Summary
A number of polymorphisms in immune‐regulatory genes have been identified as risk factors for the development of autoimmune disease. PTPN22 (that encodes a tyrosine phosphatase) has been associated with the development of several autoimmune diseases, including type 1 diabetes, rheumatoid arthritis and systemic lupus erythematosus. PTPN22 regulates the activity and effector functions of multiple important immune cell types, including lymphocytes, granulocytes and myeloid cells. In this review, we describe the role of PTPN22 in regulating T‐cell activation and effector responses. We discuss progress in our understanding of the impact of PTPN22 in autoimmune disease in humans and mouse models, as well as recent evidence suggesting that genetic manipulation of PTPN22 expression might enhance the efficacy of anti‐tumour T‐cell responses.
Keywords: autoimmunity, signal transduction, T‐cell, tumour immunology
Abbreviations
- PTPN22
protein tyrosine phosphatase, non‐receptor type, 22
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- SNP
single nucleotide polymorphism
- T1D
type 1 diabetes
- TCR
T‐cell receptor
- TGFβ
transforming growth factor β
- ZAP70
zeta chain associated protein kinase of 70 kDa
Introduction
Regulation of T‐cell receptor (TCR) signalling and activation is essential for the maintenance of immunological tolerance and homeostasis. Disruption of these complex signalling networks can lead to undesirable outcomes, such as unregulated inflammation, autoimmunity or ineffective anti‐tumour responses. The identification of HLA class II alleles as strong risk factors for the development of autoimmune diseases1 clearly implicates T‐cell activation as an important driver of auto‐reactive immune responses. Furthermore, polymorphisms in genes encoding molecules involved in the regulation of T‐cell signalling and activation, such as CTLA42 and IL‐2,1 have been linked to autoimmunity, highlighting the need for appropriate T‐cell responses in order to maintain tolerance.
In the past 15 years, polymorphisms in PTPN22 (that encodes a cytoplasmic tyrosine phosphatase) have been identified as risk factors for the development of autoimmune diseases, such as type 1 diabetes (T1D), rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).3 Subsequently, important cell‐intrinsic roles for PTPN22 (protein tyrosine phosphatase, non‐receptor type #bib22) in a number of immune cell populations, including B‐cells,4, 5 neutrophils,6 dendritic cells7, 8 and myeloid cells,9 have been identified. In this review, we focus on the mechanisms by which PTPN22 regulates T‐cell activation. We describe recent progress in the field highlighting the importance of PTPN22 in regulating both autoimmune and anti‐tumour T‐cell responses.
T‐cell activation and PTPN22
T‐cell activation results when the TCR engages antigenic peptide presented by major histocompatibility complex (MHC) molecules on the surface of antigen‐presenting cells (APCs). TCR signalling is initiated by the Src tyrosine kinases, Lck and Fyn, that phosphorylate immunoreceptor tyrosine‐based activatory motifs (ITAMs) in TCR‐associated CD3 and zeta chains. ITAM phosphorylation enables the recruitment and phosphorylation of the zeta‐associated protein kinase of 70 kDa (ZAP70), and the propagation of downstream signals ultimately leading to gene expression and effector functions (reviewed in10, 11).
The activity and function of Src family kinases is regulated by phosphorylation of key tyrosine residues within the kinase domain (e.g. Lck Y394) and the C‐terminus (e.g. Lck Y505; reviewed in11). In this regard, when phosphorylated by Csk, the inhibitory Y505 residue forms an intermolecular association with the Lck src‐homology (SH)2 domain keeping Lck in a ‘closed’ conformation.11 Dephosphorylation of Lck Y505 by CD45 enables Lck to attain an ‘open’ or ‘primed’ conformation.11 By contrast, auto‐ or transphorylation of the active site Y394 is essential for optimal Lck kinase activity.
PTPN22 is a cytoplasmic, non‐receptor protein tyrosine phosphatase that is expressed predominantly in cells of haematopoietic origin.12 In T‐cells, PTPN22 as well as additional phosphatases including SHP‐1 (PTPN6) and PTPN2 can dephosphorylate Lck Y394, thereby limiting TCR proximal signalling.13, 14 A number of additional PTPN22 substrates have been identified, including ZAP70, TCRζ and VAV1.15 In order to dephosphorylate TCR proximal kinases Lck and ZAP70, cytoplasmic PTPN22 is thought to be recruited to the cell membrane through association with C‐terminal Src kinase (CSK) that in turn binds to transmembrane adapter proteins such as phosphoprotein associated with glycosphingolipid‐enriched microdomains (PAG).12, 16 Interestingly, a well‐described autoimmune‐associated polymorphism in PTPN22 (C1858T) results in an amino acid substitution that disrupts PTPN22‐CSK interactions (described in further detail below).
As well as impacting upon canonical TCR signalling pathways, PTPN22 also influences ‘inside‐out’ signalling to integrins such as LFA‐1. In the absence of PTPN22, TCR triggering results in enhanced activation of the small GTPase Rap1 and a subsequent increase in LFA‐1‐dependent adhesion.17 As a consequence, PTPN22‐deficient T‐cells have an increased propensity to form productive conjugates with APCs.18 Interestingly, PTPN22 also regulates integrin ‘outside‐in’ signalling. In migrating T‐cells, PTPN22 localizes to the leading edge and regulates the activation of Lck, ZAP70 and VAV1 following LFA‐1‐ICAM‐1 engagement.19
The generation of PTPN22‐deficient mice has increased our understanding of the role PTPN22 plays in limiting T‐cell activation and the maintenance of T‐cell homeostasis. Under resting conditions, Ptpn22‐deficient mice accumulate increased numbers of effector/memory phenotype T‐cells as compared with wild‐type counterparts.17, 20 Initial studies using TCR cross‐linking antibodies indicated that increased activation was seen for effector T‐cells lacking PTPN22, whereas the responses of naïve WT and Ptpn22 −/− T‐cells were indistinguishable.20 These data suggested that PTPN22 was important for the regulation of effector but not naïve T‐cell activation. Consistent with these findings, PTPN22 expression is elevated in effector and memory T‐cells relative to naïve T‐cells.21, 22 More recently, studies using the OT‐I TCR transgenic mouse strain [that expresses an MHC class I restricted ovalbumin (ova)‐specific TCR] have shown that naïve T‐cell activation is regulated by PTPN22. Thus, initial T‐cell activation in response to high‐affinity ova‐peptide antigens was unaffected by loss of PTPN22 expression, consistent with previous data using cross‐linking antibodies.18 By contrast, PTPN22 was important for limiting naïve T‐cell responses to weak agonist ova‐peptide variants. Furthermore, several studies have determined that the extent of T‐cell proliferation and activation under lymphopenic conditions in vivo is regulated by PTPN22.18, 23 Interestingly, in effector CD8+ cytotoxic T‐cells, the absence of PTPN22 reduced the threshold for activation in response to very low affinity, self‐antigen.18 These data are consistent with a central role for PTPN22 in maintaining T‐cell homeostasis and in limiting autoreactive T‐cell responses.
PTPN22 polymorphisms in autoimmunity
PTPN22 single‐nucleotide polymorphisms (SNPs) have been identified as risk factors for the development of autoimmunity in humans.24 Bottini and colleagues first identified a link between the PTPN22 C1858T SNP, which results in the substitution of tryptophan for arginine at position 620 (R620W), and type 1 diabetes.3 Many studies have subsequently confirmed an association of the PTPN22 R620W variant with T1D and other autoimmune conditions such as RA and SLE.25, 26, 27 Whilst much work has focused on the relatively common R620W PTPN22 variant, other PTPN22 SNPs have also been linked with some degree to autoimmunity. For example, a SNP in the PTPN22 promoter region (rs2488457) has been identified as a risk factor for the development of RA in Asian populations (reviewed in28). By contrast, a rare missense SNP in the PTPN22 catalytic domain (rs33996649) serves to lessen the risk of RA and SLE.29 PTPN22 R620W is not associated with all autoimmune conditions, for example multiple sclerosis30; there seems to be a particularly strong link of R620W with diseases characterized by the presence of autoantibodies.31 Interestingly, in mice, PTPN22 regulates the numbers of follicular T helper (Tfh) cells that are critical for B‐cells to make antibody responses within the germinal centre.32 In these experiments, the absence of PTPN22 permitted increased Tfh cell proliferation and elevated levels of IL‐21 production.32
The location of R620W (corresponding to position R619W in mouse) within the regulatory P1 PEST [proline (P), glutamic acid (E), serine (S), threonine (T)‐rich] domain of PTPN22 abrogates its interaction with CSK, a kinase that together with PAG and Dok adaptors also negatively regulates TCR signalling.16, 33 The loss of this interaction could predict a loss of normal PTPN22 function; however, the findings from a number of studies are more complex. An early study suggested that PTPN22 R620W had elevated phosphatase activity indicating gain‐of‐function, whilst T‐cells from carriers of the C1858T SNP produced lower levels of IL‐2 upon TCR stimulation.34 While some studies support this initial description of PTPN22 R620W function,35, 36, 37, 38 other studies have reported the converse to be true; that R620W confers a loss of PTPN22 function.19, 39, 40 Knock‐in mice engineered to express the PTPN22 R619W variant display an overall phenotype similar to that of PTPN22 knock‐out mice,8, 41 including T‐cell hyper‐responsiveness, suggesting that in mice the variant results in loss of PTPN22 function. However, it should be noted that mass spectrometry analysis indicates that the complete absence of PTPN22 has distinct effects on the mouse T‐cell phosphoproteome as compared with PTPN22 R619W expression.41 It is therefore likely that the precise impact of the R619W/R620W polymorphism on signalling pathways is highly context dependent.
Mouse models of PTPN22 function in autoimmunity
Researchers have attempted to clarify the role of PTPN22 in autoimmunity using mouse models. Expression of PTPN22 R619W does not result in the development of spontaneous autoimmunity on a C57BL/6 genetic background yet, when expressed in an autoimmune‐prone strain (129/Sv), knock‐in mice develop systemic autoimmunity.41 Similarly, deletion of PTPN22 in C57BL/6 mice does not result in overt autoimmunity;17, 20 however, when combined with a mutation in an additional phosphatase CD45 (E613R) the mice succumb to a lupus‐like disease.40 Thus, in mice, the absence of PTPN22 or expression of disease‐associated variants pre‐disposes to spontaneous autoimmunity only in a permissive genetic background.
PTPN22‐deficient mice have been crossed to additional autoimmune‐prone genetic backgrounds, such as the non‐obese diabetic (NOD)42, 43, 44 and the ZAP70‐mutant SKG strains.45 A summary of autoimmune models carried out in PTPN22‐mutant mouse strains is shown in Table 1. The results of these studies paint a complicated picture of the role of PTPN22 in the regulation of autoimmunity. Under some circumstances, the absence of PTPN22 confers a protective effect6, 43, 45, 46 whilst, in others, PTPN22‐deficiency or PTPN22 R619W expression enhances the severity of autoimmunity.17, 32, 40, 41, 42, 47 These apparently contradictory results are likely explained, at least in part, by the fact that PTPN22 regulates both inflammatory and anti‐inflammatory T‐cell responses. For example, PTPN22‐deficient mice have increased numbers of peripheral regulatory T‐cells (Tregs),17, 46, 48 and these Tregs are more suppressive.17 Consistent with these data, diminished autoimmune inflammation in PTPN22‐mutant animals in the EAE and NOD models was associated with enhanced regulatory T‐cell numbers and activity.43, 46
Table 1.
PTPN22 and models of autoimmunity
| Model | PTPN22 status | Genetic background | Impact on disease | Immunological features | References |
|---|---|---|---|---|---|
| EAE | KO | C57Bl/6 | Protection | Protection involves increased Tregs | 46 |
| Colitis | KO | C57Bl/6 | Exacerbated | Increased T‐cell expansion | 17 |
| Lupus like disease | KO + CD45 E613R | C57Bl/6 | Exacerbated | Enhanced effector/memory T‐cells | 40 |
| Diabetes | Knock‐down | NOD | Protection | Protection involves increased Tregs | 43 |
| Diabetes | Overexpression | NOD | Protection | Reduced Th1 functionality | 44 |
| Diabetes | R619W KI (CRISPR) | NOD | Exacerbated | Not assessed | 42 |
| Diabetes RIP‐LCMV | KO | C57Bl/6 | Exacerbated | Increased T effector function | 47 |
| Systemic autoimmunity | R619W (and STZ diabetes) | C57Bl/6x129/Sv | Exacerbated | B‐cell restricted R619W expression sufficient to induce autoimmunity | 41 |
| SKG arthritis | KO | SKG | Protection | Biasing of Th17 differentiation toward Th1/Treg | 45 |
| SKG arthritis | Transgenic R620W | SKG | No difference | – | 50 |
| KBxN arthritis | KO | C57Bl/6 | Exacerbated | Increased T follicular helper cells | 32 |
| KBxN arthritis | KO | C57Bl/6 | Protected | Reduced neutrophil activation | 6 |
PTPN22‐dependent regulation of Th differentiation also impacts upon disease severity in mouse models. For example, combined deletion of PTPN22 with the ZAP70 SKG mutation, a hypomorphic mutant allele of ZAP70 that gives rise to a CD4+ T‐cell‐driven model of arthritis,49 resulted in less severe disease.45 PTPN22 deficiency appeared to bias CD4+ Th cell differentiation away from the Th17 lineage, which is pathogenic in the SKG model, to a more Th1/Treg biased response, resulting in lower levels of inflammation.45 At a mechanistic level, it is possible that elevated IL‐2 secretion by Ptpn22 −/− T‐cells biases against Th17 polarization, instead favouring Th1/Treg differentiation. Similarly, the PTPN22 R620W variant favoured Th1 differentiation and diminished Th17 differentiation in human T‐cells.38 Thus, the precise nature of the disease‐driving T‐cell response and the balance between inflammatory and regulatory CD4+ T‐cells populations is critical for the outcome of disease in PTPN22‐deficient and knock‐in mouse models.
A number of studies have sought to determine the role of PTPN22 in T‐cell development and central tolerance. Overall numbers and distributions of thymocyte subsets are unaffected in Ptpn22 −/− mice expressing a polyclonal T‐cell repertoire.17, 20 PTPN22‐deficiency resulted in a small increase in the positive selection of DO11·10 and HY TCR transgenic single‐positive thymocytes;20 however, no increases in absolute numbers of single‐positive thymocytes were apparent in the OT‐1 system.18 There was no impact of PTPN22 on negative selection of HY thymocytes20 or in transgenic mice expressing PTPN22 R620W.50 Finally, TCR sequencing analyses suggested that the absence of PTPN22 did not impact upon thymocyte selection processes in the ZAP70 SKG mouse model.45 Together, these studies suggest that PTPN22 plays only a minor role in the thymus, and that disease‐associated PTPN22 polymorphisms likely impact on peripheral rather than central tolerance mechanisms.
Non‐cell intrinsic effects of PTPN22 on T‐cells
PTPN22 function in additional cell types likely has an important effect on T‐cell‐driven inflammation. For example, in myeloid cells, rather than act as a negative regulator, PTPN22 enhances production of type 1 interferons (IFNs).9, 51 Conversely, PTPN22 negatively regulates type 1 IFN‐receptor signalling pathways.23, 52 A clear example of cell‐extrinsic effects of PTPN22 on T‐cell function comes from studies of chronic viral infection. In this regard, several studies reported that PTPN22‐deficient mice were more efficient at clearing chronic lymphocytic choriomeningitis virus (LCMV) infection.51, 53 Ptpn22 −/− mice infected with the persistent LCMV clone 13 had increased numbers and function of virus‐specific CD4+ T‐cells51 and CD8+ T‐cells.53 However, cell transfer studies and mixed bone marrow chimera experiments indicated that the ability of Ptpn22 −/− T‐cells to resist exhaustion, and therefore clear virus load more efficiently, was not cell‐intrinsic.51, 53 Excessive production of type 1 IFN following infection can result in T‐cell exhaustion.54, 55 Thus, reduced production of type 1 IFN by PTPN22‐deficient myeloid cells enabled prolonged T‐cell responses to clone 13 LCMV. Therefore, in addition to modulating TCR signalling directly, PTPN22 influences T‐cell activation in a cell‐extrinsic manner, complicating our experimental interpretation of both infectious disease and autoimmune mouse models.
PTPN22 and anti‐tumour responses
There are several parallels between the regulation of autoimmunity and effective tumour immunosurveillance,56, 57 and while one is detrimental to the host, the other is desirable. Autoimmune T‐cells respond to self‐antigens and resist immune‐regulatory mechanisms, such as those mediated by Tregs.58, 59 By contrast, anti‐tumour T‐cell responses are frequently hampered by a failure to respond to low‐affinity tumour‐associated antigens (TAA) adequately, and a hostile tumour microenvironment, characterized by the presence of suppressive cell types (Tregs) and ligands (PD‐L1/2), limited nutrient and oxygen levels and high levels of immunosuppressive cytokines such as transforming growth factor β (TGFβ).
Adoptive cell transfer (ACT) of genetically engineered tumour‐reactive T‐cells or ex vivo expanded tumour‐infiltrating lymphocytes (TILs) has had substantial success as a cancer immunotherapy.60 Furthermore, modulation of intracellular signalling pathways in T‐cells has the potential to improve the efficacy of anti‐tumour ACT. Importantly, data indicate that, similar to their role in the regulation of auto‐reactivity, inhibitory phosphatases limit T‐cell anti‐tumour activity.61, 62 In a recent study, we investigated the impact of PTPN22‐deficiency on anti‐tumour T‐cell responses. Previous data showed that TGFβ plays a critical role in controlling autoreactive and anti‐tumour T‐cell responses, particularly to weak, self‐ligand‐mediated responses.59, 63 Interestingly, PTPN22‐deficient CD8+ T‐cells were highly resistant to the suppressive effects of TGFβ.64 This reduced susceptibility to TGFβ was not a consequence of alterations in canonical TGFβ‐receptor signalling. Rather, enhanced TCR‐driven IL‐2 production in the absence of PTPN22 interfered with the suppressive function of TGFβ (Fig. 1). As a consequence of enhanced TCR signalling and concomitant reduced susceptibility to TGFβ, upon adoptive transfer, tumour‐reactive PTPN22‐deficient CD8+ T‐cells were better able to control the growth of established tumours that secreted TGFβ than wild‐type T‐cells.64 These data suggest that deleting PTPN22 in human TILs or TAA‐specific T‐cells may improve the efficiency of T‐cell immunotherapy of human cancer.
Figure 1.
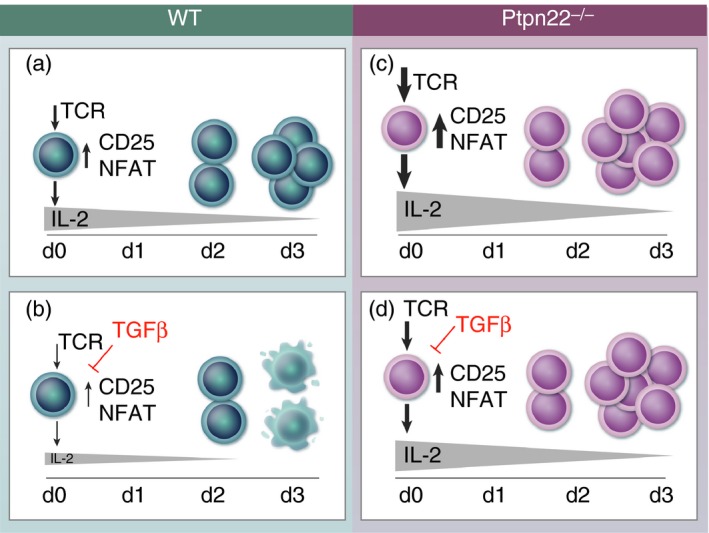
CD8+ T‐cells lacking PTPN22 have sufficiently strong T‐cell receptor (TCR) signals to overcome transforming growth factor β (TGFβ)‐mediated suppression. (a) Control T‐cells stimulated through the TCR upregulate expression of transcription factors (TFs), activation markers such as CD25 and translocate NFAT into the nucleus within 24 hr. Subsequently, cells start to secrete IL‐2 and proliferate. (b) TGFβ added at the start of control cell culture inhibits TCR‐driven TFs, activation marker upregulation and NFAT translocation, resulting in lower levels of IL‐2 production, thereby less cell proliferation and more cell death by d3. (c) TCR stimulation is stronger in Ptpn22 −/− cells, resulting in more IL‐2 and more cell proliferation. (d) TGFβ is less able to suppress strong TCR signals, allowing Ptpn22 −/− cells to secrete enough IL‐2 to proliferate and survive by d3.
Concluding remarks
PTPN22 has emerged as a key regulator of T‐cell activation, and effector responses in infection, autoimmunity and anti‐tumour immunity. The deleterious role of PTPN22 polymorphisms in autoimmunity is well established, yet recent evidence in mice suggests that deletion of PTPN22 could also be harnessed as an approach to improve anti‐tumour immunity. Future studies targeting PTPN22 in human T‐cells will be required to determine the utility of such approaches in human disease. Furthermore, fundamental questions regarding the role of PTPN22 in T‐cell memory and longevity remain outstanding. Thus, it is clear that deletion of PTPN22 enhances T‐cell effector responses. Does this push T‐cells to a short‐lived effector phenotype and is development of T‐cell memory affected? Finally, it has become apparent that PTPN22 has complex positive‐ and negative‐regulatory effects in different immune cell types and signalling pathways. In future studies, the use of lineage‐specific knockout or mutant mice should help clarify the precise role of PTPN22 in T‐cells and other immune populations.
Funding
This work was supported by a grant from Cancer Research UK to RS (23269).
Disclosures
None.
References
- 1. McDevitt H. The discovery of linkage between the MHC and genetic control of the immune response. Immunol Rev 2002; 185:78–85. [DOI] [PubMed] [Google Scholar]
- 2. Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol Rev 2005; 204:102–15. [DOI] [PubMed] [Google Scholar]
- 3. Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol 2014; 32:83–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C et al The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest 2011; 121:3635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schickel JN, Kuhny M, Baldo A, Bannock JM, Massad C, Wang H et al PTPN22 inhibition resets defective human central B cell tolerance. Sci Immunol 2016; 1:aaf7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vermeren S, Miles K, Chu JY, Salter D, Zamoyska R, Gray M. PTPN22 is a critical regulator of fcgamma receptor‐mediated neutrophil activation. J Immunol 2016; 197:4771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Purvis HA, Clarke F, Jordan CK, Blanco CS, Cornish GH, Dai X et al Protein tyrosine phosphatase PTPN22 regulates IL‐1beta dependent Th17 responses by modulating dectin‐1 signaling in mice. Eur J Immunol 2017; 48:306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang J, Zahir N, Jiang Q, Miliotis H, Heyraud S, Meng X et al The autoimmune disease‐associated PTPN22 variant promotes calpain‐mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet 2011; 43:902–7. [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z et al The autoimmunity‐associated gene PTPN22 potentiates toll‐like receptor‐driven, type 1 interferon‐dependent immunity. Immunity 2013; 39:111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol 2013; 13:257–69. [DOI] [PubMed] [Google Scholar]
- 11. Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T‐cell receptor proximal signaling via the Src‐family kinases, Lck and Fyn, influences T‐cell activation, differentiation, and tolerance. Immunol Rev 2009; 228:9–22. [DOI] [PubMed] [Google Scholar]
- 12. Cloutier JF, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J 1996; 15:4909–18. [PMC free article] [PubMed] [Google Scholar]
- 13. Wiede F, Shields BJ, Chew SH, Kyparissoudis K, van Vliet C, Galic S et al T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Invest 2011; 121:4758–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiang GG, Sefton BM. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr‐394 by the SHP‐1 protein‐tyrosine phosphatase. J Biol Chem 2001; 276:23 173–8. [DOI] [PubMed] [Google Scholar]
- 15. Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J et al Identification of substrates of human protein‐tyrosine phosphatase PTPN22. J Biol Chem 2006; 281:11 002–10. [DOI] [PubMed] [Google Scholar]
- 16. Davidson D, Zhong MC, Pandolfi PP, Bolland S, Xavier RJ, Seed B et al The Csk‐associated adaptor PAG inhibits effector T cell activation in cooperation with phosphatase PTPN22 and Dok adaptors. Cell Rep 2016; 17:2776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal 2012; 5:ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat Immunol 2014; 15:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burn GL, Cornish GH, Potrzebowska K, Samuelsson M, Griffie J, Minoughan S et al Superresolution imaging of the cytoplasmic phosphatase PTPN22 links integrin‐mediated T cell adhesion with autoimmunity. Sci Signal 2016; 9:ra99. [DOI] [PubMed] [Google Scholar]
- 20. Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain‐enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science 2004; 303:685–9. [DOI] [PubMed] [Google Scholar]
- 21. Salmond RJ, Brownlie RJ, Zamoyska R. Multifunctional roles of the autoimmune disease‐associated tyrosine phosphatase PTPN22 in regulating T cell homeostasis. Cell Cycle 2015; 14:705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho JH, Kim HO, Ju YJ, Kye YC, Lee GW, Lee SW et al CD45‐mediated control of TCR tuning in naive and memory CD8(+) T cells. Nat Commun 2016; 7:13 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jofra T, Di Fonte R, Hutchinson TE, Dastmalchi F, Galvani G, Battaglia M et al Protein tyrosine phosphatase PTPN22 has dual roles in promoting pathogen versus homeostatic‐driven CD8 T‐cell responses. Immunol Cell Biol 2017; 95:121–8. [DOI] [PubMed] [Google Scholar]
- 24. Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol 2012; 13:439–47. [DOI] [PubMed] [Google Scholar]
- 25. Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC et al A missense single‐nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet 2004; 75:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee AT, Li W, Liew A, Bombardier C, Weisman M, Massarotti EM et al The PTPN22 R620W polymorphism associates with RF positive rheumatoid arthritis in a dose‐dependent manner but not with HLA‐SE status. Genes Immun 2005; 6:129–33. [DOI] [PubMed] [Google Scholar]
- 27. Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE et al Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet 2004; 75:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanford SM, Bottini N. PTPN22: the archetypal non‐HLA autoimmunity gene. Nat Rev Rheumatol 2014; 10:602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Orru V, Tsai SJ, Rueda B, Fiorillo E, Stanford SM, Dasgupta J et al A loss‐of‐function variant of PTPN22 is associated with reduced risk of systemic lupus erythematosus. Hum Mol Genet 2009; 18:569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M et al Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet 2005; 76:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cambier JC. Autoimmunity risk alleles: hotspots in B cell regulatory signaling pathways. J Clin Invest 2013; 123:1928–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maine CJ, Marquardt K, Cheung J, Sherman LA. PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. J Immunol 2014; 192:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cloutier JF, Veillette A. Cooperative inhibition of T‐cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med 1999; 189:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P et al Autoimmune‐associated lymphoid tyrosine phosphatase is a gain‐of‐function variant. Nat Genet 2005; 37:1317–9. [DOI] [PubMed] [Google Scholar]
- 35. Rieck M, Arechiga A, Onengut‐Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol 2007; 179:4704–10. [DOI] [PubMed] [Google Scholar]
- 36. Aarnisalo J, Treszl A, Svec P, Marttila J, Oling V, Simell O et al Reduced CD4 + T cell activation in children with type 1 diabetes carrying the PTPN22/Lyp 620Trp variant. J Autoimmun 2008; 31:13–21. [DOI] [PubMed] [Google Scholar]
- 37. Cao Y, Yang J, Colby K, Hogan SL, Hu Y, Jennette CE et al High basal activity of the PTPN22 gain‐of‐function variant blunts leukocyte responsiveness negatively affecting IL‐10 production in ANCA vasculitis. PLoS ONE 2012; 7:e42783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vang T, Landskron J, Viken MK, Oberprieler N, Torgersen KM, Mustelin T et al The autoimmune‐predisposing variant of lymphoid tyrosine phosphatase favors T helper 1 responses. Hum Immunol 2013; 74:574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lefvert AK, Zhao Y, Ramanujam R, Yu S, Pirskanen R, Hammarstrom L. PTPN22 R620W promotes production of anti‐AChR autoantibodies and IL‐2 in myasthenia gravis. J Neuroimmunol 2008; 197:110–3. [DOI] [PubMed] [Google Scholar]
- 40. Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non‐autoimmune background. J Immunol 2009; 182:4093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai X, James RG, Habib T, Singh S, Jackson S, Khim S et al A disease‐associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest 2013; 123:2024–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin X, Pelletier S, Gingras S, Rigaud S, Maine CJ, Marquardt K et al CRISPR‐Cas9‐mediated modification of the NOD mouse genome with Ptpn22R619W mutation increases autoimmune diabetes. Diabetes 2016; 65:2134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng P, Kissler S. PTPN22 silencing in the NOD model indicates the type 1 diabetes‐associated allele is not a loss‐of‐function variant. Diabetes 2013; 62:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yeh LT, Miaw SC, Lin MH, Chou FC, Shieh SJ, Chuang YP et al Different modulation of Ptpn22 in effector and regulatory T cells leads to attenuation of autoimmune diabetes in transgenic nonobese diabetic mice. J Immunol 2013; 191:594–607. [DOI] [PubMed] [Google Scholar]
- 45. Sood S, Brownlie RJ, Garcia C, Cowan G, Salmond RJ, Sakaguchi S et al Loss of the protein tyrosine phosphatase PTPN22 reduces mannan‐induced autoimmune arthritis in SKG mice. J Immunol 2016; 197:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maine CJ, Hamilton‐Williams EE, Cheung J, Stanford SM, Bottini N, Wicker LS et al PTPN22 alters the development of regulatory T cells in the thymus. J Immunol 2012; 188:5267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fousteri G, Jofra T, Di Fonte R, Kuka M, Iannacone M, Battaglia M. PTPN22 controls virally‐induced autoimmune diabetes by modulating cytotoxic T lymphocyte responses in an epitope‐specific manner. Clin Immunol 2015; 156:98–108. [DOI] [PubMed] [Google Scholar]
- 48. Fousteri G, Jofra T, Debernardis I, Stanford SM, Laurenzi A, Bottini N et al The protein tyrosine phosphatase PTPN22 controls forkhead box protein 3 T regulatory cell induction but is dispensable for T helper type 1 cell polarization. Clin Exp Immunol 2014; 178:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S et al Altered thymic T‐cell selection due to a mutation of the ZAP‐70 gene causes autoimmune arthritis in mice. Nature 2003; 426:454–60. [DOI] [PubMed] [Google Scholar]
- 50. Wu DJ, Zhou W, Enouz S, Orru V, Stanford SM, Maine CJ et al Autoimmunity‐associated LYP‐W620 does not impair thymic negative selection of autoreactive T cells. PLoS ONE 2014; 9:e86677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maine CJ, Teijaro JR, Marquardt K, Sherman LA. PTPN22 contributes to exhaustion of T lymphocytes during chronic viral infection. Proc Natl Acad Sci USA 2016; 113:E7231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Holmes DA, Suto E, Lee WP, Ou Q, Gong Q, Smith HR et al Autoimmunity‐associated protein tyrosine phosphatase PEP negatively regulates IFN‐alpha receptor signaling. J Exp Med 2015; 212:1081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jofra T, Galvani G, Kuka M, Di Fonte R, Mfarrej BG, Iannacone M et al Extrinsic protein tyrosine phosphatase non‐receptor 22 signals contribute to CD8 T cell exhaustion and promote persistence of chronic lymphocytic choriomeningitis virus infection. Front Immunol 2017; 8:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M et al Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 2013; 340:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G et al Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 2013; 340:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Joseph CG, Darrah E, Shah AA, Skora AD, Casciola‐Rosen LA, Wigley FM et al Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 2014; 343:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maueroder C, Munoz LE, Chaurio RA, Herrmann M, Schett G, Berens C. Tumor immunotherapy: lessons from autoimmunity. Front Immunol 2014; 5:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 2000; 12:171–81. [DOI] [PubMed] [Google Scholar]
- 59. Zhang N, Bevan MJ. TGF‐β signaling to T cells inhibits autoimmunity during lymphopenia‐driven proliferation. Nat Immunol 2012; 13:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Johnson LA, June CH. Driving gene‐engineered T cell immunotherapy of cancer. Cell Res 2017; 27:38–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stromnes IM, Fowler C, Casamina CC, Georgopolos CM, McAfee MS, Schmitt TM et al Abrogation of SRC homology region 2 domain‐containing phosphatase 1 in tumor‐specific T cells improves efficacy of adoptive immunotherapy by enhancing the effector function and accumulation of short‐lived effector T cells in vivo. J Immunol 2012; 189:1812–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Watson HA, Dolton G, Ohme J, Ladell K, Vigar M, Wehenkel S et al Purity of transferred CD8(+) T cells is crucial for safety and efficacy of combinatorial tumor immunotherapy in the absence of SHP‐1. Immunol Cell Biol 2016; 94:802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thomas DA, Massagué J. TGF‐β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005; 8:369–80. [DOI] [PubMed] [Google Scholar]
- 64. Brownlie RJ, Garcia C, Ravasz M, Zehn D, Salmond RJ, Zamoyska R. Resistance to TGFbeta suppression and improved anti‐tumor responses in CD8(+) T cells lacking PTPN22. Nat Commun 2017; 8:1343. [DOI] [PMC free article] [PubMed] [Google Scholar]


