Abstract
There is an urgent need for the discovery of effective new antimicrobial agents to combat the rise of bacterial drug resistance. High-throughput screening (HTS) in whole-animal infection models is a powerful tool for identifying compounds that show antibacterial activity and low host toxicity. In this report, we characterize the activities of four novel antistaphylococcal compounds identified from an HTS campaign conducted using Caenorhabditis elegans nematodes infected with methicillin-resistant Staphylococcus aureus (MRSA). The hit compounds included an N-hydroxy indole—1, a substituted melamine derivative—2, N-substituted indolic alkyl isothiocyanate—3, and p-difluoromethylsulfide analog—4 of the well-known protonophore carbonyl cyanide m-chlorophenyl hydrazone. Minimal inhibitory concentrations (MICs) of the four compounds ranged from 2 to 8 μg/ml against MRSA-MW2 and Enterococcus faecium and all were bacteriostatic. The compounds were mostly inactive against Gram-negative pathogens, with only 1 and 4 showing slight activity (MIC = 32 μg/ml) against Acinetobacter baumanii. Compounds 2 and 3 (but not 1 or 4) were found to perturb MRSA membranes. In phagocytosis assays, compounds 1, 2, and 4 inhibited the growth of internalized MRSA in macrophages, whereas compound 3 showed a remarkable ability to clear intracellular MRSA at its MIC (p < 0.001). None of the compounds showed hemolytic activity at concentrations below 64 μg/ml (p = 0.0021). Compounds 1, 2, and 4 (but not 3) showed synergistic activity against MRSA with ciprofloxacin, while compound 3 synergized with erythromycin, gentamicin, streptomycin, and vancomycin. In conclusion, we describe four new antistaphylococcal compounds that warrant further study as novel antibacterial agents against Gram-positive organisms.
Keywords: : antibiotic, bioactive compounds, high-throughput screening, isothiocyanate, melamine, MRSA infection, N-hydroxy indoles, protonophores
Introduction
Antibiotic resistance is a major current and future threat to the global population, and new antibiotics are urgently needed to combat the inexorable rise of multidrug-resistant bacteria. Methicillin-resistant Staphylococcus aureus (MRSA) is a major nosocomial pathogen1 that can cause localized and systemic infections.2 Drug resistance in MRSA occurs primarily through the production of β-lactamases or altered penicillin binding proteins.3 According to the Center for Disease Control and Prevention, in the United States there are more than 11,000 deaths and 80,000 severe cases of MRSA infection each year.4 Vancomycin has typically been the choice of antibiotic against serious multidrug-resistant Gram-positive bacterial infections but reports of vancomycin-resistant S. aureus are now common.5 Combination antimicrobial treatment is a promising strategy.6
Development of new antimicrobial agents has significantly declined in the past two decades due to challenging regulatory guidelines, perceptions around poor financial returns, and difficulties in discovering the mechanism of action of new compounds.7 But, the whole animal Caenorhabditis elegans-based high-throughput screening (HTS) provides a powerful tool for identifying new antimicrobial agents, antivirulence agents and immunomodulators. To identify novel antibacterial leads, we have employed C. elegans as a simple whole-animal host for studying infections of human pathogens.8 We recently completed a C. elegans HTS to identify small molecules that are active against MRSA and show low host toxicity.9 This report details the broader antibacterial properties of four novel antistaphylococcal hit compounds discovered during an MRSA–C. elegans HTS campaign.
Materials and Methods
Bacterial and nematode strains
Bacteria were all from the Mylonakis laboratory collection (Table 1). S. aureus MW2 and Enterococcus faecium ATCC E007 were grown in tryptic soy broth (TSB; BD Biosciences, Franklin Lakes, NJ); Klebsiella pneumoniae ATCC 77326, Acinetobacter baumannii ATCC 17978, Pseudomonas aeruginosa PA14, and Enterobacter aerogenes EAE 2625 strains were grown in Luria-Bertani broth (BD Biosciences). All strains were grown at 37°C. The C. elegans glp-4(bn2);sek-1(km4) double mutant strain was maintained at 15°C on lawns of Escherichia coli HB101 on 10 cm plates.9 The glp-4(bn2) mutation renders the strain unable of producing progeny at 25°C,10 and the sek-1(km4) mutation increases sensitivity to pathogens,11 reducing assay time.
Table 1.
Antibacterial Activity (μg/ml) of Compounds 1–4 Against ESKAPE Pathogens
| 1 | 2 | 3 | 4 | Vancomycin | Polymyxin B | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Staphylococcus aureus | 4 | 64 | 8 | >64 | 8 | >64 | 2 | 32 | 4.0 | 16 | >64 | >64 |
| Enterococcus faecium | 8 | >64 | 8 | >64 | 8 | >64 | 2 | >64 | 4.0 | 64 | >64 | >64 |
| Acinetobacter baumanii | 32 | >64 | >64 | >64 | >64 | >64 | 32 | >64 | >64 | >64 | 4 | 8 |
| Enterobacter aerogens | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | 8 | 8 |
| Klebsiella pneumoniae | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | 8 | 8 |
| Pseudomonas aeruginosa | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | 2 | 4 |
MIC, minimal inhibitory concentration; MBC, minimal bactericidal concentration.
C. elegans-MRSA liquid infection assays
The C. elegans-MRSA infection assay has been described previously.9 In brief, C. elegans glp-4(bn2);sek-1(km4) worms were grown at 25°C and harvested with M9 buffer. MRSA-MW2 was grown overnight at 37°C in TSB under aerobic conditions and then transferred to anaerobic conditions at 37°C. Bacteria were added at a final OD600 of 0.04 to 384-well assay plates (Corning; Corning, NY) containing test compounds at a final concentration of 2.86 μg/ml. Adult sterile worms (15 worms) were then added to each well using a Complex Object Parameter Analyzer and Sorter (COPAS) (Union Biometrica, Holliston, MA). After 5-days of incubation at 25°C, the plates were washed (to remove bacteria) with a microplate washer and Sytox Orange (Life Technologies, Carlsbad, CA) was added to selectively stain dead worms. After overnight incubation at 25°C, the wells were imaged using an Image Xpress Micro automated microscope (Molecular Devices, Sunnyvale, CA), capturing both transmitted light and TRITC (535 nm excitation, 610 nm emission) fluorescent images with a 2× objective. Images were processed using the open source image analysis software CellProfiler (http://cellprofiler.org/). The ratio of Sytox worm area to bright field worm area and the resultant percentage survival data were calculated by the software for each well.9 Assays were completed in duplicate.
Hit compounds
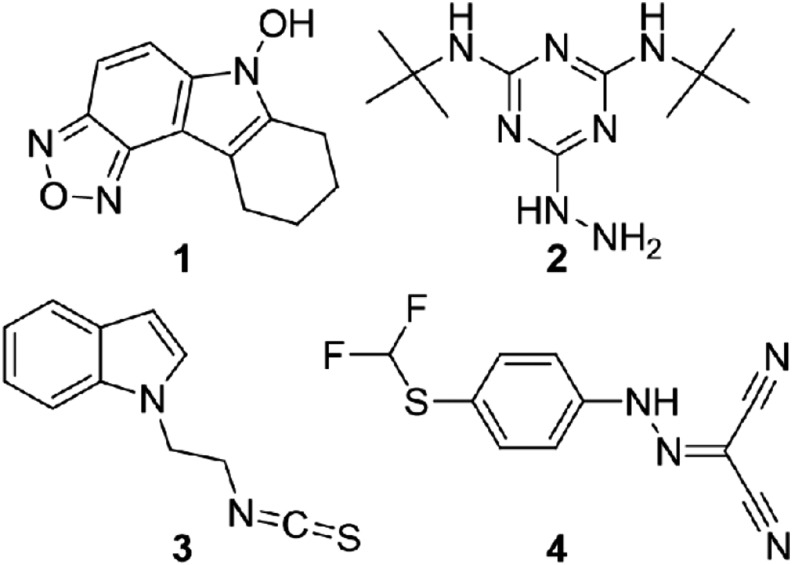
The compounds were an N-hydroxy indole (NHI) 1, a melamine derivative 2, indole isothiocyanate (ITC) 3 and a protonophore 4 related to carbonyl cyanide m-chlorophenyl hydrazone (CCCP). Compounds 1 (6-hydroxy-7,8,9,10-tetrahydro-[1,2,5]oxadiazolo[3,4-c]carbazole) and 2 (2-N,4-N-ditert-butyl-6-hydrazinyl-1,3,5-triazine-2,4-diamine) were purchased from Asinex (Winston-Salem, NC). Compound 3 (1-(2-isothiocyanatoethyl)-1H-indole) was purchased from Lifechemicals (Burlington, Canada) and compound 4 (3, 2-[[4-(difluoromethylsulfanyl)phenyl] hydrazinylidene]propanedinitrile) was purchased from Enamine (Monmouth, NJ). All compounds were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) to obtain 10 mg/ml stock solutions that were diluted for experiments.
Antibacterial susceptibility assays
In vitro antibacterial activities were tested using the broth microdilution method.12 Assays were carried out in triplicate using Müller-Hinton broth (BD Biosciences) in 96-well plates (BD Biosciences) with a total assay volume of 100 μl. Two-fold serial dilutions were prepared over the concentration range 0.01–64 μg/ml. An initial bacterial inoculum was adjusted to OD600 = 0.06 and incubated with test compounds at 35°C for 18 hr. OD600 was measured and the lowest concentration of compound that suppressed bacterial growth was reported as its minimal inhibitory concentration (MIC).13 Broth cultures (10 μl) from the MIC assays were plated onto Müller-Hinton agar (BD Biosciences) and after overnight incubation at 37°C the lowest concentration at which colonies were not observed was reported as the minimal bactericidal concentration (MBC).
Time to kill assays
The antibacterial properties of 1–4 against MRSA-MW2 were further examined using time to kill assays, as previously described.14 Briefly, overnight cultures of S. aureus MW2 were diluted in fresh TSB to a density of 108 cells/ml and placed into 10 ml tubes (BD Biosciences). Test compounds at 4× MIC were added and the tubes incubated at 37°C, with agitation. Aliquots were periodically drawn from the tubes over a 4 hr period, serially diluted with TSB and plated onto tryptic soy agar (TSA; BD Biosciences). Colony forming units (CFUs) were then enumerated after overnight incubation at 37°C. Assays were carried out in triplicate.
Membrane permeabilization assays
Sytox Green (Life Technologies) was used to probe the effects of 1–4 on MRSA-MW2 membrane permeabilization, as previously described.15 Assays were carried out in duplicate in 96-well plates (Corning). Bacterial cells were harvested from logarithmically growing cultures by centrifugation at 3,724 g for 5 min, washed twice with phosphate-buffered saline (PBS, pH 7.4), and resuspended in PBS to OD595 nm = 0.2. Sytox Green was added at a final concentration of 5 μM and cells were incubated in the dark for 30 min. Cell suspensions (50 μl) were added to 50 μl of compound (64 μg/ml in PBS), and the fluorescence intensity was measured (excitation 485 nm, emission 530 nm) periodically over 60 min. DMSO was included as the vehicle control. Membrane effects of compounds were indicated by an increase in cellular fluorescence caused by enhanced permeability of the DNA staining, membrane impermeable dye.
Intracellular MRSA killing assays
RAW 264.7 macrophages were used to examine intracellular killing of MRSA-MW2 by 1–4, as described by Schmitt et al.16 Macrophages were grown in Dulbecco's modified Eagle medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (Gibco) and maintained at 37°C in 5% CO2.17,18 Cells (50,000) in antibiotic and serum-free DMEM were seeded in 12-well plates 24 hr before infection. MRSA-MW2 (multiplicity of infection = 50) were added to macrophages and phagocytosis allowed to proceed. Planktonic bacteria were removed after 2 hr and DMEM supplemented with 200 μg/ml gentamicin was added for 2 hr to eliminate extracellular bacteria. Antibiotic and serum-free DMEM with and without test compounds was added and the cells incubated in a 5% CO2. After 4, 8, 12, or 24 hr sodium dodecyl sulfate was added to a final concentration of 0.02% to lyse the macrophages only (i.e., not ingested bacteria). Cell lysates were diluted serially with TSB, plated onto TSA plates and CFUs enumerated. Vancomycin (8 μg/ml) was used as a positive control and DMSO 0.1% as the negative control. Assays were carried out in triplicate.19
Human blood cell (RBC) hemolysis assays
Human erythrocytes (Rockland Immunochemicals, Limerick, PA) were used to measure the hemolytic activity of the compounds, as described by Isnansetyo and Kamei.20 Briefly, human erythrocytes (4%, in PBS, 50 μl) were added to 50 μl of serially diluted test compounds in PBS in 96-well plates. After incubating at 37°C for 1 hr, the plates were centrifuged at 500 g for 5 min and 50 μl of the supernatant from each well was transferred to a second 96-well plate. Absorbance (540 nm) was used as a measure of hemolytic activity. Assays were carried out in triplicate.
Cytotoxicity assay
Mammalian cell lines HepG2 (hepatic cell line), MKN-28 (gastric cell line), and HKC-8 (renal cell line) were used to determine the cytotoxicity of the compound, as detailed elsewhere.17,21,22 Cells were grown in DMEM (Gibco) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Gibco) and maintained at 37°C in 5% CO2. Cells were harvested and suspended in DMEM, and 100 μl of cells were added to each well at a final concentration of 5 × 104 cells. The compound was serially diluted in serum- and antibiotic-free DMEM and added to the monolayer and incubated at 37°C in 5% CO2 for 24 hr. For the last 4 hr of this 24 hr incubation period, 10 μl of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium (WST-1) solution (Roche, Mannheim, Germany) was added to each well. The WST-1 reduction was measured at 450 nm using Vmax microplate reader (Molecular Device). This assay was done in triplicate, and the percentage of survival was calculated by comparing with DMSO-treated vehicle control.
Checkerboard assays
Antibacterial synergy for combinations of compounds 1–4 with each other and clinical antibiotics from various class of antibacterial agents such as fluoroquinolone, tetracycline, aminoglycosides, macrolides, and glycopeptides (ciprofloxacin, doxycycline, erythromycin, gentamicin, streptomycin, and vancomycin) was tested for using checker board assays. Cultures of MRSA-MW2 were adjusted to OD600 = 0.06 and added to compound pairs that had been serially diluted in the same 96-well plates, vertically for one compound and horizontally for the other. Assays were carried out in triplicate as described for antibacterial susceptibility assays. The inhibitory concentration of compound in combination with clinical antibiotic or other compounds was evaluated using the fractional inhibitory concentration index (FICI) that was calculated using the formula: MICA combination/MICA alone + MICB combination/MICB alone.23
Statistical analysis
Statistical analysis (Two-way analysis of variance followed by Bonfererroni post-test) was carried out using GraphPad Prism version 6.04 (GraphPad Software, La Jolla, CA) and p-values of <0.05 were considered significant.
Results
HTS assay
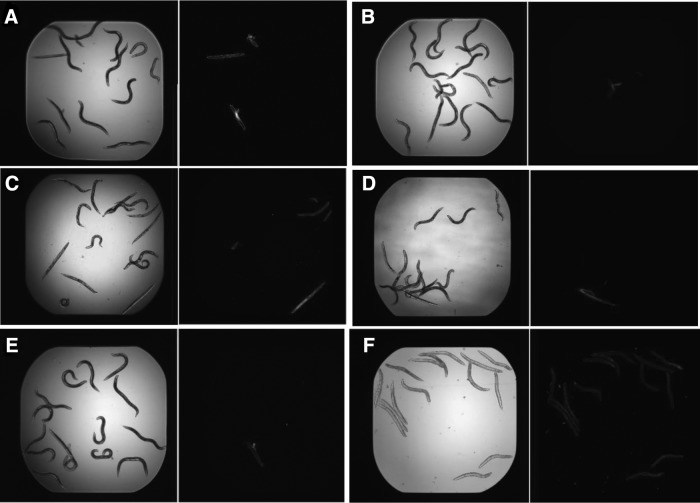
We previously reported a C. elegans HTS assay for the identification of novel antibacterial hits against MRSA9 and screened 3,930 compounds in Asinex 1 library and 3,892 compounds in Life chemicals library.9,15 During the screening, we identified that compounds 1–4 (Fig. 1) prolonged the survival of C. elegans infected with MRSA-MW2 at a concentration of 2.86 μg/ml compared to the DMSO control (Fig. 2A–D).
FIG. 1.
Chemical structures of compounds 1–4.
FIG. 2.
Images from Caenorhabditis elegans-MRSA HTS. Worms observed in light microscope (left) were stained with Sytox Orange (right) that specifically stains dead worms. Compounds were classified as hits based on extension of survival of MRSA-MW2 infected worms. (A) compound 1; (B) compound 2; (C) compound 3; (D) compound 4; (E) Vancomycin; (F) DMSO. DMSO, dimethyl sulfoxide; MRSA, methicillin-resistant Staphylococcus aureus; HTS, high-throughput screening.
Antibacterial susceptibility
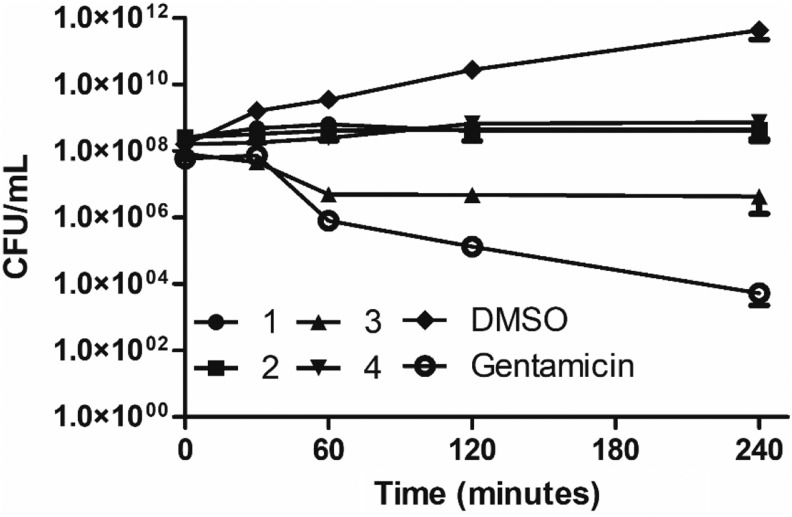
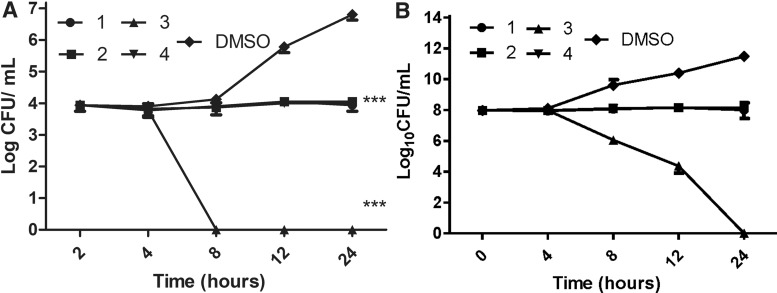
The antibacterial activity of the four hits was evaluated against a panel of ESKAPE pathogens. All four compounds were found to inhibit the growth of the Gram-positives MRSA-MW2 and E. faecium (MICs 2–8 mg/ml, Table 1). Compounds 1 and 4 were slightly active against A. baumannii (MIC = 32 μg/ml) but no other activity was observed against Gram-negatives. The MIC of vancomycin was 4 μg/ml against Gram-positives and polymyxin B was 2–8 μg/ml against Gram-negatives in the ESKAPE panel (Table 1). The MBC of 1 and 4 against MRSA-MW2 were 64 and 32 μg/ml, respectively, while the MBC of compounds 2 and 3 was >64 μg/ml. The MIC of oxacillin, vancomycin, and polymyxin B, was tested with various clinical S. aureus strains. All the clinical strains were resistant to oxacillin. The MICs of compounds 1–4 were listed in Table 2. Time to kill assays were used to further confirm the bactericidal/bacteriostatic properties of 1–4 against MRSA-MW2. When cells were exposed to the compounds at 4× MIC all showed only bacteriostatic activity relative to DMSO controls (Fig. 3). While compounds 1, 2, and 4 inhibited bacterial growth, ITC derivative 3 was able to reduce CFU/ml counts by 2-log10.
Table 2.
Antibacterial Activity (μg/ml) of Compounds 1–4 Against Clinical Staphylococcus aureus Pathogens
| MIC (μg/ml) | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Vancomycin | PolymyxinB | Oxacillin | |
| S. aureus BF1 | 4 | 8 | 8 | 2 | 2 | >64 | >64 |
| S. aureus BF2 | 4 | 8 | 8 | 2 | 2 | >64 | >64 |
| S. aureus BF3 | 4 | 8 | 8 | 2 | 2 | >64 | 32 |
| S. aureus BF4 | 4 | 8 | 8 | 2 | 2 | >64 | 16 |
| S. aureus BF5 | 4 | 8 | 8 | 2 | 2 | >64 | >64 |
FIG. 3.
Time to kill assay. MRSA-MW2 cells were exposed to compounds 1–4 at 4 × MIC and cell viability was monitored over 4 hr. Data represent the mean ± SEM (n = 3). MIC, minimal inhibitory concentration; SEM, standard error of the mean.
Membrane permeabilization
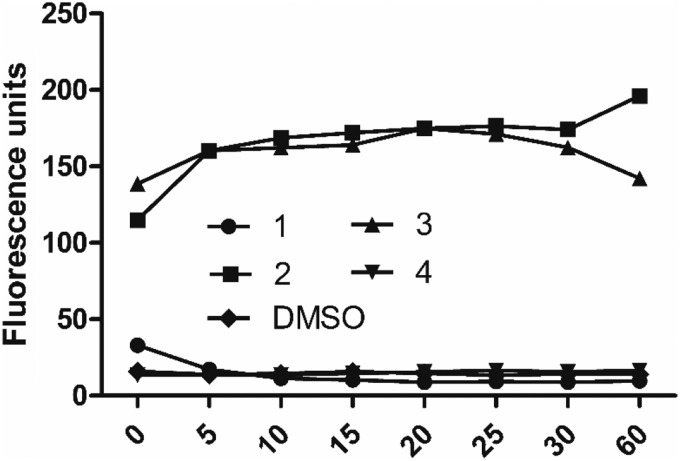
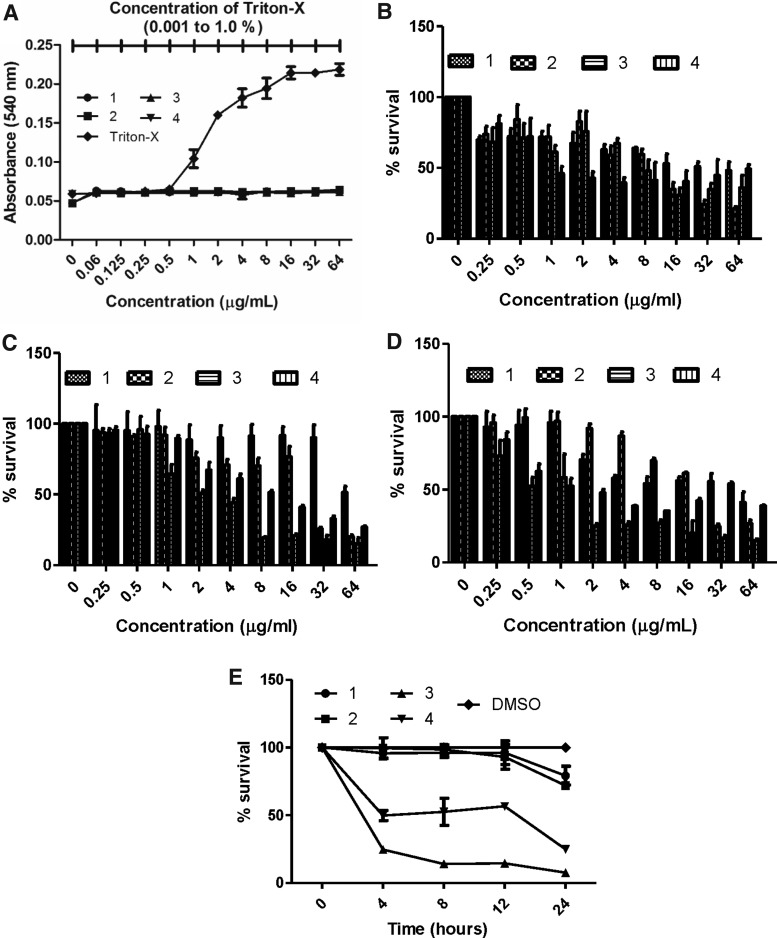
To evaluate the membrane effects of 1–4, uptake of the membrane-impermeable DNA-binding fluorescent dye Sytox Green into MRSA-MW2 cells was monitored in the presence/absence of the compounds. Exposure of cells to the compounds at 64 μg/ml identified that only 2 and 3 show effects on MRSA membranes, as indicated by increases in cellular fluorescence (Fig. 4). Observing membrane effects with 2 and 3 was in agreement with previous reports on members from the melamine24 and ITC classes.25 In contrast, compounds 1 and 4 showed no changes in cellular fluorescence (Fig. 4), indicating that they do not elicit their antibacterial effects through action on membranes.
FIG. 4.
Bacterial membrane permeabilization assay. Cellular fluorescence of MRSA-MW2 cells treated with Sytox Green and compounds 1–4 (64 μg/ml) was monitored over a 1 hr period.
Killing of intracellular MRSA in macrophages
It is known that S. aureus can act as an intracellular pathogen.26 To explore the effects of 1–4 on intracellular MRSA, RAW 264.7 macrophages were exposed to MRSA-MW2 cells and treated with test compounds at 1× MIC, vancomycin (positive control, 8 μg/ml, 2× MIC) and 0.1% DMSO (negative control). Compounds 1, 2 and 4 were found to significantly inhibit the growth of intracellular MRSA relative to DMSO (p < 0.001). While vancomycin was able to produce a slight reduction in bacterial counts, compound 3 completely cleared intracellular MRSA after 8 hr of treatment (Fig. 5A). The difference in bacterial killing between in vitro and intracellular killing by compound 3 may be a result of the limited exposure of bacterial cells to the compound (only 4 hr). However, we treated MRSA-MW2 cells with compounds 1–4, and incubated as indicated in the macrophage assay and we observed that compound 3 killed the planktonic bacteria after prolonged incubation (Fig. 5B).
FIG. 5.
(A) Killing of intracellular MRSA-MW2 in macrophages. MRSA-MW2 cells were exposed to RAW 264.7 macrophages, treated with test compounds 1–4 at 1× MIC and the killing of internalized bacteria was measured by CFU enumeration. Vancomycin (8 μg/ml) was used as a positive control and DMSO 0.1% as the negative control. Data represent the mean ± SEM (n = 3). ***p < 0.001, two-way analysis of variance with Bonfererroni post-test comparing DMSO control at 24 hr time point. (B) Killing of planktonic MRSA-MW2. MRSA-MW2 cells were exposed test compounds 1–4 at 1× MIC and the CFU was measured. CFU, colony forming unit.
Human red blood cell lysis assays and cytotoxicity
Serial dilutions of 1–4 were added to human red blood cells to establish whether they show hemolytic activity. It was found that none of the compounds showed hemolysis at concentrations up to 64 μg/ml. Serial dilutions of triton-X in PBS (0.001–1%) were added to human RBCs as a positive control (Fig. 6A). Hepatotoxicity of the test compounds 1–4 was evaluated using the liver cell line HepG2, commonly used to test the toxicity of compounds.14 In this series of experiments, the IC50 of the compound 1–4 against HepG2 was 32, 16, 8, and 1 μg/ml respectively (Fig. 6B). Also, we tested the cytotoxicity with gastric and renal cell lines and we observed similar results with hepatic cell lines. The IC50 of compounds 1–4 against MKN-28 was 64, 32, 4 and 4 μg/ml respectively (Fig. 6C); and against HKC-8 was 64, 32, 2, and 2 μg/ml respectively (Fig. 6D). The IC50 of compounds 3 and 4 were high in mammalian cell lines, however, we are working on the analogs to eliminate the cytotoxicity and sustain potent antimicrobial ability. In addition, we monitored the survival of macrophages in the presence of test compounds 1–4 at MIC level and observed that the compound 3 was harmful to macrophages (Fig. 6E) and bacteria (Fig. 5B).
FIG. 6.
Cytotoxicity of compounds 1–4. (A). Hemolytic activity. Human RBCs were exposed to two-fold serial dilutions of compounds and hemolysis was measured after 1 hr. Serially diluted triton-X (0.001–1%) was included as a positive control. (B–D) Cytotoxicity. Mammalian cells (HepG2, MKN-28, HKC-8) were treated with two-fold serial dilutions of compounds and the cytotoxicity was measured after 24 hr by WST-1. (B) HepG2 cells; (C) MKN-28; (D) HKC-8. Data represent the mean ± SEM (n = 3).
Antibacterial synergy
Use of paired combinations of drugs can reduce bacterial resistance and even restore clinical efficacy of some antibiotics.27 Checkerboard assays were performed to establish whether compounds 1–4 act synergistically against MRSA-MW2 when paired with one another and five clinical antibiotics from different class of antibacterials (i.e., ciprofloxacin, doxycycline, erythromycin, gentamicin, streptomycin, and vancomycin). Paired combinations of compounds and their observed FICIs are listed in Table 3. Synergistic effects, where the combined antibacterial activity of the two agents is more than the sum of their effects alone, were identified by FICI ≤0.5, antagonism by FICI >4.0 and “no interaction” by FICI >0.5–4.0.28
Table 3.
Fractional Inhibitory Concentration Index of Compounds 1–4 Used in Paired Combinations with Each Other and with Antibiotics
| FICI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Clinical antibiotics | |||||||||
| Compound | 1 | 2 | 3 | 4 | CIP | DOX | EMN | GMN | STN | VAN |
| 1 | 0.75 | 1.0 | 0.75 | 0.5 | 1.0 | 0.75 | 0.75 | 2.0 | 2.0 | |
| 2 | 0.75 | 1.0 | 0.75 | 0.5 | 1.0 | 1.0 | 1.0 | 0.625 | 1.0 | |
| 3 | 1.0 | 1.0 | 1.0 | 1.0 | 0.75 | 0.5 | 0.5 | 0.5 | 0.5 | |
| 4 | 0.75 | 0.75 | 1.0 | 0.5 | 0.5 | 0.75 | 1.0 | 1.0 | 1.0 | |
Synergy FICI ≤0.5, antagonism FICI >4.0, no interaction 0.5 > FICI ≤4.0.28
CIP, ciprofloxacin; DOX, doxycycline; EMN, erythromycin; GMN, gentamicin; STN, streptomycin; Van, vancomycin; FICI, fractional inhibitory concentration index.
Antagonism was not observed for any of the compound combinations. Compounds 1–4 showed no interactions when paired with one another but all four compounds showed synergy with at least one antibiotic. Ciprofloxacin was synergistic with compounds 1, 2, and 4, with compound 4 also showing synergy with doxycycline. Compound 3 showed no synergy with ciprofloxacin or doxycycline but synergized with all four of the antibiotics. Previous studies have reported that the activity of natural ITCs is enhanced by clinical antibiotics,29–31 in agreement with our observations with 3.
Discussion
Bacterial resistance to antibiotics has become a major global public health threat, with drug-resistant bacteria causing significant and increasing mortality and morbidity.32 There is an urgent need to develop new antibiotics, ideally with novel mechanisms of action to slow the onset of resistance. Lead antibacterials are usually either synthesized chemically or isolated from natural products that exhibit antibacterial activity.33,34 We completed a C. elegans-MRSA HTS study and identified four small molecules that rescued nematodes from MRSA infection at 2.86 μg/ml.9
Compound 1 represents a [1,2,5]oxadiazolo derivative from the NHI class, which are known to have antibacterial activity against Gram-positive organisms.35 Natural products bearing the NHI group, such as the nocathiacins and thiazomycins and their semi-synthetic analogs, exhibit activity against Gram-positive bacteria by inhibiting protein synthesis through direct interactions with the bacterial 50s ribosome.35 The related 7-hydroxy indole reportedly shows antivirulence effects against P. aeruginosa.36
Compound 2 was a derivative from the widely studied melamine class, whose examples have found use in antimicrobial polymers37 and as water and food disinfectants.38 Melamine derivatives related to compound 2 have found applications in biocidal polymers, in food industries, as water disinfectants and as additives in livestock feeds.37,39 Reports have described the antibacterial activity of melamine40 and Weaver et al. reported that melamine derivatives target the bacterial membrane via nonspecific interactions.24
Compound 3 contained an alkyl ITC attached to an indole nitrogen via a 2-carbon linker. ITC derivatives are known to show activity against Gram-positive and Gram-negative bacteria.41 ITCs are also present in several plant natural products42 and can produce both bactericidal and bacteriostatic activities against a range of bacterial pathogens.43 ITCs are known to react with amines and alcohols due to their highly electrophilic character,44 suggesting nontarget specific mechanisms for compound 3. However, Breier and Ziegelhoffer, reported that ITCs can selectively inhibit the ATP binding sites of P-ATPase in bacteria via reaction with a cysteine residue, suggesting the possibility of target-specific activity.45 Also, Sofrata et al., reported that benzyl isothiocyanate promotes outer membrane penetration in Gram-negative bacteria, leading to effects similar to those observed with cationic antimicrobial peptides.46
Compound 4 was a diarylacylhydrazone and close structural analog of the protonophore CCCP. Protonophores are molecules that dissipate the proton motive force in bacterial membranes leading to growth inhibition.27 Compounds of this type were recently shown to exert nonspecific (protonophoric) antibacterial effects against the Gram-positive bacterium Clostridium difficle.47 Clinically used protonophores include salicylanilide anthelmintics niclosamide, oxyclozanide, and closantel, which are also known to show antistaphylococcal activity.9,14
Characterization of the antibacterial properties of 1–4 here confirmed that they each show direct activity against two Gram-positives, inhibit intracellular growth of MRSA in macrophages, are nonhemolytic, and synergize with clinical antibiotics. Future work focusing on the specific characteristics of each compound would likely provide further insights into their mechanisms of action. For example, studies exploring the effects of compound 1 on bacterial 50s ribosomes and its antivirulence activity against S. aureus would be informative. Indole ITC 3 showed the most interesting activity of the four compounds, being able to clear intracellular MRSA from macrophages and synergizing with multiple antibiotics against MRSA, possibly due to its membrane permeabilizing properties (Fig. 4). While it is unlikely that 3 could be developed into a drug for systemic MRSA infections due to the reactive ITC group, it would be interesting to study its activity against skin and other body-surface MRSA infections in mammalian models, particularly in combination with the antibiotics it was shown here to synergize with.
In conclusion, screening for novel antibacterial compounds using a whole-animal HTS identified novel small molecule hits with antistaphylococcal activity. Combinatorial activity with clinical antibiotics might decrease the chances of emerging antimicrobial resistance and absence of antagonism with other compounds can be a valid credential of the hit compounds. Validation of activity of the compounds here suggest further investigations and warrant further evaluation in mammalian models.
Acknowledgments
This study was supported by NIH grant P01 AI083214 to E.M. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the article, apart from those disclosed. No writing assistance was utilized in the production of this article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Waness A. 2010. Revisiting methicillin-resistant Staphylococcus aureus infections. J. Glob. Infect. Dis. 2:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstein R.A., Gaynes R., Edwards J.R., and National Nosocomial Infections Surveillance System. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848–854 [DOI] [PubMed] [Google Scholar]
- 3.Stryjewski M.E., and Corey G.R. 2014. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin. Infect. Dis. 58 Suppl 1:S10–S19 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance: Biggest Threats. Available at https://www.cdc.gov/drugresistance/biggest_threats.html (accessed September8, 2016)
- 5.Gardete S., and Tomasz A. 2014. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Invest. 124:2836–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aktas G., and Derbentli S. In vitro activity of daptomycin combinations with rifampicin, gentamicin, fosfomycin and fusidic acid against MRSA strains. J. Glob. Antimicrob. Resist. 10:223–227 [DOI] [PubMed] [Google Scholar]
- 7.Rodvold K.A., and McConeghy K.W. 2014. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin. Infect. Dis. 58:S20–S27 [DOI] [PubMed] [Google Scholar]
- 8.Ewbank J.J. 2002. Tackling both sides of the host–pathogen equation with Caenorhabditis elegans. Microb. Infect. 4:247–256 [DOI] [PubMed] [Google Scholar]
- 9.Rajamuthiah R., Fuchs B.B., Jayamani E., Kim Y., Larkins-Ford J., Conery A., Ausubel F.M., and Mylonakis E. 2014. Whole animal automated platform for drug discovery against multi-drug resistant Staphylococcus aureus. PLoS One 9:e89189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beanan M.J., and Strome S. 1992. Characterization of a germ-line proliferation mutation in C. elegans. Development 116:755–766 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka-Hino M., Sagasti A., Hisamoto N., Kawasaki M., Nakano S., Ninomiya-Tsuji J., Bargmann C.I., and Matsumoto K. 2002. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 3:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikler M.A. 2006. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 13.Gwisai T., Hollingsworth N., Cowles S., Tharmalingam N., Mylonakis E., Fuchs B., and Shukla A. 2017. Repurposing niclosamide as a versatile antimicrobial surface coating against device-associated, hospital-acquired bacterial infections. Biomed. Mater. 12:045010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajamuthiah R., Fuchs B.B., Conery A.L., Kim W., Jayamani E., Kwon B., Ausubel F.M., and Mylonakis E. 2015. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS One 10:e0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim W., Conery A.L., Rajamuthiah R., Fuchs B.B., Ausubel F.M., and Mylonakis E. 2015. Identification of an antimicrobial agent effective against methicillin-resistant Staphylococcus aureus persisters using a fluorescence-based screening strategy. PLoS One 10:e0127640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt D.M., O'Dee D.M., Cowan B.N., Birch J.W., Mazzella L.K., Nau G.J., and Horzempa J. 2013. The use of resazurin as a novel antimicrobial agent against Francisella tularensis. Front. Cell Infect. Microbiol. 3:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tharmalingam N., Park M., Lee M.H., Woo H.J., Kim H.W., Yang J.Y., Rhee K.-J., and Kim J.-B. 2016. Piperine treatment suppresses Helicobacter pylori toxin entry in to gastric epithelium and minimizes β-catenin mediated oncogenesis and IL-8 secretion in vitro. Am. J. Transl. Res. 8:885. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M.H., Cho Y., Do Hyun Kim H.J.W., Yang J.Y., Kwon H.J., Yeon M.J., Park M., Kim S.-H., Moon C., and Tharmalingam N. 2016. Menadione induces G2/M arrest in gastric cancer cells by down-regulation of CDC25C and proteasome mediated degradation of CDK1 and cyclin B1. Am. J. Transl. Res. 8:5246–5255 [PMC free article] [PubMed] [Google Scholar]
- 19.Jayamani E., Tharmalingam N., Rajamuthiah R., Coleman J.J., Kim W., Okoli I., Hernandez A.M., Lee K., Nau G.J., Ausubel F.M., et al. 2017. Characterization of a Francisella tularensis–Caenorhabditis elegans pathosystem for the evaluation of therapeutic compounds. Antimicrob. Agents Chemother. 61:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isnansetyo A., and Kamei Y. 2003. MC21-A, a bactericidal antibiotic produced by a new marine bacterium, Pseudoalteromonas phenolica sp. nov. O-BC30(T), against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tharmalingam N., Jayamani E., Rajamuthiah R., Castillo D., Fuchs B.B., Kelso M.J., and Mylonakis E. 2017. Activity of a novel protonophore against methicillin-resistant Staphylococcus aureus. Future Med. Chem. 9:1401–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.-H., Lee M.H., Park M., Woo H.J., Kim Y.S., Tharmalingam N., Seo W.-D., and Kim J.-B. Regulatory effects of black rice extract on Helicobacter pylori infection-induced apoptosis. Mol. Nutr. Food Res. [Epub ahead of print]; DOI: 10.1002/mnfr.201700586 [DOI] [PubMed] [Google Scholar]
- 23.Zheng Z., Tharmalingam N., Liu Q., Jayamani E., Kim W., Fuchs B., Zhang R., Vilcinskas A., and Mylonakis E. 2017. Synergistic efficacy of Aedes aegypti antimicrobial peptide cecropin A2 and tetracycline against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 61: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver A.J., Jr., Shepard J.B., Wilkinson R.A., Watkins R.L., Walton S.K., Radke A.R., Wright T.J., Awel M.B., Cooper C., and Erikson E. 2014. Antibacterial activity of THAM trisphenylguanide against methicillin-resistant Staphylococcus aureus. PLoS One 9:e97742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borges A., Abreu A.C., Ferreira C., Saavedra M.J., Simões L.C., and Simões M. 2015. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 52:4737–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouillette E., Grondin G., Shkreta L., Lacasse P., and Talbot B.G. 2003. In vivo and in vitro demonstration that Staphylococcus aureus is an intracellular pathogen in the presence or absence of fibronectin-binding proteins. Microb. Pathog. 35:159–168 [DOI] [PubMed] [Google Scholar]
- 27.Torella J.P., Chait R., and Kishony R. 2010. Optimal drug synergy in antimicrobial treatments. PLoS Comput. Biol. 6:e1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odds F.C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser S.J., Mutters N.T., Blessing B., and Günther F. 2017. Natural isothiocyanates express antimicrobial activity against developing and mature biofilms of Pseudomonas aeruginosa. Fitoterapia 119:57–63 [DOI] [PubMed] [Google Scholar]
- 30.Palaniappan K., and Holley R.A. 2010. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 140:164–168 [DOI] [PubMed] [Google Scholar]
- 31.Tajima H., Kimoto H., and Taketo A. 2001. Specific antimicrobial synergism of synthetic hydroxy isothiocyanates with aminoglycoside antibiotics. Biosci. Biotechnol. Biochem. 65:1886–1888 [DOI] [PubMed] [Google Scholar]
- 32.Davies J., and Davies D. 2010. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74:417–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tharmalingam N., Kim S.-H., Park M., Woo H.J., Kim H.W., Yang J.Y., Rhee K.-J., and Kim J.B. 2014. Inhibitory effect of piperine on Helicobacter pylori growth and adhesion to gastric adenocarcinoma cells. Infect. Agents Cancer 9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S.-H., Park M., Woo H., Tharmalingam N., Lee G., Rhee K.-J., Eom Y.B., Han S.I., Seo W.D., and Kim J.B. 2012. Inhibitory effects of anthocyanins on secretion of Helicobacter pylori CagA and VacA toxins. Int. J. Med. Sci. 9:838–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rani R., and Granchi C. 2015. Bioactive heterocycles containing endocyclic N-hydroxy groups. Eur. J. Med. Chem. 97:505–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J., Attila C., Cirillo S.L., Cirillo J.D., and Wood T.K. 2009. Indole and 7‐hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb. Biotechnol. 2:75–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z., and Sun Y. 2005. Antimicrobial polymers containing melamine derivatives. II. Biocidal polymers derived from 2-vinyl-4,6-diamino-1,3,5-triazine. J. Polym. Sci. A Polym. Chem. 43:4089–4098 [Google Scholar]
- 38.Stewart M.L., Bueno G.J., Baliani A., Klenke B., Brun R., Brock J.M., Gilbert I.H., and Barrett M.P. 2004. Trypanocidal activity of melamine-based nitroheterocycles. Antimicrob. Agents Chemother. 48:1733–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kandelbauer A., and Widsten P. 2009. Antibacterial melamine resin surfaces for wood-based furniture and flooring. Prog. Org. Coat. 65:305–313 [Google Scholar]
- 40.Sun X., Cao Z., Porteous N., and Sun Y. 2010. Amine, melamine, and amide N-halamines as antimicrobial additives for polymers. Ind. Eng. Chem. Res. 49:11206–11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou Y., Jung L.S., Lee S.H., Kim S., Cho Y., and Ahn J. 2013. Enhanced antimicrobial activity of nisin in combination with allyl isothiocyanate against Listeria monocytogenes, Staphylococcus aureus, Salmonella Typhimurium and Shigella boydii. Int. J. Food Sci. Technol. 48:324–333 [Google Scholar]
- 42.Kim M., and Lee H. 2009. Growth‐inhibiting activities of phenethyl isothiocyanate and its derivatives against intestinal bacteria. J. Food Sci. 74:M467–M471 [DOI] [PubMed] [Google Scholar]
- 43.Tajima H., Kimoto H., Taketo Y., and Taketo A. 1998. Effects of synthetic hydroxy isothiocyanates on microbial systems. Biosci. Biotechnol. Biochem. 62:491–495 [DOI] [PubMed] [Google Scholar]
- 44.Dufour V., Stahl M., and Baysse C. 2015. The antibacterial properties of isothiocyanates. Microbiology 161:229–243 [DOI] [PubMed] [Google Scholar]
- 45.Breier A., and Ziegelhoffer A. 2000. “ Lysine is the Lord,” thought some scientists in regard to the group interacting with fluorescein isothiocyanate in ATP-binding sites of P-type ATPases. But, is it not cysteine? (Mini review). Gen. Physiol. Biophys. 19:253–264 [PubMed] [Google Scholar]
- 46.Sofrata A., Santangelo E.M., Azeem M., Borg-Karlson A.-K., Gustafsson A., and Pütsep K. 2011. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PLoS One 6:e23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C., Dolla N.K., Casadei G., Bremner J.B., Lewis K., and Kelso M.J. 2014. Diarylacylhydrazones: clostridium-selective antibacterials with activity against stationary-phase cells. Bioorg. Med. Chem. Lett. 24:595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]