Abstract
Background
Papulopustular rosacea and rosacea‐like demodicosis have numerous similarities, but they are generally considered as two distinct entities, mainly because the causal role of the Demodex mite in the development of rosacea is not yet widely accepted. Several clinical characteristics are traditionally considered to differentiate the two conditions; for example, papulopustular rosacea is typically characterized by central facial papulopustules and persistent erythema, whereas small superficial papulopustules and follicular scales rather suggest rosacea‐like demodicosis. However, none of these characteristics is exclusive to either entity.
Objective
To explore differences in Demodex densities according to clinical characteristics traditionally associated with these two conditions.
Methods
Retrospective, observational, case–control study of 242 patients with central face papulopustules. Demodex densities were measured on two consecutive standardized skin surface biopsies.
Results
In the whole cohort, Demodex densities were greater in patients with persistent erythema than in those without. In 132 patients without recent treatment or other facial dermatoses, 120 (91%) had persistent erythema, 119 (90%) small superficial papulopustules and 124 (94%) follicular scales; 116 (88%) simultaneously had clinical characteristics traditionally associated with both papulopustular rosacea and rosacea‐like demodicosis. Higher Demodex densities were linked to the presence of follicular scales, but not to papulopustules size, nor to the presence/absence of persistent erythema.
Conclusion
Our observations highlight the difficulty differentiating between these entities and suggest that rosacea‐like demodicosis and papulopustular rosacea should no longer be considered as two separate entities, but rather as two phenotypes of the same disease.
Introduction
Rosacea and demodicosis are common dermatoses, but their nosologic classification and diagnostic criteria are still controversial.1, 2, 3 The National Rosacea Society (NRS) expert committee defines rosacea as a central face distribution of at least one of four primary features: flushing, persistent erythema, papules and pustules and telangiectasia.4 The global ROSacea COnsensus (ROSCO) panel recently suggested a more phenotype‐based approach with two features only–phymatous changes and persistent centrofacial erythema–considered as diagnostic, and other features considered as major (flushing, inflammatory papules and pustules, telangiectasia, ocular manifestations) or secondary (burning, stinging and dry sensation of the skin, oedema).5
Four subtypes of rosacea–erythematotelangiectatic rosacea, papulopustular rosacea (PPR), phymatous rosacea and ocular rosacea–and one variant (granulomatous rosacea) were defined by the NRS consensus, each associated with specific groups of symptoms.4 Using the NRS definitions, diagnosis of subtype II, PPR, requires the presence of two features: persistent erythema and papulopustules.4 Hence, patients with central face papulopustules have rosacea, but if this is not associated with persistent erythema, they do not have PPR according to the NRS consensus definitions. Chen and Plewig consider that these patients probably have rosacea‐like demodicosis (which they called ‘primary papulopustular demodicosis’).6
Rosacea is usually considered to be a primary disorder of innate and adaptative immunity, which can be stimulated by diverse triggers.4, 5 Some experts consider that these immune reactions are not primary, but are the consequence of an abnormal proliferation of Demodex mite, itself probably induced by a local immunosuppressive factor, hypervascularization or sebaceous hyperplasia.2, 3, 7, 8, 9 However, this viewpoint is not yet widely accepted, because it cannot be proved using classical methods as the Koch postulates do not apply to this parasite.7, 9, 10
In 1960, Ayres described rosacea‐like demodicosis as a facial eruption clinically resembling rosacea but caused by Demodex proliferation.11 Diagnosis is confirmed by the presence of a high density of Demodex mites1, 2, 3, 7, 9, 16, 17, 18, 19, 20, 21, 22, 23, 24 and by clinical cure with normalization of Demodex density (Dd) after acaricidal treatment.2, 7, 9, 11, 12, 13
Currently, most experts distinguish two separate entities: ‘PPR, not caused by Demodex’ and ‘rosacea‐like demodicosis, caused by Demodex’.1, 4, 5 Paradoxically, the central role of Demodex in demodicosis is generally well‐accepted,1 although there are no more data to support its role in demodicosis than there are to support its role in PPR. Traditionally, rosacea‐like demodicosis differs from PPR in several clinical criteria, including its unilateral distribution, the presence of more superficial and smaller papules and pustules, follicular scales and pruritus; moreover, persistent erythema may not be present.1, 2, 3, 7, 9, 16, 17, 18, 19, 20, 21, 22, 23, 24 However, none of these criteria is exclusive or absolute: for example, unilateral distribution can be encountered in PPR,5 and rosacea‐like demodicosis can have a bilateral distribution. Moreover, in clinical practice, we often observe patients with mixed characteristics from the two ‘conditions’ and the distinction can thus be very difficult.2, 7, 13, 14 In addition, histopathological analysis cannot distinguish between these conditions.15
If PPR and rosacea‐like demodicosis are two separate entities, the most logical hypothesis is that Dds should be normal in PPR and high in rosacea‐like demodicosis. However, we now know that this is not the case: patients with PPR have higher Dds than do patients with healthy skin9, 16, 17, 18, 19, 20, 21, 22, 23, 24 and cases of PPR with normal Dds are rare.3, 16, 24 There are no reports comparing Dds in these two conditions.
In this article, we explore Dds in patients with central face papulopustules, according to clinical characteristics usually attributed to either PPR or rosacea‐like demodicosis, and report a case history that strongly supports the hypothesis that these two ‘entities’ are phenotypes of the same disease.
Methods
Patients
This retrospective study was approved by Erasme Hospital Ethics committee. In an earlier study, all patients attending our dermatology practice in Brussels between 2002 and 2010 with clinical rosacea with central facial papulopustules were included (n = 254).24 This study is a secondary analysis of 242 patients included in that study: the 215 patients with central facial papulopustules and persistent erythema and the 27 with central facial papulopustules without persistent erythema.
Two consecutive standardized skin surface biopsies (SSSBs) were performed in each patient. For each patient, the date of consultation, age, sex, clinical diagnosis, symptoms, other potential facial dermatoses, recent treatment for the facial condition, location of the SSSBs and Dd values were recorded.
To avoid confounding factors that could potentially influence the facial cutaneous symptoms, we also evaluated a subgroup of 132 patients who had received no treatment during the previous 3 months and had no concomitant facial dermatoses (such as acne vulgaris or seborrhoeic dermatitis).
Sampling method
The SSSB is a sampling method in which 1 cm² of the superficial part of the horny layer and of the follicular content of the skin is collected (Fig. 1b).16 Full details of the method, including an online video, are available in our earlier publication.24 The technique is simple and uses tools that are readily available in most dermatology clinics.
Figure 1.

Rosacea‐like demodicosis and papulopustular rosacea occurring successively in the same patient. Panels (a), (b) and (c): right cheek and full‐face images of a 19‐year‐old woman consulting for a facial papulopustular eruption that had been present for 1 year. Panel a: careful examination showed no comedones, but discreet follicular scales at the hair roots (arrows), together with small superficial papulopustules unilaterally, enabling us to diagnose rosacea‐like demodicosis. Panels (b) and (c): two standardized skin surface biopsies (SSSBs) were performed at the same site on each cheek during the consultation and Demodex densities (Dds) measured; results of the two biopsies are shown in the lower left and right corners for left and right cheek, respectively. Panels (d) and (e): full‐face images after 5 weeks and 3 months of topical acaricidal treatment, showing progressive clearance of the eruption and normalization of Dds on the right cheek (SSSBs were not performed on the left cheek at these time points). Panel (f): full‐face images of the same patient more than 3 years after the first presentation, 27 months after stopping the maintenance treatment. At this time, the papulopustules were larger than at the initial consultation and were spread across both her cheeks; she also suffered from flushing and persistent erythema. The clinical diagnosis was PPR. This time, the Dds were high on both cheeks. Panels (g) and (h): full‐face images after 2 months and 4 months of the same topical acaricidal treatment as after the first episode, again showing progressive clearance of the eruption and normalization of Dds. Panels (a) and (c) reprinted from ref 2 (Forton FM, et al. Demodicosis: descriptive classification and status of Rosacea, in response to prior classification proposed. J Eur Acad Dermatol Venereol 2015;29:829‐32). The patient has provided written consent for publication.
In our study, the patient's skin and the microscope slides were first cleaned with ether, and two SSSBs were then performed consecutively at the same place, allowing measurement of two Dds (D/cm²) (superficial [SSSB1] and deep [SSSB2]). The sum of these two values (SSSB1+2) was also noted.
The SSSBs were performed at the site of the main skin lesions, preferably on the cheek if affected (because the highest Dds have been observed here).17, 19
Statistical analysis
Continuous variables are summarized by their mean ± standard error of the mean (SEM) and qualitative variables by numbers and percentages. Differences in continuous variables between groups were compared using Student's t‐tests. Differences in qualitative variables were compared between groups using Pearson's exact chi‐squared tests. Statistical significance was considered when P was <0.05. All statistical tests were performed using IBM‐SPSS (version 23.0 to 24.0) software (IBM Corp, Armonk, NY, USA).
Results
Among the 215 patients with persistent erythema, the mean age was 48 years (range: 46–50; SEM: 1); 158 (73.5%) were women. Among the 27 patients without persistent erythema, the mean age was 43 years (range: 36–50; SEM: 3); 17 (63%) were women. There were no statistically significant differences between these groups in age (P = 0.084) or sex (P = 0.259). The cheek was the most frequent biopsy site (215/242, 89%), with no statistically significant difference between the groups (196/215 and 19/27; P = 0.099).
Patients with persistent erythema had higher Dds than those without persistent erythema, the differences being statistically significant for SSSB2 and SSSB1+2 (respective values: SSSB1: 91 ± 8 D/cm² and 60 ± 17 D/cm² (P = 0.081); SSSB2: 208 ± 14 D/cm² and 130 ± 23 D/cm² (P = 0.031); SSSB1+2: 298 ± 19 D/cm² and 191 ± 36 D/cm² (P = 0.025)).
Among the subgroup of 132 patients who had not received any recent treatment and did not have another concomitant dermatosis, 120 (91%) had persistent erythema, 119 (90%) had small superficial papulopustules and 124 (94%) had follicular scales; 105 (80%) patients had all three of these signs. Eight patients had persistent erythema, follicular scales and large papulopustules, three patients had persistent erythema and small superficial papulopustules without follicular scales and 11 patients had follicular scales and small superficial papulopustules without persistent erythema. For three patients with persistent erythema and no follicular scales, the papulopustule size was unknown. One patient had persistent erythema with large papulopustules and no follicular scales, and one had large papulopustules, with no persistent erythema or follicular scales.
In this subgroup of 132 patients, those with persistent erythema had higher Dds than those without persistent erythema, but this difference was not statistically significant (Table 1); patients with follicular scales had higher SSSBs than those without follicular scales, the differences being statistically significant for SSSB2 and SSSB1+2 (Table 1). There was no statistically significant difference in mean Dd according to the size of the papulopustules.
Table 1.
Demodex densities in a subgroup of patients who had received no treatment during the previous 3 months and had no concomitant facial dermatosis (n = 132)
| Clinical symptoms | Patients | SSSB 1 | SSSB 2 | SSSB 1 + 2 | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | Mean ± SEM | P | Mean ± SEM | P | Mean ± SEM | P | |
| Persistent erythema | ||||||||
| Present* | 120 | 91 | 96 ± 12 | 0.361 | 215 ± 17 | 0.270 | 311 ± 26 | 0.253 |
| Absent | 12 | 9 | 81 ± 34 | 162 ± 36 | 243 ± 65 | |||
| Papulopustules † | ||||||||
| Small | 119 | 90 | 101 ± 12 | 0.065 | 212 ± 17 | 0.764 | 312 ± 26 | 0.531 |
| Large | 10 | 8 | 49 ± 11 | 231 ± 81 | 279 ± 88 | |||
| Follicular scales | ||||||||
| Present | 124 | 94 | 99 ± 12 | 0.092 | 220 ± 17 | 0.015 | 319 ± 26 | 0.018 |
| Absent | 8 | 6 | 23 ± 14 | 62 ± 18 | 85 ± 26 | |||
| All | 132 | 100 | 95 ± 11 | 210 ± 16 | 305 ± 25 | |||
Significant differences are highlighted by the bold printout of their P‐value.
SSSB, standardized skin surface biopsy.
* PPR according to the consensus of the National Rosacea Society (NRS).
† for three patients (2%), the size of the papulopustules was unknown.
Case report
A 19‐year‐old woman consulted in September 2010 for a facial papulopustular eruption that had been present for 1 year. Careful examination enabled us to diagnose rosacea‐like demodicosis (Fig. 1a,c).
Two SSSBs were performed at the same site on each cheek (Fig. 1b): on the right cheek, where follicular scales and papulopustules were observed, the Dds were 108 and 216 D/cm²; on the left cheek, where the skin was clinically normal, the Dds were 12 and 20 D/cm².
The clinical diagnosis of rosacea‐like demodicosis was thus confirmed and the patient was prescribed topical acaricidal treatment: she was told to wash her face twice a day with a soft soap, and then to apply a moisturizing cream in the morning and a cream composed of benzyl benzoate 20% and crotamiton 10% in cetomacrogol in the evening. She was also told to apply a lotion (benzyl benzoate 10% in isopropyl alcohol) to the scalp twice a week in the evening, and to use a sulphur shampoo the following morning.
After 5 weeks, the eruption had improved and the Dds were normal on the right cheek (Fig. 1d); by 3 months, she was clinically cured (Fig. 1e). The patient continued maintenance treatment, applying the acaricidal cream once a week and the sulphur shampoo once a month. 5 months later, her skin was still healthy and she decided to stop the treatment.
In July 2013, 27 months after stopping the maintenance treatment, the eruption reappeared. The patient attended our clinic 6 months later, in January 2014, more than 3 years after the initial consultation: at this time, the papulopustules were larger than at her initial consultation and involved both cheeks (Fig. 1f); she also suffered from flushing and persistent erythema. The clinical diagnosis was typical PPR. This time, the Dds were high on the two cheeks. She was prescribed the same treatment as for her initial presentation.
Two months later, the eruption had improved and the Dds were normal on both cheeks (Fig. 1g). The patient continued the treatment and by 4 months, the eruption had nearly completely cleared, with just postinflammatory macules persisting on her left cheek (Fig. 1h). She was told to continue the same maintenance therapy as after her first episode.
Discussion
The NRS expert committee and the global ROSCO panel define PPR as central face papulopustules with persistent erythema.4, 5 According to this definition, most of our patients had PPR (89% in the group of 242 patients, 91% in the subgroup of 132 patients), whereas the other patients would be considered as ‘not PPR’. According to Chen and Plewig, these latter patients could be considered as having rosacea‐like demodicosis.1 In our patients, mean Dds were higher in those with persistent erythema than in those without. The same tendency was observed when patients with recent treatment or concomitant facial dermatoses were excluded, but this difference was no longer statistically significant, possibly because of the smaller number of patients compared in the statistical analysis. Our findings therefore demonstrate that a disease usually considered as not being caused by Demodex (PPR) has similar (and perhaps even slightly higher) Dds than a disease in which the role of the mite is accepted (rosacea with papulopustules without persistent erythema). It is difficult to understand how the presence of mites at similar density in these two clinically similar diseases can be considered to have a causative role in one condition, but to be only an epiphenomenon in the other. A more probable hypothesis is that the numerous mites are responsible for both conditions and that these two ‘entities’ should therefore be considered as two phenotypes of a single disease. This would explain the similar symptoms, similar histology, similar Dds and the similar response to the same acaricidal treatment.2, 9, 15, 25, 26 This hypothesis is also compatible with other arguments that support the active role of Demodex in rosacea.9
If in addition to consider only the presence of persistent erythema to differentiate PPR from rosacea‐like demodicosis, we also take into account two other clinical characteristics usually considered as suggestive of rosacea‐like demodicosis rather than PPR (i.e. follicular scales and small size of papulopustules), we observe that 88% (n = 116) of our patients had characteristics of both entities (105 with all three characteristics studied, eight with persistent erythema and follicular scales and three with persistent erythema and small superficial papulopustules). This finding further highlights the difficulty in distinguishing clinically between these conditions in patients with central face papulopustules.2, 7, 13, 14
Patients with visible follicular scales had higher mean Dds than those without follicular scales; this is not surprising because follicular scales correspond to clusters of Demodex mites protruding externally. Based on this difference in Dds, one hypothesis could be that only patients with visible follicular scales (and higher Dds) could be considered as having rosacea‐like demodicosis (94% of our patients with central face papulopustules), and the other patients considered as PPR (despite the presence of persistent erythema in 91% of the patients and Dds much higher than normal in the two groups). A more likely hypothesis is that Demodex mites are responsible for both conditions, and that the more numerous the mites, the more often follicular scales are visible.
Patients with large papulopustules tended to have a lower SSSB1 but higher SSSB2 than those with small papulopustules, although these differences were not statistically significant: this observation suggests that large papulopustules may be caused by more deeply situated D. folliculorum mites. Indeed, the depth of the inflammatory reaction may determine the size of the papulopustules: more superficial mites (D. folliculorum identified on the SSSB1) may induce more superficial inflammation, resulting in smaller and more superficial papulopustules; however, more deeply situated mites (deeply situated D. folliculorum identified on the SSSB2 or D. brevis) may induce inflammation at deeper levels, resulting in larger, deeper papulopustules. This hypothesis is compatible with the observation that among patients with chalazia, the prevalence of D. brevis was higher than that of D. folliculorum, and the prevalence and density of D. brevis were higher than in patients without chalazia.27
All our observations therefore highlight the nosological confusion that persists between PPR and rosacea‐like demodicosis and the need to update the consensus concerning the definition and classification of rosacea. Moreover, they suggest that PPR and rosacea‐like demodicosis may be phenotypes of the same disease. This concept is supported by our case report, with many features indicating that the second presentation was an evolution of the first: for example, the same area was affected in both presentations, but the lesions were more dispersed in the second; the same symptoms were present but they were more intense in the second presentation; Dds were high on both occasions; and finally, both conditions responded to the same acaricidal treatment, with associated normalization of the Dds. Interestingly, our case report suggests that Demodex proliferation may initially be unilateral, but become bilateral with time.
Among patients with PPR, papulopustules usually appear after the vascular symptoms,28, 29 suggesting that hypervascularized skin may favour Demodex proliferation. However, in the patient of our case report, vascular symptoms (flushes, persistent erythema) were only present on the second presentation, whereas the inflammatory symptoms (papulopustules) were present during the first episode, suggesting that proliferation of Demodex may also induce vasodilation, thus creating a vicious cycle in which hypervascularization favours Demodex proliferation, which induces an inflammatory reaction,9, 22, 30, 31 leading to further vasodilation (Fig. 2). There may also be a second vicious circle: immunosuppression can favour proliferation of Demodex mites, but the mites probably secrete local immunosuppressant factors to facilitate their survival.2, 32 At the beginning of the disease, the clinical presentation probably depends on which factors are present that favour Demodex proliferation2, 9, 33: hypervascularization gives rise to ‘PPR with persistent erythema’28, 29; whereas other factors (specific immune defect against the mite,32 general immunosuppression,34 seborrhoeic hyperplasia2, 9, 35) may initially present as ‘PPR without persistent erythema’. Later in the course of the disease, these clinical patterns quickly overlap (Fig. 2).
Figure 2.
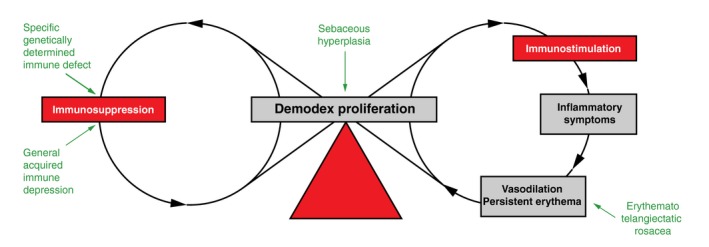
Pathophysiological hypothesis. Different underlying conditions (in green) may explain why there can be different phenotypes at the initial presentation. The two vicious circles of Demodex proliferation form a chain of eight explaining why phenotypes later often overlap and become largely indistinguishable. Finally, the balance (in red) between the two opposite actions of the parasite on immunity determines whether the predominant clinical manifestation of the disease is inflammatory or not.
In conclusion, while our observations do not prove a causative role of Demodex in rosacea, they nevertheless support the idea that PPR and rosacea‐like demodicosis should no longer be considered as two separate entities, but rather as two phenotypes of the same disease. As such, the definition of rosacea subtype II (PPR) should be reconsidered and simplified to include all patients with central face papulopustules–with or without persistent erythema –and thus also patients with ‘rosacea‐like demodicosis’, which is a term that should therefore disappear.
Acknowledgements
We thank Drs M Parmentier, V del Marmol and C Verhoeven, for their constructive remarks, and K Pickett for proofreading.
Conflicts of Interest
Dr Forton occasionally works as a consultant for Galderma; Prof De Maertelaer has no conflict of interest to declare.
Funding sources
None.
The study was approved by our local institutional review board (Erasme Hospital, P 2014/117)
References
- 1. Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol 2014; 170: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 2. Forton FM, Germaux M‐AE, Thibaut SC et al Demodicosis: descriptive classification and status of Rosacea, in response to prior classification proposed. J Eur Acad Dermatol Venereol 2015; 29: 829–832. [DOI] [PubMed] [Google Scholar]
- 3. Forton F, Germaux MA, Brasseur T et al Demodicosis and rosacea: epidemiology and significance in daily dermatological practice. J Am Acad Dermatol 2005; 52: 74–87. [DOI] [PubMed] [Google Scholar]
- 4. Wilkin J, Dahl M, Detmar M et al Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol 2002; 46: 584–587. [DOI] [PubMed] [Google Scholar]
- 5. Tan J, Almeida L, Bewley A et al Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol 2017; 176: 431–438. [DOI] [PubMed] [Google Scholar]
- 6. Chen WC, Plewig G. Are demodex mites principal, conspirator, accomplice, witness or bystander in the cause of rosacea? Am J Clin Dermatol 2015; 16: 67–72. [DOI] [PubMed] [Google Scholar]
- 7. Elston DM. Demodex mites as a cause of human disease. Cutis 2005; 76: 294–296. [PubMed] [Google Scholar]
- 8. Bevins ChL, Liu F‐T. Rosacea: skin innate immunity gone awry? Nat Med 2007; 13: 904–906. [DOI] [PubMed] [Google Scholar]
- 9. Forton F. Papulopustular rosacea, skin immunity and demodex: pityriasis folliculorum as a missing link. J Eur Acad Dermatol Venereol 2012; 26: 19–28. [DOI] [PubMed] [Google Scholar]
- 10. Nutting WMB, Andrews JRH, Desch CE. Studies in symbiosis: hair follicle mites of mammals and man. J Biol Educ 1979; 13: 315–321. [Google Scholar]
- 11. Ayres S Jr, Ayres S III. Demodectic eruptions (Demodicidosis) in the human. Arch Dermatol 1961; 83: 816–827. [DOI] [PubMed] [Google Scholar]
- 12. Shelley WB, Shelley ED, Burmeister V. Unilateral demodectic rosacea. J Am Acad Dermatol 1989; 20: 915–917. [DOI] [PubMed] [Google Scholar]
- 13. Hoekzema R, Hulsebosch HJ, Bos JD. Demodicidosis or rosacea: what did we treat? Br J Dermatol 1995; 133: 294–299. [DOI] [PubMed] [Google Scholar]
- 14. Hsu CK, Hsu MM, Lee JY. Demodicosis: a clinicopathological study. J Am Acad Dermatol 2009; 60: 453–462. [DOI] [PubMed] [Google Scholar]
- 15. Cribier B. Rosacea under the microscope: characterisctic histological findings. J Eur Acad Derm Venereol 2013; 27: 1336–1343. [DOI] [PubMed] [Google Scholar]
- 16. Forton F, Seys B. Density of Demodex folliculorum in rosacea: a case control study using standardized skin surface biopsy. Br J Dermatol 1993; 128: 650–659. [DOI] [PubMed] [Google Scholar]
- 17. Bonnar E, Eustace P, Powell FC. The Demodex mite population in rosacea. J Am Acad Dermatol 1993; 28: 443–448. [DOI] [PubMed] [Google Scholar]
- 18. Abd‐El‐Al AM, Bayoumy AM, Abou Salem EA. A study on Demodex folliculorum in rosacea. J Egypt Soc Parasitol 1997; 27: 183–195. [PubMed] [Google Scholar]
- 19. Erbagci Z, Ozgoztasi O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol 1998; 37: 421–425. [DOI] [PubMed] [Google Scholar]
- 20. El Shazly AM, Ghaneum BM, Morsy TA, Abdel Aaty HE. The pathogenesis of Demodex folliculorum (hair follicular mites in females with and without rosacea. J Egypt Soc Parasitol 2001; 31: 867–875. [PubMed] [Google Scholar]
- 21. Sattler EC, Maier T, Hoffmann VS, Hegyi J, Ruzicka T, Berking C. Non‐invasive in vivo detection and quantification of Demodex mites by confocal laser scanning microscopy. Br J Dermatol 2012; 62: 1050–1052. [DOI] [PubMed] [Google Scholar]
- 22. Casas C, Paul C, Lahfa M et al Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol 2012; 21: 906–910. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Ya, Wu LP, Peng Y, Cheng H. Retrospective analysis of the association between Demodex infestation and rosacea. Arch Dermatol 2010; 146: 896–902. [DOI] [PubMed] [Google Scholar]
- 24. Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol 2017; 97: 242–248. [DOI] [PubMed] [Google Scholar]
- 25. Taieb A, Ortonne JP, Ruzicka T et al Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator‐blinded trial. Br J Dermatol 2015; 172: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 26. Schaller M, Gonser L, Belge K et al Dual anti‐inflammatory and antiparasitic action of topical ivermectin 1% in papulopustular rosacea. J Eur Acad Dermatol Venereol 2017; 31: 1907–1911. [DOI] [PubMed] [Google Scholar]
- 27. Liang L, Ding X, Tseng SC. High Prevalence of Demodex brevis Infestation in Chalazia. Am J Ophthalmol 2014; 157: 342–348. [DOI] [PubMed] [Google Scholar]
- 28. Tan J, Blume‐Peytavi U, Ortonne JP et al An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol 2013; 169: 555–562. [DOI] [PubMed] [Google Scholar]
- 29. Aroni K, Tsagroni E, Lazaris AC, Patsouris E, Agapitos E. Rosacea: a clinicopathological approach. Dermatology 2004; 209: 177–182. [DOI] [PubMed] [Google Scholar]
- 30. Forton F. Demodex et inflammation périfolliculaire chez l'homme: revue et observation de 69 biopsies. Ann Dermatol Venereol 1986; 113: 1047–1058. [PubMed] [Google Scholar]
- 31. Georgala S, Katoulis AC, Kylafis GD, Koumantaki‐Mathioudaki E, Georgala C, Aroni K. Increased density of Demodex folliculorum and evidence of delayed hypersensitivity reaction in subjects with papulopustular rosacea. J Eur Acad Dermatol Venereol 2001; 15: 441–444. [DOI] [PubMed] [Google Scholar]
- 32. Akilov OE, Mumcuoglu KY. Immune response in demodicosis. J Eur Acad Dermatol Venereol 2004; 18: 440–444. [DOI] [PubMed] [Google Scholar]
- 33. Aydingöz IE, Mansur T, Dervent B. Demodex folliculorum in renal transplant patients. Dermatology 1997; 195: 232–234. [DOI] [PubMed] [Google Scholar]
- 34. Aquilina C, Viraben R, Sire S. Ivermectin‐responsive Demodex infestation during human immunodeficiency virus infection. Dermatology 2002; 205: 394–397. [DOI] [PubMed] [Google Scholar]
- 35. Zhao Ya E, Guo N, Xun M, Xu JR, Wang M, Wang DL. Sociodemographic characteristics and risk factor analysis of Demodex infestation (Acari: Demodicidae). J Zhejiang Univ‐Sci B 2011; 12: 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]


