Abstract
Every year billions of chickens are shipped thousands of miles around the globe in order to meet the ever increasing demands for this cheap and nutritious protein source. Unfortunately, transporting chickens internationally can also increase the chance for introducing zoonotic viruses, such as highly pathogenic avian influenza A (H5N1) to new countries. Our study used a retrospective analysis of poultry trading data from 2003 through 2011 to assess the risk of H5N1 poultry infection in an importing country. We found that the risk of infection in an importing country increased by a factor of 1.3 (95% CI: 1.1–1.5) for every 10-fold increase in live chickens imported from countries experiencing at least one H5N1 poultry case during that year. These results suggest that the risk in a particular country can be significantly reduced if imports from countries experiencing an outbreak are decreased during the year of infection or if biosecurity measures such as screening, vaccination, and infection control practices are increased. These findings show that limiting trade of live chickens or increasing infection control practices during contagious periods may be an important step in reducing the spread of H5N1 and other emerging avian influenza viruses.
Keywords: Highly pathogenic avian influenza A (H5N1), Poultry trade, Transmission model, Zoonotic disease
Highly pathogenic avian influenza A (H5N1) was first identified in Chinese poultry in 1996 and has spread to 53 countries as of July 2014 (Organization for Animal Health, 2014a). Previous studies suggest that both poultry trade and bird migration are the main drivers spreading this virus to previously uninfected countries (Kilpatrick et al., 2006). Unfortunately, due to limited or poor infectious disease surveillance, identified and reported H5N1 cases are likely to only represent the tip of the iceberg of the true number of cases occurring in a particular country. Consequently, limiting trade of live birds or increasing prevention efforts during this time may have greater impacts on reducing the spread of this disease.
The zoonotic nature and pandemic potential of avian influenza viruses, especially H5N1 (de Jong, Claas, Osterhaus, Webster, & Lim, 1997), makes their introduction into new countries especially troublesome. As of December 2015, there have been a total of 844 identified human cases, and 449 deaths, giving a case fatality rate in humans of 53% (World Health Organization, 2016). Although it is not currently easily transmittable in mammals, and only a couple cases of human-to-human transmission have occurred, there is concern that this could easily be changed by a few genetic mutations (Russell et al., 2012). Additionally, H5N1 can have devastating economic impacts from mass culling of sick birds, decreased market demand during an outbreak, and loss of trade (Chmielewski & Swayne, 2011).
Gaining a better understanding of the drivers that spread avian influenza globally will be important for targeting and improving future prevention efforts and policies, such as vaccination, increased biosecurity and limiting trade during epidemic periods. Identifying the mechanism for H5N1 will also be important for understanding the spread of similar avian influenza viruses of concern, such as H7N9 which recently emerged in China in February 2013 (Centers for Disease Control and Prevention, 2013).
A previous study found that poultry trading and bird migration, played a role in the introductions of avian flu to new countries, with the magnitude of risk from these two factors differing by region. However, this study was done in 2006 and didn't assess some of the more recent introductions of avian influenza into new countries and did not assess the specific role of different species (Kilpatrick et al., 2006).
Other social network analysis models have looked at Live Bird Market (LBM) trade and the spread of H5N1 at the regional or country level (Fournié et al, 2013, Soares Magalhães et al, 2010, Soares Magalhães et al, 2012). One study found that counties in China with H5N1 positive birds identified in LBMs had significantly higher centrality measures than counties without H5N1 in poultry (Martin et al., 2011), indicating that hub areas may be at greatest risk. Another network study found that avian influenza disease transmission can be reduced if the network is fragmented through increasing prevention efforts or limiting trade at the hub sites (Fournié et al., 2013). Additionally, temporal changes in network connectivity, due to increased demands for poultry consumption, have also been used to identify time periods that may be associated with greater risk (Soares Magalhães et al., 2012). Unlike previous studies that have only looked at the local or county level networks, our study is unique in that it identifies networks of poultry trading at the global level.
The goal of this paper is to determine if the international spread of highly pathogenic H5N1 in poultry have been influenced by the magnitude of poultry traded from infected countries. This study assessed potential covariates on this association such as year, gross domestic product (GDP), and population size of the importing and exporting country and also examined the role of different poultry species, including ducks, chickens, and turkeys, in driving H5N1 global spread. Our final model shows how much risk could be reduced if imports are limited from countries experiencing an H5N1 poultry case or if prevention efforts are increased during these time periods.
Results
In 2003, two countries reported an H5N1 poultry case to the WHO, six years after the last case was identified. This number of countries with poultry cases increased and peaked to 37 countries in 2006, and declined to 14 by 2011. The number of countries importing chickens from an infected country followed a similar trend, starting with 7 in 2003, peaking to 87 in 2006, and declining back to 9 in 2011. The percentage of countries with an outbreak in poultry that imported chickens from an infected country also peaked during 2006. The number of chicken imports from any country appears to have slightly declined over time from 2003 through 2011; however the quantity of chicken imports has steadily increased. Interestingly, H5N1 cases in humans followed a similar trend, with the number of cases and number of countries with a case peaking around the same time as in poultry (Table 1).
Table 1.
Descriptive statistics of chicken trade and outbreaks of avian influenza A (H5N1) in poultry and humans.
| Year | No. of countries with an H5N1 case in poultry | No. of countries importing chickens from an infected country | % of countries with an outbreak that imported chickens from an infected country | No. of chicken trades between countries | Thousands of chickens imported from infected countries | Thousands of chickens imported from all countries | % of all imported chickens that were from infected countries | No. of human H5N1 cases4 | Human case fatality rate | No. of countries with an H5N1 case in humans18 |
|---|---|---|---|---|---|---|---|---|---|---|
| 2003 | 2 | 7 | 0 | 702 | 2180 | 687,671 | 0.3 | 4 | 100 | 2 |
| 2004 | 9 | 7 | 22 | 682 | 35,161 | 787,585 | 4.5 | 46 | 70 | 2 |
| 2005 | 9 | 19 | 44 | 672 | 27,866 | 854,789 | 3.3 | 98 | 44 | 5 |
| 2006 | 37 | 87 | 68 | 581 | 308,001 | 807,714 | 38.1 | 115 | 69 | 9 |
| 2007 | 29 | 80 | 55 | 627 | 374,537 | 952,675 | 39.3 | 88 | 67 | 9 |
| 2008 | 22 | 66 | 41 | 598 | 190,883 | 967,539 | 19.7 | 44 | 75 | 6 |
| 2009 | 10 | 5 | 20 | 599 | 6181 | 1,116,485 | 0.6 | 73 | 44 | 5 |
| 2010 | 15 | 13 | 13 | 597 | 8544 | 1,203,651 | 0.7 | 48 | 50 | 5 |
| 2011 | 14 | 9 | 14 | 581 | 10,321 | 1,273,598 | 0.8 | 62 | 55 | 5 |
We used statistical models to assess the relationship between poultry trade and transmission of H5N1 from one country to another (see Materials and Methods). This study found significant and positive interaction between quantity of chickens traded and H5N1 poultry infection in the exporting country, in both the non-adjusted (p = 0.0013) and full adjusted models (p = 0.0005) (Table 2, Table 3). For every unit increase in the log of the number of chickens that were imported from an infected country, the poultry infection in the importing country increased by a factor of 1.3 (95% CI: 1.1–1.5) for every 10-fold increase in live chickens imported when adjusting for year, import population and export population. The quantity of chickens traded (not part of the interaction term) was not significant (p = 0.34), indicating that increased trading of non-infected chickens did not change risk of infection. We also found that human population in the importing and exporting country was positively associated with the outcome (Table 3). GDP and GDP per capita were also assessed in the model but were not statistically significant. Ducks and turkeys were also assessed in a similar model, but ducks did not have a significant interaction (Supplementary Table 1) and turkeys had a significantly negative coefficient for the log of the quantity of turkeys traded (Supplementary Table 2).
Table 2.
GEE model regressing H5N1 infection in the importing country on H5N1 infection in the exporting country, thousands of chickens traded, and their interaction. Errors clustered on import country. N = 365,418, QIC = 195,618.3, Null QIC = 206,928.9
| Effect | OR | 95% CI | p-value |
|---|---|---|---|
| Log (chicken) × H5N1 in export country | 1.22 | 1.08, 1.39 | 0.0013 |
| Log (chicken) | 1.13 | 0.98, 1.30 | 0.093 |
| H5N1 in export country | 0.93 | 0.92, 1.06 | <0.0001 |
| Year | |||
| 2003 | 0.13 | 0.03, 0.54 | 0.0051 |
| 2004 | 0.63 | 0.34, 1.15 | 0.13 |
| 2005 | 0.63 | 0.29, 1.34 | 0.23 |
| 2006 | 3.03 | 1.82, 5.05 | <0.0001 |
| 2007 | 2.25 | 1.35, 3.74 | 0.0018 |
| 2008 | 1.65 | 1.04, 2.59 | 0.032 |
| 2009 | 0.70 | 0.45, 1.07 | 0.101 |
| 2010 | 1.08 | 0.78, 1.49 | 0.65 |
| 2011 | – | – | – |
Table 3.
Full GEE modela regressing H5N1 infection in the importing country on H5N1 infection in the exporting country, thousands of chickens traded, and their interaction. Errors clustered on import country, N = 365,418, QIC = 150,089.7
| Effect | OR | 95% CI | p-value |
|---|---|---|---|
| Log (chicken) × H5N1 in export country | 1.30 | 1.12, 1.51 | 0.0005 |
| Log (chicken) | 0.93 | 0.80, 1.08 | 0.34 |
| H5N1 in export country | 0.92 | 0.91, 0.93 | <0.0001 |
| Year | |||
| 2003 | 0.10 | 0.02, 0.49 | 0.0041 |
| 2004 | 0.57 | 0.27, 1.21 | 0.14 |
| 2005 | 0.57 | 0.23, 1.40 | 0.22 |
| 2006 | 4.06 | 2.20, 7.46 | <0.0001 |
| 2007 | 2.77 | 1.48, 5.21 | 0.0015 |
| 2008 | 1.84 | 1.06, 3.19 | 0.029 |
| 2009 | 0.65 | 0.39, 1.08 | 0.10 |
| 2010 | 1.09 | 0.74, 1.62 | 0.65 |
| 2011 | – | – | – |
| Log (export population) | 1.01 | 1.00, 1.01 | 0.0001 |
| Log (import population) | 7.17 | 4.14, 12.43 | <0.0001 |
Full model is adjusted for log (export population) and log (import population).
The triple interaction models showed significant negative associations for the interaction term of H5N1 in the export country, log (chickens) and time period (Supplementary Table 3), as well as H5N1 in the export country, log (chickens) and Southeast Asia or East Asia region (Supplementary Tables 4–5). A third region model with South Asia was also assed but not found to be significant.
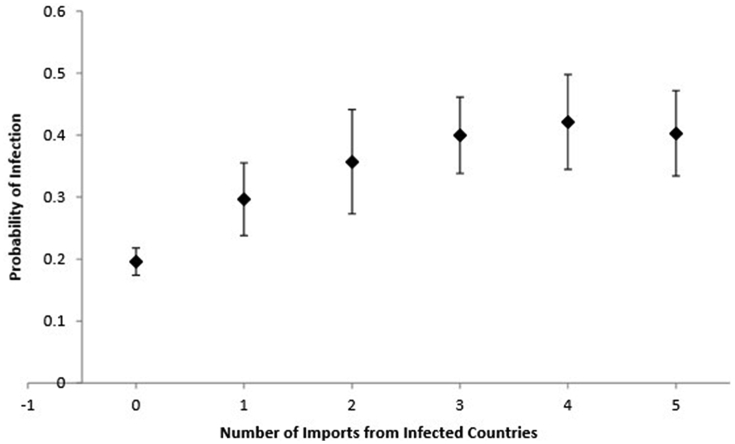
The FAO trade data and WHO reported case data also showed that as the number of imports from infected countries increased, the probability of infection (as determined from the model in Table 1) also increased, with significantly higher risk in countries importing from one or more infected countries compared to none in 2006. There was a small baseline probability of infection in countries not importing infectious chickens (Fig. 1).
Fig. 1.
Probability of infection (SD) in the importing country in 2006 based on the number of infected countries from which they imported chickens.
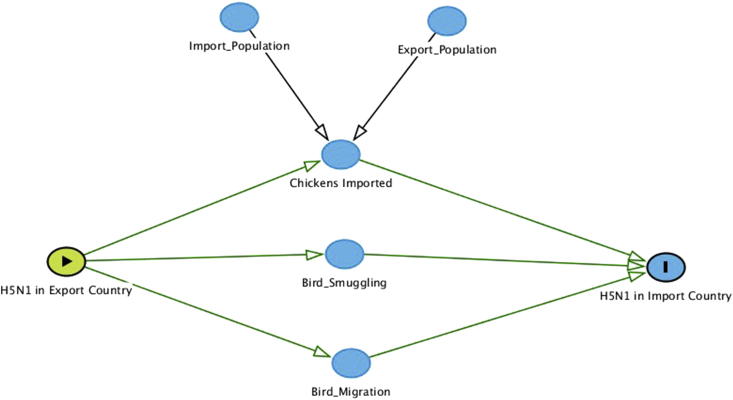
Using our infection model, we created a Directed Acyclic Graph showing the relationships between all the variables in our model and additional variables that we were not able to account for due to lack of data (Fig. 2). It is possible that chicken trade, bird migration and smuggling all play a role in the spread of H5N1 infection between countries.
Fig. 2.
Directed acyclic graph showing the relationships between variables included in the model and additional variables that could have played a role in the relationship between spread of H5N1 between countries.
Discussion
The results of this study suggest that the more chickens a country imports from an infected country, the greater the risk for having an H5N1 case in poultry. Unfortunately, it may sometimes be difficult for a country to identify infected chickens in a flock that is being traded. Avian influenza has an incubation period of 3–5 days in chickens (Organization for Animal Health, 2014b), during which time infected birds may unknowingly be traded before they show illness. Additionally, increased use of avian influenza vaccination in poultry may reduce symptom severity and thus delay detection among a flock (Savill, St Rose, Keeling, & Woolhouse, 2006). H5N1 also has non-specific symptoms in chickens, which may be misdiagnosed as a similar looking infection, such as Newcastle disease, unless laboratory tests confirm H5N1 viruses (Organization for Animal Health, 2014b). Infectious disease surveillance and laboratory resources are also limited in some countries and it is likely that many cases are missed. Consequently, if a country has an identified avian influenza outbreak within its borders, it may be beneficial to limit all exports for that year or longer, as there are likely to be other undetected cases still circulating. If reducing trade is not possible, increased prevention efforts such controlling movement of birds and culling of sick animals, combined with heightened screening and active surveillance of poultry that is being traded will be important for preventing disease transmission.
Our results also suggest a relatively large risk increase with human population size in the importing country. Since population size is correlated with GDP, more populous countries may have better resources to identify imported cases. However, GDP was not found to be significant in the model. A small increase in risk was also associated with an increase in export population size (Table 3).
Interestingly, we did not find that the trade of turkeys or ducks significantly impacted H5N1 disease spread across borders (Supplementary Tables 1 and 2). Although, ducks are commonly asymptomatic carriers of avian influenza and shed the virus for longer (Hulse-Post et al., 2005), the magnitude of duck and turkey trading worldwide was much smaller than that of chickens. Additionally, the demand for and trade of turkeys was especially low in Asia where H5N1 cases predominate. As production and demand for ducks and turkeys increase, it will be important to reassess their role in transmission of avian influenza globally.
Our study did find significant association for the triple interaction terms with the dummy variables time period and region. This suggests that risk varied by region and time. However, in all models, the main interaction term of H5N1 in the export country and log (chickens) remained significant (Supplementary Tables 3–5).
One weakness of this study is the timeliness and completeness of reported H5N1 poultry cases. Countries sometimes delay or don't report H5N1 poultry cases due to fear about the negative impact it could have on their economy. Additionally, public health authorities may not be aware of an outbreak until several weeks later, since many outbreaks occur in people's backyard where they may not recognize the signs and symptoms of avian influenza or may be afraid to report cases to their government. Despite this, we found that countries with endemic H5N1 avian flu, such as Egypt and Indonesia, continued to report cases to the WHO through 2011.
Another weakness of this paper is that it includes ecological level data; therefore we were not able to tie a specific bird trade to the introduction of avian influenza to a new country. We also only used import data which is sometimes different than reported export data; however, in most cases they were the same or similar. Additionally, we only knew the year of the poultry trade: consequently, we did not know for sure whether the trade happened before or after an identified case. Moreover, it was impossible to know if consecutive yearly H5N1 cases in the same country were the result of continued infections within the country or reintroductions from outside countries. However, by the end of our study period, only a few countries were considered to have endemic H5N1 influenza in poultry (FAO-OIE-WHO, 2011).
It is highly likely that other variables not accounted for in our model, also played a significant role in driving H5N1 spread as seen in our Directed Acyclic Graph (see Fig. 2). One potential risk factor that was not accounted for in our model is smuggling of birds across borders through illegal trade (van den Berg, 2009). This is not captured by the FAOSTAT database but could have contributed to the spread of H5N1, especially in some regions where borders are not highly regulated. Additionally, since we only looked at poultry trade, we do not know the role that migratory birds played in spreading avian influenza across borders. Avian influenza has been found in many migratory birds and other studies have found that both poultry trade and migratory birds likely played a key role in transmission of H5N1 around the globe (Kilpatrick et al., 2006). The elevated baseline probability of H5N1 poultry infection in countries that didn't trade infected chickens, as seen in Fig. 1, is likely explained by these other factors.
In an effort to feed the current seven billion people on the globe today, it is necessary to reevaluate the risks of shipping our food thousands of miles around the globe. Gaining a better understanding of how H5N1 spreads internationally is especially important, as poultry has become one of the fastest-growing livestock industries, due to high consumer demand and low price compared to other meat sources (Food and Agriculture Organization, 2014). Eating food that is produced locally may greatly decrease the spread of infectious diseases, such as H5N1 which can threaten economies, livelihoods, and human and animal health. If a reduction in trade from infectious countries is not possible, improved screening and infection control practices during epidemic periods can also help reduce transmission across borders.
Methods
Data collection
A historical prospective network design was used, starting with the first cases of sustained H5N1 transmission in poultry in 2003 and following the spread forward until 2011. There were two earlier reported cases of H5N1 identified in poultry in 1996 and 1997; however these were not included due to the six year period of no reported poultry outbreaks from 1998 to 2003. Poultry H5N1 cases were identified from the World Health Organization's avian influenza timeline of events (World Health Organization, 2012). Binary coding was used for H5N1 infection in each country: countries were considered infected for each year that they reported an H5N1 case in poultry, regardless of outbreak size, production type (backyard, small farm, industrial), or species. Reports of H5N1 amongst wild birds were not included. Human avian influenza data was collected from the World Health Organization's cumulative case report (World Health Organization, 2016).
Poultry importing and exporting data were collected from the Food and Agriculture Organization's (FAO) FAOSTAT database (Food and Agriculture Organization, 2013). Data was collected from 1996 to 2011 (the most recent year) for live chickens, ducks, and turkeys. This database includes the value and number of live poultry (1000 heads) that were imported between each country by year. In some instances, there was a trade reported with a value, but no poultry count because the magnitude of the trade was less than 1000 birds, therefore the exact number of the trade could not be determined as the value per bird varied by country. In these cases, we recoded the trade as 500 poultry heads.
This study included 202 countries, regardless of whether they traded any poultry during the time period of the study. Country population size, density, and GDP in 2011 were collected from the World Bank's database (The World Bank, 2014).
Statistical analysis
The following Generalized Estimating Equation (GEE) model was used to predict H5N1 poultry infection in an importing country for a given year: Ijt = α + β1Iit*Cijt + β2*Cijt + β3Iit + T2003…2010, where Ijt is H5N1 poultry infection in importing country j at year t, Iit is H5N1 poultry infection in exporting country i at year t, Cijt is thousands of heads of poultry imported from country i to country j at year t, and T2003…2010 as year fixed effects. A dummy variable was used to create year fixed effects. This variable was included because reporting of H5N1 cases is likely to have fluctuated over the study period as countries became more aware of this new emerging disease and gained resources for surveillance, identification, and prevention. The GEE model was selected because our data consisted of repeated measures of imports from each country over time. This model type allowed us to cluster our errors on import country since trading amongst the same countries was often correlated from year to year (Table 2, Table 3). The same model clustering on export country was also evaluated and yielded substantively identical results. The role of ducks and turkeys were also assessed separately in an identical model (Supplementary Tables 1–2).
In our adjusted model we assessed the role of human density and population, since previous studies have found an increased number of poultry H5N1 outbreaks are associated with higher human population density in the same region (Si, de Boer, & Gong, 2013). We also assessed the model controlling for GDP and GDP per capita since developing countries usually have less public health resources to investigate outbreaks. Human population, density, GDP, and GDP per capita were log-transformed since they were not normally distributed. Variables with p < 0.05 were included in our final adjusted model (Table 3).
Additional models with a triple interaction term for H5N1 in export country*log (quantity of chickens*Time Period) and one with H5N1 the export country*log (quantity of chickens)*(region) were assessed to determine if risk varied by region. The dummy variable for time period was coded as 2006–2008 versus all other years and the dummy variable for region was coded as SE Asia versus all other regions and East Asia versus all other regions (Supplementary Tables 3–5).
SAS version 9.3 was used for all statistical analyses: PROC GENMOD was used for the GEE models with a repeated statement for import country, link = logit and a binary distribution. An independent correlation structure was used. DAGitty was used to create the Directed Acyclic Graph (Textor, Hardt, & Knüppel, 2011).
Author contributions
J.M.R. collected the data and wrote the manuscript. J.M.R. analyzed the data with input and supervision from J.H.F and R.R. R.A.S, S.P.L, M.R.G.A, R.R., and J.H.F edited the manuscript and provided design input.
Competing financial interests
There were no competing financial interests.
Acknowledgments
This work is sponsored in part by the Robert Wood Johnson Foundation Pioneer Grant 67919.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.idm.2017.09.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- van den Berg T. The role of the legal and illegal trade of live birds and avian products in the spread of avian influenza. Revue scientifique et technique (International Office of Epizootics) 2009;28:93–111. doi: 10.20506/rst.28.1.1878. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Emergence of avian influenza A(H7N9) virus causing severe human illness – China, February-April 2013. Morbidity and Mortality Weekly Report. 2013;62:366–371. [PMC free article] [PubMed] [Google Scholar]
- Chmielewski R., Swayne D. Avian influenza: Public health and food safety concerns. Annual Review of Food Science and Technology. 2011;2:37–57. doi: 10.1146/annurev-food-022510-133710. [DOI] [PubMed] [Google Scholar]
- FAO-OIE-WHO technical update: current evolution of avian influenza H5N1 viruses. 7 September 2011. http://www.who.int/influenza/human_animal_interface/tripartite_notes_H5N1.pdf Available at: [Google Scholar]
- Food and Agriculture Organization (FAO) FAOSTAT: Detailed world agricultural grade flows. Available at: http://faostat.fao.org/. Accessed 23 August 2013.
- Food and Agriculture Organization (FAO). Food outlook: Biannual report of global food markets. Available at: http://www.fao.org/docrep/018/al999e/al999e.pdf. Accessed 6 September 2014.
- Fournié G., Guitian J., Desvaux S., Cuong V.C., Dungdo H., Pfeiffer D.U. Interventions for avian influenza A (H5N1) risk management in live bird market networks. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9177–9182. doi: 10.1073/pnas.1220815110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse-Post D.J., Sturm-Ramirez K.M., Humberd J., Seiler P., Govorkova E.A., Krauss S. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J.C., Claas E.C., Osterhaus A.D., Webster R.G., Lim W.L. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A.M., Chimura A.A., Gibbons D.W., Fleischer R.C., Marra P.P., Daszak P. Predicting the global spread of H5N1 avian influenza. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19368–19373. doi: 10.1073/pnas.0609227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin V., Zhou X., Marshall E., Jia B., Fusheng G., FrancoDixon M.A. Risk-based surveillance for avian influenza control along poultry market chains in South China: The value of social network analysis. Preventive Veterinary Medicine. 2011;102:196–205. doi: 10.1016/j.prevetmed.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization for Animal Health (OIE). Outbreaks of highly pathogenic avian influenza (subtype H5N1) in poultry notified to the OIE from the end of 2003 to 17 July 2014. Available at: http://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/graph_avian_influenza/graphs_HPAI_17_07_2014.pdf. Accessed 6 September 2014.
- Organization for Animal Health (OIE). Bird flu avian influenza. Available at: http://www.oie.int/doc/ged/D10302.PDF. Accessed 27 June 2014.
- Russell C.A., Fonville J.M., Brown A.E., Burke D.F., Smith D.L., James S.L. The potential for respiratory droplet-transmissible A/H5N1 virus to evolve in a mammalian host. Science. 2012;336:1541–1547. doi: 10.1126/science.1222526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill N.J., St Rose S.G., Keeling M.J., Woolhouse M.E. Silent spread of H5N1 in vaccinated poultry. Nature. 2006;442:757. doi: 10.1038/442757a. [DOI] [PubMed] [Google Scholar]
- Si Y., de Boer W.F., Gong P. Different environmental drivers of highly pathogenic avian influenza H5N1 outbreaks in poultry and wild birds. PLoS One. 2013;8:e53362. doi: 10.1371/journal.pone.0053362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares Magalhães R.J., Ortiz-Pelaez A., Thi K.L., Dinh Q.H., Otte J., Pfeiffer D.U. Associations between attributes of live poultry trade and HPA1 H5N1 outbreaks: A descriptive and network analysis study in northern Vietnam. BMC Veterinary Research. 2010;6:1–13. doi: 10.1186/1746-6148-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares Magalhães R.J., Zhou X., Jia B., Guo F., Pfeiffer D.U., Martin V. Live poultry trade in Southern China provinces and HPAIV H5N1 infection in humans and poultry: The role of Chinese new year festivities. PLoS One. 2012;7:e49712. doi: 10.1371/journal.pone.0049712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J., Hardt J., Knüppel S. DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology. 2011;5(22):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- The World Bank. Data: Indicators. Available at: http://data.worldbank.org/indicator. Accessed 6 September 2014.
- World Health Organization (WHO) 25 January 2012. H5N1 avian influenza: Timeline of major events.http://www.who.int/influenza/human_animal_interface/H5N1_avian_influenza_update.pdf Available at: [Google Scholar]
- World Health Organization (WHO). Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. Available at: http://www.who.int/influenza/human_animal_interface/EN_GIP_20151214cumulativeNumberH5N1cases.pdf?ua=1. Accessed 19 January 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.