Summary
Stroke is one of the leading causes of death and disability worldwide. The long‐standing dogma that stroke is exclusively a vascular disease has been questioned by extensive clinical findings of immune factors that are associated mostly with inflammation after stroke. These have been confirmed in preclinical studies using experimental animal models. It is now accepted that inflammation and immune mediators are critical in acute and long‐term neuronal tissue damage and healing following thrombotic and ischaemic stroke. Despite mounting information delineating the role of the immune system in stroke, the mechanisms of how inflammatory cells and their mediators are involved in stroke‐induced neuroinflammation are still not fully understood. Currently, there is no available treatment for targeting the acute immune response that develops in the brain during cerebral ischaemia. No new treatment has been introduced to stroke therapy since the discovery of tissue plasminogen activator therapy in 1996. Here, we review current knowledge of the immunity of stroke and identify critical gaps that hinder current therapies. We will discuss advances in the understanding of the complex innate and adaptive immune responses in stroke; mechanisms of immune cell‐mediated and factor‐mediated vascular and tissue injury; immunity‐induced tissue repair; and the importance of modulating immunity in stroke.
Keywords: cytokines, inflammation, neuroinflammation
Abbreviations
- BBB
blood–brain barrier
- CCL
CC‐chemokine ligand
- CCR
CC‐chemokine receptor
- CD
cluster of differentiation
- CNS
central nervous system
- CXCL
CXC‐chemokine ligand
- CXCR
CXC‐chemokine receptor
- DAMP
damage‐associated molecular pattern
- DC
dendritic cell
- ICA
internal carotid artery
- IFN
interferon
- IL
interleukin
- MCP‐1
monocyte chemoattractant protein 1
- MMP
matrix metalloproteinase
- PBS
phosphate‐buffered saline
- pMCAO
permanent middle cerebral artery occlusion
- PRX
peroxiredoxin
- tMCAO
transient middle cerebral artery occlusion
- TNF‐α
tumour necrosis factor α
- Treg
regulatory T
Introduction
Stroke was first described by Hippocrates as a disease of ‘struck down by violence’ or apoplexy (Greek) over 2400 years ago. Later studies in the mid‐1600s identified apoplexy as a vascular disease associated with bleeding in the brain and blockage of main blood vessels. Until the pioneering work of John M. Hallenbeck,1, 2, 3, 4 it was accepted that stroke was a vascular and thrombotic disease. Currently, however, the role of the immune system in stroke‐induced injuries and tissue regeneration processes is more appreciated.
Experimental studies in animal models have revealed that innate and adaptive cellular immune responses following ischaemic stroke occur over a time course spanning from minutes to weeks or even months after the injury.5 Whether these immune responses are beneficial or detrimental to tissue damage and healing is still a controversial question. Supporting the detrimental role for the early inflammatory immune cell responses, it has been shown that immune‐deficient nude rats have reduced infarct volume after transient middle cerebral artery occlusion (tMCAO) compared with those of the control Sprague Dawley strain.6 However, different experimental stroke models might have different outcomes. It has been shown that, in contrast to the previous observations, infarct volumes in immune‐deficient rats are similar to controls following permanent middle cerebral artery occlusion (pMCAO).
Here, we will address the complex innate and adaptive immune responses involved in influencing ischaemic stroke pathology. We will describe the different types of innate myeloid cells that are involved during ischaemic stroke and the challenges in differentiating brain‐resident and peripherally derived cells. Furthermore, we will highlight the diversity of lymphocytic phenotype, the myriad of factors that they can produce, and how antigen specificity can influence their impact on ischaemic stroke pathology. Overall, we aim to shed light on the molecular mechanisms of immune‐induced tissue injury and functional repair following ischaemic stroke with the hope that this information might lead to beneficial immunomodulatory treatments in stroke.
Innate immune responses to ischaemic injuries within the central nervous tissue: microglia provide the first level of defence within the ischaemic brain
Microglia are a type of resident myeloid cell in the central nervous system (CNS) derived from yolk‐sac progenitors.7 After neurodevelopment, microglial cells serve as the regulators of homeostasis in the CNS and are thought to play a variety of roles in neuronal injury and survival.8 Besides the presence of some brain‐resident macrophages, microglia are the majority of myeloid cells in the CNS during steady‐state conditions.8, 9 In response to disease or injury, other myeloid cells such as bone‐marrow‐derived monocytes are recruited to the CNS from the periphery and may exhibit similar morphology and expression patterns to microglia.10, 11 This has made it difficult to distinguish resident microglia from other myeloid cells and to delineate the specific roles of microglia in CNS disease and injury. Current methods of microglial identification rely on cell surface marker expression, morphological distinctions or bone marrow transplantation. The combination of cell surface markers and novel technologies such as two‐photon imaging, cytometry by time‐of‐flight (CyTOF; Fluidigm, San Francisco, CA), cytometry, and whole‐genome transcriptomic and epigenomic analysis using bioinformatics has led to a better understanding of microglia functions during stroke. Analysis of cell surface marker expression of CD45 intermediate cells (CD45int) by flow cytometry or morphological distinctions with immunohistochemistry are frequently used to identify microglia populations.12 CD45, also known as protein tyrosine phosphatase receptor type C, is an enzyme that is a member of the protein tyrosine phosphatases family. Due to the existence of various isoforms of CD45, the application of correct anti‐CD45 antibody is crucial in the characterization of these cells. Another challenge is that CD45 expression and cell morphology can change during pathological states, indicating that these methods may not be reliable markers for microglia detection. The use of bone marrow chimeric mouse models is problematic as well. For this method, mice undergo irradiation to eliminate resident bone‐marrow‐derived cells and are then injected with fluorescence‐labelled bone marrow from a donor mouse. This allows for distinction between host‐derived microglia and donor bone‐marrow‐derived myeloid cells.13 However, irradiation therapy prompts inflammatory responses14, 15 and may obscure the ability to define specific microglial roles in stroke. Recently, the discovery of new microglia‐specific markers, such as transmembrane protein 119 (Tmem119), has helped to distinguish microglia contributions from those of other myeloid cell populations.10
The primary inflammatory responses by microglia are to clear up debris and repair injured tissue. However, they may also promote secondary inflammation‐associated damage as multiple studies have indicated that microglia serve as pro‐inflammatory responders and increase damage following stroke.16, 17, 18, 19 Specifically, evidence suggests that microglia contribute to increased neurovascular breakdown and permeability by prompting activation of platelet‐derived factors,17 and up‐regulating expression of matrix metalloproteinases (MMPs) such as MMP‐9,18 during the early stages of stroke. Additionally, reactive microglia are substantial producers of pro‐inflammatory mediators such as tumour necrosis factor‐α (TNF‐α), interleukin‐1β (IL‐1β), reactive oxygen species and inducible nitric oxide synthase following stroke.16, 19 The CR3 complement receptor, CD11b/CD18 is also frequently used as a microglia marker. The morphology of CD11b+ CD45int microglia following stroke is highly dynamic and can produce varying levels of TNF‐α and IL‐1β in a time‐dependent fashion (Fig. 1). These inflammatory factors are thought to contribute to post‐ischaemic neuronal damage and apoptosis, so worsening the outcome after stroke.16 Furthermore, genetic or pharmacological inhibition of certain microglial functions, such as proliferation and release of cytokines, may result in dampened inflammatory responses and neuronal damage after ischaemic insult.20, 21, 22, 23 Importantly, factors such as Fas ligand released by ischaemic neurons may induce a specific pro‐inflammatory phenotype in microglia.24 A recent study by Meng et al. suggests that neuronal soluble Fas ligand promotes microglial polarization to the ‘M1’ classical activation phenotype after cerebral ischaemia; and that ‘M1‐microglia’ release pro‐inflammatory factors leading to reduced cell viability and survival.24
Figure 1.
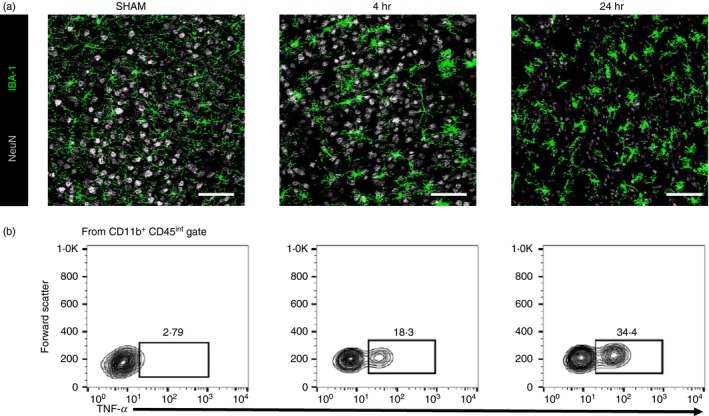
Myeloid cells damage neurons following brain ischaemia. (a) Representative immunohistological staining of myeloid cells (IBA‐1) and neurons (NeuN) in the ipsilateral transient middle cerebral artery occlusion (tMCAO) brain in SHAM mice (left), at 4 hr tMCAO (middle) and 24 hr tMCAO (right). Scale bar = 20 μm. (b) Representative flow cytometry staining of microglia (CD11b+ CD45int) and their relative expression of tumour necrosis factor‐α (TNF‐α) in the ipsilateral tMCAO brain in SHAM mice (left), at 4 hr tMCAO (middle) and 24 hr tMCAO (right).
Findings from several studies already suggest a potential anti‐inflammatory role of microglial cells that may contribute to neuroprotection after stroke. Microglial expression of P2X7 receptor,25 triggering receptors expressed on myeloid cells (TREM2),26 ST2/IL‐33,27 and B‐cell activating factor28 could be involved in limiting ischaemic neuronal injury. Activation of these microglial‐dependent signalling factors results in the release of anti‐inflammatory cytokines, such as IL‐10, IL‐4 and transforming growth factor‐β, which may contribute to neuronal survival.26, 27 Microglial secretion of these neuroprotective factors, as well as phagocytic activity, are characteristic of the protective ‘M2’ phenotype of myeloid cells.29 Notably, polarization of microglia to an ‘M2’ state may be driven by signals such as IL‐10 and IL‐4 from damaged neurons and regulatory B cells after stroke.28, 29 Accordingly, current research focuses on the discovery of methods to induce a switch from pro‐inflammatory to protective phenotype in myeloid cells.29, 30, 31 In fact, a study by Narantuya et al. suggests that microglial transplant enhances neuroprotection through production and release of neurotrophic factors and M2‐related anti‐inflammatory cytokines from these donor microglia.31 Cautionary concerns were also raised regarding microglia polarization and characterization into M1 or M2 phenotypes.32 It appears that microglia phenotype after stroke is dynamic and influenced by the local environment. Although it was found that MI/M2 polarization is concurrent following traumatic brain injury,33 our understanding regarding dynamic microglia activation is still limited. A deeper understanding of complex microglia responses to stroke would be critical for developing novel therapies.
Although resident microglia are an attractive target for stroke treatment, null findings from studies suggest that microglia may not be directly involved in worsening the outcome after stroke.34, 35 For example, a study by Harrison et al. reports that blocking the major signalling pathway of fractalkine‐CX3CR1 between neurons and microglia36 does not directly contribute to ischaemic damage or protection. Conflicting results from other studies on microglia‐specific deficiency of CX3CR1 further complicate our current understanding of microglial roles in cerebral ischaemia.30, 37 Importantly, Tang et al. indicate that infiltrating monocytes/macrophages also express CX3CR1 and may be a major source of neuroinflammation and secondary damage following ischaemia. This could account for the contrasting findings, and further emphasizes the importance of distinguishing myeloid cell populations. In summary, the knowledge of specific roles of microglia following cerebral ischaemia is currently incomplete and requires more study. The ability to distinguish between resident microglia from infiltrating monocytes will be crucial in research that aims to target myeloid cells as a therapeutic strategy against stroke.
Myeloid cells from the periphery respond to stress signals released from the ischaemic brain
Early responses against danger‐associated molecular patterns following ischaemic injuries in the CNS are mediated with the remarkably diverse groups of innate immune cells developed within the myeloid cell pathway. The differentiation of these cells is controlled by transcription factors, transcriptional co‐regulators and post‐transcriptional mechanisms, which have been recently reviewed by others.38 These ‘early’ cells rush to the danger site to restore homeostasis and assist in repair. Along with these desirable functions, there are also damage‐associated molecular patterns (DAMPs) ‐induced cellular activities that are detrimental to the tissue. Nonetheless, our understanding about the contribution of innate myeloid cells to beneficial and detrimental responses within the CNS following ischaemic stroke is still limited.
How innate myeloid cells are activated by DAMPs through pattern recognition receptors is a subject of intense study.39, 40 These receptors not only recognize pathogen‐derived molecules, but also endogenous molecules that are released in stressed tissues. It is well known that the release of DAMPs from dead cells in ischaemic tissues induces sterile inflammation that contributes to pathogenesis.41 There are several well‐characterized DAMPS in the CNS such as high mobility group box 1 (HMGB1), the S100A8 and S100A9 (S100A8/A9) proteins, and the peroxiredoxin (PRX) family proteins. One of the earliest DAMPs after stroke is the HMGB1,42, 43 which contributes to the damage of the blood–brain barrier (BBB).44, 45 The S199A8/A9 proteins can be produced by infiltrating cells in the brain46 and contribute to inflammation post‐injury.47, 48 Finally, the role of PRX family members is more controversial, but they are known to contribute to numerous tissue injuries.49, 50, 51, 52, 53 Dying cells release PRX, inducing immune cell activation through Toll‐like receptor 2 and Toll‐like receptor 4 receptors. This leads to the production of pro‐inflammatory mediators including IL‐1β and IL‐23. Interestingly, PRXs can also contribute to neuroprotection following ischaemia.54 More recently, it was shown that clearance of DAMPs by mononuclear phagocytes through Mrs1, Marco and Mafb gene‐regulated mechanisms contributes to tissue protection following stroke.55 This supports that acute tissue damage following stroke triggered by DAMPs exacerbates tissue injury and contributes to the induction of T‐cell‐mediated inflammation.53, 56, 57 The impact of ischaemic neurons on local and peripheral immune cells is summarized in Fig. 2.
Figure 2.
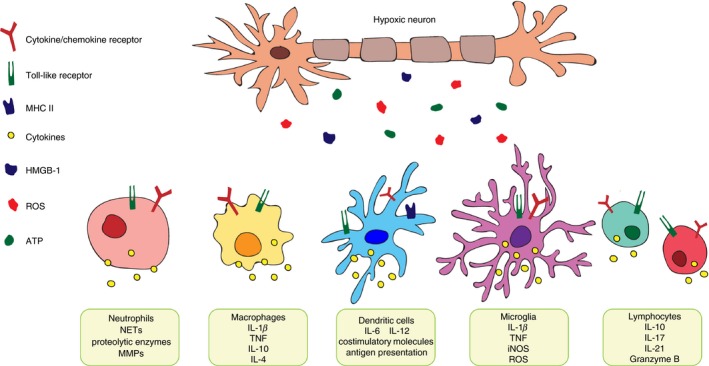
Influence of hypoxic neurons on immune cells. Following brain hypoxia, ischaemic neurons release damage associated molecular patterns such as reactive oxygen species (ROS), ATP, and high mobility group box 1 (HMGB1). This effect can influence local microglia and infiltrating leucocytes to produce inflammatory cytokines to further influence stroke pathology.
There are multiple subtypes of innate myeloid cells including circulating monocytes, tissue macrophages, dendritic cells (DCs), circulating granulocytes (neutrophils, basophils and eosinophils) and mast cells. Circulating granulocytes, specifically neutrophils, are considered as the first infiltrating immune cell type within the stroke‐affected tissues. However, the contribution of these cells to tissue damage or regeneration after ischaemic injuries of the CNS is still controversial. The essential immune function of neutrophils in protection against pathogens and their short lifespan demand their constant production and release from the bone marrow. In humans, there are about 1011 neutrophils produced daily in a process known as granulopoiesis. This process is highly regulated during conditions of tissue injury and stress.58, 59 Granulocyte colony‐stimulating factor has been shown to be the major regulator of neutrophil trafficking from the bone marrow to the blood.60 In addition, controlled neutrophil mobilization has been shown from the bone marrow to the blood through induction of CXCR2 ligands on these cells.61 Anti‐neutrophil antibodies block the efflux of neutrophils from the bone marrow and inhibit their infiltration into the stroke tissues, which has been shown to be protective in a rat model of hypoxic injury.62
Due to early neutrophil infiltration into ischaemic brain tissues, neutrophils were considered as cargo vehicles to deliver macromolecular drugs to block reactive oxygen species‐mediated apoptosis following stroke.63 Clinical studies demonstrated that the neutrophil‐to‐lymphocyte ratio might be a strong prognostic marker in acute ischaemic stroke64 and that neutrophil extracellular traps are increased in patients with stroke.65 Neutrophil extracellular traps are produced by activated myeloid cells, including neutrophils, as networks of DNA, histones and proteolytic enzymes, and are capable of activating platelets and contribute to the thrombotic processes.66 Taken together, these data indicate that early neutrophil infiltration is detrimental to ischaemic tissue injury following stroke and call for novel therapies to target neutrophil infiltration.
Peripheral monocytes can be recruited to the ischaemic brain within hours after insult as the BBB becomes compromised. As mentioned previously, there is some difficulty in identifying and differentiating monocytes from local microglia based on their similar morphology and phenotypes. However, transplantation techniques and assessment of relative expression of various surface markers have allowed researchers to distinguish peripherally derived myeloid cells from microglia with more confidence. These studies have identified that the two cell types are functionally very different. Further discussion of this subject will continue in the next section. One model to differentiate the two cell types uses the CX3CR1GFP/+ CCR2RFP/+ bone marrow chimeric mice. These studies have indicated that peripheral CCR2+ Ly6Chigh monocytes infiltrate the ischaemic brain to potentiate stroke damage.67 Interestingly, there is evidence that these cells could differentiate into microglia‐like CX3CR1− Ly6Clo cells that participate in the post‐stroke repair process.13, 68
One of the major cytokines involved in monocyte recruitment to the ischaemic brain is monocyte chemoattractant protein 1 (MCP‐1), also known as CCL2. Several groups have characterized the expression of MCP‐1 in the stroke brain identifying it in the cortex and mainly expressed on brain endothelial cells or astrocytes.69, 70, 71 Evidence suggests that MCP‐1‐deficient mice are protected from stroke following pMCAO due to the limited amount of recruited monocytes in the stroke brain as well as the lower levels of IL‐1β production compared with littermate mice.72 Interestingly, in CCR2‐deficient (the cognate receptor for MCP‐1) mice that have reduced numbers of infiltrating macrophages in the stroke brain, Schilling et al. was not able to detect a difference in infarct volume compared with littermate controls. This suggests that the receptor may have conflicting effects depending on whether it is expressed on microglia or macrophages.73 Aside from MCP‐1, macrophage inflammatory protein 3α, also known as CCL20, has been suggested to be involved in pro‐inflammatory macrophage recruitment to the ischaemic brain and subsequent TNF‐α and IL‐1β production.74, 75, 76
There is also evidence that macrophages can be anti‐inflammatory following stroke. Chu et al. observed that Ly6Chigh monocytes can exert a protective effect following tMCAO by polarizing to an M2 phenotype based on local damage signals.77 Furthermore, Liu et al. showed that IL‐4 is important for M2 phenotype induction and suggested IL‐4 therapy as a potential approach for long‐term functional recovery after stroke.78 In addition, Korhonen et al. indicated that IL‐33 administration could steer local macrophages to produce T helper type 2 cytokines such as IL‐4, and induce stroke recovery.79 Lastly, it was also shown that macrophages could produce growth factors such as basic fibroblast growth factor, glial cell‐derived neurotrophic factor, insulin growth factor 1, and vascular endothelial growth factor to promote functional recovery following stroke.80
Dendritic cells are the bridge to adaptive immunity following ischaemic stroke
Dendritic cells are an adaptive immune cell recognized for their antigen‐presentation capability and importance in facilitating a T‐cell response during disease. Multiple groups have shown that following acute cerebral injury in both rats and mice, there is an increase in the number of infiltrating DCs into the CNS.81, 82 In the murine stroke models, DCs are visualized in both the core infarct region as well as the border region. Interestingly, bone marrow transplantation experiments have helped to identify how DCs originate, giving us a clue as to how they function. DCs from the periphery mainly reside in the core infarct area whereas resident DCs lie in the border region.81 In addition, these dendritic cells have an increased expression in major histocompatibility complex II and co‐stimulatory molecule CD80, suggesting that there is important T‐cell interaction occurring in these areas.81 Moreover, pMCAO in rats presents similar results. An increase in DC number is observed as early as 1 hr following pMCAO, remains elevated for 24 hr, and increases over the next 6 days. To assess the functionality of these DCs, in situ hybridization has been performed to detect cytokine mRNA expression in the infiltrating DCs. At 1 hr following pMCAO, the mRNA levels of IL‐1β, IL‐12, IL‐6, IL‐10, TNF‐α and interferon‐γ (IFN‐γ) in DCs were increased compared with DCs in both sham controls and in the non‐ischaemic hemisphere.82 The positive correlation of the number of DCs and their cytokine‐producing potential between the infarct volume suggests a damaging role for DCs following stroke in rodents.
In humans, however, the role of DCs in ischaemic brain injuries is not so well‐described. One group found that following stroke in human patients, the relative and absolute number of DC precursors in circulation is decreased compared with healthy controls. They show that this effect only lasts for a few days, with the number of DC precursors soon returning to normal. Interestingly, patients with the lowest levels of DC precursors in circulation had larger infarct sizes assessed through computed tomography scan. To further investigate this, post‐mortem brain tissue was analysed for the presence of DCs and T cells. Patients with larger infarct sizes had more DC–T‐cell clusters compared with patients with smaller infarcts, and they were located near intracerebral vessels. These data suggest that following cerebral injury, DC precursors quickly leave the circulation and infiltrate the CNS to interact with other infiltrating immune cells.83
Many groups have shown that DCs infiltrate the CNS following stroke in both rodents and humans. Recent work has exploited these findings to develop new therapies for stroke. By deriving DCs ex vivo and engineering them to express specific proteins of interest, it allows for a novel way to administer pharmaceuticals following stroke.84 Works et al. expanded on these findings to derive DCs to over‐express soluble TNF receptor 1, which ultimately blocks TNF‐α bioavailability. They injected these DCs into rats who had undergone tMCAO 6 hr earlier and saw reduced infarct volume compared with rats who received DCs expressing GFP lentivirus.85 Using the quick infiltration of DCs and the prolonged time they reside in the CNS may provide a unique and novel approach to drug delivery in human stroke patients.
Lymphocytes encompass adaptive immune‐mediated responses following ischaemic stroke
Recently, T lymphocytes have been identified to play a major role in acute ischaemic stroke pathology. Within hours after tMCAO there is an influx of T lymphocytes, which aggregate around the border of the infarcted region. Particularly, cytotoxic CD8+ lymphocytes have been indicated to be recruited into the stroke brain as early as 3 hr following stroke while CD4+ T cells and natural killer cells are recruited within 24 hr and peak at 72 hr post‐reperfusion.5, 86 In order to further characterize the timeline of T‐cell infiltration to the stroke brain, novel techniques such as multiphoton laser scanning microscopy have been applied to monitor immune responses in real time ex vivo.87, 88, 89 The first study by Stoll's group described the spatial and temporal infiltration of T cells following MCAO.90 Since these studies, there is strong support for the role of immune cells in promoting inflammation that cause secondary tissue injury in the brain following stroke.91 T‐cell infiltration is becoming increasingly acknowledged as a key mediator of the acute phase of stroke pathology in both human disease and rodent models.92 It was shown that in murine models of both permanent and temporary cerebral ischaemia, T‐cell populations can be found throughout the parenchyma as early as 3 hr post infarction and lasting up to 5 days.5, 92, 93 Following ischaemic events, CD4+ T‐cell populations have two spikes in infiltration: following the first 3 hr and again after 24 hr.
Yilmaz et al., provided a comprehensive picture of the important role played by T lymphocytes in cerebral ischaemia. Using T‐cell‐deficient mice, they evaluated infarct volume and neurological recovery following 60 min of tMCAO and observed reduced infarct size, indicating a novel role of T cells in brain injury and the neurological deficit seen with stroke.94 In addition, Hurn et al. were able to recapitulate that data in 90‐min tMCAO mice showing that both T and B cells have a role in the early damage within 24 hr of reperfusion.95 In one of the first papers to inhibit T‐cell migration to the brain during the acute phase of stroke, Liesz et al. administered very late antigen‐4 and its counterpart vascular cell adhesion molecule‐1 via monoclonal antibodies, which improved the outcome of stroke lesions by inhibiting lymphocyte invasion.96 Moreover, recombinant T‐cell receptor ligands and lymphocyte egress inhibitors such as fingolimoid have also been successful in murine models of stroke.97, 98, 99
Given the heterogeneity of T‐cell phenotype, over the past 10 years researchers have been trying to elucidate the T‐cell‐specific factors responsible for contributing to stroke pathology. Shichita et al. showed that γδ T cells, but not conventional CD4+ T cells, can produce IL‐17 following tMCAO, which contributes to ischaemic brain injury in an IL‐23‐dependent manner.57 To clarify the mechanism for γδ T‐cell‐produced‐IL‐17 contribution to stroke damage, Zhang et al. described that IL‐17 causes reperfusion damage through the Calpain‐transient reporter potential canonical (subtype) 6 pathway.100 Following this, Arunachalam et al. clarified that the migration of these IL‐17‐producing cells to the stroke brain happens via CCR6 signalling as the ischaemic brains of CCR6‐deficient mice are mostly devoid of IL‐17.101
In their initial evaluation of lymphocyte influence to stroke damage, Yilmaz et al. also revealed the importance of IFN‐γ, as splenocytes from IFN‐γ‐deficient mice did not confer the same levels of damage as splenocytes from wild‐type mice when transferred into Rag1−/− mice following tMCAO.94 More recently. Seifert et al. showed that administration of IFN‐γ‐neutralizing antibodies could block interferon‐inducible protein 10 and subsequent brain damage and neurodegeneration following MCAO.102 Another T‐cell‐derived cytokine that has been implicated in stroke damage is IL‐21. We have shown that CD4+ T‐cell‐derived IL‐21 is a major contributor of reperfusion damage following tMCAO as CD4+ T cells from IL‐21‐deficient mice do not cause the same levels of ischaemic damage compared with CD4+ T cells from wild‐type mice when transferred intravenously into T‐cell‐deficient mice.103 Other cytokines such as IL‐1β, TNF‐α, and particularly IL‐23 have also been shown to influence T cells following stroke indirectly.104, 105
Recently, there has been a lot of excitement for the potential role of T regulatory (Treg) cells for protection against stroke damage. Liesz et al. showed that transfer of Foxp3+ T cells into T‐cell‐deficient mice was protective and that Treg cells were present surrounding ischaemic brain areas at 7, 14 and 30 days following 30‐min tMCAO.106 Furthermore, Brea et al. administered a CD28 superagonist to target Treg proliferation, which reduced stroke‐induced brain damage.107 The migration of Treg cells during stroke pathology has been clarified by Li et al., who suggest that CCR5‐mediated recruitment of Treg cells is crucial to protect against blood–brain barrier disruption following stroke.108 Lee et al. also suggested that CXCL14 can promote Treg differentiation and activation.109 In contrast, Kleinshnitz et al. surprisingly showed that depletion of Treg cells using the DEREG mouse model reduces brain infarct size, suggesting that Treg cells can further brain damage by inducing BBB dysfunction.110 Currently, the Treg cell contribution to ischaemic brain damage is still a highly debated topic.111, 112
As lymphocytes are typically associated with adaptive immunity and antigen‐specific immune responses, their dependency on specific antigens for contributing to stroke pathology has recently been evaluated. It was shown that adoptive transfer of myelin basic protein‐tolerized splenocytes contribute to MCAO damage in rats.113 This study concluded that immunological tolerance towards myelin basic protein could be transferred and induce neuroprotective effects potentially in a transforming growth factor‐β 1‐dependent fashion. In contrast, however, if rats are immunized with myelin basic protein and or peptides, they mainly induce T helper type 1 responses, resulting in worse stroke outcome.114 The role of another myelin peptide‐induced immunity, myelin oligodendrocyte glycoprotein (MOG)35–55 has also been studied by several groups in the context of stroke. It was shown that mucosal induced tolerance by MOG35–55 reduced infarct size by almost 70% following MCAO in an IL‐10‐dependent manner.115 Conversely, Ren et al. demonstrated that MOG‐specific splenocytes transferred into SCID mice can exacerbate infarct volume and associated neurological deficits, suggesting that antigen‐specific T cells need to be tolerized to be beneficial.116 More recently, Wang et al. evaluated the effect of administering DRα1‐MOG35–55, a compound that can inhibit neuroantigen‐specific T cells and block the binding of chemokine‐mediated migration. This study concluded that inhibiting MOG35–55‐specific T cells reduces brain damage following distal MCAO by shifting myeloid cells from a pro‐inflammatory to an anti‐inflammatory phenotype.117
The contribution to cytotoxic CD8+ lymphocytes has also been assessed with regards to stroke pathology. In a clinical study, Schwab et al. identified the presence of CD8+ T cells around infarcted lesions in patients with focal cerebral infarctions.118 Furthermore, in aged mice inflicted with MCAO, a population of memory CD8 T cells were identified that contribute to injury by influencing microglia homeostasis.16 CD8+ T‐cell‐released factors have also been identified to contribute to stroke injury as cytotoxic protease granzyme‐B has been identified to up‐regulate pro‐apoptotic proteins in neurons following tMCAO119 and is also present in human stroke lesions.120 Furthermore, cytotoxic lymphocyte‐produced perforin has also been identified to contribute to stroke damage in an antigen‐dependent manner.121 On the other hand, an anti‐inflammatory population of IL‐10‐producing CD8+ CD122+ T cells was identified following MCAO in the ischaemic brain.122
Several groups have demonstrated that administration of B cells could be protective following stroke. Administration of B cells directly into striatum reduced infarct volume in tMCAO mice 48 hr after reperfusion.123, 124 The protective role of these B cells was later found to be dependent on IL‐10 by the same group.123 Furthermore, Monson et al. showed that generation of these protective B cells could be induced by hypoxic preconditioning and they are potentially recruited to the ischaemic brain by CXCL13.125 Nonetheless, the role of B cells in contributing to stroke injury is somewhat controversial as their phenotype can be heterogeneous126 and it has been suggested that they have a minor, if any, role in contributing to ischaemic stroke during the acute phase.127 Interestingly however, others have suggested that B cells have a role in mediating cognitive impairment in the weeks after stroke by producing harmful autoantibodies that inhibit long‐term potentiation.128 These studies highlight the diversity of lymphocyte contribution to ischaemic stroke pathology. The influence of lymphocytes on ischaemic pathology is summarized in Table 1.
Table 1.
Influence of T cells on the ischaemic brain in murine models of stroke
| Cell type | Cytokine released | Mouse model | Effect on stroke volume | Reference |
|---|---|---|---|---|
| γδ T cell | IL‐17a | tMCAO | Increase | Shichita et al.57 |
| CD4 T cell | IL‐17a | tMCAO | Increase | Zhang et al.18 |
| CD4 T cell | IL‐17a | tMCAO | Increase | |
| Treg | N/A | tMCAO | Decrease | |
| Treg | IL‐10 | tMCAO | Decrease | Liesz et al.106 |
| Treg | N/A | tMCAO | Increase | Kleinschnitz et al.111 |
| CD4 T cell | IFN‐γ | tMCAO | Increase | Yilmaz et al.94 |
| CD4 T cell | IFN‐γ | pMCAO | Increase | Seifert et al.102 |
| CD4 T cell | IL‐21 | tMCAO | Increase | Clarkson et al.103 |
| CD8 T cell | Granzyme B | tMCAO | Increase | Chaitanya et al.119 |
| CD8 T cell | Perforin | pMCAO | Increase | Mracsko et al.121 |
| B cell | IL‐10 | tMCAO | Decrease | Chen et al.124 |
| B cell | N/A | tMCAO | Decrease | Monson et al. (2013)125 |
| B cell | N/A | dMCAO | Increase | Doyle et al.128 |
dMCAO, distal middle cerebral artery occlusion; IFN‐γ, interferon‐γ; IL‐17a, interleukin‐17a; pMCAO, permanent middle cerebral artery occlusion; tMCAO, transient middle cerebral artery occlusion.
Immune cells orchestrate dynamic changes to the neurovascular unit following ischaemic stroke
The cells that make up the neurovascular unit play a complex role in mediating BBB function, immune cell trafficking and injury after stroke129, 130, 131 (Fig. 3). Several immune cells including neutrophils, DCs, lymphocytes and microglia, have been shown to directly mediate vascular injury during the acute phase of stroke to facilitate BBB dysfunction and consequent neuronal death. During both tMCAO and pMCAO, activated neutrophils can be seen within the leptomeninges as early as 6 hr, within the perivascular space by 15 hr, and within the CNS parenchyma by 24 hr.132 IL‐1 up‐regulates the neutrophil‐selective chemokines CXCL1 and CXCL2 within the plasma to recruit neutrophils into the brain during ischaemia.133 In conjunction with this, intercellular adhesion molecule 1 has been shown to facilitate neutrophil adhesion to endothelial cells during MCAO,134 and consequently to induce BBB dysfunction by disrupting the tight junction protein Claudin‐5 through the production of MMP‐9.135, 136, 137, 138 Inhibition of either neutrophil infiltration or neutrophil‐derived MMP‐9 reduces inflammatory‐mediated cerebral damage and risk of haemorrhagic stroke,135 suggesting that neutrophils can mediate BBB dysfunction and consequently affect neuronal health.
Figure 3.
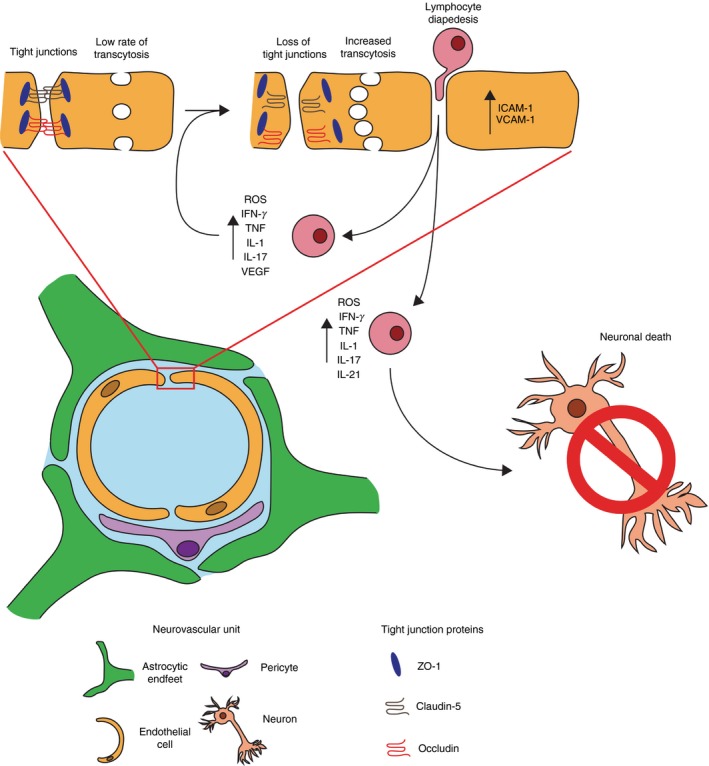
Schematic for how pro‐inflammatory T cells impact the neurovascular unit (NVU) following brain ischaemia. Following brain ischaemia, damage to the endothelium results in the loss of tight junctions such as occludin, ZO‐1 and Claudin‐5. This process facilitates increased lymphocyte diapedesis through paracellular and transcellular pathways. Upon entry into the brain parenchyma T cells prorogate ischaemic damage by releasing pro‐inflammatory factors and cytokines such as reactive oxygen species (ROS), interferon‐γ (IFN‐γ), tumour necrosis factor‐α (TNF α), interleukin‐1β (IL‐1β), IL‐17 and IL‐21, which can act by further damaging cells of the NVU or by damaging neurons directly.
The role of infiltrating macrophages during ischaemic stroke is controversial, partly because both microglia and infiltrating macrophages were almost indistinguishable by cell surface markers as described above. CD11b‐positive immune cells, which encompass both microglia and infiltrating macrophages, express abundant levels of angiopoietin‐like protein 2 within the brain during tMCAO, which contributes to increased levels of the pro‐inflammatory cytokines IL‐1β and TNF‐α to promote endothelial dysfunction and subsequent neurological deficits.16, 75 Additionally, CD11b+ cells (expressed by both infiltrating macrophages and microglia) can signal through the platelet‐derived factor receptor on arterioles to promote BBB permeability and the incidence of haemorrhage during stroke.17 In contrast, depleting CCR2+ monocyte infiltration increases BBB permeability and risk of haemorrhage after stroke, suggesting that infiltrating macrophages may play a role in maintaining brain vasculature.77, 139 In support of this, it has recently been shown that the myeloid cell and astrocyte‐derived matricellular glycoprotein osteopontin is critically involved in astrocyte‐mediated protection of the neurovascular unit after ischaemia; macrophage‐specific depletion of matricellular glycoprotein osteopontin resulted in the failure of astrocytes to properly maintain cerebral blood vessels and BBB integrity after ischaemic stroke.140 Macrophages are also hypothesized to promote vascular endothelial growth factor‐mediated angiogenesis to promote neuroprotection and remove necrotic debris, termed the ‘clean‐up’ hypothesis141; however, newly formed vessels are often leaky and it is unknown whether this is beneficial or detrimental following ischaemia.
As mentioned previously in this review, T cells play an intimate and complex role with the neurovascular unit during ischaemic stroke. Mechanistically, T cells have been shown to induce vascular dysfunction by promoting leucocyte/endothelial interactions through lymphocyte function‐associated antigen 1/ intercellular adhesion molecule 1.110 In this context, it is hypothesized that T cells play an important role in reducing neutrophil infiltration across the BBB, lymphocyte invasion, microglial activation and the production of pro‐inflammatory cytokines through IL‐10. It is still unclear how T‐cell subtypes directly influence the neurovascular unit during cerebral ischaemia.
Conclusion
Many questions remain with regards to the roles and interactions of immune cells during stroke. Although there is a general consensus that in the acute phase of ischaemic stroke immune cells are primarily damaging, anti‐inflammatory myeloid cells and lymphocytes can infiltrate the brain as well to influence functional recovery. It appears that the chronic phase after ischaemic stroke is a little more complex as lymphocytes can develop tolerance, immunity and memory to various signals released from the ischaemic brain damage, which can determine how lymphocytes function. Nonetheless, recent experimental murine studies unveiling the molecular mechanisms of immune cell contribution to stroke have led to exciting findings that could be used at the clinic. Although these studies have been valuable and promising for future stroke therapies, an ongoing challenge will be the differences in gene changes across unbiased studies between humans and animal models that can arise due to heterogeneity between human samples, analyses of circulating factors in blood versus tissue parenchyma, and the timing of sample collection.
Overall, given the heterogeneous nature of immune cells and their influence on stroke pathology, it is essential to identify and target the specific cytokines that they produce to limit their detrimental effects and harness their benefits. Pursuing this will be critical to move forward and revolutionize the field. Apparent contradictions and current gaps in our knowledge of cellular immunity during stroke can now be further addressed in greater detail than ever before by using novel and emerging technologies such as single‐cell RNA sequencing, cytometry by time‐of‐flight, two‐photon imaging, whole‐genome transcriptomic and epigenetic analysis with complementary bioinformatics, and unbiased proteomics among others. The use of these methods will not only serve to answer some the most vexing questions in the field, but will also lead to the discovery of novel pathways and a deeper understanding of the complex cellular interactions that occur during stroke. This will be critical in the development of new immunomodulatory therapies and novel treatments to mitigate, reverse and prevent the damage done during stroke.
Materials and methods
Mice
Wild‐type C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Transient middle cerebral artery occlusion
Focal cerebral ischaemia in mice was induced by occlusion of the left MCA, as described previously.142 Operators performing surgeries were masked to experimental groups. In brief, the left common carotid artery was exposed, and the occipital artery branches of the external carotid artery (ECA) were isolated and coagulated. After coagulation of the superior thyroid artery, the ECA was dissected distally and coagulated along with the terminal lingual and maxillary artery branches. The internal carotid artery (ICA) was isolated, and the extracranial branch of the ICA was then dissected and ligated. A standardized polyamide resin glue‐coated 6·0 nylon monofilament (3021910; Doccol Corp.,Sharon, MA) was introduced into the ECA lumen, and then advanced ~ 9–9·5 mm in the ICA lumen to block middle cerebral artery blood flow. During the entire procedure, mouse body temperature was kept between 37° and 38° with a heating pad. The suture was withdrawn 60 min after occlusion. The incision was closed, and the mice underwent recovery.
Lymphocyte isolation, intracellular cytokine staining and FACS
Mice were deeply anaesthetized with ketamine/xylazine and then transcardially perfused with cold phosphate‐buffered saline (PBS). Single‐cell suspensions were made from cervical lymph nodes and spleens by grinding the tissues between the frosted ends of glass slides. Red blood cells were lysed using ACK lysis buffer, and cells were washed with Hanks’ balanced salt solution. Brains were minced with razor blades and pushed through 70‐μm nylon cell strainers. Cells were washed, resuspended in 70% Percoll and overlaid with 30% Percoll. The gradient was centrifuged at 1200 rcf for 30 min at 4° without brake. The interface was removed and washed before plating. All collected organs were weighed, and live cells were counted using a haemocytometer.
Fluorescent microscopy
For frozen sections, mice were first perfused with cold PBS, followed by perfusion with 4% paraformaldehyde/PBS. Harvested tissues were left in 25% sucrose/PBS overnight at 4° and then embedded in Tissue‐Tek OCT Compound (Sakura Finetek USA, Inc., Torrance, CA) before freezing at −80°. Five‐micrometre‐thick tissue cryosections were cut and stored at −80° until staining. Frozen sections were thawed for 10 min at room temperature and then placed in acetone for 10 min at −20°. Next, sections were incubated in PBS for 30 min at room temperature and then blocked with 2.4G2 antibody in 0·1% Triton X‐100/PBS (1 : 50) for 30 min before applying anti‐GFP‐FITC primary antibody in 0·1% Triton X‐100/PBS (1 : 100) for 1 hr at 37°. Sections were then washed three times for 10 min each time with PBS and mounted with ProLong Gold antifade reagent containing diamidino‐phenyl indole (Invitrogen, Carlsbad, CA). All images were acquired with a camera (Optronics Inc., Goleta, CA) mounted on a fluorescence microscope (Olympus BX41, Leeds Precision Instruments). Individual fluorescent channel images were merged using pictureframe software (Optronics Inc., Muskogee, OK). The brightness/contrast of the acquired digital images was applied equally across the entire image and equally to control images and analysed using adobe photoshop CS4 software (Adobe Systems Inc., San Jose, CA).
Antibodies
The following antibodies were purchased from BD Biosciences (Franklin Lakes, NJ): anti‐CD4 Alx700 (RM4‐5), anti‐CD4 Alx647 (RM4‐5), anti‐CD8 PerCP (53‐6.7), anti‐B220 (RA3‐6B2), anti‐CD45 PerCP (20‐F11); from eBioscience (San Diego, CA): anti‐CD11b (M1/70); from Wako (Richmond, VA): anti‐IBA‐1 (019‐19741); from EDM Millipore (Darmstadt, Germany): anti‐GFAP (AB5541); from Millipore (Darmstadt, Germany): anti‐NeuN (MAB377B). All secondary antibodies were purchased from Thermo Fisher Scientific (Waltham, MA).
Statistics
One‐tailed unpaired Student's t‐tests were computed using instat software (GraphPad Software, La Jolla, CA) to make statistical comparisons between groups. Results are given as means plus or minus one standard deviation. Multiple comparisons were made using one‐way analysis of variance. Where appropriate, two‐sided Student's t‐test analysis was used to compare measures made between two groups. P‐values < 0·05 were considered significant.
Ethics statement
All animal procedures used in this study were conducted in strict compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Wisconsin Center for Health Sciences Research Animal Care Committee. All mice (~25 g) were anaesthetized with ketamine and xylazine for procedures, and all efforts were made to minimize pain and discomfort.
Funding
NIH/NIGMS grant T32 GM007507 (Neuroscience Training Program). NIH grant RO1‐NS37570. AHA Predoc 1525500022.
Disclosures
The author declares no competing interests.
References
- 1. Hallenbeck JM. Cytokines, macrophages, and leukocytes in brain ischemia. Neurology 1997; 49(5 Suppl 4):S5–9. [DOI] [PubMed] [Google Scholar]
- 2. Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl 1996; 66:27–31. [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Jacobowitz DM, Barone F, McCarron R, Spatz M, Feuerstein G et al Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. J Cereb Blood Flow Metab 1994; 14:348–52. [DOI] [PubMed] [Google Scholar]
- 4. Hallenbeck JM, Dutka AJ. Background review and current concepts of reperfusion injury. Arch Neurol 1990; 47:1245–54. [DOI] [PubMed] [Google Scholar]
- 5. Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA et al Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009; 40:1849–57. [DOI] [PubMed] [Google Scholar]
- 6. Xiong X, Gu L, Zhang H, Xu B, Zhu S, Zhao H. The protective effects of T cell deficiency against brain injury are ischemic model‐dependent in rats. Neurochem Int 2013; 62:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L et al Tissue‐resident macrophages originate from yolk‐sac‐derived erythro‐myeloid progenitors. Nature 2015; 518:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 2011; 11:775–87. [DOI] [PubMed] [Google Scholar]
- 9. Prinz M, Erny D, Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol 2017; 18:385–92. [DOI] [PubMed] [Google Scholar]
- 10. Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB et al New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 2016; 113:E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia 1993; 7:19–24. [DOI] [PubMed] [Google Scholar]
- 12. Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein‐reactive CD4+ T cells compared. J Immunol 1995; 154:4309–21. [PubMed] [Google Scholar]
- 13. Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 2003; 183:25–33. [DOI] [PubMed] [Google Scholar]
- 14. Capotondo A, Milazzo R, Politi LS, Quattrini A, Palini A, Plati T et al Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc Natl Acad Sci U S A 2012; 109:15018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M et al Microglia in the adult brain arise from Ly‐6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 2007; 10:1544–53. [DOI] [PubMed] [Google Scholar]
- 16. Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER et al Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation 2015; 12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su EJ, Cao C, Fredriksson L, Nilsson I, Stefanitsch C, Stevenson TK et al Microglial‐mediated PDGF‐CC activation increases cerebrovascular permeability during ischemic stroke. Acta Neuropathol 2017; 134:585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang C, An J, Haile WB, Echeverry R, Strickland DK, Yepes M. Microglial low‐density lipoprotein receptor‐related protein 1 mediates the effect of tissue‐type plasminogen activator on matrix metalloproteinase‐9 activity in the ischemic brain. J Cereb Blood Flow Metab 2009; 29:1946–54. [DOI] [PubMed] [Google Scholar]
- 19. Zhou M, Wang CM, Yang WL, Wang P. Microglial CD14 activated by iNOS contributes to neuroinflammation in cerebral ischemia. Brain Res 2013; 1506:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JH, Kam EH, Kim JM, Kim SY, Kim EJ, Cheon SY et al Intranasal administration of interleukin‐1 receptor antagonist in a transient focal cerebral ischemia rat model. Biomol Ther (Seoul) 2017; 25:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pires PW, Girgla SS, Moreno G, McClain JL, Dorrance AM. Tumor necrosis factor‐α inhibition attenuates middle cerebral artery remodeling but increases cerebral ischemic damage in hypertensive rats. Am J Physiol Heart Circ Physiol 2014; 307:H658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Z, Han K, Chen J, Wang C, Dong Y, Yu M et al Vascular endothelial growth factor is neuroprotective against ischemic brain injury by inhibiting scavenger receptor A expression on microglia. J Neurochem 2017; 142:700–9. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Q, Chen C, Lü J, Xie M, Pan D, Luo X et al Cell cycle inhibition attenuates microglial proliferation and production of IL‐1β, MIP‐1α, and NO after focal cerebral ischemia in the rat. Glia 2009; 57:908–20. [DOI] [PubMed] [Google Scholar]
- 24. Meng HL, Li XX, Chen YT, Yu LJ, Zhang H, Lao JM et al Neuronal soluble Fas ligand drives M1‐microglia polarization after cerebral ischemia. CNS Neurosci Ther 2016; 22:771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yanagisawa D, Kitamura Y, Takata K, Hide I, Nakata Y, Taniguchi T. Possible involvement of P2X7 receptor activation in microglial neuroprotection against focal cerebral ischemia in rats. Biol Pharm Bull 2008; 31:1121–30. [DOI] [PubMed] [Google Scholar]
- 26. Wu R, Li X, Xu P, Huang L, Cheng J, Huang X et al TREM2 protects against cerebral ischemia/reperfusion injury. Mol Brain 2017; 10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Y, Liu H, Zhang H, Ye Q, Wang J, Yang B et al ST2/IL‐33‐dependent microglial response limits acute ischemic brain injury. J Neurosci 2017; 37:4692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li K, Yu W, Cao R, Zhu Z, Zhao G. Microglia‐mediated BAFF‐BAFFR ligation promotes neuronal survival in brain ischemia injury. Neuroscience 2017; 363:87–96. [DOI] [PubMed] [Google Scholar]
- 29. Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H. Role for microglia in sex differences after ischemic stroke: importance of M2. Metab Brain Dis 2015; 30:1515–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fumagalli S, Perego C, Ortolano F, De Simoni MG. CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice. Glia 2013; 61:827–42. [DOI] [PubMed] [Google Scholar]
- 31. Narantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S et al Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS ONE 2010; 5:e11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 2016; 19:987–91. [DOI] [PubMed] [Google Scholar]
- 33. Morganti JM, Riparip LK, Rosi S. Call off the dog(ma): M1/M2 polarization is concurrent following traumatic brain injury. PLoS ONE 2016; 11:e0148001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dénes A, Ferenczi S, Halász J, Környei Z, Kovács KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab 2008; 28:1707–21. [DOI] [PubMed] [Google Scholar]
- 35. Heldmann U, Mine Y, Kokaia Z, Ekdahl CT, Lindvall O. Selective depletion of Mac‐1‐expressing microglia in rat subventricular zone does not alter neurogenic response early after stroke. Exp Neurol 2011; 229:391–8. [DOI] [PubMed] [Google Scholar]
- 36. Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK et al Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1‐expressing microglia. Proc Natl Acad Sci U S A 1998; 95:10896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang Z, Gan Y, Liu Q, Yin JX, Liu Q, Shi J et al CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J Neuroinflammation 2014; 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Monticelli S, Natoli G. Transcriptional determination and functional specificity of myeloid cells: making sense of diversity. Nat Rev Immunol 2017; 17:595–607. [DOI] [PubMed] [Google Scholar]
- 39. Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008; 8:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10:826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 2011; 14:1363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hayakawa K, Miyamoto N, Seo JH, Pham LD, Kim KW, Lo EH et al High‐mobility group box 1 from reactive astrocytes enhances the accumulation of endothelial progenitor cells in damaged white matter. J Neurochem 2013; 125:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI et al Early release of HMGB‐1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab 2008; 28:927–38. [DOI] [PubMed] [Google Scholar]
- 44. Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I et al Anti‐high mobility group box‐1 antibody therapy for traumatic brain injury. Ann Neurol 2012; 72:373–84. [DOI] [PubMed] [Google Scholar]
- 45. Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T et al Anti‐high mobility group box‐1 monoclonal antibody protects the blood–brain barrier from ischemia‐induced disruption in rats. Stroke 2011; 42:1420–8. [DOI] [PubMed] [Google Scholar]
- 46. Ziegler G, Prinz V, Albrecht MW, Harhausen D, Khojasteh U, Nacken W et al Mrp‐8 and ‐14 mediate CNS injury in focal cerebral ischemia. Biochim Biophys Acta 2009; 1792:1198–204. [DOI] [PubMed] [Google Scholar]
- 47. Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W et al The Toll‐like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med 2010; 16:713–7. [DOI] [PubMed] [Google Scholar]
- 48. Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA et al Mrp8 and Mrp14 are endogenous activators of Toll‐like receptor 4, promoting lethal, endotoxin‐induced shock. Nat Med 2007; 13:1042–9. [DOI] [PubMed] [Google Scholar]
- 49. Klichko VI, Orr WC, Radyuk SN. The role of peroxiredoxin 4 in inflammatory response and aging. Biochim Biophys Acta 2016; 1862:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salzano S, Checconi P, Hanschmann EM, Lillig CH, Bowler LD, Chan P et al Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin‐2, which acts as a danger signal. Proc Natl Acad Sci U S A 2014; 111:12157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Riddell JR, Wang XY, Minderman H, Gollnick SO. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J Immunol 2010; 184:1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riddell JR, Bshara W, Moser MT, Spernyak JA, Foster BA, Gollnick SO. Peroxiredoxin 1 controls prostate cancer growth through Toll‐like receptor 4‐dependent regulation of tumor vasculature. Cancer Res 2011; 71:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I et al Peroxiredoxin family proteins are key initiators of post‐ischemic inflammation in the brain. Nat Med 2012; 18:911–7. [DOI] [PubMed] [Google Scholar]
- 54. Rashidian J, Rousseaux MW, Venderova K, Qu D, Callaghan SM, Phillips M et al Essential role of cytoplasmic cdk5 and Prx2 in multiple ischemic injury models, in vivo . J Neurosci 2009; 29:12497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shichita T, Ito M, Morita R, Komai K, Noguchi Y, Ooboshi H et al MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat Med 2017; 23:723–32. [DOI] [PubMed] [Google Scholar]
- 56. Ito M, Shichita T, Okada M, Komine R, Noguchi Y, Yoshimura A et al Bruton's tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun 2015; 6:7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I et al Pivotal role of cerebral interleukin‐17‐producing γδ T cells in the delayed phase of ischemic brain injury. Nat Med 2009; 15:946–50. [DOI] [PubMed] [Google Scholar]
- 58. Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest 1976; 58:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol 2014; 14:302–14. [DOI] [PubMed] [Google Scholar]
- 60. Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G‐CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 2002; 17:413–23. [DOI] [PubMed] [Google Scholar]
- 61. Köhler A, De Filippo K, Hasenberg M, Van Den Brandt C, Nye E, Hosking MP et al G‐CSF‐mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 2011; 117:4349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Doycheva DM, Hadley T, Li L, Applegate RL II, Zhang JH, Tang J. Anti‐neutrophil antibody enhances the neuroprotective effects of G‐CSF by decreasing number of neutrophils in hypoxic ischemic neonatal rat model. Neurobiol Dis 2014; 69:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang C, Ling CL, Pang L, Wang Q, Liu JX, Wang BS et al Direct macromolecular drug delivery to cerebral ischemia area using neutrophil‐mediated nanoparticles . Theranostics 2017; 7:3260–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T et al Neutrophil‐to‐lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis 2017; 26:650–7. [DOI] [PubMed] [Google Scholar]
- 65. Vallés J, Lago A, Santos MT, Latorre AM, Tembl JI, Salom JB et al Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb Haemost 2017; 117:1919–29. [DOI] [PubMed] [Google Scholar]
- 66. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–5. [DOI] [PubMed] [Google Scholar]
- 67. Garcia‐Bonilla L, Faraco G, Moore J, Murphy M, Racchumi G, Srinivasan J et al Spatio‐temporal profile, phenotypic diversity, and fate of recruited monocytes into the post‐ischemic brain. J Neuroinflammation 2016; 13:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tanaka R, Komine‐Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M et al Migration of enhanced green fluorescent protein expressing bone marrow‐derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience 2003; 117:531–9. [DOI] [PubMed] [Google Scholar]
- 69. Tei N, Tanaka J, Sugimoto K, Nishihara T, Nishioka R, Takahashi H et al Expression of MCP‐1 and fractalkine on endothelial cells and astrocytes may contribute to the invasion and migration of brain macrophages in ischemic rat brain lesions. J Neurosci Res 2013; 91:681–93. [DOI] [PubMed] [Google Scholar]
- 70. Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA et al Expression of monocyte chemoattractant protein‐1 and macrophage inflammatory protein‐1 after focal cerebral ischemia in the rat. J Neuroimmunol 1995; 56:127–34. [DOI] [PubMed] [Google Scholar]
- 71. Wang X, Yue TL, Barone FC, Feuerstein GZ. Monocyte chemoattractant protein‐1 messenger RNA expression in rat ischemic cortex. Stroke 1995; 26:661–5; discussion 665–6. [DOI] [PubMed] [Google Scholar]
- 72. Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C et al Monocyte chemoattractant protein‐1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab 2002; 22:308–17. [DOI] [PubMed] [Google Scholar]
- 73. Schilling M, Strecker JK, Ringelstein EB, Schäbitz WR, Kiefer R. The role of CC chemokine receptor 2 on microglia activation and blood‐borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res 2009; 1289:79–84. [DOI] [PubMed] [Google Scholar]
- 74. Terao Y, Ohta H, Oda A, Nakagaito Y, Kiyota Y, Shintani Y. Macrophage inflammatory protein‐3α plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neurosci Res 2009; 64:75–82. [DOI] [PubMed] [Google Scholar]
- 75. Amadatsu T, Morinaga J, Kawano T, Terada K, Kadomatsu T, Miyata K et al Macrophage‐derived angiopoietin‐like protein 2 exacerbates brain damage by accelerating acute inflammation after ischemia‐reperfusion. PLoS ONE 2016; 11:e0166285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 2000; 20:53–65. [DOI] [PubMed] [Google Scholar]
- 77. Chu HX, Broughton BR, Kim HA, Lee S, Drummond GR, Sobey CG. Evidence that Ly6Chi monocytes are protective in acute ischemic stroke by promoting M2 macrophage polarization. Stroke 2015; 46:1929–37. [DOI] [PubMed] [Google Scholar]
- 78. Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M et al Interleukin‐4 is essential for microglia/macrophage M2 polarization and long‐term recovery after cerebral ischemia. Stroke 2016; 47:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Korhonen P, Kanninen KM, Lehtonen Š, Lemarchant S, Puttonen KA, Oksanen M et al Immunomodulation by interleukin‐33 is protective in stroke through modulation of inflammation. Brain Behav Immun 2015; 49:322–36. [DOI] [PubMed] [Google Scholar]
- 80. Smirkin A, Matsumoto H, Takahashi H, Inoue A, Tagawa M, Ohue S et al Iba1+/NG2+ macrophage‐like cells expressing a variety of neuroprotective factors ameliorate ischemic damage of the brain. J Cereb Blood Flow Metab 2010; 30:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Felger JC, Abe T, Kaunzner UW, Gottfried‐Blackmore A, Gal‐Toth J, McEwen BS et al Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun 2010; 24:724–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke 2002; 33:1129–34. [DOI] [PubMed] [Google Scholar]
- 83. Yilmaz A, Fuchs T, Dietel B, Altendorf R, Cicha I, Stumpf C et al Transient decrease in circulating dendritic cell precursors after acute stroke: potential recruitment into the brain. Clin Sci (Lond) 2009; 118:147–57. [DOI] [PubMed] [Google Scholar]
- 84. Manley NC, Caso JR, Works MG, Cutler AB, Zemlyak I, Sun G et al Derivation of injury‐responsive dendritic cells for acute brain targeting and therapeutic protein delivery in the stroke‐injured rat. PLoS ONE 2013; 8:e61789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Works MG, Koenig JB, Sapolsky RM. Soluble TNF receptor 1‐secreting ex vivo‐derived dendritic cells reduce injury after stroke. J Cereb Blood Flow Metab 2013; 33:1376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gill D, Veltkamp R. Dynamics of T cell responses after stroke. Curr Opin Pharmacol 2016; 26:26–32. [DOI] [PubMed] [Google Scholar]
- 87. Ortolano F, Maffia P, Dever G, Rodolico G, Millington OR, De Simoni MG et al Advances in imaging of new targets for pharmacological intervention in stroke: real‐time tracking of T‐cells in the ischaemic brain. Br J Pharmacol 2010; 159:808–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fumagalli S, Coles JA, Ejlerskov P, Ortolano F, Bushell TJ, Brewer JM et al In vivo real‐time multiphoton imaging of T lymphocytes in the mouse brain after experimental stroke. Stroke 2011; 42:1429–36. [DOI] [PubMed] [Google Scholar]
- 89. Kislin M, Sword J, Fomitcheva IV, Croom D, Pryazhnikov E, Lihavainen E et al Reversible disruption of neuronal mitochondria by ischemic and traumatic injury revealed by quantitative two‐photon imaging in the neocortex of anesthetized mice. J Neurosci 2017; 37:333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol 1994; 55:195–203. [DOI] [PubMed] [Google Scholar]
- 91. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhou W, Liesz A, Bauer H, Sommer C, Lahrmann B, Valous N et al Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol 2013; 23:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule‐1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab 1995; 15:42–51. [DOI] [PubMed] [Google Scholar]
- 94. Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon‐gamma in ischemic stroke. Circulation 2006; 113:2105–12. [DOI] [PubMed] [Google Scholar]
- 95. Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA et al T‐ and B‐cell‐deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab 2007; 27:1798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M et al FTY720 reduces post‐ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS ONE 2011; 6:e21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhu W, Dotson AL, Libal NL, Lapato AS, Bodhankar S, Offner H et al Recombinant T‐cell receptor ligand RTL1000 limits inflammation and decreases infarct size after experimental ischemic stroke in middle‐aged mice. Neuroscience 2015; 288:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Akiyoshi K, Dziennis S, Palmateer J, Ren X, Vandenbark AA, Offner H et al Recombinant T cell receptor ligands improve outcome after experimental cerebral ischemia. Transl Stroke Res 2011; 2:404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Czech B, Pfeilschifter W, Mazaheri‐Omrani N, Strobel MA, Kahles T, Neumann‐Haefelin T et al The immunomodulatory sphingosine 1‐phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem Biophys Res Commun 2009; 389:251–6. [DOI] [PubMed] [Google Scholar]
- 100. Zhang J, Mao X, Zhou T, Cheng X, Lin Y. IL‐17A contributes to brain ischemia reperfusion injury through calpain‐TRPC6 pathway in mice. Neuroscience 2014; 274:419–28. [DOI] [PubMed] [Google Scholar]
- 101. Arunachalam P, Ludewig P, Melich P, Arumugam TV, Gerloff C, Prinz I et al CCR6 (CC Chemokine Receptor 6) is essential for the migration of detrimental natural interleukin‐17‐producing γδ T cells in stroke. Stroke 2017; 48:1957–65. [DOI] [PubMed] [Google Scholar]
- 102. Seifert HA, Collier LA, Chapman CB, Benkovic SA, Willing AE, Pennypacker KR. Pro‐inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol 2014; 9:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Clarkson BD, Ling C, Shi Y, Harris MG, Rayasam A, Sun D et al T cell‐derived interleukin (IL)‐21 promotes brain injury following stroke in mice. J Exp Med 2014; 211:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Arango‐Dávila CA, Vera A, Londono AC, Echeverri AF, Cañas F, Cardozo CF et al Soluble or soluble/membrane TNF‐α inhibitors protect the brain from focal ischemic injury in rats. Int J Neurosci 2015; 125:936–40. [DOI] [PubMed] [Google Scholar]
- 105. Amantea D, Certo M, Russo R, Bagetta G, Corasaniti MT, Tassorelli C. Early reperfusion injury is associated to MMP2 and IL‐1β elevation in cortical neurons of rats subjected to middle cerebral artery occlusion. Neuroscience 2014; 277:755–63. [DOI] [PubMed] [Google Scholar]
- 106. Liesz A, Suri‐Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S et al Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009; 15:192–9. [DOI] [PubMed] [Google Scholar]
- 107. Brea D, Agulla J, Rodríguez‐Yáñez M, Barral D, Ramos‐Cabrer P, Campos F et al Regulatory T cells modulate inflammation and reduce infarct volume in experimental brain ischaemia. J Cell Mol Med 2014; 18:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li P, Wang L, Zhou Y, Gan Y, Zhu W, Xia Y et al C‐C Chemokine Receptor Type 5 (CCR5)‐mediated docking of transferred Tregs protects against early blood–brain barrier disruption after stroke. J Am Heart Assoc 2017; 6:e006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee HT, Liu SP, Lin CH, Lee SW, Hsu CY, Sytwu HK et al A crucial role of CXCL14 for promoting regulatory T cells activation in stroke. Theranostics 2017; 7:855–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Göbel K, Schuhmann MK et al Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 2013; 121:679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kleinschnitz C, Wiendl H. Con: Regulatory T cells are protective in ischemic stroke. Stroke 2013; 44:e87–8. [DOI] [PubMed] [Google Scholar]
- 112. Hu X, Li P, Chen J. Pro: Regulatory T cells are protective in ischemic stroke. Stroke 2013; 44:e85–6. [DOI] [PubMed] [Google Scholar]
- 113. Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein‐tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke 2003; 34:1809–15. [DOI] [PubMed] [Google Scholar]
- 114. Zierath D, Kunze A, Fecteau L, Becker K. Promiscuity of autoimmune responses to MBP after stroke. J Neuroimmunol 2015; 285:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA et al Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL‐10‐producing CD4+ T cells. J Immunol 2003; 171:6549–55. [DOI] [PubMed] [Google Scholar]
- 116. Ren X, Akiyoshi K, Grafe MR, Vandenbark AA, Hurn PD, Herson PS et al Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metab Brain Dis 2012; 27:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang J, Ye Q, Xu J, Benedek G, Zhang H, Yang Y et al DRα1‐MOG‐35‐55 reduces permanent ischemic brain injury. Transl Stroke Res 2017; 8:284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schwab JM, Nguyen TD, Meyermann R, Schluesener HJ. Human focal cerebral infarctions induce differential lesional interleukin‐16 (IL‐16) expression confined to infiltrating granulocytes, CD8+ T‐lymphocytes and activated microglia/macrophages. J Neuroimmunol 2001; 114:232–41. [DOI] [PubMed] [Google Scholar]
- 119. Chaitanya GV, Schwaninger M, Alexander JS, Babu PP. Granzyme‐b is involved in mediating post‐ischemic neuronal death during focal cerebral ischemia in rat model. Neuroscience 2010; 165:1203–16. [DOI] [PubMed] [Google Scholar]
- 120. Chaitanya GV, Eeka P, Munker R, Alexander JS, Babu PP. Role of cytotoxic protease granzyme‐b in neuronal degeneration during human stroke. Brain Pathol 2011; 21:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mracsko E, Liesz A, Stojanovic A, Lou WP, Osswald M, Zhou W et al Antigen dependently activated cluster of differentiation 8‐positive T cells cause perforin‐mediated neurotoxicity in experimental stroke. J Neurosci 2014; 34:16784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bodhankar S, Chen Y, Lapato A, Vandenbark AA, Murphy SJ, Saugstad JA et al Regulatory CD8+CD122+ T‐cells predominate in CNS after treatment of experimental stroke in male mice with IL‐10‐secreting B‐cells. Metab Brain Dis 2015; 30:911–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Treatment of experimental stroke with IL‐10‐producing B‐cells reduces infarct size and peripheral and CNS inflammation in wild‐type B‐cell‐sufficient mice. Metab Brain Dis 2014; 29:59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen Y, Bodhankar S, Murphy SJ, Vandenbark AA, Alkayed NJ, Offner H. Intrastriatal B‐cell administration limits infarct size after stroke in B‐cell deficient mice. Metab Brain Dis 2012; 27:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Monson NL, Ortega SB, Ireland SJ, Meeuwissen AJ, Chen D, Plautz EJ et al Repetitive hypoxic preconditioning induces an immunosuppressed B cell phenotype during endogenous protection from stroke. J Neuroinflammation 2014; 11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Selvaraj UM, Poinsatte K, Torres V, Ortega SB, Stowe AM. Heterogeneity of B cell functions in stroke‐related risk, prevention, injury, and repair. Neurotherapeutics 2016; 13:729–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schuhmann MK, Langhauser F, Kraft P, Kleinschnitz C. B cells do not have a major pathophysiologic role in acute ischemic stroke in mice. J Neuroinflammation 2017; 14:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Doyle KP, Quach LN, Solé M, Axtell RC, Nguyen TV, Soler‐Llavina GJ et al B‐lymphocyte‐mediated delayed cognitive impairment following stroke. J Neurosci 2015; 35:2133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rustenhoven J, Jansson D, Smyth LC, Dragunow M. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci 2017; 38:291–304. [DOI] [PubMed] [Google Scholar]
- 130. LeCuyer EA, Christensen JJ, Kreher D, Kearney MH, Kitzman HJ. African American mothers’ self‐described discipline strategies with young children in 1992 and 2012. J Pediatr Health Care 2015; 29:28–37. [DOI] [PubMed] [Google Scholar]
- 131. Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci 2012; 1268:21–5. [DOI] [PubMed] [Google Scholar]
- 132. Perez‐de‐Puig I, Miró‐Mur F, Ferrer‐Ferrer M, Gelpi E, Pedragosa J, Justicia C et al Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 2015; 129:239–57. [DOI] [PubMed] [Google Scholar]
- 133. McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin‐1‐ and neutrophil‐dependent mechanisms. J Neurosci 2007; 27:4403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Connolly ES, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD et al Cerebral protection in homozygous null ICAM‐1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest 1996; 97:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci 2008; 28:9451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gautier S, Ouk T, Tagzirt M, Lefebvre C, Laprais M, Pétrault O et al Impact of the neutrophil response to granulocyte colony‐stimulating factor on the risk of hemorrhage when used in combination with tissue plasminogen activator during the acute phase of experimental stroke. J Neuroinflammation 2014; 11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase‐9 reduces infarct size. Stroke 1998; 29:1020–30. [DOI] [PubMed] [Google Scholar]
- 138. Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD et al Leukocyte‐derived matrix metalloproteinase‐9 mediates blood–brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 2005; 289:H558–68. [DOI] [PubMed] [Google Scholar]
- 139. Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP et al Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol 2012; 71:743–52. [DOI] [PubMed] [Google Scholar]
- 140. Gliem M, Krammes K, Liaw L, van Rooijen N, Hartung HP, Jander S. Macrophage‐derived osteopontin induces reactive astrocyte polarization and promotes re‐establishment of the blood–brain barrier after ischemic stroke. Glia 2015; 63:2198–207. [DOI] [PubMed] [Google Scholar]
- 141. Manoonkitiwongsa PS, Jackson‐Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean‐up hypothesis. J Cereb Blood Flow Metab 2001; 21:1223–31. [DOI] [PubMed] [Google Scholar]
- 142. Chu HX, Kim HA, Lee S, Moore JP, Chan CT, Vinh A et al Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J Cereb Blood Flow Metab 2014; 34:450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


