Abstract
Background
Atrophy and fatty infiltration of muscle with aging are associated with fractures and falls, however, their direct associations with muscle function are not well described. It was hypothesized that participants with lower quadriceps muscle attenuation, area, and greater intramuscular adipose tissue (IMAT) will exhibit slower rates of torque development (RTD) and lower peak knee extension torques.
Methods
Data from 4,842 participants (2,041 men, 2,801 women) from the Age Gene/Environment Susceptibility Reykjavik Study (mean age 76 ± 0.1 years) with complete thigh computed tomography and isometric knee testing. Regression models were adjusted for health, behavior, and comorbidities. Muscle attenuation was further adjusted for muscle area and IMAT; muscle area adjusted for IMAT and attenuation; and IMAT adjusted for muscle area and attenuation. Standardized betas (β) indicate association effect sizes.
Results
In the fully-adjusted models, attenuation (men β = 0.06, 95% CI: 0.01, 0.11; women β = 0.07, 95% CI: 0.03, 0.11) and muscle area (men β = 0.13, 95% CI: 0.07, 0.19; women β = 0.10, 95% CI: 0.06, 0.15) were associated with knee RTD. Attenuation (men β = 0.12, 95% CI: 0.08, 0.16; women β = 0.12, 95% CI: 0.09, 0.16) and muscle area (men β = 0.38, 95% CI: 0.33, 0.43; women β = 0.33, 95% CI: 0.29, 0.37) were associated with peak torque.
Conclusions
These data suggest that muscle attenuation and area are independently associated with RTD and peak torque; and that area and attenuation demonstrate similar contributions to RTD.
Keywords: Power, Physical function, Fat, Knee extension, Rate of force development
In addition to losses in lean mass, the fatty infiltration of muscle, known as myosteatosis, is a characteristic of aging populations (1,2) associated with mortality (3,4), falls (5,6), fractures (7,8), and poor balance (9). Imaging-based measures of myosteatosis include visible depots of adipose tissue between (inter-) or within (intra-) muscles, and smaller stores of fat and lipids within the muscle fibers estimated via muscle attenuation or proton spectroscopy (10,11). Interventions with exercise alone, or exercise and nutritional supplementation arms (12) have proven effective for reducing measures of myosteatosis in older adults, suggesting them to be modifiable aspects of muscle, with clinical implications for physical function and mortality (13). Healthy older adults have demonstrated associations between thigh muscle attenuation and quadriceps peak torque after adjustment of age, race, and height (14). Although torque is an important risk factor for falls and fall-related injuries (15), muscle power or a rapid rate of torque development are critical for fall avoidance and may better explain associations between myosteatosis and injury. Recent ex vivo (16) and in vivo (16,17) evidence supports a relationship between myosteatosis and the rate of torque development. However, these data are from select samples of older adults, unadjusted for established confounders such as pain, physical activity, and inflammation. Due to methodological limitations, prior investigations of myosteatosis and the rate of torque development isolated either intermuscular adipose (17) or myocellular lipids (16). Inter/intramuscular adipocytes are thought to secrete proinflammatory cytokines and alter the overall architecture of the muscle organ; whereas myocellular lipids reflect metabolic processes within the myocytes. Although distinct properties of myosteatosis, muscle adipocytes and myocellular lipids are both entangled with muscle function and should be studied together (14). A better understanding of how compositional aspects of muscle directly associate with function—in particular, the rate of torque development—is warranted. As a large, well-described population-based cohort of older adults with both computed tomography (CT) and direct measures of quadriceps performance, the Age Gene/Environment Susceptibility (AGES) Reykjavik Study provides a unique opportunity to address prior limitations and investigate the direct associations of muscle size, intramuscular adipose tissue area (IMAT), and muscle attenuation with muscle torque properties. Describing these associations will further our understanding of the protective effects of reducing myosteatosis in older adults (4,13).
The purpose of this analysis is to describe associations of the size and composition of the quadriceps muscle with peak torque and rate of torque development in older adults, while adjusting for covariates related to muscle size and function, health, behavior, and comorbidities. We hypothesize that older adults with lower quadriceps attenuation, smaller muscle mass, and greater quadriceps IMAT will exhibit slower rates of torque development and lower peak isometric torques.
Methods
Sample Description and Design
The AGES Reykjavik Study is a sample nested within the Reykjavik Study, a birth-cohort of persons born in Reykjavik, Iceland between 1907 and 1935 (18). This analysis utilized cross-sectional data collected from Reykjavik Study participants recruited from 2002 to 2006 (N = 5,764). Eligible observations (n = 4,842) had complete isometric knee extension testing with CT imaging on the same thigh. The AGES Reykjavik Study was approved by the National Bioethics Committee for the Icelandic Heart Association (VSN-00-063) and by the National Institute on Aging Intramural Institutional Review Board.
Isometric Knee Extension
Quadriceps function was assessed with a dynamometer chair (Good Strength, Mettitur Ltd; Palokka, Finland). Participants with ischemic heart conditions (ie, angina) and/or contraindications in both legs were excluded (n = 696). The testing protocol involved three trials, visually cued with a 30-second count-down, each lasting 4 seconds, with a 30-second rest between trials. Participants were securely seated with a pelvic stabilization belt and their knee at 60° flexion. They were asked to push their lower leg forward “as hard as possible” against a strain-gauge cuff as if they were straightening their knee up to the maximum. A practice trial ensured comprehension of the instructions. Force signals were amplified and sampled at 100 Hz. To prevent selection of fast peaks, force-time data was median filtered, and processed through a filter window where the minimum value in each window was recorded. Peak torque (Nm) was derived as the product of the maximum value (N) of all possible filter windows and lower leg length (m). Rate of torque development (Nm/s)—an isometric measure of power—was the product of the maximum derivative (N/s) of the force-time curve (prior to the peak torque) and lower leg length (m). For each outcome, the trial with the maximum value was analyzed. The precision error (coefficient of variation [CV] of 9.5–14.1%) of knee extension measures from the dynamometer chair have been previously demonstrated in older adults (19).
CT
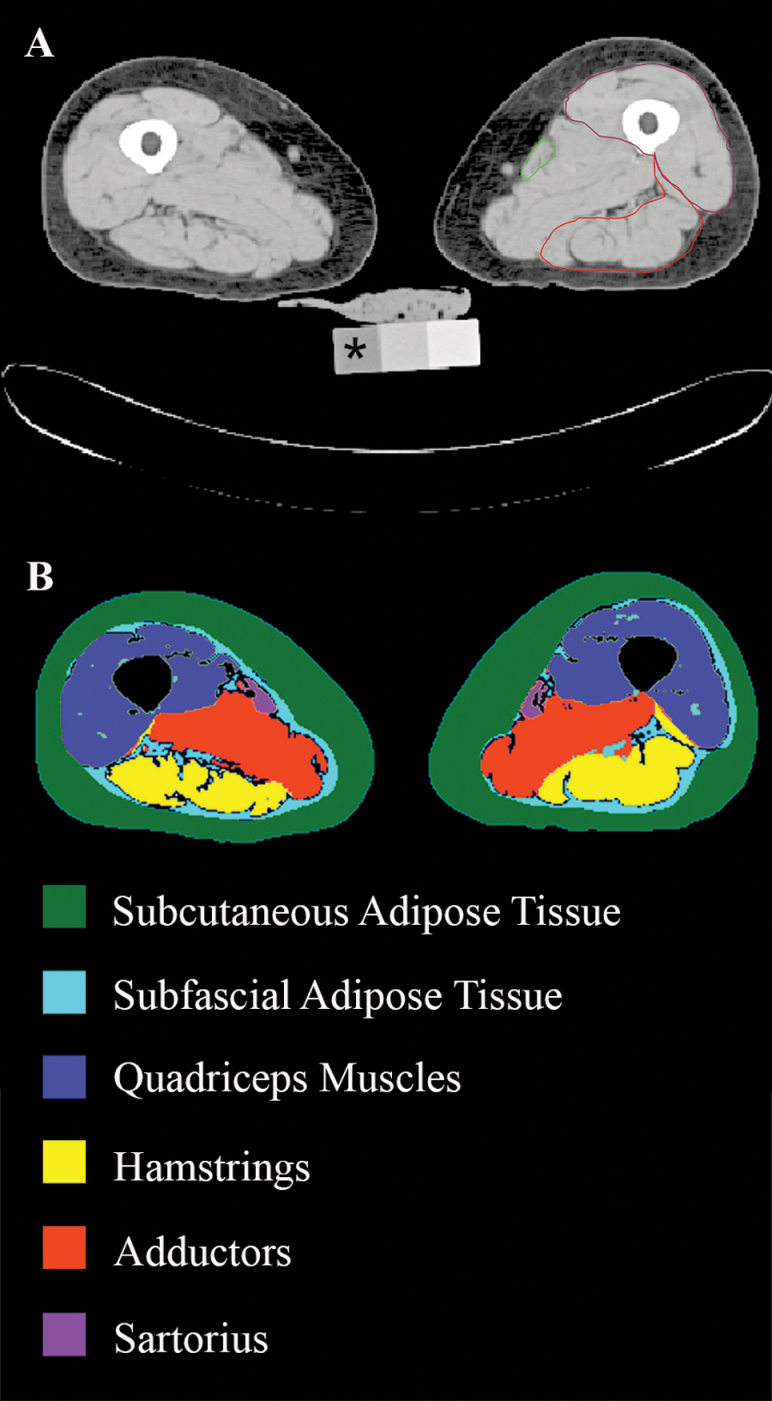
Mid-femur images were acquired using a four-row detector system (Somatom Sensation 4, Siemens AG, Erlangen, Germany) at 120kVp with a 512 × 512 image matrix. Participants unable to lay flat on the scanner bed or had metal implants at the scan site (n = 846) were excluded. A 10mm slice was reconstructed with a standard kernel medium smooth filter. Images were processed in Advanced Visual Systems 5 visualization program (Advanced Visual Systems, Waltham, MA) via a manual contour trace of the right and left quadriceps muscles, followed by a segmentation algorithm for the separation of subcutaneous fat, muscle, bone, and IMAT as described in Lang et al. (8). In brief, the attenuation values corresponding to upper and lower fat and lean segmentation thresholds are a function of the value of a water-equivalent cell in the CT calibration phantom. Muscle area and attenuation were derived as the cross-sectional area and average attenuation of voxels above the attenuation threshold for fat and below the threshold for bone. Relative quadriceps IMAT was defined as the area of muscle below the lean voxel threshold and expressed as a percentage of the total area of the quadriceps muscle (Figure 1: Subfascial adipose tissue within the Quadriceps). Attenuation is inversely related to the lipid content of the muscle tissue, therefore a higher attenuation reflects lower myocellular lipid content (10,11), whereas IMAT is positively associated with the quantity of intramuscular adipose tissue. We analyzed data from the same thigh tested in the dynamometer chair. Participants with large discrepancies between their right and left thighs were excluded (n = 57). Precision error for thigh muscle area (CV = 3.5%) was determined by repositioning and rescanning of 26 participants (20).
Figure 1.
(A) Scan of the left and right thigh. Asterisk indicates water equivalent cell of computed tomography calibration phantom. Manual contour tracings of the sartorius (outlined in green), quadriceps (purple) and hamstrings (red). (B) Soft tissue mappings of processed images; intra-muscular adipose tissue area defined as area of subfascial adipose tissue (light blue) within the quadriceps map, relative to the size of the quadriceps. Please see online version for color maps. Images courtesy of Dr. Thomas Lang, University of California, San Francisco.
Covariates
Covariates were selected based on their biological relevance to muscle size, function, and known effects on health in aging. These included demographic data such as age, height, body mass index (BMI), postsecondary education, cognitive function (assessed via Mini-Mental Status Exam), depression (assessed via Geriatric Depression Scale), general pain (pain/discomfort domain of the European Quality of Life Five Domain (EQ5D) questionnaire), recent history of knee or hip pain in tested leg (self-report), diabetes (determined from self-report, medications and clinical assessment); self-reported behavioral variables such as smoking status, alcohol intake (g/week) and self-reported moderate to vigorous physical activity (minutes/week). General health covariates included acute inflammation (serum C-reactive protein), EQ5D health status, and the number of deficits in five basic Activities of Daily Living (ADL; walking, dressing bathing, transferring and self-feeding). Comorbidities (osteoarthritis, hypertension, chronic obstructive pulmonary disorder, congestive heart failure, stroke, kidney disease, and cancer) were determined from self-report, medications, and/or clinical assessments.
Statistical Analysis
Characteristics were compared between men and women. Continuous variables were reported as medians and interquartile ranges (IQR). Categorical variables were reported as counts and percentages. Continuous variable p values were derived from Wilcoxon Rank-Sum Tests, whereas categorical variables were compared using chi-square tests; adjusted for multiple comparisons. Nested multiple linear regression models were constructed to study the associations between dependent variables (maximum knee extensor torque and rate of torque development) and independent variables (muscle area, attenuation, and IMAT) with adjustment for covariates. Standardized beta values and 95% confidence intervals were estimated. In Model 1, associations were adjusted for: age, height, BMI, cognition, depression, health status, general pain, diabetes, knee or hip pain, and acute inflammation. Model 2 included covariates of Model 1, as well as smoking status, alcohol consumption, physical activity, education. Model 3 included covariates of Model 2 with adjustment for the number of deficits in activities of daily living and the number of comorbidities. To explore the independence of each muscle size/composition variable, Model 3 was mutually adjusted for other muscle size/composition variables separately and combined. To account for missing covariate data, model results were derived using five multiple imputed datasets generated with 20 iterations using the R package “MICE” (version 2.25). Analyses were performed using R version 3.3.2 for Macintosh (R Foundation for Statistical Computing, Vienna, Austria).
Results
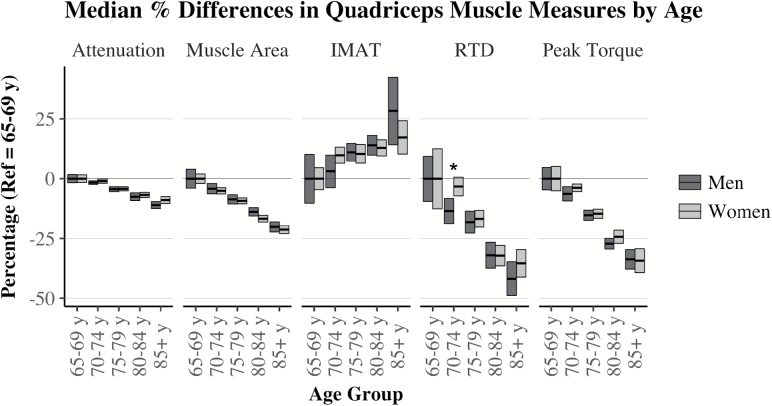
In our sample, men and women shared similar distributions for age, depression scale, and health status with similar frequencies for hypertension, stroke, chronic obstructive pulmonary disorder (COPD), kidney disease and cancer (Table 1). A higher percentage of women were underweight or obese, never smoked, and reported osteoarthritis and history of pain. Women also had better cognitive test scores, more ADL impairments, comorbidities, greater acute inflammation, and IMAT. Proportionally, more men were overweight, had postsecondary education, former smokers, physically active, diabetic or had congestive heart failure. Men also consumed more alcohol, demonstrated higher maximum rates of torque development, peak torque, time to peak torque values, greater muscle attenuation and muscle area. When analyzed within sex across 5-year age groups, both men and women demonstrated differences (p < .001) relative to their 65–69 years median values for all muscle composition and torque outcomes (Figure 2). Men ages 70–74 years appeared to have slower (p < .01) rates of torque development relative to women of the same age, but this sex difference was no longer apparent beyond 74. Linear models for peak torque and rate of torque development demonstrated several sex interactions (p < .01) across muscle area, attenuation and IMAT.
Table 1.
Analytical Sample Descriptive Statistics
| Male | Female | p Valuea | |
|---|---|---|---|
| N (%) | 2,041 (42) | 2,801 (58) | <.001 |
| Age (years) | 76 (72–81) | 76 (72–80) | 1.000 |
| Body Mass Index (Kg/m2) | 26.54 (24.26–28.99) | 26.79 (23.95–29.88) | 1.000 |
| Body Mass Index categories (n, %) | <.001 | ||
| Underweight | 21 (1) | 46 (2) | |
| Normal Weight | 637 (31) | 910 (32) | |
| Overweight | 1,003 (49) | 1,164 (42) | |
| Obese | 379 (19) | 681 (24) | |
| Height (cm) | 175.4 (171.3–179.7) | 161 (157.2–164.8) | <.001 |
| Mini Mental Status Exam Score | 27 (25–28) | 27 (26–29) | <.001 |
| Geriatric Depression Scale Score | 2 (1–3) | 2 (1–3) | 1.000 |
| Education (postsecondary) (n, %) | 633 (31) | 657 (23) | <.001 |
| Smoking status (n, %) | <.001 | ||
| Never | 579 (28) | 1,471 (53) | |
| Former | 1,219 (60) | 966 (35) | |
| Current | 241 (12) | 360 (13) | |
| Alcohol Intake (g/week) | 6.4 (0–26.4) | 1.6 (0–8) | <.001 |
| Physical activity, (>240 min/wk) (n, %) | 711 (35) | 777 (28) | <.001 |
| ADL impairment (five-item score) | 0 (0–0) | 0 (0–1) | <.001 |
| Serum C-Reactive Protein (mg/L) | 1.7 (0.9–3.5) | 1.9 (1–4) | .014 |
| EQ5D - Health Status (0–100) | 89 (75–95) | 87 (70–95) | 1.000 |
| Diabetes Mellitus (n, %) | 308 (15) | 264 (9) | <.001 |
| Number of comorbidities (max=7) | 1 (1–2) | 2 (1–2) | <.001 |
| Osteoarthritis (n, %) | 497 (25) | 1311 (48) | <.001 |
| Hypertension (n, %) | 1,640 (80) | 2,265 (81) | 1.000 |
| Congestive Heart Failure (n, %) | 116 (6) | 98 (4) | .008 |
| Stroke (n, %) | 138 (7) | 164 (6) | 1.000 |
| COPD (n, %) | 196 (10) | 298 (11) | 1.000 |
| Kidney Disease (n, %) | 80 (4) | 152 (5) | .534 |
| Cancer (n, %) | 311 (15) | 413 (15) | 1.000 |
| EQ5D - Moderate or Extreme Pain (n, %) | 478 (30) | 1119 (53) | <.001 |
| History of pain in tested knee or hip (n, %) | 367 (18) | 781 (28) | <.001 |
| Attenuation (HU) | 46.72 (43.47–49.42) | 43.54 (40.53–46.24) | <.001 |
| Muscle Area (cm2) | 60.47 (53.94–66.94) | 42.3 (37.26–47.05) | <.001 |
| Intra-muscular Fat Area (%) | 5.734 (3.846–7.521) | 9.011 (6.945–11.05) | <.001 |
| Maximum Rate of Torque Development (Nm/s) | 524.6 (352.7–749.9) | 270.9 (185–397) | <.001 |
| Peak torque (Nm) | 166.5 (134.9–200.7) | 95.38 (75.64–115.8) | <.001 |
Note: ADL = Activities of Daily Living; COPD = Chronic Obstructive Pulmonary Disorder; EQ5D = European Quality of Life 5 Domain Questionnaire. Continuous variables are described as medians and inter-quartile ranges. Binary and categorical variables are counts and proportions.
a p Values adjusted for multiple comparisons using Benjamini-Hochberg Procedure.
Figure 2.
Muscle composition and function by 5-year age groupings and sex. Bars are 95% confidence intervals of median % differences from the reference group (65–69 years). Attenuation is inversely related to myocellular lipid content (10,11). IMAT = Percentage Intra-muscular Adipose Tissue Area; RTD = Rate of Torque Development. Differences across age groups within sex were all p < .001. Wilcoxon Rank Sum tests compared men and women within age group. *p < .01 adjusted for multiple comparisons.
After adjustment for the total number of model parameters, male and female models explained 14% and 16% of the variance in rate of torque development, and 46% and 38% of peak torque, respectively. Muscle attenuation, area, and IMAT remained independent predictors of the rate of torque development and peak torque after adjustment for covariates in Models 1–3. Standardized beta estimates (which reflect the effect size of an association) and their 95% CIs are reported for each model in Tables 2 and 3. Muscle attenuation, a surrogate measure of myocellular lipids, explained variance in rate of torque development (95% CI range β = 0.01–0.11) and peak torque (95% CI range β = 0.08–0.16) independent of muscle area and IMAT. Considering the separation of the 95% CIs across similar models, associations for muscle attenuation were larger for predicting peak torque (Model 3: 95% CI range β = 0.17–0.26) compared to rate of torque development (Model 3: 95% CI range β = 0.05–0.15). However, after adjustment for muscle area (Tables 2 and 3: Attenuation Model 3 + Muscle Area), effect sizes for muscle attenuation overlapped with rate of torque development and peak torque. Beta estimates for muscle area were more than twice as large for peak torque (95% CI range β = 0.29–0.61) when compared to rate of torque development (95% CI range β = 0.06–0.29). In men, IMAT was no longer an independent predictor for rate of torque development after additional adjustment for muscle area. In women, IMAT was no longer an independent predictor of peak torque after the additional adjustment for both attenuation and muscle area.
Table 2.
Male Standardized Betas and 95% Confidence Intervals
| Torque Properties | |||
|---|---|---|---|
| Muscle | Model | Rate of Torque Development (Nm/s) | Peak Torque (Nm) |
| Attenuation | |||
| Bivariate | 0.20 (0.16 to 0.25) | 0.33 (0.29 to 0.37) | |
| 1 | 0.11 (0.06 to 0.16) | 0.23 (0.19 to 0.27) | |
| 2 | 0.10 (0.06 to 0.15) | 0.23 (0.19 to 0.27) | |
| 3 | 0.10 (0.05 to 0.14) | 0.22 (0.18 to 0.26) | |
| 3 + Muscle Area | 0.06 (0.02 to 0.11) | 0.13 (0.09 to 0.17) | |
| 3 + IMAT | 0.07 (0.02 to 0.12) | 0.16 (0.12 to 0.20) | |
| 3 + Muscle area and IMAT | 0.06 (0.01 to 0.11) | 0.12 (0.08 to 0.16) | |
| Area | |||
| Bivariate | 0.24 (0.20 to 0.29) | 0.57 (0.54 to 0.61) | |
| 1 | 0.17 (0.12 to 0.23) | 0.45 (0.41 to 0.49) | |
| 2 | 0.17 (0.11 to 0.22) | 0.45 (0.40 to 0.49) | |
| 3 | 0.16 (0.11 to 0.21) | 0.44 (0.40 to 0.48) | |
| 3 + Attenuation | 0.14 (0.09 to 0.20) | 0.40 (0.36 to 0.45) | |
| 3 + IMAT | 0.14 (0.08 to 0.20) | 0.40 (0.35 to 0.45) | |
| 3 + Attenuation & IMAT | 0.13 (0.07 to 0.19) | 0.38 (0.33 to 0.43) | |
| IMAT | |||
| Bivariate | −0.12 (−0.16 to −0.08) | −0.19 (−0.24 to −0.15) | |
| 1 | −0.09 (−0.14 to −0.05) | −0.23 (−0.27 to −0.20) | |
| 2 | −0.09 (−0.14 to −0.04) | −0.23 (−0.27 to −0.19) | |
| 3 | −0.09 (−0.13 to −0.04) | −0.23 (−0.27 to −0.19) | |
| 3 + Attenuation | −0.06 (−0.11 to −0.01) | −0.17 (−0.21 to −0.13) | |
| 3 + Muscle Area | −0.04 (−0.09 to 0.01) | −0.08 (−0.12 to −0.04) | |
| 3 + Attenuation and Muscle Area | −0.02 (−0.07 to 0.03) | −0.05 (−0.09 to −0.00) | |
Note: IMAT = % Intramuscular Adipose Tissue area. Estimates are pooled results from imputed data. Models adjusted for:
Model 1: age, height, BMI, cognitive status, depression, health status, general pain, diabetes, knee or hip pain, acute inflammation;
Model 2: covariates in Model 1 + smoking status, alcohol consumption, physical activity, education;
Model 3: covariates in Model 2 + number of deficits in activities of daily living, number of comorbidities.
Table 3.
Female Standardized Betas and 95% Confidence Intervals
| Torque Properties | |||
|---|---|---|---|
| Muscle | Model | Rate of Torque Development (Nm/s) | Peak Torque (Nm) |
| Attenuation | |||
| Bivariate | 0.20 (0.16 to 0.23) | 0.31 (0.28 to 0.35) | |
| 1 | 0.12 (0.08 to 0.16) | 0.21 (0.18 to 0.25) | |
| 2 | 0.11 (0.07 to 0.15) | 0.21 (0.17 to 0.24) | |
| 3 | 0.11 (0.07 to 0.15) | 0.20 (0.17 to 0.24) | |
| 3 + Muscle Area | 0.08 (0.04 to 0.12) | 0.13 (0.10 to 0.16) | |
| 3 + IMAT | 0.08 (0.04 to 0.12) | 0.17 (0.13 to 0.20) | |
| 3 + Muscle area and IMAT | 0.07 (0.03 to 0.11) | 0.12 (0.09 to 0.16) | |
| Area | |||
| Bivariate | 0.25 (0.22 to 0.29) | 0.49 (0.46 to 0.52) | |
| 1 | 0.15 (0.11 to 0.19) | 0.37 (0.34 to 0.41) | |
| 2 | 0.14 (0.10 to 0.19) | 0.37 (0.33 to 0.41) | |
| 3 | 0.14 (0.10 to 0.19) | 0.37 (0.33 to 0.41) | |
| 3 + Attenuation | 0.12 (0.08 to 0.17) | 0.33 (0.30 to 0.37) | |
| 3 + IMAT | 0.12 (0.07 to 0.16) | 0.35 (0.31 to 0.39) | |
| 3 + Attenuation & IMAT | 0.10 (0.06 to 0.15) | 0.33 (0.29 to 0.37) | |
| IMAT | |||
| Bivariate | −0.14 (−0.17 to −0.10) | −0.19 (−0.23 to −0.15) | |
| 1 | −0.11 (−0.14 to −0.07) | −0.15 (−0.19 to −0.12) | |
| 2 | −0.10 (−0.14 to −0.07) | −0.15 (−0.18 to −0.12) | |
| 3 | −0.11 (−0.14 to −0.07) | −0.15 (−0.18 to −0.12) | |
| 3 + Attenuation | −0.08 (−0.12 to −0.04) | −0.10 (−0.13 to −0.06) | |
| 3 + Muscle Area | −0.07 (−0.11 to −0.04) | −0.05 (−0.09 to −0.02) | |
| 3 + Attenuation & Muscle Area | −0.06 (−0.10 to −0.02) | −0.02 (−0.05 to 0.01) | |
Note: IMAT = % Intramuscular Adipose Tissue area. Estimates are pooled results from imputed data. Models adjusted for:
Model 1: age, height, BMI, cognitive status, depression, health status, general pain, diabetes, knee or hip pain, acute inflammation;
Model 2: covariates in Model 1 + smoking status, alcohol consumption, physical activity, education;
Model 3: covariates in Model 2 + number of deficits in activities of daily living, number of comorbidities.
All reported model parameters are pooled estimates from five multiple imputation data sets; however, interpretation of results were similar in a complete case (n = 3,386) analysis (data not shown). To test whether the associations were driven by extreme cases, sensitivity analyses identified and removed 86 unique observations across all male and female models that exerted high influence, leverage, or large studentized residuals on model parameter estimates. Reported parameter estimates and the interpretation of the results were robust to removal of these cases (data not shown). Additional sensitivity analyses considered use of waist circumference instead of BMI; or abdominal and thigh subcutaneous adipose tissue area with visceral adipose tissue area instead of BMI. All model parameter estimates for men and women were robust to these alternate covariates, with the exception of the fully adjusted (IMAT Model 3 + Attenuation and Muscle Area). Fully adjusted IMAT models indicated no significant associations with rate of torque development or peak torque when adjusted for waist circumference instead of BMI (Supplementary Table 1A,1B), and an association between IMAT and rate of torque development for women only when adjusted for subcutaneous and visceral adipose tissue areas instead of BMI (Supplementary Table 2A,2B).
Discussion
Similar to reports in other cohort of older adults (1,2,14), our data indicate progressively higher IMAT and lower muscle area, attenuation, and peak torque across a much wider and older age range (Figure 2). For the first time, we investigated these associations with the rate of torque development. Men had lower rates (p < .01) than women at ages 70–74, but were similar across all other age brackets. Sex-differences in quadriceps size, composition and peak torque values were similar to those reported in a sample of healthy adults age 70–79 years (14); however, IMAT was higher in our population-based sample. In prior studies, myocellular lipids were negatively associated with myofibril contraction velocity (16) and IMAT demonstrated associations with muscle power (17). However, the independent roles of IMAT and attenuation on rate of torque development were unknown. Our results demonstrate that muscle attenuation—an estimate of myocellular lipid—is equally and independently associated with peak torque and rate of torque development for both men and women. This novel result extends our understanding beyond peak torque (14) and elucidates previous linkages observed between muscle attenuation and events such as falls and fall-related injuries where the rapid generation of torques are important (5,6,8,21,22). Our results narrow the importance of IMAT as a predictor of torque properties independent of muscle attenuation and area. Previous investigations have linked IMAT to both torque and power (1,17). However, Delmonico et al. reported that 5-year changes in quadriceps IMAT were not associated with changes in the peak torque (1). Similar to our sex-specific results, Goodpaster et al. indicated a trend (p < .10) in men but not women, for an independent effect of thigh IMAT on quadriceps peak torque (14). With some beta estimates crossing zero after full adjustment, our initial BMI-adjusted results appeared to confirm a sex-specific association of IMAT with peak torque and rate of torque development that was not always independent of muscle attenuation and area. However, our sensitivity analyses and supplemental data suggest this sex specificity to be unstable. Alternative measures of obesity and adiposity appear to specifically shrink the associations of IMAT with peak torque and rate of torque development, potentially nullifying the sex-specific results reported from models adjusted for BMI.
There are both biomechanical and biological mechanisms that may explain associations between muscle size, attenuation, IMAT, and torque properties. Biomechanically, large quantities of adipose tissue may have detrimental effects on muscle architecture and the overall muscle function. Computer simulations have suggested deteriorations in muscle function (23) and steeper, less efficient muscle fiber pennation angles have been reported with greater myosteatosis (24). Beyond structural changes, proinflammatory cytokines secreted from adipocytes are known to inhibit production of myofilament proteins, increase oxidative stress, and reduce contractility (25). Myosteatosis has been observed to increase with age (1,2), regardless of changes in weight (1). Furthermore, associations with obesity, diabetes (2,26), and reductions in mitochondrial efficiency in older adults (27,28) implicate an energy-surplus milieu. Surplus nutrient conditions prolong the post-fission state of the mitochondrial lifecycle characterized by inefficient function and heightened reactive oxygen species production (26,29). The slower turnover rate of myosin molecules make them susceptible to oxidative stress-induced post-translational modifications that alter molecule organization and reduce contractile motility. Interestingly, these modifications do not appear to affect overall myofibril force, but do reduce contraction velocity across fiber types (30). Using both in vivo and ex vivo methods, Choi et al. recently confirmed associations of reduced single-fiber-specific power and maximum shortening velocity with myocellular lipids in the muscles of obese older adults (16). Measures of muscle attenuation reflect myocellular lipids (10,11) and may provide an indirect glimpse into the myocellular respiratory environment with implications for myosin function, overall muscle power, and the physical function of older adults.
Very few investigations provide direct analyses of myosteatosis and torque properties (1,14,16,17). As the largest, most well-described population-based cohort analyzed, our results verify and expand upon earlier findings by analyzing IMAT, muscle area and attenuation, as well as reporting on the rate of torque development. Our key results contrasted beta estimates between peak torque and the rate of torque development. These data show muscle area to be an important parameter for peak torque—a well-known association (14). However, our results also contribute novel data demonstrating that both muscle area and attenuation contribute equally to the rate of torque development—a surrogate measure of muscle power with greater clinical implications for physical function and injury avoidance in older adults.
Strengths and Limitations
Our results address many of the limitations of prior research with adjustment for many known factors that can influence physical performance and body composition in older adults. Furthermore, we have reduced the potential bias of missing data, and verified the robustness of our results. Our sample size exceeded design estimates required to detect a small effect size when stratified by sex. We accounted for factors that can introduce variability into physical function results by adjusting our analyses for measures of cognition, pain, depression, and acute inflammation as well as chronic diseases known to reduce muscle power (31). However, our data should be interpreted in consideration of several design limitations. Our population-based data is from a relatively homogenous Icelandic community of European descent. Prior American studies have demonstrated race to be a factor in the association between thigh muscle composition and peak torque (14). Although we collected measures of cognition, depression and pain, a direct measure of neuromuscular activation may have better described variability in volitional effort. In addition, performance on unfamiliar motor tasks like the isometric knee extension test improves with practice. Therefore, motor-learning effects may have inflated our unexplained variance because the testing protocol provided a single practice trial before recording three maximal attempts. Lastly, our study did not measure patellar tendon stiffness. Although contractile components are a key determinant of a muscle’s rate of torque development, connective tissue compliance also increases with age and known to reduce knee extensor rate of torque development (32).
Conclusions
These results provide the most comprehensive description of the role of muscle size and composition in peak muscle torque and the rate at which that torque can be generated—a key factor in protective counter-movements for injury avoidance in older adults. Older adults with lower quadriceps attenuation and smaller muscle mass, will exhibit slower rates of torque development and lower peak torques. Muscle attenuation is equally and independently associated with the rate of torque development and peak torque. Furthermore, muscle attenuation and area contribute equally to the rate of torque development. These results further elucidate a potential pathway underpinning previously described relationships of muscle size, composition, and function with falls, fractures, and poor balance among older adults.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by National Institutes of Health (N01-AG-12100); the National Institute on Aging Intramural Research Program; Hjartavernd (the Icelandic Heart Association); and the Althingi (the Icelandic Parliament).
Acknowledgments
We would like to thank Osorio Meirelles (NIA) for his assistance preparing the data, the editorial assistance of the NIH Fellows Editorial Board, the study participants, and the Icelandic Heart Association clinic staff for their invaluable contribution.
References
- 1. Delmonico MJ, Harris TB, Visser M et al. ; Health, Aging, and Body Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi:10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miljkovic I, Kuipers AL, Cvejkus R et al. . Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity (Silver Spring). 2016;24:476–482. doi:10.1002/oby.21328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reinders I, Murphy RA, Brouwer IA et al. ; Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study Muscle quality and myosteatosis: novel associations with mortality risk: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Am J Epidemiol. 2016;183:53–60. doi:10.1093/aje/kwv153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santanasto AJ, Goodpaster BH, Kritchevsky SB et al. . Body composition remodeling and mortality: the Health Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2016:glw163. doi:10.1093/gerona/glw163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frank-Wilson AW, Farthing JP, Chilibeck PD et al. . Lower leg muscle density is independently associated with fall status in community-dwelling older adults. Osteoporos Int. 2016. Jul;27(7):2231–2240. doi:10.1007/s00198-016-3514-x. Epub 2016 Feb 15 [DOI] [PubMed] [Google Scholar]
- 6. Inacio M, Ryan AS, Bair WN, Prettyman M, Beamer BA, Rogers MW. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC Geriatr. 2014;14:37. doi:10.1186/1471-2318-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB; Health ABC Study Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi:10.1359/jbmr.090807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lang T, Koyama A, Li C et al. . Pelvic body composition measurements by quantitative computed tomography: association with recent hip fracture. Bone. 2008;42:798–805. doi:10.1016/j.bone.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 9. Anderson DE, Quinn E, Parker E et al. . Associations of computed tomography-based trunk muscle size and density with balance and falls in older adults. J Gerontol A Biol Sci Med Sci. 2015:glv185–glv186. doi:10.1093/gerona/glv185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR; Look AHEAD Adipose Research Group Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring). 2006;14:73–87. doi:10.1038/oby.2006.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89:104–110. doi:10.1152/jappl.2000.89.1.104 [DOI] [PubMed] [Google Scholar]
- 12. Englund DA, Kirn DR, Koochek A et al. . Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, the VIVE2 Study: a randomized, double-blind, placebo-controlled trial. J Gerontol A Biol Sci Med Sci. 2017;73:95–101. doi:10.1093/gerona/glx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570. doi:10.1155/2014/309570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodpaster BH, Carlson CL, Visser M et al. . Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985). 2001;90:2157–2165. doi:10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 15. Benichou O, Lord SR. Rationale for strengthening muscle to prevent falls and fractures: a review of the evidence. Calcif Tissue Int. 2016;98:531–545. doi:10.1007/s00223-016-0107-9 [DOI] [PubMed] [Google Scholar]
- 16. Choi SJ, Files DC, Zhang T et al. . Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71:557–564. doi:10.1093/gerona/glv169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reid KF, Pasha E, Doros G et al. . Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014;114:29–39. doi:10.1007/s00421-013-2728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris TB, Launer LJ, Eiriksdottir G et al. . Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi:10.1093/aje/kwk115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bieler T, Magnusson SP, Kjaer M, Beyer N. Intra-rater reliability and agreement of muscle strength, power and functional performance measures in patients with hip osteoarthritis. J Rehabil Med. 2014;46:997–1005. doi:10.2340/16501977-1864 [DOI] [PubMed] [Google Scholar]
- 20. Johannesdottir F, Aspelund T, Siggeirsdottir K et al. . Mid-thigh cortical bone structural parameters, muscle mass and strength, and association with lower limb fractures in older men and women (AGES-Reykjavik Study). Calcif Tissue Int. 2012;90:354–364. doi:10.1007/s00223-012-9585-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frank AW, Farthing JP, Chilibeck PD, Arnold CM, Olszynski WP, Kontulainen SA. Community-dwelling female fallers have lower muscle density in their lower legs than non-fallers: evidence from the Saskatoon Canadian Multicentre Osteoporosis Study (CaMos) cohort. J Nutr Health Aging. 2015;19:113–120. doi:10.1007/s12603-014-0476-6 [DOI] [PubMed] [Google Scholar]
- 22. Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB; Health ABC Study Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi:10.1359/jbmr.090807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rahemi H, Nigam N, Wakeling JM. The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. J R Soc Interface. 2015;12:20150365. doi:10.1098/rsif.2015.0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rastelli F, Capodaglio P, Orgiu S et al. . Effects of muscle composition and architecture on specific strength in obese older women. Exp Physiol. 2015;100:1159–1167. doi:10.1113/EP085273 [DOI] [PubMed] [Google Scholar]
- 25. Michaud M, Balardy L, Moulis G et al. . Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14:877–882. doi:10.1016/j.jamda.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 26. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:2237–2238. doi:10.1056/NEJMc1412427 [DOI] [PubMed] [Google Scholar]
- 27. Petersen KF, Befroy D, Dufour S et al. . Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi:10.1126/science.1082889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johannsen DL, Conley KE, Bajpeyi S et al. . Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab. 2012;97:242–250. doi:10.1210/jc.2011-1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi:10.1016/j.cmet.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li M, Ogilvie H, Ochala J et al. . Aberrant post-translational modifications compromise human myosin motor function in old age. Aging Cell. 2015;14:228–235. doi:10.1111/acel.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strollo SE, Caserotti P, Ward RE, Glynn NW, Goodpaster BH, Strotmeyer ES. A review of the relationship between leg power and selected chronic disease in older adults. J Nutr Health Aging. 2015;19:240–248. doi:10.1007/s12603-014-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quinlan JI, Maganaris CN, Franchi MV et al. . Muscle and tendon contributions to reduced rate of torque development in healthy older males. J Gerontol A Biol Sci Med Sci. 2017. doi:10.1093/gerona/glx149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.