Abstract
Transfusion-transmitted leishmaniasis has been a concern in regions endemic for the disease. Whether immediate or delayed, the risks posed by this mode of transmission call for careful assessment. The purpose of this study was to detect Leishmania infection in blood donors living in an endemic area and to investigate progression to the disease in these individuals. Immunofluorescent antibody test, enzyme-linked immunosorbent assay, leishmaniasis rapid test, and the polymerase chain reaction were applied to 430 donors in an initial evaluation. Of those donors with at least one positive test, 50 were reevaluated four years later by the same methods, as were 25 controls who had been negative on the same tests. In the first evaluation, Leishmania infection was detected in 41.4% (95% CI: 36.7–46.1) of donors (n = 430). None of the 75 reevaluated individuals had developed the disease, but retesting revealed positivity in at least one test in 36.0% (95% CI: 25.1–46.9) of donors. Of the 50 initially testing positive, 50% remained so on retesting. Of the 25 initially negative controls, two tested positive in the subsequent evaluation. The severity of the parasitosis and the risk of transfusion transmission warrant investigation of the potential inclusion of methods for Leishmania detection into blood banks for effective screening of infected donors.
Introduction
The ratio of asymptomatic Leishmania infections versus clinical cases is 13:1 in Iran [1] and 50:1 in Spain [2]. In Brazil, this ratio ranges from 8:1 to 18:1 [3], with evidence that one in every six infected individuals develops visceral leishmaniasis (VL) [4].
Leishmania transmission during blood transfusion has been a major concern in endemic areas. Although carriers may not exhibit clinical evidence of the disease, the parasite can become active and multiply in the mononuclear phagocytic system in response to factors such as patient immunological and nutritional status [5,6].
Infectivity among blood donors and the risk that latent infection becomes manifest appear to be self-perpetuating traits [7]. Asymptomatic infection in seemingly healthy donors promotes transfusion transmission [8] if parasite load is sufficiently high and amastigotes survive blood processing and storage until transfusion time [5]. This is a concern particularly among recipients exhibiting unfavorable immunological conditions, irrespective of exposure time [9].
The purpose of this study was to employ serological testing and identification of parasite DNA to detect cases of Leishmania infection in blood donors, thus providing support for discussions on the inclusion of laboratory methods for donor screening and selection in blood banks located in endemic regions. To this end, donors who initially tested positive on at least one of four diagnostic techniques were reevaluated after four years for observation of progression to the disease.
Materials and methods
Study population
The investigation was conducted at the José Scaff Hematology and Hemotherapy Center of Mato Grosso do Sul (Hemosul), in Campo Grande, the capital city of Mato Grosso do Sul state, in Midwest Brazil.
Study design
The study comprised two evaluations. In the first, conducted in 2011, the indirect fluorescent antibody test (IFAT), enzyme-linked immunosorbent assay (ELISA rk39), rK39 rapid test, and polymerase chain reaction (kDNA-PCR) were applied to 430 donors. For the calculation of the sample, the average of 100 donors/day, five days per week was considered. For an approximate population of 14,000 individuals in the seven-month period, the sample size was recommended according to http://www.raosoft.com/samplesize.html, with a margin of error of 5% and confidence level equal to 95%. Systematic sampling was employed.
Subjects considered clinically fit for blood donation (Hemosul criteria) and having no signs, symptoms, or history of leishmaniasis were enrolled. Individuals seropositive for Trypanosoma cruzi were excluded. Infection with Leishmania sp., defined as positivity on at least one test, was detected in 178 subjects (41.4%).
Four years later, these 178 donors were invited for clinical evaluation by an infectologist and collection of a new blood sample at the Hospital Dia (a division of the teaching hospital of the Universidade Federal de Mato Grosso do Sul—UFMS). Changes of address or unavailability for the appointment, however, greatly reduced attendance to 75 individuals—namely, 50 donors who had tested positive on PCR, IFAT, or on both tests four years earlier, plus 25 donors testing negative on all exams (control group).
Blood collection
A 10 mL blood sample was collected from each individual (7 mL for serum separation and 3 mL, preserved in EDTA, for DNA isolation). The samples were centrifuged and serum stored at –20 °C. DNA aliquots were maintained at –70 °C. The serological and molecular tests were performed in the UFMS Clinical Immunology Laboratory and the UFMS Molecular Biology and Cell Culture Laboratory, with support from the Oswaldo Cruz Institute Interdisciplinary Laboratory of Medical Research (LIPMed-Fiocruz, Rio de Janeiro) and the Seroepidemiology and Immunobiology Laboratory of the São Paulo Institute of Tropical Medicine (IMT-USP, São Paulo). All samples were subjected to IFAT, rK39 ELISA, rK39 rapid test, and kDNA-PCR.
Four years later, 75 blood samples were drawn and all the exams were repeated to assess the dynamics of infection.
Indirect fluorescent antibody test (IFAT)
Sera were subjected to IFAT [10] using a kit from the Instituto Biomanguinhos (Oswaldo Cruz Institute, Rio de Janeiro), following manufacturer’s instructions. Samples with IFAT titers of 1:80 or higher were considered positive. Leishmania major–like antigens obtained from cell culture were employed. The assay involved titration of the conjugate and inclusion of a negative and a positive control on each slide. The slides were read by two independent laboratory technicians [10].
Enzyme-linked immunosorbent assay (rK39 ELISA)
Leishmania infantum rK39 antigen was employed [11], using a modified technique [12]. Briefly, Costar High Binding 3690 polystyrene plates (Corning, Corning, NY, USA) were sensitized with 50 μL per well of 0.5 μg/mL of K39 recombinant antigen and blocked with 5% skimmed milk. Duplicate serum samples diluted 1:100 and anti-human IgG—peroxidase conjugate (A-0170, Sigma-Aldrich, St. Louis, USA) diluted 1:30 000 were incubated at 37 °C for 30 min. For color development, the samples were incubated in the dark with tetramethylbenzidine/H2O2 (Novex-Life Technologies, Carlsbad, CA, USA) (50 μL per well) at ambient temperature for 7 min. The reaction was quenched by adding 2 N H2SO4 (Merck KGaA). Absorbance was read at 450 nm on a Multiskan GO device (Thermo Scientific, Vantaa, Finland).
Cutoff was calculated based on a receiver operating characteristic (ROC) curve constructed from the absorbance values of 110 serum samples from patients with symptomatic, parasitologically confirmed VL who resided in an endemic area and 110 serum samples from São Paulo—based controls [13,14]. rK39 ELISA exhibited 99.1% sensitivity (95% CI: 95.0–100.0) and 100.0% specificity (95% CI: 99.1–100.0), considering a 0.110 cutoff. A reactivity index (RI) was calculated for each sample as RI = (sample absorbance)/cutoff. Samples with RI ≥ 1 were considered positive.
Anti-Leishmania rK39 rapid test
The rapid test employed a Kalazar Detect kit (Inbios International, Seattle, WA, USA), according to manufacturer’s instructions. Briefly, 10 μL of serum were placed in the specific area on the test strip and three drops of chase buffer were added. Results, read at 15 min, were considered positive or negative depending on the presence or absence of a test line, respectively. All tests were read by two technicians.
Polymerase chain reaction (PCR)
DNA isolation from blood was performed using a Wizard Genomic DNA Purification kit (Promega), according to manufacturer’s instructions. A human β-actin gene was used as a control to verify DNA integrity and the presence of possible PCR inhibitors [15].
Primers HM1 (5'-CCG CCC CTA TTT TAC ACC AAC CCC-3'), HM2 (5'-GGG GAG GGG CGT TCT GCG AA-3'), and HM3 (5'-GGC CCA CTA TAT TAC ACC AAC CCC-3') were employed to amplify the 120-bp fragment of the conserved region of Leishmania kDNA minicircle [16].
kDNA-PCR was performed with a final 25 μL volume containing 1x Colorless GoTaq Flexi buffer (Promega, Madison, USA), 200 μM dNTPs (dATP, dCTP, dGTP, and dTTP; Promega), 1.5 mM MgCl2, a 1 μM concentration of each primer, roughly 150 ng of extracted DNA, and water to complete the reaction. In all reactions, 2 μL of Leishmania (Viannia) braziliensis genomic DNA (MHOM/BR/1975/M2903, LIPMed-Fiocruz, Rio de Janeiro) served as the positive control. The negative control was a sample containing the reagent mixture devoid of DNA. Cycling started at 95 °C for 2 min, followed by 35 cycles at 95 °C for 30 s, 54 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 7 min.
Amplification was verified by performing 2% agarose gel electrophoresis in 0.5x TBE buffer, followed by gel staining with GelRed (1:500).
Statistical analysis
BioEstat v. 5.3 software (Sociedade Mamirauá, Belém, Brazil) was employed to evaluate agreement between Leishmania detection tests, based on the kappa statistic (κ) at a 5% significance level, as follows: κ < 0.00, poor; κ = 0.00–0.20, slight; κ = 0.21–0.40, fair; κ = 0.41–0.60, moderate; κ = 0.61–0.80, substantial; κ = 0.81–1.00, almost perfect [17]. In the second evaluation, the clinical background and clinical status of participants were investigated by means of semistructured interviews, treatment history records, and general and specific physical examination.
Ethical considerations
The study was approved by the Human Research Ethics Committee of the Universidade Federal de Mato Grosso do Sul (permit 0037.0.049.049–11). All subjects voluntarily signed a statement of informed consent for the collection of data and received their exam results, along with clarifications on clinical and epidemiological aspects of the infection.
Results
Of the initial 430 blood donors, 70.2% were male and 29.8% female. Age ranged from 18 to 68 years, with a mean of 32 ± 10 years (SD). Of the 430 subjects, 131 (30.5%) were first- or second-time donors. The remaining 299 had been donors for 1 to 35 years, with a mean of 7 ± 6 years (SD). Of these, only 13.0% (39/299) did not donate blood on a regular basis.
In the first evaluation (n = 430), Leishmania sp. detection rates were as follows: 15.6% (95% CI: 12.2–19.0) on IFAT, 5.8% on rK39 ELISA (95% CI: 3.6–8.0), 12.1% on the rK39 rapid test (95% CI: 9.0–15.2), and 22.3% on PCR (95% CI: 18.4–26.3). Only one donor tested positive on all four methods.
Of the 96 donors who tested positive on PCR, 37 (38.5%) were also positive on IFAT. Of the 67 who were positive on IFAT, 37 (55.2%) were also positive on PCR (Table 1). Agreement between these techniques was fair (κ = 0.331, p < 0.001). Of the 67 donors positive on IFAT, seven (10.4%) were also positive on rK39 ELISA. Of the 25 positive on rK39 ELISA, seven (28.0%) were also positive on IFAT. Slight agreement was observed between these techniques (κ = 0.074, p = 0.039). No agreement was detected between the other tests.
Table 1. Distribution of blood donors in the initial evaluation, by test employed for Leishmania sp. detection (n = 430).
| Test | N | κ | p | |
|---|---|---|---|---|
| Positive | Negative | |||
| PCR | ||||
| IFAT | ||||
| Positive | 37 | 30 | 0.331 | <0.001 |
| Negative | 59 | 304 | ||
| Rapid test | ||||
| Positive | 12 | 40 | 0.006 | 0.445 |
| Negative | 84 | 294 | ||
| rK39 ELISA | ||||
| Positive | 7 | 18 | 0.026 | 0.241 |
| Negative | 89 | 316 | ||
| IFAT | ||||
| Rapid test | ||||
| Positive | 8 | 44 | -0.002 | 0.483 |
| Negative | 59 | 319 | ||
| ELISA-rK39 | ||||
| Positive | 7 | 18 | 0.074 | 0.039 |
| Negative | 60 | 345 | ||
| Rapid test | ||||
| ELISA-rK39 | ||||
| Positive | 1 | 24 | -0.057 | 0.101 |
| Negative | 51 | 354 | ||
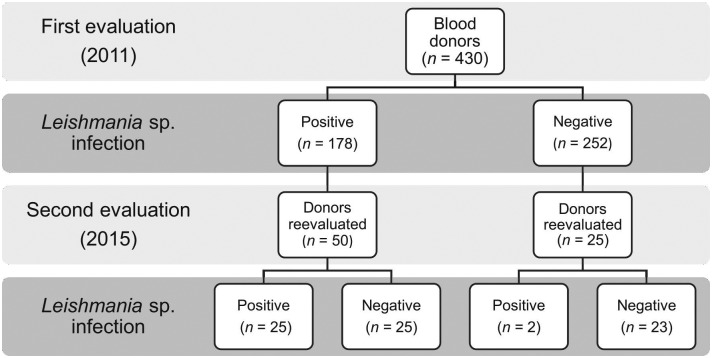
In the initial evaluation, 41.4% of subjects (178/430; 95% CI: 36.7–46.1) were positive for at least one Leishmania test (Fig 1). Four years later, none had developed the disease, but infection was detected in 36.0% (27/75; 95% CI: 25.1–46.9). Of the 50 who initially tested positive in at least one test, 50.0% (95% CI: 36.6–63.4) retained this status. Of the 25 initially negative, two (8.0%) tested positive on the second evaluation.
Fig 1. Distribution of blood donors, by positivity for Leishmania sp. on at least one test (IFAT, rK39 ELISA, rK39 rapid test, PCR).
Of the 50 subjects positive in the first evaluation, in at least one test, 16 (32%) were positive on IFAT, two (4%) on rk39 ELISA, and 14 (28%) on PCR, while all tested negative on the rK39 rapid test. Of the 25 individuals initially negative on all tests, two (8%) were positive on the IFAT test alone four years later.
Of those 14 positive on PCR in the second evaluation, two (14.3%) tested positive on rk39 ELISA, but a fair agreement was found between these techniques (κ = 0.213, p = 0.001). No agreement was detected between other tests (Table 2).
Table 2. Distribution of blood donors in the second evaluation, by test employed for Leishmania sp. detection (n = 75).
| Test | N | κ | p | |
|---|---|---|---|---|
| Positive | Negative | |||
| PCR | ||||
| IFAT | ||||
| Positive | 5 | 13 | 0.130 | 0.128 |
| Negative | 9 | 48 | ||
| Rapid test | ||||
| Positive | - | - | - | - |
| Negative | 14 | 61 | ||
| rK39 ELISA | ||||
| Positive | 2 | - | 0.213 | 0.001 |
| Negative | 12 | 61 | ||
| IFAT | ||||
| Rapid test | ||||
| Positive | - | - | - | - |
| Negative | 18 | 57 | ||
| rK39 ELISA | ||||
| Positive | 1 | 1 | 0.055 | 0.191 |
| Negative | 17 | 56 | ||
| Rapid test | ||||
| rK39 ELISA | ||||
| Positive | - | 2 | - | - |
| Negative | - | 73 | ||
Comparison of both evaluations for each test (Table 3) revealed a fair agreement for rK39 ELISA (κ = 0.309, p = 0.002) and IFAT (κ = 0.302, p = 0.003), but slight agreement for PCR (κ = 0.171, p = 0.015). For presence of infection, defined by positivity on at least one of the four tests, a fair agreement was observed between first and second evaluations (κ = 0.341, p < 0.001).
Table 3. Distribution of blood donors for each test employed for Leishmania sp. detection in comparison of year of evaluation 2011 vs. 2015 (n = 75).
| Test | 2015 | κ | p | |
|---|---|---|---|---|
| Positive | Negative | |||
| 2011 | N | N | ||
| rK39 ELISA | ||||
| Positive | 1 | 3 | 0.309 | 0.002 |
| Negative | 1 | 70 | ||
| IFAT | ||||
| Positive | 11 | 15 | 0.302 | 0.003 |
| Negative | 7 | 42 | ||
| PCR | ||||
| Positive | 12 | 33 | 0.171 | 0.015 |
| Negative | 2 | 28 | ||
| Rapid test | ||||
| Positive | - | 11 | - | - |
| Negative | - | 64 | ||
| Leishmania infection* | ||||
| Positive | 25 | 25 | 0.341 | <0.001 |
| Negative | 2 | 23 | ||
*Defined as positivity on at least one test (IFAT, rK39 ELISA, rK39 rapid test, PCR).
The second evaluation revealed no subjects with a history of VL diagnosis or clinical treatment in the four-year study period investigated. No subjects developed visceromegaly or lymphadenopathy.
Discussion
Among healthy populations, the seroprevalence of asymptomatic leishmaniasis ranges from <10.0% in regions where transmission rates are low or moderate [18], to >30.0% in areas with high transmission rates or among close contacts [19,20].
The first evaluation revealed infection in 41.4% of blood donors—a surprisingly high rate, not only for Brazil (Salvador: 5.4% of 700; Paraná: 11.4% of 176; Fortaleza: 17.1% of 431) [21–23], but also globally (France: 13.4% of 565; Spain: 3.1% of 1437; Nepal: 1% of 507) [24–26].
These disparate rates may be explained not only by regional variability in transmission dynamics, but also by differences in type and sensitivity of diagnostic methods [27] and the age range and specificities of the population investigated [20,28].
In the present study, four diagnostic methods were applied to all samples, whereas performing only molecular analysis of serologically positive samples can lead to underestimation of infection rates. In fact, prevalence and distribution of asymptomatic infection can serve as indicators of transmission, facilitating disease monitoring and control [29].
Presence of seropositive individuals in a population may indicate recent infection, followed by spontaneous cure [28,30], as seems to have occurred in the present sample, since none of the infected subjects developed the disease during the four-year study period investigated. However, longer exposure to the parasite may increase resistance against the disease, yielding positive tests in individuals with no history of VL [31]. In endemic areas, detection of antibodies can be interpreted as transient protection acquired from previous exposure, not necessarily indicating risk of disease progression [32]. As the receptors could not be investigated and the duration of the presence of these antibodies is not known, this possibility was not discussed.
Positivity on IFAT was defined as titers of 1:80 or higher—a criterion advocated by the Brazilian Ministry of Health [33] and adopted by a number of studies [20,31,32,34]—whereas adopting a 1:40 threshold [8,35] would have yielded a much higher prevalence rate.
In the present study, IFAT yielded higher positivity than other serological methods. Of the four tests employed for reevaluation, IFAT also yielded the highest rate of detection, despite the possibility of reinfection, with a consequent effect on anti-Leishmania antibody production. Differences in detection rates were also found for ELISA (25 positive cases, or 5.8%) and the rapid test (52, or 12.1%), both of which employed the rK39 antigen, which demonstrates that antigen choice can also influence antibody identification results [21]. Validated in Brazil and elsewhere [36,37], the rk39 rapid test has been used to detect asymptomatic and subclinical infections [29]. In the reevaluation, only two samples were positive on ELISA, while all were negative on the rapid test—a detection failure also observed in other studies [38,39].
The poor agreement observed among serological tests may stem from the fact that each detects a different stage of infection [32], with variable performance, given the diversity exhibited by the parasite and differences in antibody concentration, immune response, age range, and host nutritional status [40]. To overcome the limitations of serological detection and difficulties in parasite visualization, an association of methods has been recommended as an approach to enhance sensitivity and improve the detection of carriers [9,24,41].
Presence of amastigotes in peripheral blood of the donors was revealed by detection of Leishmania sp. DNA by PCR. Owing to its high specificity and sensitivity, the technique can detect low parasitic loads [42], even before clinical manifestation [43].
Amplification of Leishmania kDNA in serologically negative samples may indicate prior contact with the parasite, suggesting that kDNA presence, identified by PCR, is not always sufficient to elicit detectable humoral response [25,44–46]. On the other hand, adaptive immunity with antibody production, found in donors simultaneously positive on serology and negative on PCR, confirmed that L. infantum circulates intermittently, despite undetectable DNA [5,9,24].
No cases of progression to leishmaniasis were identified. Disease development, or lack thereof, may be related to exposure risk, geographic differences, or genetic susceptibility or resistance [46–48]. Although the mechanism of Leishmania survival in carriers remains unknown, the process is believed to dependent primarily on an equilibrium between host immune system and parasite virulence [24,41].
Although conversion rates can be significantly higher among individuals with positive results in more than one type of test [49], in the present study only one subject was positive on all tests in the first evaluation. Four years later, positivity in this donor was found on only two tests (ELISA and PCR), but no clinical manifestation was observed.
The high percentage of donors who were positive on both serology and PCR is a disquieting finding, compounded by their continued positive status four years later, irrespective of whether reinfection had occurred. Asymptomatic donors pose a silent threat to blood recipients—potential transmission has been illustrated by detection of free parasites in blood products following monocyte damage during fractionation [25]. Amastigote viability in blood under normal storage conditions [50] and experimental transmission of Leishmania [51,52] have both been confirmed.
The rising number of immunocompromised individuals is now a major contributor to the spread of infection, given their increased vulnerability to primary and reactivated infection with Leishmania [5,9]. In the present study it was not possible to obtain information on blood recipients, for the tracing and examination of them, since this process is totally confidential.
If better elucidated, asymptomatic cases should help in characterizing the epidemiology of leishmaniasis more accurately, serving as markers of potential progression to the disease, particularly in view of the frequency of seropositivity among the general population. Deeper knowledge of asymptomatic leishmaniasis can provide a fuller understanding of Leishmania transmission, dissemination, and survival in carriers, facilitating patient follow-up and ultimately reducing morbidity and mortality rates [34].
The implementation of routine screening methods in blood banks should improve the quality of donor selection, with immediate gains in the safety of blood products. For immunosuppressed recipients, inactivation techniques for a range of pathogens should be made mandatory [28], as deployed in the form of leucodepletion filters and other methods for Leishmania [53–56].
Special attention should be devoted to asymptomatic infection in blood donors recruited for simultaneous donation of bone marrow, given the potential presence of amastigotes in this type of tissue. Recent data on bone marrow recipients [38] suggest the advantages of employing PCR to identify higher risks of VL reactivation or protozoan transmission. Transmission by blood transfusion further complicates the clinical course of organ transplant, with possibly fatal outcomes [57].
The severity of this parasitosis and the risk of transfusion transmission, particularly in endemic areas, warrant investigation into the potential inclusion of methods for Leishmania detection into the routine of blood banks to ensure proper screening of donors. Although asymptomatic carriers are potential reservoirs, molecular studies are needed to evaluate the risks of parasite viability and development in the blood of donors and subsequent establishment of active disease in recipients.
Supporting information
(XLS)
(XLSX)
Acknowledgments
The authors wish to thank the management board and technicians of Hemosul and the blood donors who agreed to participate in the study. Thanks are also extended to Steve Reed and Greg Ireton of the Infectious Diseases Research Institute (IDRI, Seattle, USA), for donating Leishmania infantum rK39 antigen; and the Brazilian Ministry of Health, for donating the rK39 rapid test kits.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work received financial support from the Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (Fundect), http://fundect.ledes.net/, termos de outorga n° 0191/12, SIAFEM:020919, Processo: 23/200.686/2012 (to MAP) and n° 114/2014, SIAFEM:023670, Processo: 23/200.262/2014 (to MEMCD). e-mail:projetos@fundect.ms.gov.br.
References
- 1.Davies CR, Gavgani ASM. Age, acquired immunity and the risk of visceral leishmaniasis: a prospective study in Iran. Parasitology. 1999;119(3): 247–257. [DOI] [PubMed] [Google Scholar]
- 2.Moral L, Rubio EM, Moya M. A leishmanin skin test survey in the human population of l’Alacanti region (Spain): implications for the epidemiology of Leishmania infantum infection in Southern Europe. Trans R Soc Trop Med Hyg. 2002;96(2): 129–132. [DOI] [PubMed] [Google Scholar]
- 3.Evans TG, Teixeira MJ, Mcauliffe I, Vasconcelos IAB, Vasconcelos AW, Souza AQ, et al. Epidemiology of visceral leishmaniasis in Northeast Brazil. J Infect Dis. 1992;166(5): 1124–1132. [DOI] [PubMed] [Google Scholar]
- 4.Caldas AJM, Silva DRC, Pereira CCR, Nunes PMS, Silva BP, Silva AAM, et al. Infecção por Leishmania (Leishmania) chagasi em crianças de uma área endêmica de leishmaniose visceral americana na Ilha de São Luis-MA, Brasil. Rev Soc Bras Med Trop. 2001;34(5): 445–451. [DOI] [PubMed] [Google Scholar]
- 5.Cardo LJ. Leishmania: risk to the bood supply. Tranfusion. 2006;46: 1641–1645. [DOI] [PubMed] [Google Scholar]
- 6.Dey A, Singh S. Transfusion transmitted leishmaniasis: a case report and review of literature. Indian J Med Microbiol. 2006;24(3): 165–170. [PubMed] [Google Scholar]
- 7.Guevara P, Ramírez JL, Rojas E, Scorza JV, González N, Añez N. Leishmania braziliensis in blood 30 years after cure. Lancet. 1993;341: 1341. [DOI] [PubMed] [Google Scholar]
- 8.Urias EVR, Carvalho SFG, Oliveira CL, Carvalho MLM, Teles LF, Rodrigues MC, et al. Prevalência de adultos infectados por Leishmania (Leishmania) chagasi entre doadores de sangue do Hemocentro regional de Montes Claros, Minas Gerais, Brasil. Rev Bras Hematol Hemoter. 2009;31(5): 348–354. [Google Scholar]
- 9.Michel G, Pomares C, Ferrua B, Marty P. Importance of world asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Trop. 2011;19(2–3): 69–75. [DOI] [PubMed] [Google Scholar]
- 10.França AO, Castro VL, Lima-Júnior MSC, Pontes ERJC, Dorval MEC. Anti-Leishmania antibodies in blood donos from the Midwest region of Brazil. Transf Apher Sci. 2013;49: 627–630. [DOI] [PubMed] [Google Scholar]
- 11.Burns JM, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci. 1993;90(2): 775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houghton RL, Petrescu M, Benson DR, Skeiky YA, Scalone A, Badaró R, et al. A cloned antigen (recombinant K39) of Leishmania chagasi diagnostic for visceral leishmaniasis in human Immunodeficiency Virus type 1 patients and a prognostic indicator for monitoring patients undergoing drug therapy. J Infect Dis. 1998;177(5): 1339–1344. [DOI] [PubMed] [Google Scholar]
- 13.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45(1–2): 23–41. [DOI] [PubMed] [Google Scholar]
- 14.Martinez EZ, Louzada-Neto F, Pereira BB. A curva ROC para testes diagnósticos. Cad Saude Colet 2003;11(1): 7–31. [Google Scholar]
- 15.Rodrigues KM, Oliveira MP, Maretti-Mira AC, Oliveira-Neto MP, Mattos M S, Silva L, et al. Influence of the notch system in the therapeutic response of American tegumentary leishmaniasis. Br J Dermatol. 2011;164(6): 1228–1234. doi: 10.1111/j.1365-2133.2011.10240.x [DOI] [PubMed] [Google Scholar]
- 16.Pirmez C, Trajano VS, Oliveira-Neto MP, Da-Cruz AM, Gonçalves-da-Costa SC, Catanho M, et al. Use of PCR in diagnosis of human American tegumentary leishmaniasis in Rio de Janeiro, Brazil. J Clin Microbiol. 1999;37(6): 1819–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1): 159–174. [PubMed] [Google Scholar]
- 18.Schenkel K, Rijal S, Koirala SI, Koirala S, Vanlerberghe V, Stuyf PV, et al. Visceral leishmaniasis in Southeastern Nepal: a cross-sectional survey on Leishmania donovani infection and its risk factors. Trop Med Int Health. 2006;11(12):1792–1799. doi: 10.1111/j.1365-3156.2006.01735.x [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim ME, Lambson B, Yousif AO, Deifalla NS, Alnaiem DA, Ismail A, et al. Kala-azar in a high transmission focus: an ethnic and geographic dimension. Am J Trop Med Hyg. 1999;61(6): 941–944. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira ALL, Paniago AMM, Sanches M, Dorval MEC, Oshiro ET, Leal CRB, et al. Asymptomatic infection in family contacts of patients with human visceral leishmaniasis in Três Lagoas, Mato Grosso do Sul State, Brazil. Cad Saud Publ. 2008;24(12): 2827–2833. [DOI] [PubMed] [Google Scholar]
- 21.Fukutani KF, Figueiredo V, Celes FS, Cristal JR, Barral A, Barral-Neto M, et al. Serological survey of Leishmania infection in blood donos in Salvador, Northeastern Brazil. BMC Infect Dis. 2014;14: 422 doi: 10.1186/1471-2334-14-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braga LS, Navasconi TR, Leatte EP, et al. Presence of anti-Leishmania (Viannia) braziliensis antibodies in blood donors in the West-Central region of the State of Paraná, Brazil. Rev Soc Bras Med Trop. 2015(5);48: 622–625. doi: 10.1590/0037-8682-0043-2015 [DOI] [PubMed] [Google Scholar]
- 23.Monteiro DCS, Sousa AQ, Lima DM, et al. Leishmania infantum infection in blood donos, Northeastern Brazil. Emerg Infect Dis. 2016;22(4): 739–740. doi: 10.3201/eid2204.150065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, et al. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an área of endemicity in southern France. J Clin Microbiol. 1999;37(6): 1953–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riera C, Fisa R, Chejade-Lopez P, Serrra T, Girona E, Jiménez MT, et al. Asymptomatic infection by Leishmania infantum in blood donors from the Balearic Islands (Spain). Transfusion. 2008;48: 1383–1389. doi: 10.1111/j.1537-2995.2008.01708.x [DOI] [PubMed] [Google Scholar]
- 26.Timilsina S, Bhattarai RN, Khanal B, Rijal S. Serological assesment for Leishmania donovani infection blood donors of Sunsari District, Dharan, Nepal. Indian J Hematol Blood Transfus. 2016;32(1): 95–99. doi: 10.1007/s12288-015-0505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werneck GL. Forum: geographic spread and urbanization of visceral leishmaniasis in Brazil. Introduction. Cad Saud Publ. 2008;24(12): 2937–2940. [DOI] [PubMed] [Google Scholar]
- 28.Scarlata F, Vitale F, Saporito L, Reale S, Li Vecchi V, Giordano S et al. Asymptomatic Leishmania infantum chagasi infection in blood donors of Western Sicily. Trans R Soc Trop Med Hyg. 2008;102(4): 394–396. doi: 10.1016/j.trstmh.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 29.Barão SC, Camargo-Neves VLF, Resende MR, Silva LJ. Human asymptomatic infection in visceral leishmaniasis: a seroprevalence study in urban area of low endemicity. Preliminary results. Am J Trop Med Hyg. 2007;77(6): 1051–1053. [PubMed] [Google Scholar]
- 30.Lima ID, Queiroz JW, Lacerda HG, Queiroz PVS, Pontes NN, Barbosa JDA, et al. Leishmania infantum chagasi in Northeastern Brazil: asymptomatic infection at the urban perimeter. Am J Trop Med Hyg. 2012; 86(1): 99–107. doi: 10.4269/ajtmh.2012.10-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crescente JAB, Silveira FT, Lainson R, Gomes CMC, Laurenti MD, Corbett CEP. A cross-sectional study on the clinical and immunological spectrum of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon region. Trans R Soc Trop Med Hyg. 2009;103(12): 1250–1256. doi: 10.1016/j.trstmh.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 32.Silva LA, Romero HD, Nascentes GAN, Costa RT, Rodrigues V, Prata A. Antileishmania immunological tests for asymptomatic subjects living in a visceral leishmaniasis endemic area in Brazil. Am J Trop Med Hyg. 2011;84(2): 261–266. doi: 10.4269/ajtmh.2011.10-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de vigilância e controle da leishmaniose visceral. Brasília:Ministério da Saúde,Brasil; 2014. 120 p.
- 34.Romero HD, Silva lA, Silva-Vergara ML, Rodrigues V, Costa RT, Guimarães SF, et al. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am J Trop Med Hyg. 2009;81(1): 27–33. [PubMed] [Google Scholar]
- 35.Moreno EC, Melo MN, Lambertucci JR, Serufo JC, Andrade ASR, Antunes CMF, et al. Diagnosing human asymptomatic visceral leishmaniasis in an urban area of the state of Minas Gerais, using serological and molecular biology techniques. Rev Soc Bras Med Trop. 2006;39(5): 421–427. [DOI] [PubMed] [Google Scholar]
- 36.Peruhype-Magalhães V, Assis TSM, Rabello A. Use of the Kala-Azar Detect® and IT-LEISH® rapid tests for the diagnosis of visceral leishmaniasis in Brazil. Mem Inst Oswaldo Cruz. 2012;107(7): 951–952. [DOI] [PubMed] [Google Scholar]
- 37.Cañavate C, Herrero M, Nieto J, Cruz I, Chicharro C, Aparicio P, et al. Evaluation of two rK39 dipstick tests, direct agglutination test and indirect fluorescent antibody test for diagnosis of visceral leishmaniasis in a new epidemic site in Highland Ethiopia. Am J Trop Med Hyg. 2011;84(1): 102–106. doi: 10.4269/ajtmh.2011.10-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemente WT, Rabello A, Faria LC, Peruhype-Magalhães V, Gomes LI, Silva TAM, et al. High prevalence of asymptomatic Leishmania spp. infection among liver transplant recipients and donors from an endemic area of Brazil. Am J Transplant. 2014;14: 96–101. doi: 10.1111/ajt.12521 [DOI] [PubMed] [Google Scholar]
- 39.Fraga TL, Fernandes MF, Pontes ERJC, Levay APS, Cunha EBA, França AO, et al. Antissaliva antibodies of Lutzomyia longipalpis in area of visceral leishmaniasis. Pediatr Infect Dis J. 2016;35(7): 805–807. doi: 10.1097/INF.0000000000001172 [DOI] [PubMed] [Google Scholar]
- 40.Maia Z, Lírio M, Mistro S, Mendes CMC, Mehta SR, Badaro R. Comparative study of rK39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: systematic review with meta-analysis. PLoS Negl Trop Dis. 2012;6(1): e1484 doi: 10.1371/journal.pntd.0001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riera C, Fisa R, Udina M, Gállego M, Portus M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands,Spain) by different diagnostic methods. Trans R Soc Trop Med Hyg. 2004;98(2): 102–110. [DOI] [PubMed] [Google Scholar]
- 42.Fakhar M, Motazedian MH, Hatam GR, Asgari Q, Kalantari M, Mohebali M. Asymptomatic human carriers of Leishmania infantum: possible reservoirs for Mediterranean visceral leishmaniasis in Southern Iran. Ann Trop Med Parasitol 2008;102(7): 1–7. [DOI] [PubMed] [Google Scholar]
- 43.Singh S, Sivakumar R. Recent advances in the diagnosis of leishmaniasis. J Postgrad Med. 2003;49(1): 55–60. [DOI] [PubMed] [Google Scholar]
- 44.Costa CH, Stewart JM, Gomes RB, Garcez LM, Ramos PKS, Bozza M, et al. Asymptomatic human carriers of Leishmania chagasi. Am J Trop Med Hyg. 2002;66(4): 334–337. [DOI] [PubMed] [Google Scholar]
- 45.Martín-Sánchez J, Pineda JÁ, Morillas-Márquez F, García-García JA, Acedo C, Macías J. Detection of Leishmania infantum kinetoplast DNA in peripheral blood from asymptomatic individuals at risk for parenterally transmited infections: relationship between polymerase chain reaction results and other Leishmania infection markers. Am J Trop Med Hyg. 2004;70(5): 545–548. [PubMed] [Google Scholar]
- 46.Viana LG, Assis TSM, Orsini M, Silva AR, Souza GF, Caligiorne R, et al. Combined diagnostic methods identify a remarkable proportion of asymptomatic Leishmania (Leishmania) chagasi carriers who present modulated cytokine profiles. Trans R Soc Trop Med Hyg. 2008;102(6): 548–555. doi: 10.1016/j.trstmh.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 47.Singh S, Kumari V, Singh N. Predicting kala-azar disease manifestation in asymptomatic patients with latent Leishmania donovani infection by detection of antibody against recombinant k39 antigen. Clin Diagn Lab Immunol. 2002;9(3): 568–572. doi: 10.1128/CDLI.9.3.568-572.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeronimo SMB, Duggal P, Ettinger NA, Nascimento ET, Monteiro GR, Cabral AP, et al. Genetic predisposition to self-curing infection with the protozoan Leishmania chagasi: a genomewide scan. J Infect Dis. 2007;196(8): 1261–1269. doi: 10.1086/521682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das VN, Siddiqui NA, Verma RB, Topno RK, Singh D, Das S, et al. Asymptomatic infection of visceral leishmaniasis in hyperendemic areas of Vaishali district, Bihar, India: a challenge to ka-lazar elimination programs. Trans R Soc Trop Med Hyg. 2011;105(11): 661–666. doi: 10.1016/j.trstmh.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 50.Grogl M, Daugirda JL, Hoover DL, Magill AJ, Berman JD. Survivability and infectivity of viscerotropic Leishmania tropica from operation desert storm participants in human blood products maintained under blood bank conditions. Am J Trop Med Hyg. 1993;49(3): 308–315. [DOI] [PubMed] [Google Scholar]
- 51.Kubar J, Quaranta JF, Marty P, et al. Transmission of L. infantum by blood donors. Nat Med. 1997;3(4): 368 [DOI] [PubMed] [Google Scholar]
- 52.Morillas-Márquez F, Martín-Sánchez J, Acedo-Sanchez C, Pineda JA, Macias J, Sanjuan-Garcia J. et al. Leishmania infantum (Protozoa, Kinetoplastida): transmission from infected patients to experimental animal under conditions that simulate needle-sharing. Exp Parasitol. 2002;100(1): 71–74. doi: 10.1006/expr.2001.4678 [DOI] [PubMed] [Google Scholar]
- 53.Cardo LJ, Salata J, Harman R, Mendez J, Weina PJ. The use of leukodepletion filters at collection to reduce the risk of transfusion transmission of Leishmania donovani infantum and Leishmania major. Transfusion. 2005;45: 30A. [Google Scholar]
- 54.Kyriakou DS, Alexandrakis MG, Passam FH, Kourelis TV, Foundouli P, Matalliotakis E, et al. Quick detection of Leishmania in peripheral blood by flow cytometry. Is prestorage leucodepletion necessary for leishmaniasis prevention in endemic areas? Transfus Med. 2003;13(2): 59–62. [DOI] [PubMed] [Google Scholar]
- 55.Jimenez-Marco T, Fisa R, Riera C, Girona-Llobera E, Sedeño M, Saura A, et al. Pathogen inactivation technology applied to a blood componente collected from an asymptomatic carrier of Leishmania infantum: a case report. Vox Sang. 2012;103: 356–358. doi: 10.1111/j.1423-0410.2012.01622.x [DOI] [PubMed] [Google Scholar]
- 56.Mansueto P, Seidita A, Vitale G, Cascio A. Transfusion transmitted leishmaniasis. What to do with blood donos from endemic areas? Travel Med Infect Dis. 2014;12: 617–627. doi: 10.1016/j.tmaid.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 57.Mestra L, Lopez L, Robledo SM, Muskus CE, Nicholls RS, Vélez ID. Transfusion-transmitted visceral leishmaniasis caused by Leishmania (Leishmania) mexicana in an immunocompromised patient: a case report. Transfusion. 2011;51: 1919–1923. doi: 10.1111/j.1537-2995.2011.03092.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.