Abstract
Background
Disruption of insulin-like growth factor-I (IGF-I) increases health and life span in animal models, though this is unconfirmed in humans. If IGF-I stability indicates homeostasis, the absolute level of IGF-I may be less clinically relevant than maintaining an IGF-I setpoint.
Methods
Participants were 945 U.S. community-dwelling individuals aged ≥65 years enrolled in the Cardiovascular Health Study with IGF-I levels at 3–6 timepoints. We examined the association of baseline IGF-I level, trajectory slope, and variability around the trajectory with mortality.
Results
There were 633 deaths over median 11.3 years of follow-up. Lower IGF-I levels, declining or increasing slope, and increasing variability were each individually associated with higher mortality (all p < .001). In an adjusted model including all three trajectory parameters, baseline IGF-I levels <70 ng/mL (hazard ratio [HR] 1.58, 95% CI 1.28–1.96 relative to IGF-I levels of 170 ng/mL), steep declines and steep increases in trajectory slope (HR 2.22, 1.30–3.80 for a 15% decline; HR 1.40, 1.07–1.84 for a 10% decline; HR 1.80, 1.12–2.89 for a 15% increase; HR 1.31, 1.00–1.72 for a 10% increase, each vs no change), and variability ≥10% (HR 1.59, 1.09–2.32 for ≥ 30%; HR 1.36, 1.06–1.75 for 20%; and HR 1.17, 1.03–1.32 for 10% variability, each vs 0%) in IGF-I levels were independently associated with mortality.
Conclusions
In contrast to data from animal models, low IGF-I levels are associated with higher mortality in older humans. Irrespective of the actual IGF-I level, older individuals with stability of IGF-I levels have lower mortality than those whose IGF-I levels fluctuate over time.
Keywords: Aging, Insulin like growth factor, Longevity, Trajectory, Longitudinal
Both researchers and the public are interested in clues as to what determines longevity. Life-span and healthspan extension are observed in invertebrate and vertebrate models in which insulin-like growth factor-I (IGF-I) pathways are disrupted, including the nematode Caenorhabditis elegans, fruit fly Drosophila, and several strains of mice (1). Caloric restriction also leads to greater life span in rodent models, and a key change observed with caloric restriction is a decrease in IGF-I levels (2). These studies in model systems imply that altering IGF signaling throughout the life span, typically by decreasing signaling, may promote longevity. In humans, it has been observed that community-dwelling older adults have lower IGF-I levels compared to younger persons (3–8), leading to the similar hypothesis that higher IGF-I levels in older age may be associated with increased mortality.
Previous studies have focused on the association between longevity and absolute IGF-I level, typically either lowered throughout the lifespan (as in experimental studies of model systems), or with cross sectional measurements of IGF-I (as in most observational studies of humans). Nonetheless, to fully understand any factor which changes throughout the life span, one must model not only its absolute level but the way it changes over time, that is, its trajectory. Beyond the absolute IGF-I level, the way that an IGF-I level changes over time may provide more insight about how an individual ages by illustrating homeostatic control or stress response. Previous studies have not simultaneously modeled absolute IGF-I level and IGF-I trajectory and therefore may have poorly represented IGF-I biology, obscuring the relevance of this factor to human health and life span. We alternatively hypothesized that, irrespective of the actual level of IGF-I, older individuals with stability in IGF-I levels over time would have better survival than those with significant deviations, such as a steep decline or extreme variability. We analyzed trajectories of IGF-I to examine the biology of aging at the population level and more completely understand the potential role of IGF-I in human longevity.
Methods
Study Population
These analyses are based on data from the Cardiovascular Health Study (CHS) (9). The CHS is a population-based, longitudinal study of risk factors for the development of cardiovascular disease in 5,888 adults aged 65 years and older. Enrollment of an original cohort of 5,201 adults occurred between May 1989 and June 1990, and an additional cohort of 687 African Americans, was enrolled in 1992–1993. Eligible individuals were identified from an age- and gender-stratified random sample of the Medicare eligibility rosters in four U.S. communities: Washington County, Maryland; Pittsburgh (Allegheny County), Pennsylvania; Sacramento County, California; and Forsyth County, North Carolina. To be eligible, individuals had to be noninstitutionalized; expecting to remain in the area for the following 3 years; not under active treatment for cancer; not wheelchair-bound in the home; and not requiring a proxy respondent at entry. Household members of the sampled individual were recruited, if eligible. The institutional review boards of all four sites and the coordinating center at the University of Washington in Seattle approved the study. All participants gave informed consent.
At the initial visit, a detailed medical history, physical examination, and health status assessment were performed. Blood was drawn after a 12-hour fast and serum was frozen in −70°C freezers for future investigations. Participants in the original cohort subsequently returned annually for nine additional interviews and examinations. African American participants underwent a comparable baseline examination and returned annually for six additional interviews and examinations. Fasting plasma specimens were collected at the 1989–1990, 1992–1993, 1993–1994, 1994–1995, 1996–1997, and 1997–1998 visits.
After exclusion of individuals taking corticosteroid preparations, estrogens, dehydroepiandrosterone, or growth hormone (GH) at any phlebotomy timepoint, a random sample of 1,250 participants (500 men and 500 women from the original cohort and 125 men and 125 women from the new cohort) was selected for measurement of IGF-I levels. Sampling was otherwise performed without knowledge of baseline health status, blood availability, or subsequent survival.
IGF-I Measurement
IGF-I assays were performed at the Cancer Prevention Research Unit of the Lady Davis Research Institute of Jewish General Hospital and McGill University, Montreal, Quebec in 2008. Plasma total IGF-I levels were measured in duplicate from frozen specimens using enzyme-linked immune sorbent assay methods (Diagnostics Systems Laboratory of Beckman Coulter, Webster, TX) as previously described (10). All kits and reagents were purchased from the same lot to reduce interassay variability. All samples from a given participant were retrieved from storage and assayed in the same batch. The assay has a detection range of 11–637 ng/mL (11–637 μg/L). Within-batch and between-batch coefficients of variation were determined to be 2.9% and 4.3%. Previous data show low within-subject variability in IGF-I levels in healthy subjects over years, supporting its use as an indicator of long-term IGF-I status (11).
Ascertainment of Events
Deaths were ascertained through participant surveillance that has occurred every 6 months from study inception. Confirmation of deaths was conducted through reviews of obituaries, medical records, death certificates, and the Health Care Financing Administration’s health care utilization database for hospitalizations. Contacts and proxies were also interviewed for participants unavailable for follow-up. Ascertainment of mortality in the CHS is 100%. The incident events in this report occurred through June 30, 2009.
Assessment of Covariates
Age, sex, and race were self-reported. Five assessed comorbid conditions were included as covariates: cardiovascular disease, defined as a history of coronary heart disease, claudication, congestive heart failure, stroke, or transient ischemic attack; lung disease, defined by self-report of physician diagnosis of emphysema, chronic bronchitis, or asthma; diabetes, defined by a fasting serum glucose of at least 126 mg/dL or the use of insulin or oral hypoglycemic medications; cancer in the past 5 years, defined by self-report of physician diagnosis; and depression, defined as a Center for Epidemiological Studies Depression scale (CES-D) score of ≥8 on a modified 10 item scale (12). These comorbid conditions were chosen because they are common, strongly age-associated, and strongly associated with mortality.
Statistical Analysis
Because a minimum of three measurements are required to define a trajectory, we examined data from the 950 men and women who had plasma available at 3–6 timepoints over the first 8 years of CHS for analysis. Five of these participants were subsequently excluded: a man with IGF-I levels consistently >700 ng/dL, suggestive of acromegaly; a man whose IGF-I was below the assay detection limit of 11 ng/dL; and three participants with extreme outlying values (a man whose IGF-I levels declined 34% per year on average, a woman with high variability in IGF-I [coefficient of variation 55%], and a woman with both steep decline [38% per year] and high variability [coefficient of variation 53%]). The analysis included the remaining 945 participants.
To assess the potential for selection bias, we compared the 945 individuals included in the current analysis to (1) the overall CHS cohort (n = 5,888) and (2) the men and women in our random sample with only one or two IGF-I measurements (n = 300). Compared to the full CHS cohort, our analytic sample was of slightly lower age but had similar baseline comorbid status. Consistent with the requirement of surviving long enough to have at least three blood measures collected, the mortality rate was lower in our analytic sample than in those with only one or two IGF-I levels obtained, and lower than the full CHS cohort.
To allow interpretation on a relative scale, IGF-I levels were log-transformed. We subsequently examined the distributions of baseline lnIGF-I level, trajectory slope, and variability around the trajectory slope. The slope on the log-scale can be interpreted as the annual relative change in the original, nontransformed scale. The variability around the trajectory was calculated by determining the standard deviation of the error in a model with a linear trajectory, which is equivalent to the coefficient of variation (CV) in the original scale.
Cox proportional hazard models were performed to estimate the relative hazard of death. Slope and CV were modeled as time-varying covariates; at each IGF-I measure, slope and CV were recalculated using all measures through that date. Robust standard errors and 95% confidence intervals were estimated to account for repeated observations per participant. Because three IGF-I measures were required for inclusion, participants entered the analysis on the date of the third IGF-I measurement. Median time at risk for analysis was 11.3 years. The proportional hazards assumption was confirmed using a test based on Schoenfeld residuals. There was no evidence of confounding by the number of IGF-I timepoints. Relative hazards of death by sex-specific decile of baseline ln(IGF-I) level, slope, and CV were estimated and visual inspection performed to examine for potential nonlinear effects. Both baseline ln(IGF-I) and slope were found to have nonlinear associations with mortality. Quadratic terms for each were significant (Wald test p < .05) and retained in the final models along with the linear terms. The relation of CV to mortality was linear, and CV was modeled as a linear term in all models. The overall significance of each set of IGF-I terms (linear and quadratic for baseline IGF-I and slope, linear for CV) was assessed using the Wald test. Interactions of baseline IGF-I, slope, and CV with covariates were assessed using the Wald test; no notable interactions were found. Final models included both sexes. All models were adjusted for age, race, and sex and additionally adjusted for five comorbid conditions (cardiovascular disease, pulmonary disease, diabetes, cancer, and depression). To provide clinically meaningful interpretations, hazard ratios were evaluated at selected IGF-I levels and specific values. Estimates at specific values of each parameter are displayed in Table 2. The specific baseline IGF-I values were selected to represent points throughout the reference range. The values of slope and variability were selected to represent the distribution of values for these parameters. Scatter plots were created by sex for each IGF-I point versus the age- and race-adjusted predicted hazard ratio, centering the covariates at their means. Mortality rates were displayed based on groups defined by variability and slope, again using specific cutpoints. Minimal variability was defined as a CV < 10%, moderate variability as a CV of 10%–20% (inclusive), and severe variability as a CV > 20%. Steep decline was defined as an annual decline of −5% or steeper, no steep decline was defined as an annual rate of −5 to +5%, and steep increase was defined as an annual increase of +5% or steeper.
Table 2.
Mortality Risk by IGF-I Trajectory Parameter
| Model | Measure | Value | HR (95% CI)a |
|---|---|---|---|
| Baseline only | Baseline IGF-I (ng/mL) | 70 | 1.72 (1.43, 2.08) |
| 120 | 1.09 (1.02, 1.17) | ||
| 170 | 1 (reference) | ||
| 220 | 1.04 (0.96, 1.12) | ||
| 270 | 1.14 (0.97, 1.34) | ||
| Slope only | Average annual change | 15% decline | 2.51 (1.50, 4.21) |
| 10% decline | 1.47 (1.13, 1.92) | ||
| 5% decline | 1.10 (0.99, 1.22) | ||
| No change | 1 (reference) | ||
| 5% increase | 1.08 (0.97, 1.21) | ||
| 10% increase | 1.37 (1.05, 1.77) | ||
| 15% increase | 1.96 (1.24, 3.10) | ||
| Variability only | CV | 0% | 1 (reference) |
| 10% | 1.21 (1.07, 1.36) | ||
| 20% | 1.46 (1.15, 1.86) | ||
| 30% | 1.77 (1.23, 2.54) | ||
| Baseline, slope, and variability | Baseline IGF-I (ng/mL) | 70 | 1.58 (1.28, 1.96) |
| 120 | 1.07 (0.99, 1.15) | ||
| 170 | 1 (reference) | ||
| 220 | 1.05 (0.97, 1.14) | ||
| 270 | 1.16 (0.98, 1.38) | ||
| Average annual change | 15% decline | 2.22 (1.30, 3.80) | |
| 10% decline | 1.40 (1.07, 1.84) | ||
| 5% decline | 1.08 (0.97, 1.21) | ||
| No change | 1(reference) | ||
| 5% increase | 1.07 (0.96, 1.20) | ||
| 10% increase | 1.31 (1.003, 1.72) | ||
| 15% increase | 1.80 (1.12, 2.89) | ||
| CV | 0% | 1 (reference) | |
| 10% | 1.17 (1.03, 1.32) | ||
| 20% | 1.36 (1.06, 1.75) | ||
| 30% | 1.59 (1.09, 2.32) |
Note: CI = Confidence interval; CV = Coefficient of variation; HR = Hazard ratio. The specific baseline IGF-1 values were selected to represent points throughout the reference range. The values of slope and variability were selected to represent the distribution of values for these parameters.
aAdjusted for sex, age, race, and the following comorbid conditions at baseline: current lung disease, history of cardiovascular disease, diabetes, cancer in the past 5 years, depression.
Results
At study entry, the mean age of men was 72.5 years and of women was 73.1 years (Table 1). The study sample was 24% African American and had a distribution of comorbidity that was similar to community-dwelling older adults (3–8). As expected, men had higher IGF-I levels, on average, than did women. The death rate was 65 per 1,000 person-years, (633 deaths/945 participants).
Table 1.
Baseline Characteristics of Participants
| Characteristics | Men (N = 463) | Women (N = 482) |
|---|---|---|
| Age, years, mean (SD) | 72.5 (5.1) | 73.1 (5.6) |
| African American, number (%) | 103 (22) | 121 (25) |
| Cardiovascular disease, number (%) | 142 (31) | 98 (20) |
| Lung disease, number (%) | 34 (7) | 45 (9) |
| Diabetes, number (%) | 88 (19) | 71 (15) |
| Cancer, number (%) | 32 (7) | 20 (4) |
| Depression, number (%) | 64 (14) | 105 (22) |
| IGF-I (ng/mL), mean (SD) | 183 (59) | 168 (55) |
Overall, there was a slight decrease in IGF-I levels over time, at −0.96 ng/mL/y.
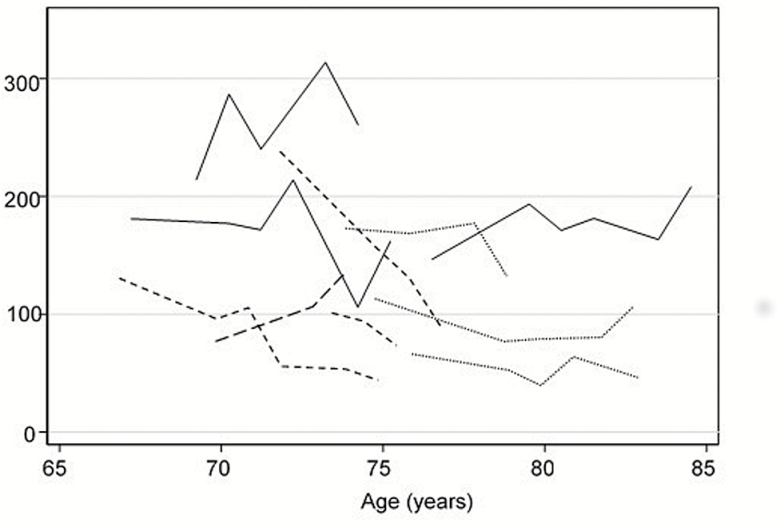
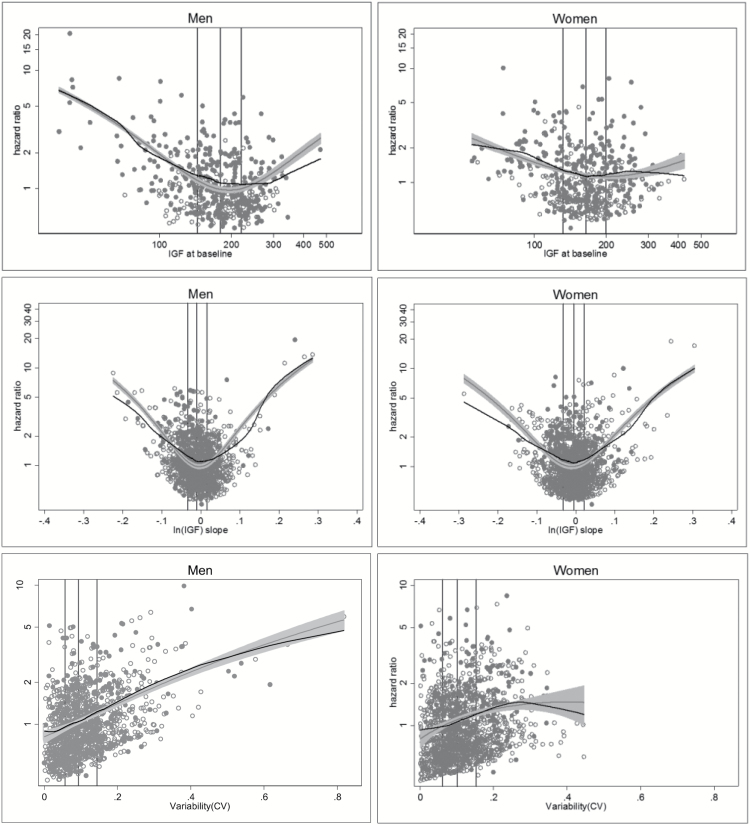
However, there was significant heterogeneity in the pattern of IGF-I levels over time, as shown by a subsample of trajectory plots in Figure 1. We examined three different aspects of the trajectory in all subsequent analyses: the baseline IGF-I value, the trajectory slope, and the trajectory variability. Inspection of plots of mortality revealed nonlinear associations of baseline IGF-I level and the slope of IGF-I levels with mortality, with higher mortality at extremes of IGF-I values and with increasing and decreasing IGF-I levels (Figure 2A and B). Increasing variability of IGF-I values was linearly associated with mortality (Figure 2C).
Figure 1.
Representative trajectory plots of IGF-I level by age. Each line represents a different participant from the study.
Figure 2.
Mortality risk by sex for each IGF-I trajectory parameter. (A) IGF-I at baseline, (B) Slope, (C) Variability. For plot (A), the x-axis is labeled as IGF-I, though plotted values are for ln(IGF-I). Models were adjusted for age and race. The black line represents a lowess smoothed line and the gray line represents the fitted model with 95% confidence intervals. Vertical lines represent the 25th, 50th, and 75th percentiles (from left to right) of the IGF-I values. Open circles are participants who were alive and closed circles are participants who died during follow-up.
When examined individually as continuous measures, IGF-I levels, the slope of IGF-I levels, and variability were each associated with higher mortality in models both unadjusted and adjusted for demographics and comorbidity (all p ≤ .002), with estimates at specific values of each parameter displayed in Table 2. In adjusted models including all three trajectory parameters (baseline IGF-I, slope, and variability), only very low baseline IGF-I levels (<70 ng/mL vs 170 ng/mL) were associated with mortality (hazard ratio [HR] 1.58, 95% CI 1.28–1.96) (Table 2). Only 1.8% of the cohort had a baseline IGF-I level <70 ng/mL, and higher baseline levels of IGF-I were not associated with mortality. Although the data suggested higher mortality at higher IGF-I levels, this was not statistically significant. A steep decline in IGF-I (HR 2.22, 95% CI 1.30–3.80 for a 15% decline; HR 1.40, 95% CI 1.04–1.84 for a 10% decline) or a steep increase (HR 1.80, 95% CI 1.12–2.89 for a 15% increase; HR 1.31, 95% CI 1.003–1.72 for a 10% increase) independently predicted mortality, compared to no change in slope (Table 2). There was a 17% increase in mortality for each 10% higher CV. None of these associations differed in men versus women (interaction p > .05).
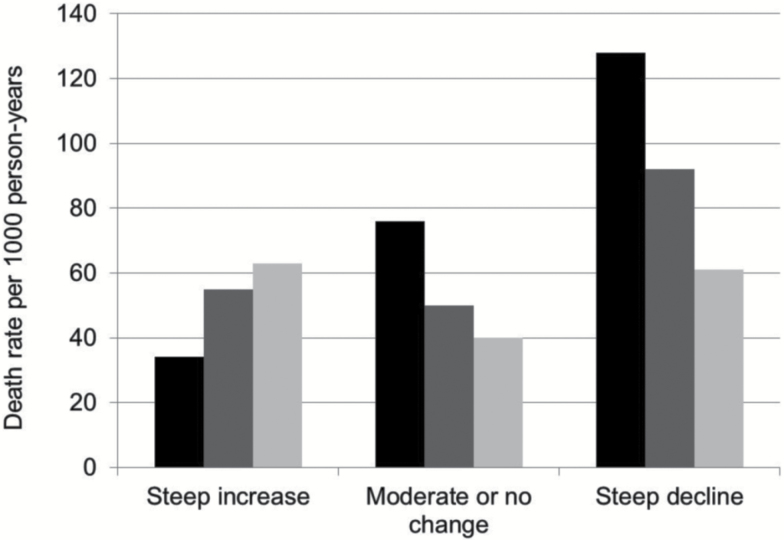
When the joint effects of slope and variability were examined, individuals with both a steep decline and severe variability had the highest mortality (128 deaths per 1,000 person-years; Figure 3). In those with steep decline, moderate decline, or no change in slope, increasing variability was associated with increasing mortality. This was not found in the group with increasing slope, in whom greater variability had no additional impact on mortality.
Figure 3.
Death rate by slope and coefficient of variation (CV). Minimal variability was defined as a CV < 10%, moderate variability as a CV = 10%–20%, and severe variability as a CV > 20%. Steep decline was defined as an annual decline of −5% or steeper, no steep decline was defined as an annual rate of −5 to +5 %, and steep increase was defined as an annual increase of +5% or steeper.
Discussion
In contrast to findings from animal models of longevity which show extended life span due to disruption of the IGF-I pathway, our data suggest that older humans with lower IGF-I levels have higher mortality. Furthermore, the trajectory of IGF-I level is a stronger predictor of mortality than baseline IGF-I level and predicts mortality independent of the baseline level, age, sex, race, and five common age-associated comorbidities. The trajectory slope and variance also appear to act together in individuals with a more severe decline in IGF-I, in whom greater variability signaled higher mortality.
The study of dynamics in complex systems like the human body demands incorporation of variability, as we have done here. This has been shown mathematically and experimentally (13). Complexity is a fundamental factor in how these systems effectively operate basally and in response to stress. At a basal level, a setpoint may appear stable on the surface while the underlying complex system adjusts many components on small timescales. As compared to a simple system, when a complex system is perturbed, the complex system has the ability to finely alter many components rather than a few, resulting in a response that is smaller in magnitude and less variable from moment to moment. The human body’s complexity degrades with age. As complexity degrades, the system loses its ability to respond smoothly, leading to greater heterogeneity of basal and stimulated responses characterized by differences in both the mean and standard deviation compared to younger populations (13).
Geroscience has increasingly focused on homeostatic control as a marker and/or target for longevity, though data from humans over long time scales is lacking. These empiric analyses represent a new paradigm in studying human aging at the population level and IGF-I in particular. This is somewhat analogous to the frequent sampling paradigms in which blood is collected every few minutes, hours, days, weeks, or months to assess fluctuations, such as with GH, leptin, and luteinizing hormone (14–18), though with a different time scale amenable to studying aging. Although we did not focus on measuring IGF-I at specific time points during the day, or at rigidly defined time intervals, we do show that as long as the IGF-I levels are not very low (and only 1.8% of the population had a baseline IGF-I level <70 ng/mL), the absolute level in older age may be clinically unimportant. Instead, how well an individual maintains their given IGF-I level might provide more clinically relevant information. This emphasizes the value of homeostasis throughout the life span, which should be envisioned not only as the maintenance of a setpoint, but the controlled variability around that setpoint as it undergoes a natural change with age. Using data on IGF-I as an example, we argue that geroscience must incorporate absolute level, slope, and variability of biomarkers to fully appreciate their role in longevity.
IGF-I is closely linked to GH in the GH/IGF-I axis whereby pituitary-released GH stimulates hepatic secretion of IGF-I, which suppresses GH in a negative feedback loop. The population-level decline in mean IGF-I level with age may in part reflect changes in the GH/IGF-I axis, including an age-related decline in GH. Because the symptoms of GH deficiency overlap with the phenotype of aging that includes decreased muscle mass, increased fat mass, decreased bone mineral density, and decreased well-being, there has been speculation that lower levels of IGF-I in older people, whether due to declining GH or not, may actually contribute to age-related morbidity and mortality rather than indicate or promote longevity. Moreover, this population-level decline in IGF-I level and the potential physiologic consequences of relative GH deficiency have led to numerous clinical trials exploring the benefit of GH, IGF-I, and related secretagogue administration as an “anti-aging” therapy (19). However, these trials have not consistently shown benefit, and GH supplementation is not approved for age-associated IGF-I decline (19–22), despite large sums spent for off-label use of GH as an antiaging drug (20).
Observationally, lower baseline IGF-I was associated with higher mortality in the Framingham Heart Study (23), though this was not seen in the Cardiovascular Health Study (24), Women’s Health and Aging Study (25), National Health and Nutrition Examination Survey (26), Rancho Bernardo Study (27), or Seven Countries Study (28). In the CHS All Stars study, Kaplan and colleagues reported that baseline IGF-I and change in IGF-I levels measured 9 years apart were not significantly associated with subsequent mortality (29). It is possible that observational studies and trials altering the GH/IGF-I pathway failed to demonstrate clinical benefit or significant associations with mortality because they were conducted under the assumption that the hormonal setpoint (baseline value) was the most important factor contributing to adverse effects seen with aging. We show that tight regulation of IGF-I, regardless of the level, is more important. If experimental alteration of the GH/IGF-I pathway are attempted in humans, either through direct replacement or inhibition or through more general means (eg, dietary restriction), it may be prudent to target tighter control of slope and variability rather than specific hormonal levels, though we cannot advocate for these practices therapeutically at this time.
IGF binding proteins (IGFBPs) also regulate the availability of biologically-active IGF-I (30). Thus, binding proteins may be a significant homeostatic control which explain IGF-I associations with health outcomes. In CHS, Kaplan and colleagues found IGFBPs were differentially associated with a composite outcome of first incident myocardial infarction, stroke, heart failure, hip fracture, or death independent of IGF-I (31). Also in CHS, Sanders and colleagues showed that a change in IGF-I or IGFBP-3 measured at two times 9 years apart were significantly associated with concurrent change in the Modified Mini-Mental Status Examination, though not with concurrent change in other functional outcomes (32). Trajectories of IGFBPs are unknown. Joint modeling of trajectories of both IGF-I and IGFBPs would help untangle their possible independent associations with aging outcomes.
Very recently, facets of network medicine are also being applied in this area. For example, genome-wide studies of IGF-I and IGFBPs found new associations with single nucleotide polymorphisms which may influence their respective concentrations, as well as associations with other longevity pathways, such as the FOXO pathway (33). In the Health, Aging, and Body Composition study, a targeted metabolomic profiling paired with graph theory analysis found new associations between metabolites and lean mass and adiposity in older black men (34). Twenty-five metabolites clustered into a single network, an example of how network medicine may find particularly rich biologic nodes to investigate. If network data is paired with measurement of trajectories and novel longevity phenotypes, it will be possible clarify the pathways by which IGF may mediate aging.
A major strength of our study is the use of a large, multicenter, population-based cohort comprised of older men and women who had serial examinations and phlebotomy. Follow-up was long, with up to 17 years of data, and the cohort had 100% outcome ascertainment. We measured IGF-I using a validated approach and consistent laboratory materials and excluded participants using medications that would likely result in unreliable physiologic measurements of IGF-I. The magnitude of annual decline in IGF-I level found here is similar to that found in cross-sectional studies (3–8), suggesting generalizability to similarly aged populations. Additionally, we engaged in several model checking strategies to strengthen confidence in the data’s robustness. However, our results may not be generalizable to younger populations, as we had no data on individuals who are less than 65 years of age. Also, because we used observational data, we could not determine whether the hormonal trajectory was a sensitive marker of physiologic dysregulation, or whether alterations in the dynamics of IGF-I mediated physiologic effects. The hazard ratios presented at selected IGF-I levels are estimates based on our data and should not be used clinically to form therapeutic recommendations. There is data that specifically patterned intermittent or prolonged fasting in humans, as well as changes in dietary amino acids, can alter IGF-I levels directly or through changes in weight (35). It is unlikely that weight fluctuations in community-dwelling older adults in our study mirrored the rigid and more extreme fasting strategies in dietary restriction studies that have been associated with changes in IGF-I. Adjustment for weight and body mass index has not substantially altered associations between IGF-I and mortality in prior community-based observational studies. Nonetheless, modeling co-existing changes may provide more insight on this potential confounding.
In summary, we found that in community-dwelling older adults, there was not an optimal IGF-I level, and that only very low IGF-I levels found in a small portion of the population were associated with higher mortality. Furthermore, steeper IGF-I trajectory slope and greater variability in trajectory were even more strongly associated with mortality than the actual IGF-I level, supporting the importance of hormonal stability. It remains unknown whether IGF-I is a marker or in the causal pathway of aging in humans, and whether interventions could leverage the IGF-I pathway to promote human longevity. Answering this question requires further observational and experimental human studies measuring how changes in the IGF-I pathway track with changes in age-related phenotypes over the human life span.
Funding
The research reported in this article was supported by R01-027058, R01-023629, and R01-031890-01 from the National Institute on Aging (NIA) and NHLBI contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grant HL080295, with additional contribution from NINDS. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. See also http://www.chs-nhlbi.org/pi.htm. J.L.S. was supported by a National Research Service Award 1F30-AG038093-01 from the NIA. Role of sponsor(s): The investigators retained full independence in the conduct of this research.
Conflict of Interest
None reported.
Acknowledgments
Author contributions: conception and design (A.R.C.); acquisition of data (M.N.P., A.B.N., L.P.F., A.R.C.); analysis and interpretation of data (J.L.S., W.G., E.S.O., R.C.K., M.N.P., T.M.B., A.B.N., L.P.F., A.R.C.); drafting of the manuscript (J.L.S., A.R.C.); critical revision of the manuscript (J.L.S., W.G., E.S.O., R.C.K., M.N.P., T.M.B., A.B.N., L.P.F., A.R.C.); statistical analysis (W.G., E.S.O., T.M.B.); obtaining funding (A.B.N., L.P.F., A.R.C.); administrative, technical, or materials support (A.R.C.); supervision (A.R.C.).
Author access to data: All authors had access to data at all times.
References
- 1. Sonntag WE, Csiszar A, deCabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598. doi:10.1093/gerona/gls115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326. doi:10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landin-Wilhelmsen K, Wilhelmsen L, Lappas G et al. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf). 1994;41:351–357. [DOI] [PubMed] [Google Scholar]
- 4. Papadakis MA, Grady D, Tierney MJ, Black D, Wells L, Grunfeld C. Insulin-like growth factor 1 and functional status in healthy older men. J Am Geriatr Soc. 1995;43:1350–1355. [DOI] [PubMed] [Google Scholar]
- 5. Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol. 1997;145:970–976. [DOI] [PubMed] [Google Scholar]
- 6. Harris TB, Kiel D, Roubenoff R et al. Association of insulin-like growth factor-I with body composition, weight history, and past health behaviors in the very old: the Framingham Heart Study. J Am Geriatr Soc. 1997;45:133–139. [DOI] [PubMed] [Google Scholar]
- 7. O’Connor KG, Tobin JD, Harman SM et al. Serum levels of insulin-like growth factor-I are related to age and not to body composition in healthy women and men. J Gerontol A Biol Sci Med Sci. 1998;53:M176–M182. [DOI] [PubMed] [Google Scholar]
- 8. Boonen S, Lysens R, Verbeke G et al. Relationship between age-associated endocrine deficiencies and muscle function in elderly women: a cross-sectional study. Age Ageing. 1998;27:449–454. [DOI] [PubMed] [Google Scholar]
- 9. Fried LP, Borhani NO, Enright P et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 10. Kaplan RC, McGinn AP, Pollak MN et al. Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. J Clin Endocrinol Metab. 2007;92:1319–1325. doi:10.1210/jc.2006-1631 [DOI] [PubMed] [Google Scholar]
- 11. Borofsky ND, Vogelman JH, Krajcik RA, Orentreich N. Utility of insulin-like growth factor-1 as a biomarker in epidemiologic studies. Clin Chem. 2002;48:2248–2251. [PubMed] [Google Scholar]
- 12. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 13. Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:B115–B125. [DOI] [PubMed] [Google Scholar]
- 14. Chan JL, Williams CJ, Raciti P et al. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-I in leptin deficiency states. J Clin Endocrinol Metab. 2008;93:2819–2827. doi:10.1210/jc.2008-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ionescu M, Frohman LA. Pulsatile secretion of growth hormone (GH) persists during continuous stimulation by CJC-1295, a long-acting GH-releasing hormone analog. J Clin Endocrinol Metab. 2006;91:4792–4797. doi:10.1210/jc.2006-1702 [DOI] [PubMed] [Google Scholar]
- 16. Kasa-Vubu JZ, Ye W, Borer KT, Rosenthal A, Meckmongkol T. Twenty-four hour growth hormone and leptin secretion in active postpubertal adolescent girls: impact of fitness, fatness, and age at menarche. J Clin Endocrinol Metab. 2006;91:3935–3940. doi:10.1210/jc.2005-2841 [DOI] [PubMed] [Google Scholar]
- 17. Loucks AB. The response of luteinizing hormone pulsatility to 5 days of low energy availability disappears by 14 years of gynecological age. J Clin Endocrinol Metab. 2006;91:3158–3164. doi:10.1210/jc.2006-0570 [DOI] [PubMed] [Google Scholar]
- 18. Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab. 2011;96:150–158. doi:10.1210/jc.2010-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64:1039–1044. doi:10.1093/gerona/glp091 [DOI] [PubMed] [Google Scholar]
- 20. Perls TT, Reisman NR, Olshansky SJ. Provision or distribution of growth hormone for “antiaging”: clinical and legal issues. JAMA. 2005;294:2086–2090. doi:10.1001/jama.294.16.2086 [DOI] [PubMed] [Google Scholar]
- 21. Blackman MR. Use of growth hormone secretagogues to prevent or treat the effects of aging: not yet ready for prime time. Ann Intern Med. 2008;149:677–679. [DOI] [PubMed] [Google Scholar]
- 22. Liu H, Bravata DM, Olkin I et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–115. [DOI] [PubMed] [Google Scholar]
- 23. Roubenoff R, Parise H, Payette HA et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. [DOI] [PubMed] [Google Scholar]
- 24. Kaplan RC, McGinn AP, Pollak MN et al. Total insulinlike growth factor 1 and insulinlike growth factor binding protein levels, functional status, and mortality in older adults. J Am Geriatr Soc. 2008;56:652–660. doi:10.1111/j.1532-5415.2007.01637.x [DOI] [PubMed] [Google Scholar]
- 25. Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–2025. doi:10.1210/jc.2002-021694 [DOI] [PubMed] [Google Scholar]
- 26. Saydah S, Graubard B, Ballard-Barbash R, Berrigan D. Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol. 2007;166:518–526. doi:10.1093/aje/kwm124 [DOI] [PubMed] [Google Scholar]
- 27. Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–120. doi:10.1210/jc.2003-030967 [DOI] [PubMed] [Google Scholar]
- 28. Harrela M, Qiao Q, Koistinen R et al. High serum insulin-like growth factor binding protein-1 is associated with increased cardiovascular mortality in elderly men. Horm Metab Res. 2002;34:144–149. doi:10.1055/s-2002-23198 [DOI] [PubMed] [Google Scholar]
- 29. Kaplan RC, Bùzková P, Cappola AR et al. Decline in circulating insulin-like growth factors and mortality in older adults: cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97:1970–1976. doi:10.1210/jc.2011-2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimasaki S, Shimonaka M, Zhang HP, Ling N. Identification of five different insulin-like growth factor binding proteins (IGFBPs) from adult rat serum and molecular cloning of a novel IGFBP-5 in rat and human. J Biol Chem. 1991;266:10646–10653. [PubMed] [Google Scholar]
- 31. Kaplan RC, Strizich G, Aneke-Nash C et al. Insulinlike growth factor binding protein-1 and ghrelin predict health outcomes among older adults: CHS cohort. J Clin Endocrinol Metab. 2016:jc20162779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanders JL, Ding V, Arnold AM et al. Do changes in circulating biomarkers track with each other and with functional changes in older adults?J Gerontol A Biol Sci Med Sci. 2014;69:174–181. doi:10.1093/gerona/glt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teumer A, Qi Q, Nethander M et al. ; CHARGE Longevity Working Group; Body Composition Genetics Consortium. Genomewide meta-analysis identifies loci associated with IGF-I and IGFBP-3 levels with impact on age-related traits. Aging Cell. 2016;15:811–824. doi:10.1111/acel.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy RA, Moore SC, Playdon M et al. Metabolites associated with lean mass and adiposity in older black men. J Gerontol A Biol Sci Med Sci. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Longo VD, Antebi A, Bartke A et al. Interventions to slow aging in humans: Are we ready?Aging Cell. 2015;14:497–510. doi:10.1111/acel.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]