generation family study. Journal of Affective Disorders, 227, 398–405. 10.1016/J.JAD.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Harrewijn, A. , van der Molen, M. J. W. , van Vliet, I. M. , Tissier, R. L. , & Westenberg, P. M. (2018). Behavioral and EEG responses to social evaluation: A two‐generation family study on social anxiety. NeuroImage: Clinical, 17, 549–562. 10.1016/j.nicl.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrewijn, A. , Van der Molen, M. J. W. , & Westenberg, P. M. (2016). Putative EEG measures of social anxiety: Comparing frontal alpha asymmetry and delta–beta cross‐frequency correlation. Cognitive, Affective, & Behavioral Neuroscience, 16, 1086–1098. 10.3758/s13415-016-0455-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg, R. G. , Horner, K. J. , Juster, H. R. , Safren, S. A. , Brown, E. J. , Schneier, F. R. , & Liebowitz, M. R. (1999). Psychometric properties of the Liebowitz Social Anxiety Scale. Psychological Medicine, 29, 199–212. 10.1017/S0033291798007879 [DOI] [PubMed] [Google Scholar]
- Isomura, K. , Boman, M. , Rück, C. , Serlachius, E. , Larsson, H. , Lichtenstein, P. , & Mataix‐Cols, D. (2015). Population‐based, multi‐generational family clustering study of social anxiety disorder and avoidant personality disorder. Psychological Medicine, 45, 1581–1589. 10.1017/S0033291714002116 [DOI] [PubMed] [Google Scholar]
- Jacobi, F. , Wittchen, H.‐U. , Hölting, C. , Höfler, M. , Pfister, H. , Müller, N. , & Lieb, R. (2004). Prevalence, co‐morbidity and correlates of mental disorders in the general population: Results from the German Health Interview and Examination Survey (GHS). Psychological Medicine, 34, 597–611. 10.1017/S0033291703001399 [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Petukhova, M. , Sampson, N. A. , Zaslavsky, A. M. , & Wittchen, H.‐U. (2012). Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research, 21, 169–184. 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs, M. (1983). The Childrens' Depression Inventory: A self‐rated depression scale for school‐aged youngsters. Pittsburgh: University of Pittsburgh School of Medicine. [Google Scholar]
- Kovacs, M. (1985). The Children's Depression Inventory (CDI). Psychopharmacology Bulletin, 21, 995–998. [PubMed] [Google Scholar]
- La Greca, A. M. , & Lopez, N. (1998). Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology, 26, 83–94. 10.1023/A:1022684520514 [DOI] [PubMed] [Google Scholar]
- Leary, M. R. (1983). A brief version of the Fear of Negative Evaluation Scale. Personality and Social Psychology Bulletin, 9, 371–375. 10.1177/0146167283093007 [DOI] [Google Scholar]
- Lenzenweger, M. F. (2013a). Endophenotype, intermediate phenotype, biomarker: Definitions, concept comparisons, clarifications. Depression and Anxiety, 30, 185–189. 10.1002/da.22042 [DOI] [PubMed] [Google Scholar]
- Lenzenweger, M. F. (2013b). Thinking clearly about the endophenotype‐intermediate phenotype‐biomarker distinctions in developmental psychopathology research. Development and Psychopathology, 25, 1347–1357. 10.1017/S0954579413000655 [DOI] [PubMed] [Google Scholar]
- Mack, S. , Jacobi, F. , Beesdo‐Baum, K. , Gerschler, A. , Strehle, J. , Höfler, M. , … Wittchen, H.‐U. (2015). Functional disability and quality of life decrements in mental disorders: Results from the Mental Health Module of the German Health Interview and Examination Survey for Adults (DEGS1‐MH). European Psychiatry : The Journal of the Association of European Psychiatrists, 30, 793–800. 10.1016/j.eurpsy.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Meier, S. M. , Petersen, L. , Mattheisen, M. , Mors, O. , Mortensen, P. B. , & Laursen, T. M. (2015). Secondary depression in severe anxiety disorders: A population‐based cohort study in Denmark. The Lancet Psychiatry, 2, 515–523. 10.1016/S2215-0366(15)00092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin, D. S. , Fresco, D. M. , Heimberg, R. G. , Schneier, F. R. , Davies, S. O. , & Liebowitz, M. R. (2002). Screening for social anxiety disorder in the clinical setting: Using the Liebowitz Social Anxiety Scale. Journal of Anxiety Disorders, 16, 661–673. 10.1016/S0887-6185(02)00134-2 [DOI] [PubMed] [Google Scholar]
- Miers, A. C. , Blöte, A. W. , de Rooij, M. , Bokhorst, C. L. , & Westenberg, P. M. (2013). Trajectories of social anxiety during adolescence and relations with cognition, social competence, and temperament. Journal of Abnormal Child Psychology, 41, 97–110. 10.1007/s10802-012-9651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miers, A. C. , Blöte, A. W. , Heyne, D. A. , & Westenberg, P. M. (2014). Developmental pathways of social avoidance across adolescence: The role of social anxiety and negative cognition. Journal of Anxiety Disorders, 28, 787–794. 10.1016/j.janxdis.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Miers, A. C. , Blöte, A. W. , & Westenberg, P. M. (2010). Peer perceptions of social skills in socially anxious and nonanxious adolescents. Journal of Abnormal Child Psychology, 38, 33–41. 10.1007/s10802-009-9345-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. A. , & Rockstroh, B. (2013). Endophenotypes in psychopathology research: Where do we stand? Annual Review of Clinical Psychology, 9, 177–213. 10.1146/annurev-clinpsy-050212-185540 [DOI] [PubMed] [Google Scholar]
- Munafò, M. R. , & Flint, J. (2014). The genetic architecture of psychophysiological phenotypes. Psychophysiology, 51, 1331–1332. 10.1111/psyp.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris, P. , Meesters, C. , de Kanter, E. , & Timmerman, P. E. (2005). Behavioural inhibition and behavioural activation system scales for children: Relationships with Eysenck's personality traits and psychopathological symptoms. Personality and Individual Differences, 38, 831–841. 10.1016/j.paid.2004.06.007 [DOI] [Google Scholar]
- Norton, A. R. , & Abbott, M. J. (2017). The role of environmental factors in the aetiology of social anxiety disorder: A review of the theoretical and empirical literature. Behaviour Change, 34, 76–97. 10.1017/bec.2017.7 [DOI] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Peeters, F. , Ponds, R. , & Vermeer, M. (1996). Affectiviteit en zelfbeoordeling van depressie en angst. Tijdschrift voor Psychiatrie, 38, 240–250. [Google Scholar]
- Puls, I. , & Gallinat, J. (2008). The concept of endophenotypes in psychiatric diseases meeting the expectations? Pharmacopsychiatry, 41(Suppl 1), S37–S43. 10.1055/s-2008-1081462 [DOI] [PubMed] [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Roeyers, H. , Thys, M. , Druart, C. , De Schryver, M. , & Schittekatte, M. (2011). Screeningslijst voor autismespectrumstoornissen (SRS) handleiding. Amsterdam: Hogrefe uitgevers bv. [Google Scholar]
- Ruscio, A. M. , Brown, T. A. , Chiu, W. T. , Sareen, J. , Stein, M. B. , & Kessler, R. C. (2008). Social fears and social phobia in the USA: Results from the National Comorbidity Survey Replication. Psychological Medicine, 38, 15–28. 10.1017/S0033291707001699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytwinski, N. K. , Fresco, D. M. , Heimberg, R. G. , Coles, M. E. , Liebowitz, M. R. , Cissell, S. , … Hofmann, S. G. (2009). Screening for social anxiety disorder with the self‐report version of the Liebowitz Social Anxiety Scale. Depression and Anxiety, 26, 34–38. 10.1002/da.20503 [DOI] [PubMed] [Google Scholar]
- Sanislow, C. A. , Pine, D. S. , Quinn, K. J. , Kozak, M. J. , Garvey, M. A. , Heinssen, R. K. , … Cuthbert, B. N. (2010). Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology, 119, 631–639. 10.1037/a0020909 [DOI] [PubMed] [Google Scholar]
- Schiele, M. A. , & Domschke, K. (2017). Epigenetics at the crossroads between genes, environment and resilience in anxiety disorders. Genes, Brain and Behavior.. 10.1111/gbb.12423 [DOI] [PubMed] [Google Scholar]
- Schwartz, C. E. , Wright, C. I. , Shin, L. M. , Kagan, J. , & Rauch, S. L. (2003). Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science, 300, 1952–1953. 10.1126/science.1083703 [DOI] [PubMed] [Google Scholar]
- Schwartz, C. E. , Wright, C. I. , Shin, L. M. , Kagan, J. , Whalen, P. J. , McMullin, K. G. , & Rauch, S. L. (2003). Differential amygdalar response to novel versus newly familiar neutral faces: A functional MRI probe developed for studying inhibited temperament. Biological Psychiatry, 53, 854–862. 10.1016/S0006-3223(02)01906-6 [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , … Dunbar, G. C. (1998). The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. The Journal of Clinical Psychiatry, 59(Suppl 2), 22–33. [PubMed] [Google Scholar]
- Sheehan, D. V. , Sheehan, K. H. , Shytle, R. D. , Janavs, J. , Bannon, Y. , Rogers, J. E. , … Wilkinson, B. (2010). Reliability and Validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI‐KID). The Journal of Clinical Psychiatry, 71, 313–326. 10.4088/JCP.09m05305whi [DOI] [PubMed] [Google Scholar]
- Smoller, J. W. (2015). The genetics of stress‐related disorders: PTSD, depression and anxiety disorders. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 41, 297–319. 10.1038/npp.2015.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence, S. H. , & Rapee, R. M. (2016). The etiology of social anxiety disorder: An evidence‐based model. Behaviour Research and Therapy, 86, 50–67. 10.1016/j.brat.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , & Lushene, R. E. (1970). STAI manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Spielberger, C. D. , & Vagg, P. R. (1984). Psychometric properties of the STAI: A reply to Ramanaiah, Franzen, and Schill. Journal of Personality Assessment, 48, 95–97. 10.1207/s15327752jpa4801_16 [DOI] [PubMed] [Google Scholar]
- Stein, M. B. , Chartier, M. J. , Lizak, M. V. , & Jang, K. L. (2001). Familial aggregation of anxiety‐related quantitative traits in generalized social phobia: clues to understanding “disorder” heritability? American Journal of Medical Genetics, 105, 79–83. [DOI] [PubMed] [Google Scholar]
- Stein, M. B. , & Stein, D. J. (2008). Social anxiety disorder. Lancet, 371, 1115–1125. 10.1016/S0140-6736(08)60488-2 [DOI] [PubMed] [Google Scholar]
- Steinert, C. , Hofmann, M. , Leichsenring, F. , & Kruse, J. (2013). What do we know today about the prospective long‐term course of social anxiety disorder? A systematic literature review. Journal of Anxiety Disorders, 27, 692–702. 10.1016/j.janxdis.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Storch, E. A. , Masia‐Warner, C. , Dent, H. C. , Roberti, J. W. , & Fisher, P. H. (2004). Psychometric evaluation of the Social Anxiety Scale for Adolescents and the Social Phobia and Anxiety Inventory for Children: Construct validity and normative data. Journal of Anxiety Disorders, 18, 665–679. 10.1016/j.janxdis.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Timbremont, B. , & Braet, C. (2002). Children's Depression Inventory: Nederlandstalige versie [Children's Depression Inventory: Dutch version]. Lisse: Swets & Zeitlinger. [Google Scholar]
- Tissier, R. , Tsonaka, R. , Mooijaart, S. P. , Slagboom, E. , & Houwing‐Duistermaat, J. J. (2017). Secondary phenotype analysis in ascertained family designs: Application to the Leiden longevity study. Statistics in Medicine, 36, 2288–2301. 10.1002/sim.7281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does, A. (2002). Handleiding bij de Nederlandse versie van Beck Depression Inventory—second edition (BDI—II—NL) [Manual for the Dutch version of the Beck Depression Inventory—second edition (BDI—II—NL).]. Amsterdam: Harcourt. [Google Scholar]
- Van der Molen, M. J. W. , Poppelaars, E. S. , Van Hartingsveldt, C. T. A. , Harrewijn, A. , Gunther Moor, B. , & Westenberg, P. M. (2014). Fear of negative evaluation modulates electrocortical and behavioral responses when anticipating social evaluative feedback. Frontiers in Human Neuroscience, 7, 936 10.3389/fnhum.2013.00936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleur, C. L. , Fassassi, S. , Castelao, E. , Glaus, J. , Strippoli, M.‐P. F. , Lasserre, A. M. , … Preisig, M. (2017). Prevalence and correlates of DSM‐5 major depressive and related disorders in the community. Psychiatry Research, 250, 50–58. 10.1016/j.psychres.2017.01.060 [DOI] [PubMed] [Google Scholar]
- van Vliet, I. M. , & de Beurs, E. (2007). The MINI‐International Neuropsychiatric Interview. A brief structured diagnostic psychiatric interview for DSM‐IV en ICD‐10 psychiatric disorders. Tijdschrift voor Psychiatrie, 49, 393–397. [PubMed] [Google Scholar]
- Watson, D. , Clark, L. A. , & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1991). Manual for the Wechsler Intelligence Scale for Children—Third edition (WISC‐III). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler, D. , Coalson, D. , & Raiford, S. (2008). WAIS‐IV technical and interpretive manual. San Antonio, TX: Pearson. [Google Scholar]
- Westenberg, P. M. , Gullone, E. , Bokhorst, C. L. , Heyne, D. A. , & King, N. J. (2007). Social evaluation fear in childhood and adolescence: Normative developmental course and continuity of individual differences. British Journal of Developmental Psychology, 25, 471–483. 10.1348/026151006X173099 [DOI] [Google Scholar]
- Williams, J. T. , & Blangero, J. (1999). Power of variance component linkage analysis to detect quantitative trait loci. Annals of Human Genetics, 63, 545–563. 10.1017/S0003480099007848 [DOI] [PubMed] [Google Scholar]
- Wittchen, H.‐U. , & Fehm, L. (2003). Epidemiology and natural course of social fears and social phobia. Acta Psychiatrica Scandinavica, 108, 4–18. 10.1034/j.1600-0447.108.s417.1.x [DOI] [PubMed] [Google Scholar]
- Wong, Q. J. J. , & Rapee, R. M. (2016). The aetiology and maintenance of social anxiety disorder: A synthesis of complimentary theoretical models and formulation of a new integrated model. Journal of Affective Disorders, 203, 84–100. 10.1016/j.jad.2016.05.069 [DOI] [PubMed] [Google Scholar]
- Wray, N. , & Visscher, P. (2008). Estimating trait heritability. Nature Education, 1, 29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information


