Abstract
The study aimed to review the etiology of failed back surgery syndrome (FBSS) and to propose a treatment algorithm based on a systematic review of the current literature and individual experience. FBSS is a term that groups the conditions with recurring low back pain after spine surgery with or without a radicular component. Since the information on FBSS incidence is limited, data needs to be retrieved from old studies. It is generally accepted that its incidence ranges between 10% and 40% after lumbar laminectomy with or without fusion. Although the etiology of FBSS is not completely understood, it is possibly multifactorial, and the causative factors may be categorized into preoperative, operative, and postoperative factors. The evaluation of patients with FBSS symptoms should ideally initiate with reviewing the patients’ clinical history (observing “red flags”), followed by a detailed clinical examination and imaging (whole-body X-ray, magnetic resonance imaging, and computed tomography). FBSS is a complex and difficult pathology, and its accurate diagnosis is of utmost importance. Its management should be multidisciplinary, and special attention should be provided to cases of recurrent disc herniation and postoperative spinal imbalance.
Keywords: Failed back surgery syndrome, Electrostimulation, Postural balance
Introduction
First described by North et al. [1] in 1991, failed back surgery syndrome (FBSS) is a term that groups the conditions with recurring low back pain after spine surgery with or without a radicular component. This is in fact a misnomer because the clinical presentation may be caused due to a mismatch between the patient’s and surgeon’s expectations prior to the surgery [2]. Moreover, the high-quality evidence on the medical and surgical management of FBSS is limited. This review discusses the incidence and economic burden of this syndrome, studies its different etiologies, focuses on its organic causes, and elaborates the different evaluation and treatment methods.
Epidemiology
Low back pain, a common symptom among adults, has a lifetime prevalence ranging between 60% and 85% [3]. Among orthopedic and chronic pain conditions, back pain has the highest elevated indirect cost with an average estimate of 19.8 billion dollars [4]. On the other hand, the incidence of spine surgery in general and spinal fusion, particularly among adult, has been exponentially increasing in recent years [5]. Since FBSS incidence is not wellstudied, the available data is from old, ill-designed studies [2]. Because the definition of FBSS is not precise, its exact incidence is unknown. Nonetheless, it is an accepted fact that FBSS incidence ranges between 10% and 40% after lumbar laminectomy with or without fusion [2]. Lumbar microdiscectomy seems to be associated with a lower incidence of FBSS, and some authors have reported a failure rate of 8.4% in a single cohort study [6], but other randomized controlled trials have shown a higher incidence of failed surgery, i.e., reaching 19% by postoperative 2 years [7]. Decompressive surgery seemingly has a success rate between 65% and 75% [8,9].
Etiology
Although the etiology of FBSS is not clearly understood, several reports are in agreement that its origin is multifactorial and that the causative factors may be categorized into preoperative, operative, and postoperative factors (Table 1).
Table 1.
Summary of the etiologies of FBSS
| Etiology of FBSS | |
|---|---|
| Preoperative | Patient-related factors: psychological, social |
| Surgery-related factors: poor candidate selection, revision surgery, improper planning | |
| Operative | Inadequate decompression of lateral recesses and foramina |
| Instability with excessive decompression | |
| Incorrect level surgery | |
| Postoperative | Recurrent disc herniation |
| Adjacent segment disease | |
| Sagittal balance-related problems | |
| Pelvic incidence and lumbar lordosis mismatch | |
| Battered root syndrome | |
| Nerve root entrapment syndrome |
FBSS, failed back surgery syndrome.
1. Preoperative factors
Preoperative causal factors may be further divided into surgery- and patient-related factors. Patient psychological factors such as anxiety, depression, and hypochondriasis or social characteristics such as salary or wages and litigation may have a negative impact on surgical outcomes [2]. Surgical preoperative pitfalls include poor candidate selection such as selecting patients with predominant axial pain for microdiscectomy, revision surgery, and improper surgical planning such as the use of inadequate decompression levels. Reportedly, psychological factors are significantly more substantial than structural abnormalities in predicting low back pain [10]. Notably, the presence of these factors does not exclude the presence of an organic problem; rather, they require special attention and optimization before surgery [11]. Further, patients with lumbar disc disease and poorer psychometric scores may benefit from an earlier surgery because prolonged distress and pain may reduce the benefits of surgical intervention [12].
2. Operative factors
Poor surgical technique may be an equally significant cause of FBSS compared with other factors such as psychological and social factors, with the most frequent reason being the failure to achieve surgical goal with inadequate decompression in the lateral recess or in neural foramens [13]. Inadequate decompression in the lateral recess and particularly in the neural foramens is the most common cause of poor surgical technique leading to FBSS, representing 25%–29% of the cases [14]. However, judicious decompression may also lead to instability if >33% of the articular surface is bilaterally removed or if 100% is unilaterally removed [13]. Notably, the incidence of incorrect surgery is approximately 2.1%–2.7% [2]. Using minimally invasive and microscopic techniques, the resultant limited exposure can result in a greater incidence of incorrect surgery [2]. Nachemson [15] showed that success rates decrease with each reoperation of spinal fusion on the same patient with 50% success in the first reoperation, 30% in the second, and 15% in the third. Spinal instability increases with the number of surgeries, from 12% after the first reoperation to 50% after the fourth [16]. Due to all these factors, a reoperation after lumbar discectomy has a higher success rate of 70% [17].
3. Postoperative factors
Postoperative causes may be divided into disease progression and surgery-related factors. Recurrent disc herniation is known to occur in approximately 6%–23% of patients who have undergone microdiscectomy at the same site or the adjacent level [18,19]. Adjacent segment disease, a known complication of lumbar fusion with radiological and clinical subcategories, is a significant risk factor for reoperation post primary microendoscopic discectomy [19]. On the other hand, the incidence of clinical adjacent segment disease is believed to be approximately 27% [20]. Sagittal balance plays an important role because unbalanced and compensated balanced spines predispose to adjacent disc degeneration [21]. Furthermore, pelvic incidence–lumbar lordosis mismatch was recently found to be a predisposing risk factor for adjacent segment disease [22] (Fig. 1). Equally importantly, nerve root irritation may be a cause of FBSS. Nerve root entrapment in epidural fibrosis is believed to be a causative or contributing factor for postoperative pain in 20%–36% of FBSS cases [2]. “Battered root syndrome,” caused by excessive bleeding or aggressive root retraction, may also be a cause of postoperative radicular pain [2].
Fig. 1.
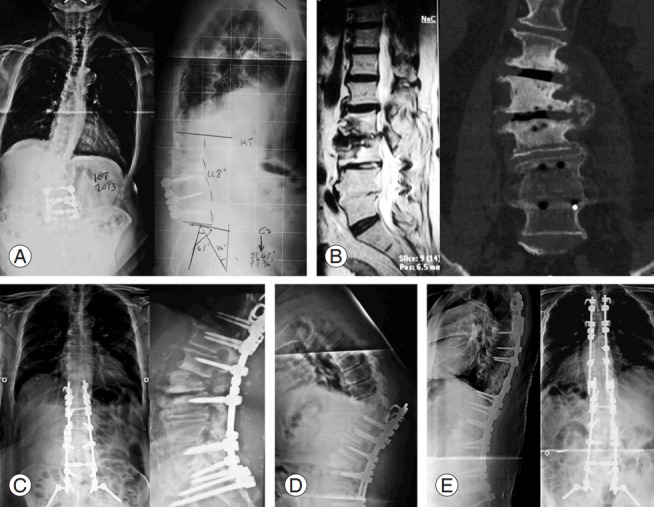
An 81-year-old male underwent laminectomy with L3–L5 fusion 15 years ago. He presented with lumbar back pain with bilateral numbness and paresthesia in his 5th toes aggravated over the past 2 years and was resistant to medical treatment. (A) Complete spine X-ray and (B) computed tomography scanning and magnetic resonance imaging showed adjacent segment disease and degenerative scoliosis. (C) He underwent corrective osteotomy, and the postoperative course was unremarkable. (D) One month later, he presented with back pain and lower limb weakness following an abrupt movement. X-ray showed proximal junctional kyphosis. (E) He underwent an extension of his arthrodesis to T2.
Evaluation
The evaluation of a patient with FBSS symptoms should ideally initiate with a detailed history assessment and clinical examination. The assessment should compare the preoperative symptomatology with the recent one. If the pain is of an early onset or is the same as the preoperative one, an intraoperative cause or a postoperative complication may be suspected as the cause [23]. Centralization is referred to as pain moving toward or from the lumbar spine due to repetitive movements, and it is suggestive of discogenic pain. Radicular pain may be caused by foraminal stenosis, inadequate decompression, epidural fibrosis, or recurrent disc herniation [23]. In contrast, a new onset of radicular pain is more suggestive of an instrumentation issue such as pedicle screw misplacement (Table 2, Fig. 2) [23].
Table 2.
Summary of the evaluation of FBSS
| Evaluation of FBSS | |
|---|---|
| Detailed history and clinical examination | Compare preoperative symptomatology to the current one |
| Look for radicular pain vs. centralization | |
| Red flags (organic signs and symptoms) | |
| Yellow flags (psychological stressors) | |
| Waddell signs | |
| Laboratory studies | Complete blood count, C-reactive protein, and erythrocyte sedimentation rate |
| Plain X-ray and dynamic, whole-spine anteroposterior, and lateral X-ray | |
| Gadolinium-enhanced magnetic resonance imaging | |
| Computed tomography scan+reconstruction | |
| Facet injection±myelography |
FBSS, failed back surgery syndrome.
Fig. 2.
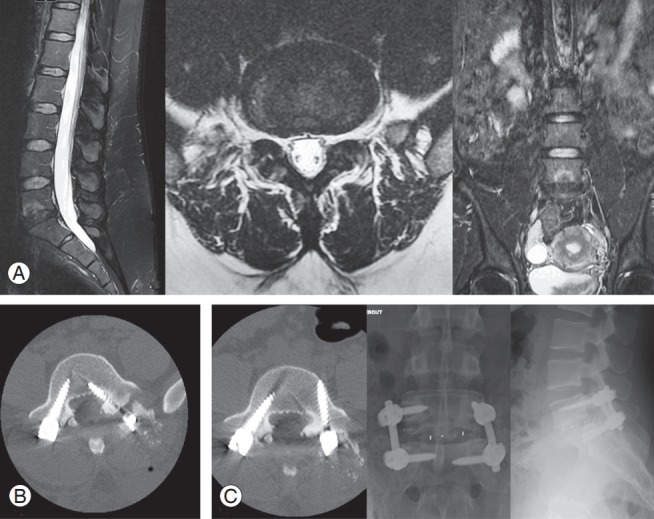
A 24-year-old woman presented 2 years after L5–S1 discectomy. She had intense low back pain with no lower limb involvement. (A) MRI showed Modic 1 changes at the L5–S1 level, with no neural element compression. Diagnostic discography was positive at this level. She was operated on via a posterior minimally invasive transforaminal lumbar interbody fusion and arthrodesis. She had intense left lower limb radicular pain on postoperative day 1. (B) Emergent computed tomography scanning showed an intracanalar left L5 screw. (C) She underwent repositioning of the incriminated screw. At the last follow-up, she had persistent moderate low back pain (Visual Analog Scale=3/10), which did not affect her daily activities. MRI, magnetic resonance imaging.
1. History and clinical examinations
History and clinical examinations should search for “red flags” such as saddle (perianal/perineal) anesthesia or paresthesia, a recent onset of bladder or anal dysfunction, and severe or progressive neurological deficit in the lower extremities, suggesting the presence of cauda equina syndrome. The onset of pain in >50- or <20-year-old individuals, a history of cancer, constitutional symptoms such as fever, chills, or unexplained weight loss, recent bacterial infection, intravenous drug abuse, immune suppression, and the pain that persists while lying in the supine position suggest the presence of cancer or infection. Significant muscle weakness or wasting, the loss of tendon reflexes, or the presence of a positive Babinski reflex suggest a high risk of permanent damage to the compressed nerve [2].
History and clinical examinations should also assess “yellow flags” or psychological stressors that were first described by Nicholas A. S. Kendall [24]. These psychosocial factors are indicative of long-term chronicity and disability and are known to be critically involved in chronic pain syndromes (in general) and FBSS (in particular) [2]. These include the following: (1) fear-avoidance behavior and reduced activity; (2) back pain-related negative attitude stating that it is harmful or potentially severely disabling; (3) an expectation that passive, rather than active, treatment will be beneficial; (4) tendency toward depression, low morale, and social withdrawal; (5) social or financial problems; and (6) salary or wages.
Physical examination may completely show normal results and mislead the diagnosis. Therefore, special attention should be paid to Waddell signs [25], which include the signs of superficial or non-anatomic pain on palpation, the reports of pain during painless-designed evaluations, and an overreaction to stimuli. The presence of ≥2 Waddell signs is associated with poorer outcome, regardless of spinal pathology [23]. Although their significance is controversial, recent studies have shown their association with psychological perturbation [26]. The examination should also focus on other adjacent joints because sacroiliac or hip pathologies could mimic low back pain. Lastly, a dehiscent wound or sinus tract symptom could alert the physician about a current infection.
2. Laboratory studies
Laboratory studies should comprise a complete blood count, including white blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein level, and should be used to eliminate postoperative infection. Plain radiographs with flexion–extension films and whole-spine anteroposterior and lateral views should be ordered; they are used to evaluate the surgical site, spinal alignment, the presence or absence of spinal imbalance, and degenerative changes. The chief advantage of plain radiographs over other modalities is their dynamic nature and ability to detect an instability that may otherwise not appear [27]. Since magnetic resonance imaging (MRI) provides the most suitable information regarding the origin of the symptoms, it should be performed with gadolinium enhancement to help differentiate epidural fibrosis (which appears enhanced) and recurrent disc herniation (which does not appear enhanced) [28]. Nerve root enhancement indicates a radicular origin of the symptoms and when associated with recurrent disc herniation, it may require surgical revision in FBSS [28]. Recently, Yamada et al. [29] showed that conventional MRI had low sensitivity in detecting intra/extra foraminal stenosis, indicating the risk of overlooking such stenosis while using conventional MRI alone. In contrast, they showed threedimensional (3D) MRI-associated high reliability for the detection of this type of stenosis and recommended its use for foraminal stenosis detection [29].
Computed tomography (CT), another excellent modality to assess spinal instrumentation-related complications, can help qualify and quantify a fusion mass in an instrumented spine when instrumentation removal is contemplated [30]. In patients with contraindications for MRI, CT myelography can be used that may show the compression of neural structures by bony elements or others [23]. Further, lumbar discography can be used as an adjunct for the diagnosis of discogenic pain. Discography is considered positive when disc injection elicits the same pain that the patient feels while experiencing symptoms; however, 40% of asymptomatic patients also present with positive results. Further, the patients with psychological predispositions may also present false positive results [31]. Ultimately, posterior facets are an unrecognized generators of pain in FBSS because posterior facet-originated pain is present in 15%–40% of patients with chronic low back pain [2]. Diagnostic facet injection determines the exact implication of the facets in pain generation.
At Hôtel Dieu de France Hospital, Beirut, every patient with FBSS undergoes plain radiography and gadoliniumenhanced MRI and CT scanning with 3D reconstruction. If these examinations are inconclusive but an organic cause of the symptoms is present, facet injection or myelography is performed to further delineate the cause.
Treatment
The treatment of FBSS presents a major, similar dilemma to the patient and physician. It needs to be emphasized that FBSS treatment should be multidisciplinary. As a team, pain physicians, spine surgeons, physical therapists, and psychiatrists should target demystifying the pain for the patient. Further, the alternative definition of FBSS that “it results when the outcome of lumbar spinal surgery does not meet the pre-surgical expectations of the patient and surgeon” [32], leads to patient education being considered paramount; patients should be an integral part of the treatment process (Table 3).
Table 3.
Summary of possible strategies for the treatment of FBSS
| Treatment of FBSS | |
|---|---|
| Non-surgical treatment | Multimodal anesthesia: |
| Nonsteroidal anti-inflammatory drugs | |
| Paracetamol+tramadol | |
| Muscle relaxants | |
| Opioids and their derivatives | |
| Antidepressants and antiepileptics | |
| Spinal infiltration (with precautions) | |
| Spinal cord stimulation (specific candidates) | |
| Surgical treatment | Documented anatomic or pathologic causes: |
| Recurrent disc herniation: | |
| First recurrence: microdiscectomy | |
| Second recurrence: fusion+grafting | |
| Restore sagittal and coronal spinal balance |
FBSS, failed back surgery syndrome.
1. Non-operative management
A complete assessment must be conducted to define the pain characteristics (the mode of onset, irradiations, and neuropathic or nociceptive components) and its repercussions on social life. Clearly, the management of this pain is based on the combination of diverse therapeutic classes of drugs. First-line treatment includes nonsteroidal antiinflammatory drugs (NSAIDs), with oral NSAIDs being shown to be effective for persistent low back pain [33]. However, a recent Cochrane review highlighted the lack of NSAID efficacy in chronic radicular pain [34]. Particularly, the absence of NSAID is considered superior, but recent studies have focused on cyclooxygenase 2 (Cox 2) inhibitors. In fact, Cox 2 inhibitors inhibit prostaglandin E2 production and have been shown to upregulate the regeneration of articular cartilage. However, to the best of our knowledge, no human studies have yet shown the advantage of Cox 2 inhibitors over other NSAIDs [35].
In contrast, the efficacy and safety of paracetamol in patients with spinal pain are controversial. A recent metaanalysis by Machado et al. [36] showed that paracetamol is ineffective in reducing pain and disability in patients with chronic low back pain. Despite this observation, combining paracetamol with tramadol is considered an efficient approach in low back pain [37]. Further, paracetamol continues to be the cornerstone of multimodal analgesia in chronic back pain management.
Muscle relaxants such as thiocolchicoside, a competitive gamma-aminobutyric acid (GABA) receptor antagonist, are a good treatment option for patients with muscle spasm-associated low back pain [38]. Similarly, tolperisone, a piperidine derivative and centrally acting muscle relaxant, is an effective option in the presence of skeletal muscle spasm [39]. Although muscle relaxants are regularly prescribed, no randomized trial has been conducted testing their use in the treatment of chronic low back pain.
In the majority of FBSS cases, opioids and their derivatives are frequently required. Weak opioid agonists such as tramadol and codeine may improve pain and disability, particularly in elderly individuals, while avoiding the adverse effects of NSAIDs such as gastrointestinal and renal toxicity [40]. On the other hand, major oral opiates such as morphine, oxycodone, and methadone can be an option in refractory pain, with the combination of oxycodone and naloxone being associated with a lower risk of constipation and better analgesic efficacy than oxycodone alone or morphine [41]. Notably, the risk of dependence is considerably reduced with careful prescriptions and frequent control by health professionals [42]. There is some growing evidence indicating that psychosocial factors can strongly influence spine surgery outcomes and can be considered one of the diverse underlying etiologies of FBSS [43]. Antidepressants such as amitriptyline, duloxetine, and venlafaxine are recommended owing to their antidepressant and specific analgesic effects [44]. Lastly, the neuropathic component of pain can be targeted using antiepileptics. Currently, pregabalin and gabapentin, which are GABA analogues, are the most commonly used antiepileptics [45]; they bind to voltage-dependent calcium channel sub-units, consequently inhibiting the release of neurotransmitters such as glutamate, noradrenaline, and substance P. Pregabalin or gabapentin should be gradually introduced to avoid somnolence and dizziness, the common dose-dependent adverse effects. However, a recent randomized controlled trial showed the ineffectiveness of gabapentin for analgesia in chronic low back pain with or without a radicular component [46]. Therefore, its use should be reserved for patients with neuropathic pain.
Pain localization-guided spinal infiltration can be performed in the absence of contraindications such as bleeding disorders and the use of anticoagulants or platelet aggregation inhibitors. Local infiltration into ≥1 facet joints via fluoroscopic guidance may be suitable in case of low back pain caused by facet joint arthropathy [47]. Nociceptive radicular pain related to recurrent disc hernia, foraminal stenosis, or secondary spinal canal stenosis may benefit from epidural infiltration [48]. An epidural steroid block may provide limited and short-term pain relief and improvement in activity. The risk of infection and contamination while performing epidural injection is present (predominantly in fragile patients), particularly while using glucocorticoids [49]. In addition, several cases of serious neurological complications, including paraplegia and quadriplegia after fluoroscopy-guided cervical and lumbar foraminal injections, were reported by the AFSSAPS (Agence Française de Sécurité Sanitaire des Produits de Santé, French medicines agency) [48]. Further, the US Food and Drug Administration has declared an alert regarding the rare but serious risks of neurological complications such as stroke, the loss of vision, paralysis, and sometimes death after epidural steroid administration, and it has required a drug label change for injectable longterm corticosteroids [50]. Therefore, infiltration should be considered as a last resort in view of the risk of serious neurological complications. Lastly, recent reports have evaluated the efficacy of intradiscal glucocorticoid injections for chronic back pain. These showed promising results, but no randomized controlled trials have corroborated such outcomes [51]. Therefore, infiltration should be prudently used, particularly due to the risk of postinjection discitis, which is approximately 2.5% [51].
Spinal cord stimulation (SCS), one of the medicosurgical options for FBSS management [2], involves the implantation of electrodes in the epidural space with the production of an electrical current by a subcutaneously inserted pulse generator [2]. Its exact underlying mechanism of action is poorly understood, but analgesia is believed to occur via a gate-control mechanism and variation in neurotransmitter release in the dorsal horns [52]. Several randomized controlled trials have shown the superiority of SCS in analgesia and the cases with functional outcomes compared with repeated back surgery or conventional medical management alone [2,53]. However, this has only been demonstrated in cases wherein the pain was mainly radicular in nature and there was no anatomical cause for the recurrent symptoms [2]. Despite greater healthcare costs associated with the insertion and maintenance of the device in SCS, the gain in health-related quality of life was significantly greater in SCS compared with that in conventional medical management [54]. In addition, cost analysis results have been in the favor of SCS over repeated back surgery [53]. The American Academy of Pain Medicine has defined the practice parameters for the selection of SCS-eligible patients; a patient eligible for a permanent implant is the one with a successful 3–8-day screening trial period. For the trial period to be successful, the patient should have experienced a minimum of 50% pain relief that should be sustained despite adequate provocative physical therapy. The patient should also be satisfied with the results with stable or reduced analgesic consumption during the trial period and should be familiarized with the technical issues of the device [55].
2. Surgical management
Surgical management of FBSS should be reserved for patients with a documented anatomic or pathologic cause for their pain and/or with failed medical treatment [2]. As stated above, the success rate of the surgery decreases with every reoperation [15]. A detailed description of each surgical treatment for FBSS is beyond the scope of this review, but specific attention is paid to two special conditions that are encountered by every spine surgeon: recurrent disc herniation and postoperative sagittal imbalance (Fig. 3).
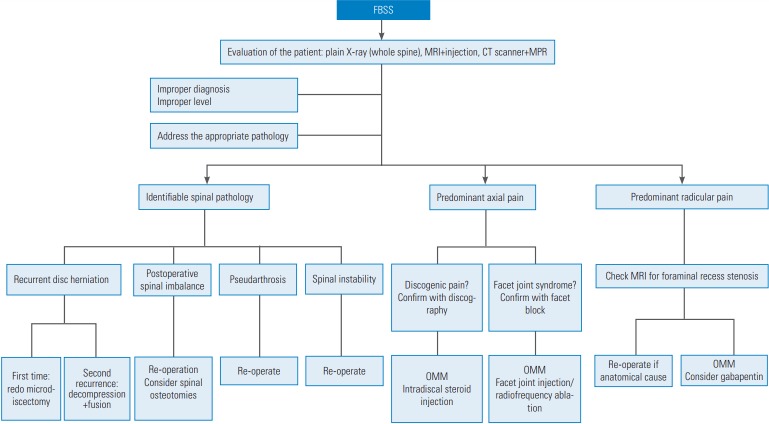
Fig. 3.
Proposed algorithmic approach for FBSS. FBSS, failed back surgery syndrome; MRI, magnetic resonance imaging; CT, computed tomography; MPR, multiplanar reconstruction; OMM, optimal medical management.
Recurrent disc herniation may occur in up to 23% of patients operated using microdiscectomy, at the operated or adjacent level [18,19]. Pain is intensely perceived during recurrences, particularly in the presence of a radicular component [56]. Nonetheless, there is no consensus weather decompressive surgery or decompression with fusion is the best treatment option. In fact, Kogias et al. [57] found that minimally invasive redo discectomy (microdiscectomy and endoscopic discectomy) for recurrent lumbar disc herniations is a safe and efficient treatment option with good success and low complication rates. On the other hand, El Shazly et al. [58] compared microdiscectomy and fusion for recurrent disc disease; they concluded that revision discectomy is effective in patients with recurrent lumbar disc herniation, whereas fusion with revision discectomy improves postoperative low back pain, decreases intraoperative risk of dural tear or neural damage, and decreases postoperative incidence of mechanical instability or further recurrence. We consider the algorithm proposed by Assaker and Zairi [59] to be the best option for recurrent disc herniation, with the first recurrence treated with microdiscectomy and the second recurrence treated with a more radical solution, posterior fusion with anterior grafting.
A global understanding of spinal balance is mandatory while performing spinal instrumentation and fusion. Coronal balance restoration was shown to have critical importance by Glassman et al. [60], with a C7 vertical axis lying <4 cm from the central sacral vertical line, and to be an excellent predictor of postoperative clinical outcomes. On the other hand, the restoration of a sagittal vertical axis (SVA) to <5 cm [61], a lumbar lordosis that fits the patient’s pelvic incidence [62], a pelvic tilt and thoracic kyphosis that fit the patient’s age [63], and pelvic incidence [64] have been found to have a direct correlation with the patient’s postoperative quality of life. Further, Schwab et al. [65] described an age-adjusted alignment threshold, accounting for pelvic tilt, lumbar lordosis, and SVA [66]. When surgeons abide by these principles, good results are expected; if not, postoperative pain and possible complications are possible. In fact, spinal imbalance has been shown to increase the risk of adjacent segment degeneration [67], distal junctional pathology [68], and proximal junctional kyphosis and failure [68,69]. Therefore, evaluating postoperative sagittal balance is imperative. Although a detailed description of the management of this imbalance is beyond the scope of this review, it frequently requires reoperation via an osteotomy of the spine (posterior column or pedicle subtraction osteotomy) with the associated morbidities and complications [70,71].
Conclusions
In summary, FBSS is a complex and difficult pathology with multiple known causes and largely unknown etiologies. An accurate diagnosis is of utmost importance, and its management should be multidisciplinary. Although the medical management of FBSS is challenging, the prescription of an antidepressant or antiepileptic is recommended as first-line treatment. Tramadol is recommended as firstline treatment in case of a major nociceptive component and in acute pain episodes. Morphine should be reserved for second-line treatment after the failure of first-line therapy. Special attention should be paid to the cases of recurrent disc herniation and postoperative spinal imbalance.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.North RB, Campbell JN, James CS, et al. Failed back surgery syndrome: 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery. 1991;28:685–90. [PubMed] [Google Scholar]
- 2.Chan CW, Peng P. Failed back surgery syndrome. Pain Med. 2011;12:577–606. doi: 10.1111/j.1526-4637.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt CO, Raspe H, Pfingsten M, et al. Back pain in the German adult population: prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine (Phila Pa 1976) 2007;32:2005–11. doi: 10.1097/BRS.0b013e318133fad8. [DOI] [PubMed] [Google Scholar]
- 4.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–54. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30:1441–5. doi: 10.1097/01.brs.0000166503.37969.8a. [DOI] [PubMed] [Google Scholar]
- 6.Shamim MS, Parekh MA, Bari ME, Enam SA, Khursheed F. Microdiscectomy for lumbosacral disc herniation and frequency of failed disc surgery. World Neurosurg. 2010;74:611–6. doi: 10.1016/j.wneu.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Peul WC, van den Hout WB, Brand R, Thomeer RT, Koes BW, Leiden-The Hague Spine Intervention Prognostic Study Group Prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: two year results of a randomised controlled trial. BMJ. 2008;336:1355–8. doi: 10.1136/bmj.a143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson A, Lurie J, Tosteson T, Zhao W, Abdu W, Weinstein JN. Who should have surgery for spinal stenosis?: treatment effect predictors in SPORT. Spine (Phila Pa 1976) 2012;37:1791–802. doi: 10.1097/BRS.0b013e3182634b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fokter SK, Yerby SA. Patient-based outcomes for the operative treatment of degenerative lumbar spinal stenosis. Eur Spine J. 2006;15:1661–9. doi: 10.1007/s00586-005-0033-4. [DOI] [PubMed] [Google Scholar]
- 10.Carragee EJ, Alamin TF, Miller JL, Carragee JM. Discographic, MRI and psychosocial determinants of low back pain disability and remission: a prospective study in subjects with benign persistent back pain. Spine J. 2005;5:24–35. doi: 10.1016/j.spinee.2004.05.250. [DOI] [PubMed] [Google Scholar]
- 11.Voorhies RM, Jiang X, Thomas N. Predicting outcome in the surgical treatment of lumbar radiculopathy using the Pain Drawing Score, McGill Short Form Pain Questionnaire, and risk factors including psychosocial issues and axial joint pain. Spine J. 2007;7:516–24. doi: 10.1016/j.spinee.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Carragee EJ. Psychological screening in the surgical treatment of lumbar disc herniation. Clin J Pain. 2001;17:215–9. doi: 10.1097/00002508-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Phillips FM, Cunningham B. Managing chronic pain of spinal origin after lumbar surgery: the role of decompressive surgery. Spine (Phila Pa 1976) 2002;27:2547–53. doi: 10.1097/00007632-200211150-00029. [DOI] [PubMed] [Google Scholar]
- 14.Slipman C. Posterior joints of the lumbar spine as a potential cause of low back pain. Pain Med. 2004;5:287–8. doi: 10.1111/j.1526-4637.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- 15.Nachemson AL. Evaluation of results in lumbar spine surgery. Acta Orthop Scand Suppl. 1993;251:130–3. doi: 10.3109/17453679309160143. [DOI] [PubMed] [Google Scholar]
- 16.Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome: reasons, intraoperative findings, and longterm results: a report of 182 operative treatments. Spine (Phila Pa 1976) 1996;21:626–33. doi: 10.1097/00007632-199603010-00017. [DOI] [PubMed] [Google Scholar]
- 17.Asch HL, Lewis PJ, Moreland DB, et al. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J Neurosurg. 2002;96(1 Suppl):34–44. doi: 10.3171/spi.2002.96.1.0034. [DOI] [PubMed] [Google Scholar]
- 18.Parker SL, Mendenhall SK, Godil SS, et al. Incidence of low back pain after lumbar discectomy for herniated disc and its effect on patient-reported outcomes. Clin Orthop Relat Res. 2015;473:1988–99. doi: 10.1007/s11999-015-4193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong X, Liu L, Bao J, Shi R, Fan Y, Wu X. Characterization and risk factor analysis for reoperation after microendoscopic diskectomy. Orthopedics. 2015;38:e490–6. doi: 10.3928/01477447-20150603-57. [DOI] [PubMed] [Google Scholar]
- 20.Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1701–7. doi: 10.1097/BRS.0b013e31817bb956. [DOI] [PubMed] [Google Scholar]
- 21.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314–9. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothenfluh DA, Mueller DA, Rothenfluh E, Min K. Pelvic incidence-lumbar lordosis mismatch predisposes to adjacent segment disease after lumbar spinal fusion. Eur Spine J. 2015;24:1251–8. doi: 10.1007/s00586-014-3454-0. [DOI] [PubMed] [Google Scholar]
- 23.Guyer RD, Patterson M, Ohnmeiss DD. Failed back surgery syndrome: diagnostic evaluation. J Am Acad Orthop Surg. 2006;14:534–43. doi: 10.5435/00124635-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Crawford C, Ryan K, Shipton E. Exploring general practitioner identification and management of psychosocial Yellow Flags in acute low back pain. N Z Med J. 2007;120:U2536. [PubMed] [Google Scholar]
- 25.Waddell G, McCulloch JA, Kummel E, Venner RM. Nonorganic physical signs in low-back pain. Spine (Phila Pa 1976) 1980;5:117–25. doi: 10.1097/00007632-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Carleton RN, Kachur SS, Abrams MP, Asmundson GJ. Waddell’s symptoms as indicators of psychological distress, perceived disability, and treatment outcome. J Occup Rehabil. 2009;19:41–8. doi: 10.1007/s10926-009-9165-4. [DOI] [PubMed] [Google Scholar]
- 27.Kizilkilic O, Yalcin O, Sen O, Aydin MV, Yildirim T, Hurcan C. The role of standing flexion-extension radiographs for spondylolisthesis following single level disk surgery. Neurol Res. 2007;29:540–3. doi: 10.1179/016164107X164166. [DOI] [PubMed] [Google Scholar]
- 28.Lee YS, Choi ES, Song CJ. Symptomatic nerve root changes on contrast-enhanced MR imaging after surgery for lumbar disk herniation. AJNR Am J Neuroradiol. 2009;30:1062–7. doi: 10.3174/ajnr.A1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada H, Terada M, Iwasaki H, et al. Improved accuracy of diagnosis of lumbar intra and/or extraforaminal stenosis by use of three-dimensional MR imaging: comparison with conventional MR imaging. J Orthop Sci. 2015;20:287–94. doi: 10.1007/s00776-014-0677-1. [DOI] [PubMed] [Google Scholar]
- 30.Mazzie JP, Brooks MK, Gnerre J. Imaging and management of postoperative spine infection. Neuroimaging Clin N Am. 2014;24:365–74. doi: 10.1016/j.nic.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Carragee EJ, Chen Y, Tanner CM, Truong T, Lau E, Brito JL. Provocative discography in patients after limited lumbar discectomy: a controlled, randomized study of pain response in symptomatic and asymptomatic subjects. Spine (Phila Pa 1976) 2000;25:3065–71. doi: 10.1097/00007632-200012010-00014. [DOI] [PubMed] [Google Scholar]
- 32.Waguespack A, Schofferman J, Slosar P, Reynolds J. Etiology of long-term failures of lumbar spine surgery. Pain Med. 2002;3:18–22. doi: 10.1046/j.1526-4637.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 33.Wong JJ, Cote P, Ameis A, et al. Are non-steroidal anti-inflammatory drugs effective for the management of neck pain and associated disorders, whiplash-associated disorders, or non-specific low back pain?: a systematic review of systematic reviews by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Eur Spine J. 2016;25:34–61. doi: 10.1007/s00586-015-3891-4. [DOI] [PubMed] [Google Scholar]
- 34.Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst Rev. 2008;(1):CD000396. doi: 10.1002/14651858.CD000396.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Dijk B, Potier E, Licht R, Creemers L, Ito K. The effect of a cyclooxygenase 2 inhibitor on early degenerated human nucleus pulposus explants. Global Spine J. 2014;4:33–40. doi: 10.1055/s-0033-1359724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tetsunaga T, Tetsunaga T, Tanaka M, Ozaki T. Efficacy of tramadol-acetaminophen tablets in low back pain patients with depression. J Orthop Sci. 2015;20:281–6. doi: 10.1007/s00776-014-0674-4. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Rani S, Siwach R, Verma P. To compare the efficacy and safety of fixed dose combination of thiocolchicoside and aceclofenac versus chlorzoxazone, aceclofenac and paracetamol in patients with acute lower backache associated with muscle spasm. Int J Appl Basic Med Res. 2014;4:101–5. doi: 10.4103/2229-516X.136789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao R, Panghate A, Chandanwale A, et al. Clinical comparative study: efficacy and tolerability of tolperisone and thiocolchicoside in acute low back pain and spinal muscle spasticity. Asian Spine J. 2012;6:115–22. doi: 10.4184/asj.2012.6.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imamura T. Significant efficacy of tramadol/acetaminophen in elderly patients with chronic low back pain uncontrolled by NSAIDs: an observational study. Open Orthop J. 2015;9:120–5. doi: 10.2174/1874325001509010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueberall MA, Mueller-Schwefe GH. Safety and efficacy of oxycodone/naloxone vs. oxycodone vs. morphine for the treatment of chronic low back pain: results of a 12 week prospective, randomized, open-label blinded endpoint streamlined study with prolonged-release preparations. Curr Med Res Opin. 2015;31:1413–29. doi: 10.1185/03007995.2015.1047747. [DOI] [PubMed] [Google Scholar]
- 42.Kraychete DC, Sakata RK. Use and rotation of opioids in chronic non-oncologic pain. Rev Bras Anestesiol. 2012;62:554–62. doi: 10.1016/S0034-7094(12)70155-1. [DOI] [PubMed] [Google Scholar]
- 43.Pakpour AH, Nikoobakht M, Campbell P. Association of pain and depression in those with chronic low back pain: the mediation effect of patient sexual functioning. Clin J Pain. 2015;31:44–51. doi: 10.1097/AJP.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 44.Williamson OD, Sagman D, Bruins RH, Boulay LJ, Schacht A. Antidepressants in the treatment for chronic low back pain: questioning the validity of meta-analyses. Pain Pract. 2014;14:E33–41. doi: 10.1111/papr.12119. [DOI] [PubMed] [Google Scholar]
- 45.Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;(4):CD007938. doi: 10.1002/14651858.CD007938.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atkinson JH, Slater MA, Capparelli EV, et al. A randomized controlled trial of gabapentin for chronic low back pain with and without a radicula component. Pain. 2016;157:1499–507. doi: 10.1097/j.pain.0000000000000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sae-Jung S, Jirarattanaphochai K. Outcomes of lumbar facet syndrome treated with oral diclofenac or methylprednisolone facet injection: a randomized trial. Int Orthop. 2016;40:1091–8. doi: 10.1007/s00264-016-3154-y. [DOI] [PubMed] [Google Scholar]
- 48.Durand G, Girodon J, Debiais F. Medical management of failed back surgery syndrome in Europe: evaluation modalities and treatment proposals. Neurochirurgie. 2015;61 Suppl 1:S57–65. doi: 10.1016/j.neuchi.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Shamliyan TA, Staal JB, Goldmann D, Sands-Lincoln M. Epidural steroid injections for radicular lumbosacral pain: a systematic review. Phys Med Rehabil Clin N Am. 2014;25:471–89. doi: 10.1016/j.pmr.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 50.U.S. Food and Drug Administration . Silver Spring (MD): U.S. Food and Drug Administration; 2014. FDA drug safety communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain [Internet] [cited 2016 Apr 10]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm394280.htm. [Google Scholar]
- 51.Goodman BS, Sowa GA, Buzanowska M, et al. Intradiskal steroids: a viable treatment for low back pain? PM R. 2014;6:547–55. doi: 10.1016/j.pmrj.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage. 2006;31(4 Suppl):S6–12. doi: 10.1016/j.jpainsymman.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 53.North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. 2007;61:361–8. doi: 10.1227/01.NEU.0000255522.42579.EA. [DOI] [PubMed] [Google Scholar]
- 54.Manca A, Kumar K, Taylor RS, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial) Eur J Pain. 2008;12:1047–58. doi: 10.1016/j.ejpain.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 55.North R, Shipley J, Prager J, et al. Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8 Suppl 4:S200–75. doi: 10.1111/j.1526-4637.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 56.Rigoard P, Assaker R. Failed back surgery syndrome: from pathophysiology to recent therapeutic advances in neurostimulation: introduction. Neurochirurgie. 2015;61 Suppl 1:S5. doi: 10.1016/j.neuchi.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Kogias E, Franco Jimenez P, Klingler JH, Hubbe U. Minimally invasive redo discectomy for recurrent lumbar disc herniations. J Clin Neurosci. 2015;22:1382–6. doi: 10.1016/j.jocn.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 58.El Shazly AA, El Wardany MA, Morsi AM. Recurrent lumbar disc herniation: a prospective comparative study of three surgical management procedures. Asian J Neurosurg. 2013;8:139–46. doi: 10.4103/1793-5482.121685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assaker R, Zairi F. Failed back surgery syndrome: to re-operate or not to re-operate?: a retrospective review of patient selection and failures. Neurochirurgie. 2015;61 Suppl 1:S77–82. doi: 10.1016/j.neuchi.2014.10.108. [DOI] [PubMed] [Google Scholar]
- 60.Glassman SD, Berven S, Bridwell K, Horton W, Dimar JR. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976) 2005;30:682–8. doi: 10.1097/01.brs.0000155425.04536.f7. [DOI] [PubMed] [Google Scholar]
- 61.Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005;30:2024–9. doi: 10.1097/01.brs.0000179086.30449.96. [DOI] [PubMed] [Google Scholar]
- 62.Schwab F, Ungar B, Blondel B, et al. Scoliosis Research Society-Schwab adult spinal deformity classification: a validation study. Spine (Phila Pa 1976) 2012;37:1077–82. doi: 10.1097/BRS.0b013e31823e15e2. [DOI] [PubMed] [Google Scholar]
- 63.Protopsaltis TS, Lafage R, Henry J. Do optimal spinal alignment targets result in less PJK for vulnerable elderly spinal deformity patients?; Proceedings of the International Meeting on Advanced Spine Techniques (IMAST); 2014 Jul 16-19; Valencia, Spain. Milwaukee (WI): Scoliosis Research Society; 2014. [Google Scholar]
- 64.Lafage V, Schwab F, Vira S, Patel A, Ungar B, Farcy JP. Spino-pelvic parameters after surgery can be predicted: a preliminary formula and validation of standing alignment. Spine (Phila Pa 1976) 2011;36:1037–45. doi: 10.1097/BRS.0b013e3181eb9469. [DOI] [PubMed] [Google Scholar]
- 65.Schwab FJ, Diebo B, Lafage V. Prevention of PJK: can appropriate surgical planning reduce the risk?; Proceedings of the Scoliosis Research Society 50th Annual Meeting and Courses; 2015 Sep 30-Oct 3; Minneapolis, USA. Milwaukee (WI): Scoliosis Research Society; 2015. pp. 48–51. [Google Scholar]
- 66.Lafage R, Schwab F, Challier V, et al. Defining spinopelvic alignment thresholds: should operative goals in adult spinal deformity surgery account for age? Spine (Phila Pa 1976) 2016;41:62–8. doi: 10.1097/BRS.0000000000001171. [DOI] [PubMed] [Google Scholar]
- 67.Baek SW, Kim C, Chang H. The relationship between the spinopelvic balance and the incidence of adjacent vertebral fractures following percutaneous vertebroplasty. Osteoporos Int. 2015;26:1507–13. doi: 10.1007/s00198-014-3021-x. [DOI] [PubMed] [Google Scholar]
- 68.Arlet V, Aebi M. Junctional spinal disorders in operated adult spinal deformities: present understanding and future perspectives. Eur Spine J. 2013;22 Suppl 2:S276–95. doi: 10.1007/s00586-013-2676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yagi M, Akilah KB, Boachie-Adjei O. Incidence, risk factors and classification of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Spine (Phila Pa 1976) 2011;36:E60–8. doi: 10.1097/BRS.0b013e3181eeaee2. [DOI] [PubMed] [Google Scholar]
- 70.Enercan M, Ozturk C, Kahraman S, Sarıer M, Hamzaoglu A, Alanay A. Osteotomies/spinal column resections in adult deformity. Eur Spine J. 2013;22 Suppl 2:S254–64. doi: 10.1007/s00586-012-2313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolff S, Kheirredine W, Riouallon G. Surgical dural tears: prevalence and updated management protocol based on 1359 lumbar vertebra interventions. Orthop Traumatol Surg Res. 2012;98:879–86. doi: 10.1016/j.otsr.2012.06.016. [DOI] [PubMed] [Google Scholar]