Abstract
Background
For almost 50 years sub-Saharan Africa, including Uganda, has experienced several outbreaks due to Vibrio cholerae. Our aim was to determine the genetic relatedness and spread of strains responsible for cholera outbreaks in Uganda.
Methodology/Principal findings
Sixty-three V. cholerae isolates collected from outbreaks in Uganda between 2014 and 2016 were tested using multiplex polymerase chain reaction (PCR), multi-locus variable number of tandem repeat analysis (MLVA) and whole genome sequencing (WGS). Three closely related MLVA clonal complexes (CC) were identified: CC1, 32% (20/63); CC2, 40% (25/63) and CC3, 28% (18/63). Each CC contained isolates from a different WGS clade. These clades were contained in the third wave of the 7th cholera pandemic strain, two clades were contained in the transmission event (T)10 lineage and other in T13. Analysing the dates and genetic relatedness revealed that V. cholerae genetic lineages spread between districts within Uganda and across national borders.
Conclusion
The V. cholerae strains showed local and regional transmission within Uganda and the East African region. To prevent, control and eliminate cholera, these countries should implement strong cross-border collaboration and regional coordination of preventive activities.
Author summary
Cholera, an acute diarrheal disease, essentially was eliminated in the western world many decades ago, but has continued to cause many deaths in sub-Saharan Africa, South America and Asia. Cholera diagnosis in most countries in sub-Saharan Africa, including Uganda, is by stool culture, serology and biochemical methods. These testing methods are unable to establish the relatedness, virulence and spread of Vibrio cholerae in region. To determine the spread, relatedness and virulence of V. cholerae responsible for the various cholera outbreaks in Uganda, we used DNA-based testing methods. We tested 63 V. cholerae isolates from samples collected in Uganda from 2014–2016. Our results showed three distinct lineages of genetically related cholera-causing bacteria. These organisms showed internal spread in Uganda and cross-border spread to neighboring countries in East Africa. These findings provide a valuable baseline and help define the context for directing control measures and technologies for cholera prevention in East Africa.
Introduction
Vibrio cholerae remains a major cause of morbidity and mortality globally [1]. There have been seven cholera pandemics since the disease was recognized as a global threat [2]. The English record of pandemics of cholera started in 1816, but cholera as a disease goes back centuries in Indian literature [3]. The organism responsible for cholera outbreaks, V. cholerae, was cultured over 130 years ago by Robert Koch (1884) in India [4] and its epidemiology in England was described by John Snow in 1886 [5].
Over time, considerable knowledge and skills in the management of this deadly infectious disease have accumulated leading to better prevention and control of epidemics [6–8]. Industrialized countries essentially have eliminated cholera as a public health problem through improved water and sanitation [9]. Nonetheless, this enteric bacterium continues to cause deaths and suffering in many countries [10–12]. Sub-Saharan Africa bears the highest reported cholera disease burden [13]. The ongoing outbreaks in Africa and elsewhere in the world are part of the seventh pandemic caused by the V. cholerae O1, El Tor lineage [14,15]. Genetic differences among isolates allow for a greater understanding of the transmission of the bacteria within and between geographic regions and time periods [16].
Two methods, multilocus variable-number tandem-repeat analysis (MLVA) [17,18] and whole genome sequencing (WGS) [19], provide sufficient genetic differentiation to distinguish between the isolates across different places and times. Less complex methods such as culture, biochemical and serological tests to detect, confirm and describe V. cholerae [20], do not permit accurate tracking of the spread of specific genetic lineages. Yet these are the only methods available in most African countries including Uganda [21]. The goal of this study was to analyze V. cholerae isolates responsible for cholera outbreaks that occurred between 2014–2016 in Uganda using multiplex PCR, MLVA and WGS to determine the genetic relatedness and spread of V. cholerae isolates from different outbreaks in Uganda.
Materials and methods
Study design
A cross-sectional study was conducted using all available viable V. cholerae isolates collected during cholera outbreaks in Uganda between 2014 and 2016 and kept frozen (-80°C) at the Central Public Health Laboratory (CPHL) in Kampala. In addition, aggregated epidemiological cholera surveillance data for the years 2014–2016 were reviewed and used to generate Epi-maps that contextualized the epidemic spread and transmission of cholera.
Ethical considerations
Permission to conduct the study was obtained from the Makerere University School of Public Health Institution Review Board (IRB number, 00011353). The isolates were collected through the Ministry of Health disease surveillance system and stored at the CPHL. Personal identifiers were removed by labeling the isolates using the district name and district codes.
Data management
Data used to create the disease distribution over the period 2014–2015 were from the Uganda Ministry of Health epidemic disease surveillance system which is part of the national health management information system (S1 Dataset). Data were analyzed to calculate percentages and proportions. Aggregated cholera cases and deaths were analyzed and used to generate maps. Shapefiles used to create the Uganda maps were obtained from the Uganda Bureau of Statistics. The maps were created using the Arc View Geographical Information System (GIS).
Recovery of frozen isolates
V. cholerae isolates were recovered from frozen storage. During this process safety precautions were observed as described in standard laboratory manuals for epidemic dysentery and cholera diagnosis [22,23]. The recovered isolates were packaged and shipped to Baltimore, Maryland, USA, for genetic testing.
PCR test
To confirm the isolates as V. cholerae and to determine their virulence by PCR tests, primers targeting ompW (outer membrane protein), ctxA (cholera enterotoxin sub-unit A) and toxR (transcription activator controlling cholera toxin) were used. DNA was extracted and amplified using primers as described previously [24].
Multi-locus variable tandem repeat analysis (MVLA)
DNA was genotyped for five MLVA loci: VC0147, VC0436-7 (intergenic), VC1650, VCA0171 and VCA0283 [18]. Each of the five loci was amplified as described previously [17,18]. The presence of amplified products was confirmed by gel electrophoresis.
The fluorescently labeled amplified products were separated using a 3730xl Automatic Sequencer with the size determined from internal lane standards (LIZ600) by the GeneScan program (Applied Biosystems, Foster City, CA). The genotypes for each isolate are in supplementary table 1 (S1 Table) EBURST (www.mlst.net) was used to define the genetic relatedness between genotypes. Genotypes within a clonal complex were related by a series of single locus variants.
Whole genome sequencing
Three or four representative samples were selected from each of the 3 MLVA clonal complexes identified during the period 2014–2016 for testing by WGS. Libraries for Illumina sequencing were prepared from DNA fragmented with Covaris E210 (Covaris, Wolburn, MA) using the KAPA High Throughput Library Preparation Kit (Millipore-Sigma, St. Louis MO). The libraries were enriched and barcoded in ten cycles of PCR amplification with primers containing an index sequence. Subsequently, the libraries were sequenced using a 100 bp paired-end run on an Illumina HiSeq2500 (Illumina, San Diego, CA).
The quality of the 101-base paired-end reads was assured by a quality trimming procedure using Sickle (v1.33), with a minimum read length after trimming of 75nt, and a quality threshold of 20. High quality reads were assembled with “Spades” software (v.3.6.2). Annotation was performed using the RAST server [25]. The annotated sequences were submitted to Genbank Accession number PYRD00000000-PYRM00000000. The BioProjectID is PRJNA439310.
Nucleotide variation was identified and compared to V. cholerae O1 El Tor strain to identify single nucleotides variants (SNVs). Parsnp (v1.2) was used to align the variable nucleotides from the core-genome using the option ‘–c’ to constrain the use of all input genomes and generate the ‘.vcf’ variant description file and ‘.ggr’ alignment description file. The ‘.ggr’ file was loaded in Gingr (v1.2) to visualize the alignments and export the variable nucleotide alignment ‘.mfa’ file [26]. The ‘.vcf’ file was then used to remove all variable nucleotides from the ‘.mfa’ file detected near the edge of the contigs (less than 1 kb of the contigs edges) using an in-house script. Information about each genome sequence is in Supplemental Table 1 (S1 Table). No regions with an excess density of SNPs were detected.
To understand the relatedness of the Ugandan strains to those from the seventh pandemic, 41 representative African isolates with known WGS from the wave 3 transmissions T10, T11 and T12 were selected (S2 Table) and analyzed with the Ugandan sequences in FastTree2 (v2.1.9) [27] with default parameters to generate the maximum-likelihood tree. Data were displayed and visualized using Interactive Tree of Life (iTOL) [28].
Results
A total of 63 V. cholerae isolates for the years 2014–2016 were tested. The isolates were from 9 locations: 8 districts in Uganda and a ninth from patients who acquired their illness in Juba, South Sudan, and were treated in Uganda. All 63 isolates tested positive for ompW, toxR and ctxA indicating the presence of V. cholerae virulence genes. The isolates included both V. cholerae Inaba (63%) and Ogawa (34%) serotypes as shown in Table 1.
Table 1. District of origin, number of isolates by year of isolate identification and serotype of V. cholerae isolates tested using PCR, MLVA and WGS.
| Location | Number of isolates by year of isolation | Serotype | Total | ||
|---|---|---|---|---|---|
| District | 2014 | 2015 | 2016 | 2014–2016 | |
| Moyo | 12 | 0 | 0 | Inaba | 12 |
| Arua | 3 | 2 | 0 | Inaba | 5 |
| Hoima | 0 | 5 | 0 | Inaba | 5 |
| Kasese | 0 | 15 | 0 | Ogawa & Inaba | 15 |
| Kampala | 0 | 5a | 1 | Ogawa | 6 |
| Mbale | 0 | 0 | 15 | Ogawa | 15 |
| Moroto | 0 | 1 | 0 | Ogawa | 1 |
| Mityana | 0 | 0 | 2 | Ogawa | 2 |
| Juba, South Sudan | 0 | 2 | 0 | Inaba | 2 |
| Total | 15 | 30 | 18 | 63 | |
a—includes Wakiso district.
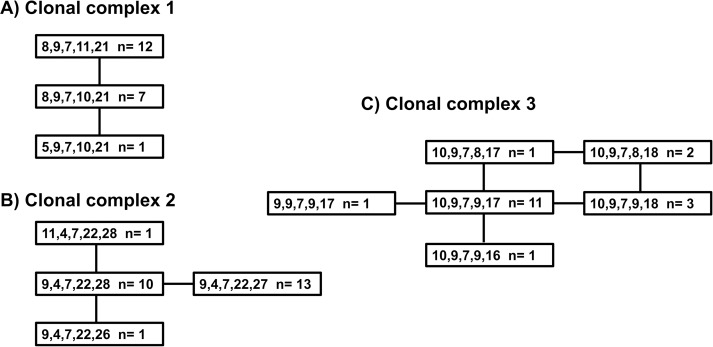
All 63 V. cholerae isolates were genotyped using MLVA. Three clonal complexes (CC) were identified circulating in Uganda. MLVA CC1 contained 32% (20/63); MLVA CC2, 40% (25/63); and MLVA CC3, 28% (18/63) of the isolates. The three MLVA CCs are shown in Fig 1.
Fig 1. MLVA CC for V. cholerae associated with outbreaks in Uganda.
Each genotype is represented by five numbers indicating the number of repeats at the five loci, VC0147, VC0436-7 (intergenic), VC1650, VCA0171 and VCA0283. ‘N = ‘ reports the number of isolates with that genotype. The lines connecting the boxes indicate variation at a single locus. Part A is Clonal Complex 1, Part B is Clonal Complex 2, and Part C is Clonal Complex 3.
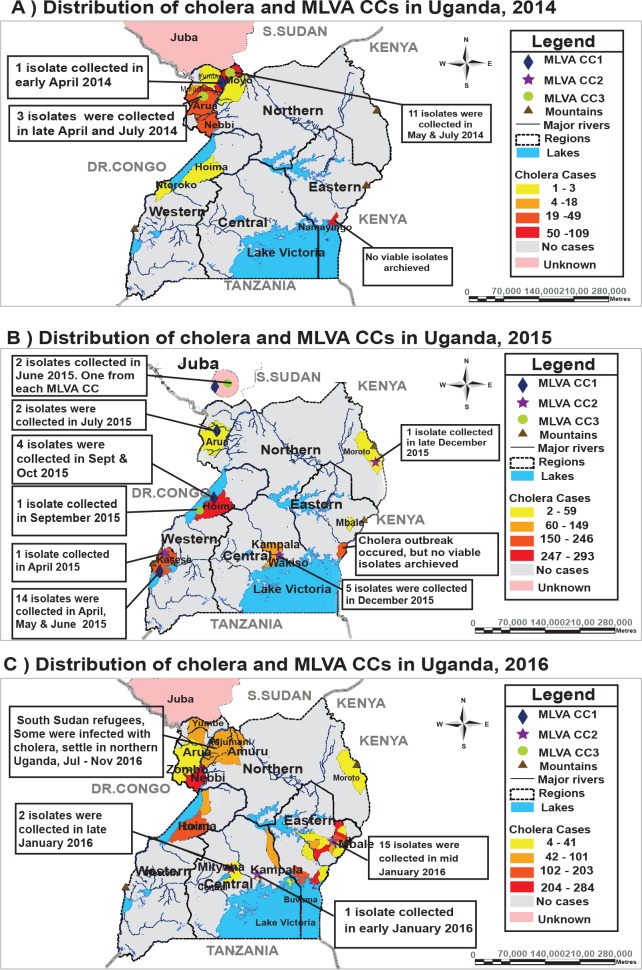
The spatial distribution of MLVA CCs in Uganda reveals the presence of multiple genetic lineages within outbreaks and genetically defined connections between outbreaks (Fig 2). Two lineages were observed in 2014, when CCs 1 & 3 were isolated in Arua and Moyo districts in northwest Uganda. In 2015, CCs 1 & 3 were observed in Hoima and CCs 1 & 2 were isolated in Kasese district in southwest Uganda.
Fig 2.
Shows the spatial distribution of MLVA clonal complexes and location of cholera outbreaks in Uganda: Part A during 2014, Part B during 2015, and Part C during 2016. Clonal Complex 1 are green circles, CC2 are purple stars, and CC3 are dark blue diamonds. The number of cases reported from each area varies by the year, yellow is the fewest number of cases, orange, then red-orange and red is the largest number of reported cases. Grey color indicates that there were no reported cases and blue indicates the Great Lakes of Africa.
Each separate CC identified one of three genetically related series of outbreaks. First, isolates from CC3 were observed in June 2015 in individuals from Juba, South Sudan, and later in July 2015 in nearby Arua district, Uganda. Additional isolates were seen further south in September 2015 in Hoima on Lake Albert in Uganda. A second outbreak, defined by CC2, was initially identified in April 2015 in Kasese district in western Uganda, and subsequently in November 2015 in Wakiso district in central Uganda, in December 2015 in Kampala district in central Uganda and in December 2015 in Moroto district in northeastern Uganda. This outbreak persisted into January 2016 when it was found in Kampala and Mityana in central Uganda and in Mbale district in eastern Uganda. A third outbreak, defined by CC1, contained isolates collected in May and July 2015 in Kasese district, Uganda, and in June 2015 in individuals from Juba, South Sudan.
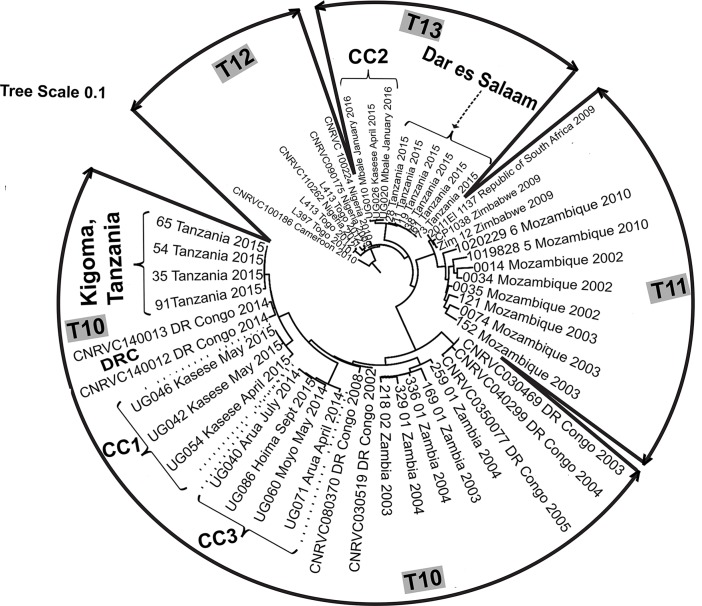
WGS genotyping of ten isolates indicated that the DNA was typical of the third wave of the seventh pandemic containing the classical allele of ctxA (S2 Table). The Ugandan DNA sequences belonged to three distinct clades. Within these distinct clades, the Ugandan sequences differed by five or fewer nucleotides (Fig 3). Two clades were contained in the transmission event (T)10 lineage and the other was contained in T13; no Ugandan isolate sequences were contained in a third African lineage T12.
Fig 3. Phylogram of V. cholerae WGS data.
Forty-one sequences from African isolates representing T10, T11 and T12 were included. Solid lines and black arrows demarcate the boundaries of the transmission events (T). Dotted lines and outlined arrows demarcate the boundaries of the clonal complexes (CC) in Uganda. Dashed arrows identify specific isolates from locations outside Uganda inferred to be examples of cross-border spread. The sequences within the Ugandan clades were less than five nucleotides apart. Those sequences in the Tanzanian clades were less than nine nucleotides from the Ugandan sequences of the closest clade. The radial lines are proportional to the number of nucleotide differences.
The Ugandan clades were closely related to each other and to sequences from Democratic Republic of Congo and Tanzania (Fig 3). Clade 2 sequences from Kasese district in April 2015 were related most closely to sequences from Mbale district in January 2016 and secondarily to sequences from i) the Democratic Republic of Congo and ii) epidemic isolates from Dar es Salaam, Tanzania in August 2015 which spread across Tanzania during 2015. Clade 3 sequences from Arua and Moyo districts, Uganda in April and May 2014 and Clade 1 sequences from Kasese district, Uganda in April and May 2015 were related closely to sequences from an outbreak in January 2015 in Kigoma, Tanzania. The distance between the Ugandan and Tanzanian clades was nine or fewer nucleotides.
Discussion
Our data are consistent with the spread of multiple genetic lineages of V. cholerae within Uganda and across its borders during 2014, 2015 and 2016. We found three CCs identified by MLVA that corresponded to the three clades of sequences by WGS. Each of these three genetic lineages displayed cross-border spread and spread within Uganda. The cross-border spread was both into and out of Uganda. These three clades circulating in East Africa belong to wave 3 of the seventh cholera pandemic, ctx carrying V. cholerae El Tor strain and belong to the T10 and T13 introductions of V. cholerae into East Africa [29].
Our data do not change the fundamental topology of the phylogenetic tree for V. cholerae. However, our WGS data revealed incidences of cross-border spread and of spread within Uganda. One example of cross-border spread was demonstrated by the close relationship between isolates (CC1, Clade 1, T10) from i) the Democratic Republic of Congo in 2014, ii) an outbreak in January 2015 in Kigoma, Tanzania, on the shores of Lake Tanganyika, iii) isolates from an outbreak in April and May 2015 in Kasese district on the western border of Uganda about 600 kilometers north of Kigoma, and iv) extended based on MLVA data to include the travelers seeking medical care in Uganda from, Juba, South Sudan. Cross-border spread between the Democratic Republic of Congo, South Sudan and Uganda was previously inferred from epidemiological evidence alone [30,31]. A second cross-border spread was revealed by the close relationship between isolates from an outbreak (CC2, Clade 2, T13) in April 2015 in Kasese district and those from Dar es Salaam, Tanzania, in August 2015 [32]. This lineage also spread from Kasese district to Mbale district in January 2016 or perhaps the seeding of these early 2016 cases came from Tanzania. The genetic distances between the various isolates was too small for the origin to be determined with certainty. Although these two incidences of cross-border spread included isolates from Kasese district in April 2015, the isolates that spread were from two distinct genetic lineages. This finding implies that the two distinct genetic lineages were present at the same time in the cholera outbreak in Kasese district similar to the cholera outbreak in Kenya in January 2009 –May 2010 in which two distinct lineages were also found [33]. A third example of cross border spread comes from MLVA CC3 (Clade 3, T10), the genetically related isolates included isolates from Kigoma, Tanzania and Kasese district, Uganda in January and April 2015. Additional isolates were collected in June 2015 among the fishing community in Hoima district on Lake Albert, Uganda indicating spread within Uganda. A fourth example of cross-border spread comes from the presence of South Sudanese refugees in Uganda in the last half of 2016 seeking health care for cholera, although no isolates were available for testing.
Examples of spread within Uganda included CC3 that was found in April and May 2014 in Arua and Moyo districts respectively, 125 kilometers apart, in northwest Uganda; and was found in July 2015 again in Arua district and in September 2015 in Hoima district, 250 kilometers to the southwest. A second example of spread within Uganda is CC2, initially identified in Kasese district in April 2015 and identified subsequently in December 2015 in Kampala and Moroto districts, in central and eastern Uganda respectively, although the latter could have come from Tanzania, as the genetic data are insufficient to distinguish between the two alternatives.
Tracking the spread of V. cholerae requires genetic identification as demonstrated by the presence of multiple genetic lineages occurring simultaneously in the same region. Multiple lineages were collected in Moyo, Kasese and Hoima districts in Uganda. Multiple lineages were found despite our analyses being limited to a small number of isolates.
Analyses of additional isolates may identify even more cases of multiple lineages in a single location. Each genetic lineage in a given location probably represents an independent introduction event to that location. The caveat to that hypothesis are the reports of multiple lineages within a single person [18], a phenomenon that has not been explored in Africa.
The spread of cholera inferred by this study is consistent with the documented movement of populations including refugees and traders affecting communities located along the great lakes, rivers, fishing villages, and trade and communication routes [30,34]. This is supported by evidence from the 2016 cholera outbreak in northern Uganda that was confined to districts hosting refugees from or bordering South Sudan.
These findings have several implications for cholera control in the region. Apart from providing a baseline for future molecular studies in Uganda, they demonstrate the need for approaches to disease prevention and control that cross national boundaries. In addition to strengthening interventions within countries, an approach similar to that taken to contain Ebola in West Africa [35,36] should be adopted. An outbreak in one country should elicit support from neighbors to ensure timely control [37]. Cross-border collaboration and joint interventions between neighboring countries should be implemented and sustained over an extended period to promote cholera elimination.
Study limitation
No V. cholerae isolates were collected and tested from a cholera outbreak in 2016 in northwestern Uganda that started with the influx of South Sudan refugees and was restricted to districts where the refugees settled and their immediate neighborhoods. However, since this outbreak was restricted to a few districts in northwestern Uganda with refugees, it is unlikely that this had an effect on the findings of this study.
Conclusion
The cholera outbreaks in Uganda were due to genetically diverse V. cholerae O1 isolates from two introductions from wave 3 of the seventh pandemic carrying the classical El Tor toxin gene. The V. cholerae strains showed local and regional transmission within Uganda and East Africa. Interventions to prevent, control, and eliminate cholera in Uganda and throughout East Africa should be strengthened with a focus on regional collaboration.
Supporting information
(PDF)
"The genomes were submitted to NCBI and accessible under the BioProject ID PRJNA439310." Annotated genomes are available at Genbank accession numbers PYRD00000000-PYRM00000000.
(XLSX)
(XLSX)
(XLS)
Acknowledgments
We thank the management of the Ministry of Health Uganda and staff of the Central Public Health Laboratory, Kampala, Uganda, for guidance and support during the implementation of this study. We are particularly grateful to the following individuals: Dr. Asuman Lukwago and Prof. AK. Mbonye.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financed by Bill and Melinda Gates Foundation (BMGF) through Grant number OPP1148763 received by Delivering Oral Vaccine Effectively (DOVE) project. DOVE Project administered by administered through the Johns Hopkins Bloomberg School of Public Health. BMGF had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dick MH, Guillerm M, Moussy F, Chaignat C-L. Review of two decades of cholera diagnostics—how far have we really come? PLoS Negl. Trop. Dis. [Internet]. Public Library of Science; 2012. [cited 2017 Mar 2];6:e1845 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23071851 doi: 10.1371/journal.pntd.0001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jusatz HJ. 150 years pandemics of Asiatic cholera, 1831–1981. Zentralblatt fur Bakteriol. Mikrobiol. und Hyg. 1 Abt Orig. A Medizinische Mikrobiol. Infekt. und Parasitol. Int. J. Microbiol. Hyg. A Med. Microbiol. Infect. 1982;252:257–67. [PubMed] [Google Scholar]
- 3.Pollitzer R. Cholera studies. 1. History of the disease. Bull. World Health Organ. 1954;10:421–61. [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterji BP. On the discovery of the nature of cholera toxin in India. Vesalius acta Int. Hist. Med. 2010;16:16–8. [PubMed] [Google Scholar]
- 5.Ball L. Cholera and the Pump on Broad Street: Available from: http://www.ph.ucla.edu/epi/snow/Snow_Laura_Ball.pdf
- 6.Van Loon FPL. Cholera: Developments in prevention and cure. Trop. Geogr. Med. 1993;45:269–73. [PubMed] [Google Scholar]
- 7.Charles RC, Ryan ET. Cholera in the 21st century. Curr. Opin. Infect. Dis. 2011;24:472–7. doi: 10.1097/QCO.0b013e32834a88af [DOI] [PubMed] [Google Scholar]
- 8.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012. p. 2466–76. doi: 10.1016/S0140-6736(12)60436-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagen KS. Cholera: The Biography. Emerg. Infect. Dis. 2011. p. 159–159. [Google Scholar]
- 10.Ali M, Lopez AL, Ae You Y, Eun Kim Y, Sah B, Maskery B, et al. The global burden of cholera. Bull World Heal. Organ. 2012;90:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murugaiah C. The burden of cholera. Crit. Rev. Microbiol. 2011;37:337–48. doi: 10.3109/1040841X.2011.603288 [DOI] [PubMed] [Google Scholar]
- 12.Zuckerman JN, Rombo L, Fisch A. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 2007. p. 521–30. doi: 10.1016/S1473-3099(07)70138-X [DOI] [PubMed] [Google Scholar]
- 13.Gaffga NH, Tauxe R V., Mintz ED. Cholera: A new homeland in Africa? Am. J. Trop. Med. Hyg. 2007;77:705–13. [PubMed] [Google Scholar]
- 14.Langa JP, Sema C, De Deus N, Colombo MM, Taviani E. Epidemic waves of cholera in the last two decades in mozambique. J. Infect. Dev. Ctries. 2015;9:635–41. doi: 10.3855/jidc.6943 [DOI] [PubMed] [Google Scholar]
- 15.Hu D, Liu B, Feng L, Ding P, Guo X, Wang M, et al. Origins of the current seventh cholera pandemic. Proc. Natl. Acad. Sci. 2016;113:E7730–9. doi: 10.1073/pnas.1608732113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stine OC, Morris JG Jr. Circulation and Transmission of Clones of Vibrio cholerae During Cholera Outbreaks. Curr. Top. Microbiol. Immunol. 2014;379:181–93. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4243509/pdf/nihms642311.pdf doi: 10.1007/82_2013_360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh R, Nair GB, Tang L, Morris JG, Sharma NC, Ballal M, et al. Epidemiological study of Vibrio cholerae using variable number of tandem repeats. FEMS Microbiol. Lett. 2008;288:196–201. doi: 10.1111/j.1574-6968.2008.01352.x [DOI] [PubMed] [Google Scholar]
- 18.Kendall EA, Chowdhury F, Begum Y, Khan AI, Li S, Thierer JH, et al. Relatedness of Vibrio cholerae O1/O139 isolates from patients and their household contacts, determined by multilocus variable-number tandem-repeat analysis. J. Bacteriol. [Internet]. American Society for Microbiology; 2010. [cited 2017 Aug 29];192:4367–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20585059 doi: 10.1128/JB.00698-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S, et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature. 2011;477:462–5. doi: 10.1038/nature10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yahaya AA, Ndihokubwayo JB, Coulibaly SO, Akanmori B, Mwenda J, Dosseh A, et al. Laboratory capacity in 2012 for diagnosis of epidemic prone diseases in the context of Integrated Disease Surveillance and Response in the WHO African Region. African Heal. Monit. [Internet]. 2013. [cited 2017 Jul 25];November:44–8. Available from: https://www.aho.afro.who.int/en/ahm/issue/18/reports/laboratory-capacity-2012-diagnosis-epidemic-prone-diseases-context-integrated [Google Scholar]
- 21.De R, Ghosh JB, Sen Gupta S, Takeda Y, Nair GB. The Role of Vibrio cholerae Genotyping in Africa. J. Infect. Dis. 2013. 208:S32–8. Available from: doi: 10.1093/infdis/jit199 [DOI] [PubMed] [Google Scholar]
- 22.WHO and CDC. Laboratory Methods for the Diagnosis of Epidemic Dysentery and Cholera. Prevention 1999, Available from: https://www.cdc.gov/cholera/pdf/Laboratory-Methods-for-the-Diagnosis-of-Epidemic-Dysentery-and-Cholera.pdf [Google Scholar]
- 23.World Health Organization. Laboratory Processing of Fecal Specimens. Man. Identif. Antimicrob. Susceptibility Test. 2003;299–300. Available from: http://www.who.int/csr/resources/publications/drugresist/VIAMRManual.pdf [Google Scholar]
- 24.Nandi B, Nandy RK, Mukhopadhyay S, Nair GB, Shimada T, Ghose AC. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein Omp W. J. Clin. Microbiol. [Internet]. 2000. [cited 2017 Jul 12];38:4145–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11060082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75 doi: 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treangen TJ, Ondov BD, Koren S, Phillippy AM. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. [Internet]. 2014;15:524 Available from: http://github.com/marbl/harvest. doi: 10.1186/s13059-014-0524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price MN, Dehal PS, Arkin AP. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. [Internet]. Nucleic Acids Res. 2016. [cited 2017 Aug 2]. p. gkw290–. Available from: https://academic.oup.com/nar/article-lookup/doi/10.1093/nar/gkw290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weill F-X, Domman D, Njamkepo E, Tarr C, Rauzier J, Fawal N, et al. Genomic history of the seventh pandemic of cholera in Africa. Science 2017;358:785–9. Available from: doi: 10.1126/science.aad5901 [DOI] [PubMed] [Google Scholar]
- 30.Bwire G, Mwesawina M, Baluku Y, Kanyanda SSE, Orach CG. Cross-Border Cholera Outbreaks in Sub-Saharan Africa, the Mystery behind the Silent Illness: What Needs to Be Done? Carpenter DO, editor. PLoS One. Public Library of Science; 2016;11: e0156674 doi: 10.1371/journal.pone.0156674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abubakar A, Bwire G, Azman AS, Bouhenia M, Deng LL, Wamala JF, et al. Cholera Epidemic in South Sudan and Uganda and Need for International Collaboration in Cholera Control. Emerg. Infect. Dis. 2018. 24:883–7. Available from: doi: 10.3201/eid2405.171651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kachwamba Y, Mohammed AA, Lukupulo H, Urio L, Majigo M, Mosha F, et al. Genetic Characterization of Vibrio cholerae O1 isolates from outbreaks between 2011 and 2015 in Tanzania. BMC Infect. Dis. 2017;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohamed AA, Oundo J, Kariuki SM, Boga HI, Sharif SK, Akhwale W, et al. Molecular epidemiology of geographically dispersed Vibrio Cholerae, Kenya, January 2009-may 2010. Emerg. Infect. Dis. 2012;18:925–31. doi: 10.3201/eid1806.111774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebaudet S, Sudre B, Faucher B, Piarroux R. Environmental determinants of cholera outbreaks in inland africa: A systematic review of main transmission foci and propagation routes J. Infect. Dis. 2013. 208 Suppl 1:S98–106. Available from: https://academic.oup.com/jid/article-lookup/doi/10.1093/infdis/jit195 [DOI] [PubMed] [Google Scholar]
- 35.Dixon MG, Schafer IJ. Ebola viral disease outbreak—West Africa, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2014. p. 548–51. [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander KA, Sanderson CE, Marathe M, Lewis BL, Rivers CM, Shaman J, et al. What factors might have led to the emergence of ebola in West Africa? PLoS Negl. Trop. Dis. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey A, Atkins KE, Medlock J, Wenzel N, Townsend JP, Childs JE, et al. Strategies for containing Ebola in West Africa. Science (80-.). 2014;346:991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
"The genomes were submitted to NCBI and accessible under the BioProject ID PRJNA439310." Annotated genomes are available at Genbank accession numbers PYRD00000000-PYRM00000000.
(XLSX)
(XLSX)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.