Abstract
Background
Disrupted gait has been associated with an increased risk of frailty, disability, and death, but the causal molecular pathways are not well understood. Sphingolipids, including ceramides, are associated with multiple age-related diseases. Ceramides promote atrophy, necrosis, and proteolysis in cellular and animal models, and ceramide C16:0 levels are negatively correlated with muscle mass in men. However, there is a paucity of evidence examining sphingolipids and physical function.
Methods
We examined the cross-sectional association between plasma ceramides, sphingosine-1-phosphate (S1P), and ceramide/S1P ratios and gait, a robust measure of physical function, in 340 clinically normal participants aged 70 years and older enrolled in the Mayo Clinic Study of Aging. GAITRite® instrumentation was used to measure gait speed, cadence, step width, double support time, and intra-individual stride time variability. Based on previous studies, we hypothesized that higher plasma levels of ceramide C16:0 would be associated with worse gait.
Results
Multivariable adjusted linear regression models revealed that higher levels of ceramide C16:0 were associated with slower gait speed, decreased cadence, and increased double support time.
Conclusions
These results suggest an association between plasma ceramide C16:0 and physical function. Longitudinal studies are needed to determine whether elevated ceramide C16:0 can be utilized as a prognostic marker for functional decline.
Keywords: Ceramides, Atrophy, Functionality
Slow gait speed is a robust marker of functional decline and serves as a useful prognostic assessment for risk of disability and death (1). While the relationships between gait and adverse health outcomes are consistent across studies, the causal molecular pathways are not well understood. Functional decline and disability in the elderly have been associated with hormonal dysregulation, immune system dysfunction, increased coagulation, and inflammation (2). Understanding other biological processes involved will be important for identifying ways to prevent or delay subsequent functional decline and to identify which individuals are at greatest risk of sarcopenia, frailty, disability, and death.
Ceramides, the central molecular species of the sphingolipid pathway, function both as structural lipids and as second messengers for intra- and inter-cellular signaling. These bioactive lipids are important for multiple age-related cellular processes including senescence, apoptosis, inflammation, immune cell trafficking, and the generation of reactive oxygen species (3). Ceramides can be metabolized to sphingosine and then sphingosine-1-phosphate (S1P). In contrast to ceramide, S1P promotes cell migration, proliferation, survival, and angiogenesis (4). Therefore, the balance of ceramide to S1P, also termed the “rheostat”, regulates cellular growth and survival in response to cellular and environmental stressors or cues; a higher ratio is linked with apoptosis (5). Perturbations in sphingolipid metabolism are associated with the development and progression of multiple age-related conditions, including dementia, sarcopenia, and cardiovascular disease (6–9).
Both ceramides and S1P have been associated with skeletal muscle mass and performance. In skeletal muscle cells and animal models, ceramide accumulation decreases lean mass and muscle strength (10,11). In contrast, S1P enhances muscle contractility, fiber growth, regeneration, and adaptation (12). Moreover, very long chain length ceramides may increase white matter decline (13), thus leading to disrupted gait (14). However, the relationship between sphingolipids and gait has not been extensively examined.
We examined the cross-sectional association between plasma ceramides and S1P with gait among clinically normal (CN) individuals enrolled in the Mayo Clinic Study of Aging (MCSA). Based on previous research identifying specificity of circulating carbon chain length (C) 16:0-ceramide with lower muscle mass in younger and older men (11), we initially focused on ceramide C16:0, S1P, and the ceramide C16:0/S1P ratio. However, we also explored the association between other ceramide chain lengths and gait. Inflammatory markers have also been previously associated with poorer physical function and disability in older adults (15), thus we additionally examined the association between plasma levels of tumor necrosis factor alpha (TNFα) and gait parameters. Lastly, because TNFα can activate sphingomyelinase to increase ceramide levels (16–18), we also examined TNFα as a mediator or modifier in the relationship between ceramide and gait.
Methods
Study Design and Setting
The MCSA is a prospective population-based cohort study designed to assess the incidence and prevalence of mild cognitive impairment (MCI) in Olmsted County, MN. The study began in 2004, when Olmsted County residents between the ages of 70 and 89 were identified for recruitment using an age- and sex-stratified random sampling design to ensure that men and women were equally represented in each 10-year age strata (19).
Participants
The present study included 340 CN participants with complete plasma sphingolipid measures and gait parameters. Participants with a history of stroke, alcoholism, Parkinson’s disease, subdural hematoma, traumatic brain injury, or normal pressure hydrocephalus were excluded from analyses. The study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participants provided written informed consent.
Gait Measures
GAITRite® instrumentation (CIR systems Inc. Havertown, PA) was used to assess gait parameters (20). GAITRite® is an electronic walkway 5.6 m in length and 0.9 m wide. Each subject completed two walks and was instructed to walk at their normal pace without gait aids on the walkway, initiating and terminating their walk 1 m before and after the walkway. Details on GAITRite® analysis are reported elsewhere (20). Hollman and colleagues (20) identified five domains of gait—pace, rhythm, phase, variability, and base of support. In this study, we examined the association between sphingolipid levels and gait parameters in each of those domains. We assessed participant’s gait speed (m/s) (pace); cadence (rhythm), which is the rate of walking (steps per minute); step width (base of support), which is the distance (cm) between the midpoint of the current footprint to the midpoint of the previous footprint of the opposite foot; double support time (phase), which is the amount of time (seconds) both feet are on the walking surface between steps, expressed as a percentage of gait cycle; and intraindividual stride time variability, expressed as the standard deviation (SD) across multiple stride times, which is represented as the time (seconds) between the first contacts of two successive footfalls of the same foot. We created z-scores for each of the gait parameters to make them more directly comparable with each other.
Ceramide and Inflammatory Cytokine Analyses
During the in-clinic exam, participants’ blood (serum and EDTA plasma) was collected. The blood was centrifuged, aliquoted, and stored at –80°C. LC/ESI/MS/MS analysis of ceramides and sphingolipids was performed using a AB Sciex quadrupole mass spectrometer 6500 (Sciex, Framingham, MA) equipped with an ESI probe and interfaced with the Agilent 1290 infinity LC system (Agilent, Palo Alto, CA). The UPLC system consisted of an Agilent 1290 binary pump, thermostat, TCC, and sampler. The injection volume was 10 μL for extracted sample. Sphingolipids were separated with a Poroshell 120 EC- C8 column, 2.1 × 50 mm, 2.7 µm (Agilent, Palo Alto, CA). Mobile phase A was water:methanol:formic acid:ammonium formate (45/55/0.5%/5 mM by v/v). Mobile phase B was acetonitrile:methanol:formic acid:ammonium formate (50/50/0.5%/5 mM by v/v). The valve, sample loop, and needle were washed with acetonitrile:methanol (50/50 by v/v) for 20 seconds. Mass spectrometric analyses were performed online using electrospray ionization tandem mass spectrometry in the positive mode.
Samples were prepared using Biomek FX (Beckman Coulter, Brea, CA). Small amount of plasma sample was added to a 2-mL 96-well plate. Internal standard mixture was added to the samples. Sphingolipids were extracted using 1 phase extraction with methanol-dichloromethane. Lipid levels were quantified by the ratio of analyte and internal standard and calibration curves obtained by serial dilution of a mixture of lipid standards. Pure synthetic standards of sphingolipids were purchased from Avanti Lipids. Isotope labeling synthetic standards were synthesized internally at Eli Lilly and Company. In our analyses, we examined levels of saturated C14:0-, 16:0-, 18:0-, 20:0-, 22:0-, 23:0-, and 24:0-ceramide, unsaturated C24:1-ceramide, and total ceramide; S1P; and the sphingolipid rheostat (ie, ceramide/S1P ratios).
TNFα high sensitivity is measured by a quantitative two-site enzyme immunoassay from R & D Systems (Minneapolis, MN). As determined by R&D Systems, intra-assay CVs are 8.8, 5.9, and 5.3% at 2.6, 7.2, and 14.0 pg/mL, respectively. Inter-assay CVs are 16.7, 12.6, and 10.8% at 2.4, 6.7, and 13.5 pg/mL, respectively.
Other Covariates
Demographics (eg, age, sex, and education) were recorded at the in-clinic interview. Participants’ height (cm) and weight (kg) were measured during the in-clinic exam; these measures were used to calculate body mass index (BMI). Participants also completed the Beck Depression Inventory (BDI); participants were considered depressed if they had a score of ≥13 (21). A medication inventory was taken at each examination and cross-checked with the medical record. Medical comorbidities (eg, diabetes and hypertension) and the Charlson comorbidity index (22) were ascertained by medical record abstraction using the medical records-linkage system of the Rochester Epidemiology Project (19,23).
Cognition Diagnosis
Cognitive status was based on consensus agreement between the study coordinator, examining physician, and neuropsychologist who evaluated the participant. The diagnosis considered education, prior occupation, visual or hearing deficits, informant interview, and all other participant clinical information (19). Cognitive test performance in four domains (memory, executive function, language, and visual-spatial) and a global average of the four was compared with the age-adjusted scores of CN individuals previously obtained using Mayo’s Older American Normative Studies (24). This approach relies on prior normative work and extensive experience with the measurement of cognitive abilities in an independent sample of subjects from the same population. Individuals who performed in the normal range and did not meet criteria for MCI or dementia, which was diagnosed using Diagnostic and Statistical Manual of Mental Disorders, Fourth Addition (DSM-IV) criteria (25), were deemed CN.
Statistical Analysis
We natural log-transformed sphingolipid and TNFα levels to create more normally distributed data. We used linear regression models to determine the cross-sectional association between log-transformed sphingolipid levels and z-scored continuous gait parameters. Model 1 was unadjusted. Model 2 adjusted for age and sex. Model 3 was adjusted for age, sex, years of education, depression, medical comorbidities, BMI, and number of medications. All analyses were completed using Stata versions 12.0 and 13.0 (Stata Corp, College Station, TX).
Results
Participant Characteristics
The study population consisted of 340 CN MCSA participants, aged 70–95, of whom 210 (61.8%) were men. Table 1 summarizes the main characteristics of the study participants. The number of comorbidities among participants was typical of this age group with a median (interquartile range, IQR) of 7 (5,9) and with a median number of medications of 8 (5,10). Seventy-five (22%) had a diagnosis of diabetes and 264 (78%) had a diagnosis of hypertension.
Table 1.
Characteristics of the 340 Participants, Expressed as Median (Interquartile Range) or N (%)
| Age | 80.3 (77.2, 83.7) |
|---|---|
| Men | 210 (61.8%) |
| Years of education | 14 (12,16) |
| Depression | 24 (7.1%) |
| Diabetes | 75 (22.1%) |
| Hypertension | 264 (77.7%) |
| BMI (kg/m2) | 26.9 (24.3, 30.0) |
| Number of comorbidities | 7 (5,9) |
| Number of medications | 8 (5,10) |
| TNFα (pg/mL) | 3.3 (2.6, 4.4) |
| Gait speed (m/s) | 1.1 (0.94, 1.2) |
| Cadence (steps/min) | 106.0 (100.6, 112.6) |
| Step width (cm) | 61.3 (55.0, 68.3) |
| Double support time (%) | 30.0 (27.1, 32.8) |
| Stride time SD | 0.04 (0.03, 0.05) |
| Ceramides (nmol/mL) | |
| C14:0 | 7.5 (5.7, 9.7) |
| C16:0 | 88.4 (76.1, 107.1) |
| C18:0 | 83.3 (66.4, 104.3) |
| C20:0 | 191.5 (147.5, 233.7) |
| C22:0 | 618.6 (482.3, 749.7) |
| C23:0 | 574.4 (473.4, 722.6) |
| C24:0 | 940.9 (779.8, 1144.6) |
| C24:1 | 260.0 (214.6, 323.1) |
| Sphingosine-1-phosphate (nmol/mL) | 152.7 (133.6, 174.2) |
Note: Depression defined as a score of ≥13 on the Beck Depression Inventory.
Plasma Ceramide C16:0, S1P, and the Ceramide C16:0/S1P Ratio and Gait
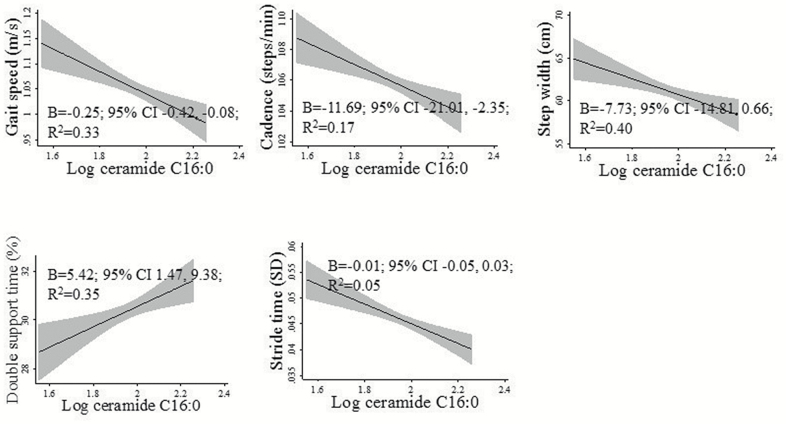
Higher levels of ceramide C16:0 were associated with slower gait speed, decreased cadence, and increased double support time in multivariable models (Table 2; Figure 1). For instance, in Model 3 a one log unit increase in C16:0 ceramide is associated with 1.07 reduction in z-scored gait speed (Table 2) or a one log unit increase in C16:0 ceramide is associated with 0.25 m/s decrease in gait speed (Figure 1). Higher levels of S1P were associated with better performance on most gait measures (gait speed, cadence, double support time, and stride time SD) adjusting for age and sex. However, the results were no longer significant after adjustment for variables in Model 3. The results for the ceramide C16:0/S1P ratio were similar to ceramide C16:0 with higher levels of the ratio associated with slower gait speed, decreased cadence, and increased double support time across the models.
Table 2.
Linear Regression: Association Between Ceramide C16:0, Sphingosine-1-Phosphate (S1P), Their Ratio, and TNFα and z-Scored Gait Parameters
| Log Lipid, Lipid ratio, or TNFα | Gait Speed | Cadence | Step Width | Double Support Time % | Stride Time SD |
|---|---|---|---|---|---|
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | |
| Ceramide C16:0 | |||||
| Model 1 | –0.70 (–1.57, 0.18) | –0.38 (–1.17, 0.40) | –0.70 (–1.63, 0.23) | 0.56 (–0.36, 1.48) | –0.25 (–1.18, 0.69) |
| Model 2 | –0.74 (–1.54, 0.05) | –0.70 (–1.47, 0.07) | –0.35 (–1.15, 0.44) | 0.54 (–0.36, 1.43) | –0.24 (–1.17, 0.69) |
| Model 3 | –1.07 (–1.84, –0.30)** | –0.95 (–1.72, –0.18)* | –0.71 (–1.43, 0.03) | 0.93 (0.16, 1.71)* | –0.05 (–0.98, 0.88) |
| S1P | |||||
| Model 1 | 1.18 (–0.01, 2.38) | 1.70 (0.65, 2.76)** | 0.48 (–0.79, 1.74) | –1.79 (–3.04, –0.53)** | –1.47 (–2.74, –0.20)* |
| Model 2 | 1.38 (0.29, 2.47)* | 1.31 (0.28, 2.35)* | 0.82 (–0.26, 1.90) | –1.63 (–2.86, –0.40)** | –1.34 (–2.62, –0.06)* |
| Model 3 | 0.58 (–0.52, 1.67) | 0.95 (–0.13, 2.02) | –0.18 (–1.22, 0.85) | –0.44 (–1.54, 0.66) | –0.77 (–2.09, 0.54) |
| Ceramide 16:0/S1P Ratio | |||||
| Model 1 | –0.44 (–0.77, –0.11)** | –0.43 (–0.72, –0.13)** | –0.31 (–0.66, 0.04) | 0.50 (0.15, 0.84)** | 0.18 (–0.18, 0.53) |
| Model 2 | –0.47 (–0.76, –0.17)** | –0.45 (–0.73, –0.16)** | –0.26 (–0.55, 0.04) | 0.45 (0.12, 0.79)** | 0.15 (–0.20, 0.50) |
| Model 3 | –0.43 (–0.71, –0.15)** | –0.46 (–0.74, –0.17)** | –0.20 (–0.47, 0.08) | 0.38 (0.09, 0.67)* | 0.11 (–0.24, 0.46) |
| TNFα | |||||
| Model 1 | –0.47 (–0.88, –0.07)* | –0.13 (–0.49, 0.24) | –0.53 (–0.96, –0.10)* | 0.37 (–0.06, 0.80) | –0.12 (–0.56, 0.32) |
| Model 2 | –0.15 (–0.51, 0.21) | –0.19 (–0.53, 0.16) | –0.52 (–0.88, –0.15)** | 0.38 (–0.04, 0.80) | –0.11 (–0.55, 0.32) |
| Model 3 | –0.14 (–0.49, 0.20) | –0.20 (–0.54, 0.14) | –0.52 (–0.85, –0.18)* | 0.45 (0.10, 0.81)* | –0.13 (–0.56, 0.30) |
Note: Model 1 unadjusted. Model 2 adjusted for age and sex. Model 3 adjusted for age, sex, years of education, depression, medical comorbidities, BMI, and number of medications. Bold values are statistically significant.
*p ≤ .05, **p ≤ .01, ***p ≤ .001.
Figure 1.
Fitted linear regression estimates and 95% confidence intervals between the log-transformed levels of ceramide C16:0 and gait parameters. Regression models were adjusted for age, sex, years of education, depression, medical comorbidities, BMI, and number of medications.
TNFα and Gait
The relationship between log TNFα and gait was also examined with linear regression models. Higher levels of TNFα were associated with reduced strep width, greater double support time in Model 3, and reduced gait speed in univariate regression (Table 2; Supplementary Figure 1). Notably, the association between TNFα and double support time (Model 3: B = 0.45, 95% CI 0.10, 0.81) was approximately half the magnitude of the association between C16:0 ceramide and double support time (Model 3: B = 0.93, 95% CI 0.16, 1.71) (Table 2).
Relationship Between Other Plasma Ceramides and Gait
Because our previous results showed that ceramide C16:0 was as robustly associated with gait as the ceramide C16:0/S1P ratio, we focused only on the ceramide level in subsequent analyses. The relationship between ceramides of other carbon chain lengths and gait are shown in Table 3. Overall, there were few associations. Higher levels of ceramides C14:0, C18:0, C20:0, and C24:1 were associated with longer double support time in multivariable regression models. Interestingly, higher levels of ceramide C24:0 were associated with faster gait speed and reduced intraindividual stride time variability in multivariable regression models.
Table 3.
Linear Regression: Association Between Other Ceramide Chain Lengths and z-Scored Gait Parameters
| Log Ceramide | Gait Speed | Cadence | Step Width | Double Support Time % | Stride Time SD |
|---|---|---|---|---|---|
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | |
| C14:0 | |||||
| Model 1 | –0.35 (–0.86, 0.17) | 0.24 (–0.22, 0.70) | –0.64 (–1.18, –0.10)* | 0.45 (–0.08, 0.99) | –0.04 (–0.58, –0.51) |
| Model 2 | –0.06 (–0.54, 0.43) | –0.03 (–0.49, 0.43) | –0.00006 (–0.48, 0.48) | 0.33 (–0.21, 0.87) | –0.08 (–0.64, 0.48) |
| Model 3 | –0.23 (–0.70, 0.25) | –0.16 (–0.63, 0.31) | –0.23 (–0.68, 0.22) | 0.52 (0.05, 0.99)* | 0.06 (–0.51, 0.63) |
| C16:0 | |||||
| Model 1 | –0.70 (–1.57, 0.18) | –0.38 (–1.17, 0.40) | –0.70 (–1.63, 0.23) | 0.56 (–0.36, 1.48) | –0.25 (–1.18, 0.69) |
| Model 2 | –0.74 (–1.54, 0.05) | –0.70 (–1.47, 0.07) | –0.35 (–1.15, 0.44) | 0.54 (–0.36, 1.43) | –0.24 (–1.17, 0.69) |
| Model 3 | –1.07 (–1.84, –0.30)** | –0.95 (–1.72, –0.18)* | –0.71 (–1.43, 0.03) | 0.93 (0.16, 1.71)* | –0.05 (–0.98, 0.88) |
| C18:0 | |||||
| Model 1 | –0.33 (–0.91, 0.25) | 0.40 (–0.12, 0.91) | –0.77 (–1.38, –0.16)* | 0.70 (0.10, 1.31)* | 0.21 (–0.41, 0.83) |
| Model 2 | 0.02 (–0.52, 0.56) | 0.03 (–0.49, 0.54) | –0.25 (–0.79, 0.28) | 0.69 (0.09, 1.29)* | 0.24 (–0.39, 0.87) |
| Model 3 | 0.33 (–0.20, 0.85) | 0.16 (–0.36, 0.67) | 0.12 (–0.38, 0.61) | 0.23 (–0.29, 0.76) | 0.09 (–0.54, 0.72) |
| C20:0 | |||||
| Model 1 | –0.51 (–1.18, 0.16) | 0.33 (–0.27, 0.92) | –0.92 (–1.62, –0.22)** | 0.87 (0.17, 1.57)* | –0.14 (–0.85, 0.57) |
| Model 2 | –0.06 (–0.68, 0.56) | –0.17 (–0.76, 0.43) | –0.44 (–1.05, –0.18) | 0.94 (0.25, 1.63)** | –0.09 (–0.81, 0.64) |
| Model 3 | –0.10 (–0.69, 0.49) | –0.19 (–0.77, 0.40) | –0.47 (–1.04, 0.09) | 0.94 (0.35, 1.53)** | –0.07 (–0.78, 0.65) |
| C22:0 | |||||
| Model 1 | –0.10 (–0.80, 0.60) | 0.46 (–0.16, 1.08) | –0.57 (–1.31, 0.17) | 0.49 (–0.24, 1.22) | –0.71 (–1.45, 0.02) |
| Model 2 | 0.50 (–0.13, 1.13) | 0.09 (–0.53, 0.70) | –0.14 (–0.78, 0.50) | 0.52 (–0.20, 1.25) | –0.69 (–1.43, 0.06) |
| Model 3 | 0.56 (–0.06, 1.18) | 0.07 (–0.56, 0.69) | –0.02 (–0.62, 0.58) | 0.18 (–0.46, 0.81) | –0.62 (–1.38, 0.14) |
| C23:0 | |||||
| Model 1 | –0.08 (–0.77, 0.61) | 0.78 (0.17, 1.39) | –0.78 (–1.51, –0.05)* | 0.43 (–0.30, 1.16) | –0.68 (–1.41, 0.05) |
| Model 2 | 0.45 (–0.20, 1.09) | 0.33 (–0.29, 0.95) | 0.01 (–0.63, 0.66) | 0.39 (–0.35, 1.12) | –0.71 (–1.47, 0.05) |
| Model 3 | 0.49 (–0.14, 1.13) | 0.33 (–0.30, 0.96) | 0.11 (–0.50, 0.72) | 0.04 (–0.61, 0.68) | –0.59 (–1.36, 0.18) |
| C24:0 | |||||
| Model 1 | 0.51 (–0.24, 1.24) | 0.88 (0.23, 1.54)** | –0.08 (–0.87, 0.70) | 0.02 (–0.76, 0.80) | –1.14 (–1.92, –0.36)** |
| Model 2 | 0.83 (0.15, 1.51)* | 0.60 (–0.05, 1.26) | 0.16 (–0.51, 0.84) | 0.09 (–0.68, 0.86) | –1.10 (–1.88, –0.31)** |
| Model 3 | 0.69 (0.03, 1.36)* | 0.51 (–0.15, 1.18) | 0.03 (–0.60, 0.66) | 0.06 (–0.61, 0.73) | –0.89 (–1.69, –0.09)* |
| C24:1 | |||||
| Model 1 | –0.64 (–1.42, 0.15) | 0.10 (–0.60, 0.80) | –0.88 (–1.70, –0.05)* | 0.87 (0.05, 1.70)* | 0.08 (–0.76, 0.92) |
| Model 2 | –0.23 (–0.94, 0.48) | –0.17 (–0.85, 0.52) | –0.34 (–1.05, 0.37) | 0.76 (–0.05, 1.56) | 0.05 (–0.79, 0.89) |
| Model 3 | –0.27 (–0.95, 0.41) | –0.22 (–0.90, 0.45) | –0.44 (–1.10, 0.21) | 0.85 (0.16, 1.54)* | 0.11 (–0.73, 0.94) |
Note: Model 1 unadjusted. Model 2 adjusted for age and sex. Model 3 adjusted for age, sex, years of education, depression, medical comorbidities, BMI, and number of medications.
*p ≤ .05, **p ≤ .01.
Further Examination of Possible Mediators and Modifiers
Because evidence has suggested that TNFα and ceramide may interact to promote cell death leading to atrophy (26), we examined TNFα as an effect modifier of the association between plasma ceramides and gait. Plasma TNFα was not found to be a modifier or a confounder. We similarly investigated whether either sex or diabetes modified the association between sphingolipids and gait, but again found no evidence of effect modification. In sensitivity analyses, we investigated whether additionally adjusting for APOE genotype, cognitive test performance (global z-score), statin use, diabetes, and dyslipidemia impacted the association between sphingolipids and gait parameters, but found that they did not (results not shown). Finally, because gait speed is correlated with cadence (Spearman r = 0.58, p < .001), double support time (r = –0.69, p < .001), stride time SD (r = –0.47, p < .001), and step width (r = 0.88, p < .001), in models specifying these parameters as dependent variables we additionally adjusted for gait speed. In these analyses, the association between plasma ceramides and gait attenuated to non-significance.
Discussion
In this cross-sectional study, we examined the association between sphingolipids and gait among CN participants aged 70 and older in the MCSA. Higher levels of plasma ceramide C16:0 and ceramide C16:0/S1P, as compared to other carbon chain lengths, were most strongly associated with poorer performance across multiple gait parameters. The associations were even stronger after adjustment for multiple factors including comorbidities, suggesting an independent association between higher plasma ceramide C16:0 levels and disrupted gait.
Our observed relationship between the C16:0 chain length and gait parameters is consistent with findings from a small sample of men (N = 19) which showed that intramuscular C16:0 ceramide levels were negatively correlated with lean leg mass, and positively correlated with fat mass and increased phosphorylation and concentration of NFκB, a pro-inflammatory transcription factor (11). Together, these findings suggest that C16:0 ceramide levels may be associated with functional decline.
Our findings are supported by evidence from cellular and animal model studies, which suggest that ceramides are associated with muscle atrophy and decreased physical function. In cellular models, ceramides induce atrophy, proteolysis, and necrosis (27,28), and mimic the atrophic effects of TNFα. Conversely, S1P protected against these atrophic effects in myotubes and in a murine model of cachexia (27). Moreover, evidence suggests that increases in long chain length ceramides (eg, C16:0, C18:0, C20:0) are anti-proliferative, compared to very long chain length ceramides (eg, C24:0, C24:1) (29,30). This may explain both our primary findings driven by C16:0 chain lengths, and our finding that higher levels of very long ceramide chain lengths appear to be associated with faster gait speed. Of note, very long chain length ceramides have also been associated with white matter microstructure decline (13), which in turn has been associated with poorer gait performance (14). This may be another potential mechanism linking sphingolipids and gait, and future research should investigate the association between sphingolipids, atrophy, cerebrovascular pathology, and gait.
This study has a number of strengths, including the large population-based sample and the multiple sphingolipid and gait measures. However, limitations of the study also warrant consideration. First, because this study is cross-sectional, directionality cannot be inferred. Future studies will need to examine the longitudinal relationship between ceramides and gait to determine whether ceramides either predict change in gait, or whether changes in ceramides coincide with changes in gait. Second, some individuals without a diagnosis of diabetes likely have insulin resistance, which we were not able to consider. Previous studies have shown that muscle ceramides enhance insulin resistance (31), and that plasma ceramides are associated with insulin resistance (32). Future research should determine whether the association between plasma ceramides and gait vary by severity of insulin resistance and diabetes. Third, Olmsted County, MN residents are largely of northern European ancestry, thus our findings may not be directly generalizable to other populations. Indeed, we previously reported higher ceramide levels in African Americans compared to Caucasians (6). However, this difference does not indicate that the relationship between ceramides and gait will differ by race. In this same study we showed that women had higher levels of ceramides than men; however, this difference has not been shown consistently (33). In the current study, we did not find an interaction between sex and ceramides in relation to gait performance. Thus, higher ceramide levels in either sex may be equitably associated with disrupted gait, regardless of differences in absolute values. Fourth, because the sample used was relatively healthy, most participants had gait speeds greater than 1.0 m/s. Therefore we were unable to robustly estimate the association between ceramide, S1P, and inflammatory cytokines levels and risk of sarcopenia as defined by the International Working Group on Sarcopenia (34). Additionally, muscle performance data are not available in the MCSA, so we were unable to examine the association between plasma ceramide levels and muscle atrophy via a measure of muscle strength. Similarly, neuroimaging measures of white matter decline were not available, so we were unable to investigate whether white matter microstructure decline mediates the association between plasma sphingolipid levels and gait. Lastly, the association between cell-tissue-organ aging has been hypothesized to alter the plasma composition (35,36), but plasma ceramides may not be a direct indicator of intramuscular ceramides. Additional studies are needed to understand the mechanistic connection between plasma ceramides and tissues, and how plasma ceramides are associated with functional decline.
In conclusion, higher levels of plasma ceramide C16:0 are associated with poorer performance across multiple gait parameters. These results are not attenuated after adjusting for TNFα or comorbidities. Gait parameters are a strong proxy measure for functional decline and predicting disability, sarcopenia, frailty, and mortality. Therefore, the present results suggest that high levels of plasma ceramide C16:0 may be an indicator of functional decline. Longitudinal studies are needed to determine the directionality of the association between plasma ceramides and gait, and other measures of physical function, as well as how fluctuations in ceramide levels may impact gait over time.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by grants from the National Institutes of Health/National Institute on Aging (R01 AG49704, U01 AG006786) and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Conflicts of Interest
Drs Wennberg, Schafer, LeBrasseur, Savica, and Hollman, and Mr Hagen report no disclosures. Dr. Bai is an employee of Eli Lilly. Dr. Petersen serves on scientific advisory boards for Pfizer, Inc., Janssen Alzheimer Immunotherapy, Roche, Inc., Merck, Inc., and Genentech, Inc.; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the National Institutes of Health (P50 AG016574, U01 AG006786, U01 AG024904, and R01 AG011378). Dr. Mielke served as a consultant to Lysosomal Therapeutics, Inc., and Eli Lilly; and receives research support from the National Institutes of Health (R01 AG49704, P50 AG44170, U01 AG06786), Department of Defense (W81XWH-15-1), and unrestricted research grants from Biogen and Roche.
Supplementary Material
References
- 1. Abellan van Kan G, Rolland Y, Andrieu S et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. [DOI] [PubMed] [Google Scholar]
- 2. Topinková E. Aging, disability and frailty. Ann Nutr Metab. 2008;52(suppl 1):6–11. doi:10.1159/000115340 [DOI] [PubMed] [Google Scholar]
- 3. Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi:10.1038/nature13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi:10.1074/jbc.R200007200 [DOI] [PubMed] [Google Scholar]
- 5. Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi:10.1074/jbc.R200008200 [DOI] [PubMed] [Google Scholar]
- 6. Mielke MM, Bandaru VV, Han D et al. Factors affecting longitudinal trajectories of plasma sphingomyelins: the Baltimore Longitudinal Study of Aging. Aging Cell. 2015;14:112–121. doi:10.1111/acel.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu Z, Zhai G, Singmann P et al. Human serum metabolic profiles are age dependent. Aging Cell. 2012;11:960–967. doi:10.1111/j.1474-9726. 2012.00865.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fabbri E, Yang A, Simonsick EM et al. Circulating ceramides are inversely associated with cardiorespiratory fitness in participants aged 54-96 years from the Baltimore Longitudinal Study of Aging. Aging Cell. 2016;15:825–831. doi:10.1111/acel.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pralhada Rao R, Vaidyanathan N, Rengasamy M, Mammen Oommen A, Somaiya N, Jagannath MR. Sphingolipid metabolic pathway: an overview of major roles played in human diseases. J Lipids. 2013;2013:178910. doi:10.1155/2013/178910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005;19:461–463. doi:10.1096/fj.04-2284fje [DOI] [PubMed] [Google Scholar]
- 11. Rivas DA, Morris EP, Haran PH et al. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J Appl Physiol (1985). 2012;113:1727–1736. doi:10.1152/japplphysiol.00412.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zanin M, Germinario E, Dalla Libera L et al. Trophic action of sphingosine 1-phosphate in denervated rat soleus muscle. Am J Physiol Cell Physiol. 2008;294:C36–C46. doi:10.1152/ajpcell.00164.2007 [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez CE, Venkatraman VK, An Y et al. Peripheral sphingolipids are associated with variation in white matter microstructure in older adults. Neurobiol Aging. 2016;43:156–163. doi:10.1016/j.neurobiolaging.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Annweiler C, Montero-Odasso M. Vascular burden as a substrate for higher-level gait disorders in older adults. A review of brain mapping literature. Panminerva Med. 2012;54:189–204. [PubMed] [Google Scholar]
- 15. Schaap LA, Pluijm SM, Deeg DJ et al. ; Health ABC Study. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi:10.1093/gerona/glp097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De La Monte SM. Metabolic derangements mediate cognitive impairment and Alzheimer’s disease: role of peripheral insulin-resistance diseases. Panminerva Med. 2012;54:171–178. [PMC free article] [PubMed] [Google Scholar]
- 17. Kim MY, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- 18. Mathias S, Dressler KA, Kolesnick RN. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci USA. 1991;88:10009–10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberts RO, Geda YE, Knopman DS et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi:10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture. 2011;34:111–118. doi:10.1016/j.gaitpost.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. [DOI] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 23. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi:10.1016/j.mayocp.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s older americans normative studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol. 1992;6:1–30. [Google Scholar]
- 25. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 26. Xu J, Yeh CH, Chen S et al. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J Biol Chem. 1998;273:16521–16526. [DOI] [PubMed] [Google Scholar]
- 27. De Larichaudy J, Zufferli A, Serra F et al. TNF-α- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet Muscle. 2012;2:2. doi:10.1186/2044-5040-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo YL, Kang B, Yang LJ, Williamson JR. Tumor necrosis factor-alpha and ceramide induce cell death through different mechanisms in rat mesangial cells. Am J Physiol. 1999;276(3 Pt 2):F390–F397. [DOI] [PubMed] [Google Scholar]
- 29. Hartmann D, Wegner MS, Wanger RA et al. The equilibrium between long and very long chain ceramides is important for the fate of the cell and can be influenced by co-expression of CerS. Int J Biochem Cell Biol. 2013;45:1195–1203. doi:10.1016/j.biocel.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 30. Stiban J, Perera M. Very long chain ceramides interfere with C16-ceramide-induced channel formation: a plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta. 2015;1848:561–567. doi:10.1016/j.bbamem.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 31. Adams JM 2nd, Pratipanawatr T, Berria R et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. [DOI] [PubMed] [Google Scholar]
- 32. Huang H, Kasumov T, Gatmaitan P et al. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity (Silver Spring). 2011;19:2235–2240. doi:10.1038/oby.2011.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bui HH, Leohr JK, Kuo MS. Analysis of sphingolipids in extracted human plasma using liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2012;423:187–194. doi:10.1016/j.ab.2012.01.027 [DOI] [PubMed] [Google Scholar]
- 34. International Working Group on Sarcopenia. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J Am Med Dir Assoc. 2011;12:249–256. doi:10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jové M, Maté I, Naudí A et al. Human aging is a metabolome-related matter of gender. J Gerontol A Biol Sci Med Sci. 2016;71:578–585. doi:10.1093/gerona/glv074 [DOI] [PubMed] [Google Scholar]
- 36. Jové M, Naudí A, Gambini J et al. A stress-resistant lipidomic signature confers extreme longevity to humans. J Gerontol A Biol Sci Med Sci. 2017;72:30–37. doi:10.1093/gerona/glw048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.