Summary
Due to imbalances between vascularity and cellular growth patterns, the tumour microenvironment harbours multiple metabolic stressors including hypoxia and acidosis, which have significant influences on remodelling both tumour and peritumoral tissues. These stressors are also immunosuppressive and can contribute to escape from immune surveillance. Understanding these effects and characterizing the pathways involved can identify new targets for therapy and may redefine our understanding of traditional anti‐tumour therapies. In this review, the effects of hypoxia and acidosis on tumour immunity will be summarized, and how modulating these parameters and their sequelae can be a useful tool for future therapeutic interventions is discussed.
Keywords: acidity, cancer, hypoxia, immunotherapy, microenvironment
Introduction
Tumours commonly exhibit large amounts of intratumoral heterogeneity at genomic, physiological and anatomic scales. Tumours not only contain cancer cells, but are also massively infiltrated by host stromal cells,1 angiogenic vascular cells, cancer‐associated fibroblasts and cells of the immune system (Fig. 1). The immune system has roles in both cancer surveillance and tumour promotion.2 Tumour‐associated immune responses contribute to various hallmarks of cancer including: sustaining tumour proliferative ability; resisting cell death, angiogenesis, invasion; promoting metastasis; evading growth suppressors; and avoiding immune destruction.3 To prevent hyperactivation of the immune cells, multiple suppressive mechanisms are employed to inhibit CD4+ and CD8+ T‐cell activities.4 This inhibition is accomplished by regulatory T (Treg) cells [by the secretion of inhibitory cytokines such as interleukin‐10 (IL‐10) and transforming growth factor‐β (TGF‐β)], or by cytolysis [through the secretion of granzymes by natural killer (NK) cells and CD4+ T cells], or by metabolic disruption of the effector T (Teff) cells, or targeting T‐cell functions via dendritic cells (DCs) through cytotoxic T lymphocyte antigen‐4 (CTLA‐4) or other mechanisms.5 CTLA‐4 and programmed death protein 1 (PD‐1) are the two checkpoint receptors that have critical roles in cancer immunotherapy. CTLA‐4 acts as a regulator for the early activation of naive and memory T cells. It is transported to the T‐cell surface and reduces T‐cell receptor (TCR) signalling. PD‐1 restricts the activity of T cells in the periphery to control autoimmunity. It has two known ligands, PD‐L1 (B7‐H1) and PD‐L2 (B7‐DC). PD‐L1, in particular, is up‐regulated in many cancers in response to pro‐inflammatory cytokines and results in inhibition of local anti‐tumour T‐cell responses.6 Macrophages alter their cell surface expression profile as they polarize between anti‐inflammatory (M1) and pro‐inflammatory (M2) phenotypes in response to the external milieu and contribute to immunoregulation.4 ‘Classically activated’ M1 macrophages express CD80/CD86 molecules and can activate T cells. Additionally, M1 macrophages can display cytotoxic activity against pathogenic bacteria or neoplastic cells. ‘Alternatively activated’ M2 macrophages express immunosuppressive ligands.4 During tumour progression, neoplastic cells evade immune surveillance by evolving mechanisms that subvert the normal tumour‐specific immune response, such as distorting the macrophage populations to favour the M2 phenotype7 or overexpression of checkpoint ligands.6 Up‐regulated expression of immunosuppressive ligands, such as B7H4, on both tumour‐associated macrophages (TAMs) and tumour cells are also found in the tumour‐microenvironment.8 Myeloid‐derived suppressor cells (MDSCs) are another type of immune suppressors in the tumour microenvironment that have known negative effects on T‐cell and NK cell functions.9 This inhibition of immune surveillance via alternative activation of immune cells is mediated by ligand expression and cytokines, as well as soluble and physico‐chemical factors of the tumour microenvironment.7 Herein, we consider tumour hypoxia and acidity as two important physico‐chemical factors that can inhibit anti‐tumour immune responses (Fig. 2).
Figure 1.
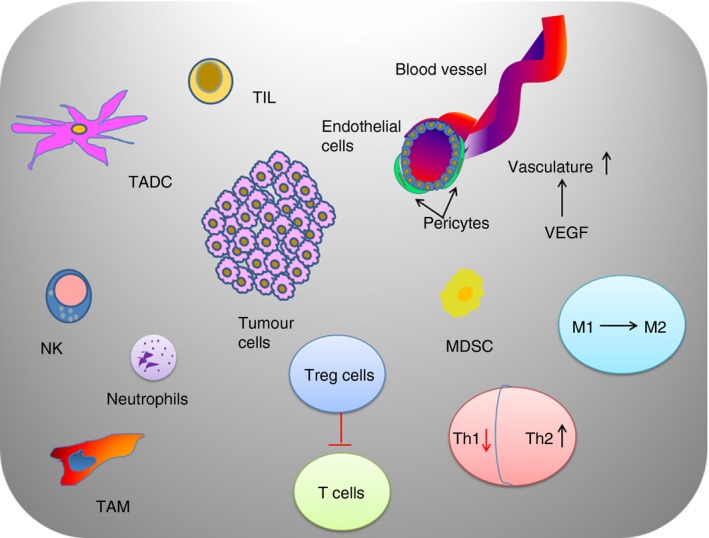
Illustration of the cellular components of tumour microenvironment.
Figure 2.
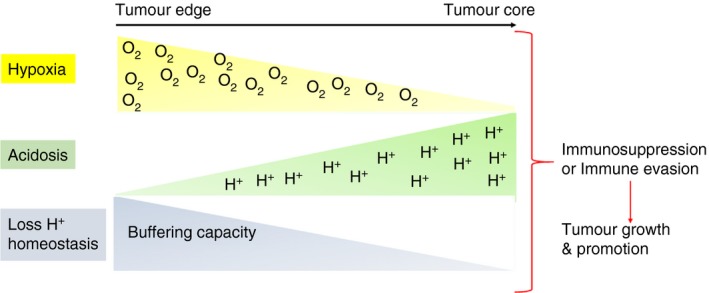
Tumour physical microenvironment (hypoxia and low pH) can modulate immune cells to enhance tumour growth.
Effect of hypoxia on immune function
Hypoxia can be simply identified as lack of oxygen, which is a consequence of increasing oxygen requirement by proliferating cancer cells and dysfunctional and insufficient blood supply resulting from tumour angiogenesis.10 As normal oxygen pressure varies between different tissues, there is not a fixed level to define hypoxia or degree of hypoxia. For instance, normoxia is a partial pressure (po 2) of 21% in the lungs, 13% in arterial blood and 5% in the human liver.11, 12 However, when the vessels are not developed and/or are blocked, e.g. in an ischaemic episode, the oxygen partial pressure decreases, leading to oxygen deficits with po 2 < 1·5% (c.10 mmHg), known as hypoxia. Although many tumours promote vessel formation through signalling via the vascular endothelial growth factor (VEGF), unregulated VEGF can actually lead to hyper‐proliferation of blood vessels, which leads to reduced perfusion due to imbalanced capillary networks.13, 14 As described below, hypoxia has dramatic effects on all cells involved in immune reactivity (Table 1).
Table 1.
Effects of hypoxia on immune cells
| Immune cell | Pathway | Effect |
|---|---|---|
| Natural killer cell | MICA29
 TGF‐β
30, 31, 32 TGF‐β
30, 31, 32
 Cx4335 Cx4335

|
Natural killer cell activation and function 
|
| Myeloid cells | VEGF, FGF2, MMP7, MMP942

|
Macrophage infiltration into hypoxic regions 
|
| Neutrophils | PHD355 NF‐κB48 | Neutrophil survival 
|
| Dendritic cells | Activity18

|
|
| T cells | Th223

|
T‐cell activity 
|
FOXP320
 TGF‐β and CCL2821, 22 TGF‐β and CCL2821, 22

|
Regulatory T cells 
|
|
| HIF1‐α 19 | CD8+ T‐cell activation, Ca2+ signalling 
|
|
cAMP25

|
Antitumour activity 
|
|
| CD4+ and CD8+ T cells | Interferon‐γ and interleukin‐226

|
|
| TAMs | HIF1‐α expression | T‐cell responses53

|
| MDSC | HIF1‐α expression | Programmed death‐ligand 128

|
FGF, fibroblast growth factor; HIF1‐α, hypoxia‐inducible factor 1‐α; MDSC, myeloid‐derived suppressor cells; MICA, MHC class I chain‐related molecule A; MMP, matrix metalloprotease; NF‐κB, nuclear factor‐κB; TAMs, tumour‐associated macrophages; TGF‐β, transforming growth factor‐β; VEGF, vascular endothelial growth factor.
Effect of hypoxia on lymphocytes
The three major types of lymphocytes are T cells, B cells and NK cells. T cells and B cells are major cellular components of the adaptive immune response to neo‐antigens, whereas NK cells are considered part of innate immunity. The effect of the hypoxia inducible factor (HIF1‐α) pathway on T lymphocytes was reviewed by Palazon et al.15 In vitro experiments have demonstrated that the concentration of oxygen in the culture media can modify the proliferation and function of T lymphocytes. Low oxygen levels significantly reduce lymphocyte proliferation, compared with normoxic conditions.16, 17 This may have a physiological role, as lymph nodes and spleen contain large regions of hypoxia in vivo,18 and this is thought to prevent CD8+ T‐cell activation by stabilization of HIF1‐α and suppression of TCR‐mediated Ca2+ signalling.19
Hypoxia increases the FOXP3 transcription factor levels, which is a potent regulator of Treg cells. 20 Hypoxia also promotes the production of TGF‐β and CCL28, both of which have a role in the up‐regulation of Treg cells, contributing to the inhibition of Teff cell responses, and also promoting angiogenesis and tumour tolerance.21, 22 Further, hypoxia appears to skew CD4+ cells towards a T helper type 2 (Th2) phenotype,23 and the resultant IL‐4 can induce alternative macrophage polarization. HIF1‐α regulates the balance between Treg cells and Th17 differentiation.24 Additionally, it has been shown that the accumulation of extracellular adenosine linked to increased elevation of cyclic adenosine monophosphate (cAMP), promoted by the cAMP adenosine receptor A2AR, causes inhibition of antitumour activity of T cells.25 Hypoxia negatively affects the production of interferon‐γ (IFN‐γ) and IL‐2 by both CD4+ and CD8+ T cells.26 It was also observed that the performance and presence of T cells are decreased in hypoxic regions in vivo.27 Hypoxia generates reactive nitrogen species by MDSC and TAMs. Nitration of TCR and CD8 cells decreases their ability for recognizing cognate MHC antigen. Besides, nitration of the chemokine CCL2 inactivates its capacity to attract effector lymphocytes while it can still attract MDSCs. HIF1‐α prompts PD‐L1 expression on tumour cells and MDSCs, which results in the suppression of Teff cells.28
In NK cells, the relation between HIF1‐α, up‐regulation of metalloproteinase ADAM10 and down‐regulation of MHC class I chain‐related molecule A (MICA), has an effect on resistance to lysis. Accordingly the interaction between natural group 2D (NK2D) and MICA plays a critical role in the direction of NK cell responses against tumour cells.29 Up‐regulation of TGF‐β by hypoxia can also decrease NKG2D receptors on NK cells, which may be due to hypoxia‐induced release of microvesicles.30, 31, 32 Further, hypoxia‐induced autophagy leads to granzyme B degradation and allows tumour cells to escape from NK‐mediated killing.33, 34 The effects of hypoxia on reduced NK activity can also be indirect. For example, in melanoma cells, hypoxic stress increases the gap junctional Connexin43 expression in a HIF1‐α dependent manner35 and Connexin43 renders cells less susceptible to NK‐cell‐mediated lysis.36 The cytotoxicity of NK cells in metastatic niches has been shown to be decreased by hypoxia, which was demonstrated by injecting mice with cells derived from hypoxic mammary tumour cells, resulting in increased MDSC infiltration and NK inhibition.37
Effect of hypoxia on myeloid cells
Macrophages are one of the most abundant types of myeloid cells in the tumour microenvironment. Their main function is to engulf and digest debris and foreign substances, including microbes, extracellular matrix remnants and tumour cells.38 However, attracted by the hypoxic environment of tumour,39 TAMs can promote malignant progression in part by inducing angiogenesis40 and matrix remodelling that supports progressively growing neoplasms.41 Hypoxia promotes the expression of VEGF, fibroblast growth factor, and matrix metallopeptidases 7 and 9 genes by macrophages, which leads to an increase of macrophage infiltration into hypoxic regions, where they reduce inflammation to promote tumour progression.42 It has been demonstrated in vivo that HIF1‐α is required in the regulation of myeloid cell glycolytic capacity, survival and function in the inflammatory microenvironment.43
Both TAMs and MDSCs often express Semaphorin 3A via Neuropilin binding, which helps both types of cells to enter hypoxic zones. As they enter, HIF1‐α down‐regulates Semaphorin 3A, resulting in the accumulation of these cells in hypoxic zones.30 Studies in adenocarcinoma models support the idea that the M1 phenotype of TAMs dominates in the normoxic niches, whereas the M2 phenotype of TAMs dominated in hypoxic niches.44 Different types of HIF activity of TAMs have also been observed. HIF1‐α is stabilized during M1 polarization, which involves nuclear factor‐κB,45, 46, 47, 48 whereas HIF2‐α is stabilized during M2 polarization.49, 50 The consequences of this differential expression of HIFs are not known, although it has been shown that HIF2‐α is an important regulator of arginase1 gene expression, a molecular marker of M2 polarization.51 During oxygen deficiency, HIF1‐α is stabilized in TAMs, which is associated with the increase of transcription and secretion in VEGF, crucial for pro‐tumoral angiogenesis. TAMs that express angiopoietin 2 (Tie2+), induced by hypoxia in monocytes, are profoundly pro‐angiogenic.52 HIF1‐α expression by TAMs is also known to be responsible for the suppression of T‐cell responses53 and promotes PD‐L1 expression on MDSCs.28
Effect of hypoxia on neutrophils
Neutrophils are phagocytic granulocytes that are mediators of innate immunity. Despite their short lifespans, they are the most abundant class of white blood cells in humans. There is a link between the high rate of tumour invasiveness and elevated neutrophils and those neutrophils are stabilized by hypoxia.54 Hypoxia promotes neutrophil survival in a HIF1‐α‐mediated manner,48 which promotes a loss of host defence function of neutrophils. Degradation of HIF1‐α is promoted by activation of prolyl hydroxylase enzymes, PHD‐2 and PHD‐3. A direct and specific role for PHD3 in promoting neutrophil survival in hypoxia has been observed using PHD3‐deficient neutrophils.55 It is possible that the effect of hypoxia on neutrophil longevity is mediated by a significant inhibition of neutrophil apoptosis, which is the major mechanism involved in the resolution of inflammation. This effect of hypoxia on neutrophil apoptosis has been shown in vitro to be a bcl‐2‐independent process.56 The mechanisms by which hypoxia regulates neutrophil survival have been studied, and it was shown that induced survival is mediated by HIF1‐α‐dependent nuclear factor‐κB activity.48 Hypoxia also appears to impair neutrophil chemotactic migration in vitro.57
Effect of hypoxia on dendritic cells
Dendritic cells are antigen‐presenting cells that play an important role in connecting innate and adaptive immunity. There are few studies investigating the effect of hypoxia on DCs. In contrast to other immune system cells, hypoxia appears to improve the activity of DCs, which may have a physiological role, as lymph nodes are known to be hypoxic.18 Hypoxia and HIF1‐α regulate DC maturation, activation and antigen‐presenting functions.58 Hypoxia regulates expression of co‐stimulatory molecules CD80 and CD86 by DCs. Dendritic cells stabilized HIF1‐α and this resulted in a stronger T‐cell activation and proliferation with PHD inhibition. HIF1‐α also hinders differentiation of bone marrow precursors into plasmacytoid DCs.31
Effect of acidosis on immune function
In contrast with hypoxia, studies on the effect of low pH on the immune system are less well developed, despite its near‐universal presence in solid tumours. Tumour acidosis is a phenomenon of tumour progression, and results from elevated fermentative carbohydrate metabolism combined with poor perfusion. During carcinogenesis, cancer cells are selected by stressful conditions to ferment glucose, even in the presence of adequate oxygen.59 Even though aerobic glycolysis (fermentation) is less efficient than respiration for energy production, it is one of the most commonly observed phenotypes in cancers. A significant consequence of increased glycolysis is acidification of the extracellular milieu and some have proposed that cancer cells that produce acid are more competitive and hence, acidity itself, rather than glycolytic ATP production or access to anabolic substrates, is the selected phenotype.60
Releasing lactate at high rates is also a strategy to overcome immune surveillance. Some studies investigate lactate without changing pH, and others focus on acidosis without changing lactate,61 whereas both are changing simultaneously in vivo. Those studies that have investigated both in concert have observed that low pH exacerbates lactate effects and vice‐versa. Lactate is a promoter of tumour acidity, local invasion and matrix remodelling.62 This acidity has direct effects on the immune system.
The acidic tumour microenvironment has multiple consequences relevant to carcinogenesis, including somatic selection of cancer cells during the initial phase of tumorigenesis for augmented cell survival, escape from apoptosis and drug resistance.63 More recent studies also demonstrated that acidosis can alter the functions of cells of the immune system, including T cells, neutrophils, macrophages and DCs.7, 64 The effect of low pH on the immune system is discussed below and summarized in Table 2.
Table 2.
Effects of lactate and acidosis on immune cells
| Immune cell | Pathway | Effect |
|---|---|---|
| Myeloid cells | Lactate | MDSC71

|
Lactate  VEGF VEGF 
|
Pro‐angiogenic factor release70 | |
Low pH  TNF‐α TNF‐α
 NF‐κB72 NF‐κB72
|
iNOS 
|
|
| Lactic acid | M1  M273 M273
|
|
| Neutrophils | Low pH  CD18 and Ca2+ CD18 and Ca2+

|
Neutrophil survival76

|
| Dendritic cells | Low pH | Antigen‐presenting capacity78

|
| T cells | Low pH  Sequestration of IFN‐γ mRNA Sequestration of IFN‐γ mRNA |
T‐cell activation and functions  61
61
|
Low pH  IFN‐γ and TNF‐α IFN‐γ and TNF‐α

|
Th1  Th2 Th2  66
66
|
|
| CD8+ T cells | Low pH  IL‐2Ra, TCR, STAT5 IL‐2Ra, TCR, STAT5 
|
Anergy68 |
| TAMs | Pro‐angiogenic phenotype70 |
IFN‐γ, interferon‐γ; IL‐2Ra, interleukin‐2 receptor; iNOS, inducible nitric oxide synthase; MDSC, myeloid‐derived suppressor cells; NF‐κB, nuclear factor‐κB; STAT5, signal transducer and activator of transcription 5; TAMs, tumour‐associated macrophages; TCR, T‐cell receptor; Th1, T helper type 1; TNF‐α, tumour necrosis factor‐α; VEGF, vascular endothelial growth factor.
Effect of acidosis on lymphocytes
Both cancer and immune cells are highly dependent on the glycolytic pathway for proliferation, survival and activity. Cancer cells rely on glycolysis even under aerobic conditions, which may be due to rapid temporal changes in energy demands for the cell membrane activities needed for division, growth and migration.65 This allows cancer cells to rapidly adapt to changing conditions more effectively than non‐glycolytic cells. Furthermore, elevated glycolysis significantly reduces glucose availability and, although cancer cells can enter quiescence in the absence of glucose, activated T cells do not survive without glucose. Thus, reduced glucose alone puts T cells at a disadvantage when attempting to expand into an acidic environment.66
An acidic tumour microenvironment does not appear to affect CD4+ Treg cells, possibly because they primarily rely on fatty acid oxidation.67 In contrast to CD4+ cells, acidification profoundly induces an anergic state in both human and mouse tumour‐specific CD8+ T lymphocytes. This results in severe reductions in cytolytic activity, cytokine secretion, down‐regulation of IL‐2Ra (CD25) and TCR followed by diminished activation of signal transducer and activator of transcription 5/extracellular signal‐regulated kinase (ERK) signalling.68 It is also reported that low pH affects plasma membrane and microtubule mobility, leading to decreased association of different TCR components with CD8 or other co‐receptors, so contributing to T‐cell anergy.69 It has been shown in vitro that raising the culture pH can reverse T‐cell anergy and that long‐term exposure to low pH can cause permanent damage or induce T‐cell apoptosis. In the short‐term, however, acidosis leads to increased persistence, presumably because of the aforementioned anergy.
Lymphocyte motility under low pH microenvironments has also been investigated. Stimulation of murine splenic lymphocytes with IL‐2 in three‐dimensional gels showed that acidic pH increases the migration of lymphocytes through the extracellular matrix. Acidic pH blocks the activation and anti‐tumour functions of T cells in vitro through sequestration of IFN‐γ mRNA and this is associated with metabolic changes that are not mediated by acidification of cytoplasmic pH.61 In this study, it was also shown that neutralization of tumour acidity in vivo with oral buffers increased the efficacy of checkpoint inhibitors and adoptive T‐cell transfer. Furthermore, tumour acidity promotes tumour progression by negatively affecting maturation and function of Th1 lymphocytes while stimulating the progression of tumour‐promoting Th2 lymphocytes by inactivation of IFN‐γ and suppression of tumour necrosis factor‐α.66
Effect of acidosis on myeloid cells
The influence of pH on macrophage polarization is less well documented. Some studies focused on the effect on tumour angiogenesis, and showed that the process of activation and transformation of the TAMs into a pro‐angiogenic phenotype in breast cancer is stimulated by low pH and high lactate concentrations.70
Lactate has been found to be responsible for promoting survival and proliferation of MDSC71 and to increase the release of pro‐angiogenic factors by the murine macrophage cell line RAW264.7 through induction of VEGF production and release. In contrast, another study has shown that there is no effect of low pH on angiogenic activity of human macrophages. Other studies of low pH effect on regulation of macrophages have been focused on activation of inducible isoform of nitric oxide synthase (iNOS). Macrophages exposed to low pH had an elevated iNOS level in a nuclear factor‐κB‐dependent manner, which can be activated by tumour necrosis factor‐α.72 Lactic acid produced by tumour cells affects signalling through the induction of VEGF and M2‐like polarization of TAMs,73 and gives rise to the expression of arginase 1 in macrophages to inhibit T‐cell proliferation and activation. Consistent with this, we have observed that adaptation of macrophages to low pH induces an M1 → M2 phenotypic switch, characterized by a loss of iNOS and induction of arginase and the mannose receptor, CD206.74
Effect of acidosis on neutrophils
The effect of acidosis on neutrophils was comprehensively reviewed in 2001 by Lardner, in which it was stated that acidic pH leads to decrease of superoxide production.75 Acidosis can also elicit human neutrophil activation through transient up‐regulation of CD18 expression, increase in Ca2+ over resting levels, and delays in rate of apoptosis.76 Acidosis‐induced neutrophil activation occurs via phosphatidylinositol 3‐kinase/Akt and ERK pathways.77
Effect of acidosis on dendritic cells
Studies investigating the effect of acidosis on DC behaviour found that extracellular acidity improved the antigen‐presenting capacity of DCs derived from murine bone marrow.78 In this study, lactic acid either alone or with combination of the tumour‐derived cytokines macrophage colony‐stimulating factor and IL‐6, significantly altered antigen presentation and functional activity of DCs, pointing to a possible metabolic effector function of lactate independent of its effects on pH.
Targeting hypoxia and acidosis to improve immunotherapy
Immunotherapy is a growing and promising field that can lead to improved control of cancers with high mutational loads (neo‐antigens). The prominent types of immunotherapy include immune checkpoint inhibitors, cancer vaccines and adoptive T‐cell transfer. Although significant progress has been made, durable response rates remain low. For instance, objective response rates in melanoma are from 18% to 27% for anti‐PD‐1 or PD‐L1 antibodies, and 11% for anti‐CTLA‐4 antibodies. Notably, the combination of PD‐1 and CTLA‐4 checkpoint blockades showed an increase in response when compared with using either checkpoint alone, from 20% to 40%. This implies, however, that 60% of patients are non‐responsive and so, other immunosuppressive activities are probably present. An underexplored inhibitor of immunotherapy is the impact of physical tumour microenvironment on immune function. Given the above evidence of the impact of hypoxia and acidosis on immune function, it is reasonable to expect that manipulating the microenvironment will enhance the outcomes of immunotherapy in some patients, especially if they are biomarker driven.
Targeting hypoxia in combination with immunotherapy
Hypoxia influences immune checkpoint receptors and their respective ligands. HIF1‐α and HIF2‐α were both investigated in relation to PD‐L1, whose promoter contains a hypoxia‐response element. Importantly, hypoxia selectively up‐regulates PD‐L1 on MDSCs through HIF1‐α.28 This was not limited to MDSCs, as hypoxia also significantly increased PD‐L1 expression on macrophages, DCs and tumour cells.28 Combinatorial approaches using HIF1‐α inhibitors with PD‐L1 blockade may boost immune responses in patients.79 Another combinatorial approach using a HIF1‐α inhibitor with DC‐based immunotherapy resulted in tumour regression and improved survival in a mouse model of breast cancer.80
Alternatively, targeting tumour hypoxia directly may enhance immunotherapy responses by decreasing the number and function of immunosuppressive cells, increasing effector T cells and improving vaccine efficacy. Tumour cells adapt to hypoxia by angiogenic switch with production of VEGFs, angiopoietin‐2, IL‐8 and other factors. As dysfunctional vessels lead to hypoxia and tumour resistance there is a strong effect to normalize vessels to reduce hypoxic volumes within the tumour microenvironment. Normalizing vessels by targeting VEGF/VEGFR promotes CTL entry to tumour mass. It also affects macrophage polarization and stimulates M1 phenotype. Inducing Th1 and triggering secretion of IFN‐γ and IL‐12 allows CTL infiltration which triggers decrease in Treg cells.81 Targeting hypoxic regions with hypoxia‐activated pro‐drugs, evofosfamide (TH‐302), improved checkpoint blockade (CTLA‐4, PD‐1) in transplantable and genetically engineered prostate cancers in preclinical models.82 Hypoxia‐specific cytotoxic activity of hypoxia‐activated pro‐drugs was observed with in vivo and in vitro models.83, 84 Based on these and other data, a clinical trial (NCT03098160) combining evofosfamide with ipilimumab in solid tumours was opened in May 2017.
Targeting acidosis in combination with immunotherapy
Therapies based on manipulation of tumour micro‐environmental acidosis using buffers have shown improved results as monotherapy in a variety of animal cancer models.85, 86, 87, 88 According to preliminary studies, neutralizing tumour acidosis with buffers in combination with immunotherapy can lead to improved durable outcomes.61 In this study, oral bicarbonate buffer (200 mm ad libitum) was used to neutralize tumour acidity and, in combination with anti‐CTLA‐4, anti PD‐1 or adoptive T‐cell therapy, led to durable responses in both B16 melanoma and Panc02 pancreatic cancer models. These findings suggest that buffer therapy may improve response rates to immune therapies in the clinic. However, a buffer therapy approach is not easy to implement clinically as three clinical trials (NCT‐1350583, NCT‐01198821 and NCT‐1846429) were not successful due to poor patient compliance and grade 2 gastrointestinal events. Another approach to increase tumour pH directly is by using the action of urease enzyme. Ureases are urea‐degrading enzymes that convert urea into more toxic ammonia and lead to a local pH rise from the generated ammonia.89 CEACAM6‐targeted Jack bean urease (L‐DOS47) has begun to be used in preclinical tumour models and clinical trials to modify the tumour microenvironment.90
There are numerous possibilities to target tumour acidity more indirectly by influencing metabolism or ion transport. For example, carbonic anhydrase IX (CA‐IX) controls intracellular and extracellular acid–base balance to maintain survival and is a key regulator of extracellular acidity. As a catalyser of reversible hydration of CO2 to bicarbonate and protons at the extracellular surface, inhibition of the catalytic activity of CA‐IX can manipulate its role in pH regulation.10 Lactate overproduction is another hallmark of cancer that is associated with acidity and can impact a cell's ability to regulate its pH in the face of acidity.91 As tumour cells need slightly alkaline intracellular pH, they overcome the accumulation of lactate and H+ ions in terms of using monocarboxylate transporters (primarily MCT‐4) that facilitate the export of lactate and H+. By reversing the molecular machinery, cancer cells provide alkaline intracellular media while having an external acidic pH, which is also beneficial for the survival and invasive characteristic of cancer cells. Furthermore, there is a mutual metabolic relationship between cancer and stromal cells. In contrast to the belief that stromal cells use only glucose as an energy source, it was demonstrated that they also can use cancer‐cell‐derived lactate, which is transported into cells primarily with MCT‐1.92 There is a strong effort to target MCTs, and these approaches may be combined with immune therapies by virtue of their effects on pH regulation.10 Targeting lactate metabolism is another promising approach for the modulation of tumour pH. High glycolysis rates in cancer cells result in lactate overproduction. As cancer cells export lactate rather than using it as a nutrient, it acidifies the tumour microenvironment.93 One such approach could be targeting lactate dehydrogenase A (LDHA), which has been shown to inhibit pyruvate to lactate in vivo conversion.94, 95 On the other hand, use of LDHA inhibitors is challenging as it was reported that LDHA deletion in CD4 T cells shows defects in IFN‐γ production.96
Conclusion
Until recently, the primary approach to treating cancer has been the identification of genetic instabilities with gene‐product‐targeted therapies, which has failed to lead to durable responses. The emergence of immune targeted therapies has led to more durable responses, although response rates remain low. The role of the tumour microenvironment's effect on the immune system has begun to be studied. Both hypoxia and tumour pH have direct and indirect effects on the immune system and are, in general, ‘immune suppressors’. There is strong evidence that hypoxia and low pH can contribute to resistance to immune therapies. Although important progress has been made to understand the mechanisms underlying the effects of hypoxia and low pH on immunotherapy, non‐negligible knowledge gaps still remain. There is strong evidence that hypoxia and acidic pH in the tumour microenvironment have dramatic effects on immune system cells and therapeutic resistance. New treatments that aim to perturb them is an intriguing avenue for further improving responses to immunotherapy.
Conflict of interest
RJG has financial interests in Molecular Templates (formerly Threshold Pharma), Helix Biopharma and HealthMyne, Inc.
Sultan Damgaci participates in a research agreement with Helix BioPharma.
References
- 1. Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007; 101:805–15. [DOI] [PubMed] [Google Scholar]
- 2. Bremnes RM, Al‐Shibli K, Donnem T, Sirera R, Al‐Saad S, Andersen S et al The role of tumor‐infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis emphasis on non‐small cell lung cancer. J Thorac Oncol 2011; 6:824–33. [DOI] [PubMed] [Google Scholar]
- 3. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309–22. [DOI] [PubMed] [Google Scholar]
- 4. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Topalian SL, Drake CG, Pardoll DM. Targeting the PD‐1/B7‐H1(PD‐L1) pathway to activate anti‐tumor immunity. Curr Opin Immunol 2012; 24:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Draghiciu O, Nijman HW, Daemen T. From tumor immunosuppression to eradication: targeting homing and activity of immune effector cells to tumors. Clin Dev Immunol 2011; 2011:439053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P et al B7‐H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 2006; 203:871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diaz‐Montero CM, Finke J, Montero AJ. Myeloid‐derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol 2014; 41:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonald PC, Chafe SC, Dedhar S. Overcoming hypoxia‐mediated tumor progression: combinatorial approaches targeting pH regulation, angiogenesis and immune dysfunction. Front Cell Dev Biol 2016; 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carreau A, El Hafny‐Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 2011; 15:1239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia‐inducible factors in cancer. Pharmacol Therapeut 2016; 164:152–69. [DOI] [PubMed] [Google Scholar]
- 13. Gillies RJ, Schornack PA, Secomb TW, Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia New York, NY. 1999; 1:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307:58–62. [DOI] [PubMed] [Google Scholar]
- 15. Palazon A, Aragones J, Morales‐Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res 2012; 18:1207–13. [DOI] [PubMed] [Google Scholar]
- 16. Atkuri KR, Herzenberg LA, Niemi AK, Cowan T, Herzenberg LA. Importance of culturing primary lymphocytes at physiological oxygen levels. P Natl Acad Sci USA 2007; 104:4547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atkuri KR, Herzenberg LA, Herzenberg LA. Culturing at atmospheric oxygen levels impacts lymphocyte function. P Natl Acad Sci USA 2005; 102:3756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohta A, Diwanji R, Kini R, Subramanian M, Ohta A, Sitkovsky M. In vivo T cell activation in lymphoid tissues is inhibited in the oxygen‐poor microenvironment. Front Immunol 2011; 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neumann AK, Yang J, Biju MP, Joseph SK, Johnson RS, Haase VH et al Hypoxia inducible factor 1α regulates T cell receptor signal transduction. P Natl Acad Sci USA 2005; 102:17071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P et al Hypoxia‐inducible factor‐1α‐dependent induction of FoxP3 drives regulatory T‐cell abundance and function during inflammatory hypoxia of the mucosa. P Natl Acad Sci USA 2012; 109:E2784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasmim M, Noman MZ, Messai Y, Bordereaux D, Gros G, Baud V et al Cutting edge: hypoxia‐induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF‐β1. J Immunol 2013; 191:5802–6. [DOI] [PubMed] [Google Scholar]
- 22. Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP et al Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011; 475:226–30. [DOI] [PubMed] [Google Scholar]
- 23. Yang M, Ma C, Liu S, Sun J, Shao Q, Gao W et al Hypoxia skews dendritic cells to a T helper type 2‐stimulating phenotype and promotes tumour cell migration by dendritic cell‐derived osteopontin. Immunology 2009; 128:e237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y et al Control of TH17/T‐reg balance by hypoxia‐inducible factor 1. Cell 2011; 146:772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK et al A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA 2006; 103:13132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG et al Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol 2001; 167:6140–9. [DOI] [PubMed] [Google Scholar]
- 27. Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P et al Targeted Deletion of HIF‐1α gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS ONE 2007; 2:e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P et al PD‐L1 is a novel direct target of HIF‐1α, and its blockade under hypoxia enhanced MDSC‐mediated T cell activation. J Exp Med 2014; 211:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barsoum IB, Hamilton TK, Li X, Cotechini T, Miles EA, Siemens DR et al Hypoxia induces escape from innate immunity in cancer cells via increased expression of ADAM10: role of nitric oxide. Cancer Res 2011; 71:7433–41. [DOI] [PubMed] [Google Scholar]
- 30. Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 2017; 36:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol 2015; 42:378–86. [DOI] [PubMed] [Google Scholar]
- 32. Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E et al Hypoxic tumor‐derived microvesicles negatively regulate NK cell function by a mechanism involving TGF‐ and miR23a transfer. Oncoimmunol 2016; 5:e1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K et al Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer‐mediated lysis under hypoxia. Proc Natl Acad Sci USA 2013; 110:17450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Viry E, Baginska J, Berchem G, Noman MZ, Medves S, Chouaib S et al Autophagic degradation of GZMB/granzyme B: a new mechanism of hypoxic tumor cell escape from natural killer cell‐mediated lysis. Autophagy 2014; 10:173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janji B, Viry E, Moussay E, Paggetti J, Arakelian T, Mgrditchian T et al The multifaceted role of autophagy in tumor evasion from immune surveillance. Oncotarget 2016; 7:17591–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tittarelli A, Janji B, Van Moer K, Noman MZ, Chouaib S. The selective degradation of synaptic connexin 43 protein by hypoxia‐induced autophagy impairs natural killer cell‐mediated tumor cell killing. J Biol Chem 2015; 290:23670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM et al Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res 2012; 72:3906–11. [DOI] [PubMed] [Google Scholar]
- 38. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol 2015; 33:643–75. [DOI] [PubMed] [Google Scholar]
- 39. Lewis C, Murdoch C. Macrophage responses to hypoxia – implications for tumor progression and anti‐cancer therapies. Am J Pathol 2005; 167:627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA et al Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 2006; 66:11238–46. [DOI] [PubMed] [Google Scholar]
- 41. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer 2005; 117:701–8. [DOI] [PubMed] [Google Scholar]
- 43. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front Immunol 2014; 5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J et al Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6Chigh monocytes. Cancer Res 2010; 70:5728–39. [DOI] [PubMed] [Google Scholar]
- 45. Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL et al Hypoxia‐inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood 2009; 114:844–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL et al HIF‐1α expression regulates the bactericidal capacity of phagocytes. J Clin Invest 2005; 115:1806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia‐inducible factor 1α . Blood 2004; 103:1124–30. [DOI] [PubMed] [Google Scholar]
- 48. Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T et al Hypoxia‐induced neutrophil survival is mediated by HIF‐1α‐dependent NF‐κB activity. J Exp Med 2005; 201:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takeda N, O'Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C et al Differential activation and antagonistic function of HIF‐α isoforms in macrophages are essential for NO homeostasis. Genes Dev 2010; 24:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rodriguez‐Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin‐Sanz P et al Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 2010; 185:605–14. [DOI] [PubMed] [Google Scholar]
- 51. Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care 2003; 6:223–8. [DOI] [PubMed] [Google Scholar]
- 52. De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M et al Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005; 8:211–26. [DOI] [PubMed] [Google Scholar]
- 53. Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG et al Macrophage expression of hypoxia‐inducible factor‐1α suppresses T‐cell function and promotes tumor progression. Cancer Res 2010; 70:7465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP et al Tumor‐recruited neutrophils and neutrophil TIMP‐free MMP‐9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 2011; 179:1455–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walmsley SR, Chilvers ER, Thompson AA, Vaughan K, Marriott HM, Parker LC et al Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Investig 2011; 121:1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hannah S, Mecklenburgh K, Rahman I, Bellingan GJ, Greening A, Haslett C et al Hypoxia prolongs neutrophil survival in vitro . FEBS Lett 1995; 372:233–7. [DOI] [PubMed] [Google Scholar]
- 57. Rotstein OD, Fiegel VD, Simmons RL, Knighton DR. The deleterious effect of reduced pH and hypoxia on neutrophil migration in vitro . J Surg Res 1988; 45:298–303. [DOI] [PubMed] [Google Scholar]
- 58. Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG et al Hypoxia and hypoxia‐inducible factor‐1α modulate lipopolysaccharide‐induced dendritic cell activation and function. J Immunol 2008; 180:4697–705. [DOI] [PubMed] [Google Scholar]
- 59. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4:891–9. [DOI] [PubMed] [Google Scholar]
- 60. Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med 2008; 49(Suppl 2):24S–42S. [DOI] [PubMed] [Google Scholar]
- 61. Pilon‐Thomas S, Kodumudi KN, El‐Kenawi AE, Russell S, Weber AM, Luddy K et al Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res 2016; 76:1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barbosa IA, Machado NG, Skildum AJ, Scott PM, Oliveira PJ. Mitochondrial remodeling in cancer metabolism and survival: potential for new therapies. Bba‐Rev Cancer 2012; 1826:238–54. [DOI] [PubMed] [Google Scholar]
- 63. Damaghi M, Tafreshi NK, Lloyd MC, Sprung R, Estrella V, Wojtkowiak JW et al Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat Commun 2015; 6:8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol 2012; 13:1129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Epstein T, Xu L, Gillies RJ, Gatenby RA. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab 2014; 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kareva I, Hahnfeldt P. The emerging “hallmarks” of metabolic reprogramming and immune evasion: distinct or linked? Cancer Res 2013; 73:2737–42. [DOI] [PubMed] [Google Scholar]
- 67. Hirschhaeuser F, Sattler UG, Mueller‐Klieser W. Lactate: a metabolic key player in cancer. Cancer Res 2011; 71:6921–5. [DOI] [PubMed] [Google Scholar]
- 68. Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A et al Modulation of microenvironment acidity reverses anergy in human and murine tumor‐infiltrating T lymphocytes. Cancer Res 2012; 72:2746–56. [DOI] [PubMed] [Google Scholar]
- 69. Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF et al Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor‐infiltrating lymphocytes. Immunity 2008; 28:414–24. [DOI] [PubMed] [Google Scholar]
- 70. Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol 2001; 70:478–90. [PubMed] [Google Scholar]
- 71. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor‐derived lactate modifies antitumor immune response: effect on myeloid‐derived suppressor cells and NK cells. J Immunol 2013; 191:1486–95. [DOI] [PubMed] [Google Scholar]
- 72. Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B et al Low environmental pH is responsible for the induction of nitric‐oxide synthase in macrophages. Evidence for involvement of nuclear factor‐κB activation. J Biol Chem 1998; 273:5086–92. [DOI] [PubMed] [Google Scholar]
- 73. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V et al Functional polarization of tumour‐associated macrophages by tumour‐derived lactic acid. Nature 2014; 513:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. El‐Kenawi AE, Ibrahim‐Hashim AA, Luddy KA, Pilon‐Thomas SA, Gatenby RA, Gillies RJ. Extracellular acidosis alters polarization of macrophages. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18–22; Philadelphia, PA Philadelphia (PA): AACR; Cancer Res 2015;75(15 Suppl):Abstract nr 3213. doi:10.1158/1538-7445AM2015-3213. 2015. [Google Scholar]
- 75. Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol 2001; 69:522–30. [PubMed] [Google Scholar]
- 76. Trevani AS, Andonegui G, Giordano M, Lopez DH, Gamberale R, Minucci F et al Extracellular acidification induces human neutrophil activation. J Immunol 1999; 162:4849–57. [PubMed] [Google Scholar]
- 77. Martinez D, Vermeulen M, Trevani A, Ceballos A, Sabatte J, Gamberale R et al Extracellular acidosis induces neutrophil activation by a mechanism dependent on activation of phosphatidylinositol 3‐kinase/Akt and ERK pathways. J Immunol 2006; 176:1163–71. [DOI] [PubMed] [Google Scholar]
- 78. Vermeulen M, Giordano M, Trevani AS, Sedlik C, Gamberale R, Fernandez‐Calotti P et al Acidosis improves uptake of antigens and MHC class I‐restricted presentation by dendritic cells. J Immunol 2004; 172:3196–204. [DOI] [PubMed] [Google Scholar]
- 79. Lazarus D, Peters C, Stockmann A, Eliasof S, Jayaraman L. CRLX101, an investigational nanoparticle–drug conjugate of camptothecin, demonstrates synergy with immunotherapy agents in preclinical models. Cancer Res 2016; 76:3209. [DOI] [PubMed] [Google Scholar]
- 80. Kheshtchin N, Arab S, Ajami M, Mirzaei R, Ashourpour M, Mousavi N et al Inhibition of HIF‐1α enhances anti‐tumor effects of dendritic cell‐based vaccination in a mouse model of breast cancer. Cancer Immunol Immunother 2016; 65:1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B et al Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am J Physiol‐Cell Physiol 2015; 309:C569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ai M, Budhani P, Sheng J, Ager CR, Dharia DD, Curran MA. Tumor hypoxia drives immune suppression and immunotherapy resistance. J Immunother Cancer 2015; 3(Suppl 2):P392. [Google Scholar]
- 83. Portwood S, Lal D, Hsu YC, Vargas R, Johnson MK, Wetzler M et al Activity of the hypoxia‐activated prodrug, TH‐302, in preclinical human acute myeloid leukemia models. Clin Cancer Res 2013; 19:6506–19. [DOI] [PubMed] [Google Scholar]
- 84. Hu J, Handisides DR, Van Valckenborgh E, De Raeve H, Menu E, Vande Broek I et al Targeting the multiple myeloma hypoxic niche with TH‐302, a hypoxia‐activated prodrug. Blood 2010; 116:1524–7. [DOI] [PubMed] [Google Scholar]
- 85. Ibrahim Hashim A, Cornnell HH, Coelho Ribeiro Mde L, Abrahams D, Cunningham J, Lloyd M et al Reduction of metastasis using a non‐volatile buffer. Clin Exp Metastasis 2011; 28:841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ibrahim‐Hashim A, Cornnell HH, Abrahams D, Lloyd M, Bui M, Gillies RJ et al Systemic buffers inhibit carcinogenesis in TRAMP mice. J Urol 2012; 188:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ibrahim‐Hashim A, Wojtkowiak JW, de Lourdes Coelho Ribeiro M, Estrella V, Bailey KM, Cornnell HH et al Free base lysine increases survival and reduces metastasis in prostate cancer model. J Cancer Sci Ther 2011; Suppl 1(4): JCST‐S1‐004. [PMC free article] [PubMed] [Google Scholar]
- 88. Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF et al Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 2009; 69:2260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wong WY, DeLuca CI, Tian B, Wilson I, Molund S, Warriar N et al Urease‐induced alkalinization of extracellular pH and its antitumor activity in human breast and lung cancers. J Exp Ther Oncol 2005; 5:93–9. [PubMed] [Google Scholar]
- 90. Tian B, Wong WY, Hegmann E, Gaspar K, Kumar P, Chao H. Production and characterization of a camelid single domain antibody‐urease enzyme conjugate for the treatment of cancer. Bioconjug Chem 2015; 26:1144–55. [DOI] [PubMed] [Google Scholar]
- 91. Zhang Y, Yang JM. Altered energy metabolism in cancer. A unique opportunity for therapeutic intervention. Cancer Biol Ther 2013; 14:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN et al Targeting lactate‐fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Investig 2008; 118:3930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest 2013; 123:3685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rajeshkumar NV, Dutta P, Yabuuchi S, de Wilde RF, Martinez GV, Le A et al Therapeutic targeting of the Warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Res 2015; 75:3355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dutta P, Le A, Vander Jagt DL, Tsukamoto T, Martinez GV, Dang CV et al Evaluation of LDH‐A and glutaminase inhibition in vivo by hyperpolarized 13C‐pyruvate magnetic resonance spectroscopy of tumors. Can Res 2013; 73:4190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell 2017; 169:570–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


