Abstract
Aim
Rheumatoid arthritis (RA) is associated with an approximately two-fold elevated risk of cardiovascular (CV)-related mortality. Patients with RA present with systemic inflammation including raised circulating myeloid cells, but fail to display traditional CV risk-factors, particularly dyslipidaemia. We aimed to explore if increased circulating myeloid cells is associated with impaired atherosclerotic lesion regression or altered progression in RA.
Methods and results
Using flow cytometry, we noted prominent monocytosis, neutrophilia, and thrombocytosis in two mouse models of RA. This was due to enhanced proliferation of the haematopoietic stem and progenitor cells (HSPCs) in the bone marrow and the spleen. HSPCs expansion was associated with an increase in the cholesterol content, due to a down-regulation of cholesterol efflux genes, Apoe, Abca1, and Abcg1. The HSPCs also had enhanced expression of key myeloid promoting growth factor receptors. Systemic inflammation was found to cause defective cellular cholesterol metabolism. Increased myeloid cells in mice with RA were associated with a significant impairment in lesion regression, even though cholesterol levels were equivalent to non-arthritic mice. Lesions from arthritic mice exhibited a less stable phenotype as demonstrated by increased immune cell infiltration, lipid accumulation, and decreased collagen formation. In a progression model, we noted monocytosis, enhanced monocytes recruitment to lesions, and increased plaque macrophages. This was reversed with administration of reconstituted high-density lipoprotein (rHDL). Furthermore, RA patients have expanded CD16+ monocyte subsets and a down-regulation of ABCA1 and ABCG1.
Conclusion
Rheumatoid arthritis impairs atherosclerotic regression and alters progression, which is associated with an expansion of myeloid cells and disturbed cellular cholesterol handling, independent of plasma cholesterol levels. Infusion of rHDL prevented enhanced myelopoiesis and monocyte entry into lesions. Targeting cellular cholesterol defects in people with RA, even if plasma cholesterol is within the normal range, may limit vascular disease.
Keywords: Atherosclerosis, Enhanced haematopoiesis, Cellular cholesterol defects, Rheumatoid arthritis
Introduction
Patients with rheumatoid arthritis (RA), have a two- to three-fold increased risk of atherosclerotic cardiovascular disease (CVD), the major cause of mortality in these individuals.1–3 Identifying CVD risk in these patients remains challenging.4–6 Currently, risk is calculated based on methodologies used for the general population, which are often an underestimate.4,6 This highlights the need to better understand the relationship between RA and CVD, first, in order to accurately identify patients at risk, and second, in order to treat and reduce CV events.
The mechanisms that cause accelerated atherosclerotic-CVD in RA are unclear, although systemic inflammation is thought to play a central role.7–11 Consistent with this hypothesis, patients with RA often have increased circulating monocytes12,13 and platelets.14,15 It is well documented that circulating monocyte levels correlate with CVD in humans,16 and animal studies have demonstrated a causal relationship between circulating levels of monocytes and platelets with atherogenesis17–23 and impaired atherosclerotic lesion regression.20 While the involvement of monocytes in joint pathology has been previously studied,24 the association of myeloid cells and atherosclerosis in RA has not yet been investigated.
Monocytes, neutrophils, and platelets are produced in the bone marrow (BM) from haematopoietic stem and progenitor cells (HSPCs) through a process termed myelopoiesis. Maintaining cellular cholesterol homeostasis in HSPCs is essential to maintain normal haematopoiesis.18,19,23 Accumulation of cellular cholesterol results in hyperproliferation, myeloid skewing and, consequently, increased myelopoiesis. Haematopoietic stem and progenitor cells and myeloid progenitors regulate cholesterol efflux through the ATP binding cassette transporters (ABC)-A1 and ABCG1, and their cell intrinsic ligand apolipoprotein-E (apoE).18,23 Interestingly, patients with RA appear to have defective cholesterol metabolism, through the down-regulation of ABCA1 and ABCG1.25,26 These defects in cholesterol metabolism may alter cellular cholesterol homeostasis, contributing to enhanced CVD risk. Moreover, deletion of key components of the cholesterol efflux pathway amplifies autoimmune-like phenotypes, revealing an intimate link between autoimmune disorders, inflammation, cholesterol metabolism and, potentially, CVD.27–31
We hypothesized that systemic inflammation associated with RA would drive enhanced and sustained myelopoiesis, associated with a central defect in HSPC cholesterol metabolism that causes increased growth factor receptor expression. Consequently, these alterations in cholesterol metabolism may impair atherosclerotic lesion regression or alter lesion progression in pre-clinical models of atherosclerosis.
Methods
See Supplementary material online for complete details.
Animals
All experiments were approved either by the Alfred Medical Research Education Precinct (AMREP) animal ethics committee or Walter and Eliza Hall Institute of Medical Research (WEHI). Wild type (WT), Ldlr−/−, and Apoe−/− mice (all C57BL/6) were purchased from Jackson Laboratories and colonies were maintained at AMREP or WEHI. Mice were fed a normal chow diet unless stated otherwise. Arthritis was induced using the collagen-induced arthritis (CIA) or K/BxN serum transfer models. Specific experimental designs are detailed in the results, figures, and Supplemental material online. Data are expressed as mean ± standard deviation (SD), along with individual data points. Exact P-values are stated.
Results
Murine models of rheumatoid arthritis exhibit monocytosis, neutrophilia, and thrombocytosis
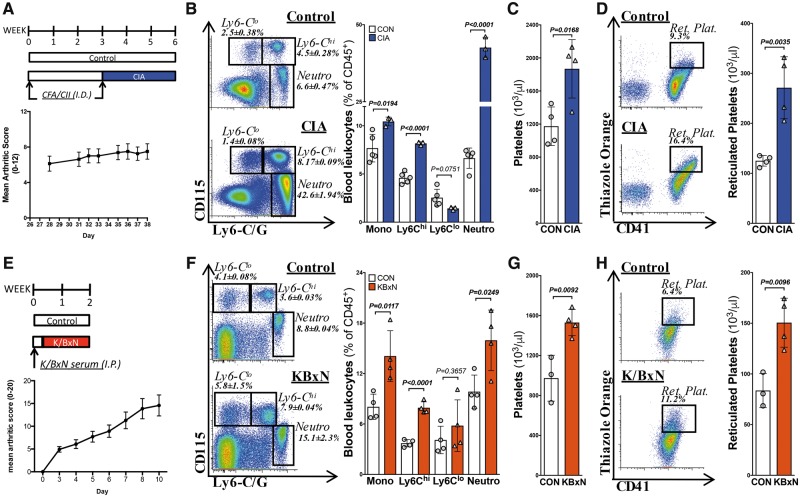
First, we confirmed leucocytosis in two murine models of RA. CIA caused Ly6-Chi-driven monocytosis and prominent neutrophilia, along with thrombocytosis and reticulated thrombocytosis (Figure 1A–D). These findings were consistent with literature24 and were replicated in the K/BxN model of RA (Figure 1E–H). Thus, animal models of RA recapitulate the blood profile observed in RA patients.
Translational perspective
Rheumatoid arthritis (RA) elevates the risk of mortality from cardiovascular disease (CVD), independent of traditional CVD risk factors. Our findings in mouse and man suggest that sustained, RA-driven inflammation could be a causal determinant of lesion severity in atherosclerosis. RA is associated with persistent leucocytosis and defects in cellular cholesterol handling. Additionally, monocytosis may present as an alternative factor to consider when determining CV risk in patients. In an experimental model of RA, we found impaired plaque regression, and altered progression of atherosclerosis, with enhanced monocyte infiltration into the plaque increasing the macrophage burden and generating an unstable plaque phenotype. This could be reduced by administration of the cholesterol acceptor, reconstituted high-density lipoprotein (rHDL). Thus, we suggest that modulation of cellular cholesterol (i.e. with rHDL or statins), could limit CVD in RA, even if cholesterol levels are not elevated.
Figure 1.
Murine models of inflammatory arthritis display leucocytosis and thrombocytosis. Experimental arthritis was induced by: (A) the collagen-induced arthritis (CIA) model or (E) the K/BxN serum transfer model. (B, F) Blood leucocytes were quantified by flow cytometry, (C, G) Circulating platelets counts, and (D, H) reticulated thrombocytes as determined by flow cytometry and normalized to platelet counts. n = 3–5. All data are mean ± standard deviation.
Rheumatoid arthritis promotes myelopoiesis and extramedullary haematopoiesis
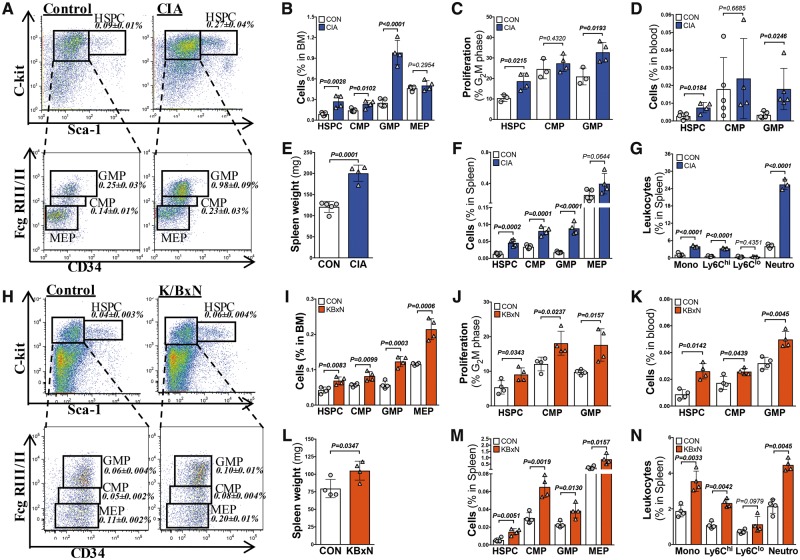
To investigate the mechanisms contributing to the increased abundance of circulating myeloid cells, we characterized the BM in both arthritis models. We observed a robust expansion and enhanced proliferation of HSPCs and myeloid progenitor subsets, common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and the megakaryocyte-erythroid progenitors (MEPs) (Figure 2A–C and H–J). Platelet production is tightly regulated by thrombopoietin (TPO), which we found to be elevated in both models of RA (Supplementary material online, Figure S1A and B).
Figure 2.
Inflammatory arthritis promotes extramedullary haematopoiesis in the spleen. Blood, spleen and bone marrow cell populations were assessed using flow cytometry in the collagen-induced arthritis (blue bars) and K/BxN (red bars) models of inflammatory arthritis. (A, B, H, I) Populations and (C,J) proliferation of bone marrow haematopoietic stem and progenitor cells, common myeloid progenitors, granulocyte-macrophage progenitors, and megakaryocyte-erythroid progenitors. (D, K) Blood stem and progenitor cells. (E, L) Spleen weights. (F, M) Haematopoietic stem and progenitor cell and progenitor cell abundance in the spleen. (G, N) Splenic leucocyte populations. n = 3–5. All data are mean ± standard deviation.
We also observed an increase in the abundance of circulating HSPCs and myeloid progenitors in arthritic mice, suggesting active stem cell mobilization (Figure 2D and K). Additionally, we noted splenomegaly, and an expansion of splenic HSPCs, CMPs, GMPs, and MEPs in both models of RA, confirming extramedullary haematopoiesis (Figure 2E, F, L, and M). As the spleen is an important organ for producing inflammatory monocyte subsets that track into atherosclerotic lesions,32 we assessed the abundance of mature splenic myeloid cells. Consistent with this atherogenic pathway, we found an increase in total monocytes, driven by the Ly6-Chi subset, and dramatically higher neutrophils in the spleens of mice with inflammatory arthritis (Figure 2G and N). These findings collectively provide evidence that both medullary and extramedullary organs are involved in enhanced myelopoiesis in the setting of polyarticular joint inflammation.
Bone marrow haematopoietic stem and progenitor cells from murine inflammatory arthritis models display defects in cholesterol metabolism
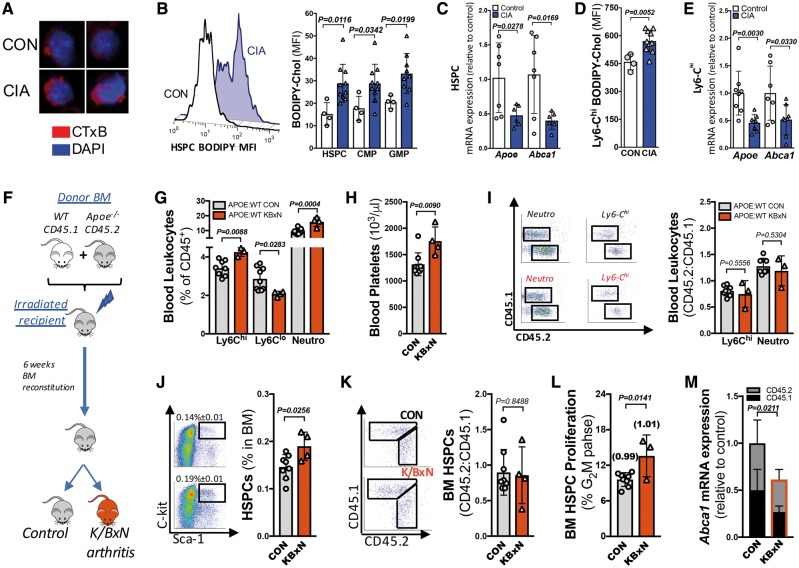
There are reports of altered cholesterol metabolism in RA,25,26 and we have previously shown that genetic deletion of cholesterol efflux in HSPCs promotes stem cell proliferation, myelopoiesis, and HSPC mobilization.18,33 Thus, we hypothesized that altered cholesterol metabolism in the HSPCs may also occur in arthritic mice. In support of this hypothesis, we observed an increase in membrane cholesterol-rich domains and cholesterol content in the HSPCs and myeloid progenitors from the arthritic mice (Figure 3A and B). This was associated with a down-regulation in the expression of the cholesterol efflux genes Apoe, Abca1, and Abcg1 in the HSPCs (Figure 3C and Supplementary material online, Figure S2A and B).
Figure 3.
Systemic inflammation causes enhanced myelopoiesis in inflammatory arthritis, and is associated with disturbed cellular cholesterol handling. (A) Membrane lipid rafts staining of bone marrow haematopoietic stem and progenitor cells from control and collagen-induced arthritis visualized by confocal microscopy (CT-xB staining; red, 4′,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI) counterstained; blue, 40× magnification). (B) Haematopoietic stem and progenitor cell representative plot and quantified membrane cholesterol on bone marrow haematopoietic stem and progenitor cells, common myeloid progenitors, and granulocyte-macrophage progenitors using boron-dipyrromethene (BODIPY)-cholesterol mean fluorescence intensity (MFI) via flow cytometry. (C) Cholesterol efflux gene expression from sorted bone marrow haematopoietic stem and progenitor cells. Ly6-Chi (D) BODIPY-cholesterol content, and (E) gene expression. n = 4–11. (F) Experimental overview. (G) Blood leucocyte abundance. (H) Platelet levels. (I) Representative flow plots, and the ratio of CD45.2 to CD45.1 cells. (J, K) Bone marrow haematopoietic stem and progenitor cell abundance and haematopoietic stem and progenitor cell CD45.2/CD45.1 cell ratio. (L) Haematopoietic stem and progenitor cell proliferation and the ratio of CD45.2/CD45.1 proliferation indicated in brackets. (M) Gene expression from isolated haematopoietic stem and progenitor cells. n = 3–8. All data are mean ± standard deviation.
Consistent with genetically deficient models with augmented membrane lipid rafts (i.e. Apoe−/− and Abca1−/−/Abcg1−/−),18,23 we observed increased cell surface levels of the common β-subunit of the interleukin (IL)-3/granulocyte-macrophage-colony stimulating factor (GM-CSF) receptor (IL-3Rβ; CD131) and M-CSF receptor (M-CSFR; CD115) on BM HSPCs, CMPs, and GMPs of arthritic mice (Supplementary material online, Figure S3A and B). We also found an increase in mRNA expression of Il-3rβ, M-csfr, G-csfr, and Gm-csfr in the CMPs isolated from mice with CIA (Supplementary material online, Figure S3C) and sustained higher expression of Il-3rβ and G-csfr in GMPs (Supplementary material online, Figure S3D). Taken together, these data suggest that defective cholesterol metabolism plays an important role in sustained proliferation and stem cell skewing towards the myeloid lineage during inflammatory arthritis.
Circulating myeloid cells from arthritic mice have persistent cholesterol dysregulation
Impaired cholesterol efflux in mature myeloid cells, including monocytes and macrophages, promotes foam cell development, contributing to enhanced atherogenesis.31,34 We postulated that this might also be occurring in mature myeloid cells in the arthritic mice, which could promote transition to lipid-laden macrophages in the atherosclerotic lesion.35 Monocytes (Ly6-Chi and Ly6-Clo) and neutrophils showed down-regulation of Abca1 and Apoe, and significantly increased membrane cholesterol (Figure 3D and E and Supplementary material online, Figure S3E–G). These findings were independent of plasma cholesterol levels (Supplementary material online, Figure S3H), suggesting that the suppression of key efflux genes in haematopoietic stem cells are retained during myelopoiesis.
Systemic inflammation drives enhanced haematopoiesis
We explored whether defects in cellular cholesterol metabolism might be responsible for driving the enhanced monocyte production or if these cholesterol disturbances are a consequence of systemic inflammation in experimental RA. To distinguish between these possibilities, we determined if HSPCs with defective cholesterol efflux would outcompete WT HSPCs in mice with inflammatory arthritis. This was achieved by performing a competitive bone marrow transplant (cBMT). Equal portions of WT CD45.1 and Apoe−/− CD45.2 BM were transplanted into irradiated WT mice. Following BM reconstitution, a group of transplanted mice were rendered arthritic using the K/BxN serum transfer model (Figure 3F and Supplementary material online, Figure S4). As expected, leucocytosis and thrombocytosis was observed in arthritic mice with WT/Apoe−/− BM (Figure 3G and H). We observed equal contribution of WT and Apoe−/− BM-derived monocytes and neutrophils in both control and arthritic mice, suggesting the Apoe−/− CD45.2 cells have no competitive advantage (Figure 3I).
Consistent with this finding, BM HSPCs were expanded in arthritic mice (Figure 3J), with an equal representation of WT CD45.1 and Apoe−/− CD45.2 cells (Figure 3K). In keeping with the lack of competitive advantage for Apoe−/− HSPCs, we also observed increased proliferation of BM HSPCs in the arthritic mice, with equal contributions from the genotypes (Figure 3L). To confirm the persistent down-regulation in cholesterol metabolism, we sorted the HSPCs based on expression of CD45.1 and CD45.2 from the control and arthritic mice and examined the level of Abca1 mRNA. Consistent with a systemic effect of inflammatory arthritis on the HSPCs, we found a significant down-regulation of Abca1 in both the WT CD45.1 and Apoe−/− CD45.2 HSPCs from the arthritic mice compared with the respective genotypes isolated from the control mice (Figure 3M). These results confirm that the disturbances in cellular homeostasis are a consequence of inflammatory arthritis.
We next explored if the inflammatory cytokines that are known to be involved in the pathogenesis of RA are directly capable of down-regulating the cholesterol efflux genes in HSPCs. To assess this, we incubated HSPC-enriched BM from WT mice in serum isolated from control or CIA mice and observed a down-regulation of Apoe and Abca1 (Supplementary material online, Figure S5A). To shed light on which cytokine(s) might be involved in this pathway, HSPC-enriched BM was treated with a cocktail of RA-associated cytokines (GM-CSF, TNF-α, IL-1β, and IL-6). This experiment confirmed that inflammatory cytokine signalling induced the down-regulation of these genes (Supplementary material online, Figure S5B). We also examined these cytokines individually and found that while they each decreased the expression of Apoe and Abca1 in HSPC-enriched BM, there were differential effects on these genes, adding to the complexity of this disease and the interplay with cellular cholesterol metabolism (Supplementary Figure S5C and D). Taken together, these data reveal that systemic inflammation found in RA down-regulates cholesterol efflux genes and promotes enhanced myelopoiesis.
Rheumatoid arthritis impairs atherosclerotic lesion regression
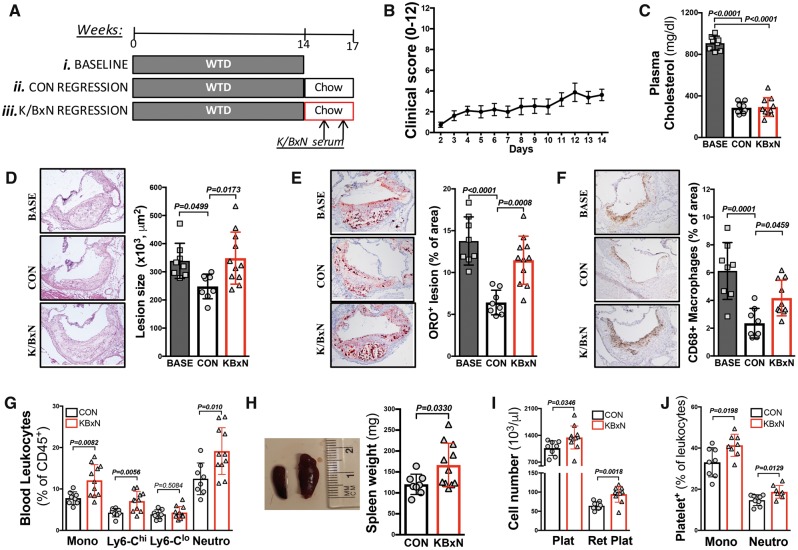
Rheumatoid arthritis generally affects middle-aged adults with existing atherosclerosis, therefore we performed a lesion regression study. Female Ldlr−/− mice were placed on a western type diet (WTD) for 14 weeks to initiate atherogenesis. At this time-point, a subset of mice was euthanized for baseline atherosclerotic lesion characterization, while the remaining mice were switched to a chow diet to reduce circulating cholesterol levels and initiate lesion regression. Following 1 week on chow, a subgroup of mice were rendered arthritic (K/BxN), while the remaining mice provided a control regression group (Figure 4A and B).
Figure 4.
Inflammatory arthritis impairs atherosclerotic regression independent of circulating cholesterol. (A) Ldlr−/− mice were fed a western type diet for 14 weeks to induce atherogenesis [(i) baseline]. The remaining mice were switched to a chow diet to induce atherosclerotic lesion regression [(ii) control regression group] and a (iii) arthritic group (K/BxN). (B) Arthritic clinical scores. (C) Plasma cholesterol. The aortic sinus was characterized for (D) lesion size, (E) lipid abundance (Oil Red O; ORO staining) and (F) macrophage content (CD68+). (G) Peripheral blood cell abundance, (H) spleen weight, (I) total and reticulated platelet counts, and (J) leucocyte–platelet interaction. n = 8–11. All data are mean ± standard deviation.
Compared to baseline, 3 weeks on a chow diet resulted in a significant reduction in plasma cholesterol in both groups (Figure 4C). While atherosclerotic lesions in the control group regressed compared with baseline, this was impaired in the arthritic mice (Figure 4D). Further examining lesion characteristics, we confirmed the expected reduction in plaque lipid content and macrophage abundance in the control regression mice (Figure 4E and F). This was not seen in the plaques of arthritic mice, where a significant lipid and macrophage burden remained (Figure 4E and F). To explore lesion remodelling, we examined the collagen content of the plaques. This revealed an increasing trend in the control regression mice, which was not observed in the arthritic mice (Supplementary material online, Figure S6).
Lesional macrophages arise predominantly from blood monocytes, which can impair lesion regression.20,36,37 Similar to our observations in WT mice (Figure 1), we confirmed enhanced myelopoiesis and splenomegaly in arthritic Ldlr−/− mice (Figure 4G and H). To provide further insight into impaired lesion regression in mice with inflammatory arthritis, we also found reticulated thrombocytosis, signifying enhanced platelet production/turnover, increased reactive immature platelets in Ldlr−/− K/BxN regression mice (Figure 4I) and enhanced platelet–leucocyte interactions (Figure 4J). Each of these features is linked to enhanced atherogenesis.17,38
Rheumatoid arthritis enhances Ly6-Chi monocyte entry and macrophage abundance in atherosclerotic lesions: reversal by reconstituted high-density lipoprotein
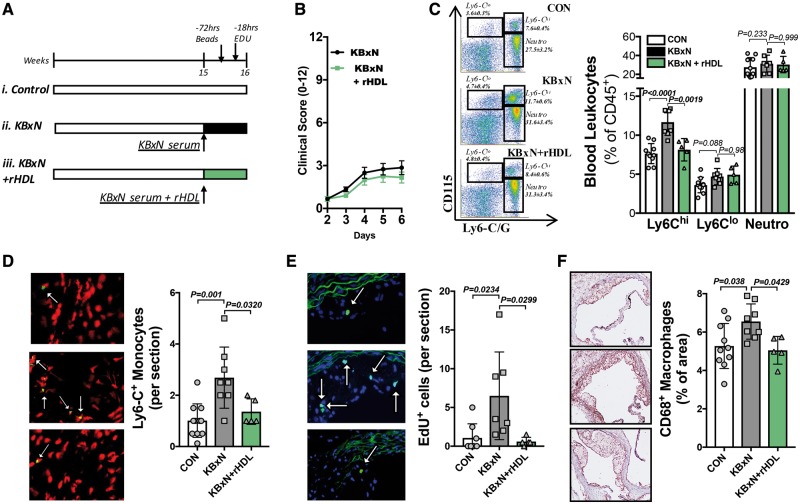
We queried whether RA-driven monocytosis directly contributed to enhanced monocyte lesion entry in an atherosclerotic progression model. Additionally, we aimed to explore if administering a bolus of reconstituted HDL (rHDL), which can promote cholesterol efflux via passive diffusion,30 could inhibit monocytosis and improve lesion outcomes. Apoe−/− mice were randomized into three experimental groups for 1 week of treatment; (i) control, (ii) K/BxN induced arthritis, and (iii) K/BxN and treated with rHDL (Figure 5A and B). To explore the role of monocytes in this context, we employed two methods of monocyte labelling. Mice were administered fluorescent tracking beads and EdU to label Ly6-Clo and Ly6-Chi monocytes, respectively20,37 (Figure 5A and Supplementary material online, Figure S7A).
Figure 5.
Reconstitute high-density lipoprotein prevents the rheumatoid arthritis-driven Ly6-Chi monocyte entry into atherosclerotic lesions and reduces macrophage abundance. (A) 15-week-old Apoe−/− mice, on a chow diet, were either (i) left as controls, (ii) made arthritic, or (iii) made arthritic and treated with reconstitute high-density lipoprotein. Mice were injected with fluorescent tracking beads on Day 4 (−72 h before sacrifice) and the following day with EdU. (B) Clinical arthritic scores. (C) Representative flow cytometry gating and quantified abundance of blood leucocytes. (D) Monocyte (Ly6-C) fluorescent staining (Ly6-C—green; nuclei—red) and (E) EdU+ cells in atherosclerotic lesions (EdU—green; nuclei—blue). Indicated with white arrows. (F) Lesional macrophage (CD68) content. n = 5–11. All data are mean ± standard deviation.
Administration of rHDL had no impact on clinical score but effectively reduced Ly6-Chi monocytes (Figure 5B and C). Exploring monocyte recruitment into atherosclerotic lesions, we observed an approximately three-fold increase in the abundance of Ly6-C+ cells in the plaques of arthritic mice, compared to control (Figure 5D). Consistent with peripheral blood monocytes, rHDL also reduced the abundance of Ly6-C+ cells within lesions (Figure 5D). The numbers of Ly6-C+ cells within lesions appeared to be due to the entry of EdU+ cells but not Ly6-Clo monocytes (Figure 5E and Supplementary material online, Figure S7B). EdU could also mark proliferative cells within lesions, but this is unlikely to occur as proliferation only occurs in advanced plaques.39 When examining the lesion characteristics, we observed no change in lesion size in this short-term experiment (Supplementary material online, Figure S7C). However, arthritic mice displayed a significant increase in plaque macrophages, which was reduced with rHDL treatment, most likely explained by modulation of monocyte production and consequently recruitment (Figure 5F). Again, there was no change in circulating cholesterol levels between the groups (Supplementary material online, Figure S7D). Together, these data show the direct involvement of monocyte entry into atherosclerotic lesions, which subsequently influences the plaque complexity in RA, and confirms the link with cholesterol metabolism, as rHDL reversed this phenotype.
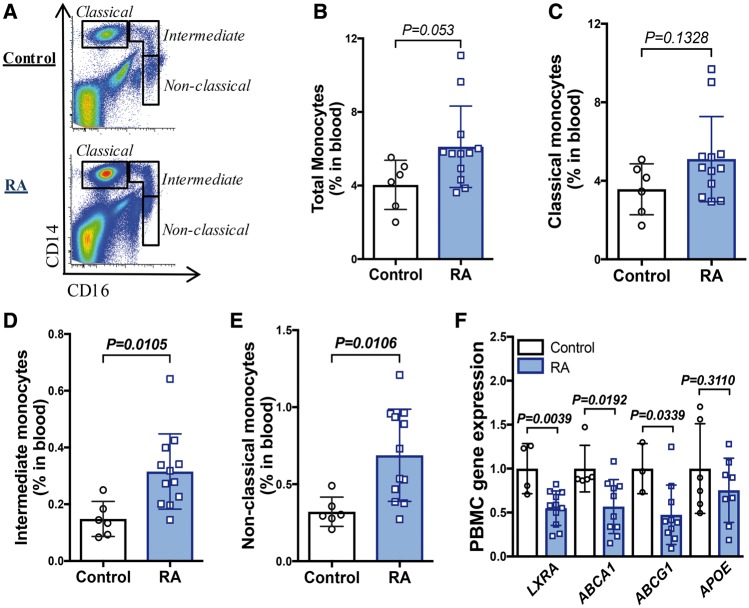
Rheumatoid arthritis patients display monocytosis and defects in cholesterol efflux genes
We next aimed to determine if our findings in pre-clinical models of arthritis, were evident in man. Patients and healthy controls were age and sex matched. Patients with RA were receiving treatment for active disease, including oral glucocorticoids and synthetic or biological disease modifying anti-rheumatic drugs (DMARDs; Table 1). Despite these therapies, and consistent with previous literature,12,13 there was an increase in total monocytes, driven by a robust, approximately two-fold increase in the intermediate (CD14+CD16+) and non-classical (CD14dimCD16++) subsets (Figure 6A–E). These monocyte subsets, have also been associated with adverse CVD-outcomes.40,41 We explored the mRNA expression of cholesterol efflux genes in peripheral blood mononuclear cells from RA patients. This revealed a significant reduction in LXR-A (NR1H3), ABCA1, and ABCG1 (Figure 6F). Taken together, these data suggest that our discoveries in the experimental models of RA in mice are clinically translatable.
Table 1.
Patient characteristics
| RA (n = 12) | Non-RA control (n = 7) | P-value | |
|---|---|---|---|
| Mean age (SD) | 50.8 (10.2) | 38.9 (15.9) | 0.109 |
| Sex (% female) | 11 (92%) | 6 (86%) | 1.00 |
| DAS28-CRP (SD) | 2.6 (1.0) | N/A | |
| Medications | |||
| Prednisone | 2 (17%) | N/A | |
| Synthetic DMARD | 7 (58%) | N/A | |
| Biologic DMARD | 8 (67%) | N/A | |
Characteristics of healthy control and patients with RA that are age and sex matched. n = 7–12.
DAS28, Disease Activity Score for 28 Joints; CRP, C-reactive protein.
Figure 6.
Patients with rheumatoid arthritis display peripheral blood monocytosis. (A) Monocyte subsets were identified using flow cytometry, from healthy controls and patients with rheumatoid arthritis. (B) Total monocytes, (C) classical CD14+CD16dim monocytes, (D) intermediate CD14+CD16+ monocytes, and (E) non-classical CD14dimCD16++ monocyte levels. (F) Gene expression in peripheral blood mononuclear cells. n = 3–12. All data are mean ± standard deviation.
Discussion
RA is a chronic inflammatory disorder that significantly increases the risk of CVD,1,9–11 independent of traditional risk factors,7,9,10 making CVD-risk assessment challenging.4–6 We found that inflammatory arthritis impairs atherosclerotic lesion regression and accelerates atherogenesis in pre-clinical models of RA, independent of plasma cholesterol (Take home figure). RA was associated with prominent leucocytosis and thrombocytosis, providing a potential explanation for the changes in atherosclerosis. We also found that the BM HSPCs from arthritic mice have dysregulated cellular cholesterol metabolism due to systemic inflammation. Furthermore, downstream myeloid cells retained this defect in cholesterol metabolism which could, in addition to enhanced myelopoiesis, contribute to foam cell development and increased CV events in RA.
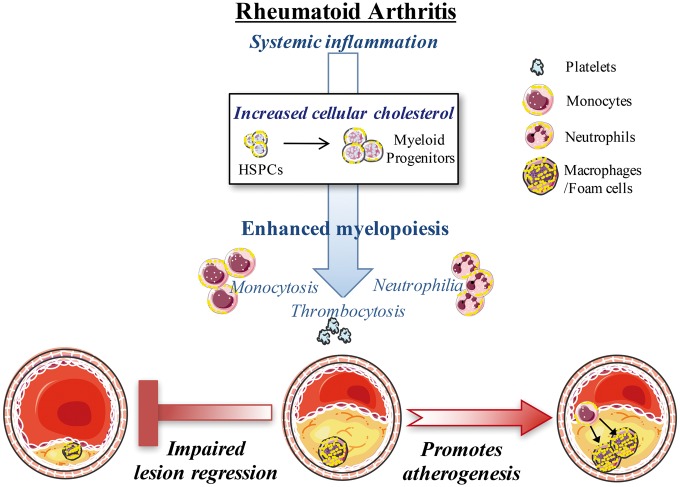
Take home figure.
Rheumatoid arthritis impairs atherosclerotic regression, independent of circulating cholesterol levels. Systemic inflammation in rheumatoid arthritis causes an accumulation of cellular cholesterol in bone marrow haematopoietic stem and progenitor cells that contributes to enhanced proliferation and myeloid skewing towards myeloid progenitors. Enhanced myelopoiesis causes increased levels of circulating monocytes (monocytosis), neutrophils (neutrophilia), and platelets (thrombocytosis). Rheumatoid arthritis promotes increased Ly6-Chi monocyte entry into lesions, and consequently increases macrophage burden. Rheumatoid arthritis impairs the regression of established atherosclerotic lesions, which contained more macrophages and lipid, even when cholesterol levels were controlled. Therefore, rheumatoid arthritis appears to impair atherosclerotic lesion regression, associated with an expansion of myeloid cells and disturbed cellular cholesterol handling, independent of plasma cholesterol levels. Promoting cholesterol efflux with reconstitute high-density lipoprotein could reverse the abundance and entry of monocytes into the lesion, and consequently reduce plaque macrophage burden.
An emerging concept is that systemic inflammation in RA could be responsible for the increased CVD risk in these patients.9 In keeping with this hypothesis, we observed increased circulating myeloid cells, platelets, and leucocyte–platelet interactions in arthritic mice, a profile previously shown to be causal in atherosclerotic development.16 The enhanced abundance of platelets was paralleled by increased TPO levels, suggesting a link between inflammation in peripheral tissues and the liver.42 Known to enter atherosclerotic lesions,32 inflammatory Ly6-Chi blood monocytes where increased in arthritic mice. Furthermore, we found an expansion of total monocytes in RA patients, predominantly due to a doubling of both the intermediate and the non-classical monocytes. This finding is consistent with previous reports that have shown an increase in CD16+ monocytes,13,41,43–45 which are inflammatory and commonly associated with CVD.41,46–48 Importantly, we have shown that cellular cholesterol dysfunction, which contributes to monocytosis in RA, is unlikely to be caused by only one particular cytokine. Thus, cellular cholesterol metabolism appears to be influenced by the multiple inflammatory cytokines which are upregulated in RA.
Cellular cholesterol handling is known to be important in atherosclerosis, independent of total circulating cholesterol.34 Cholesterol efflux in RA patients appears to be defective, potentially mediated via ABC-transporters.25,26,49 We found BM HSPCs from arthritic mice have reduced expression of Abca1, Abcg1, and Apoe, with the functional consequence of increased cell membrane cholesterol. This phenotype is similar to the genetic deletion of these genes where an increase in the cell surface expression of IL-3Rβ was observed, promoting cell intrinsic proliferation and myeloid skewing.18,23 However, disruption of the efflux pathway achieved by deletion of Apoe resulted in no competitive advantage over WT HSPCs (i.e. cell extrinsic). These finding are consistent with mechanisms of systemic inflammation, presumably originating from peripheral sites,50 instructing HSPCs to down-regulate cholesterol efflux genes in order to sustain higher rates of proliferation.
Our findings show that cellular cholesterol defects in HSPCs are retained in circulating monocytes in RA. It is possible that the impaired atherosclerotic lesion regression observed in mice with inflammatory arthritis could be initiated by early lineage-restricted changes in cholesterol metabolism. Daughter monocytes may then enter the lesion harbouring an increased cellular cholesterol, exacerbating foam cell formation.35 Consistent with this hypothesis, macrophage specific deletion of Abca1/Abcg1 promotes atherogenesis34; however, this phenotype is far more prominent when these transporters are deleted in HSPCs and the downstream myeloid cells.23 We also found that inflammatory arthritis enhances atherogenesis by increasing Ly6-Chi monocyte entry into atherosclerotic lesions, causing an increased burden of macrophages. Interestingly, a link between cellular cholesterol metabolism and inflammatory autoimmune diseases is emerging through the characterization of genetic models of impaired cholesterol efflux28,30; however, a causal relationship has not been reported in man.
Targeting cellular cholesterol metabolism (as opposed to circulating levels) may be of most importance in inflammatory diseases, such as RA. Administration of rHDL limits the autoimmune phenotype in mice lacking Abca1/Abcg1 in CD11c expressing cells30 and reduces atherogenesis in Apoe−/− mice.51 Similar to these findings, infusion of rHDL in the setting of inflammatory arthritis was also able to overcome the decrease in ABC-transporter expression and reverse the inflammation-induced changes in lesion development, independent of the progression of arthritis. However, more frequent dosing of apoA-I and rHDL has been shown to inhibit arthritis in rats,52 suggesting rHDL may also be useful adjunct therapy for acute arthritic flares. Importantly, rosuvastatin can induce carotid plaque regression in RA patients, independent of lipid lowering.53,54 This is likely due to the inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA) reductase, the molecular target of statins, that block cellular cholesterol synthesis and alter cellular metabolism, which in immune cells promotes anti-inflammatory effects.55
This study highlights that normal cholesterol levels do not necessarily equate to vascular protection. Based on our findings, we postulate that even if people with RA do not have hypercholesterolaemia, administration of a statin is worth consideration. Our findings in mouse and man suggest that sustained, RA-driven inflammation could be a causal determinant of lesion severity in atherosclerosis.17–23 We found evidence for enhanced myelopoiesis during inflammatory arthritis and also identified defects in cholesterol efflux pathways in HSPCs that were retained in mature myeloid cells. These features developed in spite of normal cholesterol levels. Thus, restoring cholesterol efflux could be a potential avenue to promote atherosclerotic lesion regression in RA. Importantly, inhibiting one cytokine alone in RA may not be sufficient to protect against atherosclerosis, as multiple cytokines can play a role. To date, simply enhancing cholesterol efflux potential by raising HDL in patients with CVD has not yielded any meaningful success with respect to lowering CV events.56,57 Thus, further understanding the mechanisms causing the suppression of cholesterol efflux genes in HSPCs and mature myeloid cells could reveal novel targets to promote lesion regression in RA, other autoimmune disorders associated with CVD, and in the general population.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
M.J.K. is a Russell Berrie Foundation Scholar in Diabetes Research from the Naomi Berrie Diabetes Centre, Columbia University, New York; NIH (R01HL1379 & R00HL1225 to P.R.N.); M.A.F. is a Senior Principal Research Fellow of the NHMRC (APP1116936); (1K23AR068450–01A1 to B.Y.H.) and the University of Kentucky Center of Research in Obesity and Cardiovascular Disease COBRE P20 (GM103527-06 to B.Y.H.); Reid Charitable Trusts, the National Health and Medical Research Council (NHMRC) Australia Clinical Practitioner Fellowship (1023407 to I.P.W.) and an NHMRC Program Grant (1016647 to I.P.W.); A.J.M. is Career Development Fellow of the NHMRC (APP1085752) and a Future Leader Fellowship from the National Heart Foundation (100440) and a recipient of a CSL Centenary Award; NHMRC project grants (APP1106154 and APP1142938 to G.I.L., J.C.-D., and A.J.M.).
Conflict of interest: none declared.
Supplementary Material
References
- 1. Gonzalez-Juanatey C, Llorca J, Testa A, Revuelta J, Garcia-Porrua C, Gonzalez-Gay MA.. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine 2003;82:407–413. [DOI] [PubMed] [Google Scholar]
- 2. McInnes IB, Schett G.. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–2219. [DOI] [PubMed] [Google Scholar]
- 3. Meune C, Touze E, Trinquart L, Allanore Y.. High risk of clinical cardiovascular events in rheumatoid arthritis: levels of associations of myocardial infarction and stroke through a systematic review and meta-analysis. Arch Cardiovasc Dis 2010;103:253–261. [DOI] [PubMed] [Google Scholar]
- 4. Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE.. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol 2012;110:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJL, Kvien TK, Dougados M, Radner H, Atzeni F, Primdahl J, Södergren A, Wallberg Jonsson S, van Rompay J, Zabalan C, Pedersen TR, Jacobsson L, de Vlam K, Gonzalez-Gay MA, Semb AG, Kitas GD, Smulders YM, Szekanecz Z, Sattar N, Symmons DPM, Nurmohamed MT.. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- 6. Arts EE, Popa C, Den Broeder AA, Semb AG, Toms T, Kitas GD, van Riel PL, Fransen J.. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis 2015;74:668–674. [DOI] [PubMed] [Google Scholar]
- 7. Kraakman MJ, Dragoljevic D, Kammoun HL, Murphy AJ.. Is the risk of cardiovascular disease altered with anti-inflammatory therapies? Insights from rheumatoid arthritis. Clin Transl Immunol 2016;5:e84.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, Pincus T, Raggi P, Gebretsadik T, Shintani A, Stein CM.. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum 2009;61:1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skeoch S, Bruce IN.. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat Rev Rheumatol 2015;11:390–400. [DOI] [PubMed] [Google Scholar]
- 10. Van Doornum S, McColl G, Wicks IP.. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum 2002;46:862–873. [DOI] [PubMed] [Google Scholar]
- 11. Faccini A, Kaski JC, Camici PG.. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J 2016;37:1799–1806. [DOI] [PubMed] [Google Scholar]
- 12. Coulthard LR, Geiler J, Mathews RJ, Church LD, Dickie LJ, Cooper DL, Wong C, Savic S, Bryer D, Buch MH, Emery P, Morgan AW, McDermott MF.. Differential effects of infliximab on absolute circulating blood leucocyte counts of innate immune cells in early and late rheumatoid arthritis patients. Clin Exp Immunol 2012;170:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klimek E, Mikołajczyk T, Sulicka J, Kwaśny-Krochin B, Korkosz M, Osmenda G, Wizner B, Surdacki A, Guzik T, Grodzicki TK, Skalska A.. Blood monocyte subsets and selected cardiovascular risk markers in rheumatoid arthritis of short duration in relation to disease activity. Biomed Res Int 2014;2014:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dahlqvist SR, Nilsson TK, Norberg B.. Thrombocytosis in active rheumatoid arthritis. Relation to other parameters of inflammatory activity and confounding effect of automated cell counting. Clin Rheumatol 1988;7:335–341. [DOI] [PubMed] [Google Scholar]
- 15. Farr M, Scott DL, Constable TJ, Hawker RJ, Hawkins CF, Stuart J.. Thrombocytosis of active rheumatoid disease. Ann Rheum Dis 1983;42:545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy AJ, Tall AR.. Disordered haematopoiesis and athero-thrombosis. Eur Heart J 2016;37:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kraakman MJ, Lee MKS, Al-Sharea A, Dragoljevic D, Barrett TJ, Montenont E, Basu D, Heywood S, Kammoun HL, Flynn M, Whillas A, Hanssen NMJ, Febbraio MA, Westein E, Fisher EA, Chin-Dusting J, Cooper ME, Berger JS, Goldberg IJ, Nagareddy PR, Murphy AJ.. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Invest 2017;127:2133–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR.. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest 2011;121:4138–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy AJ, Bijl N, Yvan-Charvet L, Welch CB, Bhagwat N, Reheman A, Wang Y, Shaw JA, Levine RL, Ni H, Tall AR, Wang N.. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med 2013;19:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ.. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 2013;17:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ.. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ.. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 2007;117:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR.. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science (New York, NY) 2010;328:1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cook AD, Turner AL, Braine EL, Pobjoy J, Lenzo JC, Hamilton JA.. Regulation of systemic and local myeloid cell subpopulations by bone marrow cell-derived granulocyte-macrophage colony-stimulating factor in experimental inflammatory arthritis. Arthritis Rheum 2011;63:2340–2351. [DOI] [PubMed] [Google Scholar]
- 25. Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, Zimetti F, Adorni MP, Bernini F, Meroni PL.. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis 2014;73:609–615. [DOI] [PubMed] [Google Scholar]
- 26. Voloshyna I, Modayil S, Littlefield MJ, Belilos E, Belostocki K, Bonetti L, Rosenblum G, Carsons SE, Reiss AB.. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood, NJ) 2013;238:1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P.. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell 2003;12:805–816. [DOI] [PubMed] [Google Scholar]
- 28. Ito A, Hong C, Oka K, Salazar JV, Diehl C, Witztum JL, Diaz M, Castrillo A, Bensinger SJ, Chan L, Tontonoz P.. Cholesterol accumulation in CD11c+ immune cells is a causal and targetable factor in autoimmune disease. Immunity 2016;45:1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. N A-G, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A.. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 2009;31:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westerterp M, Gautier EL, Ganda A, Molusky MM, Wang W, Fotakis P, Wang N, Randolph GJ, D'Agati VD, Yvan-Charvet L, Tall AR.. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab 2017;25:1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR.. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest 2007;117:3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK.. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012;125:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L.. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 2012;11:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR.. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res 2013;112:1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murphy AJ, Dragoljevic D, Tall AR.. Cholesterol Efflux Pathways Regulate Myelopoiesis: a Potential Link to Altered Macrophage Function in Atherosclerosis. Front Immunol 2014;5:490.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murphy AJ, Tall AR.. Proliferating macrophages populate established atherosclerotic lesions. Circ Res 2014;114:236–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ.. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest 2011;121:2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K.. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med 2003;9:61–67. [DOI] [PubMed] [Google Scholar]
- 39. Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo J-L, Gorbatov R, Sukhova GK, Gerhardt LMS, Smyth D, Zavitz CCJ, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK.. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hilgendorf I, Swirski FK.. Making a difference: monocyte heterogeneity in cardiovascular disease. Curr Atheroscler Rep 2012;14:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winchester R, Giles JT, Nativ S, Downer K, Zhang H-Z, Bag-Ozbek A, Zartoshti A, Bokhari S, Bathon JM.. Association of elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheum (Hoboken, NJ) 2016;68:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H.. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood 2001;98:2720–2725. [DOI] [PubMed] [Google Scholar]
- 43. Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M, Iwahashi M, Ueno A, Ohmoto Y, Makino H.. CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum 2002;46:2578–2586. [DOI] [PubMed] [Google Scholar]
- 44. Cooper DL, Martin SG, Robinson JI, Mackie SL, Charles CJ, Nam J, Consortium Y, Isaacs JD, Emery P, Morgan AW.. FcyRIIIa expression on monocytes in rheumatoid arthritis: role in immune-complex stimulated TNF production and non-response to methotrexate therapy. PLoS One 2012;7:e28918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U.. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 2012;64:671–677. [DOI] [PubMed] [Google Scholar]
- 46. Yang J, Zhang L, Yu C, Yang X-F, Wang H.. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2014;2:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Urbanski K, Ludew D, Filip G, Filip M, Sagan A, Szczepaniak P, Grudzien G, Sadowski J, Jasiewicz-Honkisz B, Sliwa T, Kapelak B, McGinnigle E, Mikolajczyk T, Guzik TJ.. CD14+CD16++ “nonclassical” monocytes are associated with endothelial dysfunction in patients with coronary artery disease. Thromb Haemost 2017;117:971–980. [DOI] [PubMed] [Google Scholar]
- 48. Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 2007;81:584–592. [DOI] [PubMed] [Google Scholar]
- 49. Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, Taylor M, McMahon M, Paulus HE, Reddy ST.. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis 2012;71:1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ.. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 2014;19:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murphy AJ, Funt S, Gorman D, Tall AR, Wang N.. Pegylation of high-density lipoprotein decreases plasma clearance and enhances antiatherogenic activity. Circ Res 2013;113:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu BJ, Ong KL, Shrestha S, Chen K, Tabet F, Barter PJ, Rye KA.. Inhibition of arthritis in the Lewis rat by apolipoprotein A-I and reconstituted high-density lipoproteins. Arterioscler Thromb Vasc Biol 2014;34:543–551. [DOI] [PubMed] [Google Scholar]
- 53. Rollefstad S, Ikdahl E, Hisdal J, Olsen IC, Holme I, Hammer HB, Smerud KT, Kitas GD, Pedersen TR, Kvien TK, Semb AG.. Rosuvastatin-induced carotid plaque regression in patients with inflammatory joint diseases: the rosuvastatin in rheumatoid arthritis, ankylosing spondylitis and other inflammatory joint diseases study. Arthritis Rheum (Hoboken, NJ) 2015;67:1718–1728. [DOI] [PubMed] [Google Scholar]
- 54. Danninger K, Hoppe UC, Pieringer H.. Do statins reduce the cardiovascular risk in patients with rheumatoid arthritis? Int J Rheum Dis 2014;17:606–611. [DOI] [PubMed] [Google Scholar]
- 55. Schonbeck U, Libby P.. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 2004;109:II18–II26. [DOI] [PubMed] [Google Scholar]
- 56. Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE;. ACCELERATE Investigators. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 57. Tardif JC, Ballantyne CM, Barter P, Dasseux JL, Fayad ZA, Guertin MC, Kastelein JJ, Keyserling C, Klepp H, Koenig W, L'Allier PL, Lesperance J, Luscher TF, Paolini JF, Tawakol A, Waters DD; Can Hdl Infusions Significantly QUicken Atherosclerosis REgression (CHI-SQUARE) Investigators. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J 2014;35:3277–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.