Abstract
Background
The use of poor quality antimalarial medicines, including the use of non-recommended medicines for treatment such as sulfadoxine-pyrimethamine (SP) monotherapy, undermines malaria control and elimination efforts. Furthermore, the use of subtherapeutic doses of the active ingredient(s) can theoretically promote the emergence and transmission of drug resistant parasites.
Methods
We developed a deterministic compartmental model to quantify the impact of antimalarial medicine quality on the transmission of SP resistance, and validated it using sensitivity analysis and a comparison with data from Kenya collected in 2006. We modelled human and mosquito population dynamics, incorporating two Plasmodium falciparum subtypes (SP-sensitive and SP-resistant) and both poor quality and good quality (artemether-lumefantrine) antimalarial use.
Findings
The model predicted that an increase in human malaria cases, and among these, an increase in the proportion of SP-resistant infections, resulted from an increase in poor quality SP antimalarial use, whether it was full- or half-dose SP monotherapy.
Interpretation
Our findings suggest that an increase in poor quality antimalarial use predicts an increase in the transmission of resistance. This highlights the need for stricter control and regulation on the availability and use of poor quality antimalarial medicines, in order to offer safe and effective treatments, and work towards the eradication of malaria.
Keywords: Deterministic compartmental model, Falsified antimalarial medicine, Substandard antimalarial treatments, Antimalarial quality, Plasmodium falciparum malaria, Drug resistance
1. Introduction
The spread of antimalarial resistance is hampering malaria control and elimination efforts globally (Ambroise-Thomas, 2012, World Health Organization, 2010a). Poor quality antimalarials can be categorised into three main groups: falsified; substandard; and degraded (WorldWide Antimalarial Resistance Network, 2010). Each of these can be a source of subtherapeutic doses of the active ingredient(s), which promote the emergence and transmission of drug resistant parasites through selection pressures (Barnes et al., 2008, Simpson et al., 2000, White et al., 2009). Falsified antimalarials are those that are fraudulently made and typically contain an incorrect amount of active ingredient, incorrect active ingredient, toxic substances, or no active ingredient. Substandard antimalarials are those made by licenced companies but use poor manufacturing practices. Degraded antimalarials degrade from their initial quality due to inadequate storage conditions, such as excessive heat. In addition, within poor quality antimalarials, we include those that are not recommended in the World Health Organization (WHO) guidelines.
Approximately 30% of antimalarial medicines in Africa and Asia are considered to be falsified or substandard (Ambroise-Thomas, 2012, Newton et al., 2009). The outcome for those receiving poor quality antimalarials ranges from prolonged malaria symptoms, unexpected side effects, financial strain due to loss of income or healthcare costs, or even death (Ambroise-Thomas, 2012, Newton et al., 2006, Tabernero et al., 2014). In Kenya, prior to 2004, sulfadoxine-pyrimethamine (SP) had been recommended as first-line for treatment of malaria. Due to increasing resistance to SP, stemming from mutations in the P. falciparum dihydrofolate reductase (DHFR) gene, which affects pyrimethamine, and the dihydropteroate synthase (DHPS) gene, which affects sulfadoxine, Kenya adopted artemether-lumefantrine (AL) as its first-line treatment in 2004. In 2001, WHO recommended the use of artemisinin-based combination therapies (ACTs) as first-line policy (World Health Organization, 2010b). In December 2007, a report was produced surveying the antimalarial medicines available in Kenya and their quality. The researchers identified a wide range of products on the market, the majority of which were not in-line with the new national guidelines, and a high proportion were either un-registered or of low quality (Ministry of Health Republic of Kenya, 2007).
The effect of antimalarial use on the transmission of resistance has been modelled previously (Hastings, 2006, Klein, 2014, Koella and Antia, 2003, Mackinnon and Hastings, 1998, Tchuenche et al., 2011). Notably, the models currently available do not take into account the quality or percentage of antimalarial active ingredient and its effect on transmission. As summarised by Koella and Antia (2003), part of the issue preventing these resistance transmission models from being developed and used is a lack of complete, comprehensive datasets for key parameters. Since their model was published, work has been carried out to look at the effect of drug quality on resistance within mice (Huijben et al., 2010a, Huijben et al., 2013) and the effect of treatment in humans with SP-resistant infections (Barnes et al., 2008, Barnes et al., 2008, Méndez et al., 2007).
Here we develop a new model to explore the impact of antimalarial quality, defined as poor quality SP, as defined above, and good quality AL, on the transmission of SP antimalarial resistance in Plasmodium falciparum. To assist in more realistic parameterisation of the model, we applied the model to Kenya in 2006, rather than Kenya being a focus for actual predictions. The model assumes that low to moderate SP-resistance conferred by mutations in the DHFR gene, the target of pyrimethamine, has already been established within both human and mosquito populations.
2. Materials and methods
2.1. Model structure
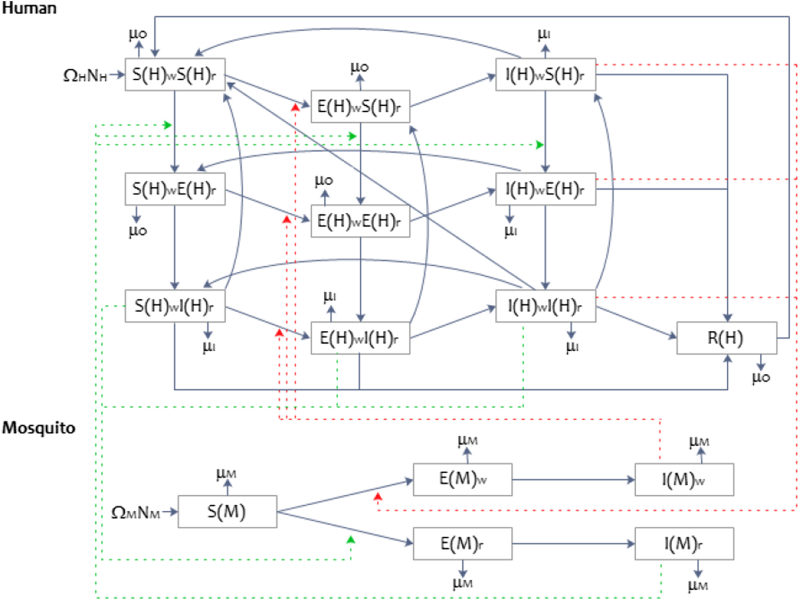
We developed a deterministic compartmental model to explore the impact of antimalarial quality on the transmission of P. falciparum SP resistance (Fig. 1). The model quantifies the transmission dynamics of SP-sensitive (denoted ) and SP-resistant (denoted ) P. falciparum between female Anopheles mosquitoes and humans. The human-mosquito system is modelled using ordinary differential equations (ODEs) (Eq. (A1), Appendix A1). Humans may be infected by SP-sensitive strains , SP-resistant strains , or both . Resistance to SP was defined as the presence of DHFR-51 and DHFR-108 pyrimethamine resistance-conferring mutations (Méndez et al., 2007), used as proxy for all low to moderate SP-resistant conferring mutations in P. falciparum (Sridaran et al., 2010). At baseline, the percentage of humans and mosquitoes with SP-resistant infections was set to 42% (Kum et al., 2013, Spalding et al., 2010) and mixed infections was set to 8% (Kum et al., 2013).
Fig. 1.
A summary of the structure of the mathematical model showing the movement between compartments of SP-sensitive and SP-resistant Plasmodium falciparum in humans and female Anopheles mosquitoes (blue solid line). The transmission of gametocytes (infected human to susceptible mosquito) and sporozoites (infected mosquito to susceptible human) during a blood meal, is depicted by the red dotted line for SP-sensitive, and a dark green dotted line for SP-resistant.
Humans free of P. falciparum were classified as susceptible and denoted by . When transmission of sporozoites occurs from female An. mosquitoes to humans during a blood meal, the human moves into the exposed class ( at the rate . The script indicates a SP-sensitive ( is ) or SP-resistant ( is ) P. falciparum infection. Due to the difference in the latent periods for asexual P. falciparum and gametocytes, it is assumed that antimalarial treatment is sought while in the exposed class to treat malaria symptoms as part of the asexual lifecycle (Poser & Bruyn, 1999). There are four types of treatment available, each used as a proxy for ‘good quality’ or ‘poor quality’ treatments. Infected humans receive each treatment type with probability , where the subscript is for a full dose of AL (good quality); for a full-dose of SP monotherapy (poor quality); for a half-dose SP monotherapy (poor quality); and for no treatment, either through no antimalarial compound within the medicine sought or choosing not to seek treatment (poor quality). Following the gametocyte latency period, those in the exposed class move into the infectious class at rate , which is assumed to be equal for both SP-sensitive and SP-resistant infections. The length of infectiousness and probability of transmission of gametocytes from infected humans to mosquitoes are specified for each strain and treatment combination. The recovery rate is defined by . Natural immunity is gained at rate , among those who do not receive treatment (Bousema & Drakeley, 2011), and lost at rate . The protective nature and rates for gaining and losing natural immunity are also assumed to be independent of P. falciparum infection type.
Female Anopheles mosquitoes may be susceptible , exposed , or infected . Movement from susceptible to an exposed class, after the transmission of P. falciparum during a blood meal, occurs at rate ; where is for SP-sensitive parasites or for SP-resistant P. falciparum. The rate of transmission is defined as the product of the daily mosquito biting rate and the probability of transmission given parasite strain and drug treatment received by the human ( or ). We assume that An. mosquitoes can only be infected by one strain of P. falciparum gametocytes (i.e., no mixed infections), and in the occurrence that a susceptible mosquito feeds on a human host with a mixed infection, the probability of the SP-sensitive strain being selected over the SP-resistant strain and proceeding through the mosquito's midgut and onto the salivary gland, is set at 0.6, assigning a relatively small fitness cost to resistance (Appendix D1). Following the latent period, the mosquito enters the infectious class at rate , and it is assumed that they do not recover from their infection due to their short lifespans (Mandal, Sarkar, & Sinha, 2011). The parameters used in the model are defined in Table 1, Table 2. Additional parameters, including details on the calculations of mosquito and human demographic turnover rates can be found in Appendices B–E. The impact of SP on the level of gametocytes in humans has been extensively researched (Barnes et al., 2008, Barnes et al., 2008, Bousema and Drakeley, 2011, Méndez et al., 2007). We calculated an estimate for the length of gametocyte carriage and the probability of transmission when treated with a full dose pyrimethamine, half dose (using 50% or 37.5% of a full dose) of pyrimethamine, or no treatment (Huijben et al., 2010b, Huijben et al., 2013, Huijben et al., 2010a), and then calibrated these scenarios against human SP monotherapy studies (Barnes et al., 2008, Barnes et al., 2008, Méndez et al., 2007) (C2, C3).
Table 1.
Human Parameters. A summary of the model parameters used to calculate the rates of change in human movement (daily) between model compartments, including: parameter definitions, symbols, parameter values used in the baseline model, and literature references or the section of the Appendices where the parameters are defined.
| Parameter description | Symbol | Value | Reference |
|---|---|---|---|
| Human population size (initial) | Updated per iteration | ||
| Birth rate | 1.1349 × 10−4 | Appendix B1 | |
| Rate of humans becoming exposed to SP-sensitive sporozoites | 0.0810 | Appendix B2 | |
| Rate of humans becoming exposed to SP-resistant sporozoites | 0.0810 | Appendix B2 | |
| Rate of humans becoming infectious (gametocytes) | 0.0556 | Appendix B3 | |
| Receiving AL (proportion, at baseline) | 0.70 | Assumed, Appendix C1 | |
| Receiving full-dose SP monotherapy (proportion, at baseline) | 0.07 | Demographic and Health Surveys (various), 2003–2012 | |
| Receiving full-dose SP monotherapy (proportion, at baseline) | 0.03 | Minzi et al., 2003, Newton et al., 2006, Tabernero et al., 2014 | |
| Receiving no treatment (proportion, at baseline) | 0.20 | Chuma et al. (2007) | |
| Rate of human recovery from SP-sensitive P. falciparum having received AL | 0.1667 | Appendix B4 | |
| Rate of human recovery from SP-sensitive P. falciparum having received full-dose SP monotherapy | 0.0588 | Appendix B4 | |
| Rate of human recovery from SP-sensitive P. falciparum having received half-dose SP monotherapy | 0.0476 | Appendix B4 | |
| Rate of human recovery from SP-resistant P. falciparum having received AL | 0.1667 | Appendix B4 | |
| Rate of human recovery from SP- resistant P. falciparum having received full-dose SP monotherapy | 0.0096 | Appendix B4 | |
| Rate of human recovery from SP- resistant P. falciparum having received half-dose SP monotherapy | 0.0096 | Appendix B4 | |
| Rate of human recovery from mixed P. falciparum infection having received AL | 0.1667 | Appendix B4 | |
| Rate of human recovery from mixed P. falciparum infection having received full-dose SP monotherapy | 0.0096 | Appendix B4 | |
| Rate of human recovery from mixed P. falciparum infection having received half-dose SP monotherapy | 0.0119 | Appendix B4 | |
| Rate of recovery having received no treatment | 0.0149 | Appendix B4 | |
| Overall transmission of SP-sensitive gametocytes (probability) | 0.1459 | Appendix D2 | |
| Overall transmission of SP-resistant gametocytes (probability) | 0.1410 | Appendix D2 | |
| Rate of acquired immunity | 6.0864 × 10−4 | Appendix B5 | |
| Rate of loss of acquired immunity | 0.0027 | Labadin, Kon, & Juan (2009) | |
| Rate of malarial mortality in humans | 8.2880 × 10−4 | Appendix B6 | |
| Rate of “other” mortality in humans | 3.1779 × 10−5 | Appendix B6 |
Table 2.
Mosquito Parameters. A summary of the model parameters used to calculate the rates of change of movement (daily) of female An. mosquitoes between model compartments, including: parameter definitions, symbols, parameter values used in the baseline model, and literature references or the section of the Appendices where the parameters are defined.
| Parameter description | Symbol | Value | Reference |
|---|---|---|---|
| Ratio of An. mosquito to human population (initial) | 0.87 | Updated per iteration | |
| Rate female An. mosquitoes reach adulthood | 0.0280 | Chitnisa et al., 2008, Labadin et al., 2009 | |
| Biting rate of female An. Mosquitoes | 0.4050 | Anderson and May, 1991, Mandal et al., 2011 | |
| Rate of mosquitoes becoming exposed to SP-sensitive gametocytes | 0.0591 | Appendix E1 | |
| Rate of mosquitoes becoming exposed to SP-resistant gametocytes | 0.0571 | Appendix E1 | |
| Rate of mosquitoes becoming infectious (sporozoites at salivary gland) | 0.2000 | Appendix E2 | |
| Rate of mortality of female An. mosquitoes | 0.0280 | Mandal et al. (2011), Appendix E3 |
The model simulations were run at the 2006 baseline level for all parameters, with the initial conditions (Appendix A2) chosen to match surveillance data observed in Kenya in 2006. The system was solved for 1 year (2006), and the results analysed. All analysis were carried out in Mathworks Matlab 2012a, using the ODE15s solver.
2.2. Gametocyte carriage and infectiousness
The parameter values for the duration of gametocyte carriage and the probability a mosquito takes up a mature (infectious) gametocyte during a blood meal, given the infection-type and treatment received by the patient, utilised a combination of data from mice malaria studies for pyrimethamine (Huijben et al., 2010b, Huijben et al., 2013, Huijben et al., 2010a) and human SP studies (Barnes et al., 2008, Barnes et al., 2008, Méndez et al., 2007). Pyrimethamine (not in combination with sulfadoxine) mice studies were used to inform parameterisation due to the availability of data on pyrimethamine, with no such data was available on SP. This method is described in C2, C3.
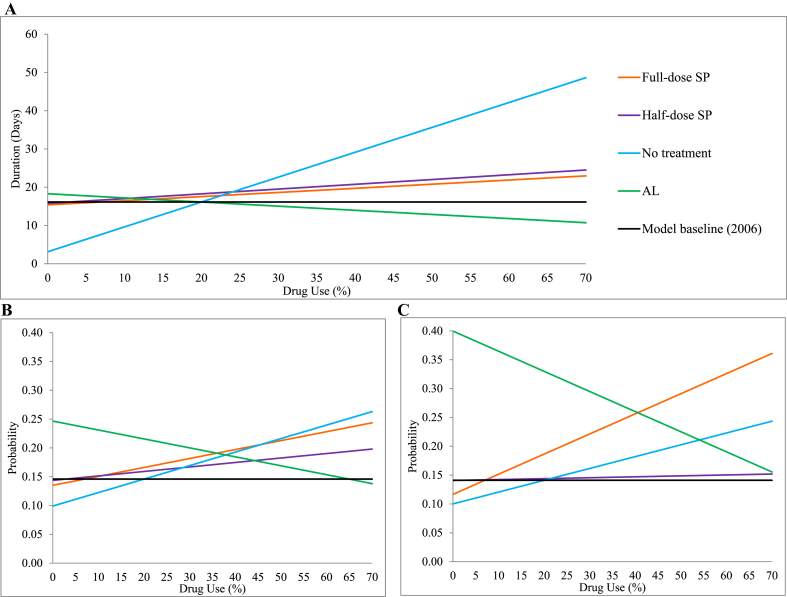
The expected duration of gametocyte carriage, given treatment scenarios, were calculated and compared to the 2006 baseline treatment level (70% AL use (assumed, Appendix C1), 7% full-dose SP monotherapy (Demographic and Health Surveys (various), 2003–2012), 3% half-dose SP monotherapy (Minzi et al., 2003, Newton et al., 2006, Tabernero et al., 2014), and 20% no treatment (Chuma, Gilson, & Molyneux, 2007)), in Fig. 2A and Table 3. An increase in the use of full-dose or half-dose SP monotherapy use, or no treatment by 1% (with a corresponding 1% decrease in AL use), resulted in an increase in the duration of gametocyte carriage from baseline (16.1 days) of 2½ hour, 3 h and 16 h, respectively. However, exclusive use of either full-dose or half-dose SP (all other treatments set to 0%), results in the duration decreasing from baseline (3.3 days and 1.6 days, respectively); whereas increasing when no treatment is exclusively used by 50.9 days; indicating no treatment has a large impact on carriage. In contrast, as the percentage of AL treatment increases, a decrease in the average duration of gametocyte carriage is observed (2 h per 1% increase), down to an eventual duration of 2 days when used exclusively.
Fig. 2.
(A) The impact of antimalarial quality on the average duration of gametocyte carriage in humans. (B–C) The impact of antimalarial quality on the infectiousness of humans to mosquitoes during a blood meal (probability), of (B) SP-sensitive and (C) SP-resistant gametocytes. Changes in the percentage use of full-dose SP monotherapy (, orange line) were adjusted for the use of 3% half-dose SP monotherapy , 20% receiving no treatment and the remainder AL treatment . Likewise, changes in half-dose SP monotherapy use (θp, purple line) were adjusted for and remainder; changes in those receiving no treatment (θn, blue line) were adjusted for and remainder; and changes in AL use (θq, green line) were adjusted for and remainder. The 2006 model baseline (black line) corresponds to and .
Table 3.
The impact of changes in the percentage use of treatments (after 365 days), with percentage change, when compared to 2006 model baseline for: the average duration of gametocyte carriage; and the probability of mosquitoes taking up infectious gametocytes. The 2006 model baseline treatment use was set to 70% AL treatment , 7% full-dose SP monotherapy , 3% half-dose SP monotherapy and 20% no treatment . Changes in the percentage use of full-dose SP monotherapy were adjusted for and = remainder; changes in the use of half-dose SP monotherapy use (θp) were adjusted for and remainder; changes in those receiving no treatment (θn) were adjusted for and remainder; and changes in AL use (θq) were adjusted for and remainder. For exclusive use of a treatment (100% use), all other treatments were set to 0%.
| Drug Use Scenarios | Duration gametocyte carriage | Probability infectious gametocytes |
|
|---|---|---|---|
| SP-sensitive | SP-resistant | ||
| 2006 model baseline | 16.1 days | 0.1459 | 0.1410 |
| +1% full-dose SP use | +2½ hours (0.6%) | +0.0016 (1.1%) | +0.0035 (2.5%) |
| +1% half-dose SP use | +3 hours (0.8%) | +0.0008 (0.5%) | +0.0002 (0.1%) |
| +1% no treatment | +16 hours (4.2%) | +0.0024 (1.6%) | +0.0021 (1.5%) |
| +1% AL use | − 2 hours (0.5%) | − 0.0015 (1.0%) | − 0.0034 (2.4%) |
| 100% full-dose SP use | − 3.3 days (20.4%) | +0.0951 (65.2%) | +0.2830 (200.7%) |
| 100% half-dose SP use | − 1.6 days (10.2%) | +0.0178 (12.2%) | −0.0498 (35.3%) |
| 100% no treatment | +50.9 days (316.1%) | +0.1741 (119.3%) | +0.1390 (98.6%) |
| 100% AL use | −14.1 days (87.6%) | −0.0600 (41.1%) | −0.0658 (46.7%) |
The calculated probability of mosquitoes taking up infectious gametocytes during a blood meal also increased in response to greater use of full- and half-dose SP monotherapy, and no treatment (Fig. 2B–C, Table 3). In settings where SP resistance is already firmly established, a 1% increase in full-dose SP monotherapy resulted in a larger percentage increase in SP-resistant infectiousness, than sensitive infectiousness (2.5% v 1.1%). This is further highlighted under the scenario of exclusive use of full-dose SP, where results indicate a 200.7% increase in the probability of mosquitoes taking up SP-resistant P. falciparum, compared to a 65.2% increase in SP-sensitive P. falciparum. In contrast, 1% increases in half-dose SP or no treatment use had a greater percentage increase in the probability of mosquitoes taking up SP-sensitive P. falciparum (0.5% and 1.6%, respectively) compared to SP-resistant P. falciparum (0.1% and 1.5%, respectively). However, when used exclusively, half-dose SP had the greatest percentage increase in SP-resistant P. falciparum compared to SP-sensitive (35.3% v 12.2%); with exclusive use of no treatment resulting in a similarly large percentage increase for both (98.6% and 119.3%, respectively). Changes in the use of AL resulted in a greater decrease in the probability of SP-resistant P. falciparum being taken up by mosquitoes, compared to SP-sensitive P. falciparum (2.4% v 1.0%, for 1% increase; and 46.7% v 41.1%, when exclusively used).
2.3. Measuring the effect on transmission
The main outcome of interest is the impact of antimalarial quality on the total proportion of SP-resistant infections (resistant and mixed infections) in the human population (, Eq. (1)):
| (1) |
Here denotes the human classes (excluding acquired immunity), where the subscript and once again denote SP-sensitive and SP-resistant P. falciparum, respectively.
In addition to the proportion of SP-resistant infections, we also measured the expected number of malaria cases in humans.
2.4. Model accuracy
To validate our results, we performed a sensitivity analysis, and compared baseline model predictions against estimates found in the literature. A one-way sensitivity analysis was carried out, where each parameter was individually changed to the minimum and maximum value in its defined parameter range, and the change in the total proportion of SP-resistant infections in the human population was calculated. Parameters that inferred a change of greater than were considered to be significant during the sensitivity analysis.
3. Results
To quantify the impact of antimalarial quality on the transmission of SP resistance in P. falciparum, we varied the amount of good and poor quality antimalarial use in the population. Any change in antimalarial use that assists the survival and propagation of antimalarial resistance within human and mosquito populations highlights the need for better control and regulations of the use and availability of these medicines in order to offer safe and effective treatment.
3.1. Malaria cases (human)
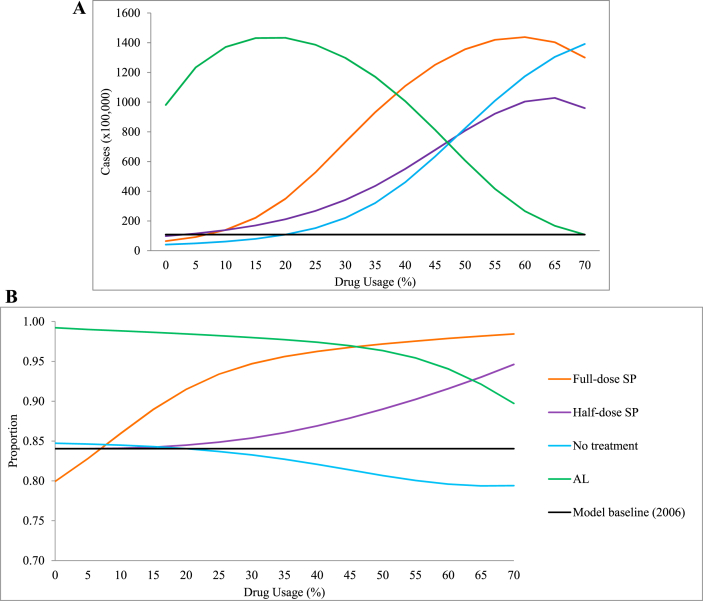
At baseline, our model predicts 10,807,000 malaria cases in Kenya during 2006 (Fig. 3A, Table 4). An increase in the use of poor quality antimalarial use predicts a greater number of malaria cases, with the greatest increase observed under the scenario of full-dose SP being exclusively used (776.9%), followed by no treatment (773.6%) and the exclusive use of half-dose SP use (558.6%). This suggests that people may experience multiple malaria infections within one calendar year (population size 36,757,498 (The World Bank., 2006–2013g)). Under the exclusive use of AL, the model predicts that the number of malaria cases in Kenya for 2006 could have been 2,978,200 (a reduction of 72.5% from baseline). The proportion of malaria cases that contained SP-resistant P. falciparum reflects these increases under each scenario of SP use, where exclusive use of full-dose SP increases by 18.4% from baseline, and half-dose increases by 13.8% (Fig. 3B, Table 4). Decreases in the proportion of SP-resistant infections were observed when no treatment was used (−5.8%) and the exclusive use of AL (−1.8%).
Fig. 3.
(A) The impact of antimalarial quality on the predicted number of human malaria cases in 2006. (B) The impact of antimalarial quality on the total proportion of SP-resistant infections in humans. Changes in the percentage use of full-dose SP monotherapy (, orange line) were adjusted for the use of 3% half-dose SP monotherapy , 20% receiving no treatment , and the remainder AL treatment . Likewise, changes in half-dose SP monotherapy use (θp, purple line) were adjusted for , and remainder; changes in those receiving no treatment (θn, blue line) were adjusted for and remainder; and changes in AL use (θq, green line) were adjusted for , and remainder. The 2006 model baseline (black line) corresponds to , and . Model simulations run for 365 days.
Table 4.
The impact of changes in the percentage use of treatments (after 365 days) on the expected number of malaria cases in Kenya for 2006 and the proportion of resistant infections (percentage change), when compared to the 2006 model baseline. The 2006 model baseline treatment use was set to 70% AL treatment , 7% full-dose SP monotherapy , 3% half-dose SP monotherapy , and 20% no treatment ; and for the exclusive use of a treatment (100% use), all other treatments were set to 0%.
| Drug Use Scenarios | Expected malaria cases (2006) | Proportion SP-resistant |
|---|---|---|
| 2006 model baseline | 10,807,000 | 0.8404 |
| 100% full-dose SP use | +83,964,000 (776.9%) | +0.1545 (18.4%) |
| 100% half-dose SP use | +60,366,000 (558.6%) | +0.1157 (13.8%) |
| 100% no treatment | +83,608,000 (773.6%) | −0.0491 (−5.8%) |
| 100% AL use | −7,831,300 (−72.5%) | −0.0148 (−1.8%) |
3.2 Results validation
Key 2006 baseline model output was compared to empirical estimates for the Kenyan population (Table 5), indicating our model predicted these outputs within an acceptable range. For the sensitivity analysis, parameters that inferred a change in the total proportion of SP-resistant infections in the human population of greater than were considered to be significant (Table 6). As seen with other malaria models (Mandal et al., 2011), our model was sensitive to mosquito parameters, such as the proportion of mosquitoes to humans, the daily rate female An. mosquitoes reach adulthood, and the probability of transmission of SP-sensitive and SP-resistant sporozoites during a blood meal. Additionally, the expected gametocyte clearances of SP-sensitive and SP-resistant gametocytes when treated with AL were found to significantly influence model outputs. The full sensitivity analysis is available in Appendix F.
Table 5.
Results validation. A comparison of the baseline model outcomes with literature estimates for 2006 and the published reference, including the percentage error in the 2006 model estimate, for: the rate of population growth; the proportion of each strain of Plasmodium falciparum malaria in humans; the number of P. falciparum cases of malaria in humans; and the human mortality (total and malaria-specific).
| Description | 2006 Model Outcome | 2006 Literature Value (Reference) | Difference (%) |
|---|---|---|---|
| Population growth, % (2006–2007) | 2.7559 | 2.7 (The World Bank, 2006–2013b) | 2 |
| Malaria cases | 10,857,000 | 8,926,058 (World Health Organization, 2010b) | 22 |
| Deaths (all) | 500,980 | 404,332 (The World Bank, 2006–2013b) | 24 |
| Malaria-specific deaths | 72,592 | 74,970 (The World Bank, 2006-2013b, World Health Organization, 2010b) | −3 |
| Proportion of SP-sensitive infections in humans | 0.1625 | 0.05–0.5 (Kum et al., 2013) | Within range |
| Proportion of SP-resistant infections in humans | 0.8356 | 0.42–0.90 (Kum et al., 2013, Spalding et al., 2010) | Within range |
| Proportion of mixed infections in humans | 0.0019 | 0–0.53 (Assumed) | Within range |
Table 6.
Sensitivity analysis summary. Results for the sensitivity analysis, where parameter range (minimum and/or maximum) resulted in a change in the proportion of SP-resistant infections in humans. Full sensitivity analysis results are available in Appendix F. A Largest value we could get a numerical solution for, actual literature range maximum value is 0.27.
| Parameter | Range (literature range or ±10%) |
Percentage change (%) |
|||
|---|---|---|---|---|---|
| Baseline | Minimum | Maximum | Minimum | Maximum | |
| Ratio of mosquito to human population (initial, humans = 1) | 0.87 | 0.5 | 40 | 0.32 | −20.55 |
| Rate female An. mosquitoes reach adulthood | 0.028 | 0.020 | 0.1406 A | 0.26 | −36.20 |
| SP-sensitive sporozoite transmission (probability) | 0.2 | 0.2 | 0.5 | 0.00 | −86.98 |
| SP-resistant sporozoite transmission (probability) | 0.2 | 0.2 | 0.5 | 0.00 | 17.90 |
| SP-sensitive gametocyte clearance in humans treated with AL | 14 | 7 | 28 | 9.20 | −13.61 |
| SP-resistant gametocyte clearance in humans treated with AL | 14 | 7 | 28 | −15.52 | 8.65 |
4. Discussion
Our model suggests that once SP resistance is widespread, as was the case in Kenya in 2006, an increase in poor quality antimalarial use (focusing on SP) results in an increase in: (i) the number of human malaria cases (Fig. 3A), and (ii) of these cases, an increase in the proportion of SP-resistant infections in humans (full- or half-dose SP used, Fig. 3B), when compared to good quality antimalarial use (AL). The predicted increase in malaria cases is of concern, where the scenario of full-dose SP being exclusively used (+776.9%), followed by no treatment (+773.6%) and the exclusive use of half-dose SP use (+558.6%), yield large increases; whereas the exclusive use of AL results in a marked decrease in the number of expected cases (−72.5%). The predicted increase in resistant-containing infections under SP drug pressure is supported by findings from Hastings (Hastings, 2006). Our findings suggest that a delay in P. falciparum clearance in humans, due to SP-resistance and/or inadequate antimalarial active ingredient, allows for prolonged transmission of SP-resistant gametocytes, hence ensuring their propagation throughout human and mosquito populations.
There are clear examples of substandard SP circulating in east Africa and elsewhere (see http://www.wwarn.org/aqsurveyor/#0). A common problem has been impaired drug dissolution due to poor manufacturing, despite having the correct amounts of SP in the tablet, which result in low blood SP drug levels (Leslie et al., 2009, White et al., 2009). The impacts described here for reduced dosage of SP will also apply to this situation of reduced bioavailability. In addition, systematic under-dosing of antimalarials, common in pregnancy and young children, has been shown to impact efficacy, with theoretical impacts of the selection of drug resistance (Barnes et al., 2008, Sambol et al., 2015). In all these cases, the key variable will be the antimalarial levels parasites are exposed to, reflecting both antimalarial content and bioavailability.
The impact of antimalarial quality on mortality could not be explicitly explored as the model assumes that the proportion of malaria-specific mortality is proportional to the prevalence of malaria and hence driven by this relationship. This additionally acts to drive the overall mortality.
The accuracy of the model indicated larger percentage errors in the predicted malaria cases and malaria deaths for 2006 (Table 5). However, this simulated number of malaria cases is below the 15 million cases estimated to have occurred in Kenya in 2006 (World Health Organization, 2010b), where under-reporting is considered a factor. This under-reporting is also assumed for malarial deaths, where there are discrepancies between the estimated (overall) deaths in Kenya in 2006 (The World Bank, 2006-2013c, World Health Organization, 2010b) (Appendix B6).
The parameterisation of the transmissibility and infectiousness of gametocytes under each treatment type utilised a combination of data from mice malaria studies for pyrimethamine (Huijben et al., 2010b, Huijben et al., 2013, Huijben et al., 2010a) and human SP studies (Barnes et al., 2008, Barnes et al., 2008, Méndez et al., 2007). The use of these calculated estimates introduces a margin of error; as well as the possible under-estimation of the transmissibility and infectiousness of those receiving a half-dose of SP (Appendix C3.2). The impact of antimalarial quality on the duration of gametocyte carriage seems plausible, with the largest increase predicted from increases in those who receive no treatment. Increases in carriage duration were observed with increases in the percentage SP use, with half-dose SP monotherapy showing more marked increases in carriage duration than full-dose SP monotherapy. This may be explained by SP-sensitive infections being cleared more slowly following sub-therapeutic concentrations of antimalarial medicine, then when using full-dose SP, thereby providing a longer period for gametocytes to remain in circulation. Interestingly, this relationship was not observed when considering pyrimethamine-resistant gametocyte density in mice, despite peak density and carriage often being correlated (Huijben et al., 2013). The limitations in approximating these parameters further highlight the need for more data in this area, as well as other more currently utilised antimalarial drugs.
The model assumed that SP-resistance is conferred by mutations in the DHFR gene, omitting other possible mutations conferring sulfadoxine-resistance, or other mutations in the DHFR gene such as C59R or the high level resistance-conferring I15L mutation (Rosenthal, 2013). The selection of low to moderate SP resistance was due to the availability of data (or lack thereof), highlighting the need for further research in this area.
It must be noted that both symptomatic and asymptomatic infections (Bousema, Okell, Felger, & Drakeley, 2014), as well as those with acquired immunity (Klein, Smith, Boni, & Laxminarayan, 2008), harbour gametocytes. The transmission potential of asymptomatic or acquired immune individuals were not included as a source of transmission, as they are outside of the scope of this study. Additionally, the model parameterised the mortality of mosquitoes irrespective of infection-status; did not allow mixed infections within the mosquito population; and a fitness-cost was assigned to SP-resistant gametocytes when mixed infections were taken up during a blood meal. A more detailed discussion of these assumptions and limitations is provided in Appendix G.
The effect of antimalarial treatment on gametogenesis and infectiousness differs depending on the antimalarial class. A key assumption in using poor quality SP as a proxy for the use of all poor quality antimalarial use is that we assume that all antimalarials have the same propensity to generate gametocytes and effect on gametocyte infectiousness, which is not the case. For example, ACT use is associated with a lower rate of gametocyte carriage (Bousema & Drakeley, 2011), highlighting the need for further studies.
5. Conclusions
The model predicts that an increase in the use of poor quality antimalarials, for which SP is an appropriate proxy, results in an increase in the transmission of antimalarial resistant malaria, providing insight into the link between poor quality antimalarial medicine use and resistance. The loss of antimalarial effectiveness is hampering malaria eradication efforts worldwide, and the continued availability and use of falsified, substandard, degraded and non-WHO recommended antimalarials are highly likely to facilitate the spread of resistance. In order to continue to effectively eradicate malaria, the availability and use of these antimalarials must be addressed by drug regulatory authorities and international organisations.
Competing interests
We declare no competing interests.
Authors' contributions
ARB, AE and JVR conceived the study, SP and AG assisted with further refinement. ARB, JVR, SG, DPD and AG contributed to the model design. ARB parameterised the model with assistance from JVR and SP. ARB, JVR, SG, DPD and AG contributed to the production of the results, which were interpreted by ARB, JVR, SP and AE. ARB drafted the manuscript. AE, JVR, SP, SG, DPD and AG reviewed and suggested modifications to the manuscript. All authors reviewed and approved the final version.
Funding
AR Brock was supported by a University of South Australia stipend.
Acknowledgements
Thank you to Professors Paul Newton and Ric Price from the WorldWide Antimalarial Resistance Network (WWARN) for your invaluable input and feedback.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Appendix A. Defining the deterministic model
A1. Ordinary differential equations
| (A1) |
A2. Initial conditions
The set of initial conditions for each class of the model are defined in Table A2.1.
Table A2.1.
Initial Conditions. The initial values of each class in the model (i.e. when time is set to 0).
| Population | Model Class | Initial Value |
|---|---|---|
| Human | ||
| Mosquito | ||
B. Human parameters
The model parameters for the human population are defined in Table B.1, along with the parameter values used, the parameter range, and the published reference or the section of the Appendices where this parameter is defined.
Table B.1.
Human Parameters. A description of the parameters specific to the human population, in Kenya (2006). For parameter values where literature values were readily available, these values, along with the range of values and references are provided. For parameters that required further manipulation from the original source, the section of the Appendices where this parameter is discussed is noted. All parameter units are in days, unless otherwise stated.
| Parameter description | Symbol | Value [range] | Reference |
|---|---|---|---|
| Human Kenyan population (count) | 36,757,498 | The World Bank (2006–2013g) | |
| Number of deaths in Kenya in 2006 (count) | 11 per 1000 | The World Bank (2006-2013c) | |
| Births per year in Kenya | 38 per 1000 | The World Bank (2006-2013a) | |
| Range of child-bearing ages (years of age): initial, final | , | 15, 49 | Ikamari, Izugbara, and Ochako (2013) |
| Fertility rate (births per woman) | The World Bank (2006-2013d) | ||
| Proportion of population that are female | 0.501 | The World Bank (2006-2013f) | |
| Life expectancy of humans (days) | 20,454 | The World Bank (2006-2013e) | |
| Kenyan 2006 malaria cases (count) | 8,926,058 | World Health Organization (2010b) | |
| Kenyan 2006 malarial deaths (count) | 74,970 | The World Bank, 2006-2013c, World Health Organization, 2010b | |
| Latency period of asexual parasites in humans (days) | 9 [9, 14] | Bloland and Williams (2002) | |
| Delay in seeking treatment (days) | 1 [0, 2] | Sumba, Wong, Kanzaria, Johnson, and John (2008) | |
| Time to initial wave of gametocytes after the initial wave of asexual parasites (days) | 7 [7, 15] | Bousema and Drakeley (2011) | |
| Time for gametocytes to mature (days) | 2 [2, 3] | Bousema and Drakeley (2011) | |
| Total time until infectious gametocytes (days) from time of transmission from mosquito | 18 [18, 32] | Appendix B3 | |
| Rate of the loss of acquired immunity | 0.0027 | Labadin et al. (2009) | |
| Allele frequency of SP-sensitive P. falciparum | 0.50 [0.05, 0.50] | Kum et al. (2013) | |
| Allele frequency of SP-resistant P. falciparum | 0.42 [0.42, 0.90] | Kum et al., 2013, Spalding et al., 2010 | |
| Allele frequency of mixed P. falciparum | 0.08 [0, 0.08] | Kum et al. (2013) | |
| The rate of building effective immunity | 27.3774 | Labadin et al. (2009) | |
| The rate of recovery of P. falciparum infection | 0.0018 | Labadin et al. (2009) |
B1. Human birth rate
Equations (B1.1), (B1.2) were used to calculate a parameter range for the daily birth rate of humans , resulting parameter range for daily birth rate of humans was -.
| (B1.1) |
| (B1.2) |
where denotes the number of births in Kenya, in 2006; denotes the average number of births per female; denotes the daily birth rate of humans; denotes the proportion of Kenyan population that are female; denotes the probability of being of childbearing age; and denotes the expected human lifespan (days).
B2. Movement from susceptible to exposed classes
The expected rate of movement of humans from being susceptible to exposed to P. falciparum, during a blood meal when sporozoites are introduced through the salivary gland of the female An. mosquito, is defined by
| (B2.1) |
where denotes the P. falciparum strain ( for SP-sensitive or for SP-resistant); denotes the female An. mosquito daily biting rate; and denotes the transmission probability of strain from mosquito to human.
B3. Movement from exposed to infected
The expected time to infectious gametocytes, , in days is defined by
| (B3.1) |
where denotes the latency period of asexual parasites in humans (days); denotes the time until the initial wave of asexual P. falciparum (days); and denotes the time for gametocytes to mature in order to be infectious to female An. mosquitoes (days).
Therefore, the rate of human movement from being exposed to P. falciparum (during a blood meal), to being infectious (mature gametocytes), is given by
| (B3.2) |
where denotes the expected time to infectious gametocytes (days).
B4. Movement from infected to susceptible
The estimated daily rate of recovery is defined by
| (B4.1) |
where for SP-sensitive P. falciparum, for SP-resistant P. falciparum, for mixed P. falciparum infection; for good quality AL treatment, for full-dose SP treatment, for half-dose SP treatment and for no antimalarial treatment; denotes the latency period of asexual P. falciparum in humans (days); denotes the delay in seeking treatment (days); denotes the estimated time to recovery in days (see Appendix C2); and denotes the expected time to infectious gametocytes (days) (Eq. (B3.1)).
B5. Movement from infected to acquired immunity
Immunity to malaria is defined by Bruce-Chwatt (1980) as “the state of resistance to infection brought about by all those processes which are involved in destroying the plasmodia or by limiting their multiplication”, and can be passive or active. Passive immunity is conferred from mother to child or through vaccinations (Doolan, Dobano, & Baird, 2009). For the purposes of this model, we assume passive immunity cannot be gained. Actively acquired immunity is assumed to be temporarily gained after continuous exposure, as used in the model by Labadin et al. (2009). Acquired immunity has been found to be delayed in the presence of intermittent or prophylaxis treatment (Doolan et al., 2009), and additionally in full dose treatment, as parasites are eradicated (Long, Nakazawa, Huaman, & Kanbara, 2002). In research carried out by Long et al. (2002) on mice, lower doses of antimalarial treatment better enabled the mice to acquire protective immunity than the higher doses. We assume that once acquired immunity is gained, protection is afforded against both SP-sensitive and SP-resistant infections.
The maximum expected rate of acquired immunity gained is defined by Eq. (B5.1) (Labadin et al., 2009), resulting in the parameter range for of [0, 0.0018].
| (B5.1) |
where denotes the rate acquired immunity is gained; denotes the daily rate of building effective immunity; and denotes the daily rate of recovery.
B6. Human mortality rates ( and )
The two different reported values for total human mortality in Kenya in 2006 are:
-
•
The World Bank states 11 deaths per 1000 population, equating to a count of 404,332 deaths (The World Bank, 2006-2013c).
-
•
The WHO World Malaria Report states that there were 216,158 deaths in total, with 40,079 as a result of malaria (World Health Organization, 2010b).
The difference in estimates of mortality provided by the World Bank and the WHO World Malaria Report indicates the possibility of under-reporting in the estimates obtained by the WHO, for both overall and malaria-specific mortality. The number of malaria deaths reported by the WHO was adjusted by an “under-reporting” factor calculated in Eq. (B6.1).
| (B6.1) |
The daily rate of mortality is defined into two groups: (i) malaria-specific mortality and (ii) other mortality . Malaria-specific mortality occurs once humans are within the infected class; and other deaths occur in all other classes of the model, where these non-malarial associated deaths may be due to other illnesses, old age, accidents, among other causes.
The calculation for the daily rate of malaria-specific mortality is defined below. The proportion of those infected with P. falciparum who die, , in 2006 is
| (B6.2) |
An estimate of the overall rate of recovery from infected to susceptible classes, , at baseline is:
| (B6.3) |
Therefore, the daily rate of malaria-specific mortality while the human is infected (i.e. has infectious gametocytes) is approximately:
| (B6.4) |
It must be noted that the estimate of malaria-specific mortality does not include the time period the patient is in the exposed class and symptomatic (asexual P. falciparum) but not infectious (mature gametocytes). In addition to this, the calculation for mortality is affected by the percentage use of antimalarial medicines. For this reason, it was defined at the 2006 baseline and cannot be explored under different drug use scenarios (results section). Finally, this calculation assumes that the “total population minus malaria cases” is an accurate estimate of the number of people who do not get malaria. It must be noted that people can get more than one malaria infection within the calendar year.
The other mortality is assumed to be approximately equal to the overall mortality rate in humans , defined as 1/(life expectancy), because the proportion of those with malaria who die is so small (see Eq. (B6.4)).
| (B6.5) |
C. Treatment parameters
The treatment-based model parameters are defined in Table C.1, along with the parameter values used, the parameter range, and the published reference or the section of the Appendices where this parameter is defined.
Table C.1.
Treatment parameters. A description of the treatment parameters used in the model. For parameter values where literature values were readily available, these values, along with the range of values and references are provided. For parameters that required further manipulation from the original source, the section of the Appendices where this parameter is discussed is noted. All parameter units are in days, unless otherwise stated.
| Symbol | Parameter description | Value [range or ] | Reference |
|---|---|---|---|
| Probability of receiving treatment | 0.80 [0.80, 0.91] | Chuma et al. (2007) | |
| Receiving no treatment (proportion, at baseline) | 0.20 [0.09, 0.20] | Chuma et al. (2007) | |
| Receiving full-dose SP monotherapy (proportion, at baseline) | 0.07 [0.063, 0.077] | Demographic and Health Surveys (various) (2003–2012) | |
| Receiving half-dose SP monotherapy (proportion, at baseline) | 0.03 [0.027, 0.33] | Appendix C1 | |
| Receiving AL (proportion, at baseline) | 0.70 [0.63, 0.77] | Appendix C1 | |
| SP-sensitive gametocyte clearance in humans treated with AL | 14 [12.6, 15.4] | Appendix C2.1 | |
| SP-resistant gametocyte clearance in humans treated with AL | 14 [12.6, 15.4] | Appendix C2.1 | |
| Mixed infection gametocyte clearance in humans treated with AL | 14 [12.6, 15.4] | Appendix C2.1 | |
| SP-sensitive gametocyte clearance in humans treated with full-dose SP monotherapy | 25 [21, 119] | Appendix C2.2 | |
| SP-resistant gametocyte clearance in humans treated with full-dose SP monotherapy | 112 [112, 882] | Appendix C2.2 | |
| Mixed infection gametocyte clearance in humans treated with full-dose SP monotherapy | 25 [21, 119] | Appendix C2.2 | |
| SP-sensitive gametocyte clearance in humans treated with half-dose SP monotherapy | 29 [29, 162] | Appendix C2.2 | |
| SP-resistant gametocyte clearance in humans treated with half -dose SP monotherapy | 112 [112, 882] | Appendix C2.2 | |
| Mixed infection gametocyte clearance in humans treated with half -dose SP monotherapy | 92 [92, 772] | Appendix C2.2 | |
| Gametocyte clearance in humans not treated | 75 [0, 730] | Anderson and May (1991) | |
| SP-sensitive gametocyte transmission when treated with AL (probability) | 0.053705 [0.0183335, 0.053705] | Appendix C3.3 | |
| SP-resistant gametocyte transmission when treated with AL (probability) | 0.053705 [0.0183335, 0.053705] | Appendix C3.3 | |
| Mixed infection gametocyte transmission when treated with AL (probability) | 0.053705 [0.0183335, 0.053705] | Appendix C3.3 | |
| SP-sensitive gametocyte transmission when treated with full-dose SP (probability) | 0.055 [0.0495, 0.0605] | Appendix C3.2 | |
| SP-resistant gametocyte transmission when treated with full-dose SP (probability) | 0.3 [0.424485, 0.4999] | Appendix C3.2 | |
| Mixed infection gametocyte transmission when treated with full-dose SP (probability) | 0.31 [0.452, 0.527375] | Appendix C3.2 | |
| SP-sensitive gametocyte transmission when treated with half-dose SP (probability) | 0.0489 [0.04401, 0.05379] | Appendix C3.2 | |
| SP-resistant gametocyte transmission when treated with half-dose SP (probability) | 0.0147 [0.0125, 0.0147] | Appendix C3.2 | |
| Mixed infection gametocyte transmission when treated with half-dose SP (probability) | 0.1913 [0.1639, 0.1913] | Appendix C3.2 | |
| Gametocyte transmission with no treatment (probability) | 0.2 [0.2, 0.5] | Mandal et al. (2011), Appendix C3.1 |
C1. Percentage drug use
Chuma et al. (2007) investigated treatment seeking behaviour of those residing in Kenya. During the two week period of the study, the percentage of people with acute illnesses that did not use treatment was 9.3% in urban areas and 20.1% in rural areas. Therefore, we parameterised the proportion of those who do not seek treatment as ranging from 0.09 to 0.20. This leads to the understanding that those who seek treatment ranges from 0.80 to 0.91.
The 2003 Demographic Health Survey (DHS) was used to estimate the percentage of SP used within the community. The survey reported the treatment practises of parents with children under five years old, and it was identified that 10.9% received SP (Demographic and Health Surveys (various), 2003–2012).
Insight into the quality of antimalarial medicines, especially the percentage of falsified medicines, has been informed by numerous studies; however, accurate estimates are hard to come by. In 2006, Newton et al. (2006) estimated the percentage of falsified medicines ranges from 1% to 50% worldwide. An analysis of the WorldWide Antimalarial Resistance Network database found that out of 9348 antimalarial medicines sampled, 30.1% failed chemical or packaging quality tests, of which: 39.3% were classified as falsified; 2.3% as substandard medicines; and 58.3% poor quality without evidence available to classify them as substandard or falsified (Tabernero et al., 2014). A study released in 2003, analysing the quality of SP tablets sold by private wholesale pharmacies in Dar Es Salaam, Tanzania, found that 8/18 (44%) of SP samples failed assay tests for content and dissolution tests (Minzi et al., 2003).
Following this evidence, the probability of receiving full-dose SP was set to 0.07; the probability of receiving half-dose SP was set to 0.03; the probability of not receiving any antimalarial treatment was set to 0.20; and the probability of receiving AL treatment was assumed to be the remainder (Eq. (C1.1)), at baseline.
| (C1.1) |
C2. Gametocyte clearance (days) post-treatment
C2.1. Artemether-Lumefantrine treatment sought
In the literature, Bousema et al. (2006) found 16.0% (12 out of 75) of children treated with AL had gametocytemia on day; and Sutherland et al. (2005) found 7.94% (30 out of 378) treated children were carriers of gametocytes on day 28 post-treatment. However, Sawa et al. (2013) found an average of days of gametocyte carriage post-treatment (95% CI: 3.6–8.5 days) of children treated with AL who remained asexual parasite free during follow-up.
For this reason, we feel it is a reasonable assumption that the patient has gametocytemia until day 14 and then moves back to the susceptible class, given that the number of humans carrying gametocytes past day 28 is small.
| (C2.1.1) |
where denotes the estimated days for gametocyte clearance post-treatment, for P. falciparum strain and drug treatment ; and the parameter range is denoted [minimum, maximum].
C2.2. Sulfadoxine-Pyrimethamine treatment sought
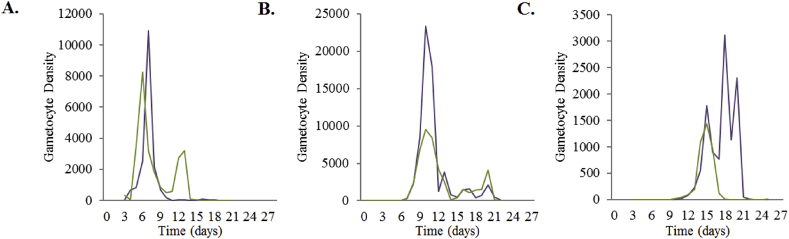
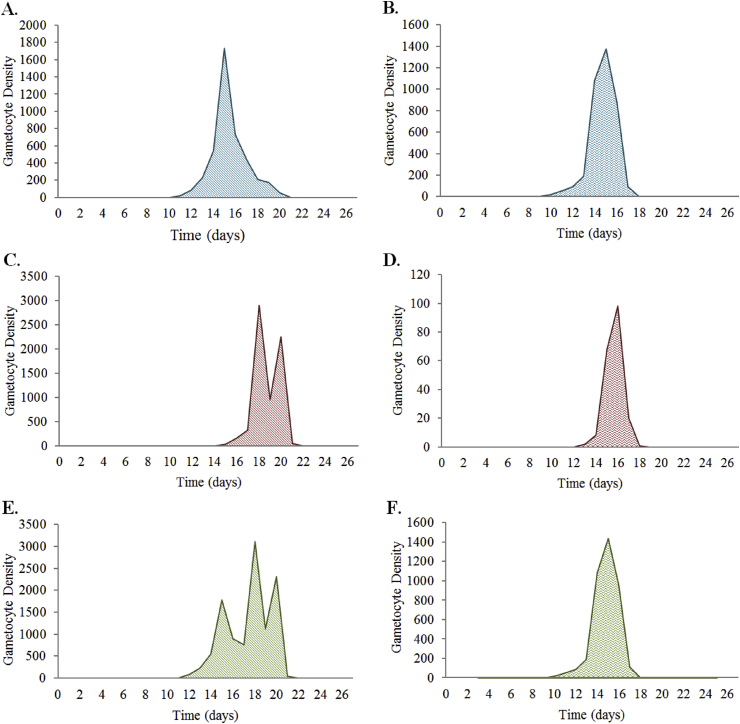
The data used to inform the asexual and gametocyte clearance rates when treated with SP were calculated using the combination of mice data (pyrimethamine treatment) (Huijben et al., 2010b, Huijben et al., 2013, Huijben et al., 2010a) and human SP studies carried out in South Africa (Barnes et al., 2008, Barnes et al., 2008) and Columbia (Méndez et al., 2007). The mice studies researched the effect of pyrimethamine (not in combination with sulfadoxine) on gametocyte densities and the length of infectivity over a maximum of 28 days (Huijben et al., 2010a, Huijben et al., 2013). The experimental design was developed to simulate the emergence of resistance, using two genetically distinct P. chabaudi clonal lineages in each study: drug-resistant AS12265 (pyr-1A) and drug-sensitive AJ5154 (Huijben et al., 2010a); and drug-susceptible clone AJ5p and pyrimethamine-resistant clone AS6p(pyr1A) (Huijben et al., 2013). The experiment was carried out in six to eight week-old female C57Bl/6J laboratory mice, with treatment taking place six days post-inoculation. In mixed infections, 106 pyrimethamine-sensitive parasites were inoculated, followed by ∼25 pyrimethamine-resistant parasites five days after the experiment began, to simulate the emergence of resistance. The estimates for half-dose SP treatments were calculated from experimental data using on 50% of a full dose of pyrimethamine (Huijben et al., 2013) and 37.5% of a full dose of pyrimethamine (Huijben et al., 2010a). Figure C2.2.1 shows a summary of data obtained.
Fig. C2.2.1.
The daily P. chabaudi gametocyte density in mice post-pyrimethamine treatment for (A) pyrimethamine-sensitive gametocytes, (B) pyrimethamine-resistant gametocytes, and (C) mixed infection gametocytes. The purple line denotes the gametocyte density for a 100% pyrimethamine treatment. The green line denotes the gametocyte density of 37.5% of a full dose of pyrimethamine treatment for (A) and 50% of a full dose of pyrimethamine treatment for (B) and (C). Data provided by Huijben et al., 2013, Huijben et al., 2010a, Huijben et al., 2010b.
Estimates for the expected gametocyte clearance time for humans in days, corresponding to each P. falciparum infection type when using half-dose SP monotherapy did not exist at the time of developing this model, so multiple studies and assumptions were used to calculate these values. Estimates of the time to recover were calculated using Eq. (C2.2.1), where the ratio of expected recovery time of mice treated with half-dose pyrimethamine to full-dose pyrimethamine treatment is multiplied by the expected time to recovery in humans treated with full-dose SP. A summary of the parameter values obtained from the human and mice studies are provided in Table C2.2.1.
| (C2.2.1) |
where denotes the gametocyte clearance time in humans for each P. falciparum strain when treated with full-dose SP, as found by Barnes et al., 2008, Barnes et al., 2008; denotes the gametocyte clearance time in mice for each P. chabaudi strain when treated with half-dose SP or no treatment , as found by Huijben et al. (2010a) (for ) or Huijben et al. (2013) (for or ); and denotes the gametocyte clearance time in mice for each P. chabaudi strain when treated with full-dose pyrimethmaine, as found by Huijben et al. (2010a) (for ) or Huijben et al. (2013) (for or ).
Table C2.2.1.
Summary findings from gametocyte clearance studies. Gametocyte clearance time in human and mice studies, along with the parameter range ([minimum, maximum]), for SP-sensitive , SP-resistant and mixed infections in humans (Barnes et al., 2008, Barnes et al., 2008, Méndez et al., 2007); and pyrimethamine-sensitive , pyrimethamine-resistant and mixed infections in mice Huijben et al., 2013, Huijben et al., 2010a, Huijben et al., 2010b.
| Strain | Human Studies |
Mice Studies |
||
|---|---|---|---|---|
| Full-dose SP | Full-dose SP | Full-dose Pyrimethamine | Half-dose Pyrimethamine | |
| Drug Sensitive | 49 [21, 119] | 14 | 11 | 15 |
| Drug resistant | 315 [112, 882] | >28 | 22 | 22 |
| Mixed Infection | 315 [112, 882] | – | 22 | 18 |
| Reference(s) | Barnes et al., 2008, Barnes et al., 2008 | Méndez et al. (2007) | Huijben et al., 2013, Huijben et al., 2010a, Huijben et al., 2010b | Huijben et al., 2013, Huijben et al., 2010a, Huijben et al., 2010b |
The expected gametocyte recovery time in days used to parameterise the model, along with the parameter range ([minimum, maximum]), is provided in Table C2.2.2.
Table C2.2.2.
Expected gametocyte clearance in humans (days). The expected clearance of P. falciparum gametocytes in humans, using a linear interpolation of SP treatment in humans (Barnes et al., 2008, Barnes et al., 2008) and pyrimethamine treatment in mice studies (Huijben et al., 2010b, Huijben et al., 2013, Huijben et al., 2010a), using Eq. (C2.2.1). AEstimated using mice data where the 37.5% of a full-dose of pyrimethamine treatment used (Huijben et al., 2010b, Huijben et al., 2010a), whereas estimates from 50% of a full-dose of pyrimethamine treatment were used for the other parameter calculations (Huijben et al., 2013).
| Strain | Treatment |
||
|---|---|---|---|
| AL | Full-dose SP monotherapy | Half-dose SP monotherapy | |
| Drug Sensitive | 28 | 49 [21, 119] | 67 [29, 162] A |
| Drug resistant | 28 | 315 [112, 882] | 315 [112, 882] |
| Mixed Infection | 28 | 315 [112, 882] | 258 [92, 722] |
C3. Infectiousness of humans to mosquitoes
Barnes et al., 2008, Barnes et al., 2008 identified a log-sigmoidal relationship between gametocyte density and infectivity to mosquitoes, with the probability of mosquito infection dependent on the prevalence, duration and density of gametocyte carriage in the human host. They further discussed that the infectivity given a “particular antimalarial treatment can be characterised as a function of blood gametocyte density and time, summing these over the acute and all subsequent recrudescence of that infection”. Bousema et al. (2006) comment that the infectious mosquito reservoir determines the force of infection.
C3.1. No treatment sought
When no treatment is sought the expected probability of transmission is assumed equal for each infection type,
| (C3.1.1) |
C3.2. Sulfadoxine-Pyrimethamine treatment
No specific dataset exists to inform the effect of SP treatment on infectiousness, so multiple studies were used to calculate estimates. A study carried out by Méndez et al. (2007) was used to calculate the probability of gametocyte transmission when treated with full-dose SP monotherapy. The study recorded the proportion of infected mosquitoes with oocysts after treatment with SP, and the infectivity of those with and without SP-conferring mutations were calculated using membrane feeding assays. Resistance was defined as the presence of DHFR-51 and DHFR-108 SP resistance-conferring mutations. Figure C3.2.1 shows the summary of the data produced in this study.
To calculate the expected probability of transmission from humans to mosquitoes, the area under the curve was calculated and then averaged over the number of days humans were found to be infectious (see Eq. (C3.2.1) and Fig. C3.2.1, results in Table C3.2.1). The calculation assumes a uniform average transmission probability, not taking into account days that humans are more “infectious” to mosquitoes than others; and that infectiousness to mosquitoes over the 28 day experimental period is indicative of the infectiousness of gametocytes in humans to mosquitoes over the timeframe of gametocytes being present (i.e. > 28 days).
| (C3.2.1) |
where denotes the average probability of transmission from humans to mosquitoes for SP-sensitive (w) or SP-resistant (r) infections treated with SP monotherapy; denotes the area under the gametocyte density-time curve, over 28 days; and denotes the count of the number of days gametocytes are seen.
Fig. C3.2.1.
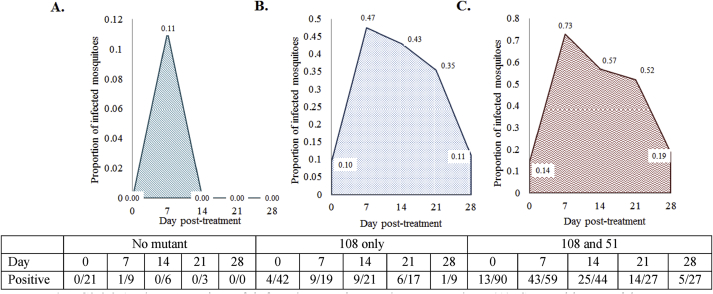
The proportion of infected mosquitoes when exposed to (A) SP-sensitive P. falciparum gametocytes, (B) SP-resistant P. falciparum gametocytes (108 mutants only) and (C) SP-resistant P. falciparum gametocytes (51 and 108 mutants), from infected humans over five years of age on the Pacific Coast of Columbia, who were treated with SP monotherapy. Estimates obtained from Fig. 1 of Méndez et al. (2007). Note: length of data collection was 28 days.
Table C3.2.1.
Total area of average gametocyte density in Fig. C3.2.1, produced using estimates from Méndez et al. (2007).
| Strain | Average gametocyte density |
|---|---|
| SP-sensitive | 0.055 |
| SP-resistant (r)108-mutatnt | 0.424485 |
| SP-resistant (r) 51 & 108 mutant | 0.4999 |
These estimates were then used to linearly interpolate the probability of transmission for mixed infections when full-dose SP monotherapy is used, and for all infections when half-dose SP monotherapy is used. As with the calculations for gametocyte clearance (Appendix C2), raw mice data provided by Huijben et al. (2013) was used (Fig. C3.2.2 and Fig. C3.2.3). Figure C3.2.2 shows that the mixed infection (dotted green line) appears to behave as the sum of the gametocyte densities of the SP drug-sensitive (, purple line) and SP drug-resistant (, blue line).
Fig. C3.2.2.
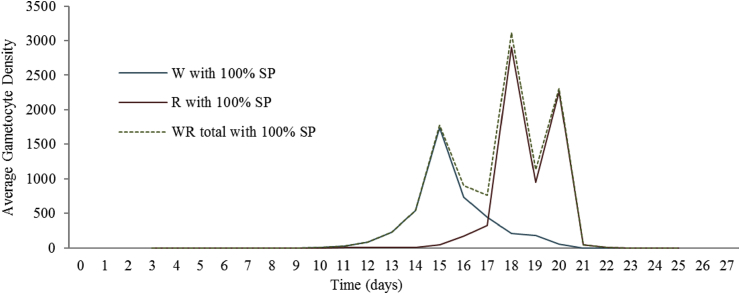
The average gametocyte density per day in mice infected with P. Chabaudi and treated with pyrimethamine. The blue line denotes the pyrimethamine-sensitive (W) gametocyte density associated with pyrimethamine treatment. In like manner, the red line denotes the pyrimethamine-resistant (R) gametocyte density, and the green dotted line denotes the mixed infection (WR) gametocyte density. Data provided by Huijben et al. (2013).
Fig. C3.2.3.
Average gametocyte density of P. Chabaudi infected mice with (A) pyrimethamine-sensitive gametocyte treated with full-dose pyrimethamine; (B) pyrimethamine-sensitive gametocyte treated with half-dose pyrimethamine; (C) pyrimethamine-resistant gametocyte treated with full-dose pyrimethamine; (D) pyrimethamine-resistant gametocyte treated with half-dose pyrimethamine; (E) mixed infection gametocyte treated with full-dose pyrimethamine; (F) mixed infection gametocyte treated with half-dose pyrimethamine. These graphs are produced using data from Huijben et al. (2013).
As the mixed infection predominates with the resistant peaks in the figure, it seems safe to assume that even with the data simulation where the mice is first infected with SP drug-sensitive P. falciparum first, then SP drug-resistant P. falciparum are added after treatment has resumed at a vastly reduced number, the transmission probabilities, .
Assuming the probability of transmission is directly proportional to gametocyte density (Fig. C3.2.3 and Table C3.2.2), the infectivity to mosquitoes is calculated by multiplying the “known” infectivity for treatment with full-dose SP for strain , by the ratio of gametocytes when treated with full- or half-dose pyrimethamine in the mice models (Huijben et al., 2010a, Huijben et al., 2013), see Eq. (C3.2.2). It must be noted, that initially 1 × 106 pyrimethamine-sensitive parasites were inoculated followed five days later with ∼25 pyrimethamine-resistant parasites. We believe this experiment skews some of the results below, however a measure of the degree of skewing in uncertain. For this reason, we must clearly indicate that these values are our best estimate.
| (C3.2.2) |
where denotes the infectiousness of humans to mosquitoes calculated using values from Méndez et al. (2007) (Eq. (C3.2.1)); denotes the area under curve of the P. falciparum gametocytes density of strain , treated with half-dose pyrimethamine from mice models (Huijben et al., 2013); denotes the area under curve of the P. falciparum gametocytes density of strain , treated with full-dose pyrimethamine from mice models (Table C3.2.2) (Huijben et al., 2010a, Huijben et al., 2013).
Table C3.2.2.
The calculated total area of average gametocyte density in Fig. C3.2.3, produced using data from Huijben et al. (2013).
| Strain | Treatment |
|
|---|---|---|
| Full-dose pyrimethamine | Half-dose pyrimethamine | |
| Pyrimethamine-sensitive | 4241.723 | 3769.852 |
| Pyrimethamine-resistant | 6698.292 | 197.569 |
| Mixed infection | 10940.015 | 3967.421 |
C3.3. Artemether-Lumefantrine treatment
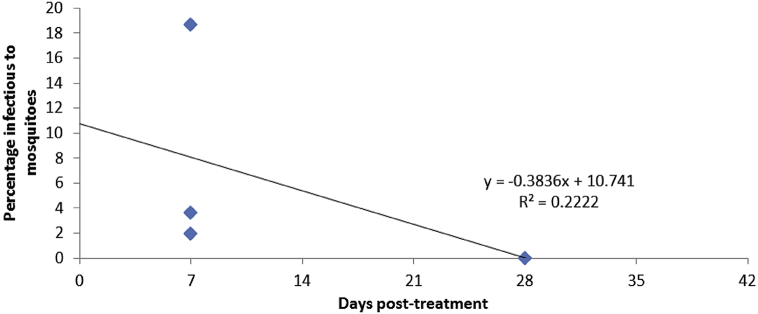
In the literature, Bousema et al. (2006) found 3.6% (27 out of 750) of randomly selected children were infectious to mosquitoes on day seven (Table C3.3.1), whereas the children tested over two years of age were found to be 18.7% infectious to mosquitoes. Sawa et al. (2013) found that 1.9% (42 out of 2292) of those treated with AL who had gametocytes were infectious to mosquitoes on day seven post treatment (Table C3.3.2). In addition, Sutherland et al. (2005) found 0% (0 out of 195) children treated with AL were infectious to mosquitoes on day 28. This data has been amalgamated to produce Fig. C3.3.1, which explores the infectivity of children treated with AL over time.
Fig. C3.3.1.
Combining past studies results for gametocyte infectivity to mosquitoes following AL treatment in children, as reported by Bousema et al., 2006, Sawa et al., 2013, Sutherland et al., 2005.
Table C2.3.3.1.
Infectiousness of gametocytes to mosquitoes after AL and SP treatment. Percentage of mosquitoes that become infected in membrane-feeding assays using blood samples from randomly selected children on day 7 post-treatment, by treatment arm. (Obtained from Table B.4 of Bousema et al. (2006).)
| Treatment arm | Infected mosquitoes, % (proportion) | RR (95% CI) |
|---|---|---|
| SP | 6.9 (52/750) | 1 |
| AL | 3.6 (27/750) | 0.52 (0.33–0.82) |
Table C3.3.2.
Infectiousness of gametocytes to mosquitoes after AL treatment. Gametocyte infectiousness among mosquitoes, by study arm. Blood samples taken on day 7 after initiation of treatment, with mosquitoes examined on day 7 after feeding. (Obtained from Table B.3 of Sawa et al. (2013).)
| Variable | Proportion of Participants (%) |
|---|---|
| Individuals participating in membrane-feeding assays, no. | 77 |
| Microscopy finding on feeding day | |
| Gametocyte prevalence | 4.2 (3/72) |
| Gametocyte density, gametocytes/, geometric mean (95% CI) | 39.5 (18.2–85.4) |
| Pfs25 QT-NASBA finding on feeding day | |
| Gametocyte prevalence | 21.7 (5/23) |
| Individuals infecting mosquito | 31.1 (24/77) |
| Infected mosquitoes, % (proportion) | 1.9 (44/2293) |
| Oocysts in infected mosquitoes, no., mean [range] | 1.3 [1, 2] |
Calculating the average area (daily) under the line of best fit of Fig. C3.3.1,
| (C3.3.1) |
where denotes the expected probability of transmission for SP-sensitive gametocytes from an infected human to mosquito; and denotes the percentage use of AL treatment; denotes the percentage use of full-dose SP; and denotes the percentage who receive no treatment.
The line of best fit in Fig. C3.3.1 indicates a poor fitting line (low R2); however the calculated estimates of infectiousness are consistent with a study by Méndez et al. (2007), where they found that SP-resistant parasites are 7–10 times more likely to infect mosquitoes after SP treatment, compared to no resistance.
A summary of the expected probability of transmission of gametocytes of strain from human to mosquito during a blood meal, given the human drug treatment received by the human, is provided in Table C3.3.3.
Table C3.3.3.
A summary of the expected probability of transmission of each P. falciparum strain from human to mosquito during a blood meal, given the antimalarial treatment. ALow values believed to be a product of the experimental design to collect data.
| Strain | Treatment |
|||
|---|---|---|---|---|
| AL | Full-dose SP | Half-dose SP | No Treatment | |
| SP-sensitive | 0.05705 | 0.055 | 0.0489 | 0.200 |
| SP-resistant | ||||
| 108 mutant | 0.05705 | 0.424485 | 0.0125 A | |
| 58 & 108 mutant | 0.4999 | 0.0147 A | 0.200 | |
| Mixed infection (wr) | ||||
| 108 mutant | 0.05705 | 0.452 | 0.1639 A | |
| 58 & 108 mutant | 0.527375 | 0.1913 A | 0.200 | |
D. Transmission parameters
The transmission-based model parameters are defined in Table D.1, along with the parameter values used, the parameter range, and the published reference or the Appendix reference where this parameter is defined.
Table D.1.
Transmission parameters. A description of the transmission parameters used in the model. For parameter values where literature values were readily available, these values, along with the range of values and references are provided. For parameters that required further manipulation from the original source, the section of the Appendices where this parameter is discussed is noted. All parameter units are in days, unless otherwise stated.
| Symbol | Parameter description | Value [range] | Reference |
|---|---|---|---|
| Biting rate of female An. Mosquitoes | 0 4050 [0.01, 0.5] | Anderson and May, 1991, Mandal et al., 2011 | |
| SP-sensitive sporozoite transmission (probability) | 0.2 [0.2, 0.5] | Mandal et al. (2011) | |
| SP-resistant sporozoite transmission (probability) | 0.2 [0.2, 0.5] | Mandal et al. (2011) | |
| Overall transmission of SP-sensitive gametocytes (probability) | 0.1459 [0.1313, 0.1605] | Appendix D2 | |
| Overall transmission of SP-resistant gametocytes (probability) | 0.1410 [0.1269, 0.1551] | Appendix D2 | |
| Fitness cost of resistance | 0.6 [0.54, 0.66] | Appendix D1 |
D1. Fitness cost in mosquito midgut
The benefit that antimalarial resistance affords to a Plasmodium parasite may also cause a fitness cost (Fröberg et al., 2013). A study conducted by Mharakurwaa et al. (2011) identified a prevalence between 2% and 12% of antifolate resistant P. falciparum in the midguts of Anopheles arabiensis mosquitoes, which they discussed was very low, when in comparison, there was a high prevalence of resistance within the human population. In contrast, Costanzo and Hartl (2011) discusses that there is no discernible cost associated with maintaining resistance afforded by highly resistant triple or quadruple mutations in P. falciparum.
The model assumes that the female An. mosquito can only be infected with one strain of P. falciparum. In the occasion when the mosquito feeds on a human containing a mixed infection, the probability of P. falciparum SP-sensitive gametocytes being selected over SP-resistant gametocytes in the An. mosquitoes midgut is defined by , and set to 0.60 (where ). This value for assigns a small cost to resistance. This assumption was made in order to simplify the model.
D2. . Transmission from humans to mosquitoes
The overall probability of gametocyte transmission from human to mosquito, for each P. falciparum infection , treatment combination, are as follows:
| (D2.1) |
| (D2.2) |
where and denote the overall expected probability of transmission of SP-sensitive and SP-resistant gametocytes from human to mosquito, respectively; denotes the percentage of drug use for each treatment type, ( for AL, for ful-dose SP, for half-dose SP, and for no treatment); denotes the probability of SP-sensitive gametocyte transmission under each treatment, ; denotes the probability of SP-resistant gametocyte transmission under each treatment, ; denotes the probability of a mixed infection gametocyte transmission under each treatment, ; and denotes the fitness cost (see Appendix D1).
E. Mosquito parameters
The model parameters for the female An. mosquito are defined in Table E.1, along with the parameter values used, the parameter range, and the published reference or the Appendix reference where this parameter is defined.
Table E.1.
Anopheles mosquito parameters. A description of the parameters specific to female An. mosquitoes used in the model. For parameter values where literature values were readily available, these values, along with the range of values and references are provided. For parameters that required further manipulation from the original source, the section of the Appendices where this parameter is discussed is noted. All parameter units are in days, unless otherwise stated.
| Symbol | Parameter description | Value [range] | Reference |
|---|---|---|---|
| Initial ratio of mosquitoes to humans (humans = 1) | 0.87 [0.5, 40] | Mandal et al. (2011) | |
| Average life span of a female Anopheles mosquito in Kenya (days) | 8–21 | Labadin et al., 2009, Olayemi and Ande, 2008, Tchuinkam et al., 2010, Wanji et al., 2003 | |
| Daily mortality rate of female mosquitoes | 0.0280 [0.05, 0.5] | Mandal et al. (2011), Appendix E3 | |
| Daily rate female An. mosquitoes reach adulthood | 0.0280 [0.020, 0.27] | Chitnisa et al., 2008, Labadin et al., 2009 | |
| Latent period of mosquitoes (days) | 5 [5, 15] | Mandal et al. (2011) | |
| Proportion of mosquitoes that are infected with P. falciparum | 0.40 [0.38, 0.83] | Mbogo et al. (2003) |
E1. Movement from susceptible to exposed
The P. falciparum transmission rate of female An. mosquitoes becoming exposed to P. falciparum gametocytes during a blood meal is defined by
| (E1.2) |
| (E1.3) |
where and denote the rate of female Anopheles mosquitoes becoming exposed to SP-sensitive and SP-resistant P. falciparum, respectively; denotes the daily biting rate of female An. mosquitoes; and and denote the overall expected probability of transmission of SP-sensitive and SP-resistant gametocytes from human to mosquito, respectively (defined in Appendix D2).
E2. Movement from exposed to infected
The daily rate of female An. mosquitoes movement from exposed to P. falciparum during a blood meal, to being infectious (sporozoites in salivary glands), is given by Eq. (E2.1). The model assumes that infected female An. mosquito do not recover from an infection due to their short lifespan, as assumed in many mathematical models (Mandal et al., 2011).
| (E2.1) |
where denotes the daily rate of female An. mosquitoes movement from exposed to infected; denotes the latency period for female Anopheles mosquitoes; and the range is defined [min, max].
A To stabilise the model, we had to use a value less than the ranges identified in the literature.
E3. Mortality rates
The mortality rate of female An. mosquitoes is assumed equal irrespective of P. falciparum infection status. In order to stabilise the model, we had to use a value less than the ranges identified in the literature, a range of 0.05-0.5.
F. Sensitivity analysis
The sensitivity analysis results are found in Table F.1, where parameters that inferred a change in the total proportion of SP-resistant infections in the human population of greater than were considered to be significant.
Table F.1.
Sensitivity analysis results. The changes in the predicted percentage of SP resistant-containing infections in humans during 2006, due to changes in parameter values. When required, values are reported to 4 d.p. A The literature parameter range is 0.020–0.27, however there were computational restrictions that only permitted a range of 0.020–0.1406 days for the sensitivity analysis.
| Symbol | Description | Range (known range or ±10%) |
Percentage change |
|||
|---|---|---|---|---|---|---|
| Baseline | Minimum | Maximum | Minimum | Maximum | ||
| Human population size (initial) | 1 | 0.9 | 1.1 | 0.10 | −0.12 | |
| Ratio of mosquito to human population (initial) | 0.87 | 0.5 | 40 | 0.32 | −20.55 | |
| Fitness cost in mosquito midgut | 0.6 | 0.5 | 0.7 | 5.12 | −6.60 | |
| Mortality rate of female An. mosquitoes | 0.028 | 0.0476 | 0.125 | 0.29 | 0.24 | |
| Rate female An. mosquitoes reach adulthood | 0.028 | 0.020 | 0.1406A | 0.26 | −36.20 | |
| Latent period of An. mosquitoes | 5 | 5 | 15 | 0.00 | 0.20 | |
| Kenyan 2006 malaria deaths | 74,970 | 67,473 | 82467 | 0.00 | 0.00 | |
| Kenyan 2006 malarial cases | 8,926,058 | 8,033,452.2 | 9818663.8 | 0.00 | 0.00 | |
| Birth rate for humans | 1.1349 × 10−4 | 1.0411 × 10−4 | 7.8811 × 10−4 | 0.00 | −0.11 | |
| Rate of malarial mortality in humans | 0.0011 | 0.0010 | 0.0012 | 0.00 | 0.00 | |
| Rate of “other” mortality in humans | 3.1779 × 10−5 | 2.86 × 10−5 | 3.50 × 10−5 | 0.00 | 0.00 | |
| Latency period of asexual parasites in humans | 9 | 9 | 14 | 0.00 | −0.04 | |
| Maturing of gametocytes | 2 | 2 | 3 | 0.00 | −0.01 | |
| Delay in seeking treatment | 1 | 0 | 2 | −0.01 | −0.04 | |
| Biting rate of female An. mosquitoes | 0.405 | 0.01 | 0.5 | 0.19 | −1.43 | |
| SP-sensitive sporozoite transmission (probability) | 0.2 | 0.2 | 0.5 | 0.00 | −86.98 | |
| SP-resistant sporozoite transmission (probability) | 0.2 | 0.2 | 0.5 | 0.00 | 17.90 | |
| Receive no treatment (proportion, at baseline) | 0.2 | 0.09 | 0.2 | 0.57 | 0.00 | |
| Receiving full-dose SP monotherapy (proportion, at baseline) | 0.07 | 0.063 | 0.077 | −0.52 | 0.54 | |
| Receiving half-dose SP monotherapy (proportion, at baseline) | 0.03 | 0.027 | 0.033 | 0.00 | 0.00 | |
| SP-sensitive gametocyte clearance in humans treated with AL | 14 | 7 | 28 | 9.20 | −13.61 | |
| SP-resistant gametocyte clearance in humans treated with AL | 14 | 7 | 28 | −15.52 | 8.65 | |
| Mixed infection gametocyte clearance in humans treated with AL | 14 | 7 | 28 | 0.05 | −0.04 | |
| SP-sensitive gametocyte clearance in humans treated with full-dose SP monotherapy | 25 | 21 | 119 | 0.52 | −1.56 | |
| SP-resistant gametocyte clearance in humans treated with full-dose SP monotherapy | 112 | 112 | 882 | 0.00 | 0.26 | |
| Mixed infection gametocyte clearance in humans treated with full-dose SP monotherapy | 112 | 21 | 119 | 0.01 | 0.00 | |
| SP-sensitive gametocyte clearance in humans treated with half-dose SP monotherapy | 29 | 29 | 162 | 0.00 | −0.52 | |
| SP-resistant gametocyte clearance in humans treated with half-dose SP monotherapy | 112 | 112 | 882 | 0.00 | 0.12 | |
| Mixed infection gametocyte clearance in humans treated with half-dose SP monotherapy | 92 | 92 | 772 | 0.00 | 0.00 | |
| Gametocyte clearance in humans not treated | 75 | 30 | 720 | −0.04 | 0.02 | |
| SP-sensitive gametocyte transmission when treated with AL (probability) | 0.0537 | 0.0183 | 0.0537 | 5.69 | 0.00 | |
| SP-resistant gametocyte transmission when treated with AL (probability) | 0.0537 | 0.0183 | 0.0537 | 5.69 | 0.00 | |
| Mixed infection gametocyte transmission when treated with AL (probability) | 0.0537 | 0.0183 | 0.0537 | 5.69 | 0.00 | |
| SP-sensitive gametocyte transmission when treated with full-dose SP monotherapy (probability) | 0.055 | 0.0495 | 0.0605 | 0.11 | −0.11 | |
| SP-resistant gametocyte transmission when treated with full-dose SP monotherapy (probability) | 0.3 | 0.4245 | 0.4999 | 2.30 | 3.58 | |
| Mixed infection gametocyte transmission when treated with full-dose SP monotherapy (probability) | 0.31 | 0.452 | 0.5274 | −0.56 | −0.87 | |
| SP-sensitive gametocyte transmission when treated with half-dose SP monotherapy (probability) | 0.0489 | 0.0440 | 0.0538 | 0.05 | −0.04 | |
| SP-resistant gametocyte transmission when treated with half-dose SP monotherapy (probability) | 0.0147 | 0.0125 | 0.0147 | −0.01 | 0.00 | |
| Mixed infection gametocyte transmission when treated with half-dose SP monotherapy (probability) | 0.1913 | 0.1639 | 0.1913 | 0.05 | 0.00 | |
| Gametocyte transmission with no treatment (probability) | 0.2 | 0.2 | 0.5 | 0.00 | −4.27 | |
| Rate of acquired immunity | 6.0864 × 10−4 | 5.4778 × 10−4 | 6.6950 × 10−4 | 0.00 | 0.00 | |
| Rate of loss of acquired immunity | 0.0027 | 0.0024 | 0.0030 | 0.00 | 0.00 | |
G. Model Limitations
There are key assumptions and limitations in the model that have an impact on the findings; these are discussed in detail below.
G1. Estimating the transmissibility and infectiousness of gametocytes
The transmissibility of gametocytes is estimated from the duration and density of gametocyte carriage in humans over time. A well-known property of SP is its propensity to increase gametogenesis, which in turn increases the transmission of P. falciparum between humans and mosquitoes (Barnes et al., 2008, Barnes et al., 2008, Bousema and Drakeley, 2011, Hastings, 2006). The data to inform the asexual and gametocyte clearance rates were calculated using a combination of mice malaria data for pyrimethamine (Huijben et al., 2010b, Huijben et al., 2013, Huijben et al., 2010a), with human SP studies carried out in South Africa (Barnes et al., 2008, Barnes et al., 2008) and Columbia (Méndez et al., 2007).
The mice studies looked at the effect of pyrimethamine (not in combination with sulfadoxine) on the gametocyte densities and length of infectivity over a maximum of 28 days (Huijben et al., 2010a, Huijben et al., 2013). In using these data, we had to assume that the estimates provided from pyrimethamine are a fair approximation of the estimates that would occur when used in combination with sulfadoxine.
We feel that the large differences in gametocyte densities and infectivity, as a result of half- and full-dose SP, are due to the experimental design of the mice studies. The pyrimethamine-sensitive infections were simulated by the injected the mice with sensitive parasites on Day One. Mixed infections had the same inoculation of pyrimethamine-sensitive parasites on Day One, followed by the introduction a small number of pyrimethamine-resistant parasites (approximately 25 parasites) on Day Five. In the pyrimethamine-resistant only studies, resistant parasites were inoculated on Day Five (25 parasites). Following this, we expect that this experimental design drives the lower SP-resistant estimates for the probability of gametocyte transmission from humans to mosquitoes for each treatment type , calculated in Appendix C3.2. This limitation highlights the need for more data in this field.
The infectiousness of SP-induced gametocytes may be less than gametocytes that differentiate without the stress of SP treatment, as found with the infectiousness of SP-induced gametocytes when compared to chloroquine-induced gametocytes. However, this decrease is thought to be offset by a higher prevalence and density of gametocytes following SP treatment (Barnes & White, 2005).
For the above reasons, the accuracy of gametocyte-parameter estimates used in our model are unknown but are thought to underestimate the true value. An additional area of uncertainty in using these estimates was introduced by slight differences in experimental design between the two mice studies, when calculating estimates for a half-dose SP monotherapy treatment. These parameter estimates were calculated using data in which 50% of a full-dose of pyrimethamine (Huijben et al., 2013) and 37.5% of a full-dose of pyrimethamine (Huijben et al., 2010a) was used.
G2. Transmissibility in asymptomatic infections and acquired immunity
Both symptomatic and asymptomatic infections harbour gametocytes (Bousema et al., 2014). The ability to transmit during asymptomatic infections are not included as they are outside of the scope of this study, as asymptomatic individuals have no reason to seek antimalarial treatment.
In a similar manner, the model does not account for the possibility of gametocyte carriage, and hence transmission to mosquitoes, in those with acquired immunity, in contrast to work carried out by Klein et al. (2008). We deemed this outside the scope of the study, as these individuals also would not need to seek treatment. A summary of the effect of acquired immunity on gametocyte density has been carried out by Carter and Graves (1988) and Bousema and Drakeley (2011). Both papers surmise a conflict in findings by researchers; some conclude that patients with acquired immunity may be able to control asexual parasite densities better, and hence have lower gametocyte densities. However, others have shown that gametocyte densities may be at their highest in acquired immune populations. Bousema and Drakeley (2011) recommend longitudinal studies to further explore this relationship. Additionally, we do not account for any passive (maternal) immunity to malaria in infants.
G3. Patient adherence
Patient adherence is commonly identified as a confounding factor. In our case, SP is a single dose regimen, so we assumed perfect patient adherence. Artemether-lumefantrine in adults requires 24 tablets administered over 3 days. However, the model assumes that all AL treatment is of good quality, obtained from a reputable source and that all patients complete the treatment regime. However, there have recently been severe problems with the quality of AL in Africa (Newton et al., 2014, World Health Organization, 2015).
G4. Defining antimalarial resistance
The model assumes there is only one kind of resistance within the P. falciparum population, to SP. In reality, resistance has emerged to nearly all antimalarial compounds currently in use, and molecular markers associated with AL resistance have been described (Newton et al., 2014).
When defining SP resistance within our model, tolerance and low levels of resistance were excluded, but are expected to have an impact on the spread of resistance in P. falciparum (Tchuenche et al., 2011).
G5. Mortality rates of mosquitoes
A constant mortality rate of mosquitoes was assumed, irrespective of P. falciparum-infection status, unlike the model produced by Tchuenche et al. (2011). Our model also assumed that mosquitoes could not harbour a mixed infection , and that the selection of SP-sensitive over resistant gametocytes during the bloodmeal, proceeding through to the mosquito's midgut and then salivary gland, occurs at . In their work, Huijben, Sim, Nelson, and Read (2011) found that resistant parasites are suppressed in the absence of treatment (irrespective of the number of competitors in multi-clonal environments), but have a marked advantage following drug treatment. They described a pattern of transient fitness advantage but it did not appear to have an effect on the overall transmission potential. Costanzo and Hartl (2011) found no discernible cost associated with maintaining resistance afforded by highly resistant triple or quadruple mutations in P. falciparum. However, in a study conducted by Mharakurwaa et al. (2011) a lower prevalence (2%–12%) of antifolate resistant parasites were found in the midgut's of Anopheles arabiensis mosquitoes, when compared to the high prevalence of resistance within the human population.
G6. Seasonality
Although the temporal resolution of the parameters were daily, the model outcomes were produced as yearly measures. For this reason, seasonality was not accounted for within the model structure.
References
- Ambroise-Thomas P. The tragedy caused by fake antimalarial drugs. Mediterranean Journal of Hematology and Infectious Diseases. 2012;4(1) doi: 10.4084/MJHID.2012.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., May R.M. Oxford University Press; 1991. Infectious diseases of humans: Dynamics and control. [Google Scholar]
- Barnes K.I., White N.J. Population biology and antimalarial resistance: The transmission of antimalarial drug resistance in Plasmodium falciparum. Acta Tropica. 2005;94:230–240. doi: 10.1016/j.actatropica.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Barnes K.I., Little F., Mabuza A., Mngomezulu N., Govere J., Durrheim D.…White N.J. Increased gametocytemia after treatment: An early parasitological indicator of emerging Sulfadoxine-Pyrimethamine resistance in falciparum malaria. The Journal of Infectious Diseases. 2008;197:1605–1613. doi: 10.1086/587645. [DOI] [PubMed] [Google Scholar]
- Barnes K., Watkins W., White N. Antimalarial dosing regimens and drug resistance. Trends in Parasitology. 2008;24(3):127–134. doi: 10.1016/j.pt.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Bloland P.B., Williams H.A. The National Academies Press; Washington, DC: 2002. Malaria control during mass population movements and natural disasters: National research council. Roundtable on the Demography of Forced Migration. Committee on Population; Program on Foced Migration and Health at the Mailman School of Public Health, Columbia University. [PubMed] [Google Scholar]
- Bousema T., Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical Microbiology Reviews. 2011;24(2):377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema J.T., Schneider P., Gouagna L.C., Drakeley C.J., Tostmann A., Houben R.…Sauerwein R.W. Moderate effect of Artemisinin-based combination therapy on transmission of Plasmodium falciparum. Journal of Infectious Diseases. 2006;193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- Bousema T., Okell L., Felger I., Drakeley C. Asymptomatic malaria infections: Detectability, transmissibility and public health relevance. Nature Reviews Microbiology. 2014;12:833–840. doi: 10.1038/nrmicro3364. [DOI] [PubMed] [Google Scholar]
- Bruce-Chwatt L.J. Hodder Education Publishers; London: 1980. Essential malariology. [Google Scholar]
- Carter R., Graves P.M. Gametocytes. In: Wernsdorfer W.H., McGregor I., editors. Vol. 1. Churchill Livingstone; Edinburgh: 1988. (Malaria: Principles and practice of malariology). [Google Scholar]
- Chitnisa N., Hymanb J.M., Cushing J.M. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bulletin of Mathematical Biology. 2008 doi: 10.1007/s11538-008-9299-0. [DOI] [PubMed] [Google Scholar]
- Chuma J., Gilson L., Molyneux C. Treatment-seeking behaviour, cost burdens and coping strategies among rural and urban households in Coastal Kenya: An equity analysis. Tropical Medicine & International Health. 2007;12(5):673–686. doi: 10.1111/j.1365-3156.2007.01825.x. [DOI] [PubMed] [Google Scholar]
- Costanzo M.S., Hartl D.L. The evolutionary landscape of antifolate resistance in Plasmodium falciparum. Journal of Genetics. 2011;90(2):187–190. doi: 10.1007/s12041-011-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demographic and Health Surveys (various) In: Calverton, Maryland, USA: ICF international, 2012. I. International, editor. 2003-2012. https://dhsprogram.com/ [Google Scholar]
- Doolan D.L., Dobano C., Baird J.K. Acquired immunity to malaria. Clinical Microbiology Reviews. 2009;22(1):13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröberg G., Ferreira P.E., Mårtensson A., Ali A., Björkman A., Gil J.P. Assessing the cost-benefit effect of a Plasmodium falciparum drug resistance mutation on parasite growth in vitro. Antimicrobial Agents and Chemotherapy. 2013;57(2):887–892. doi: 10.1128/AAC.00950-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings I.M. Gametocytocidal activity in antimalarial drugs speeds the spread of drug resistance. Tropical Medicine & International Health. 2006;11(8):1206–1217. doi: 10.1111/j.1365-3156.2006.01668.x. [DOI] [PubMed] [Google Scholar]
- Huijben S., Nelson W.A., Wargo A.R., Sim D.G., Drew D.R., Read A.F. Chemotherapy, within-host ecology and the fitness of drug-resistant malaria parasites. Evolution. 2010;64(10):2952–2968. doi: 10.1111/j.1558-5646.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijben S., Nelson W.A., Wargo A.R., Sim D.G., Drew D.R., Read A.F. 2010. Data from: Chemotherapy, within-host ecology and the fitness of drug-resistant malaria parasites. Dryad Digital Repository. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijben S., Sim D.G., Nelson W.A., Read A.F. The fitness of drug-resistant malaria parasites in a rodent model: Multiplicity of infection. The Journal of Evolutionary Biology. 2011;24:2410–2422. doi: 10.1111/j.1420-9101.2011.02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijben S., Bell A.S., Sim D.G., Tomasello D., Mideo N., Day T. Aggressive chemotherapy and the selection of drug resistant pathogens. PLOS Pathogens. 2013;9(9) doi: 10.1371/journal.ppat.1003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikamari L., Izugbara C., Ochako R. Prevalence and determinants of unintended pregnancy among women in Nairobi, Kenya. BMC Pregnancy and Childbirth. 2013;13(69) doi: 10.1186/1471-2393-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E.Y., Smith D.L., Boni M.F., Laxminarayan R. Clinically immune hosts as a refuge for drug-sensitive malaria parasites. Malaria Journal. 2008;7(67) doi: 10.1186/1475-2875-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E.Y. The impact of heterogeneous transmission on the establishment and spread of antimalarial drug resistance. Journal of Theoretical Biology. 2014;340:117–185. doi: 10.1016/j.jtbi.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koella J., Antia R. Epidemiological models for the spread of anti-malarial resistance. Malaria Journal. 2003;2(3) doi: 10.1186/1475-2875-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kum C.K., Thorburn D., Ghilagaber G., Gil P., Björkman A. On the effects of malaria treatment on parasite drug resistance – probability modelling of genotyped malaria infections. The International Journal of Biostatistics. 2013;9(1):1–14. doi: 10.1515/ijb-2012-0016. [DOI] [PubMed] [Google Scholar]
- Labadin J., Kon C.M.L., Juan S.F.S. Paper presented at the proceedings of the World congress on engineering and computer science, san Francisco, USA. 2009. Deterministic malaria transmission model with acquired immunity. [Google Scholar]
- Leslie T., Kaur H., Mohammed N., Kolaczinski K., Ord R.L., Rowland M. Epidemic of Plasmodium falciparum malaria involving substandard antimalarial drugs, Pakistan, 2003. Emerging Infectious Diseases. 2009;15(11):1753–1759. doi: 10.3201/eid1511.090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long T.T.A., Nakazawa S., Huaman M.C., Kanbara H. Influence of antimalarial treatment on acquisition of immunity in Plasmodium berghei NK65 malaria. Clinical and Diagnostic Laboratory Immunology. 2002;9(4) doi: 10.1128/CDLI.9.4.933-934.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon M.J., Hastings I.M. The evolution of multiple drug resistance in malaria parasites. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92:188–195. doi: 10.1016/s0035-9203(98)90745-3. [DOI] [PubMed] [Google Scholar]
- Mandal S., Sarkar R.R., Sinha S. Mathematical models of malaria - a review. Malaria Journal. 2011;10(202) doi: 10.1186/1475-2875-10-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbogo C.M., Mwangangi J.M., Nzovu J., Gu W., Yan G., Gunter J.T.…Beier J.C. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. The American Journal of Tropical Medicine and Hygiene. 2003;68(6):734–742. [PubMed] [Google Scholar]
- Méndez F., Herrera S., Murrain B., Gutiérrez A., Moreno L.A., Manzano M.…Plowe C.V. Selection of antifolate-resistant Plasmodium falciparum by Sulfadoxine-Pyrimethamine treatment and infectivity to Anopheles mosquitoes. The American Journal of Tropical Medicine and Hygiene. 2007;77(3):438–443. [PubMed] [Google Scholar]
- Mharakurwaa S., Kumwendaa T., Mkulamaa M.A.P., Musapaa M., Chishimbaa S., Shiffb C.J.…Agreb P. Malaria antifolate resistance with contrasting Plasmodium falciparum dihydrofolate reductase (DHFR) polymorphisms in humans and Anopheles mosquitoes. PNAS. 2011;108(46):18796–18801. doi: 10.1073/pnas.1116162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Republic of Kenya Antimalarial medicines in Kenya. Availability, quality and registration status. A baseline study undertaken prior to nationwide distribution of artemether-Lumefantine (AL) in Kenya. 2007. http://apps.who.int/medicinedocs/en/d/Js16424e/
- Minzi O.M.S., Moshi M.J., Hipolite D., Massele A.Y., Tomson G., Ericsson O. Evaluation of the quality of amodiaquine and sulphadoxine/pyrimethamine tablets sold by private wholesale pharmacies in Dar Es Salaam Tanzania. The Journal of Clinical Pharmacy and Therapeutics. 2003;28:117–122. doi: 10.1046/j.1365-2710.2003.00470.x. [DOI] [PubMed] [Google Scholar]
- Newton P.N., Green M.D., Fernández F.M., Day N.P.J., White N.J. Counterfeit anti-infective drugs. The Lancet Infectious Diseases. 2006;6:602–613. doi: 10.1016/S1473-3099(06)70581-3. [DOI] [PubMed] [Google Scholar]
- Newton P., Green M., Fernandez F. Impact of poor quality-quality medicines in the 'developing' world. Trends in Pharmacological Sciences. 2009;31(3):99–101. doi: 10.1016/j.tips.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton P.N., Tabernero P., Dwivedi P., Culzoni M.J., Monge M.E., Swamidoss I.…Fernández F.M. Falsified medicines in Africa: All talk, no action. Lancet. 2014;2 doi: 10.1016/S2214-109X(14)70279-7. [DOI] [PubMed] [Google Scholar]
- Olayemi I.K., Ande A.T. Survivorship of Anopheles gambiae in relation to malaria transmission in Ilorin, Nigeria. Online Journal of Health and Allied Sciences. 2008;7(3) [Google Scholar]
- Poser C.M., Bruyn G.W. Parthenon; New York: 1999. An illustrated history of malaria. [Google Scholar]
- Rosenthal P.J. The interplay between drug resistance and fitness in malaria parasites. Molecular Microbiology. 2013;89(6):1025–1038. doi: 10.1111/mmi.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambol N., Yan L., Creek D., McCormack S., Arinaitwe E., Bigira V.…Parikh S. Population pharmacokinetics of piperaquine in young Ugandan children treated with dihydroartemisinin-piperaquine for uncomplicated malaria. Clinical Pharmacology & Therapeutics. 2015;98(1):87–95. doi: 10.1002/cpt.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa P., Shekalaghe S.A., Drakeley C.J., Sutherland C.J., Mweresa C.K., Baidjoe A.Y.…Bousema T. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: A randomized trial. The Journal of Infectious Diseases. 2013;207:1637–1645. doi: 10.1093/infdis/jit077. [DOI] [PubMed] [Google Scholar]
- Simpson J.A., Watkins E.R., Price R.N., Aarons L., Kyle D.E., White N.J. Mefloquine pharmacokinetic-pharmacodynamic models: Implications for dosing and resistance. Antimicrobial Agents and Chemotherapy. 2000;44(12):3414–3424. doi: 10.1128/aac.44.12.3414-3424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M.D., Eyase F.L., Akala H.M., Bedno S.A., Prigge S.T., Coldren R.L.…Waters N.C. Increased prevalence of the pfdhfr/phdhps quintuple mutant and rapid emergence of pfdhps resistance mutations at codons 581 and 613 in Kisumu, Kenya. Malaria Journal. 2010;9(338) doi: 10.1186/1475-2875-9-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridaran S., McClintock S.K., Syphard L.M., Herman K.M., Barnwell J.W., Udhayakumar V. Anti-folate drug resistance in Africa: meta-analysis of reported dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutant genotype frequencies in African Plasmodium falciparum parasite populations. Malaria Journal. 2010;9(247) doi: 10.1186/1475-2875-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumba P.O., Wong S.L., Kanzaria H.K., Johnson K.A., John C.C. Malaria treatment-seeking behaviour and recovery from malaria in a highland area of Kenya. Malaria Journal. 2008;7(245) doi: 10.1186/1475-2875-7-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C.J., Ord R., Dunyo S., Jawara M., Drakeley C.J., Alexander N.…Targett G.A.T. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of Co-Artemether. PLOS Medicine. 2005;2(4) doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero P., Fernández F.M., Green M., Guerin P.J., Newton P.N. Mind the gaps - the epidemiology of poor-quality anti-malarials in the malarious world - analysis of the WorldWide Antimalarial Resistance Network database. Malaria Journal. 2014;13(139) doi: 10.1186/1475-2875-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuenche J.M., Chiyaka C., Chan D., Matthews A., Mayer G. A mathematical model for antimalarial drug resistance. Mathematical Medicine and Biology. 2011;28:335–355. doi: 10.1093/imammb/dqq017. [DOI] [PubMed] [Google Scholar]
- Tchuinkam T., Simard F., Lélé-Defo E., Téné-Fossog B., Tateng-Ngouateu A., Antonio-Nkondjio C.…Awono-Ambéné P. Bionomics of Anopheline species and malaria transmission dynamics along an altitudinal transect in Western Cameroon. BMC Infectious Diseases. 2010;10(119) doi: 10.1186/1471-2334-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Bank Birth rate, crude (per 1,000 people) 2006-2013. http://data.worldbank.org/indicator/SP.DYN.CBRT.IN/countries?page=1 Washington, DC, USA.
- The World Bank Data (various) 2006-2013. http://data.worldbank.org/ D. Washington, USA.
- The World Bank Death rate, crude (per 1,000 people) 2006-2013. http://data.worldbank.org/indicator/SP.DYN.CDRT.IN/countries?page=1 Washington, DC, USA.
- The World Bank Fertility rate, total (births per woman) 2006-2013. http://data.worldbank.org/indicator/SP.DYN.TFRT.IN/countries?page=1 Washington, DC, USA.
- The World Bank Life expectancy at birth, total (years) 2006-2013. http://data.worldbank.org/indicator/SP.DYN.LE00.IN/countries?page=1 Washington, DC, USA.
- The World Bank Population, female (% of total) 2006-2013. http://data.worldbank.org/indicator/SP.POP.TOTL.FE.ZS/countries?page=1 Washington, DC, USA.
- The World Bank Population, total. 2006-2013. http://data.worldbank.org/indicator/SP.POP.TOTL/countries?page=1 Washington, DC, USA.
- Wanji S., Tanke T., Atanga S.N., Ajonina C., Nicholas T., Fontenille D. Anopheles species of the mount Cameroon region: Biting habits, feeding behaviour and entomological inoculation rates. Tropical Medicine & International Health. 2003;8(7):643–649. doi: 10.1046/j.1365-3156.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- White N.J., Pongtavornpinyo W., Maude R.J., Saralamba S., Aguas R., Stepniewska K.…Day N.P. Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance. Malaria Journal. 2009;8 doi: 10.1186/1475-2875-8-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Global Malaria Programme, World Health Organization; 2010. Global report on antimalarial drug efficacy and drug resistance:2000 -2010. Switzerland: Drug Resistance and Containment Unit. [Google Scholar]
- World Health Organization . World Health Organization; Geneva, Switzerland: 2010. World malaria report 2009 (pp. 111–113) [Google Scholar]
- World Health Organization . 2015. Medical product alert No. 1/2015: Falsified anti-malarial medicine circulating in west Africa Retrieved 02/2015. [Google Scholar]
- WorldWide Antimalarial Resistance Network . In: Drug quality and the fight against malaria. WWARN, editor. 2010. www.wwarn.org [Google Scholar]