Abstract
Background
Ample evidence implicates cellular senescence as a contributor to frailty and functional decline in rodents, but considerable effort remains to translate these findings to human aging.
Methods
We quantified senescence biomarker p16INK4a-expressing cells in thigh adipose tissue obtained from older women previously enrolled in a 5-month resistance training intervention, with or without caloric restriction (RT ± CR, n = 11 baseline, 8 pre–post-intervention pairs). Women in this subsample were older (72.9 ± 3.4 y) and overweight/obese (body mass index: 30.6 ± 2.4 kg/m2). p16INK4a+ cells were identified from 12 to 20 random visual fields/sample at 20× magnification (immunohistochemical, nuclear staining) and were present in all adipose samples.
Results
Cross-sectional associations were observed between p16INK4a+ cell burden and physical function, including grip strength (r = –0.74), 400-m walk time (r = 0.74), 4-m gait speed (r = –0.73), and self-perceived mobility (r = –0.78) (p ≤ .05). These relationships remained significant after independent adjustments for age and adiposity (p ≤ .05). p16INK4a+ cell abundance was lower following the intervention (pre: 5.47 ± 3.4%, post: 2.17 ± 1.1% count p16INK4a+ cells, p ≤ .05).
Conclusions
These results provide proof-of-concept that p16INK4a+ cells in thigh adipose are associated with physical function, and may be sensitive to change with RT ± CR in overweight/obese older women.
Keywords: Exercise, Caloric restriction, Mobility, Biology of aging
A key strategy advanced by the interdisciplinary field of geroscience is to therapeutically target the biologic aging process to prevent or reverse declines in health and function (1,2). One such biological process, cellular senescence, has emerged as a promising target for drug development based on a growing body of animal literature (3,4). Cellular senescence entails cell cycle arrest triggered by DNA damage, oxidative stress, or metabolic stressors (5,6). This molecular damage can engage cellular signaling cascades and activate p53 and/or p16INK4a cellular senescence programs leading to a senescent phenotype demonstrated to have deleterious consequences on health and function in animal models of aging (3,6–8).
Senescent cells can accumulate within mitotic tissues such as adipose leading to detrimental local and systemic effects that can compromise function (3,4,8,9). For example, up to 30-fold greater senescent cell accumulation is observed in visceral adipose tissue from obese compared with non-obese adults (9), possibly owing to a combination of replicative, cytokine-induced, and metabolic stresses coupled with reduced apoptosis (4,9,10). Elevated adipose tissue senescent cell burden is hypothesized to contribute to local and systemic dysfunction as adipose is frequently the largest tissue in the human body with important immune and endocrine functions (10,11). In rodents, elevated adipose senescent cell burden induced by aging or “fast-food” westernized-diet accompanies systemic inflammation and physical dysfunction (4,11), but even modest reductions in the number of senescent cells can positively impact health and function. For example, adipose senescent cell abundance in middle-aged mice is attenuated from 12% in sedentary to ~2% in exercised animals exposed to a fast-food diet, which is attended by robust improvements in function and physical endurance (4). However, the physical function and clinical health consequences of cellular senescence in human aging have yet to be determined.
Determining the abundance and functional consequences of cellular senescence in humans is necessary to evaluate the translational potential of therapeutically targeting senescent cells (12–14). We hypothesized that elevated burden of senescent cell biomarker cyclin-dependent kinase inhibitor p16INK4a in adipose tissue is uniquely associated with poorer physical function in older persons. We further hypothesized that p16INK4a+ senescent cell burden can be quantified serially as a biologic outcome for clinical trials in humans, and may be sensitive to change with intervention. To gain initial insight, we leveraged previously collected cryopreserved thigh adipose tissue (intermuscular or perimuscular) from well-characterized overweight/obese older women previously enrolled in a clinical study (15). We sought to demonstrate proof-of-concept that: (1) senescent cells could be detected by immunohistochemical (IHC) staining of p16INK4a+ cells in intermuscular/perimuscular thigh adipose of older women, (2) p16INK4a senescent cell abundance would be associated with poor physical function, and (3) adipose p16INK4a+ cell burden may be sensitive to change following a 5-month resistance training with or without caloric restriction intervention (RT ± CR).
Materials and Methods
Participants and Study Design
This study took advantage of cryopreserved adipose samples collected coincident to biopsy of the vastus lateralis during a previously conducted randomized clinical trial of resistance exercise (3 d/wk) either with –600 kcal/day caloric restriction or without caloric restriction in overweight/obese (body mass index [BMI] ≥ 28–35 kg/m2) older adults (65–79 y) (Improving Muscle for Functional Independence Trial, I’M FIT; clinicaltrials.gov NCT01049698) (15). The study was approved by the Wake Forest School of Medicine Institutional Review Board and all participants provided written informed consent.
Detailed description parent study design, participants, and measures of body composition and adiposity are provided in Supplementary Methods and previous publication (15). Main outcome measures in the parent I’M FIT study that are relevant to the present investigation are: (a) body composition (height, weight, body mass index, dual-energy X-ray [DEXA]), (b) thigh intermuscular adipose tissue volume (IMAT, by computed tomography [CT scan]), (c) physical function (grip strength, 400-m walk performance, 4-m usual gait speed, and self-perceived mobility), and (d) biopsy of thigh intermuscular or perimuscular adipose tissue coincident to harvest of vastus lateralis; when adipose tissue (deep to fascia) was harvested with the skeletal muscle sample, it was immediately dissected and stored at –80°C (n = 56 total, n = 40 with ≥20 mg adipose sample needed for analysis). A subset of these cryopreserved thigh adipose samples obtained at baseline (n = 11) and follow-up (n = 8 pre-post intervention pairs with n = 4 RT and n = 4 RT + CR) was analyzed, using selection criteria to minimize impact on biorepository resources, and to ensure heterogeneity in physical function (See Supplementary Methods for detail).
Physical Function
Physical function was measured as grip strength, time to complete 400-m walk at a brisk pace, 4-m usual gait speed and self-perceived mobility (Mobility Assessment Tool short form, MAT-sf). Grip strength was measured twice to the nearest kilogram by using an isometric hydraulic hand dynamometer (Jamar); the maximal value by the right hand was used in analyses. Performance on 400-m walk was determined as time to complete the distance (10 laps on an indoor 20-m long course) as quickly as possible without running. Gait speed was determined as the usual walking speed on a 4-m course. The MAT-sf consisted of a 10-item computer-based assessment of mobility using animated video clips and covers a broad range of functioning, with score range 0–60 (60 best).
Quantification of Adipose p16INK4a
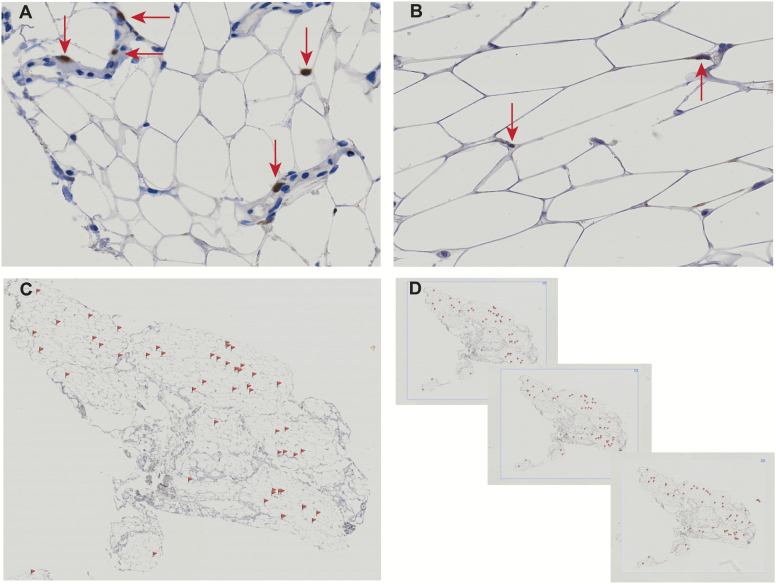
Biopsy of vastus lateralis and coincident adipose was performed in the morning following an overnight fast following standard procedures (Supplementary Methods). Briefly, a modified Bergstrom needle was used to harvest skeletal muscle and surrounding tissue, including intermuscular and/or perimuscular (subfascial) adipose. The residual adipose was manually teased apart, flash frozen, and stored at –80°C. Cryopreserved adipose samples were thawed, paraffin embedded, and IHC nuclear staining for p16INK4a as detailed in Supplemental Methods. Cells expressing p16INK4a were quantified by an investigator blinded to function and pre-post status via manual visual inspection of 12–20 visual fields at 20× mag (representative images Supplementary Figure 1), with indices reported as the average across serial sections. Abundance of p16INK4a+ cells was quantified as: (a) percent of the total fields analyzed containing p16INK4a+ cells, (b) average number of p16INK4a+ cells counted per visual field, and (c) percent of the total cells counted identified as p16INK4a+. p16INK4a+ cells were quantified by investigators blind to subject physical function via manual visual inspection at 20× magnification (positive stained nuclei confirmed at 40× magnification); total nuclei and p16INK4a+ cells were counted in each visual field (Figure 1). In each section, 12–20 visual fields were analyzed and 3–4 serial sections were analyzed per subject sample. Indices of p16INK4a abundance are reported as the average across serial sections for each subject sample (Figure 1C and D). To assess the intra-examiner reliability, the visual analysis and quantification of p16INK4a+ cell abundance was repeated in randomized order by the same reader blinded to original values separated by ~6–8 weeks.
Figure 1.
Representative images. p16INK4a+ nuclear cell staining (IHC) of representative adipose tissue sample obtained from single subject. All thigh adipose samples were obtained during biopsy of the vastus lateralis, and were harvested deep to the fascia and likely of intermuscular or perimuscular origin. Upper panels (A, B) 20 × magnification single visual fields with p16INK4a+ cells (red arrows); bottom panels p16INK4a+ cell (red flags) distribution across sample section (2×mag, C), and three sections from one sample (1.2× mag, D).
Statistical Analyses
All statistical analyses were performed with SPSS software (version 23.0, IBM); α = 0.05 was used to assess significance. Descriptive characteristics are reported as means ± SD and ranges. Intra-examiner reliability was determined by intraclass correlation coefficient using a one-way random effects model, and inter-item Pearson correlations across analyses were examined. Pearson correlations coefficients were calculated to assess cross-sectional relations at baseline and post-intervention between p16INK4a abundance (percent fields with p16INK4a-positive cells, average p16INK4a-positive cells per field, and percent of total cells that were p16INK4a-positive) and (a) age, (b) body composition (body weight, BMI, waist circumference, total fat mass, total lean mass, thigh IMAT volume), and (c) physical function (grip strength, 400-m walk time, 4-m usual gait speed, MAT-sf score, knee extensor power, five repetition chair-rise time, and SPPB). To examine potential confounders of the observed significant relationships between p16INK4a+ cell abundance with the physical function measures (grip strength, 400-m walk time, 4-m gait speed, MAT-sf score), partial correlations were calculated (individually adjusted for age, BMI, waist circumference, total fat mass, total lean mass, or thigh IMAT volume). To determine change in indices of p16INK4a+ cell abundance, body composition and physical function over time and estimates of intervention effects, the statistical differences within subjects were calculated using repeated measures ANOVA (RT and RT + CR intervention arms collapsed). Finally, to gain exploratory insight into possible relations between change in p16INK4a+ cell abundance and change in physical function and body composition, Pearson correlations between the absolute change (post-RT ± CR–pre-RT ± CR) in each variable were calculated.
Results
Subject Characteristics and Senescent Cell Abundance in Thigh Adipose Samples
Subject characteristics at baseline are summarized in Table 1, including age, body composition, and adiposity (total body fat, fat mass, and lean mass by DEXA, intermuscular adipose tissue volume, IMAT by CT scan), and measures of physical function and self-perceived mobility. Descriptive characteristics for primary indices of p16INK4a+ abundance are shown in Table 1. The intraclass correlation coefficients between repeat analyses ranged from 0.87 to 0.92, indicating strong reliability in p16INK4a+ cell identification and quantification (Table 2).
Table 1.
Subject characteristics (age, body composition/adiposity, and physical function) and indices of thigh adipose p16INK4a+ cell abundance
| Mean ± SD | Range | |
|---|---|---|
| n (women) | 11 | |
| Age (y) | 72.9 ± 3.4 | 67–77 |
| Body composition and adiposity | ||
| Body weight (kg) | 79.5 ± 7.2 | 64.9–90.0 |
| BMI (kg/m2) | 30.6 ± 2.4 | 27.0–34.7 |
| Waist circumference (cm) | 88.4 ± 5.0 | 78.9–98.9 |
| Total body fat (%) | 45.5 ± 2.6 | 40.3–48.8 |
| Fat mass (kg) | 36.8 ± 4.7 | 26.1–44.6 |
| Lean mass (kg) | 43.8 ± 3.6 | 37.1–47.2 |
| IMAT (cm3) | 24.5 ± 16.6 | 9.9–69.3 |
| Physical function | ||
| Grip strength (kg) | 23.9 ± 6.0 | 11–32 |
| 400-m walk time (s) | 332 ± 46 | 271–421 |
| 4-m usual gait speed (m/s) | 1.06 ± 0.16 | 0.81–1.39 |
| MAT-sf (score) | 62.8 ± 4.0 | 54.7–67.6 |
| Leg muscle power (W) | 103 ± 47 | 44–193 |
| rep chair rise time (s) | 11.5 ± 4.0 | 8.1–21.9 |
| SPPB (score) | 11.2 ± 1.2 | 9.0–12.0 |
| Adipose p16INK4a+ cell abundance | ||
| Fields w p16INK4a+ cell (%) | 75.7 ± 24.6 | 20–100 |
| Avg. p16INK4a+ cell count/field | 2.2 ± 1.1 | 0.3–4.3 |
| Total cell count | 771 ± 220 | 358–1122 |
| Cell count p16INK4a+ (%) | 5.6 ± 2.9 | 0.6–11.1 |
Note: Fat mass as whole body fat from DEXA. IMAT, thigh intermuscular adipose volume from CT scan. MAT-sf, Mobility Assessment Tool short form (higher score indicative of greater mobility). For indices of p16INK4a+ cell abundance, 3–4 sections per sample were mounted, stained for p16INK4a (immunohistochemistry), and manually visually analyzed; averages across the sample sections are shown.
Table 2.
Intra-examiner reliability (intraclass correlation coefficient) and inter-item correlation matrix for primary indices of adipose p16INK4a+ cell abundance across repeat analyses
| Initial Analyses | ||||
|---|---|---|---|---|
| ICC | Fields w p16INK4a+ Cells (%) | Avg. p16INK4a+ Cells/Field | Cell Count p16INK4a+ (%) | |
| Fields with p16INK4a+ cells (%) | 0.87* | 0.91* | 0.78* | 0.81* |
| Avg. p16INK4a+ cells/field | 0.87* | 0.84* | 0.88* | 0.85* |
| Cell count p16INK4a+ cells (%) | 0.92* | 0.85* | 0.90* | 0.97* |
Note: ICC, single measure intraclass correlation coefficient. Pearson (r) correlations; *p < .05 (n = 19). p16INK4a+ cell quantification was repeated 6–8 wk following initial analyses under same procedures, by investigator blind to physical function status and cell quantifications from initial analyses.
Associations Among Thigh Adipose p16INK4a+ Cell Burden and Age, Body Composition, and Physical Function
Overall, at baseline, cross-sectional correlations indicate that elevated p16INK4a+ cell burden is associated with poorer physical function, even with adjustment for age and body composition. Significant negative correlations exist between p16INK4a+ abundance with grip strength, usual gait speed on 4-m course, and MAT-sf, and though not statistically significant, moderate correlations were also observed for leg power and SPPB score (Table 3, Supplementary Figure 1). Likewise, 400-m walk time was positively correlated with percent total cell count p16INK4a+ and a moderate (but non-significant) correlation was observed with five-repetition chair rise time, such that higher p16INK4a+ cell burden was associated with poorer endurance walk and chair rise performance (Table 3, Supplementary Figure 1). p16INK4a+ cell burden was significantly associated with waist circumference, but was only modestly associated with age and other indices of body composition, such that elevated p16INK4a+ cell burden was either unrelated or moderately related to older age, lower lean body mass, and greater adiposity (Table 3). Partial correlations between physical function measures with significant correlations with p16INK4a+ burden are shown in Supplementary Table 1 (independent adjustments for age, BMI, total fat mass, total lean mass, and IMAT); adjusting for age, adiposity, and lean mass did not affect the magnitude or direction of the associations.
Table 3.
Associations between indices of thigh adipose p16INK4a+ cells with age and body composition, and physical function measures
| Fields with p16INK4a+ Cells (%) | Avg. p16INK4a+ Cells/Field | Cell couNt p16INK4a+ (%) | |
|---|---|---|---|
| Age | 0.37 | 0.34 | 0.31 |
| Body weight (kg) | 0.29 | 0.39 | 0.24 |
| BMI (kg/m2) | 0.40 | 0.49 | 0.55 |
| Waist circumference (cm) | 0.54 | 0.74* | 0.63* |
| Total body fat (%) | 0.54 | 0.38 | 0.38 |
| Fat mass (kg) | 0.49 | 0.49 | 0.38 |
| Lean mass (kg) | –0.02 | 0.17 | 0.04 |
| IMAT (cm3) | 0.07 | –0.06 | 0.02 |
| Grip strength (kg) | –0.45 | –0.65* | –0.74* |
| 400-m walk time (s) | 0.50 | 0.53 | 0.74* |
| 4-m gait speed (m/s) | –0.73* | –0.57 | –0.54 |
| MAT-sf (score) | –0.55 | –0.66* | –0.78* |
| Leg power (W) | –0.36 | –0.46 | –0.53 |
| 5-rep chair rise time (s) | 0.34 | 0.43 | 0.45 |
| SPPB (score) | –0.41 | –0.36 | –0.57 |
Note: Pearson correlations (r); *p < .05 (n = 11). Whole body fat and lean mass from DEXA. IMAT, thigh intermuscular adipose volume from CT scan. 400-m walk time (s) performed at brisk pace; 4-m Gait Speed performed at usual walking speed. MAT-sf, Mobility Assessment Tool, short form. Leg muscle power (W) of right leg from Biodex. SPPB, short physical performance battery.
At intervention follow-up, despite the smaller sample size, overall moderate correlations were observed between p16INK4a+ cell abundance and indices of adiposity, and physical function (Supplementary Table 2). The relationships between p16INK4a+ burden and grip strength, and chair-rise performance remained statistically significant (p < .05), and though not significant, moderate relations with 400-m walk speed and MAT-sf, were observed (r > 0.50), but not with 4-m usual gait speed, leg power, and SPPB (Supplementary Table 2).
Thigh Adipose p16INK4a+ Cell Burden Before and After RT ± CR
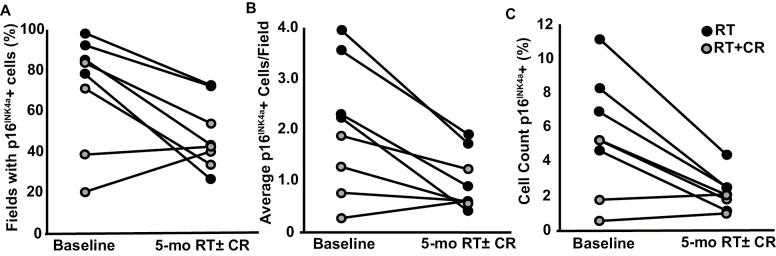
Eight subjects with thigh adipose tissue p16INK4a+ cells quantified at baseline had samples obtained at intervention follow-up for paired pre–post intervention p16INK4a+ cell analyses. Overall, p16INK4a+ cell abundance was lower following the 5-months RT ± CR intervention, with attenuated burden in 6 of the 8 sample pairs (individual data shown in Figure 2, effect estimates in Supplementary Table 4). In this study subset, the 5-month intervention elicited 3 ± 4% total weight loss and reduced adiposity (baseline vs 5-mo RT ± CR, p < .05 all), but lean mass, physical function and self-perceived mobility were unchanged (Supplementary Table 4). Bivariate correlations were assessed between absolute changes in p16INK4a+ cell abundance and changes in body composition (Supplementary Table 5), with moderate correlations observed with change in indices of adiposity including body weight, total fat mass, and percent body fat (p ≤ .05), but not physical function, though modest and inconclusive given the small sample size.
Figure 2.
p16INK4a+ cell burden before and after 5-mo RT ± CR intervention in older overweight/obese women. p16INK4a+ cell burden, measured as percent of visual fields with p16 INK4a+ cells (A), average p16INK4a+ cells per field (B), and percent cell count p16INK4a+ (C), from thigh adipose tissue samples collected before and after a 5-month RT (3 d/wk on weight-stack resistance machines), with a CR intervention designed to elicit moderate weight-loss (–600 kcal/d) or without CR. Black circles: RT only; gray: RT + CR.
Discussion
This study provides proof-of-concept that p16INK4a+ cell abundance from thigh adipose is associated with worse physical function in community-dwelling older overweight/obese women. Moreover, these relations were not mediated by age, adipose quantity, or lean mass, suggesting that p16INK4a+ cell burden derived from thigh adipose tissue is uniquely related to physical function. We demonstrate that p16INK4a+ cell abundance can be quantified serially and may be sensitive to change with an intervention. Additionally, though our data suggest a possible association between change in p16INK4a+ expression and reduction in body weight and total fat with intervention—but not with change in physical function—the evidence is not conclusive and must be replicated in a larger sample with a control group for reference. These preliminary observations require replication by a larger prospective study with supplementary senescence biomarkers and senescence-associated secretory products, however, we do provide the first evidence for translational utility of an in vivo cellular senescence biomarker and its associations with physical function and adiposity in older humans.
We found a robust cross-sectional association between thigh adipose tissue p16INK4a+ cell abundance and several subdomains of physical function, including grip strength, 400-m walk performance, gait speed, and self-perceived mobility that predict higher rates of disability, institutionalization, and mortality (16–18). Preclinical investigations in rodents suggest depot specificity, with elevated senescent cells accumulation in visceral adipose tissue compared with subcutaneous adipose (4,10). While we do not have access to visceral adipose tissue in this study, our findings do suggest that p16INK4a expressing cells do accumulate in thigh adipose harvested deep to fascia (not subcutaneous), and this depot may serve as a source of senescent cells that are relatively easier to access than visceral depots. Excess adipose tissue is associated with worse physical function in older adults (19), but thigh intermuscular fat in particular is more closely associated with physical dysfunction and annual gait speed declines in older persons, such that every 5.75 cm2 increase in intermuscular fat is associated with a 0.01 ± 0.0 m/s annual decline in gait speed, and this decline is exacerbated with intermuscular adipose gain over time in older persons (20). That robust cross-sectional associations were observed between physical function and p16INK4a+ cells harvested from thigh intermuscular or perimuscular adipose, an adipose depot known to independently predict gait speed and functional decline, is intriguing and warrants further investigation to determine depot-specificity and possible systemic mediators.
We were able to detect p16INK4a+ cells in adipose tissue biopsies performed before and after a clinical intervention, which suggests the potential of thigh adipose tissue p16INK4a-positive cell abundance as in vivo senescence biomarker for clinical trials in humans. Though senescent cells are known to accumulate in aging rodents, the relative abundance and longitudinal quantification in humans by histochemical detection methods has been limited by the rarity of these cells, potential non-specificity, and other practical issues (21). Our findings also suggest potential modulation in p16INK4a senescent cell abundance with an RT ± CR intervention, which is in accord with preclinical evidence from rodents (4,22) though conclusions from the present study are dampened by absence of a control group. Nevertheless, we illustrate that p16INK4a can be measured serially in biopsies in the context of a clinical trial in aging, and provide a conceptual foundation for future work to determine the appropriateness of p16INK4a+ cells as a surrogate marker of senescence with interventions (3). This is especially important with discovery of senolytic drugs that reduce senescent cell burden and alleviate age- and disease-related phenotypes, including frailty and declines in physical endurance in mice (12–14), and could potentially be translated to humans.
Though mechanisms have yet to be defined, we provide direct evidence linking p16INK4a+ cell abundance in thigh adipose tissue to physical function in humans for the first time, and our findings are substantiated by foundational evidence in rodents and association studies in humans. p16INK4a acts to establish retinoblastoma protein (pRB)-regulated growth arrest and is commonly used as an in vivo senescence marker owing largely to its function as a tumor suppressor and highly dynamic expression (21,23). p16INK4a is expressed by many (though not all) senescent cells, and ample evidence demonstrates elevated p16INK4a expression in mammals with advanced chronologic age across multiple tissue types (24,25). The physiologic effect of p16INK4a-expressing cells has been examined in a transgenic mouse model (INK-ATTAC), in which p16INK4a-expressing cell apoptosis can be induced (26,27). p16INK4a-expressing cell ablation partially rescues vascular reactivity and several age-related phenotypes and disorders in these mice and may extend median, but not maximum lifespan, though the effects on lifespan are not definitive (4,13,26,27). Clearance of p16INK4a-expressing cells beginning at mid-life in naturally-aged rodents attenuated age-dependent adipose tissue and stem cell dysfunction, and delays in the trajectory of age-related declines in spontaneous and exploratory behavior were noted (28,29). This is further supported by the discovery that senolytic drugs reduce p16INK4a-expressing cells in tandem with declines in other markers of cellular senescence, and affects on multiple age- and senescence-related phenotypes parallel those of genetic clearance from INK-ATTAC mice (4,12,13). p16INK4a+ cells isolated from adipose tissue exhibit elevated expression of pro-inflammatory senescence-associated secretory phenotype (SASP) products compared to cells from the same tissues not expressing p16INK4a (3,11,26,28). However, the relation between p16INK4a and the SASP is nuanced and by p16INK4a overexpression may not consistently activate a SASP (30,31). Whether driven through SASP-dependent mechanisms or not, collectively these data support a model in which p16INK4a+ cells shorten healthy lifespan and negatively impact function with age in rodents.
The negative health consequences of increased p16INK4a+ cell burden may extend to humans. For example, peripheral blood T-lymphocyte expression of p16INK4a, but not other INK4/ARF transcripts, was found to be uniquely related to chronologic age and associated with health behaviors such as tobacco use and physical inactivity (24). Unbiased human association studies demonstrate that the genotype of single nucleotide polymorphisms near the INK/ARF locus coding for p16INK4a are associated with chronic age-related diseases (32–34). A strong association between a common inherited variant of the p16INK4a genetic region and reduced limitation in physical function was found in a heterogeneous older adult population (35). Collectively, these works and our present findings implicate p16INK4a senescent cells in maintenance of health and physical function in mammalian species from rodents to humans.
We acknowledge several limitations. Important next steps should confirm whether these p16INK4a+ cells are indeed senescent and express SASP factors and determine cell types through secondary staining. In future work, adipose samples should be harvested from different depots and tissues, as p16INK4a+ cells may be differentially-expressed and could vary in their systemic effects and functional consequences on age-related adipose dysfunction, physical function declines, and frailty (8,10,36). The quantification of p16INK4a+ cell burden from adipose tissue biopsies should also be coupled with circulating SASP products, senescence biomarkers, and T-cell p16INK4expression, as blood-based biomarkers could have particular utility in clinical trials (3,24). Finally, p16INK4a+ cell burden should be assessed longitudinally in both women and men not undergoing an intervention to determine p16INK4a accumulation with normal human aging. A non-treatment reference group (which was not part of the parent trial) is critically important to determine relations between change in p16INK4a+ senescent cell burden and change in functional outcomes over time. Thus, longitudinal assessment without intervention will enable calculation of effect size that will be critical for future clinical trials with senolytics and other interventions designed to directly or indirectly alleviate senescent cell burden.
In conclusion, this study provides the first direct evidence that the senescence biomarker p16INK4a is associated with physical dysfunction that could increase risk of disability, loss of independence, and mortality in older adults, and these relations are independent of age and adiposity or lean mass. Additionally, p16INK4a+ cell burden in biopsied thigh intermuscular or perimuscular adipose tissue can be quantified serially in the context of a clinical trial in older persons, and is potentially modifiable with intervention. These results are preliminary and require replication and validation, including determination of p16INK4a+ cell type, confirmation of permanent cell-cycle arrest, and correlation with additional senescence and SASP markers. Though additional data are needed to identify the mechanisms of action, this study provides novel evidence that thigh adipose tissue p16INK4a+ cell burden may have functional consequences in community-dwelling older women, and opens a fertile area for future research seeking to translate groundbreaking insights on cellular senescence from the lab to the clinic.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332), NIH grants (R01AG020583 [BJN], T32-AG33534 [SKB, BJN; JNJ trainee], AG52958 [NKL], and R37AG13925 [JLK]), the Connor Group, and the Noaber and Glenn Foundations (JLK, NKL). This work was conducted while J.N.J. was a Glenn/AFAR postdoctoral fellow.
Conflict of Interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
We thank the women who volunteered for this study as well as the research staff who conducted recruitment and assessments. We also thank Drs. Jahmehl Demons and Mary Lyles for performing the biopsy procedures, and Karin Murphy and John Stone for assistance in handling biorepository samples.
References
- 1. Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594:2001–2024. doi:10.1113/jphysiol.2014.282665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kennedy BK, Berger SL, Brunet A et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi:10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schafer MJ, White TA, Evans G et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65:1606–1615. doi:10.2337/db15-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi:10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi:10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 7. Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17:324–328. doi:10.1097/MCO.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 8. LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–18. doi:10.1159/000382054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, Villaret A, Bouloumie A et al. Cellular senescence and inflammation in obesity. Obesity. 2009;17:S57. [Google Scholar]
- 10. Tchkonia T, Morbeck DE, Von Zglinicki T et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi:10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu M, Tchkonia T, Ding H et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA. 2015;112:E6301–E6310. doi:10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Y, Tchkonia T, Pirtskhalava T et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi:10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roos CM, Zhang B, Palmer AK et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi:10.1111/acel.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schafer MJ, White TA, Iijima K et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi:10.1038/ncomms14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101:991–999. doi:10.3945/ajcn.114.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Studenski S, Perera S, Patel K et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi:10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, White L. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr). 2012;34:563–570. doi:10.1007/s11357-011-9256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11:568–579. doi:10.1111/j.1467-789X.2009.00703.x [DOI] [PubMed] [Google Scholar]
- 20. Beavers KM, Beavers DP, Houston DK et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi:10.3945/ajcn.112.047860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15:397–408. doi:10.1038/nrc3960 [DOI] [PubMed] [Google Scholar]
- 22. Wang C, Maddick M, Miwa S et al. Adult-onset, short-term dietary restriction reduces cell senescence in mice. Aging (Albany NY). 2010;2:555–566. doi:10.18632/aging.100196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohtani N, Yamakoshi K, Takahashi A, Hara E. The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J Med Invest. 2004;51:146–153. doi:10.2152/jmi.51.146 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Sanoff HK, Cho H et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi:10.1111/j.1474-9726.2009.00489.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krishnamurthy J, Torrice C, Ramsey MR et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi:10.1172/JCI22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baker DJ, Wijshake T, Tchkonia T et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi:10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baker DJ, Childs BG, Durik M et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi:10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu M, Palmer AK, Ding H et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. doi:10.7554/eLife.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmitz ML, Kracht M. Cyclin-dependent kinases as coregulators of inflammatory gene expression. Trends Pharmacol Sci. 2016;37:101–113. doi:10.1016/j.tips.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 30. Coppé JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286:36396–36403. doi:10.1074/jbc.M111.257071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maas BM, Francis O, Mollan KR, Lee C, Cottrell ML, Prince HM et al. Concentrations of pro-inflammatory cytokines are not associated with senescence marker p16INK4a or predictive of intracellular emtricitabine/tenofovir metabolite and endogenous nucleotide exposures in adults with HIV infection. PLoS One. 2016;11:e0168709. doi:10.1371/journal.pone.0168709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helgadottir A, Thorleifsson G, Magnusson KP et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi:10.1038/ng.72 [DOI] [PubMed] [Google Scholar]
- 33. Wellcome Trust Case Control C. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi:10.1038/nature05911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeggini E, Weedon MN, Lindgren CM et al. ; Wellcome Trust Case Control Consortium (WTCCC) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi:10.1126/science.1142364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melzer D, Frayling TM, Murray A et al. A common variant of the p16(INK4a) genetic region is associated with physical function in older people. Mech Ageing Dev. 2007;128:370–377. doi:10.1016/j.mad.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda). 2017;32:9–19. doi:10.1152/physiol.00012.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.