Abstract
Background
To confirm treatment-resistant hypertension (TRH), ambulatory blood pressure measurement (ABPM) must exclude white-coat hypertension (WCH), three or more medications should be prescribed at the optimal doses tolerated, and non-adherence and lifestyle should be examined. Most previous studies have not adequately considered pseudo-resistance and merely provide an apparent TRH (aTRH) prevalence figure.
Aim
To conduct a cross-sectional study of the prevalence of aTRH in general practice, and then consider pseudo-resistance and morbidity.
Design and setting
With support, 16 practices ran an anatomical therapeutic chemical (ATC) drug search, identifying patients on any possible hypertensive medications, and then a search of individual patients’ electronic records took place.
Method
ABPM was used to rule out WCH. The World Health Organization-defined daily dosing guidelines determined adequate dosing. Adherence was defined as whether patients requested nine or more repeat monthly prescriptions within the past year.
Results
Sixteen practices participated (n = 50 172), and 646 patients had aTRH. Dosing was adequate in 19% of patients, 84% were adherent to medications, as defined by prescription refill, and 43% had ever had an ABPM. Using a BP cut-off of 140/90 mmHg, the prevalence of aTRH was 9% (95% confidence interval [CI] = 9.0 to 10.0). Consideration of pseudo-resistance further reduced prevalence rates to 3% (95% CI = 3.0 to 4.0).
Conclusion
Reviewing individual patient records results in a lower estimate of prevalence of TRH than has been previously reported. Further consideration for individual patients of pseudo-resistance additionally lowers these estimates, and may be all that is required for management in the vast majority of cases.
Keywords: adherence, cross-sectional studies, dosing, hypertension, primary care, pseudo-resistances
INTRODUCTION
Treatment-resistant hypertension (TRH) is defined by the American Heart Association and European Society of Cardiology (AHA/ESC) as high blood pressure (BP) in patients taking three or more differing groups of antihypertensive medications (one of which must be a diuretic-type medication), or patients who are taking four or more antihypertensive medications regardless of type and blood pressure level.1,2 BP levels need to be adapted to specific morbidity (for example, diabetes), ambulatory blood pressure measurement (ABPM) must exclude white-coat hypertension (WCH), doses should be the optimal tolerated for each particular medication, and both non-adherence and lifestyle should be examined.1,3–5 When these pseudo-resistance issues6 have not yet been ruled out as a potential cause for the ongoing high BP, the term apparent treatment-resistant hypertension (aTRH) is used. Apparent TRH can be considered as an overdiagnosis — a concept that is gaining increasing attention.7 True treatment-resistant hypertension (tTRH) estimates will clearly be lower.
A recent meta-analysis8 in various hypertensive populations suggests a prevalence of aTRH of 13.7% for 20 observational cohorts, and of 16.3% for four randomised trials.9 However, the individual studies were very heterogeneous (I2 >90%) with prevalence estimates varying between 4% and 25%.10,11 There were many reasons for this. First, the definition of aTRH varied, as some simply included those on three medications without specifying the need, as required, for a diuretic,12 whereas others ignored those on four or more medications with a normal BP.4 Second, many studies used large health Insurance databases12 or BP registries,4 and this can create a selection bias. Furthermore, some population studies are based on questionnaire and survey approaches only.10,13 Third, a general cut-off was often used to define hypertension (for example, ≥140/90 mmHg), without considering individual morbidities, such as diabetes or kidney disease, which require a lower threshold (130/80 mmHg). It is now also accepted that patients >80 years should have a higher threshold of 150/80 mmHg.14 Fourth, ABPM is essential to rule out WCH,15 but in most studies manual BP readings are solely used. Additional criticism includes the lack of consideration of optimal medication dosing and patient adherence.16
The authors therefore conducted a cross-sectional study of the prevalence of TRH in general practice, using the correct AHA/ESC definition, and with consideration of individual patient morbidity, and the three key aspects of pseudo-resistance — WCH, inadequate dosing, and non-adherence.
How this fits in
Both the American Heart Association (2008) and the UK National Institute for Health and Care Excellence (2011) suggest the need for further research into the prevalence, prognosis, and management of people with treatment-resistant hypertension (TRH). Prevalence estimates typically lie between 10 and 30% of patients with hypertension. However, the three main factors of pseudo-resistance (non-adherence, inadequate dosing, and white-coat hypertension) are rarely examined. The authors have shown that these factors can be reviewed for individual patients, and physician evaluation may be all that is required in considering TRH in the majority of cases.
METHOD
Forty general practices in the university-affiliated research network, WestREN, representative of the Irish population, were invited to participate.17 All used the same practice software system (Socrates®) and the International Classification for Primary Care (ICPC-2) coding of chronic diseases. Data were collected between May 2015 and October 2016, and this work fulfilled the Irish Medical Council requirement for GPs to conduct an annual audit.
Ireland does not have universal registration with a GP. Almost 45% of the population is registered through the primary care reimbursement service (PCRS), with the remainder being described as private patients and able to see any GP.18 All patients aged >80 years and those below defined income levels (<€500 [£444] gross per week for a single person; €900 [£798] gross per week for a couple) are registered with the PCRS. The authors therefore included in the cross-sectional sample all PCRS patients and those private patients who had attended the practice in the past year.
The authors supported each practice in fulfilling the annual audit requirements of the Irish Medical Council. Each practice ran a standard anatomical therapeutic chemical (ATC) drug search, identifying patients on any possible hypertensive medications as defined by the British National Formulary.19 The record of each individual patient who was reported as being on one or more hypertensive medication was reviewed and it was determined if they were hypertensive or not, and what hypertensive medications they were currently receiving. Two researchers supported each practice in this process to ensure consistency.
Patients were described as having diabetes if they had ICPC codes T89 (diabetes insulin dependent) or T90 (diabetes non-insulin dependent), or were taking insulin or oral hypoglycaemic agents. Patients were described as having chronic kidney disease (CKD) if an estimated glomerular filtration rate (eGFR) <60 mls/min/1.73 m2 was recorded. Patients were described as having cardiac failure if they had ICPC code K77 or were noted to have this condition on hospital correspondence. This figure would only include those with an initial secondary care diagnosis of heart failure.
The following BP thresholds were used for those on three or more appropriate medications, and the latest recorded manual clinic reading was the parameter used:
manual office BP for patients <80 years: ≥140/90 mmHg;
manual office BP for patients >80 years: ≥150/80 mmHg;
manual office BP for patients with diabetes or chronic kidney disease: ≥130/80 mmHg; and
24-hour ABPM (daytime mean with a minimum of 14 BP readings): ≥135/85 mmHg.
WCH was a concern, and those with elevated clinic BP readings but normal ABPM reports within the previous 6 months, without significant medication changes, were excluded at initial discovery stage.
The World Health Organization-defined daily dosing (WHO-DDD) guidelines were used to determine whether adequate dosing was achieved.20 The DDD is the assumed average maintenance dose per day for a drug, used for its main indication, in adults. Egan et al adopted a slightly different approach, as they sought to determine the numbers of patients who were receiving, for each medication, diuretics apart, at least half the maximum dose.5 The authors present both approaches.
Adherence to antihypertensive medications was determined by prescription refill data. Patients requesting nine or more repeat monthly prescriptions within the past year were deemed adherent. Prescriptions are mainly dispensed quarterly, therefore the authors chose adherence as >75% prescriptions as opposed to the standard 80%.
Statistical analysis
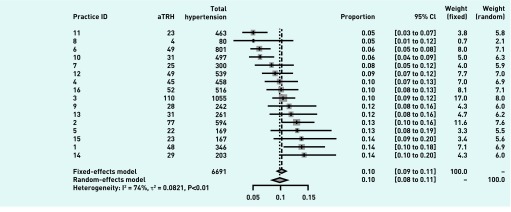
The authors used a fixed-effects (FE) model to estimate overall prevalence from the practices. The fixed-effects rather than random-effects (RE) was chosen given the cross-sectional design, the strict geographical inclusion criteria for practices, the similarity of patient covariates across practices, and the fact that estimation of prevalence of aTRH is the main objective rather than the analysis of heterogeneity. In fact, the results from both models were very similar (data available from the authors upon request). To account for adherence and adequate dosing, and various comorbidities, the authors performed regressions at the individual patient level on figures taken from the initial aTRH meta-analysis (Figure 1). Here, once again, the results from both models (FE and RE) were very similar. Statistics were analysed in R.
Figure 1.
Meta-analysis of prevalence of aTRH by practice. aTRH = apparent treatment-resistant hypertension. CI = confidence interval.
RESULTS
Sixteen practices participated, with an estimated total population of 50 172; of these, 31 157 were registered with the PCRS. Figure 2 provides a flow chart. Of the 2807 patients on three or more medications, 646 (9.6%) were deemed to have aTRH. Table 1 illustrates the characteristics of those with aTRH. They were largely older patients (mean age 71 years) and male (53.7%), with PCRS eligibility (81.7%); 43% had ever had a previous ABPM. Figure 1 illustrates a meta-analysis of prevalence by practice using a general threshold of ≥140/90 mmHg for all those without diabetes or chronic kidney disease who had a lower target BP of ≥130/80 mmHg. The FE model suggests, with significant heterogeneity, a combined prevalence of 10% (95% confidence interval [CI] = 9.3 to 10.8%).
Figure 2.
Flow chart of patients. ABPM = ambulatory blood pressure measurement. BP = blood pressure. PCRS = primary care reimbursement service. TRH = treatment-resistant hypertension.
Table 1.
Characteristics of patients with apparent treatment-resistant hypertension (n = 646)
| Mean age (SD), years | 71.1 (12.2) |
|
| |
| Sex — female, n (%) | 299 (46.3) |
|
| |
| PCRS eligibility, n (%) | 528 (81.7) |
|
| |
| Diabetes, n (%) | 237 (36.7) |
|
| |
| Chronic kidney disease, n (%) | 256 (40.0) |
|
| |
| Cardiac failure, n (%) | 88 (13.6) |
|
| |
| Mean systolic clinic BP (SD), mmHg | 142.1 (18.0) |
|
| |
| Mean diastolic clinic BP (SD), mmHg | 78.1 (11.9) |
|
| |
| Have ever had an ABPM, n (%) | 276 (42.7) |
|
| |
| Elevated ABPM (past 6 months, n= 74) | |
| Mean daytime systolic ABPM (SD), mmHg | 147.9 (21.5) |
| Mean daytime diastolic ABPM (SD), mmHg | 81.4 (14.8) |
ABPM = ambulatory blood pressure measurement. BP = blood pressure. PCRS = primary care reimbursement service. SD = standard deviation.
Table 2 illustrates participants’ medication particulars. In all, 43.7% were on three medications only, with 56.9% taking one or more combined medication; 83.9% appeared to be adherent, having had nine or more monthly prescriptions printed in the last 12 months. According to WHO-DDD guidelines, 19% achieved adequate dosing for each hypertensive medication, whereas 67.9% achieved adequate dosing when a parameter of >50% maximum dose in all medications, diuretics apart, is used. A nine-practice subset showed 93% of patients with TRH were on a stable medication regimen for ≥3 months at the study onset.
Table 2.
Medications of patients with apparent treatment-resistant hypertension (n = 646)
| Mean number of hypertensive medications (SD) | 3.7 (0.7) |
| On three hypertensive medications only, n (%) | 282 (43.7) |
| Prescribed one or more combined medication, n (%) | 368 (56.9) |
| Adequate WHO-DDD requirements for each hypertensive medication, n (%) | 123 (19.0) |
| At least half recommended dosing for all medications (not diuretics), n (%) | 439 (67.9) |
| Nine monthly PCRS prescriptions printed in the past 12 months, n (%) | 542 (83.9) |
PCRS = primary care reimbursement service. SD = standard deviation. WHO-DDD = World Health Organization-defined daily dosing.
Table 3 describes dosing according to drug class. Renin–angiotensin–aldosterone system (RAAS) drugs had high levels of adequate dosing — ACE-inhibitors (93%) and angiotensin receptor blockers (95%) — in comparison with beta-blockers (31%) and thiazides (43%). The most common thiazide drug was hydrochlorthiazide (56% of all thiazide medications), which is frequently found in combination drugs. Mineralocorticoid antagonists were rarely used (7%).
Table 3.
Dosing according to drug class of patients with apparent treatment-resistant hypertension (n = 646)
| Medication | Patients on drug group, n (%) | Patients on drug class, n (%) | Patients adequately dosed per class as per WHO-DDD, n (%) |
|---|---|---|---|
| Diuretics | 614 (95) | ||
| Thiazides | 424 (65) | 181 (43) | |
| Loop | 235 (36) | 150 (64) | |
| Potassium sparing | 50 (8) | 1 (2) | |
| Mineralocorticoid antagonists | 48 (7) | 5 (10) | |
| Renin–angiotensin–aldosterone system (RAAS) | 614 (95) | ||
| ACE-inhibitors | 286 (44) | 267 (93) | |
| Angiotensin receptor blockers | 320 (49) | 305 (95) | |
| Direct renin inhibitors | 5 (1) | 5 (100) | |
| HR reducers | 440 (68) | ||
| Beta-blockers | 412 (63) | 130 (31) | |
| Calcium channel blockers — non dihydropyridines | 41 (5) | 11 (27) | |
| CCB-DHP | 440 (68) | ||
| Calcium channel blockers — dihydropyridines | 429 (68) | 424 (99) | |
| Vasodilators | 129 (20) | ||
| Alpha-blockers | 127 (19) | 124 (98) | |
| Other vasodilators | 3 (1) | 0 (0) | |
| Centrally acting | 2 (<1) | ||
| Centrally acting drugs | 2 (1) | 0 (0) |
ACE = angiotensin-converting enzyme. CCB-DHP = calcium channel blockers — non-dihydropyridines. HR = heart rate. WHO-DDD = World Health Organization-defined daily dosing.
Table 4 illustrates the FE meta-analysis of prevalence of aTRH hypertension in the cohort, after parameters for morbidity, age, non-adherence, and inadequate dosing were applied.
Table 4.
Fixed-effects meta-analysis of apparent treatment-resistant hypertension according to additional criteria
| Analysis | Prevalence estimate (95% CI) | I2 |
|---|---|---|
| Applying a threshold of 140/90 mmHg to all patients | 0.09 (0.09 to 0.10) | 0.76 |
|
| ||
| And | ||
| Applying a threshold of 130/80 mmHg for patients with diabetes or chronic kidney disease | 0.10 (0.09 to 0.11) | 0.74 |
|
| ||
| And | ||
| Applying a threshold of 150/80 mmHg for patients >80 years regardless of morbidity | 0.09 (0.08 to 0.10) | 0.76 |
|
| ||
| And | ||
| Applying a threshold of adequate dosing in all medications (WHO-DDD) | 0.04 (0.04 to 0.05) | 0.83 |
|
| ||
| And | ||
| Applying a threshold of adequate adherence (>75% prescriptions) | 0.03 (0.03 to 0.04) | 0.80 |
CI = confidence interval. WHO-DDD = World Health Organization-defined daily dosing. I2 is the percentage of variance in the meta-analysis that is attributable to study heterogeneity.
DISCUSSION
Summary
The authors have found that reviewing individual general practice patient records results in a lower estimate of the prevalence of TRH than has generally been previously reported.8 This lower prevalence estimate is consistent with a recent estimate of 6.5% from an analysis of the electronic health records from the UK Clinical Practice Datalink in Primary Care.21 Consideration for individual patients of additional criteria such as morbidity, dosing, exclusion of WCH, and adherence lowers these estimates even more. This suggests that TRH is an uncommon condition in general practice and use of ABPM, adequate dosing, and maximising adherence may be all that is required to manage the vast majority of cases. It could be argued that optimising doses in patients with aTRH may not lead to improvements for all, as many patients may have tried more suitable doses and had medication side effects-appropriate inaction. The authors accept this point. However, Gil-Guillen et al suggest that physician inertia to up-dose is a significant problem (70% of the time).22 The WHO-DDD guidelines were used to determine whether adequate dosing was achieved.20 Egan et al adopted a different approach, as they sought to determine the numbers of patients who were receiving, for each medication, apart from diuretics, at least half the maximum dose.5 This of course had a lower threshold for adequate dosing and a greater number of patients were deemed to be optimally dosed. It could be argued that the WHO daily defined dose for many of the more side effect-prone drugs, such as beta-blockers and thiazide diuretics, are too stringent. Many older patients do not tolerate such doses and are therefore rarely escalated to these levels for fear of significant side effects such as falls. Indeed data presented in Table 3, where RAAS, calcium channel blockers (CCBs), and alpha-blockers each achieve adequate WHO dosing in excess of 90%, suggest practitioners are adopting this pragmatic approach. The authors also note that improving dosing may not actually affect BP levels, but it is hard to quantify this.23
The authors had anticipated that the outcome of this study would be to refer a significant number of patients for specialist opinion. This did not materialise, which suggests that perhaps TRH is overdiagnosed.
Strengths and limitations
In their systematic review, Achelrod et al identified a number of important limitations in studies reviewing the prevalence of resistant hypertension.8 An important contribution of the present study is how the authors have addressed these, using the AHA/ESC definition. He highlighted that the sampling frames used were often limited to those of convenience, referral centres, or specific health plans, with often a focus on high-risk patients. Reliance on large electronic insurance databases may result in the inclusion of more affluent patients. These factors constitute a source of selection bias and limited external validity. The authors have followed his recommendation in developing a prospective survey of a ‘clustered sample of participants from the general treated hypertensive population’. Also highlighted was the failure of observational studies to consider adherence and WCH. The authors have applied both the WHO-DDD guidelines and those of Egan et al 5 to determine whether adequate dosing was achieved. Recent ABPM results, where available, were used to exclude WCH at the initial patient discovery stage.
Limitations of this study include the use of 16 practices from one geographical region with an overwhelmingly white-European population; the labour-intensive approach of individual patient record review necessitated this approach. The lack of universal registration or a unique health identifier in Ireland posed difficulties in determining a population denominator. Retaining only those private patients who had attended in the past year is clearly an arbitrary but, the authors believe, pragmatic decision. It may overestimate the levels of adherence. Like most other similar studies, the authors were unable to report exercise or dietary patterns. The definition of CKD was one, rather than two, eGFR readings of <60 mls/min/1.73 m2. This would overestimate CKD prevalence, which in turn will overestimate TRH. The diagnosis of heart failure is a pragmatic one; future studies may choose to use echo criteria, which are increasingly available in primary care.
Comparison with existing literature
The operability of the AHA/ESC definition of resistant hypertension is difficult in routine practice. Even after reviewing individual general practice records, it is difficult to establish whether the dosing levels were the maximal tolerated. In addition, the routine standardised recording of lifestyle factors, such as diet and exercise, is not reliable. As a third issue, definitions should perhaps also include guidance about the duration of treatment in order for patients to qualify as having TRH (that is, ≥3 months stable on the requisite medications). Consideration could be given to developing a version of the AHA/ESC definition that can be easily applied and audited in routine general practice.
Holmqvist described in a Swedish registry-based cohort study of 48 practices the prevalence of TRH in 53 090 hypertensive patients. Applying the AHA/ESC definition, with consideration of adherence only, found a prevalence of 17%.24 They excluded all patients whose proportion of days with pharmacy refills was <80% as not having TRH. The level of morbidity (for example, diabetes) and type of medication used in this contemporaneous Swedish group is very similar to this one. Mineralocorticoid antagonists were used in 8% of Swedish patients, and 7% of Irish. The AHA guidelines2 emphasise the role that these drugs can play in the management of primary aldosteronism in patients with resistant hypertension.2 However, they require close monitoring and these data suggest that GPs are preferring, as a fourth agent, alpha-blockers. The impact of this strategy on patient outcomes requires evaluation. Daugherty et al conducted a key retrospective cohort study of 23 912 patients with hypertension enrolled in two US health plans between 2002 and 2006.12 A prevalence of 16% was determined — as with Holmqvist. However, consideration of dosing or WCH was not performed. In comparison with the Irish and Swedish groups, RAAS and alpha-blockers were less frequently used, at 69% and 10%, respectively. The use of mineralocorticoid antagonists was not reported. National variations in drug group usage patterns may provide accessible longitudinal observational data of combined drug class efficacy for patients with resistant hypertension.
Implications for research and practice
The authors have shown that reviewing individual general practice patient records is feasible and results in lower estimates of the prevalence of TRH. It also facilitates consideration of key pseudo-resistance factors, such as morbidity, dosing, exclusion of WCH, and adherence. RAAS drugs and alpha-blockers are most likely to achieve adequate doses. Future research, as suggested by Daugherty,12 Sarafidis,16 and Achelrod8 is to develop, using this individual-based methodology, a larger community-based cohort of patients with apparent resistant hypertension to assess prognosis in routine practice, ideally with consideration of diet and exercise.
Acknowledgments
The authors are indebted to all participating patients and general practices, and to the staff of the Department of General Practice at the National University of Ireland where the study was coordinated.
Funding
Funding was awarded by the Health Research Board, Ireland (reference number HRA-POR-2014-615). The funder had no role in the design of the study or the manuscript.
Ethical approval
This study was approved by the Research Ethics Committee of the Irish College of General Practitioners.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. The taskforce for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 2.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008 doi: 10.1161/hypertensionaha.108.189141. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence . Hypertension: clinical management of primary hypertension in adults. CG127. London: NICE; 2011. [Google Scholar]
- 4.de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 5.Egan BM, Zhao Y, Li J, et al. Prevalence of optimal treatment regimens in patients with apparent treatment resistant hypertension based on office blood pressure in a community-based practice network. Hypertension. 2013;62(4):691–697. doi: 10.1161/HYPERTENSIONAHA.113.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagard RH. Resistant hypertension. Heart. 2012;98(3):254–261. doi: 10.1136/heartjnl-2011-300741. [DOI] [PubMed] [Google Scholar]
- 7.Carter SM, Rogers W, Heath I, et al. The challenge of overdiagnosis begins with its definition. BMJ. 2015 doi: 10.1136/bmj.h869. [DOI] [PubMed] [Google Scholar]
- 8.Achelrod D, Wenzel U, Frey S. Systematic review and meta-analysis of the prevalence of resistant hypertension in treated hypertensive populations. Am J Hypertens. 2015;28(3):355–361. doi: 10.1093/ajh/hpu151. [DOI] [PubMed] [Google Scholar]
- 9.Gupta AK, Nasothimiou EG, Chang CL, et al. Baseline predictors of resistant hypertension in the Anglo-Scandinavian Cardiac Outcome Trial (ASCOT): a risk score to identify those at high risk. J Hypertens. 2011;29(10):2004–2013. doi: 10.1097/HJH.0b013e32834a8a42. [DOI] [PubMed] [Google Scholar]
- 10.Gee ME, Bienek A, McAlister FA, et al. Factors associated with lack of awareness and uncontrolled high blood pressure among Canadian adults with hypertension. Can J Cardiol. 2012;28(3):375–382. doi: 10.1016/j.cjca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14(12):1263–1269. doi: 10.1016/s0895-7061(01)02193-8. [DOI] [PubMed] [Google Scholar]
- 12.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57(6):1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 14.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 16.Sarafidis PA, Georgianos P, Bakris GL. Resistant hypertension — its identification and epidemiology. Nat Rev Nephrol. 2013;9(1):51–58. doi: 10.1038/nrneph.2012.260. [DOI] [PubMed] [Google Scholar]
- 17.Kavanagh KE, O’Brien N, Glynn LG, et al. WestREN: a description of an Irish academic general practice research network. BMC Fam Pract. 2010 doi: 10.1186/1471-2296-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health Service Executive Welcome to primary care reimbursement service. 2017. http://www.hse.ie/eng/staff/PCRS/ (accessed 24 Apr 2018).
- 19.Joint Formulary Committee . British national formulary. 69th edn. London: Pharmaceutical Press; 2015. [Google Scholar]
- 20.WHO Collaborating Centre for Drug Statistics Methodology . ATC/DDD Index 2018. 2017. https://www.whocc.no/atc_ddd_index/ (accessed 18 Apr 2018). [Google Scholar]
- 21.Sinnott SJ, Smeeth L, Williamson E, Douglas IJ. Trends for prevalence and incidence of resistant hypertension: population based cohort study in the UK 1995–2015. BMJ. 2017. [DOI] [PMC free article] [PubMed]
- 22.Gil-Guillen V, Orozco-Beltran D, Carratala-Munuera C, et al. Clinical inertia in poorly controlled elderly hypertensive patients: a cross-sectional study in Spanish physicians to ascertain reasons for not intensifying treatment. Am J Cardiovasc Drugs. 2013 doi: 10.1007/s40256-013-0025-4. [DOI] [PubMed] [Google Scholar]
- 23.Garg JP, Elliott WJ, Folker A, et al. Resistant hypertension revisited: a comparison of two university-based cohorts. Am J Hypertens. 2005 doi: 10.1016/j.amjhyper.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Holmqvist L, Bostrom KB, Kahan T, et al. Prevalence of treatment resistant hypertension and important associated factors — results from the Swedish Primary Care Cardiovascular Database. J Am Soc Hypertens. 2016 doi: 10.1016/j.jash.2016.08.008. [DOI] [PubMed] [Google Scholar]