Abstract
Background
Atrial fibrillation (AF) is an important and modifiable risk factor for stroke. Earlier identification may reduce stroke-related morbidity and mortality. Trial evidence shows that opportunistic pulse regularity checks in individuals aged ≥65 years increases detection of AF. However, this is not currently recommended by the National Screening Programme or implemented by most clinical commissioning groups (CCGs).
Aim
To evaluate the impact of a systematic programme to promote pulse regularity checks, the programme’s uptake in general practice, and the prevalence of AF.
Design and setting
Retrospective analysis of electronic primary care patient records in three east London CCGs (City and Hackney, Newham, and Tower Hamlets) over 10 years.
Method
Rates of pulse regularity checks and prevalence of AF in individuals aged ≥65 years were compared from the pre-intervention period, 2007–2011, to the post-intervention period, 2012–2017.
Results
Across the three CCGs, rates of pulse regularity checks increased from a mean of 7.3% pre-intervention to 66.4% post-intervention, achieving 93.1% (n = 58 722) in the final year. Age-standardised prevalence of AF in individuals aged ≥65 years increased significantly from a pre-intervention mean of 61.4/1000 to a post-intervention mean of 64.5/1000. There was a significant increase in a post-intervention trend to a final-year mean of 67.3/1000: an improvement of 9.6% (5.9/1000) with 790 additional new cases identified.
Conclusion
Organisational alignment, standardised data entry, peer-performance dashboards, and financial incentives rapidly and generally increased opportunistic screening with pulse regularity checks. This was associated with a significant increase in detection and prevalence of AF and is of public health importance.
Keywords: atrial fibrillation, pulse, screening
INTRODUCTION
Atrial fibrillation (AF) is a leading cause of stroke, resulting in significant morbidity and mortality. Stroke caused by underlying AF is twice as likely to be fatal.1 AF is common in older people, often in association with cardiovascular comorbidity, with a prevalence of 2% at age 65–74 years increasing to >13% in those aged >85 years.2 The condition is often asymptomatic and sometimes only identified at the time of acute cerebral infarction. Anticoagulation can significantly reduce the risk of stroke depending on the time spent therapeutically anticoagulated.3
Systematic and opportunistic approaches for AF detection have used manual examination of the pulse, modified sphygmomanometers,4 electrocardiogram (ECG) recording,5 and novel technologies including smartphones.6 Pulse regularity checks are cheap and sensitive but poorly specific, requiring confirmation of the diagnosis with ECG. A pulse regularity check at a point in time may miss intermittent AF. Opportunistic screening is more effective and less costly than systematic screening.7 There is ongoing debate about the benefits of systematic or opportunistic screening.8 Individuals screened opportunistically are, by the act of GP presentation, more likely than the general population to have a long-term condition and as such may have a higher rate of AF. Opportunistic screening was found to be cost-effective in a 2016 Cochrane systematic review,9 with an incremental cost per case detected of £337. Analysis of national annual opportunistic screening for AF by GPs in Ireland concluded that this would be cost-effective,10 although screening at influenza vaccination clinics may be a less effective strategy.11
In 2011, the UK National Screening Committee reviewed the case for a national AF screening programme for those aged ≥65 years.12 The committee noted that only a minority of patients eligible for anticoagulation receive treatment and argued that it would, therefore, be unethical to introduce a screening programme. Since then there has been a substantial increase in anticoagulation and 86% of eligible individuals with AF were anticoagulated nationally in 2015.13
A health technology appraisal in 2017 concluded that a national screening programme for AF would probably be cost-effective and that systematic opportunistic screening with pulse checks would be more cost-effective than systematic population screening.14 This study evaluates opportunistic screening for AF by manual pulse regularity checks in primary care, supported by standard data entry templates, performance dashboards, prompts in the electronic patient record, and financial incentives.
Setting
The study was conducted in three clinical commissioning group (CCG) areas of inner, east London: City and Hackney, Newham, and Tower Hamlets, where 136 GP practices serve a population of >1 million registered patients with a high burden of deprivation and multimorbidity. The population is relatively young and mobile compared with the rest of the UK, with those aged ≥65 years constituting approximately 6% of the population, compared with nearly 18% nationally.15 The large South Asian community that comprises around one-third of the population is known to have lower rates of AF than white ethnic groups.16
How this fits in
Opportunistic pulse regularity checks in individuals aged ≥65 years are a cheap and easy method to detect unrecognised atrial fibrillation (AF). A systematic programme rapidly improved the detection and prevalence of AF in three inner-city clinical commissioning groups.
In addition to core funding streams, practices receive payment through locally agreed enhanced services, according to their performance against indicators relating to the management of long-term conditions.
The Clinical Effectiveness Group (CEG), based at Queen Mary University of London, has access to pseudonymised data from all practices in this area through universal use of the EMIS Web clinical system. The CEG uses this resource to promote quality improvement through evidence-based guidelines, data entry templates, and clinical dashboards. Primary care clinicians in the study area use CEG data entry templates when completing annual reviews for long-term conditions.
METHOD
In 2012, a field for pulse regularity check was added to the data entry templates for individuals aged ≥65 years with long-term conditions such as hypertension, chronic obstructive pulmonary disease, diabetes, ischaemic heart disease, stroke, as well as those attending an NHS Health Check or a new patient check. Clinicians were advised to check pulse regularity in all individuals ≥65 years every 5 years and annually in those with the conditions listed above. Clinicians were also reminded about pulse regularity checks through on-screen prompts when there was no record already present. Quarterly dashboards were sent to all practices with funnel plots identifying individual practice trends and the distribution of each practice in the CCG in relation to AF register size. Pre-specified practice searches were available to identify patients who were overdue a pulse regularity check. The new programme was endorsed in educational meetings in each CCG.
Pulse regularity checks for patients with long-term conditions were financially incentivised in City and Hackney and Tower Hamlets from April 2013, and in Newham from April 2014. Practices meeting the target threshold were given additional payment that was paid directly to practices in City and Hackney and Newham, but paid to managed groups of practices in Tower Hamlets known as networks, based on network rather than individual practice performance.
In August 2017, pseudonymised coded data were retrospectively extracted from the EMIS Web electronic patient record for each financial year (1 April–31 March) for the time period 2007–2017. Rates of pulse regularity checks in individuals aged ≥65 years were examined and the prevalence of AF in this age group using a standard set of codes (see Box 1).
Box 1. Read Codes used in search strategy.
|
O/E = on examination.
The mean rate of pulse regularity checks in the pre-intervention period (2007–2012) to the post-intervention period (2012–2017) was compared. The mean prevalence of AF in the pre- and post-intervention periods was compared using a two-sample t-test with equal variances and regression analysis (change in slope and difference in intercept) was also compared. All analyses were conducted using Stata (version 14). The P-values were two-sided with statistical significance set at 0.05. Final-year pulse check and AF detection rates were also reported. The design and reporting of the study conformed to STROBE recommendations.17
RESULTS
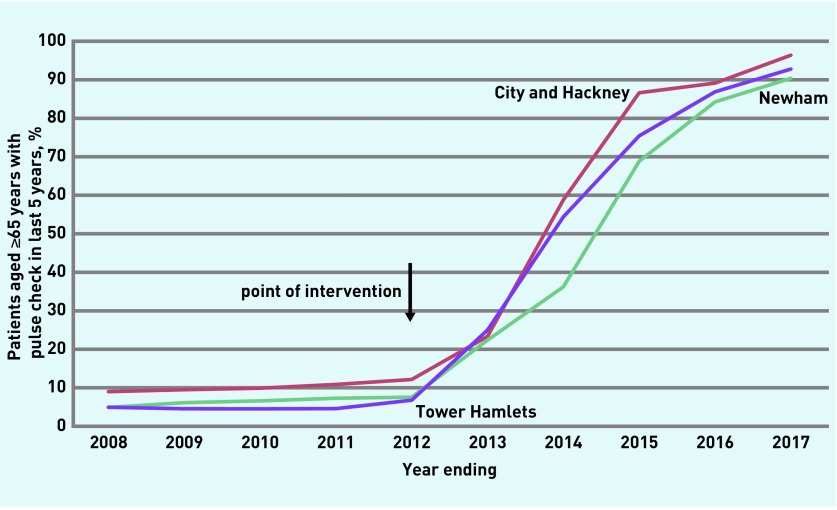
There was a large increase in patients, aged ≥65 years, coded with a pulse regularity check in the previous 5 years, from a mean of 7.3% (4118/56 314) in the pre-intervention period to a mean of 66.4% (40 346/60 723) in the post-intervention period (Table 1 and Figure 1). This rose to 93.1% (58 722/63 094)) in 2017, the final year reported.
Table 1.
Proportion of patients aged ≥65 years with pulse check in the last 5 years by clinical commissioning groups
| Year | Proportion with pulse checks | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| City and Hackney | Newham | Tower Hamlets | All CCGs | |||||
|
|
|
|
|
|||||
| % | n | % | n | % | n | % | n | |
| 2007–2008 | 9.0 | 1616 | 4.9 | 1018 | 5.0 | 850 | 6.2 | 3484 |
| 2008–2009 | 9.5 | 1705 | 6.1 | 1305 | 4.5 | 756 | 6.7 | 3766 |
| 2009–2010 | 9.9 | 1784 | 6.6 | 1408 | 4.5 | 752 | 7.0 | 3944 |
| 2010–2011 | 10.9 | 1980 | 7.3 | 1562 | 4.6 | 756 | 7.7 | 4298 |
| 2011–2012 | 12.2 | 2290 | 7.6 | 1671 | 6.8 | 1138 | 8.8 | 5099 |
| Pre-intervention mean | 10.3 | 1875 | 6.5 | 1393 | 5.1 | 850 | 7.3 | 4118 |
|
| ||||||||
| 2012–2013 | 23.3 | 4526 | 22.4 | 5029 | 25.0 | 4156 | 23.4 | 13 711 |
| 2013–2014 | 58.8 | 11 715 | 36.2 | 8234 | 54.4 | 9108 | 48.9 | 29 057 |
| 2014–2015 | 86.6 | 17 959 | 68.8 | 15 928 | 75.4 | 12 723 | 76.7 | 46 610 |
| 2015–2016 | 89.1 | 18 825 | 84.2 | 20 048 | 86.9 | 14 755 | 86.6 | 53 628 |
| 2016–2017 | 96.4 | 20 603 | 90.4 | 22 142 | 92.8 | 15 977 | 93.1 | 58 722 |
| Post-intervention mean | 71.8 | 14 726 | 61.2 | 14 276 | 67.2 | 11 344 | 66.4 | 40 346 |
CCG = clinical commissioning group.
Figure 1.
Proportion of patients aged ≥65 years with pulse check in the last 5 years by CCG.
CCG = clinical commissioning group.
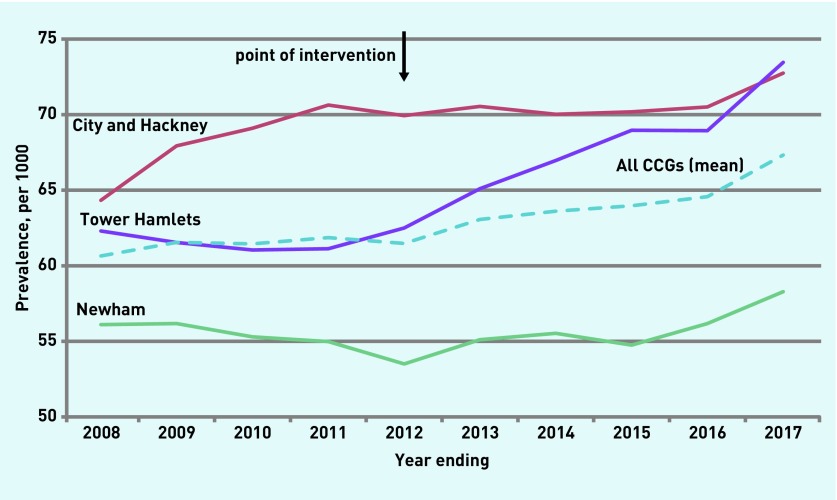
Table 2 shows changes in AF prevalence in the pre- and post-intervention periods. These were examined by comparing the mean prevalence per 1000 in the pre-intervention period 61.4 (95% confidence interval [CI] = 60.8 to 62.0) to the post-intervention period 64.5 (95% CI = 62.4 to 66.6). This difference between means of 3.1/1000 represents a mean increase of 5.0% in prevalence and 462 new AF cases. A two-sample t-test showed a significant difference between the means (T = −4.03, P = 0.01). The final-year mean prevalence of AF was 67.3/1000 and represents an increase of 9.6%; 790 new cases of AF, 5.9/1000, in comparison with the pre-intervention mean. The increases in final-year prevalence above the pre-intervention mean were 5.6%, 6.4%, and 19.1% in Newham, City and Hackney, and Tower Hamlets respectively.
Table 2.
Prevalence of atrial fibrillation per 1000 individuals ≥65 years by clinical commissioning group
| Year | Proportion with pulse checks | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| City and Hackney | Newham | Tower Hamlets | All CCGs | |||||
|
|
|
|
|
|||||
| Rate/1000 | n | Rate/1000 | n | Rate/1000 | n | Rate/1000 | n | |
| 2007–2008 | 64.3 | 1156 | 56.1 | 1167 | 62.3 | 1067 | 60.6 | 3390 |
| 2008–2009 | 67.9 | 1218 | 56.2 | 1196 | 61.5 | 1025 | 61.5 | 3439 |
| 2009–2010 | 69.1 | 1246 | 55.3 | 1180 | 61.0 | 1012 | 61.5 | 3438 |
| 2010–2011 | 70.6 | 1286 | 55.0 | 1179 | 61.1 | 1005 | 61.9 | 3470 |
| 2011–2012 | 69.9 | 1316 | 53.5 | 1184 | 62.5 | 1051 | 61.5 | 3551 |
| Pre-intervention mean | 68.4 | 1244 | 55.2 | 1181 | 61.7 | 1032 | 61.4 | 3458 |
|
| ||||||||
| 2012–2013 | 70.5 | 1368 | 55.1 | 1238 | 65.1 | 1083 | 63.1 | 3689 |
| 2013–2014 | 70.0 | 1395 | 55.5 | 1262 | 67.0 | 1121 | 63.6 | 3778 |
| 2014–2015 | 70.2 | 1455 | 54.8 | 1267 | 69.0 | 1163 | 64.0 | 3885 |
| 2015–2016 | 70.5 | 1489 | 56.2 | 1337 | 68.9 | 1171 | 64.6 | 3997 |
| 2016–2017 | 72.7 | 1555 | 58.3 | 1428 | 73.5 | 1265 | 67.3 | 4248 |
| Post-intervention mean | 70.8 | 1452 | 56.0 | 1306 | 68.7 | 1161 | 64.5 | 3919 |
CCG = clinical commissioning group.
Regression analysis confirmed that the gradient of the slope after the intervention (coefficient 0.095, 95% CI = 0.044 to 0.145) was significantly different from the pre-intervention period (0.020, 95% CI = −0.031 to 0.070) (P = 0.04).
Figure 2 shows the prevalence of AF in the ≥65 years age group over the study period. In the 5-year pre-intervention period the mean number of cases of AF per year were 3457.6 and 3919.4 in the post-intervention period; a mean increase of 461.8 cases per year. Assuming these new cases had an average CHA2DS2-VASc score of 2, and that 10% were ineligible for anticoagulation, it can be estimated that 5 years of anticoagulation would have prevented stroke in 28 individuals representing >2000 cases over the 209 CCGs in England.
Figure 2.
Prevalence of atrial fibrillation per 1000 people aged ≥65 years by CCG.
CCG = clinical commissioning group.
DISCUSSION
Summary
This study shows that a programme to increase detection of AF with opportunistic pulse regularity checks using clinical templates for structured data entry combined with peer-performance feedback, financial incentives, and educational dissemination was associated with a rapid adoption of pulse regularity checks in >75% of the target population within 3 years, and >90% by 5 years. The introduction of pulse regularity checks was associated with a significant increase in the trend of detection of AF, hence prevalence of AF had increased by 9.6% by the end of the study.
The methodology of this study was conservative and compared mean prevalence between periods rather than the mean pre-intervention compared with the final post-intervention reported rate in year 5. The estimated stroke reduction of >2000 cases over the 209 CCGs in England is of public health importance. The ease and rapidity with which this intervention was widely adopted suggests that the programme could be feasibly scaled nationally.
Strengths and limitations
This study retrospectively identified subjects based on cross-sectional samples on the registered practice population on the date the search was conducted. Patients registered at the beginning of the study period who then left the practice would not have been counted. In an area of high population mobility such as east London,18 this may be an important loss to follow-up, though less pronounced in the older population of this study. This could lead to a possible underestimate of new AF cases. The cross-sectional design precluded following individuals through the whole study period to identify new-incident cases per year. The same case definition of AF was used throughout the study, including both paroxysmal AF and atrial flutter, and relied on GP coding without further validation.
Data from all Tower Hamlets and City and Hackney practices were available for the entire study period; however, four of the 64 practices in Newham only provided information for the final study year. This may have reduced the size of the effect in Newham to a small extent, but not its direction. Some of the increase in register size may be due to demographic change but this does not account for the difference in the trends before and after the intervention. In Newham, the full programme, including financial incentives, was not instituted until April 2014 and this may have reduced the effect size, which was least in this CCG.
The study area is not representative of the UK overall. The local population is young, socioeconomically disadvantaged, and ethnically diverse, posing challenges for screening programmes. There is a large South Asian population known to have lower incidence of AF than white populations and the results may underestimate the yield from such a programme in older, less ethnically diverse CCGs.
The study examined coded pulse checks, which before the intervention may have been undocumented or entered as non-coded free-text. This study was unable to distinguish whether pulse checks were performed manually or using electronic devices, though anecdotally the vast majority were manual, with some using automated blood pressure cuffs.
In this study changes in AF prevalence were analysed and projections on potential stroke reduction were made, rather than examining anticoagulation or stroke as endpoints. Whether treatment of opportunistically detected AF in asymptomatic patients reduces stroke to the same extent as that detected by other means is uncertain, but the thromboembolic risks are similarly increased and the consensus view is to anticoagulate those with screen-detected AF.19 The proportion of patients with AF receiving anticoagulation has increased year on year, so there has been no reduction of anticoagulation performance in association with increased detection.13 The advent of direct oral anticoagulants may have increased rates of anticoagulation; however, this was beyond the scope of this study.
The prevalence of AF has been increasing nationally owing to an ageing population and, therefore, this research was unable to ascribe all the increase in local prevalence to pulse checks. However, the increase in trend since the start of the intervention is strong evidence for the impact of the intervention.
The researchers did not make any economic assessment of the human resources and infrastructure required to undertake the additional work except for anecdotal feedback: the speed and extent of adoption indicated that pulse checking fitted into existing routines for blood pressure measurement — pulse palpation is a National Institute for Health and Care Excellence-recommended component of blood pressure measurement.20 Nor did the researchers evaluate the individual components of the intervention and were unable to disaggregate the synergistic effects of local guidance, IT prompts, dashboards, and modest financial incentives. These elements follow Michie’s COM-B model, which addresses the capability, opportunity, and motivation behind behavioural change.21
Comparison with existing literature
The baseline prevalence of AF was similar to the largest relevant study on pulse regularity checks and AF by Hobbs et al.7 The present study area showed a higher uptake of opportunistic screening (93.1% versus 69.2%), although this was achieved in 5 years compared with 12 months in the former. Hobbs et al do not report prevalence but found that the incidence was higher in their intervention group compared with a control group. This observational study confirms that, in routine practice, prevalence of detected AF has increased following the commissioning, promotion, and routine provision of opportunistic pulse checks.
Implications for practice
Opportunistic pulse regularity checks can be rapidly and widely adopted in primary care, promoted by organisational alignment, IT support, peer-performance reporting, and financial incentives. This is associated with an increase in the detection of new AF cases, management of which is likely to have an impact on the public health importance of stroke reduction.
Funding
John Robson was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care North Thames at Barts Health NHS Trust.
Ethical approval
All data were depersonalised and managed according to the UK NHS information governance requirements. Ethical approval was not required for the use of anonymised data in this observational study.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27(10):1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 2.Davis RC, Hobbs FD, Kenkre JE, et al. Prevalence of atrial fibrillation in the general population and in high-risk groups: the ECHOES study. Europace. 2012;14(11):1553–1559. doi: 10.1093/europace/eus087. [DOI] [PubMed] [Google Scholar]
- 3.Sterne JA, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess. 2017;21(9):1–386. doi: 10.3310/hta21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taggar JS, Coleman T, Lewis S, et al. Accuracy of methods for detecting an irregular pulse and suspected atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(12):1330–1338. doi: 10.1177/2047487315611347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronsson M, Svennberg E, Rosenqvist M, et al. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace. 2015;17(7):1023–1029. doi: 10.1093/europace/euv083. [DOI] [PubMed] [Google Scholar]
- 6.Lowres N, Neubeck L, Freedman SB. Can screening for atrial fibrillation be implemented at scale? Europace. 2016;18(10):1449–1451. doi: 10.1093/europace/euw030. [DOI] [PubMed] [Google Scholar]
- 7.Hobbs FD, Fitzmaurice DA, Mant J, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess. 2005;9(40):iii–iv. ix–x, 1–74. doi: 10.3310/hta9400. [DOI] [PubMed] [Google Scholar]
- 8.Lown M, Garrard J, Irving G, et al. Should we screen for atrial fibrillation? Br J Gen Pract. 2017. . [DOI] [PMC free article] [PubMed]
- 9.Moran PS, Teljeur C, Ryan M, et al. Systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev. 2016;(6):CD009586. doi: 10.1002/14651858.CD009586.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran PS, Teljeur C, Harrington P, et al. Cost-effectiveness of a national opportunistic screening program for atrial fibrillation in Ireland. Value Health. 2016;19(8):985–995. doi: 10.1016/j.jval.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Rhys GC, Azhar MF, Foster A. Screening for atrial fibrillation in patients aged 65 years or over attending annual flu vaccination clinics at a single general practice. Qual Prim Care. 2013;21(2):131–140. [PubMed] [Google Scholar]
- 12.Allaby M. Screening for atrial fibrillation in people aged 65 and over. A report for the National Screening Committee. 2014. Solutions for public health. https://legacyscreening.phe.org.uk/policydb_download.php?doc=446 (accessed 8 May 2018)
- 13.NHS Digital Quality and Outcomes Framework (QOF) — 2015–16. 2016. http://www.content.digital.nhs.uk/catalogue/PUB22266 (accessed 8 May 2018)
- 14.Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1–236. doi: 10.3310/hta21290. [DOI] [PubMed] [Google Scholar]
- 15.Office for National Statistics. Population estimates for UK, England and Wales, Scotland and Northern Ireland: mid-2016. 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2016 (accessed 8 May 2018)
- 16.Mathur R, Pollara E, Hull S, et al. Ethnicity and stroke risk in patients with atrial fibrillation. Heart. 2013;99(15):1087–1092. doi: 10.1136/heartjnl-2013-303767. [DOI] [PubMed] [Google Scholar]
- 17.STROBE Statement STROBE checklist for cohort, case-control, and cross-sectional studies. 2007. https://www.strobe-statement.org/index.php?id=available-checklists (accessed 8 May 2018)
- 18.Office for National Statistics. Understanding projected population change at the local authority level. 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/compendium/subnationalpopulationprojectionssupplementaryanalysis/2014basedprojections/understandingprojectedpopulationchangeatthelocalauthoritylevel (accessed 8 May 2018)
- 19.Freedman B, Camm J, Calkins H, et al. Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation. 2017;135(19):1851–1867. doi: 10.1161/CIRCULATIONAHA.116.026693. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management CG127. 2011. https://www.nice.org.uk/guidance/cg127 (accessed 8 May 2018) [PubMed]
- 21.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]