Abstract
Background
Frailty is a key determinant of health status and outcomes of health care interventions in older adults that is not readily measured in Medicare data. This study aimed to develop and validate a claims-based frailty index (CFI).
Methods
We used data from Medicare Current Beneficiary Survey 2006 (development sample: n = 5,593) and 2011 (validation sample: n = 4,424). A CFI was developed using the 2006 claims data to approximate a survey-based frailty index (SFI) calculated from the 2006 survey data as a reference standard. We compared CFI to combined comorbidity index (CCI) in the ability to predict death, disability, recurrent falls, and health care utilization in 2007. As validation, we calculated a CFI using the 2011 claims data to predict these outcomes in 2012.
Results
The CFI was correlated with SFI (correlation coefficient: 0.60). In the development sample, CFI was similar to CCI in predicting mortality (C statistic: 0.77 vs. 0.78), but better than CCI for disability, mobility impairment, and recurrent falls (C statistic: 0.62–0.66 vs. 0.56–0.60). Although both indices similarly explained the variation in hospital days, CFI outperformed CCI in explaining the variation in skilled nursing facility days. Adding CFI to age, sex, and CCI improved prediction. In the validation sample, CFI and CCI performed similarly for mortality (C statistic: 0.71 vs. 0.72). Other results were comparable to those from the development sample.
Conclusion
A novel frailty index can measure the risk for adverse health outcomes that is not otherwise quantified using demographic characteristics and traditional comorbidity measures in Medicare data.
Keywords: Frailty, Health services, Outcomes, Medicare
Frailty, an age-related condition that affects 5–25% of community-dwelling older adults, is a vulnerability state in which there is a decreased ability to maintain homeostasis after a stressful event (1–4). Because this puts them at greater risk of adverse health outcomes (2–11) and high health care spending (12–14), measuring frailty is of great interest to clinicians, researchers, and health care organizations to generate evidence directly relevant to this population and to select a target population that requires coordinated care.
Large datasets of health care utilization (“claims” datasets) are increasingly used to study the clinical outcomes of health care interventions in older adults who are often under-represented in clinical trials (15). Data derived from such sources are often criticized for lack of detailed clinical information—in particular, frailty—that is central to the clinical management of older adults. Without adjustment for frailty, non-randomized comparative studies of health care interventions are subject to bias (16–19). Moreover, the likelihood of poor clinical outcomes after aggressive interventions may increase with greater frailty (20,21). Since claims data are readily accessible for a large number of older adults who receive routine care, there is growing interest to capture frailty in Medicare or similar administrative data (22–36).
To date, several clinical frailty assessments have been validated. Among them, frailty phenotype (2) and deficit accumulation frailty index (FI) (5) are extensively validated and widely used. The frailty phenotype generally offers better clinical operationalization based on weight loss, exhaustion, inactivity, slowness, and weakness (2). In comparison, the deficit accumulation FI quantifies frailty as a proportion of abnormalities from a list of age-associated health deficits. Since the risk of mortality goes up monotonically with the total burden of health deficits rather than specific deficit types (6,7,11), deficit accumulation FIs of different compositions offer robust and comparable prediction of mortality (5,8–10). The deficit accumulation approach has been applied to surveys, cohort studies, and clinical databases (6), including animals (11).
The objective of this study was to develop and validate a claims-based FI (CFI) in Medicare data using the deficit accumulation approach. This approach allows quantification of frailty in a continuous spectrum and provides better discrimination of the risk of adverse health outcomes in comparison with the frailty phenotype (37). We tested the predictive validity of CFI for adverse health outcomes and health care utilization in the following year.
Methods
Study Data and Sample
This study was determined as exempt by the Institutional Review Board of the Brigham and Women’s Hospital. We analyzed data from the Medicare Current Beneficiary Survey (MCBS), a rotating panel survey of a nationally representative sample of Medicare beneficiaries (38). In this survey, beneficiaries selected from Medicare enrollment files were interviewed three times a year over 4 years and survey data were linked to claims data. During the fall survey, beneficiaries or their proxy were asked about health status and physical function. This information was used to calculate a survey-based FI (SFI) which served as a reference standard (described later). We developed a CFI using MCBS 2006 claims data and examined its association with adverse health outcomes in 2007. As independent validation, we applied the CFI to MCBS 2011 claims data to predict adverse health outcomes in 2012. We included community-dwelling adults 65 years or older whose claims data were available for the entire year. Those who enrolled in the Medicare Advantage Plan or in hospice were excluded. For adequate capture of health status based on claims data, we required at least one office visit. Finally, the development sample included 5,593 beneficiaries; the validation sample included 4,424 (Supplementary Figure). The outcome analysis included 3,960 and 3,273 beneficiaries in the development and validation sample, respectively, who lived in the community during the baseline year and whose follow-up was available.
Measurements
We assessed mortality and the following outcomes of frailty from survey: (a) activities-of-daily-living (ADL) disability as any difficulty in bathing, dressing, eating, transferring, walking, and toileting; (b) instrumental-activities-of-daily-living (IADL) disability as any difficulty in using a telephone, doing light or heavy housework, preparing meals, shopping, and managing money; (c) mobility disability as any difficulty in walking 0.25 mile or transferring; and (d) recurrent falls. From claims data, we calculated the number of hospital days and skilled nursing facility days. These outcomes are used to test the predictive validity of CFI. In addition, we calculated the Combined Comorbidity Index (CCI) (39) that predicts mortality based on the diagnosis codes of the Charlson index (40) and the Elixhauser index (41). This index was used in validation to assess how CFI was different from comorbidities (see the validation section below).
SFI: Reference Standard
To develop a CFI, we needed a validated frailty measure. Using 56 self-reported symptoms, diagnosis, and functional limitations measured in the MCBS survey (see the list of variables in Supplementary Table 1), we applied the deficit accumulation approach to calculate a SFI as the proportion of abnormalities present (range: 0–1). For instance, a person who reported 12 abnormalities (of 56 items assessed) has a SFI of 0.21 (=12/56). Previous studies have shown that FIs calculated from different compositions of items from survey data, clinical examination, or medical records provide a comparable ability to predict mortality (6,7,11). Our SFI included 44 items used in published FIs (3–7) and additional 12 functional limitation items.
Development of CFI
We assumed that diagnosis and procedure codes and health care services claims in Medicare datasets (inpatient, outpatient, skilled nursing facility, home health agency, carrier, and durable medical equipment) could serve as proxies of a beneficiary’s health status and frailty (37). Below we outlined the steps to apply two alternative approaches to derive a CFI in the MCBS data:
(1) Preparation of Medicare data: The Medicare data contained almost 14,000 International Classification of Diseases (ICD)-9 diagnosis codes, 4,000 ICD-9 procedures codes, 8,000 Current Procedural Terminology (CPT-4) codes (numeric codes for medical services and procedures), and 6,000 Healthcare Common Procedure Coding System (HCPCS) level II codes (alpha-numeric codes for supplies, equipment, and devices). These codes were combined into clinically meaningful variables according to the chapters, subheadings, or categories of the coding manuals for ICD-9 diagnoses (166 variables), ICD-9 procedures (100 variables), CPT-4 services (169 variables), and HCPCS level II supplies (145 variables). These variables represent groups of conditions or symptoms within each body system or types of services.
(2) Estimation of CFI using the deficit accumulation approach: Among the variables derived from Medicare data, we considered the ones as health deficit variables, if they had positive correlation with age at false discovery rate of 0.05 (42) and prevalence higher than a threshold (0.001, 0.01, or 0.05). We calculated CFI as the proportion of health deficit variables present, assuming lack of codes during the entire year would represent the absence of the deficit.
(3) Estimation of CFI using the regression approach: As an alternative to implementation of the deficit accumulation approach, we used a lasso regression model to estimate the SFI as a function of health deficit variables derived from Medicare data. The lasso is a machine learning algorithm that applies shrinkage to the coefficients in the model; due to shrinkage, the lasso estimates are generally biased, but have better prediction accuracy due to reduced variance (43). Because variables with low prevalence may not add meaningfully to the estimation, we applied three prevalence thresholds (0.001, 0.01, or 0.05) to select candidate independent variables.
(4) Choice of our best approach: Our best approach was determined based on C statistics from logistic regression for mortality. We adjusted C statistics for optimism using 1000 bootstrap resampling (44). Since the variables from a particular data source (e.g., inpatient) may be more accurate or reflect greater severity than the same variables from other sources (e.g., outpatient), we compared whether considering the sources of claims would provide better prediction of mortality than pooling claims without considering their sources.
Validation of CFI
Clinical characteristics of development and validation samples were summarized after applying the MCBS sampling weight and compared using Wilcoxon ranksum test and chi-square test. We tested whether SFI and CFI were higher in people with older age and in women than men using chi-square test. We then compared our CFI derived from MCBS 2006 data with age and sex alone, and CCI in predicting mortality, ADL disability, IADL disability, mobility impairment, recurrent falls, and the number of hospital days and skilled nursing facility days in 2007. We only considered incident ADL disability, IADL disability, and mobility impairment by restricting analyses to those without the respective disabilities in 2006. The predictive power was evaluated using C statistics from logistic models for dichotomous outcomes and using the McFadden pseudo-R2 from zero-inflated negative binomial models for the number of hospital days and skilled nursing facility days. We estimated the odds ratio (OR) of dichotomous outcomes and their 95% confidence interval for SFI and CFI after adjusting for age, sex, and CCI, and assessed C statistics. We validated the performance of CFI in a more contemporary cohort of MCBS 2011–2012 data. Because sampling weight had little impact on prediction of adverse health outcomes, we presented the results of unweighted analysis. As a sensitivity analysis, we evaluated the performance without excluding beneficiaries who did not have any physician office visit. Analyses were performed using R version 3.2.4. A 2-sided p < .05 was considered statistically significant.
Results
Characteristics of Study Population
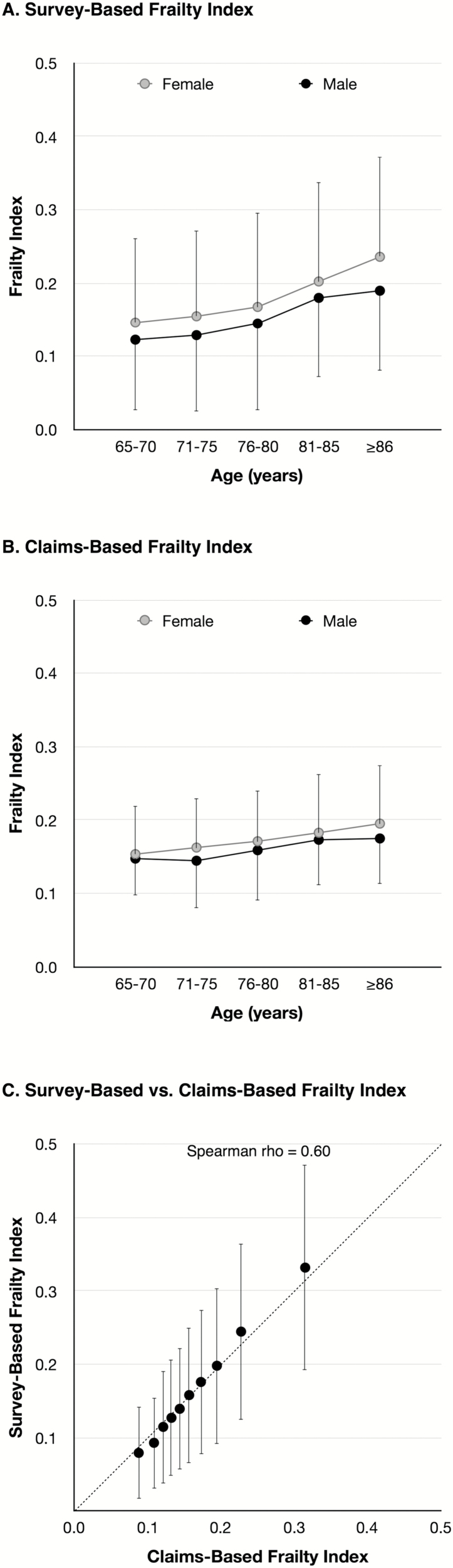
The characteristics of beneficiaries in the development and validation samples were similar (Table 1), with a few exceptions: beneficiaries in the validation sample more often reported chronic obstructive pulmonary disease, diabetes, IADL disability, but fewer strokes, recurrent falls, and hospitalization compared with those in the development sample. The median SFI was higher in the validation sample than the development sample, but the 1-year mortality was not different. The SFI increased with age and was higher in women (Figure 1A).
Table 1.
Characteristics of Beneficiaries in Medicare Current Beneficiary Survey (MCBS) 2006 and 2011.
| Characteristics | MCBS 2006 (N = 5,593) | MCBS 2011 (N = 4,424) | ||
|---|---|---|---|---|
| Unweighted | Weighted | Unweighted | Weighted | |
| Age, years, median (IQR) | 77 (71, 83) | 76 (71, 81) | 78 (71, 83) | 75 (70, 81) |
| Female | 58.4 | 58.6 | 57.4 | 57.7 |
| White race | 88.8 | 89.0 | 88.4 | 88.7 |
| SFI, median (IQR)a | 0.13 (0.08, 0.22) | 0.13 (0.08, 0.21) | 0.15 (0.10, 0.24) | 0.14 (0.09, 0.23) |
| Arthritis | 67.2 | 66.7 | 67.0 | 65.1 |
| Cancer | 21.4 | 21.1 | 22.9 | 22.3 |
| COPDa | 16.4 | 16.4 | 19.2 | 19.1 |
| Diabetesa | 21.5 | 21.8 | 25.6 | 25.9 |
| Heart disease | 42.9 | 41.4 | 41.6 | 39.6 |
| Strokea | 13.1 | 12.8 | 11.9 | 10.9 |
| Memory loss | 11.6 | 10.7 | 12.5 | 11.5 |
| ADL disability | 29.4 | 27.7 | 32.4 | 30.2 |
| IADL disabilitya | 46.5 | 44.6 | 50.4 | 47.5 |
| Mobility impairment | 52.3 | 50.0 | 53.5 | 50.1 |
| Recurrent fallsa | 14.9 | 14.6 | 11.6 | 11.1 |
| Hospitalization in past yeara | 20.2 | 19.7 | 19.1 | 18.0 |
| SNF stay in past year | 3.3 | 3.0 | 4.0 | 3.6 |
| 1-Year mortalityb | 4.0 | 3.5 | 3.6 | 3.0 |
Note. Each cell represents % unless noted otherwise. ADL = activity of daily living; COPD = chronic obstructive pulmonary disease; IADL = instrumental activity of daily living; IQR = interquartile range; SFI = survey-based frailty index; SNF = skilled nursing facility.
a p < .05 for comparison of weighted estimates between MCBS 2006 and MCBS 2011.
bSample size for outcome follow-up was 3,960 in MCBS 2006 and 3,273 in MCBS 2011 due to beneficiaries who rotated off each year.
Figure 1.
Distribution of survey-based and claims-based frailty index in medicare current beneficiary survey. (A, B) The age and sex-specific mean (node) and standard deviation (vertical bar) were displayed for survey-based frailty index (SFI) and claims-based frailty index (CFI). Both frailty indices increased with age (p < .05) and were higher in women than men (p < .05). (C) The mean (node) and standard deviation (vertical bar) of the SFI were plotted for the deciles of the CFI.
Development of CFI
When the deficit accumulation approach was directly implemented in claims data, increasing the prevalence threshold to select health deficit variables negatively affected the correlation between CFI and SFI, and C statistics for mortality (Supplementary Table 2), which suggests loss of prognostic information. The regression approach showed higher correlation with SFI and higher C statistics for mortality than the deficit accumulation approach. The correlation with SFI and C statistics for mortality decreased as the prevalence threshold was increased from 0.01 to 0.05. The sources of claims (e.g., inpatient or outpatient) made little difference in prediction. Based on these results, the regression model using the variables with prevalence ≥0.01 was chosen as our best CFI. Our final model included 52 ICD-9 diagnosis variables, 25 CPT-4 variables, and 16 HCPCS level II variables. The variables that were strongly associated with SFI included durable medical equipment claims (e.g., hospital beds, wheelchair, walking aids, and oxygen delivery devices) and diagnosis codes for degenerative diseases of the central nervous system (which includes Alzheimer’s disease and related dementia, and Parkinson’s disease), cardio-metabolic diseases, and cerebrovascular diseases (Table 2; see the full model in Supplementary Table 3). The CFI increased with age and was higher in women (Figure 1B). It matched well with SFI (Figure 1C).
Table 2.
Selected Codes Associated with Frailty in Medicare Current Beneficiary Survey
| Type | Codes | Description of Claims-Based Variables | Prevalence | Coefficient |
|---|---|---|---|---|
| HCPCS | E0250-E0373 | Hospital beds and associated supplies | 0.018 | 0.086 |
| HCPCS | K0001-K0462 K0669 |
Wheelchairs, components, and accessories | 0.035 | 0.078 |
| ICD9 Dx | 290–294 | Organic psychotic conditions | 0.052 | 0.047 |
| ICD9 Dx | 330–338 | Hereditary and degenerative diseases of the central nervous system | 0.086 | 0.040 |
| HCPCS | E0100-E0159 | Walking aids and attachments | 0.048 | 0.028 |
| HCPCS | E1353-E1406 | Accessories for oxygen delivery devices | 0.051 | 0.027 |
| HCPCS | A4244-A4290 | Other supplies including diabetes supplies and contraceptives | 0.125 | 0.024 |
| HCPCS | A5500-A5513 | Diabetic footwear | 0.029 | 0.024 |
| ICD9 Dx | 295–299 | Other psychoses | 0.036 | 0.021 |
| ICD9 Dx | 420–429 | Other forms of heart disease | 0.375 | 0.020 |
| ICD9 Dx | 890–897 | Open wound of lower limb | 0.017 | 0.020 |
| ICD9 Dx | 410–414 | Ischemic heart disease | 0.310 | 0.019 |
| ICD9 Dx | 401–405 | Hypertensive disease | 0.752 | 0.017 |
| ICD9 Dx | 430–438 | Cerebrovascular disease | 0.172 | 0.016 |
| ICD9 Dx | 300–316 | Neurotic disorders, personality disorders, and other nonpsychotic mental disorders | 0.154 | 0.014 |
| ICD9 Dx | 710–719 | Arthropathies and related disorders | 0.482 | 0.014 |
| CPT-4 | 99308 | Nursing facility care—subsequent | 0.016 | 0.014 |
| ICD9 Dx | 490–496 | Chronic obstructive pulmonary disease and allied conditions | 0.235 | 0.013 |
| ICD9 Dx | 030-041 | Other bacterial diseases | 0.031 | 0.012 |
| ICD9 Dx | 451–459 | Diseases of veins and lymphatics, and other diseases of circulatory system | 0.154 | 0.012 |
| ICD9 Dx | 480–487 | Pneumonia and influenza | 0.066 | 0.012 |
| ICD9 Dx | 250–259 | Diseases of other endocrine glands | 0.312 | 0.011 |
| ICD9 Dx | 590–599 | Other diseases of urinary system | 0.289 | 0.011 |
| ICD9 Dx | 797–799 | Ill-defined and unknown causes of morbidity and mortality | 0.046 | 0.011 |
| ICD9 Dx | 920–924 | Contusion with intact skin surface | 0.058 | 0.011 |
| ICD9 Dx | 580–589 | Nephritis, nephrotic syndrome, and nephrosis | 0.084 | 0.010 |
| HCPCS | A0021-A0999 | Transportation services including ambulance | 0.110 | 0.010 |
Note. Only claims-based variables that were associated with at least 0.01 increase in the survey-based frailty index from the lasso regression model were presented. See the full model in Supplementary Table 3. CPT = current procedural terminology; Dx = diagnosis; HCPCS = Healthcare Common Procedure Coding System; ICD = International Classification of Diseases.
Validation of CFI
After adjusting for age, sex, and CCI, SFI was associated with adverse health outcomes, which confirms the validity of SFI as a reference standard (Supplementary Table 4). The CFI (per 0.1-point increase) was associated with mortality (OR: 1.82), ADL disability (OR: 2.53), IADL disability (OR: 2.30), mobility impairment (OR: 2.45), and recurrent falls (OR: 2.21), while CCI was only associated with mortality (Supplementary Table 4).
In the development sample (Table 3), CFI was similar to CCI in predicting mortality (C statistic: 0.77 vs. 0.78) and hospital days (pseudo R2: 0.03 vs. 0.03). But CFI was better than CCI in predicting disabilities or recurrent falls (C statistic: 0.62–0.66 vs. 0.56–0.60) and skilled nursing facility days (pseudo R2: 0.04 vs. 0.02), suggesting an added value of considering CFI in addition to age, sex, and CCI. Notably, CCI was no better than demographic information in predicting disabilities, recurrent falls, and skilled nursing facility days. In the validation sample (Table 3), CFI and CCI showed similar C statistics for mortality (0.71 vs. 0.72), although C statistics were lower than those in the development sample. The findings on other outcomes were consistent with those from the development sample. When beneficiaries with no office visit were not excluded, the results were similar to the main analysis (results not shown).
Table 3.
Comparison of Claims-Based Frailty Index versus Demographic Characteristics and Combined Comorbidity Index in Predicting Adverse Health Outcomes in Medicare Current Beneficiary Survey (MCBS) 2006 and 2011
| Outcome (metric) | Model | MCBS 2006 | MCBS 2011 | ||||
|---|---|---|---|---|---|---|---|
| Age, sex | CCI | CFI | Age, sex | CCI | CFI | ||
| Mortality (C statistic) |
Base model | 0.67 | 0.78 | 0.77 | 0.68 | 0.72 | 0.71 |
| + age, sex | — | 0.80 | 0.80 | — | 0.76 | 0.75 | |
| + CCI | — | — | 0.82 | — | — | 0.77 | |
| ADL disability (C statistic) |
Base model | 0.63 | 0.60 | 0.66 | 0.65 | 0.62 | 0.68 |
| + age, sex | — | 0.65 | 0.69 | — | 0.65 | 0.69 | |
| + CCI | — | — | 0.69 | — | — | 0.69 | |
| IADL disability (C statistic) |
Base model | 0.64 | 0.56 | 0.62 | 0.61 | 0.59 | 0.62 |
| + age, sex | — | 0.64 | 0.67 | — | 0.63 | 0.65 | |
| + CCI | — | — | 0.67 | — | — | 0.65 | |
| Mobility impairment (C statistic) |
Base model | 0.58 | 0.57 | 0.63 | 0.62 | 0.57 | 0.64 |
| + age, sex | — | 0.61 | 0.64 | — | 0.64 | 0.67 | |
| + CCI | — | — | 0.64 | — | — | 0.67 | |
| Recurrent falls (C statistic) |
Base model | 0.58 | 0.57 | 0.66 | 0.54 | 0.59 | 0.64 |
| + age, sex | — | 0.60 | 0.67 | — | 0.58 | 0.64 | |
| + CCI | — | — | 0.67 | — | — | 0.64 | |
| Hospital days (Pseudo R2) |
Base model | 0.01 | 0.03 | 0.03 | 0.01 | 0.03 | 0.04 |
| + age, sex | — | 0.03 | 0.03 | — | 0.04 | 0.04 | |
| + CCI | — | — | 0.04 | — | — | 0.05 | |
| SNF days (Pseudo R2) |
Base model | 0.03 | 0.02 | 0.04 | 0.02 | 0.02 | 0.04 |
| + age, sex | — | 0.05 | 0.06 | — | 0.04 | 0.05 | |
| + CCI | — | — | 0.06 | — | — | 0.05 | |
Note. Claims-based frailty index and combined comorbidity index were measured using claims data in 2006 and 2011 and adverse health outcomes were measured in 2007 and 2012. Models were evaluated using C statistics for adverse health outcomes and using pseudo R2 for health care utilization. ADL = activity of daily living; CCI = combined comorbidity index; CFI = claims-based frailty index; IADL = instrumental activity of daily living; SNF = skilled nursing facility.
Discussion
We created a CFI using Medicare data that approximates a validated deficit accumulation FI. In the community-dwelling Medicare population, the CFI predicted mortality and number of hospital days as well as did the CCI, but the former was a better predictor of disability, mobility impairment, recurrent falls, and number of skilled nursing facility days. When added to demographic information and CCI, our CFI improved the ability to predict these outcomes. Our work contributes to the growing literature on measurement of health status of aging populations, and thus may advance observational research using claims data by enabling better risk adjustment and evaluation of treatment effect modification by frailty level.
Given the importance of identifying frail individuals for clinical care and research, several investigators developed indicators of frailty or functional limitation in administrative data that did not contain clinical information (Supplementary Table 5) (22–36). Diagnosis codes were the main data source for determination of frailty status in most studies. Only a few studies included CPT-4 and HCPCS variables as predictors (27,28,35). Frailty was determined as any presence or count of conditions that are measured by diagnosis codes selected based on clinical rationale (24,25,28–34) or from a regression model that estimated the probability of prevalent (26,27,35) or future disability (22,23). Notably, only Abrams et al. (25) and Segal et al. (36) used a validated clinical frailty assessment—the Vulnerable Elder Survey (45) and frailty phenotype (2)—to develop their frailty scale. Some studies only included special populations who were already frail (22,23,26) or who had acute myocardial infarction (28) or received intensive care (32), which may limit generalizability. In addition, all studies, except for Soong et al. (33,34), used datasets that had been collected 10–20 years ago. Therefore, it is unclear whether these indicators can be applied to more contemporary data.
Our study builds on previous effort by developing and validating a CFI using a deficit accumulation FI from the MCBS survey as the reference standard. Since this approach generates a continuous score and offers better risk discrimination than the frailty phenotype (8–10), it is suitable for our objective to identify older adults at high risk for adverse health outcomes. In the MCBS data, we found that those with greater SFI had higher risk of mortality, incident disability, mobility impairment, recurrent falls, hospitalization days, and skilled nursing facility days than those with lower SFI, which confirms the predictive validity of this approach.
In developing a CFI, we did not restrict candidate predictors to certain diagnosis codes based on clinical knowledge or to specific data sources (e.g., inpatient or outpatient datasets). Instead, we allowed all data types (diagnosis or procedure codes, CPT-4 codes, and HCPCS level II codes) from six Medicare datasets to be considered in the model. This data-driven approach was chosen to avoid omission of key predictors that may be clinically less intuitive (e.g., diagnosis codes or health service claims that are inversely associated with frailty). By comparing two alternative approaches to develop a CFI, we found that direct implementation of the deficit accumulation approach in claims data performed worse than did the regression approach. It is probably because not all claims-based variables are equally important markers of frailty. In addition, health status may be transiently impaired or improve as a result of health care services. We speculate that the regression approach which allows different weights for variables may capture the relative importance of claims-based variables on frailty. The CCI was not as good as the CFI in predicting disability, mobility impairment, recurrent falls, and skilled nursing facility days. Our CFI improved prediction when it was added to demographic information and CCI, which suggests that CFI can measure the risk for adverse health outcomes that is not captured by traditional comorbidity measures.
Our study has important implications for population health management and research. Our CFI, alone or in combination with comorbidities, may assist in the identification of older adults likely to need the greatest amount of care at the level of health care system. This is an essential step to provide preventive care expectantly and to contain health care costs. In comparative effectiveness and safety research and health services research in which health care interventions are not randomized, CFI can help reduce bias due to imbalance in the frailty level between treatment groups. Our results suggest that adjustment for CFI would be particularly important in studying disability, falls, and skilled nursing facility stay. Whether frail individuals are more likely than are their non-frail counterparts to be harmed by an intervention can be examined by conducting stratified analyses according to the different levels of CFI.
A few limitations should be considered in interpreting our results. We used a SFI as a reference standard. Objective measures of frailty (e.g., walking speed) may be more sensitive than a frailty definition based on self-reported data in predicting disability (46). Our CFI needs to be validated against objective measures of frailty in future research. When we reduced over 30,000 codes to 580 variables in Medicare data, the severity of condition was not considered. It remains to be examined whether more specific coding (e.g., codes for complications) results in better risk prediction. In addition, some codes are updated over time and health care providers’ billing practice may also change, which may be responsible for the difference in prediction accuracy between the development and validation samples. Therefore, validation using more current data is needed. We excluded beneficiaries whose health care utilization pattern might be different from fee-for-service beneficiaries (e.g., those enrolled in Medicare Advantage Plans). Accordingly, generalizability of our index to this subgroup will require further study. Our main analysis excluded a small number of beneficiaries who had no office visit, but a sensitivity analysis including them made little difference.
In conclusion, we developed a FI for use in Medicare data that can capture the risk for adverse health outcomes and higher health care utilization in community-dwelling Medicare beneficiaries. Our FI adds to the existing measures of health status by enabling measurement of the risk for incident disability and skilled nursing facility stays in aging populations that is not otherwise quantified well using traditional comorbidity measures in administrative data.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This research was funded by the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, The American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies. D.K. was supported by the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, The American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies. L.A.L. was supported by grant R01AG025037 from the National Institute on Aging. He holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. K.R. receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and has been supported in research on frailty by successive operating grants from the Canadian Institutes of Health Research. The funding sources had no role in the design, collection, analysis, or interpretation of the data, or the decision to submit the manuscript for publication. D.K., S.S., J.A. contributed to conception, design, and acquisition of data for this research. D.K. analyzed data and drafted the manuscript. All authors interpreted data, critically revised the manuscript for important intellectual content, and read and approved the final manuscript for submission.
Conflict of Interest
D.H.K. provides paid consultative services to Alosa Health, a non-profit educational organization with no relationship to any drug or device manufacturers. S.S. is a consultant to WHISCON, LLC., Newton, Massachusetts, and to Aetion, Inc., New York, New York, a software manufacturer of which he also owns equity. He is the principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis, Basel, Switzerland; Genentech, San Francisco, California; and Boehringer Ingelheim, Ingelheim am Rhein, Germany, unrelated to the topic of this study. K.R. is founder and Chief Scientific Officer of DGI Clinical, which has contracts with several companies for individualized outcome measures and advanced data analytics. Through the Dalhousie Technology Transfer Office, he has asserted copyright of the Clinical Frailty Scale, not otherwise discussed here. Other unnamed authors declare that they have no competing interests.
References
- 1. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi:10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 3. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi:10.1111/j.1532-5415.2010.02764.x [DOI] [PubMed] [Google Scholar]
- 4. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi:10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 5. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World J. 2001;1:323–336. doi:10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitnitski A, Song X, Skoog I et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi:10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 7. Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi:10.1111/j.1532-5415.2006.00738.x [DOI] [PubMed] [Google Scholar]
- 8. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. [DOI] [PubMed] [Google Scholar]
- 9. Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi:10.1111/j.1532-5415.2008.01656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi:10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- 11. Rockwood K, Blodgett JM, Theou O et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. 2017;7:43068. doi:10.1038/srep43068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bock JO, König HH, Brenner H et al. Associations of frailty with health care costs–results of the ESTHER cohort study. BMC Health Serv Res. 2016;16:128. doi:10.1186/s12913-016-1360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Comans TA, Peel NM, Hubbard RE, Mulligan AD, Gray LC, Scuffham PA. The increase in healthcare costs associated with frailty in older people discharged to a post-acute transition care program. Age Ageing. 2016;45:317–320. doi:10.1093/ageing/afv196 [DOI] [PubMed] [Google Scholar]
- 14. Peters LL, Burgerhof JG, Boter H, Wild B, Buskens E, Slaets JP. Predictive validity of a frailty measure (GFI) and a case complexity measure (IM-E-SA) on healthcare costs in an elderly population. J Psychosom Res. 2015;79:404–411. doi:10.1016/j.jpsychores.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 15. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi:10.1016/j.jclinepi.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 16. Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001;12:682–689. [DOI] [PubMed] [Google Scholar]
- 17. Ray WA. Observational studies of drugs and mortality. N Engl J Med. 2005;353:2319–2321. doi:10.1056/NEJMp058267 [DOI] [PubMed] [Google Scholar]
- 18. McGrath LJ, Cole SR, Kshirsagar AV, Weber DJ, Stürmer T, Brookhart MA. Hospitalization and skilled nursing care are predictors of influenza vaccination among patients on hemodialysis: evidence of confounding by frailty. Med Care. 2013;51:1106–1113. doi:10.1097/MLR.0b013e3182a50297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Setoguchi S, Warner Stevenson L, Stewart GC et al. Influence of healthy candidate bias in assessing clinical effectiveness for implantable cardioverter-defibrillators: cohort study of older patients with heart failure. BMJ. 2014;348:g2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puts MTE, Lips P, Ribbe MW, Deeg DJH. The effect of frailty on residential/nursing home admission in the Netherlands independent of chronic diseases and functional limitations. Eur J Ageing. 2005;2:264–274. doi:10.1007/s10433-005-0011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: A systematic review. Ann Intern Med. 2016;165:650–660. doi:10.7326/M16-0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosen A, Wu J, Chang BH, Berlowitz D, Ash A, Moskowitz M. Does diagnostic information contribute to predicting functional decline in long-term care?Med Care. 2000;38:647–659. [DOI] [PubMed] [Google Scholar]
- 23. Rosen A, Wu J, Chang BH et al. Risk adjustment for measuring health outcomes: an application in VA long-term care. Am J Med Qual. 2001;16:118–127. doi:10.1177/106286060101600403 [DOI] [PubMed] [Google Scholar]
- 24. Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. J Am Geriatr Soc. 2002;50:1108–1112. [DOI] [PubMed] [Google Scholar]
- 25. Abrams C, Lieberman R, Weiner J.. Development and Evaluation of the Johns Hopkins University Risk Adjustment Models for Medicare+Choice Plan Payment. Baltimore, MD: Johns Hopkins University Press; 2003. [Google Scholar]
- 26. Dubois MF, Dubuc N, Kroger E, Girard R, Hebert R. Assessing comorbidity in older adults using prescription claims data. J Pharm Health Serv Res. 2010;1:157–65. [Google Scholar]
- 27. Davidoff AJ, Zuckerman IH, Pandya N et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4:157–165. doi:10.1016/j.jgo.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chrischilles E, Schneider K, Wilwert J, Lessman G, O’Donnell B, Gryzlak B et al. Beyond comorbidity: expanding the definition and measurement of complexity among older adults using administrative claims data. Med Care. 2014;52 (Suppl 3):S75–84. doi:10.1097/MLR.0000000000000026 [DOI] [PubMed] [Google Scholar]
- 29. http://www.jen.com/pgs/general/jfi2.html.
- 30. Gilden DM, Kubisiak JM, Kahle-Wrobleski K, Ball DE, Bowman L. Using U.S. Medicare records to evaluate the indirect health effects on spouses: a case study in Alzheimer’s disease patients. BMC Health Serv Res. 2014;14:291. doi:10.1186/1472-6963-14-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Jonge KE, Jamshed N, Gilden D, Kubisiak J, Bruce SR, Taler G. Effects of home-based primary care on Medicare costs in high-risk elders. J Am Geriatr Soc. 2014;62:1825–1831. doi:10.1111/jgs.12974 [DOI] [PubMed] [Google Scholar]
- 32. Hope AA, Gong MN, Guerra C, Wunsch H. Frailty before critical illness and mortality for elderly medicare beneficiaries. J Am Geriatr Soc. 2015;63:1121–1128. doi:10.1111/jgs.13436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soong J, Poots AJ, Scott S, Donald K, Woodcock T, Lovett D et al. Quantifying the prevalence of frailty in English hospitals. BMJ Open. 2015;5:e008456. doi:10.1136/bmjopen-2015–008456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soong J, Poots AJ, Scott S, Donald K, Bell D. Developing and validating a risk prediction model for acute care based on frailty syndromes. BMJ Open. 2015;5:e008457. doi:10.1136/bmjopen-2015-008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Faurot KR, Jonsson Funk M, Pate V et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24:59–66. doi:10.1002/pds.3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Segal JB, Chang H-Y, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55:716–722. doi:10.1097/MLR.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23:891–901. doi:10.1002/pds.3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Centers for Medicare & Medicaid Services. Medicare Current Beneficiary Survey http://www.cms.gov/Research-Statistics-Data-and-Systems/Res earch/MCBS/index.html. Accessed September 17, 2014.
- 39. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. doi:10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 41. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 42. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 43. Tibshirani R. Regression shrinkage and selection via the lasso. J Roy Statist Soc Ser B. 1996;58:267–88. [Google Scholar]
- 44. Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York: Springer; 2015. [Google Scholar]
- 45. Saliba D, Elliott M, Rubenstein LZ et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. [DOI] [PubMed] [Google Scholar]
- 46. Pedone C, Costanzo L, Cesari M, Bandinelli S, Ferrucci L, Antonelli Incalzi R. Are performance measures necessary to predict loss of independence in elderly people?J Gerontol A Biol Sci Med Sci. 2016;71:84–89. doi:10.1093/gerona/glv096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.