Arabidopsis PRU1 is an E3 ubiquitin ligase that modulates phosphate translocation from roots to shoots by directly regulating the degradation of WRKY6 under low-Pi stress.
Abstract
Since phosphorus is an essential nutrient for plants, plants have evolved a number of adaptive mechanisms to respond to changes in phosphate (Pi) supply. Previously, we reported that the transcription factor WRKY6 modulates Pi homeostasis by downregulating PHOSPHATE1 (PHO1) expression and that WRKY6 is degraded during Pi starvation in Arabidopsis thaliana. However, the molecular mechanism underlying low-Pi-induced WRKY6 degradation was unknown. Here, we report that a ubiquitin E3 ligase, PHOSPHATE RESPONSE UBIQUITIN E3 LIGASE1 (PRU1), modulates WRKY6 protein levels in response to low-Pi stress. A pru1 mutant was more sensitive than the wild type to Pi-deficient conditions, exhibiting a reduced Pi contents in the shoot, similar to the pho1-2 mutant and WRKY6-overexpressing line. PRU1 interacted with WRKY6 in vitro and in vivo. Under low-Pi stress, the ubiquitination and subsequent degradation of WRKY6, as well as the consequential enhancement of PHO1 expression, were impaired in pru1. PRU1 complementation lines displayed no obvious differences compared with wild-type plants. Further genetic analysis showed that disruption of WRKY6 abolished the low-Pi sensitivity of pru1, indicating that WRKY6 functioned downstream of PRU1. Taken together, this study uncovers a mechanism by which PRU1 modulates Pi homeostasis, through regulating the abundance of WRKY6 in response to low-Pi stress in Arabidopsis.
INTRODUCTION
Phosphorus (P) is a major essential nutrient for plant growth and development (Raghothama, 1999), and phosphate (Pi, H2PO4−) is the main form of phosphorus that is absorbed by plants (Chiou and Lin, 2011; López-Arredondo et al., 2014). The Pi concentration in soil is typically 10 μM or less (Raghothama, 1999), which results in Pi starvation and impacts plant growth and survival. Pi uptake by plants occurs mainly through Pi transporters. The Arabidopsis thaliana genome contains at least nine members of the PHOSPHATE TRANSPORTER1 (PHT1) family (Okumura et al., 1998; Mudge et al., 2002), with PHT1;1 and PHT1;4 playing a major role in acquiring Pi from the soil (Shin et al., 2004).
Arabidopsis PHOSPHATE1 (PHO1) participates in Pi transfer from roots to shoots (Poirier et al., 1991; Hamburger et al., 2002), and the pho1 mutant only accumulates 24 to 44% as much Pi in the shoots as wild-type plants (Poirier et al., 1991). PHO1 is located in root stelar cells (Hamburger et al., 2002), which is consistent with its function in Pi efflux out of cells and into xylem. PHO1-mediated Pi export is associated with its localization to the Golgi and trans-Golgi networks (Arpat et al., 2012). Under Pi-deficient conditions, PHO1 expression increases significantly (Stefanovic et al., 2007; Ribot et al., 2008; Chen et al., 2009). The Arabidopsis transcription factors WRKY6 and WRKY42 negatively regulate PHO1 expression under Pi-sufficient conditions. Furthermore, during Pi starvation WRKY6 and WRKY42 are degraded via 26S proteosome-mediated proteolysis (Chen et al., 2009; Su et al., 2015), suggesting that a ubiquitin-proteasome system is involved in maintaining Pi homeostasis.
Ubiquitination of proteins is a multistep reaction, requiring three enzymes: E1, ubiquitin-activating enzyme; E2, ubiquitin-conjugating enzyme; and E3, ubiquitin ligase (Sadanandom et al., 2012). The Arabidopsis genome encodes two E1s, at least 37 E2s, and over 1300 potential E3s (Smalle and Vierstra, 2004). However, the role of the ubiquitin-proteasome system in the plant’s response to low-Pi stress is unclear. Arabidopsis SIZ1 encodes a small ubiquitin-like modifier E3 ligase, and its T-DNA insertion mutant, siz1, displays an exaggerated Pi starvation response (Miura et al., 2005). SIZ1 sumoylates the transcription factor PHR1 (Miura et al., 2005); however, the consequence of PHR1 sumoylation is unknown. An Arabidopsis RING-type ubiquitin E3 ligase, NITROGEN LIMITATION ADAPTATION (NLA), plays a role in maintaining nitrate-dependent Pi homeostasis (Kant et al., 2011). Recently, NLA was found to modulate the degradation of PHT1s under Pi-sufficient conditions (Lin et al., 2013; Park et al., 2014), and the nla mutant was shown to accumulate high levels of Pi due to an increase in the abundance of PHT1s (Lin et al., 2013). Arabidopsis PHO2, a ubiquitin-conjugating enzyme, mediates the degradation of PHT1s and PHO1 (Liu et al., 2012; Huang et al., 2013), and its mutant pho2 displays Pi toxicity due to enhanced Pi uptake and root-to-shoot transfer (Aung et al., 2006; Bari et al., 2006).
In this study, we found that a pru1 (phosphate response ubiquitin E3 ligase1) mutant displayed a similar phenotype to the pho1-2 mutant and WRKY6-overexpressing line, including increased sensitivity to low-Pi and reduced shoot Pi contents. Low-Pi-induced WRKY6 degradation and PHO1 accumulation were also impaired in the pru1 mutant. Further biochemical and genetic data revealed that PRU1 modulates WRKY6 degradation under low-Pi stress. This work uncovers a crucial regulatory pathway in the response to low-Pi stress in Arabidopsis.
RESULTS
The pru1 Mutant Is Defective in Pi Transfer from Roots to Shoots under Low-Pi Stress
We previously reported that under Pi-sufficient conditions, the transcription factor WRKY6 modulates Pi transfer by negatively regulating PHO1 expression and that during Pi starvation, WRKY6 is degraded by a ubiquitin-proteasome pathway, releasing the WRKY6-mediated repression of PHO1 (Chen et al., 2009). Therefore, we initiated an investigation to identify the ubiquitin E3 ligase responsible for modulating the degradation of WRKY6. We did not identify an E3 ligase that can interact with WRKY6 using a yeast two-hybrid screen. We thus obtained 459 Arabidopsis lines with T-DNA insertions in putative E3 ligases from the ABRC stock center and verified 425 of these lines as homozygous e3 mutants (Supplemental Data Set 1). Plants accumulate anthocyanin in aerial potions in response to low-Pi stress, which results in brown-colored leaves (Marschner, 2012). Our previous study showed that the pho1-2 mutant and WRKY6-overexpressing line (35S:WRKY6-9) were sensitive to low-Pi stress, with more accumulation of anthocyanin in young leaves than in wild-type plants (Chen et al., 2009). We next assessed the phenotypes of the homozygous e3 mutants under Pi-deficient conditions and selected the low-Pi-sensitive e3 mutants. The low-Pi-sensitive phenotypes of e3 mutants were confirmed with seeds from two independent harvests. We identified a T-DNA insertion line (Salk_069673C), which we named pru1, that exhibited increased sensitivity to low-Pi stress, similar to 35S:WRKY6-9. We designated the affected gene PRU1. PRU1 is an F-box/RNI-like/FBD-like domain-containing protein (TAIR, http://www.arabidopsis.org/servlets/TairObject?&id=37473type=locus). Although there are at least 41 F-box/RNI-like/FBD-like domain-containing proteins in the Arabidopsis genome, PRU1 did not have a homolog in Arabidopsis (Supplemental Figure 1A). We conducted a BLASTp analysis in NCBI (www.ncbi.nlm.nih.gov) using the PRU1 protein and identified several PRU1 orthologs in grape (Vitis vinifera), maize (Zea mays), Medicago truncatula, and soybean (Glycine max), but not in rice (Oryza sativa) (Supplemental Figure 1B).
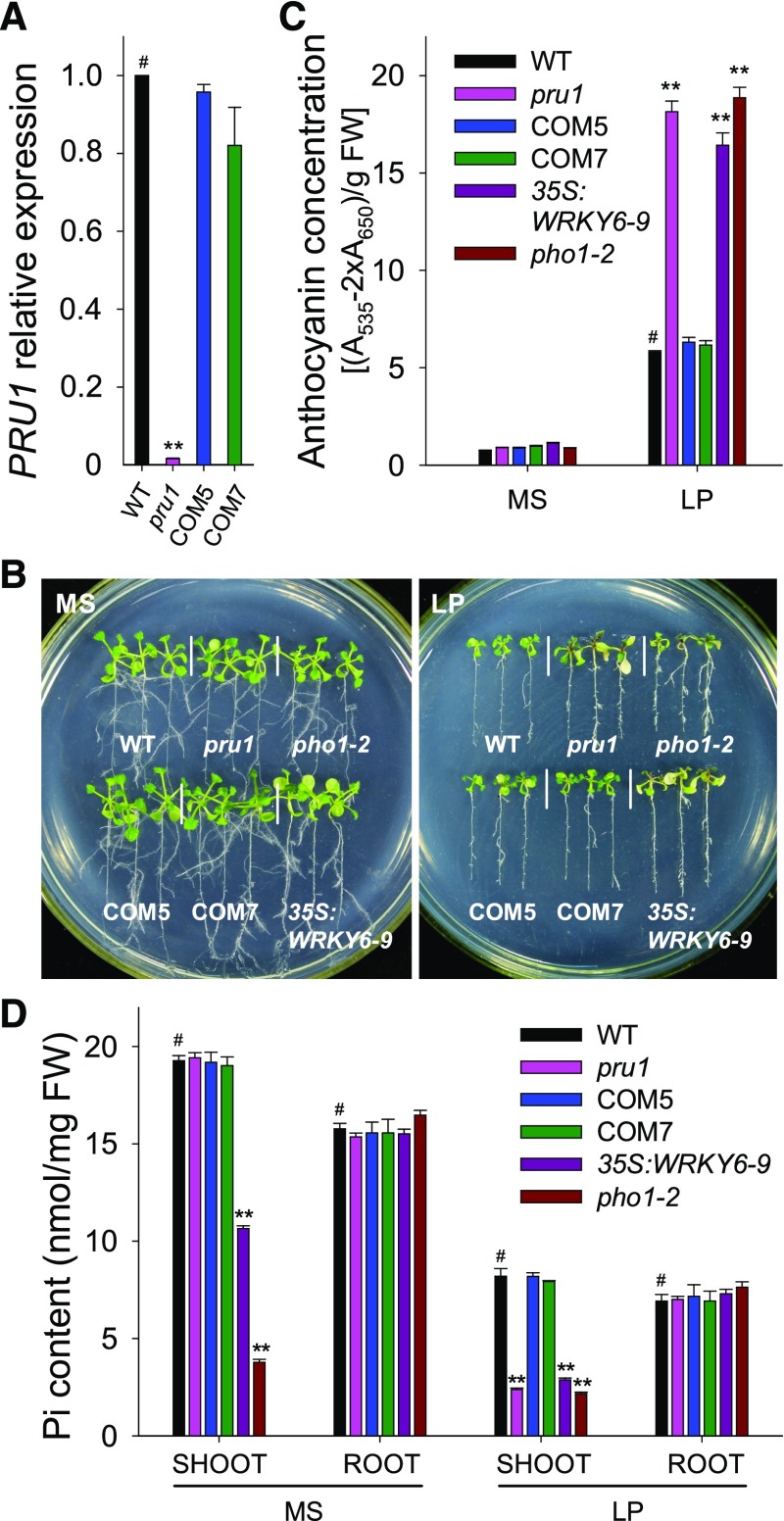
To confirm that the low-Pi sensitivity of the pru1 mutant was due to the T-DNA insertion in PRU1, we generated complementation lines. PRU1 transcript was almost absent in the pru1 mutant, and the complementation lines (COM5 and COM7) displayed similar levels of PRU1 transcript as wild-type plants (Figure 1A). Our previous study showed that the pho1-2 mutant and WRKY6-overexpressing lines were sensitive to low-Pi conditions (Chen et al., 2009). In Arabidopsis, PHO1 mediates Pi translocation from roots to shoots, and the pho1 mutants show reduced shoot growth and accumulate anthocyanin when grown in soil (Supplemental Figure 2A; Poirier et al., 1991; Hamburger et al., 2002; Liu et al., 2012). Although PHO1 transcript abundance was similar between pho1-2 and wild-type plants (Supplemental Figure 2B), the pho1-2 mutant contained a point mutation, C1018T, in PHO1, which caused a premature stop codon (Supplemental Figures 2C and 2D).
Figure 1.
Phenotypic Comparison and Pi Contents among Various Genotypes.
(A) Analysis of PRU1 expression in the pru1 mutant, complementation lines (COM5 and COM7), and wild-type plants (WT) using RT-qPCR. Data are the mean values of three biological replicates ± se.
(B) Phenotypic comparison. All genotypes were germinated and grown on MS medium for 7 d and then transferred to either MS medium or LP medium (LP, low-Pi medium containing 10 μM Pi) for another 7 d.
(C) Anthocyanin measurement. The 7-d-old seedlings were transferred to MS or LP medium for 7 d and then harvested for anthocyanin content measurements. The experiments were performed in biological triplicate, and 15 plants were measured in each replicate. FW, fresh weight.
(D) Pi content measurement. The 7-d-old seedlings were transferred to MS or LP medium for 5 d, the shoots and roots were harvested, and Pi content was measured. The experiments were conducted in biological triplicate, and a group of 15 seedlings was used as one biological sample.
Asterisks indicate significant differences compared with wild-type plants (#) by Student’s t test: **P < 0.01.
Next, we assessed pru1, pho1-2, a WRKY6-overexpressing line (35S:WRKY6-9), and wild-type plants for sensitivity to low-Pi stress. When grown on Pi-sufficient conditions (MS; MS medium with 1.25 mM Pi), no obvious phenotypic differences were observed among these genotypes (Figure 1B, left panel). When grown on Pi-deficient conditions (LP; low-Pi medium with 10 μM Pi), the pru1 mutant exhibited increased sensitivity to low-Pi stress, similar to the pho1-2 mutant and WRKY6-overexpressing line (35S:WRKY6-9) (Figure 1B, right panel). Complementation with PRU1 rescued the increased low-Pi sensitivity of pru1 (Figure 1B, right panel). Anthocyanin accumulation is one of the most striking symptoms of Pi starvation in plants. During Pi starvation, the pru1 mutant, similar to pho1-2 and 35S:WRKY6-9, accumulated more anthocyanin than wild-type plants, while complementation lines contained similar amounts of anthocyanin as wild-type plants (Figure 1C).
Both the pho1 mutant and the WRKY6-overexpressing lines are defective in Pi transfer from roots to shoots and concomitantly have reduced Pi contents in the shoot (Poirier et al., 1991; Hamburger et al., 2002; Chen et al., 2009). Consistent with this, the pho1-2 mutant and 35S:WRKY6-9 line displayed significantly lower Pi contents in the shoot relative to wild-type plants under both Pi-sufficient and Pi-deficient conditions (Figure 1D). Although the shoot Pi content was reduced, the pho1-2 mutant, similar to the PHO1-underexpressing lines, showed normal plant growth at the early seedling stage under Pi-sufficient conditions (Figures 1B and 1D; Rouached et al., 2011). Rouached et al. (2011) demonstrated that the retarded growth of the mature pho1-2 mutant was not a direct consequence of Pi deficiency, but was due to extensive gene expression reprogramming triggered by Pi deficiency (Rouached et al., 2011).
The pru1 mutant had similar levels of Pi in the shoot as wild-type plants under Pi-sufficient conditions, whereas the same line grown under Pi-deficient conditions had reduced Pi contents in the shoot, similar to what was observed for the pho1-2 and 35S:WRKY6-9 (Figure 1D). Pi levels were restored to wild-type levels in the shoots of the COM5 and COM7 complementation lines (Figure 1D). These data demonstrate that Arabidopsis PRU1 modulates Pi transfer from roots to shoots under low-Pi stress.
PRU1 Expression Pattern
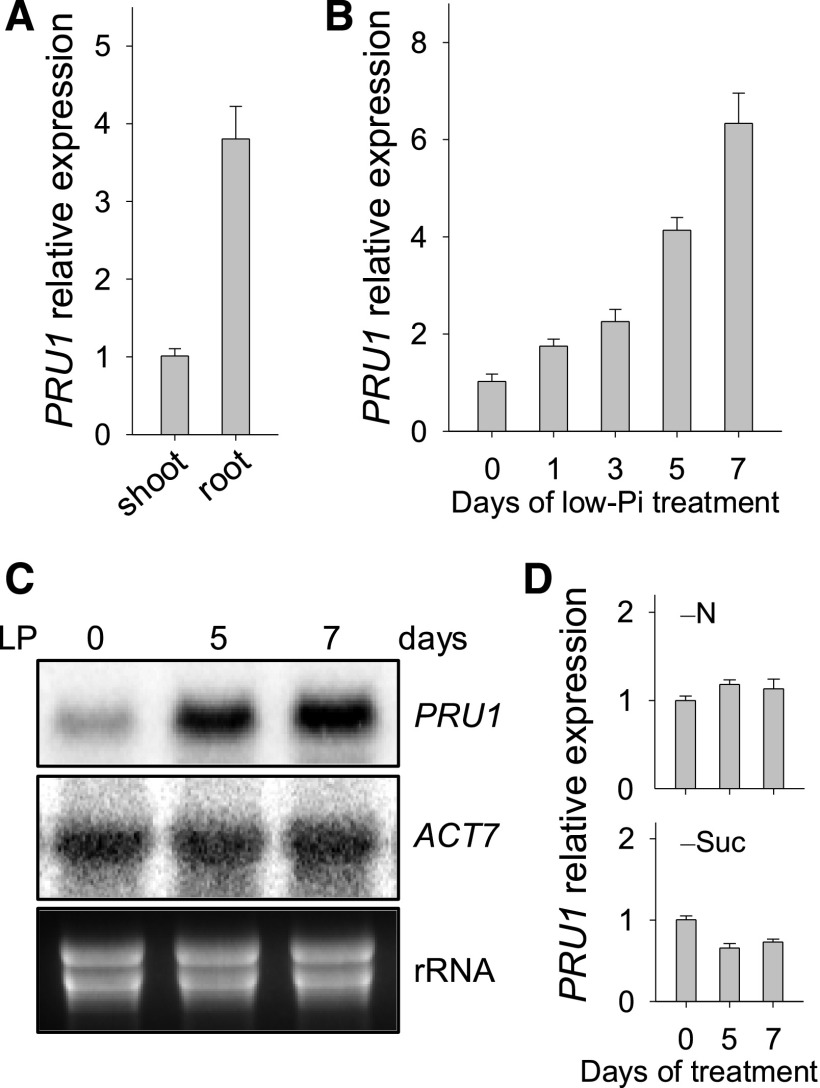
Consistent with the function of root-to-shoot Pi transfer, PRU1 was primarily expressed in the roots (Figure 2A). To measure the transcript abundance of PRU1 in wild-type roots during Pi starvation, 7-d-old plants were transferred to LP medium and then the roots were harvested at the indicated time points. During Pi starvation, the transcript abundance of PRU1 was enhanced (Figures 2B and 2C). The transcript abundance of PRU1 was not induced by nitrogen deficiency and was slightly reduced in the absence of sucrose (Figure 2D).
Figure 2.
PRU1 Expression Pattern.
(A) Analysis of PRU1 expression in wild-type plants using RT-qPCR. The wild-type plants were germinated and grown on MS medium for 7 d, and the roots and shoots were harvested separately for RNA extraction. Data are mean values of three biological replicates ± se.
(B) Analysis of PRU1 expression during Pi starvation using RT-qPCR. The 7-d-old wild-type seedlings were transferred to LP medium and then the roots were harvested at the indicated time points for RNA extraction. Data are mean values of three biological replicates ± se.
(C) Analysis of PRU1 expression during Pi starvation using RNA gel blot analysis. Seven-day-old wild-type seedlings were transferred to LP medium, and the roots were harvested at the indicated time points for RNA extraction. ACT7 and rRNA were used as loading controls.
(D) Analysis of PRU1 expression under nitrogen or sucrose deficiency using RT-qPCR. The 7-d-old wild-type seedlings were transferred to –N or –Suc medium, and then the roots were harvested at the indicated time points for RNA extraction. Data are mean values of three biological replicates ± se.
PRU1 Interacts with WRKY6 in Vitro and in Yeast
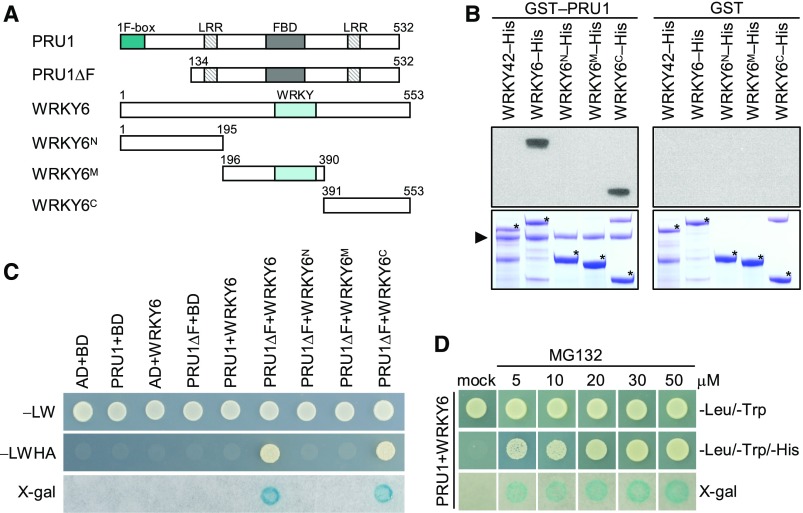
Similar to 35S:WRKY6-9, the pru1 mutant was sensitive to low-Pi stress and had reduced levels of Pi in the shoot under Pi-deficient conditions (Figure 1). Therefore, we hypothesized that the PRU1 may reduce WRKY6 accumulation during Pi starvation. First, we tested for an interaction between PRU1 and WRKY6. The predicted PRU1 protein was composed of 532 amino acids, containing an F-box domain at the N terminus, two LRR domains, and an FBD domain (Figure 3A). The F-box domain was identified as a protein-protein interaction motif that links F-box proteins to the core of the SKP1-Cullin-F-box (SCF) complex by interacting with SUPPRESSOROF KINETOCHORE PROTEIN1 (SKP1) (Bai et al., 1996). The LRR domain consisted of 20 to 30 amino acids that generally fold into a horseshoe shape (Enkhbayar et al., 2004) and appears to provide the structural framework for protein-protein interactions (Kobe and Kajava, 2001). The FBD domain is reported in F-box proteins as well as other domain-containing plant proteins and is proposed to be associated with nuclear processes (Doerks et al., 2002). To test for an interaction between PRU1 and WRKY6, WRKY6 was cleaved into three fragments, WRKY6N, WRKY6M, and WRKY6C (Figure 3A). The recombinant fusion proteins PRU1, WRKY6, and WRKY6 derivates were purified from Escherichia coli and used in pull-down assays. As shown in Figure 3B, the recombinant GST-PRU1 bound to WRKY6 as well as the C terminus of WRKY6 (WRKY6C), but not to WRKY6N, WRKY6M, or WRKY42, which is a close homolog of WRKY6 (Eulgem et al., 2000).
Figure 3.
Interaction between PRU1 and WRKY6 in Vitro and in Yeast.
(A) Schematics of full-length PRU1, WRKY6, and their deletion derivatives. Numbers refer to the positions of the first or last amino acid in the sequences.
(B) In vitro pull-down assay of the interaction between recombinant PRU1 and WRKY6. GST-PRU1 and GST were used as baits, and WRKY42-His, WRKY6-His, WRKY6N-His, WRKY6M-His, and WRKY6C-His were used as targets. The bound proteins were analyzed by immunoblotting using an anti-His antibody. The triangle indicates GST-PRU1, and asterisks indicate the target proteins.
(C) Interaction between PRU1 and WRKY6 in a yeast two-hybrid assay. Yeast cells were grown on control medium (SD/-Leu/-Trp [−LW]) or selective medium (SD/-Leu/-Trp/-His/-Ade [−LWHA]).
(D) Interaction between PRU1 and WRKY6 in a yeast two-hybrid assay with MG132 in DMSO, or DMSO only (mock).
We also tested for an interaction between PRU1 and WRKY6 using a yeast two-hybrid system; however, full-length PRU1 failed to interact with WRKY6 in yeast (Figure 3C). Within the SCF complex, the F-box protein determines the specificity of the complex. The F-box protein contains at least two domains: an F-box domain, which binds to SKP1, and a protein-protein interaction domain, which determines the substrate specificity of the SCF complex (Lechner et al., 2006). We hypothesized that the interaction between PRU1 and WRKY6 resulted in the degradation of WRKY6. In agreement with this hypothesis, the interaction could not be detected in yeast. To investigate if PRU1 interacts with SKP1 through the F-box domain (Lechner et al., 2006), we generated a truncated version of PRU1 that lacks the F-box domain (PRU1ΔF, Figure 3A). The PRU1ΔF and WRKY6 transformants grew well on SD/-Leu/-Trp/-His/-Ade medium (−LWHA) and showed strong β-galactosidase activity (Figure 3C), indicating that PRU1ΔF interacted with WRKY6 in yeast. Consistent with the pull-down results, PRU1ΔF also bound to WRKY6C in yeast (Figure 3C). MG132, a proteasome inhibitor, can impair proteasome-mediated protein degradation in yeast (Ahuja et al., 2017). To further confirm the interaction between PRU1 and WRKY6 in yeast, MG132 in DMSO (5, 10, 20, 30, or 50 μM) or DMSO only (mock) was added into the media. As shown in Figure 3D, full-length PRU1 interacted with WRKY6 in yeast in the presence of MG132. These data demonstrate that PRU1 interacts with WRKY6 in vitro and in yeast.
PRU1 Binds to WRKY6 in Vivo
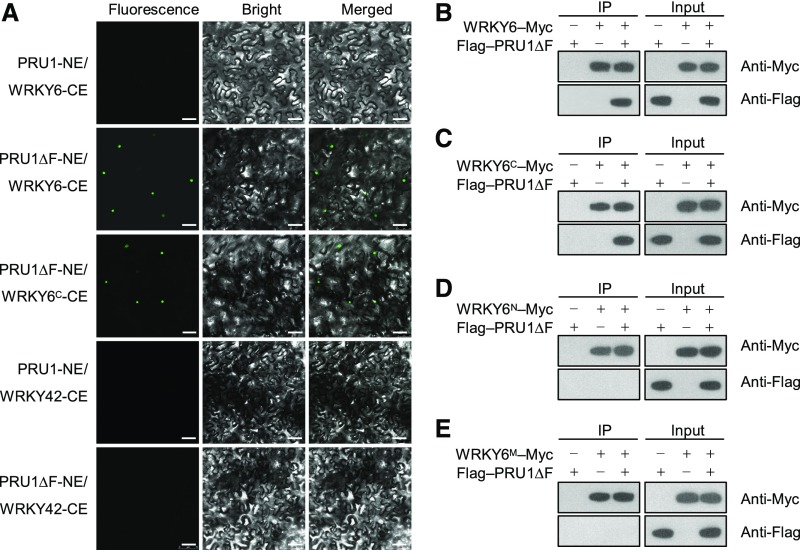
We then tested for the interaction between PRU1 and WRKY6 in Nicotiana benthamiana using a bimolecular fluorescence complementation (BiFC) assay. PRU1 and PRU1ΔF were cloned individually into the pSPYNE173 vector (NE), and WRKY6 and WRKY6C were cloned individually into the pSPYCEM vector (CE), and then various combinations of these vectors were transiently coexpressed in N. benthamiana leaves. No fluorescence signals were observed when full-length PRU1 and WRKY6 were coexpressed, whereas a strong fluorescence signal was observed in the nucleus when PRU1ΔF and WRKY6, or PRU1ΔF and WRKY6C were coexpressed (Figure 4A). This indicates that PRU1 interacted with WRKY6 in the nucleus. Neither PRU1 nor PRU1ΔF interacted with WRKY42 in N. benthamiana leaves (Figure 4A).
Figure 4.
Interaction between PRU1 and WRKY6 in Vivo.
(A) BiFC analysis of the interaction between PRU1 and WRKY6 in N. benthamiana leaves. Bars = 50 μm.
(B) to (E) Coimmunoprecipitation assay for PRU1ΔF and WRKY6 in Arabidopsis protoplasts. The Flag-PRU1ΔF construct was transiently coexpressed with WRKY6-Myc (B), WRKY6C-Myc (C), WRKY6N-Myc (D), or WRKY6M-Myc (E) in Arabidopsis protoplasts. Protein extracts were immunoprecipitated with an anti-Myc affinity gel matrix. Input proteins and immunoprecipitates (IP) were analyzed by immunoblotting using anti-Myc and anti-Flag antibodies.
A coimmunoprecipitation assay was also performed in Arabidopsis protoplasts to confirm the interaction between PRU1ΔF and WRKY6. The coimmunoprecipitation assays showed that Flag-PRU1ΔF coimmunoprecipitated with WRKY6-Myc (Figure 4B) and WRKY6C-Myc (Figure 4C), but not with WRKY6N-Myc (Figure 4D) or WRKY6M-Myc (Figure 4E). This suggests that the C terminus of WRKY6 was essential for the interaction between WRKY6 and PRU1. Together, these data indicate that PRU1 binds to WRKY6 in vivo as well as in vitro.
PRU1 Interacts with ASK Proteins and Modulates Polyubiquitination of WRKY6 in Vivo
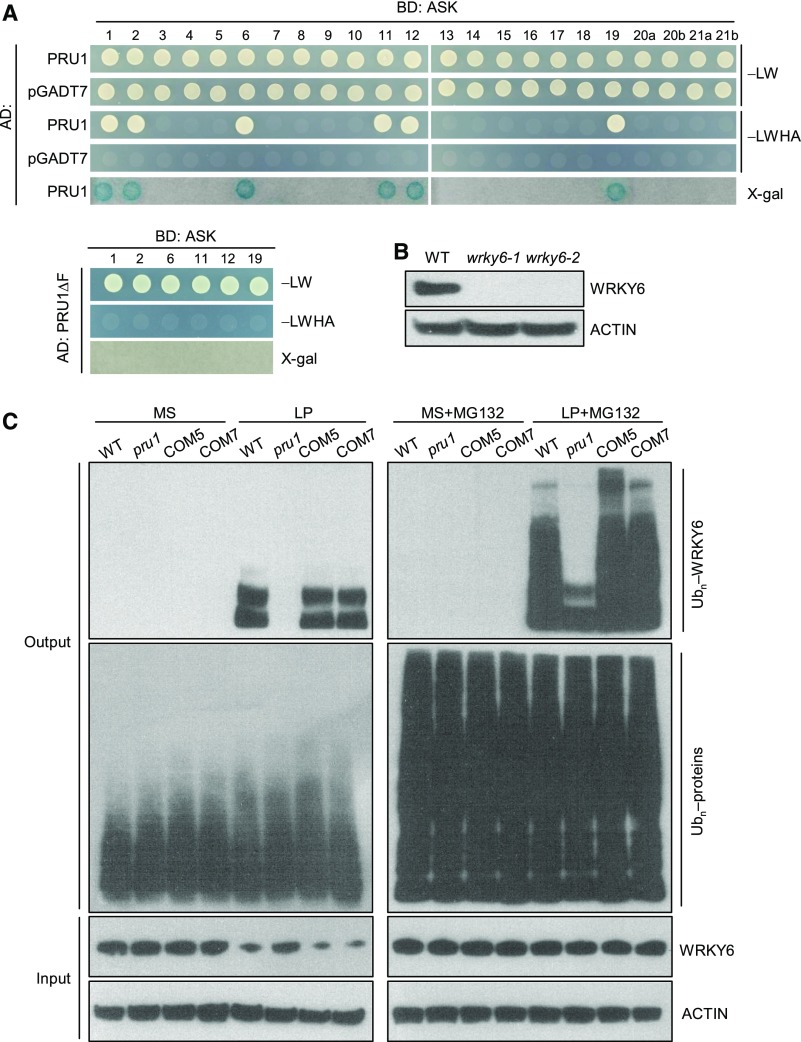
The SCF complex is composed of Cullin1 (CUL1), SKP1, RING-BOX1 (RBX1), and an F-box protein. CUL1 interacts with RBX1 at its C terminus and SKP1 at its N terminus, which in turn binds to the F-box protein (Lechner et al., 2006). In Arabidopsis, the ARABIDOPSIS SKP1-like (ASK) protein interacts with CUL1 and the F-box protein to function as an SCF E3 ubiquitin ligase (Lechner et al., 2006). There are 23 ASK proteins in the Arabidopsis genome (Risseeuw et al., 2003). To examine whether PRU1 was a component of an SCF complex, we tested for interactions between PRU1 and various ASKs using the yeast two-hybrid assay. Among the 23 Arabidopsis ASK proteins, PRU1 interacted with ASK1, 2, 6, 11, 12, and 19 (Figure 5A), while truncated PRU1 lacking the F-box domain (PRU1ΔF) did not interact with any of these six ASK proteins (Figure 5A). Not all six ASK proteins showed close phylogenetic relationships (Supplemental Figure 3A), whereas there were several conserved amino acids among these six ASK proteins (Supplemental Figure 3B), suggesting that these conserved amino acids influenced the specificity of physical interactions between PRU1 and ASK proteins.
Figure 5.
PRU1 Interacts with ASK Proteins and Modulates the Ubiquitination of WRKY6.
(A) Yeast two-hybrid assay testing for an interaction between PRU1 and multiple ASK proteins. The yeast cells were grown on control medium (SD/-Leu/-Trp [LW]) or selective medium (SD/-Leu/-Trp/-His/-Ade [LWHA]).
(B) Immunoblot analysis of WRKY6 protein in wrky6 mutants (wrky6-1 and wrky6-2) and wild-type plants with an anti-WRKY6 antibody. ACTIN was used as the loading control.
(C) Polyubiquitination of WRKY6 in the pru1 mutant, complementation lines, and wild-type plants. Seven-day-old seedlings were transferred to MS medium, LP medium, MS medium supplemented with 10 μM MG132 (MS+MG132), or LP medium supplemented with 10 μM MG132 (LP+MG132) for 3 d and then the roots were harvested and protein was extracted. Each total protein extract was incubated with a P62-agarose matrix to obtain ubiquitinated proteins. Polyubiquitinated WRKY6 was detected with an anti-WRKY6 antibody, and total ubiquitinated proteins were measured with an anti-Ub antibody in the Output. The accumulations of WRKY6 and ACTIN were tested in the Input.
Furthermore, the polyubiquitination of WRKY6 was assessed in the pru1 mutant, complementation lines, and wild-type plants. Seven-day-old seedlings were transferred to MS medium, LP medium, MS medium supplemented with 10 μM MG132 (MS+MG132), or LP medium supplemented with 10 μM MG132 (LP+MG132) for 3 d and then the roots were harvested for protein extraction. The proteasome inhibitor MG132 was added to block the degradation of ubiquitinated proteins, and the ubiquitinated proteins were enriched with P62-agarose. Polyubiquitinated WRKY6 was detected by immunoblotting with an anti-WRKY6 antibody that was known to be specific since signals corresponding to the same size of WRKY6 were not detected in two wrky6 mutants, whereas they were in wild-type plants (Figure 5B). Under Pi-sufficient conditions (MS or MS+MG132), no polyubiquitination signal of WRKY6 was detected among the various genotypes (Figure 5C). When grown under low-Pi stress (LP), WRKY6 was polyubiquitinated in the wild-type plants and complementation lines (COM5 and COM7), and no WRKY6 polyubiquitination signal was visible in the pru1 mutant (Figure 5C). Furthermore, when grown under LP+MG132 conditions, the WRKY6 polyubiquitination signal was markedly higher in the wild-type plants and complementation lines than in the pru1 mutant (Figure 5C). The ubiquitination signals of total ubiquitinated proteins were similar among all genotypes under Pi-sufficient or Pi-deficient conditions (Figure 5C). The accumulation of WRKY6 was also assessed in the same plants at the same time points. When grown under low-Pi conditions, the accumulation of WRKY6 was reduced in wild-type plants and complementation lines, and the pru1 mutant showed a relatively high level of WRKY6 protein compared with wild-type plants (Figure 5C). These data indicate that PRU1 ubiquitinates WRKY6 under low-Pi stress.
PRU1 Modulates WRKY6 Degradation during Pi Starvation
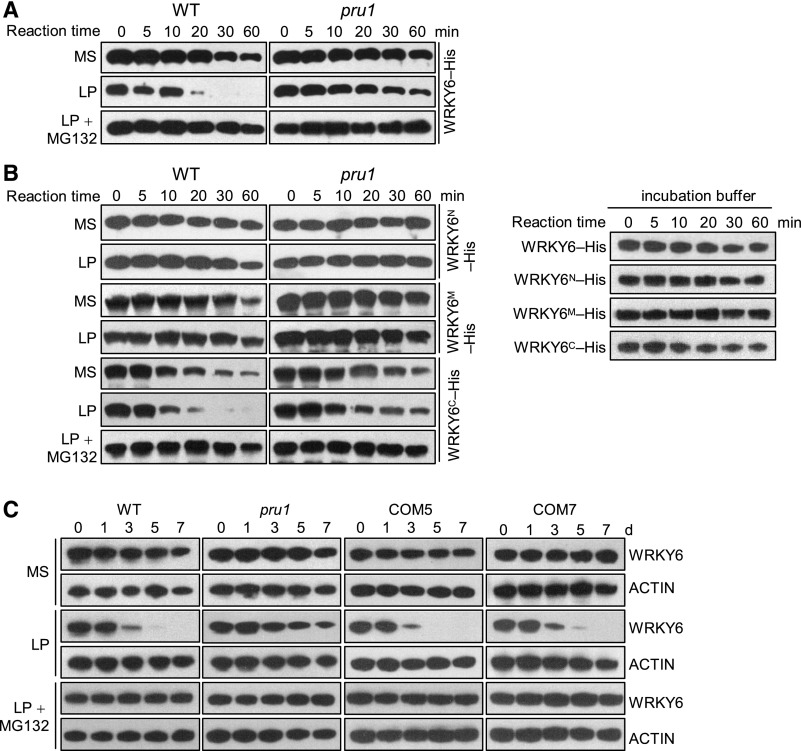
We next compared WRKY6 protein levels in the pru1 mutant and wild-type plants using a cell-free degradation assay. The 7-d-old seedlings were transferred to MS, LP, or LP+MG132 media for 3 d and then roots were collected for protein extraction. When WRKY6-His was incubated with the total proteins from plants grown on MS medium, no differences were detected in the abundance of WRKY6-His between pru1 and wild-type plants (Figure 6A). When incubated with total proteins from plants grown on LP medium, the abundance of WRKY6-His was distinctly reduced in wild-type plants, whereas WRKY6-His levels remained relatively high in the pru1 mutant (Figure 6A). Additionally, this low-Pi-induced reduction of WRKY6-His was inhibited by MG132 (Figure 6A). Since PRU1 interacted with the C terminus of WRKY6 (WRKY6C, Figures 3 and 4), we also measured the protein levels of WRKY6 derivatives using a cell-free degradation system. When incubated with the total proteins from wild-type plants grown on LP medium, only WRKY6C-His showed a low-Pi-induced decrease, which was impaired in the pru1 mutant (Figure 6B). The abundance of recombinant WRKY6 proteins was reduced when incubated with root proteins from plants grown on MS medium, similar to those incubated with incubation buffer (without root proteins) (Figures 6A and 6B), indicating that the cell-free incubation buffer influenced the stability of recombinant WRKY6.
Figure 6.
PRU1 Mediates WRKY6 Degradation Under Low-Pi Stress.
(A) and (B) Cell-free degradation assay. Seven-day-old pru1 mutant and wild-type seedlings were transferred to MS medium, LP medium, or LP medium containing 10 μM MG132 for 3 d, and then the roots were harvested and protein was extracted. The root protein extracts were incubated with recombinant WRKY6-His (A) or its deletion derivatives (WRKY6N-His, WRKY6M-His, or WRKY6C-His) (B) for the indicated periods, and the abundance of WRKY6 or its deletion derivatives was determined by immunoblotting with anti-His antibody. Recombinant WRKY6-His or its derivatives in incubation buffer (without root proteins) were used as controls.
(C) Immunoblot analysis of WRKY6 in the pru1 mutant, complementation lines (COM5 and COM7) and wild-type plants. Seven-day-old seedlings were transferred to MS medium, LP medium, or LP medium containing 10 μM MG132, and then the roots were harvested at the indicated time points and protein was extracted. The abundance of WRKY6 was analyzed by immunoblotting using an anti-WRKY6 antibody. ACTIN was used as the loading control.
Furthermore, the protein level of WRKY6 was analyzed among the pru1 mutant, complementation lines, and wild-types plants by immunoblotting with anti-WRKY6 antibody. The 7-d-old seedlings were transferred to MS, LP, or LP+MG132 medium, and the roots were harvested at the indicated time points. Consistent with a previous report, WRKY6 was degraded in wild-type roots during Pi starvation, and this degradation was inhibited by adding MG132 (Figure 6C; Chen et al., 2009). By contrast, the low-Pi-induced degradation of WRKY6 was reduced in the pru1 mutant (Figure 6C), and normal degradation of WRKY6 was restored in the complementation lines (COM5 and COM7) (Figure 6C).
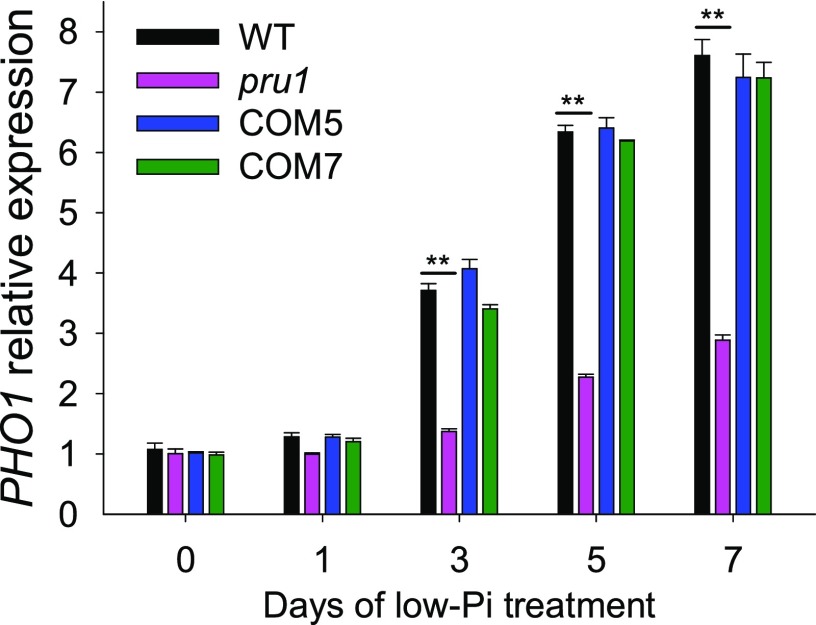
Our previous report showed that WRKY6 directly downregulates PHO1 expression under Pi-sufficient conditions and that WRKY6 is degraded under low-Pi stress, releasing its repression of PHO1 (Chen et al., 2009). Therefore, we assessed the abundance of PHO1 transcripts among pru1, complementation lines, and wild-type plants. During Pi starvation, the PHO1 transcript abundance was enhanced in wild-type plants (Figure 7; Stefanovic et al., 2007; Ribot et al., 2008; Chen et al., 2009), whereas this increase was significantly reduced in the pru1 mutant (Figure 7). The PHO1 transcript abundance in COM5 and COM7 was similar to that in wild-type plants (Figure 7). These results suggest that PRU1 modulates the degradation of WRKY6 in response to low-Pi stress.
Figure 7.
Analysis of PHO1 Expression by RT-qPCR in the pru1 Mutant, Complementation Lines (COM5 and COM7), and Wild-type Plants during Pi Starvation.
Seven-day-old seedlings were transferred to LP medium and the roots were harvested at the indicated time points for RNA extraction. Data are mean values of three biological replicates ± se. Asterisks indicate statistically significant differences compared with wild-type plants by Student’s t test: **P < 0.01.
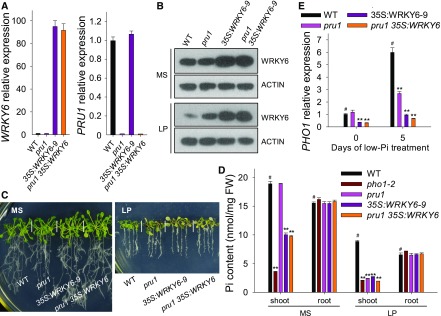
The 35S:WRKY6 and pru1 35S:WRKY6 Lines Display Similar Phenotypes in Response to Pi Starvation
To evaluate the relationship between PRU1 and WRKY6, we generated the pru1 35S:WRKY6 line by crossing pru1 with 35S:WRKY6-9 (Figure 8A). The 35S:WRKY6-9 and pru1 35S:WRKY6 lines accumulated more WRKY6 protein than either the pru1 mutant or wild-type plants under Pi-sufficient or Pi-deficient conditions (Figure 8B). When grown on LP medium, the pru1, 35S:WRKY6-9, and pru1 35S:WRKY6-9 lines displayed similar low-Pi sensitive phenotypes (Figure 8C). Under Pi-sufficient conditions (MS medium), the pru1 mutant had a similar level of Pi in the shoot as wild-type plants, whereas the pru1 35S:WRKY6 line had reduced levels of Pi in the shoot, similar to 35S:WRKY6-9 (Figure 8D). Under low-Pi stress, the pru1, pho1-2, 35S:WRKY6-9, and pru1 35S:WRKY9 lines had significantly less Pi in the shoots compared with wild-type plants (Figure 8D).
Figure 8.
The pru1 35S:WRKY6 Line Shows a Similar Phenotype to the 35S:WRKY6-9 Line.
(A) Analysis of transcript abundance of WRKY6 and PRU1 in the pru1 mutant, WRKY6-overexpressing line (35S:WRKY6-9), pru1 35S:WRKY6 line, and wild-type plants by RT-qPCR. Data are mean values of three biological replicates ± se.
(B) Immunoblot analysis of WRKY6 in the pru1 mutant, 35S:WRKY6-9, pru1 35S:WRKY6 line, and wild-type plants. Seven-day-old seedlings were transferred to MS or LP medium for 3 d, and then the roots were harvested and protein was extracted. The abundance of WRKY6 was analyzed using an anti-WRKY6 antibody. ACTIN was used as the loading control.
(C) Phenotypic comparison of various genotypes. Seven-day-old seedlings were transferred to MS or LP media for 7 d, and then photographs were taken.
(D) Pi content measurement. Seven-day-old plants were transferred to MS or LP media for 5 d, and then the shoots and roots were harvested for Pi content measurements. Three biological replicates were performed, and a group of 15 seedlings was pooled for each biological sample. Data are mean values of three biological replicates ± se.
(E) Analysis of PHO1 expression by RT-qPCR. Seven-day-old plants of various genotypes were transferred to LP medium, and the roots were harvested at the indicated time points for RNA extraction. Data are mean values of three biological replicates ± se. Asterisks in (D) and (E) indicate significant differences compared with wild-type plants (#) by Student’s t test: **P < 0.01.
Since WRKY6 influenced the expression of PHO1, we also assessed PHO1 expression in these lines. Under Pi-sufficient conditions, the PHO1 transcript abundance was significantly lower in pru1 35S:WRKY9 and 35S:WRKY6-9 lines than in wild-type plants, whereas the pru1 mutant had a similar PHO1 transcript abundance to wild-type plants (Figure 8E). During Pi starvation, the low-Pi-induced accumulation of PHO1 expression was reduced in pru1, 35S:WRKY6-9, and pru1 35S:WRKY6 plants, while the repression of PHO1 in pru1 35S:WRKY6 was similar to that in 35S:WRKY6-9 (Figure 8E). These data suggest that PRU1 targets WRKY6 to mediate Pi transfer from roots to shoots.
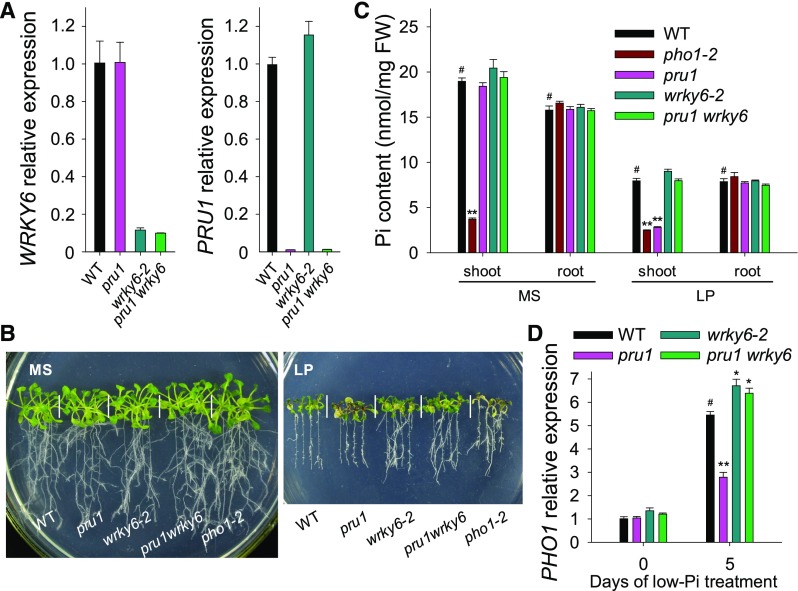
Disruption of WRKY6 Rescues the Low-Pi Sensitivity of pru1
To provide direct evidence that the enrichment of WRKY6 was responsible for the reduced Pi contents in the shoot and reduced PHO1 expression in the pru1 mutant, we generated the pru1 wrky6 double mutant by crossing pru1 with wrky6-2 (Figure 9A). There were no obvious differences among the various genotypes under Pi-sufficient conditions (Figure 9B, left panel). However, when plants were grown on LP medium, the pru1 mutant displayed a low-Pi-sensitive phenotype that was similar to pho1-2, while the pru1 wrky6 double mutant did not (Figure 9B, right panel). Furthermore, we measured the Pi contents in the shoots and roots of these lines. When grown on MS medium, only the pho1-2 mutant had lower Pi content in the shoot among all tested genotypes (Figure 9C). Under low-Pi stress, the pru1 mutant had a significantly reduced Pi content in the shoot, similar to pho1-2. This was restored in the pru1 wrky6 double mutant (Figure 9C).
Figure 9.
Disruption of WRKY6 Rescues the Low-Pi Sensitivity of the pru1 Mutant.
(A) Analysis of transcript abundance for WRKY6 and PRU1 in pru1, wrky6-2, pru1 wrky6, and wild-type plants using RT-qPCR. Data are mean values of three biological replicates ± se.
(B) Phenotype comparisons. All genotypes were germinated and grown on MS medium for 7 d and then transferred to MS or LP medium for another 7 d.
(C) Pi content measurements. Seven-day-old plants were transferred to MS or LP medium and grown for an additional 5 d, and then the shoots and roots were harvested separately for Pi content measurement. Three biological replicates were performed, and a group of 15 seedlings was pooled for each biological sample. Data are mean values of three biological replicates ± se.
(D) Analysis of PHO1 expression by RT-qPCR. Seven-day-old plants and wild-type plants were transferred to LP medium, and then the roots were harvested at the indicated time points for RNA extraction. Data are mean values of three biological replicates ± se. Asterisks in the (C) and (D) indicate significant differences compared with wild-type plants (#) by Student’s t test: *P < 0.05; **P < 0.01.
In agreement with these results, the low-Pi-induced accumulation of PHO1 transcript was repressed in the pru1 mutant, whereas the pru1 wrky6 double mutant had slightly higher PHO1 transcript abundance compared with wild-type plants under Pi-deficient conditions, similar to our observations of the wrky6-2 mutant (Figure 9D). These results indicate that PRU1 modulates Pi transfer by controlling the degradation of WRKY6 under low-Pi stress.
PUR1 Does Not Modulate the Degradation of WRKY42
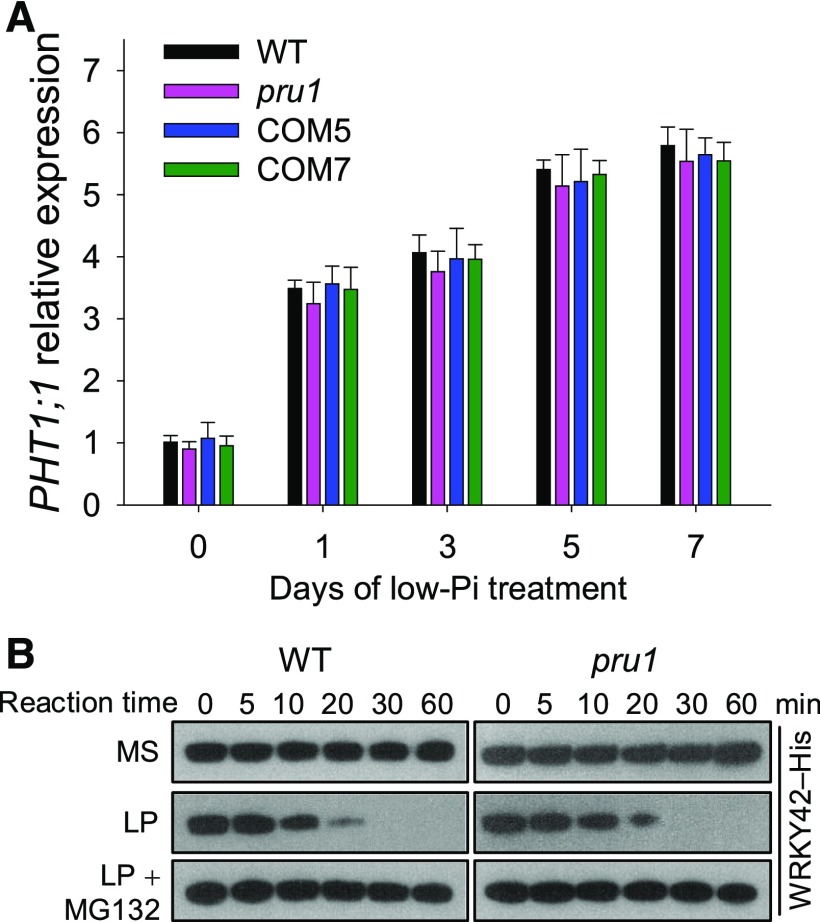
WRKY42, a homolog of WRKY6, is also degraded via the ubiquitin 26S proteasome pathway during Pi starvation (Su et al., 2015). We therefore hypothesized that PRU1 also modulated the degradation of WRKY42. WRKY42 overexpression resulted in a decrease in PHO1 expression and an increase in PHT1;1 expression, which led to an elevated Pi content in the root (Su et al., 2015). The pru1 mutant did not display an increase in Pi content in the root or in PHT1;1 expression compared with wild-type plants under either Pi-sufficient or Pi-deficient conditions (Figures 1D and 10A). The low-Pi-induced degradation of WRKY42 in the pru1 mutant was also similar to that in wild-type plants (Figure 10B). These data demonstrate that the low-Pi-induced degradation of WRKY42 is not modulated by PRU1, but by another E3 ligase.
Figure 10.
Analysis of PHT1;1 Expression and WRKY42 Protein Content in the pru1 Mutant and Wild-Type Plants.
(A) Analysis of PHT1;1 expression in pru1, complementation lines, and wild-type plants during Pi starvation using RT-qPCR. Seven-day-old seedlings were transferred to LP medium, and the roots were harvested at the indicated time points. Data are mean values of three biological replicates ± se (n = 3).
(B) Cell-free degradation of WRKY42 in pru1 and wild-type plants. Seven-day-old seedlings were transferred to MS, LP, or LP+MG132 medium and grown for an additional 3 d. The roots were harvested for a cell-free degradation assay. The root protein extracts were incubated with recombinant WRKY42-His for the indicated periods, and the abundance of WRKY42 was determined by immunoblotting with anti-His antibody.
DISCUSSION
The PRU1/WRKY6/PHO1-Regulatory Pathway Maintains Pi Homeostasis in Plants
Arabidopsis PHO1 plays an important role in Pi transfer from roots to shoots (Poirier et al., 1991; Hamburger et al., 2002), and the transcript abundance of PHO1 increases significantly during Pi starvation (Stefanovic et al., 2007; Ribot et al., 2008; Chen et al., 2009). The transcription factor WRKY6 directly downregulates PHO1 expression by binding to the W-boxes within the PHO1 promoter under Pi-sufficient conditions. During Pi starvation, WRKY6 is degraded by the ubiquitin/26S proteasome pathway (Chen et al., 2009), suggesting that a ubiquitin E3 ligase(s) participates in modulating Pi homeostasis in response to low-Pi stress.
To identify the ubiquitin E3 ligase involved in WRKY6 degradation, we assessed ∼400 T-DNA insertion mutants of putative E3 ligases for enhanced low-Pi sensitivity. The pru1 mutant showed an enhanced low-Pi sensitive phenotype and had reduced Pi levels in the shoot under low-Pi stress, and complementation with PRU1 restored these to wild-type levels (Figure 1), indicating that PRU1 is involved in modulating Pi homeostasis under low-Pi stress. PRU1 targets WRKY6 in vitro and in vivo (Figures 3 and 4). Further biochemical evidence demonstrated that PRU1 mediates the ubiquitination and degradation of WRKY6 under low-Pi stress (Figures 5 and 6). In addition, disruption of WRKY6 rescued the reduced Pi contents in the shoot and the repressed PHO1 transcript abundance of the pru1 mutant during Pi starvation (Figure 9), indicating that WRKY6 was epistatic to PRU1. In conclusion, these data show that the PRU1/WRKY6/PHO1-regulatory pathway plays an important role in the plant’s response to low-Pi stress. Under Pi-sufficient conditions, WRKY6 binds to W-box motifs within the PHO1 promoter to repress PHO1 expression, while under Pi-deficient conditions, PRU1 targets and ubiquitinates WRKY6 for proteasome-dependent degradation.
WRKY6 polyubiquitination was weak in the pru1 mutant when grown on LP+MG132 medium compared with the wild type (Figure 5C), and low-Pi-induced degradation of WRKY6 was still observed in the pru1 mutant (Figure 6C). This suggests that besides PRU1, other E3 ligases also modulate the degradation of WRKY6. The Arabidopsis genome did not contain a close homolog of PRU1; however, three F-box/RNI-like/FBD-like domain-containing proteins, encoded by AT3G58960, AT1G21990, and AT1G48400, were relatively close to PRU1 on the phylogenetic tree (Supplemental Figure 1A). We obtained the T-DNA insertion mutants for AT3G58960, AT1G21990, and AT1G48400 (Supplemental Data Set 1); however, no obvious low-Pi sensitivities were observed, indicating that these three F-box proteins did not play a major role in modulating the degradation of WRKY6.
Although WRKY42 is a close structural homolog of WRKY6, we found that PRU1 interacts with WRKY6, but not WRKY42 (Figures 3B and 4A). Furthermore, PRU1 cannot modulate the degradation of WRKY42 (Figure 10B). A pairwise sequence alignment was conducted between WRKY6 and WRKY42 using the Emboss Needle method (https://www.ebi.ac.uk/Tools/psa/emboss_needle/). The WRKY domain, an ∼60-amino acid region, was highly conserved between WRKY6 and WRKY42 (Supplemental Figure 4). Three constructs were prepared with various WRKY6 fragments: WRKY6N, WRKY6M, and WRKY6C (Figure 3A). The WRKY6M fragment contained the conserved WRKYGQK domain and the CX7CX23HXC zinc binding motif, which are required for proper DNA binding of WRKY proteins. The other two fragments, WRKY6N and WRKY6C, were variable to WRKY42 (Supplemental Figure 4). We found that PRU1 interacts with WRKY6C, but not WRKY6N or WRKY6M (Figures 3 and 4), suggesting that the WRKY6C fragment contains the sequence necessary for interaction with PRU1.
PRU1-Dependent Posttranscriptional Regulation of WRKY6 Depends on Pi Levels
Posttranscriptional regulation is known to play important roles in plant Pi homeostasis under Pi-sufficient conditions. Together, the Arabidopsis E3 ubiquitin ligase NLA and the E2 ubiquitin conjugase PHO2 modulate the ubiquitination and subsequent degradation of Pi transporters under Pi-sufficient conditions (Lin et al., 2013; Park et al., 2014). Under Pi-sufficient conditions, the CK2α3β3 protein kinase phosphorylates the PT8 Pi transporter, which inhibits localization of PT8 to the plasma membrane in rice (Chen et al., 2015). Recently, the SPX proteins AtSPX1/2 and OsSPX1/2 were reported to inhibit the transcription factors AtPHR1 and OsPHR2, respectively, under Pi-sufficient conditions (Puga et al., 2014; Wang et al., 2014).
Contrary to these reports, we found that PRU1 modulated the accumulation of WRKY6 mainly under Pi-deficient conditions. Our previous work showed that the WRKY6 transcript abundance was not very responsive to Pi starvation, whereas the WRKY6 protein level was dramatically reduced during Pi starvation (Chen et al., 2009). This suggests that WRKY6 is primarily modulated at the posttranscriptional level in response to low-Pi stress. Further biochemical and molecular data showed that the PRU1 E3 ubiquitin ligase interacted with WRKY6 (Figures 3 and 4) to modulate its ubiquitination and subsequent degradation under low-Pi stress (Figures 5 and 6). This shows that PRU1 modulates the turnover of WRKY6 under Pi-deficient conditions. Although the pru1 and pho1-2 mutants showed similar low-Pi sensitive phenotypes and reduced Pi contents in the shoot under Pi-deficient conditions, there was an obvious difference in Pi content in the shoot between pru1 and pho1-2 when grown under Pi-sufficient conditions. The pho1-2 mutant was a loss-of-function mutant and therefore displayed reduced Pi levels in the shoot under both Pi-sufficient and Pi-deficient conditions (Figure 1D; Poirier et al., 1991). However, the pru1 mutant only displayed reduced Pi levels in the shoot under Pi-deficient conditions, but not under Pi-sufficient conditions (Figure 1D), suggesting that PRU1 modulated Pi homeostasis primarily during Pi starvation.
Furthermore, when grown under Pi-sufficient conditions, no ubiquitination of WRKY6 was observed in either pru1 mutant or wild-type plants, whereas when grown under Pi-deficient conditions, WRKY6 ubiquitination was observed in wild-type plants, while little to no WRKY6 ubiquitination was observed in the pru1 mutant (Figure 5C). These data show that PRU1-mediated ubiquitination of WRKY6 depends on Pi supply levels. During Pi starvation, WRKY6 was significantly reduced in wild-type plants (Figure 6; Chen et al., 2009), and this degradation of WRKY6 was reduced in the pru1 mutant (Figure 6), indicating that PRU1 modulated the low-Pi-induced degradation of WRKY6. Consequently, the pru1 mutant had a reduced Pi content in the shoot and reduced PHO1 transcript abundance compared with wild-type plants under low-Pi stress, yet there were no obvious differences between the pru1 mutant and wild-type plants when grown on Pi-sufficient conditions (Figure 1). Together, these data demonstrate that the posttranscriptional regulation of WRKY6 by PRU1 depends on Pi levels.
Under Pi-sufficient conditions, the PRU1 was expressed (Figures 2B and 2C), whereas the ubiquitination signal of WRKY6 was not observed (Figure 5C). Also, the ubiquitinated WRKY6 was significantly accumulated in wild-type plants under Pi-deficient conditions (Figure 5C). These data suggest that PRU1 is inactive under Pi-sufficient conditions, and is activated by posttranslational modification during Pi starvation. Several E3 ligases have been reported to be phosphorylated. For instance, the SnRK2.6 kinase phosphorylates the CHY ZINC-FINGER AND RING PROTEIN1 RING-finger E3 ligase (Ding et al., 2015), and the PUB12/13 U-box E3 ligases are phosphorylated by the BRASSINOSTEROID-INSENSITIVE1 kinase (Lu et al., 2011). In humans, the ATAXIA-TELANGIECTASIA MUTATED kinase phosphorylates the MURINE DOUBLE MINUTE2 E3 ligase to inhibit E3 ligase activity (Cheng et al., 2011). By contrast, the activity of the human Cb1-b (CASITAS B-LINEAGE LYMPHOMA-B) E3 ligase is activated by phosphorylation (Kobashigawa et al., 2011). Several amino acid residues of PRU1 were predicated to be phosphorylated, and Thr-504 was shown to be phosphorylated under isoxabene treatment, and Tyr-500 was phosphorylated during nitrate starvation/nitrate resupply (PhosPhAt, http://phosphat.uni-hohenheim.de/phosphat.html?code=AT3G42770.1). We thus hypothesize that PRU1 is phosphorylated under low-Pi stress and that this phosphorylation activates its E3 ligase activity. The mechanism regulating PRU1 activity merits further investigation.
METHODS
Plant Materials and Growth Conditions
The Col-0 ecotype was used as wild-type Arabidopsis thaliana in this study. The T-DNA insertion line Salk_069673C (referred to as the pru1 mutant) was ordered from ABRC (http://www.arabidopsis.org/abrc). The pho1-2 mutant, WRKY6-overexpressing line (35S:WRKY6-9), and wrky6-1 mutant were described previously (Robatzek and Somssich, 2001; Hamburger et al., 2002). The wrky6-2 mutant (Salk_012997) was a T-DNA insertion mutant ordered from ABRC (Huang et al., 2016).
For the complementation lines, a 3599-bp DNA fragment containing the coding region of PRU1 and a 2-kb fragment of a region upstream of the start codon of PRU1 were cloned into the pCAMBIA1300 vector, designated as ProPRU1:PRU1. The ProPRU1:PRU1 construct was introduced into the pru1 mutant by Agrobacterium tumefaciens-mediated transformation (Agrobacterium strain GV3101) using the floral-dip method (Clough and Bent, 1998), and homozygous single-copy lines were obtained.
For plant growth, the Arabidopsis seeds were surface sterilized, treated at 4°C for 72 h, and then germinated and grown on MS medium containing 3% (w/v) sucrose and 0.8% (w/v) agar at 22°C for a 16-h daily light period at 80 μmol m−2 s−1 (Philips; TLD 36W/865 cool daylight), unless otherwise indicated.
The LP (low-Pi) medium used for low-Pi stress treatment was made by modifying MS medium to contain 10 μM Pi and agar instead of agarose (Promega). For sucrose or nitrogen deficiency, −Suc medium was made by preparing MS medium without sucrose, and −N medium was prepared as described previously (Rubio et al., 2001).
Pi Content Measurement
The 7-d-old seedlings were transferred to MS or LP medium for 5 d and then the shoots and roots were harvested. The Pi contents in these samples were quantified as described previously (Su et al., 2015). Each experiment was performed in triplicate, and a group of 15 seedlings was used as one biological sample. Three independent experiments were performed.
Anthocyanin Measurement
The 7-d-old seedlings were transferred to MS or LP medium for 7 d and then harvested for anthocyanin measurement. Anthocyanin contents were determined as described previously (Su et al., 2015). Each experiment was performed in triplicate, and measurements from 15 plants were pooled for one replicate. Three independent experiments were performed.
RT-qPCR
The RT-qPCR assay was conducted as described previously (Huang et al., 2016), using SYBR Green PCR Master Mix (Life Technologies) on a 7500 Real Time PCR System (Applied Biosystems). Relative quantitative results were calculated by normalization to Actin2/8. Each experiment was performed in biological triplicate, and a group of ∼120 seedlings was used as one biological replicate. At least three independent experiments were performed. The primers used are listed in Supplemental Table 1.
RNA Gel Blot Analysis
Seven-day-old wild-type seedlings were transferred to LP medium and then the roots were harvested at the indicated time points for RNA extraction. The RNA gel blot analysis was conducted as described before (Chen et al., 2009). Thirty micrograms of total RNA from roots was loaded per lane and subsequently transferred to a nylon membrane for hybridization. The probes were amplified by PCR using PRU1-specific primers (5′-ATGAATTGTCTTCCAGATGAGC-3′ and 5′-GGATCCCATGACTTGTATAATGA-3′) and ACT7-specific primers (5′-CTCAGCACCTTCCAACAGATGTGGA-3′and 5′- CCAAAAAAATGAACCAAGGACCAAA-3′) as the templates. The probes were labeled with [α-32P]dCTP using random primer labeling reagents. The probes of PRU1 and ACT7 were hybridized to RNA gel blotted onto nylon membrane. The rRNA was used as a loading control.
BiFC Assay
PRU1 and the PRU1 deletion derivative (PRU1ΔF) were cloned into the pSPYNE173 vector (NE) (Waadt and Kudla, 2008), while WRKY6, WRKY6C, and WRKY42 were cloned into the pSPYCEM vector (CE) (Waadt and Kudla, 2008). Each pair of constructs was cotransformed into Nicotiana benthamiana leaves. Five days after infiltration, YFP fluorescence signals were observed using a confocal laser scanning microscope (Leica sp5).
Yeast Two-Hybrid Assay
PRU1 and its deletion derivative (PRU1ΔF) were cloned into the pGADT7 vector (AD), while WRKY6, WRKY6 deletion derivatives, and the ASK genes were cloned into pGBKT7 vector (BD). Each pair of constructs was cotransformed into yeast strain AH109 for the yeast two-hybrid assay. Transformants growing well on SD/-Leu/-Trp medium (−LW) were considered to be positive clones. Interactions between two proteins were determined by growing transformants on SD/-Leu/-Trp/-His/-Ade medium (−LWHA) for 4 d and quantifying β-galactosidase activity. The primers used are listed in Supplemental Table 1.
Protein Expression and Antibody Generation
PRU1 and its deletion derivatives were cloned into the pGEX-4T-1 vector to generate GST fusion proteins. The WRKY42, WRKY6, and WRKY6 deletion derivatives were cloned into the pET30a vector to generate His fusion proteins. The recombinant constructs were transformed into Escherichia coli strain BL21. The E. coli cells were induced with 0.2 mM IPTG overnight at 18°C and collected by centrifugation at 4000 rpm for 15 min at 4°C. The fusion proteins were purified with glutathione-sepharose or Ni-sepharose.
The polyclonal antibody against WRKY6 was generated by inoculating mice.
Immunoblot Analysis
Total proteins were extracted according to Chen et al. (2009), and 100 μg of protein from each sample was separated on a 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride membrane. The abundances of WRKY6 and ACTIN were detected with anti-WRKY6 and anti-actin antibodies, respectively.
Cell-Free Degradation Assay
The cell-free degradation assay was conducted as described previously (Su et al., 2015). To monitor the degradation of recombinant WRKY6 or its deletion derivatives, 250 ng of each purified recombinant protein was incubated in 20 μL of root protein extract (50 μg) at 22°C for the indicated time periods. The abundance of WRKY6 or its deletion derivatives was analyzed by immunoblotting with an anti-His antibody (MBL; D291-3, lot 007).
In Vitro Pull-Down Assay
Purified GST-PRU1 or GST protein was incubated with an equal volume of glutathione-sepharose (GE Healthcare) in binding buffer (200 mM NaCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 1 mM PMSF, and 5 mM DTT) at 4°C for 2 h. The mixture was washed three times with buffer I (200 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, and 1.8 mM KH2PO4) and then aliquoted into five equal parts. Equal amounts of purified WRKY42-His, WRKY6-His, WRKY6N-His, WRKY6M-His, and WRKY6C-His were added to the mixture and incubated for 2 h at 4°C. The mixture was rinsed three times with buffer II (20 mM Tris, pH 8.0, 500 mM NaCl, and 100 mM Imidazole), and the bound proteins were boiled in 1× SDS loading buffer for 5 min and then examined by immunoblotting using an anti-His antibody.
Coimmunoprecipitation Assay
Coimmunoprecipitation assays were conducted as described previously (Feng et al., 2014). The coding sequences of WRKY6 and its deletion derivatives were cloned into the pCAMBIA1307-6myc vector yielding WRKY6-Myc, WRKY6N-Myc, WRKY6M-Myc, and WRKY6C-Myc. The coding sequence of PRU1ΔF was cloned into the pCAMBIA1307-3flag vector, yielding Flag-PRU1ΔF. The Flag-PRU1ΔF vector was cotransformed with WRKY6-Myc, WRKY6N-Myc, WRKY6M-Myc, or WRKY6C-Myc in Arabidopsis mesophyll protoplasts. After a 16-h incubation, total proteins were extracted from the protoplasts. The protoplast protein extract was incubated with anti-Myc agarose (Sigma-Aldrich) at 4°C for 2 h. The input proteins and immunoprecipitates were detected by immunoblotting with either anti-Myc (Sigma-Aldrich) or anti-Flag (MBL) antibodies.
Purification of Ubiquitinated Protein
The 7-d-old pur1 mutant, complementation lines, and wild-type seedlings were transferred to MS medium, LP medium, MS medium with 10 μM MG132 (MS+MG132), or LP medium with 10 μM MG132 (LP+MG132) for 3 d, and roots were harvested for total protein extraction. The ubiquitinated proteins were purified as described previously (Kong et al., 2015). Briefly, 100 mg of root proteins from each genotype that was grown on MS, LP, MS+MG132, or LP+MG132 medium was used in the assay. Total proteins were used as Input (2 mg), and the remaining proteins (98 mg total proteins) were incubated with P62-agarose (Enzo Life Sciences; cat. no. BML-UW9010-0500) to obtain ubiquitinated proteins. Ubiquitinated proteins were separated on a 6% SDS-PAGE gel. The ubiquitinated WRKY6 was detected with an anti-WRKY6 antibody, and total ubiquitinated proteins were detected with an anti-Ub antibody (Santa Cruz Biotechnology; cat. no. sc-8017) in the output. WRKY6 and ACTIN accumulation were measured in the input.
Phylogenetic Tree Construction
Amino acid sequences from the F-box/FBD-like domain-containing genes or Arabidopsis ASK genes were aligned in Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) (Supplemental Files 1 to 3) with default parameters. The neighbor-joining phylogenetic tree was conducted in MEGA 5.2 (Tamura et al., 2011). The tree nodes were evaluated by bootstrap analysis with 1000 replicates and were shown in the phylogenetic trees.
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under the following accession numbers: PRU1 (AT3G42770), WRKY6 (AT1G62300), PHO1 (AT3G23430), WRKY42 (AT4G04450), PHT1;1 (AT5G43350), ACT2 (AT3G18780), ACT8 (AT1G49240), ACT7 (AT5G09810), ASK1 (AT1G75950), ASK2 (AT5G42190), ASK3 (AT2G25700), ASK4 (AT1G20140), ASK5 (AT3G60020), ASK6 (AT3G53060), ASK7 (AT3G21840), ASK8 (AT3G21830), ASK9 (AT3G21850), ASK10 (AT3G21860), ASK11 (AT4G34210), ASK12 (AT4G34470), ASK13 (AT3G60010), ASK14 (AT2G03170), ASK15 (AT3G25650), ASK16 (AT2G03190), ASK17 (AT2G20160), ASK18 (AT1G10230), ASK19 (AT2G03160), ASK20a (AT2G45950.1), ASK20b (AT2G45950.2), ASK21a (AT3G61415.1), and ASK21b (AT3G61415.2).
Supplemental Data
Supplemental Figure 1. Phylogenetic tree of the F-box/FBD-like domain-containing proteins.
Supplemental Figure 2. Phenotype and mutation site of the pho1-2 mutant.
Supplemental Figure 3. Phylogenetic analysis of Arabidopsis ASK proteins.
Supplemental Figure 4. Pairwise sequence alignment of WRKY6 and WRKY42 using the emboss needle method.
Supplemental Table 1. Sequences of primers used in this study.
Supplemental Data Set 1. Analysis of putative e3 mutants.
Supplemental File 1. Alignment used to produce the phylogenetic tree shown in Supplemental Figure 1A.
Supplemental File 2. Alignment used to produce the phylogenetic tree shown in Supplemental Figure 1B.
Supplemental File 3. Alignment used to produce the phylogenetic tree shown in Supplemental Figure 3.
Acknowledgments
We thank Yves Poirier for providing the pho1-2 mutant and Imre E. Somssich for providing the WRKY6-overexpressing line (35S:WRKY6-9) and wrky6-1 mutant. This work was supported by grants from the Ministry of Agriculture of China for transgenic research (No. 2016ZX08009002 to Y.-F.C.), the National Natural Science Foundation of China (No. 31670245 to Y.-F.C. and No. 31421062 to W.-H.W.), and the ‘111’ Project of China (No. B06003 to W.-H.W.).
AUTHOR CONTRIBUTIONS
Y.-F.C. and W.-H.W. designed the research. Q.Y., H.W., and T.S. performed the research. Y.-F.C. and Q.Y. analyzed the data and wrote the article. W.-H.W. revised the article.
References
- Ahuja J.S., Sandhu R., Mainpal R., Lawson C., Henley H., Hunt P.A., Yanowitz J.L., Börner G.V. (2017). Control of meiotic pairing and recombination by chromosomally tethered 26S proteasome. Science 355: 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpat A.B., Magliano P., Wege S., Rouached H., Stefanovic A., Poirier Y. (2012). Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J. 71: 479–491. [DOI] [PubMed] [Google Scholar]
- Aung K., Lin S.I., Wu C.C., Huang Y.T., Su C.L., Chiou T.J. (2006). pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 141: 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C., Sen P., Hofmann K., Ma L., Goebl M., Harper J.W., Elledge S.J. (1996). SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86: 263–274. [DOI] [PubMed] [Google Scholar]
- Bari R., Datt Pant B., Stitt M., Scheible W.R. (2006). PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 141: 988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., et al. (2015). The rice CK2 kinase regulates trafficking of phosphate transporters in response to phosphate levels. Plant Cell 27: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.F., Li L.Q., Xu Q., Kong Y.H., Wang H., Wu W.H. (2009). The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21: 3554–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Cross B., Li B., Chen L., Li Z., Chen J. (2011). Regulation of MDM2 E3 ligase activity by phosphorylation after DNA damage. Mol. Cell. Biol. 31: 4951–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou T.J., Lin S.I. (2011). Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 62: 185–206. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Ding S., Zhang B., Qin F. (2015). Arabidopsis RZFP34/CHYR1, a ubiquitin E3 ligase, regulates stomatal movement and drought tolerance via SnRK2.6-mediated phosphorylation. Plant Cell 27: 3228–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerks T., Copley R.R., Schultz J., Ponting C.P., Bork P. (2002). Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 12: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkhbayar P., Kamiya M., Osaki M., Matsumoto T., Matsushima N. (2004). Structural principles of leucine-rich repeat (LRR) proteins. Proteins 54: 394–403. [DOI] [PubMed] [Google Scholar]
- Eulgem T., Rushton P.J., Robatzek S., Somssich I.E. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5: 199–206. [DOI] [PubMed] [Google Scholar]
- Feng C.Z., Chen Y., Wang C., Kong Y.H., Wu W.H., Chen Y.F. (2014). Arabidopsis RAV1 transcription factor, phosphorylated by SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development. Plant J. 80: 654–668. [DOI] [PubMed] [Google Scholar]
- Hamburger D., Rezzonico E., MacDonald-Comber Petétot J., Somerville C., Poirier Y. (2002). Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14: 889–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.K., et al. (2013). Identification of downstream components of ubiquitin-conjugating enzyme PHOSPHATE2 by quantitative membrane proteomics in Arabidopsis roots. Plant Cell 25: 4044–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Feng C.Z., Ye Q., Wu W.H., Chen Y.F. (2016). Arabidopsis WRKY6 transcription factor acts as a positive regulator of abscisic acid signaling during seed germination and early seedling development. PLoS Genet. 12: e1005833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S., Peng M., Rothstein S.J. (2011). Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genet. 7: e1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobashigawa Y., Tomitaka A., Kumeta H., Noda N.N., Yamaguchi M., Inagaki F. (2011). Autoinhibition and phosphorylation-induced activation mechanisms of human cancer and autoimmune disease-related E3 protein Cbl-b. Proc. Natl. Acad. Sci. USA 108: 20579–20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe B., Kajava A.V. (2001). The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11: 725–732. [DOI] [PubMed] [Google Scholar]
- Kong L., Cheng J., Zhu Y., Ding Y., Meng J., Chen Z., Xie Q., Guo Y., Li J., Yang S., Gong Z. (2015). Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat. Commun. 6: 8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner E., Achard P., Vansiri A., Potuschak T., Genschik P. (2006). F-box proteins everywhere. Curr. Opin. Plant Biol. 9: 631–638. [DOI] [PubMed] [Google Scholar]
- Lin W.Y., Huang T.K., Chiou T.J. (2013). Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 25: 4061–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.Y., Huang T.K., Tseng C.Y., Lai Y.S., Lin S.I., Lin W.Y., Chen J.W., Chiou T.J. (2012). PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24: 2168–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arredondo D.L., Leyva-González M.A., González-Morales S.I., López-Bucio J., Herrera-Estrella L. (2014). Phosphate nutrition: improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 65: 95–123. [DOI] [PubMed] [Google Scholar]
- Lu D., Lin W., Gao X., Wu S., Cheng C., Avila J., Heese A., Devarenne T.P., He P., Shan L. (2011). Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332: 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. (2012). Marschner’s Mineral Nutrition of Higher Plants. (London: Academic Press; ). [Google Scholar]
- Miura K., Rus A., Sharkhuu A., Yokoi S., Karthikeyan A.S., Raghothama K.G., Baek D., Koo Y.D., Jin J.B., Bressan R.A., Yun D.J., Hasegawa P.M. (2005). The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc. Natl. Acad. Sci. USA 102: 7760–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge S.R., Rae A.L., Diatloff E., Smith F.W. (2002). Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 31: 341–353. [DOI] [PubMed] [Google Scholar]
- Okumura S., Mitsukawa N., Shirano Y., Shibata D. (1998). Phosphate transporter gene family of Arabidopsis thaliana. DNA Res. 5: 261–269. [DOI] [PubMed] [Google Scholar]
- Park B.S., Seo J.S., Chua N.H. (2014). NITROGEN LIMITATION ADAPTATION recruits PHOSPHATE2 to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell 26: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y., Thoma S., Somerville C., Schiefelbein J. (1991). Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 97: 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga M.I., et al. (2014). SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 111: 14947–14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama K.G. (1999). Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 665–693. [DOI] [PubMed] [Google Scholar]
- Ribot C., Wang Y., Poirier Y. (2008). Expression analyses of three members of the AtPHO1 family reveal differential interactions between signaling pathways involved in phosphate deficiency and the responses to auxin, cytokinin, and abscisic acid. Planta 227: 1025–1036. [DOI] [PubMed] [Google Scholar]
- Risseeuw E.P., Daskalchuk T.E., Banks T.W., Liu E., Cotelesage J., Hellmann H., Estelle M., Somers D.E., Crosby W.L. (2003). Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 34: 753–767. [DOI] [PubMed] [Google Scholar]
- Robatzek S., Somssich I.E. (2001). A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 28: 123–133. [DOI] [PubMed] [Google Scholar]
- Rouached H., Stefanovic A., Secco D., Bulak Arpat A., Gout E., Bligny R., Poirier Y. (2011). Uncoupling phosphate deficiency from its major effects on growth and transcriptome via PHO1 expression in Arabidopsis. Plant J. 65: 557–570. [DOI] [PubMed] [Google Scholar]
- Rubio V., Linhares F., Solano R., Martín A.C., Iglesias J., Leyva A., Paz-Ares J. (2001). A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 15: 2122–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandom A., Bailey M., Ewan R., Lee J., Nelis S. (2012). The ubiquitin-proteasome system: central modifier of plant signalling. New Phytol. 196: 13–28. [DOI] [PubMed] [Google Scholar]
- Shin H., Shin H.S., Dewbre G.R., Harrison M.J. (2004). Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 39: 629–642. [DOI] [PubMed] [Google Scholar]
- Smalle J., Vierstra R.D. (2004). The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 55: 555–590. [DOI] [PubMed] [Google Scholar]
- Stefanovic A., Ribot C., Rouached H., Wang Y., Chong J., Belbahri L., Delessert S., Poirier Y. (2007). Members of the PHO1 gene family show limited functional redundancy in phosphate transfer to the shoot, and are regulated by phosphate deficiency via distinct pathways. Plant J. 50: 982–994. [DOI] [PubMed] [Google Scholar]
- Su T., Xu Q., Zhang F.C., Chen Y., Li L.Q., Wu W.H., Chen Y.F. (2015). WRKY42 modulates phosphate homeostasis through regulating phosphate translocation and acquisition in Arabidopsis. Plant Physiol. 167: 1579–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R., Kudla J. (2008). In planta visualization of protein interactions using bimolecular fluorescence complementation (BiFC). Cold Spring Harb. Protoc. 2008: pdb.prot4995. [DOI] [PubMed] [Google Scholar]
- Wang Z., et al. (2014). Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc. Natl. Acad. Sci. USA 111: 14953–14958. [DOI] [PMC free article] [PubMed] [Google Scholar]