Abstract
Objective
Resveratrol supplementation improves metabolic health in healthy obese men, but not in patients with type 2 diabetes (T2D) when given as add-on therapy. Therefore, we examined whether resveratrol can enhance metabolic health in men at risk of developing T2D. Additionally, we examined if resveratrol can stimulate brown adipose tissue (BAT).
Methods
Thirteen male first degree relatives (FDR) of patients with T2D received resveratrol (150 mg/day) and placebo for 30 days in a randomized, placebo controlled, cross-over trial.
Results
Resveratrol significantly improved ex vivo muscle mitochondrial function on a fatty acid-derived substrate. However, resveratrol did not improve insulin sensitivity, expressed as the rate of glucose disposal during a two-step hyperinsulinemic-euglycemic clamp. Also, intrahepatic and intramyocellular lipid content, substrate utilization, energy metabolism, and cold-stimulated 18F-FDG glucose uptake in BAT (n = 8) remained unaffected by resveratrol. In vitro experiments in adipocytes derived from human BAT confirmed the lack of effect on BAT.
Conclusions
Resveratrol stimulates muscle mitochondrial function in FDR males, which is in concordance with previous results. However, no other metabolic benefits of resveratrol were found in this group. This could be attributed to subject characteristics causing alterations in metabolism of resveratrol and thereby affecting resveratrol's effectiveness.
ClinicalTrials.gov ID
Keywords: Resveratrol, Type 2 diabetes, Pre-diabetes, Insulin sensitivity, Mitochondrial function, Brown adipose tissue
Abbreviations: AMPK, AMP-activated protein kinase; BAT, brown adipose tissue; DHR, dihydro-resveratrol; EGP, endogenous glucose production; F-FDG, fluoro-deoxy-glucose; FDR, first-degree relatives; IHL, intrahepatic lipid; IMCL, intramyocellular lipid content; MG, malate + glutamate; MGS, malate + glutamate + succinate; MO, malate + octanoyl-carnitine; MOG, malate + octanoyl-carnitine + glutamate; MOG, malate + octanoyl-carnitine + glutamate + succinate; MRS, magnetic resonance spectroscopy; NE, norepinephrine; OGTT, oral glucose tolerance test; OXPHOS, oxidative phosphorylation; PCr, phosphocreatine; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Ra, glucose appearance; Rd, glucose disposal; SIRT1, sirtuin 1; SUV, standard uptake value; T2D, type 2 diabetes; UCP1, uncoupling protein; WAT, white adipose tissue
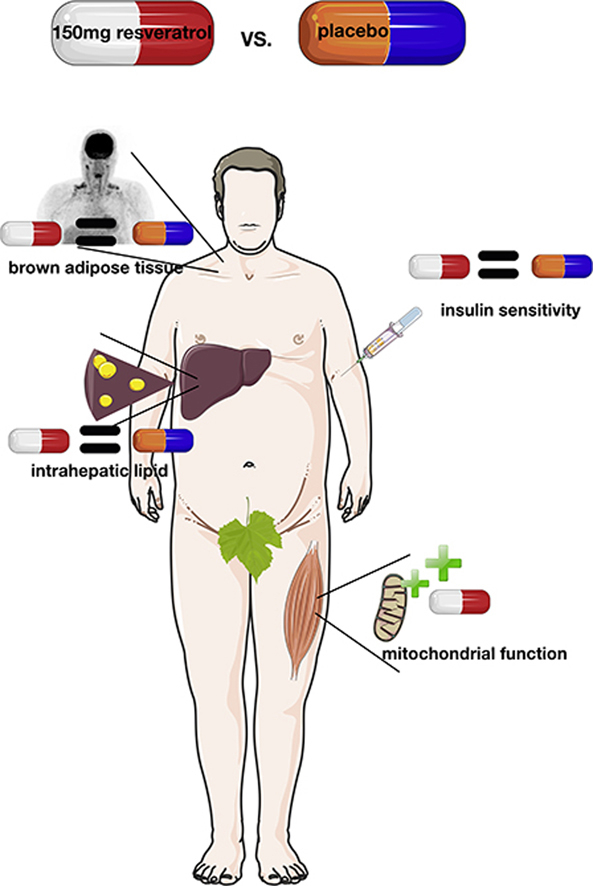
Graphical abstract
Highlights
-
•
Resveratrol supplementation improves muscle mitochondrial function.
-
•
Resveratrol does not improve insulin sensitivity in people at risk of diabetes.
-
•
Resveratrol does not affect brown adipose tissue in people at risk of diabetes.
1. Introduction
Resveratrol, a polyphenol present in various foods, has been proposed as a promising treatment in the prevention and treatment of type 2 diabetes (T2D) [1]. Beneficial effects of resveratrol are strongly related to the activation of sirtuin 1 (SIRT1) and AMP-activated protein kinase (AMPK) [2]. Both proteins are important regulators of cellular fuel metabolism and mitochondrial function, thereby making them interesting targets for combating T2D [3]. Previously, we have shown that in healthy obese men 150 mg resveratrol per day for 30 days exerted beneficial health effects, among which were improvements in ex vivo muscle mitochondrial function, energy metabolism, hepatic lipid content, and blood glucose levels [4]. However, we did not find comparable results when the same dose of resveratrol was administered to patients with T2D [5]. Although we confirmed beneficial effects of resveratrol on mitochondrial function, no improvements in insulin sensitivity or related health parameters were achieved. However, since these patients were using the oral-glucose lowering drug metformin, which may have similar targets as resveratrol, we proposed that the use of metformin might have interfered with the effectiveness of resveratrol on glucose homeostasis. To determine if resveratrol could still have a role in the prevention of diabetes, there is a need for studies in drug-naïve humans with compromised metabolic health. First-degree relatives (FDR) of T2D patients display decreased beta cell and mitochondrial function, and have reduced insulin sensitivity [6], [7], [8], [9], [10]. Thereby, they are at increased risk of developing T2D and may specifically benefit from resveratrol treatment.
Positive effects of resveratrol on metabolic health could also be related to stimulation of brown adipose tissue (BAT) thermogenesis, as represented by increased expression of uncoupling protein 1 (UCP1), SIRT1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in BAT of animals receiving resveratrol [11], [12], [13]. The main role of BAT is generation of heat, mediated via UCP1 [14]. Activation of brown adipose tissue has been associated with positive metabolic health effects [15], but so far the effect of resveratrol on BAT in humans is unknown.
Therefore, in this placebo-controlled, crossover study we investigated if 30 days of resveratrol supplementation can improve insulin sensitivity and muscle mitochondrial function in males at increased risk of developing T2D. In a sub-set of the participants, as well as in human primary brown adipocytes, we examined if resveratrol is able to stimulate BAT.
2. Material and methods
2.1. Participants
Thirteen overweight males at increased risk of developing T2D participated in the study (Table S1). The protocol was reviewed and approved by the institutional medical ethics committee (NL 47018.068.13; ClinicalTrials.gov ID: NCT02129595). An increased risk for T2D was defined as having at least one first-degree relative with T2D, BMI between 27 and 35 kg/m2, and disrupted glucose homeostasis determined by a 2-hour oral glucose tolerance test (OGTT). From the OGTT, glucose clearance was calculated using the oral glucose insulin sensitivity model [16]. Participants with a glucose clearance ≤350 mL/kg/min were regarded as having disrupted glucose homeostasis. A sub-set of eight participants was enrolled for BAT measurements. The study was carried out at Maastricht University and Medical Centre, the Netherlands. A flow chart of the enrollment process can be found in Figure S1.
2.2. Study design
Subjects participated in a double-blind, crossover trial with two conditions: a placebo and a resveratrol condition (150 mg/day trans-resveratrol [99.9%; provided by DSM Nutritional Products Ltd.]), with a washout period of at least 30 days. Each experimental condition lasted 30 or 34 days; the 34-day protocol applied only to the sub-set of eight participants who underwent BAT measurements. Participants were instructed to take the first supplement on the day baseline measurements were performed (day 0) and the last supplement in the evening before the last test day (day 29 or day 33; depending on participation in the BAT measurement). At each visit, the participants returned unused capsules. Participants were instructed to abstain from food containing substantial amounts of resveratrol (e.g. red wine, grapes and peanuts), and to maintain their normal eating, activity and sleeping pattern. Participants came to the university on a weekly basis (days 0, 7, 14, 21, 29, and 34), in the morning in overnight fasted state, for measuring body weight and drawing blood samples for the analysis of plasma resveratrol. The latter was analyzed by mass spectroscopy, as previously described [4]. Potential changes in physical activity level were evaluated by performing a maximal aerobic capacity test (VO2max) on a cycle ergometer on day 27 of both treatment periods.
2.3. Biochemical measures
2.3.1. General health parameters
On day 0 and 30 of both the resveratrol and placebo period, fasting blood samples were drawn for analysis of general safety parameters (creatinine, bilirubin, aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transferase). In addition, heart rate and blood pressure were measured in triplicate in the resting condition (Omron Healthcare, Hamburg, Germany), and a 12-lead ECG was made. Maximal aerobic capacity (VO2max) was measured on a cycle ergometer on day 27, to verify cardiorespiratory fitness (Omnical, Maastricht Instruments, Maastricht University). Body fat percentage was determined only once by dual energy X-ray absorptiometry (DXA, Hologic, the Netherlands) on day 0 of the first intervention period.
2.3.2. Cardiac function
On day 29, in the afternoon, myocardial function was measured by Doppler ultrasound. M-mode, two-dimensional and Doppler echocardiography were performed, using a Vivid 7 ultrasound system with 3.5 MHz cardiac transducer (GE Healthcare, Milwaukee, WI, USA).
2.3.3. Magnetic resonance spectroscopy
On day 29, the ultrasound was followed by a proton magnetic resonance spectroscopy (1H-MRS) measurement to quantify intrahepatic lipid (IHL) content. Proceeded by a post-exercise phosphocreatine (PCr) recovery rate measurement, by 31P-MRS, to estimate in vivo mitochondrial function in vastus lateralis muscle. MRS scans were performed on a 3T whole body scanner (Achieva Tx; Philips Heathcare, Best, The Netherlands). In short, for IHL a PRESS sequence was used with a repetition time of 4 s and an echo time of 32.5 ms. Values are given as T2 corrected ratios of the CH2 peak relative to unsuppressed water resonance, expressed as percentage (for further details see [17]). For in vivo mitochondrial function, the PCr recovery half-time in seconds was determined after submaximal knee-extension exercise performed in the scanner (for details see [18]). To standardize food intake, subjects had lunch with the same food items in the two conditions and stayed fasted until completion of the MRS scans. Afterwards a standardized evening meal was provided and subjects stayed in a respiration chamber during 10 h to allow measurement of sleeping metabolic rate [19].
2.3.4. Muscle biopsy
In the morning of day 30, a biopsy was taken from the vastus lateralis muscle. A portion of the muscle was directly frozen in melting isopentane for determination of protein expression of oxidative phosphorylation (OXPHOS) by western blotting (AB110411, abcam, Cambridge, UK), and intramyocellualr lipid content (IMCL) by Oil red O staining combined with fiber typing via immunolabeling of myosin heavy chain type I (see [20]). Another portion of ∼50 mg was immediately placed in ice-cold preservation medium for determination of ex vivo mitochondrial respiration by oxygraph (OROBOROS Instruments, Innsbruck, Austria), as previously described [4].
2.3.5. Hyperinsulinemic-euglycemic clamp
On day 30, after the muscle biopsy, a two-step hyperinsulinemic-euglycemic clamp was performed to assess whole-body and liver-specific insulin sensitivity. The clamp started with a primed continuous infusion of D-[6,6-2H2]glucose (0.04 mg/kg/min) to determine rates of endogenous glucose production (EGP), glucose appearance (Ra), and glucose disposal (Rd), as described earlier [5]. After 120 min, low dose insulin infusion was started (10 mU/m2/min) for 3 h, followed by high dose insulin infusion (40 mU/m2/min) for 2.5 h. During the last 30 min of each insulin infusion phase (0, 10, and 40 mU/m2/min), blood samples were collected, and substrate utilization was measured by indirect calorimetry (Omnical, Maastricht Instruments, Maastricht). Steele's single pool non-steady state equations were used to calculate glucose Ra and Rd [21]. The distribution volume of glucose was assumed to be 0.160 l/kg.
2.3.6. Brown adipose tissue
After the clamp, a sub-set of eight participants continued supplement intake for another four days. On day 34, these participants underwent an 18-Fluoro-Deoxy-Glucose (18F-FDG) PET/CT scan, upon acute cold exposure, to measure glucose uptake in the BAT region as described earlier [22]. The protocol started with a thermoneutral period at 36 °C for 45 min, followed by an individualized 2-hour cooling protocol using a water-perfused suit. Water temperature was decreased by 4 °C every 15 min, until shivering occurred. When shivering occurred, subjects were warmed-up again for 5 min to diminish shivering. Finally, the suit temperature was set at 2 °C above the temperature at which shivering started, for a minimum of 30 min. During this mild cold stimulation ∼74 MBq of 18F-FDG was intravenously injected. One hour after injection imaging started with a low-dose CT scan (120 kV, 30 mAs), and a static PET scan (6–7 bed positions, 6 min per bed position) (Gemini TF PET-CT, Philips). The PET image was used to determine the 18F-FDG uptake, and the CT image for PET attenuation correction and localization of the 18F-FDG uptake sites. Scans were analyzed using PMOD software (version 3.0; PMOD Technologies). PET-active areas, defined as a standard uptake value (SUV) ≥ 1.5, were selected in the supraclavicular adipose tissue region (−200 and −10 Hounsfield units) to establish the presence and volume of active BAT. The fixed-volume method [22] was used to determine the average SUV and maximum SUV of several tissues. Fixed volumes of 2.67 cm3 were placed and analyzed in supraclavicular region for BAT, subcutaneous white adipose tissue (WAT), skeletal muscle, and liver glucose uptake. The same anatomic locations were used in the analysis between the two experimental conditions.
To examine in vitro effects of resveratrol on BAT oxidative capacity, BAT and WAT biopsies were obtained from patients undergoing thyroid surgery. The protocol was reviewed and approved by the institutional ethics committee (NL31367.068.10). From the collected biopsies, the stromal vascular fraction was isolated and grown to confluence before differentiation was initiated as described previously with slight modifications [23]: bFGF and BMP4 were removed from the differentiation protocol. In vitro differentiated adipocytes were incubated for 24 h with trans-resveratrol (0, 5 and 50 μM) prior to measuring oxygen consumption in the XF96 bioanalyzer from Seahorse Bioscience. Basal oxygen consumption rate and norepinephrine (NE)-induced mitochondrial uncoupling were measured. For the latter, 1 μM NE was added after blocking ATPase by addition of 2 μM oligomycin.
2.4. Statistical analysis
Results are presented as means ± SEM when normally distributed, and as median and range when this was not the case. Shapiro–Wilk normality test was performed to evaluate normal distribution. Student's paired t-test was used to compare placebo and resveratrol treatment in normally distributed data, otherwise Wilcoxon signed-rank. Treatment comparisons of parameters measured at the beginning and end of the intervention periods were assessed by two-way repeated measures ANOVA. Linear regression analyses were conducted to identify correlations between variables. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS 22.0 for Mac OS.
3. Results
3.1. Study compliance
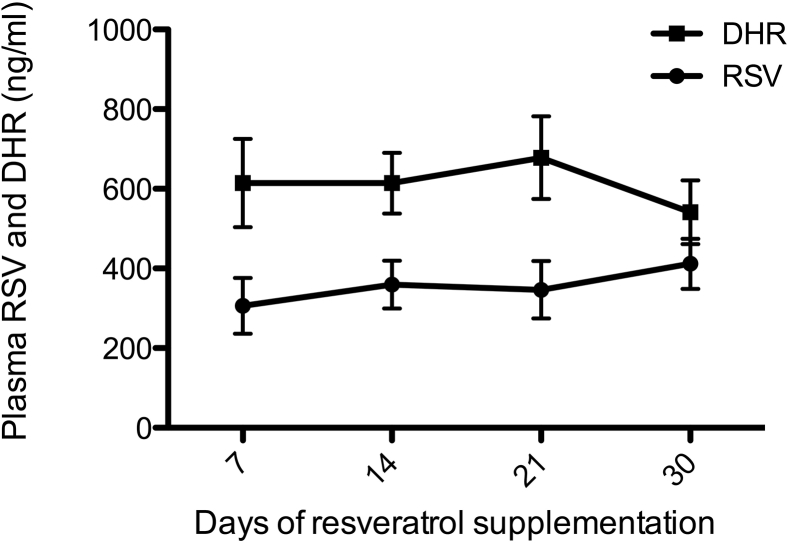
Compliance was confirmed by analysis of plasma levels of resveratrol (free + conjugated) and dihydro-resveratrol (DHR), a metabolite of resveratrol, on a weekly basis. Resveratrol and DHR levels were below detection during the placebo period, while during the resveratrol period both were elevated, with resveratrol levels of 412 ± 63 ng/mL and DHR levels of 541 ± 80 ng/mL on day 30 (Figure 1). Participants were instructed to remain their habitual physical activity level and not to lose body weight throughout the entire study. This was confirmed by VO2max (27 ± 1.5 mL/kg bw/min upon placebo vs. 28 ± 1.7 upon resveratrol, p = 0.823) and body weight on day 30 (90.2 ± 1.67 kg after placebo vs. 89.9 after resveratrol, p = 0.524).
Figure 1.
Total plasma resveratrol (RSV) and dihydro-resveratrol (DHR) values during the 30 days of resveratrol treatment period. Data are presented as mean ± SEM (n = 13).
3.2. Insulin sensitivity and substrate kinetics
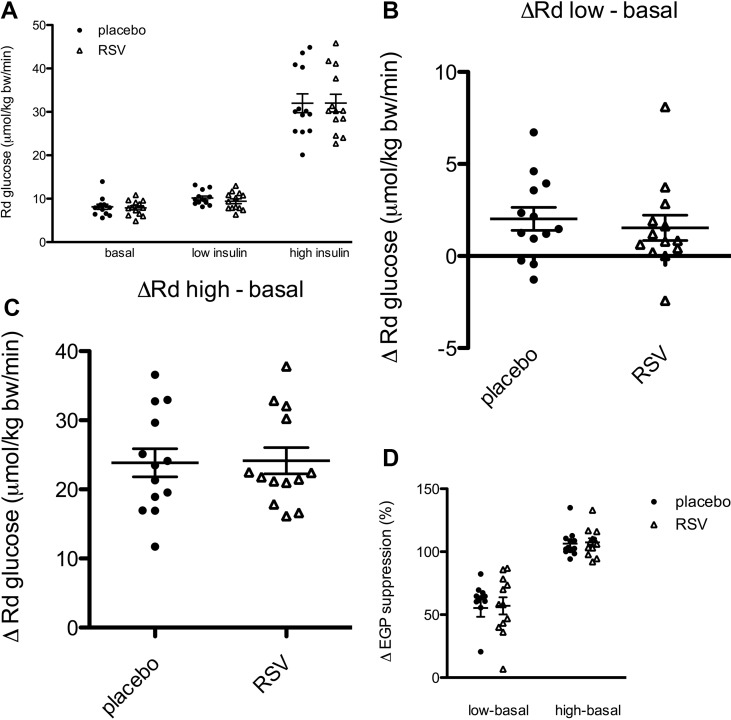
Insulin sensitivity was measured by two-step hyperinsulinemic euglycemic clamp. Basal, low, and high insulin phase Rd were not affected by resveratrol (Figure 2A). Insulin-stimulated glucose uptake, as expressed by the change in glucose disposal (delta Rd) from basal to the low or high-insulin phase, was not different between resveratrol and placebo (Figure 2B–C; Table 1). Indirect calorimetry, performed during the different stages of the clamp, revealed no effect of resveratrol on insulin-induced glucose oxidation or suppression of fat oxidation (Table 1). Also, non-oxidative glucose disposal remained unchanged (Table 1). Insulin-mediated suppression of endogenous glucose production (%EGP suppression) was similar after resveratrol and placebo administration, both during low and high-insulin infusion (Figure 2D; Table 1). In accordance with a lack of effect of resveratrol on substrate utilization during the clamp, resveratrol did not alter sleeping metabolic rate (5.1 ± 0.09 kJ/min after placebo, versus 5.0 ± 0.08 kJ/min after resveratrol) or respiratory exchange ratio, both measured overnight in a respiration chamber. Furthermore, fasting plasma FFA levels were comparable between treatments and were similarly suppressed by insulin (Table 1), and no changes in fasting insulin, glucose, HbA1c levels, or other plasma markers related to metabolic health were found upon resveratrol supplementation (Table S2).
Figure 2.
Effect of resveratrol (RSV) on peripheral and hepatic insulin sensitivity. After 30 days of resveratrol and placebo, peripheral and hepatic insulin sensitivity were assessed by a two-step hyperinsulinemic-euglycemic clamp (t = 0–120 min: D-[6,6-2H2]glucose tracer infusion; t = 120–300 min: low-insulin infusion; t = 300–420 min: high-insulin infusion). Insulin-stimulated glucose disposal, expressed as the Rd and EGP were calculated for the last 30 min of the basal, low- and high-insulin state. (A) Rd and (B–C) difference in Rd compared to basal; (D) EGP suppression (delta EGP %) upon low- and high-insulin infusion. Data are presented as individual data points, mean ± SEM (n = 13). Rd, rate of disappearance; EGP, endogenous glucose production.
Table 1.
Insulin sensitivity and substrate kinetics.
| Placebo | Resveratrol | P-value | ||
|---|---|---|---|---|
| Rd (μmol/kg body weight/min) | ||||
| Basal | 8.0 (6.86–9.42) | 7.9 (6.86–8.92) | 0.972a | |
| Low insulin | 10.2 ± 0.44 | 9.4 ± 0.56 | 0.258 | |
| High insulin | 32.0 ± 2.17 | 32.0 ± 2.03 | 0.965 | |
| Delta (Rd low insulin – Rd basal) | 1.5 (0.67–3.37) | 0.9 (0.04 - 3.02) | 0.507a | |
| Delta (Rd high insulin – Rd basal) | 23.8 ± 2.04 | 24.1 ± 1.90 | 0.810 | |
| EGP (μmol/kg body weight/min) | ||||
| Basal | 7.9 (4.48–9.16) | 8.5 (7.29–9.54) | 0.937a | |
| Low insulin | 3.0 (2.32–5.18) | 3.3 (2.36–4.41) | 0.875a | |
| High insulin | −0.3 (−1.19–−0.08) | −0.63 (−0.92–−0.03) | 0.695a | |
| % suppression (low insulin) | 62 (40.0–70.7) | 58 (42.0–72.1) | >0.99a | |
| % suppression (high insulin) | 103 (99.8–113.1) | 107 (100.4–114.6) | 0.814a | |
| NOGD (μmol/kg body weight/min) | ||||
| Basal | 2.1 ± 0.92 | 2.0 ± 0.65 | 0.894 | |
| Low insulin | 2.2 ± 0.78 | 1.0 ± 0.88 | 0.350 | |
| High insulin | 18.1 ± 2.11 | 17.6 ± 5.91 | 0.742 | |
| Carbohydrate oxidation (μmol/kg body weight/min) | ||||
| Basal | 5.3 (4.60–7.47) | 5.9 (4.27–7.38) | 0.944a | |
| Low insulin | 7.9 ± 0.53 | 8.4 ± 0.76 | 0.630 | |
| High insulin | 13.9 ± 0.51 | 14.4 ± 0.95 | 0.478 | |
| FFA oxidation (μmol/kg body weight/min) | ||||
| Basal | 3.5 ± 0.24 | 3.6 ± 0.13 | 0.978 | |
| Low insulin | 2.9 ± 0.17 | 2.7 ± 0.14 | 0.527 | |
| High insulin | 1.6 ± 0.13 | 1.5 ± 0.18 | 0.757 | |
| Plasma FFA (μmol/L) | ||||
| Basal | 552 ± 40.2 | 609 ± 59.9 | 0.333 | |
| Low insulin | 167 ± 12.2 | 174 ± 19.7 | 0.652 | |
| High insulin | 66 ± 5.3 | 65 ± 6.3 | 0.550 | |
| Respiratory Exchange Ratio | ||||
| Basal | 0.77 (0.765–0.811) | 0.78 (0.766–0.805) | 0.916a | |
| Low insulin | 0.82 ± 0.008 | 0.82 ± 0.009 | 0.634 | |
| High insulin | 0.90 ± 0.008 | 0.90 ± 0.011 | 0.833 | |
Substrate metabolism assessed by a two-step hyperinsulinemic euglycemic clamp after 30 days of placebo and resveratrol supplementation. Glucose oxidation, lipid oxidation, and respiratory quotient were determined by means of indirect calorimetry. Non-oxidative glucose disposal (NOGD) is calculated by subtracting the rate of glucose oxidation from the rate of disappearance of the glucose (Rd glucose). Data are presented as mean ± SEM when normally distributed, otherwise median and range are shown (n = 13).
aP-value relates to non-parametric Wilcoxon Signed Rank test. Rd, rate of disappearance; EGP, endogenous glucose production; NOGD, non-oxidative glucose disposal; FFA, free fatty acid.
3.3. Skeletal muscle mitochondrial function
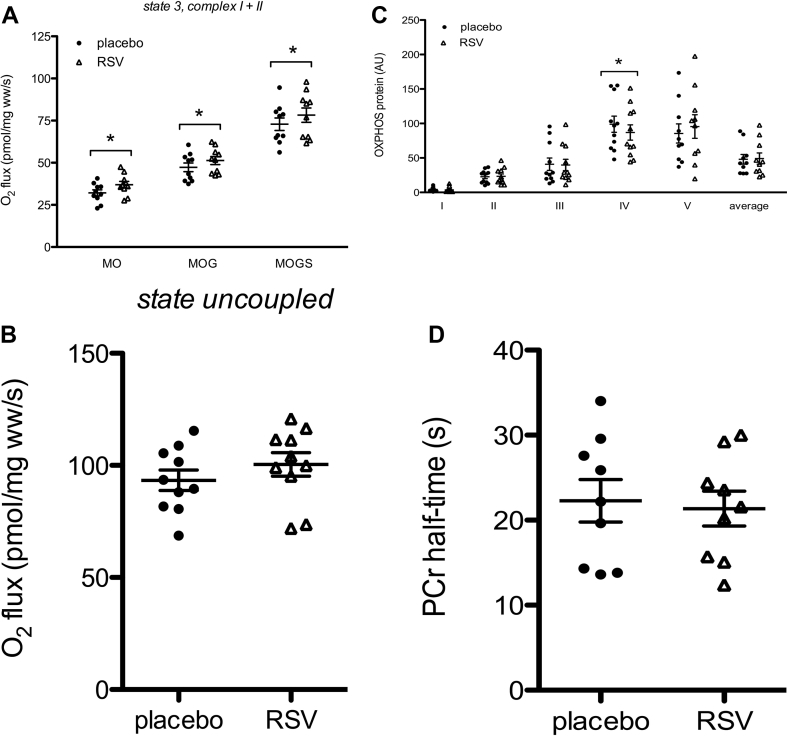
Resveratrol is expected to act on whole body metabolism via improvement of mitochondrial function. Therefore, mitochondrial state 3 respiration on a lipid-derived substrate (malate + octanoyl-carnitine, 3MO) was measured. State 3 respiration was significantly higher after resveratrol supplementation (32 ± 1.8 after placebo vs. 37 ± 1.9 pmol/mg wet weight/s after resveratrol, p < 0.001; Figure 3A). Also, state 3 respiration with parallel electron input to both complex I and II (malate + octanoyl-carnitine + glutamate + succinate, 3MOGS) was significantly higher after resveratrol supplementation (73 ± 3.7 after placebo vs. 78 ± 4.3 pmol/mg wet weight/s after resveratrol, p = 0.050; Figure 3A). Maximal FCCP-induced uncoupled respiration (sate u) also seemed to increase upon resveratrol administration but failed to reach statistical significance (p = 0.218, Figure 3B). In the absence of octanolyl-carnitine, no effects of resveratrol were observed in state 3 (3 MG, 3MGS) or state 4° respiration with complex I- and II-linked substrates, consistent with our previous reports [4], [5]. Protein content of the structural components of the individual OXPHOS complexes remained unaffected, except for complex IV that was significantly lower expressed after resveratrol supplementation (p = 0.033, Figure 3C). Finally, in vivo mitochondrial function, determined by PCr recovery half-time, was unchanged (Figure 3D).
Figure 3.
Effect of resveratrol (RSV) on ex vivo and in vivo mitochondrial function. After 30 days of resveratrol and placebo, a muscle biopsy specimen was obtained from the vastus lateralis muscle. Part of the specimen was used for evaluation of ex vivo mitochondrial function (n = 10). A: ADP stimulated respiration (state 3) upon a lipid-like substrate and upon parallel electron input into complex I and II. B: Maximally uncoupled respiration upon FCCP (carbonyl cyanide p-(trifluoro-methoxy)-phenylhydrazone). C: the protein content of the individual complexes of the electron transport chain is quantified by western blotting in vastus lateralis muscle. An antibody cocktail that detects all five complexes was used (n = 10). D: in vivo mitochondrial function expressed as PCr half-time (n = 9). Data are presented as individual data points, means ± SEM. *P < 0.05 compared to placebo. M, malate; O, octanoyl-carnitine; G, glutamate; S, succinate; ww, wet weight; OXPHOS, oxidative phosphorylation; PCr, phosphocreatine recovery.
3.4. Ectopic fat accumulation
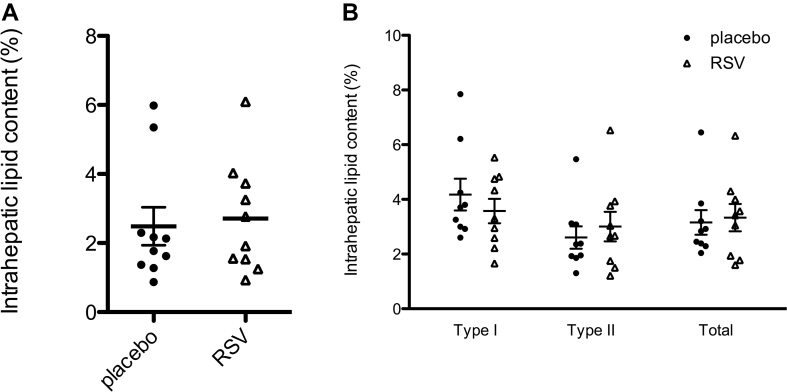
Resveratrol has been shown to be able to affect ectopic fat accumulation. However, IHL content, determined by 1H-MRS, remained unaffected by resveratrol (Figure 4A). Furthermore, IMCL, analyzed ex vivo in muscle biopsies, revealed no change in lipid area fraction in neither type I or type II muscle fibers (Figure 4B).
Figure 4.
Effect of resveratrol (RSV) on ectopic lipid storage. A: Intrahepatic lipid content quantified by 1H-MRS after 29 days of resveratrol and placebo supplementation. Box plot represents minimum, first quartile, median, third quartile, and maximum (n = 10). B: Muscle biopsy sections for the vastus lateralis muscle were stained for intramyocellular lipid content by Oil Red O staining. Intramyocellular lipid content is quantified as the percentage area of a muscle fiber that is covered by lipids. Data are presented as individuals data points, means ± SEM (n = 9).
3.5. Cardiac function
Animal studies have suggested that resveratrol may improve cardiovascular function. Nevertheless, systolic and diastolic blood pressure were unaltered by resveratrol (Table S3). Also, no changes in stroke volume, cardiac output, left ventricular end systolic diameter, or left ventricular ejection fraction were found (Table S3). Parameters of diastolic function and parameters representing structural changes of the heart also remained unchanged (Table S3).
3.6. Brown adipose tissue
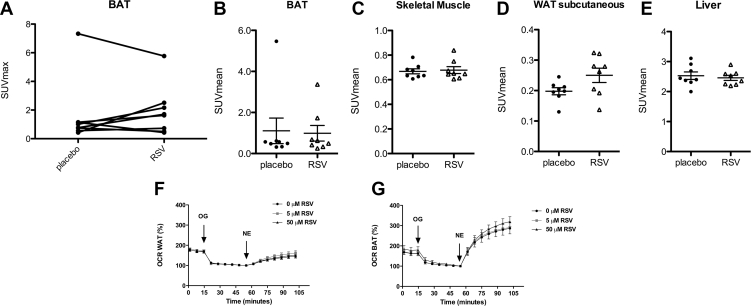
Rodent studies indicated that resveratrol could activate BAT. Here, we examined if resveratrol treatment increases cold-activated BAT activity using PET-CT scanning. Only one participant showed 18F-FDG uptake in BAT above the threshold of 1.5 SUV in both periods, as can be clearly seen in Figure 5A as the top line, indicating that these FDR participants are characterized by low levels of cold-induced BAT activity. Using the fixed volume method, we found that in the BAT region both SUVmax (1.6 ± 0.82 and 1.9 ± 0.61, Figure 5A) and SUVmean (1.1 ± 0.62 and 1.0 ± 0.38 after placebo and resveratrol respectively, Figure 5B) were not changed after resveratrol treatment. Likewise, 18F-FDG uptake did not change in skeletal muscle, subcutaneous WAT or liver (Figure 5B–E). In accordance with a lack of effect of resveratrol on BAT, resveratrol treatment did not influence cold-induced metabolic rate (5.3 ± 0.22 after placebo, versus 5.4 ± 0.22 kJ/min after resveratrol).
Figure 5.
Effects of resveratrol (RSV) on in vivo and in vitro brown adipose tissue. A–B: 18F-FDG uptake in BAT, WAT, muscle, and liver after 34 days of placebo and resveratrol intervention (n = 8). BAT SUV max (A) and BAT, SM, WATsc, and liver SUV mean (B–E) were measured under cold-stimulated conditions. (F–G) Respiration was measured in oligomycin (OG)-treated brown (n = 4) and white adipocytes (n = 6) following 1 μM norepinephrine. Data are presented as individual data points, mean ± SEM. BAT, brown adipose tissue; WAT, white adipose tissue; SM, skeletal muscle; SUV, standard uptake value; OCR, oxygen consumption rate.
In our clinical trial, the treatment duration may have been too short or the dose of resveratrol too low to accomplish effects on BAT. Therefore, to further investigate if resveratrol can stimulate human BAT activity, we examined mitochondrial uncoupling in adipocytes derived from human BAT and WAT. NE-stimulated mitochondrial uncoupling was specifically increased in adipocytes derived from human BAT. Illustrating the characteristic capacity of BAT to induce mitochondrial uncoupling. Nevertheless, incubating the cells with 5 or 50 μM trans-resveratrol for 24 h did not alter NE-stimulated mitochondrial uncoupling in adipocytes derived from BAT or WAT (Figure 5F–G).
4. Discussion
Resveratrol has been suggested to be beneficial in the prevention and treatment of T2D. Here, we investigated the effects of 30 days of resveratrol supplementation, 150 mg/day, in men at increased risk of developing T2D. However, in this population we did not see an improvement in insulin sensitivity after 30 days of resveratrol compared to placebo. Neither hepatic nor peripheral insulin sensitivity were improved, albeit we did demonstrate that 150 mg resveratrol per day was sufficient to improve ex vivo muscle mitochondrial oxidative capacity. Other markers of metabolic health and BAT metabolism also remained unchanged. Our data indicates that low-dose resveratrol supplementation does not seem to have beneficial metabolic effects in men at increased risk of developing T2D.
The potential role resveratrol could play in prevention and treatment of metabolic diseases, such as T2D, has been widely studied over the past decades. Studies in rodents show clear improvement upon resveratrol administration on among others insulin sensitivity, mitochondrial function, liver fat accumulation and indications towards increased BAT metabolism [24]. Nevertheless, human clinical trials have given inconsistent results. Some studies show clear improvement in parameters related to metabolic health [4], [25], [26], [27], [28], [29], while others see no or barely any effects [5], [30], [31], [32], [33], [34], [35], [36].
Here, we used the same dose and treatment duration of resveratrol as in our first study in healthy obese volunteers. In that study, we found beneficial effects of resveratrol on several metabolic parameters, and most importantly activation of the SIRT1-PGC-1α pathway in skeletal muscle leading to improved mitochondrial function [4], illustrating that resveratrol at this dose is indeed capable of affecting this pathway in humans. However, three other studies, with comparable resveratrol dose and treatment duration, failed to achieve improvements in glucose control [5], [35], [36]. One can therefore debate whether the dose of resveratrol used might be too low or the treatment duration too short to elicit effects on glucose metabolism in patients or subjects with disrupted glucose homeostasis. A recent clinical trial by Kjaer et al. [37] used a high (1000 mg/day) and low dose (150 mg/day) of resveratrol for a period of six months but also did not measure effects on glucose or lipid metabolism in obese men with moderate insulin resistance. Therefore, it can be speculated that differences in subject characteristics, such as age, body weight, gut microbiota composition, and metabolic status are more important influential factors for effects of resveratrol on metabolic health.
It is known that resveratrol is rapidly metabolized into its glucuronidated and sulfated form in the intestine and liver, resulting in a very low bioavailability [38]. In addition, a recent study suggests that unconjugated resveratrol, also called free resveratrol, is the active form of resveratrol [39]. Therefore, measuring plasma concentrations of free and conjugated resveratrol could shed light on why resveratrol is effective in some but not in other participants. Unfortunately, few clinical trials focused on improving metabolic health have actually measured free resveratrol concentrations. Therefore, we compared our plasma total resveratrol (free + conjugated) concentrations to two of our previous studies that used the same dose and duration of resveratrol treatment, a study with healthy obese men [4] and a study with T2D patients [5]. In all three studies, we observed positive effects of resveratrol on mitochondrial metabolism. Whereas the study with healthy obese men gave positive effects on glucose metabolism and other measures of metabolic health, our current study in FDRs and our previous study in T2D patients did not. Strikingly, in the study with healthy obese men, total resveratrol concentrations were markedly lower than those measured in the study with T2D patients and in our current FDR study (healthy obese: 183 ± 30 ng/mL; T2D patients: 379 ± 41 ng/mL; FDRs: 412 ± 63 ng/mL, p < 0.01). Higher plasma levels of resveratrol with the same intake of resveratrol could be caused by several factors: increased uptake of resveratrol from the gut, increased conjugation, diminished excretion by the kidneys and/or lower uptake of resveratrol from the plasma by the tissues. Over the 30-day resveratrol treatment period, no increase was observed in total resveratrol or DHR, indicating no accumulation occurred in the plasma. This suggests that excretion by the kidneys was not hampered. Whether potential differences in the active form of resveratrol or uptake of resveratrol in the tissues can explain differences between studies, however, cannot be deduced from the current study. It is important to note that in our first study in healthy obese men, we did not determine insulin sensitivity but only had data on fasting plasma glucose and insulin concentrations, and thus HOMA-IR.
In our current study, we found significant improvements in mitochondrial state 3 respiration on a lipid-derived substrate. These results are comparable to previous clinical trials [4], [5], [40]. We noted these improvements without changes in OXPHOS protein content, except for a decrease in complex IV, indicating that resveratrol affects predominantly mitochondrial efficiency and not abundance. An alternative explanation for the lack of effect of resveratrol on metabolic health parameters is that merely improving mitochondrial function is not sufficient to improve whole body insulin sensitivity or metabolic health in general. This suggestion is supported by findings of Hoehn et al. [41], who concluded that acute or chronic up-regulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. In addition, Wong et al. [42] previously reported that overexpressing PGC-1α in mouse skeletal muscle did not prevent high-fat diet induced insulin resistance, despite improved mitochondrial function. Only when mice were stimulated to become physically active was the PGC-1α induced increase in mitochondrial function accompanied by improved insulin sensitivity. This may suggest that just increasing mitochondrial function is not sufficient to stimulate insulin sensitivity, and it could be speculated that physical activity levels in our T2D patients [4] or FDR subjects were too low to benefit from the small increase in resveratrol-induced mitochondrial function. Future studies could test the concept that resveratrol, in addition to a physical activity program, could have beneficial effects in volunteers with disturbed glucose homeostasis.
Brown adipocytes contain a high density of mitochondria and have been suggested to also be a target of resveratrol. This hypothesis has, to our knowledge, never been examined in vivo in humans. In general our FDRs display low levels of uptake of 18F-FDG in BAT, which could be attributed to the sex, age and body weight of the participants since BAT activity declines with age and is decreased in males and overweight [43]. Despite the low starting levels of BAT, 34 days of supplementation with resveratrol did not increase BAT activity as expressed by cold-stimulated 18F-FDG uptake. Correspondingly, when in vitro cultured adipocytes derived from human BAT were incubated with 5 or 50 μM trans-resveratrol this did not increase NE-stimulated mitochondrial uncoupling.
5. Conclusion
The current study indicates that resveratrol supplementation does not affect insulin sensitivity, substrate utilization, energy expenditure, ectopic fat accumulation, or cardiac function and does not stimulate BAT in men at increased risk of developing T2D. However, the data do support our previous findings that low-dose resveratrol supplementation can improve muscle mitochondrial oxidative capacity. Future studies should unravel why there are inconsistencies in effectiveness of resveratrol when supplied to different populations, which may include investigating physical activity level of participants and bioavailability and active compounds of resveratrol.
Financial support
This study was funded by a Diabetes Fonds Project Grant (2012.00.1525). In addition, we acknowledge the support from the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation (CVON2014-02 ENERGISE). DSM Nutritional Products Ltd. for provided the resveratrol and placebo capsules.
Authors' contributions
M.d.L. designed and performed the experiments, analyzed the data, and wrote the manuscript. Y.B., J.H., M-F.H, B.H., E.N., E.M-K., and G.S. assisted during the experiments and reviewed and edited the manuscript. V.S., W.M-L., and P.S. contributed to the design of the study, interpretation of the data, and reviewed and edited the manuscript.
Acknowledgements
We are grateful to all the participants who volunteered in the study. In addition, we would like to thank DSM Nutritional Products Ltd. for providing the resveratrol and placebo capsules. Finally, we are very grateful to the Dutch Diabetes Foundation and the Dutch Heart Foundation for giving us the opportunity to execute this study, and providing us with the necessary support to perform all experiments.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.04.004.
Conflict of interest
All authors approved the final version of the manuscript. None of the authors have a potential conflict of interest to report regarding this article. P.S. is the guarantor of this work. Thus, he has full access to all the data of the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Szkudelski T., Szkudelska K. Resveratrol and diabetes: from animal to human studies. Biochimica et Biophysica Acta. 2015;1852(6):1145–1154. doi: 10.1016/j.bbadis.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni S.S., Canto C. The molecular targets of resveratrol. Biochimica et Biophysica Acta. 2015;1852(6):1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Ruderman N.B., Xu X.J., Nelson L., Cacicedo J.M., Saha A.K., Lan F. AMPK and SIRT1: a long-standing partnership? American Journal of Physiology, Endocrinology and Metabolism. 2010;298(4):E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmers S., Konings E., Bilet L., Houtkooper R.H., van de Weijer T., Goossens G.H. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metabolism. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmers S., de Ligt M., Phielix E., van de Weijer T., Hansen J., Moonen-Kornips E. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: a randomized controlled trial. Diabetes Care. 2016;39(12):2211–2217. doi: 10.2337/dc16-0499. [DOI] [PubMed] [Google Scholar]
- 6.Vaag A., Lehtovirta M., Thye-Ronn P., Groop L., European Group of Insulin R Metabolic impact of a family history of Type 2 diabetes. Results from a European multicentre study (EGIR) Diabetic Medicine. 2001;18(7):533–540. doi: 10.1046/j.1464-5491.2001.00496.x. [DOI] [PubMed] [Google Scholar]
- 7.Patti M.E., Butte A.J., Crunkhorn S., Cusi K., Berria R., Kashyap S. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the USA. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morino K., Petersen K.F., Dufour S., Befroy D., Frattini J., Shatzkes N. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. Journal of Clinical Investigation. 2005;115(12):3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Castaner M., Biarnes J., Camps I., Ripolles J., Gomez N., Soler J. Beta-cell dysfunction in first-degree relatives of patients with non-insulin-dependent diabetes mellitus. Diabetic Medicine. 1996;13(11):953–959. doi: 10.1002/(SICI)1096-9136(199611)13:11<953::AID-DIA257>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Phielix E., Schrauwen-Hinderling V.B., Mensink M., Lenaers E., Meex R., Hoeks J. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57(11):2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Alberdi G., Rodriguez V.M., Miranda J., Macarulla M.T., Churruca I., Portillo M.P. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chemistry. 2013;141(2):1530–1535. doi: 10.1016/j.foodchem.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 13.Andrade J.M., Frade A.C., Guimaraes J.B., Freitas K.M., Lopes M.T., Guimaraes A.L. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. European Journal of Nutrition. 2014 doi: 10.1007/s00394-014-0655-6. [DOI] [PubMed] [Google Scholar]
- 14.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 15.Schulz T.J., Tseng Y.H. Brown adipose tissue: development, metabolism and beyond. Biochemical Journal. 2013;453(2):167–178. doi: 10.1042/BJ20130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mari A., Pacini G., Murphy E., Ludvik B., Nolan J.J. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539–548. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 17.Lindeboom L., Nabuurs C.I., Hesselink M.K., Wildberger J.E., Schrauwen P., Schrauwen-Hinderling V.B. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. American Journal of Clinical Nutrition. 2015;101(1):65–71. doi: 10.3945/ajcn.114.094730. [DOI] [PubMed] [Google Scholar]
- 18.Schrauwen-Hinderling V.B., Kooi M.E., Hesselink M.K., Jeneson J.A., Backes W.H., van Echteld C.J. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50(1):113–120. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 19.Schoffelen P.F., Westerterp K.R., Saris W.H., Ten Hoor F. A dual-respiration chamber system with automated calibration. Journal of Applied Physiology. 1997;83(6):2064–2072. doi: 10.1152/jappl.1997.83.6.2064. [DOI] [PubMed] [Google Scholar]
- 20.Koopman R., Schaart G., Hesselink M.K. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochemistry and Cell Biology. 2001;116(1):63–68. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- 21.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 22.van der Lans A.A., Wierts R., Vosselman M.J., Schrauwen P., Brans B., van Marken Lichtenbelt W.D. Cold-activated brown adipose tissue in human adults: methodological issues. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2014;307(2):R103–R113. doi: 10.1152/ajpregu.00021.2014. [DOI] [PubMed] [Google Scholar]
- 23.Broeders E.P., Nascimento E.B., Havekes B., Brans B., Roumans K.H., Tailleux A. The bile acid chenodeoxycholic acid increases human Brown adipose tissue activity. Cell Metabolism. 2015;22(3):418–426. doi: 10.1016/j.cmet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 24.de Ligt M., Timmers S., Schrauwen P. Resveratrol and obesity: can resveratrol relieve metabolic disturbances? Biochimica et Biophysica Acta. 2015;1852(6):1137–1144. doi: 10.1016/j.bbadis.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt J.K., Thomas S., Nanjan M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutrition Research. 2012;32(7):537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Brasnyo P., Molnar G.A., Mohas M., Marko L., Laczy B., Cseh J. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. British Journal of Nutrition. 2011;106(3):383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 27.Crandall J.P., Oram V., Trandafirescu G., Reid M., Kishore P., Hawkins M. Pilot study of resveratrol in older adults with impaired glucose tolerance. Journals of Gerontology Series A Biomedical Sciences and Medical Sciences. 2012;67(12):1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Movahed A., Nabipour I., Lieben Louis X., Thandapilly S.J., Yu L., Kalantarhormozi M. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evidence Based Complementary and Alternative Medicine. 2013;2013:851267. doi: 10.1155/2013/851267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez-del Villar M., Gonzalez-Ortiz M., Martinez-Abundis E., Perez-Rubio K.G., Lizarraga-Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metabolic Syndrome and Related Disorders. 2014;12(10):497–501. doi: 10.1089/met.2014.0082. [DOI] [PubMed] [Google Scholar]
- 30.Yoshino J., Conte C., Fontana L., Mittendorfer B., Imai S., Schechtman K.B. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metabolism. 2012;16(5):658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dash S., Xiao C., Morgantini C., Szeto L., Lewis G.F. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(12):2895–2901. doi: 10.1161/ATVBAHA.113.302342. [DOI] [PubMed] [Google Scholar]
- 32.Poulsen M.M., Vestergaard P.F., Clasen B.F., Radko Y., Christensen L.P., Stodkilde-Jorgensen H. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62(4):1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chachay V.S., Macdonald G.A., Martin J.H., Whitehead J.P., O'Moore-Sullivan T.M., Lee P. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology. 2014;12(12):2092–2103. doi: 10.1016/j.cgh.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Olesen J., Gliemann L., Bienso R., Schmidt J., Hellsten Y., Pilegaard H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. The Journal of Physiology. 2014;592(8):1873–1886. doi: 10.1113/jphysiol.2013.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Made S.M., Plat J., Mensink R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: a randomized, placebo-controlled crossover trial. PLoS One. 2015;10(3):e0118393. doi: 10.1371/journal.pone.0118393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zortea K., Franco V.C., Francesconi L.P., Cereser K.M., Lobato M.I., Belmonte-de-Abreu P.S. Resveratrol supplementation in schizophrenia patients: a randomized clinical trial evaluating serum glucose and cardiovascular risk factors. Nutrients. 2016;8(2):73. doi: 10.3390/nu8020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjaer T.N., Ornstrup M.J., Poulsen M.M., Stodkilde-Jorgensen H., Jessen N., Jorgensen J.O.L. No beneficial effects of resveratrol on the metabolic syndrome: a randomized placebo-controlled clinical trial. Journal of Clinical Endocrinology & Metabolism. 2017;102(5):1642–1651. doi: 10.1210/jc.2016-2160. [DOI] [PubMed] [Google Scholar]
- 38.Walle T. Bioavailability of resveratrol. Annals of the New York Academy of Sciences. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 39.Gheldof N. Role of sulfotransferases in resveratrol metabolism in human adipocytes. Molecular Nutrition & Food Research. 2017;61(10):1–10. doi: 10.1002/mnfr.201700020. [DOI] [PubMed] [Google Scholar]
- 40.Most J., Timmers S., Warnke I., Jocken J.W., van Boekschoten M., de Groot P. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: a randomized controlled trial. American Journal of Clinical Nutrition. 2016;104(1):215–227. doi: 10.3945/ajcn.115.122937. [DOI] [PubMed] [Google Scholar]
- 41.Hoehn K.L., Turner N., Swarbrick M.M., Wilks D., Preston E., Phua Y. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metabolism. 2010;11(1):70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong K.E., Mikus C.R., Slentz D.H., Seiler S.E., DeBalsi K.L., Ilkayeva O.R. Muscle-specific overexpression of PGC-1alpha does not augment metabolic improvements in response to exercise and caloric restriction. Diabetes. 2015;64(5):1532–1543. doi: 10.2337/db14-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lecoultre V., Ravussin E. Brown adipose tissue and aging. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14(1):1–6. doi: 10.1097/MCO.0b013e328341221e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.