Summary
Persistent viruses evade immune detection by interfering with virus‐specific innate and adaptive antiviral immune responses. Fibrinogen‐like protein‐2 (FGL2) is a potent effector molecule of CD4+ CD25+ FoxP3+ regulatory T cells and exerts its immunosuppressive activity following ligation to its cognate receptor, Fcγ RIIB/RIII. The role of FGL2 in the pathogenesis of chronic viral infection caused by lymphocytic choriomeningitis virus clone‐13 (LCMV cl‐13) was assessed in this study. Chronically infected fgl2 +/+ mice had increased plasma levels of FGL2, with reduced expression of the maturation markers, CD80, CD86 and MHC‐II on macrophages and dendritic cells and impaired production of neutralizing antibody. In contrast, fgl2 −/− mice or fgl2 +/+ mice that had been pre‐treated with antibodies to FGL2 and Fcγ RIIB/RIII and then infected with LCMV cl‐13 developed a robust CD4+ and CD8+ antiviral T‐cell response, produced high titred neutralizing antibody to LCMV and cleared LCMV. Treatment of mice with established chronic infection with antibodies to FGL2 and Fcγ RIIB/RIII was shown to rescue the number and functionality of virus‐specific CD4+ and CD8+ T cells with reduced total and virus‐specific T‐cell expression of programmed cell death protein 1 leading to viral clearance. These results demonstrate an important role for FGL2 in viral immune evasion and provide a rationale to target FGL2 to treat patients with chronic viral infection.
Keywords: chronic infection, fibrinogen‐like protein 2, T‐cell exhaustion
Abbreviations
- APC
antigen‐presenting cells
- DC
dendritic cells
- FGL2
fibrinogen‐like protein 2
- FITC
fluorescein isothiocyanate
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- IFN‐γ
interferon‐γ
- LCMV cl‐13
lymphocytic choriomeningitis virus clone‐13
- PD‐1
programmed cell death protein‐1
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- PFU
plaque‐forming units
- p.i.
post infection
- Th1
T helper type 1
- TIGIT
T‐cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine‐based inhibition motif domains
- TNF‐α
tumour necrosis factor‐α
- Treg cell
CD4+ CD25+ FoxP3+ regulatory T cell
Introduction
Human chronic viral infections including, hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) represent serious global health problems.1, 2 Lymphocytic choriomeningitis virus clone‐13 (LCMV cl‐13) has been used as a model to gain insight into the mechanisms that contribute to viral persistence. In LCMV cl‐13 infection, early exposure to high viral loads leads to increased expression of inhibitory receptors, including programmed cell death protein‐1 (PD‐1),3 prostaglandin E2 receptors,4 cytotoxic T‐lymphocyte‐associated protein‐45 and T‐cell immunoglobulin mucin‐36 on activated T cells, and expression of immunosuppressive cytokines including interleukin‐10 and transforming growth factor‐β, leading to T‐cell and B‐cell dysfunction.7, 8 It is known that virus‐specific T cells are present in patients with chronic infections; however, they are functionally exhausted (T‐cell exhaustion). In T‐cell exhaustion,9 virus‐specific cytotoxic T lymphocytes have diminished cytotoxic effector activity, progressively lose effector cytokine secretion of interleukin‐2, tumour necrosis factor‐α (TNF‐α) and interferon‐γ (IFN‐γ),10 have decreased proliferative capacity11 and can be physically deleted from the immune repertoire.12 Treatment with antibodies to PD‐1 : programmed death ligand 1 or the receptor to interleukin‐10 has been reported to prevent T‐cell exhaustion and lead to clearance of LCMV cl‐13.3, 13, 14, 15 The effects of T‐cell exhaustion have been shown to be reversible and more importantly responsive to treatment, making T‐cell exhaustion a prime target for therapeutic intervention to improve treatment of patients with chronic viral infection.13, 14, 16
CD4+ CD25+ FoxP3+ regulatory T (Treg) cells are known to contribute to viral persistence through inhibition of both innate and adaptive immunity.17, 18, 19 Previous studies have shown that depletion of Treg cells enhances immunity against HBV20 and HCV.21 Recently, a subset of inducible and natural Treg cells expressing the T‐cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine‐based inhibition motif domains (TIGIT) have been identified.22 Ligation of TIGIT to CD155 on antigen‐presenting cells (APC) leads to increased expression of FGL2, resulting in suppression of both T helper type 1 (Th1) and Th17 responses in vivo.22 We and others have shown that FGL2 exerts its immunosuppressive activity following binding to its cognate receptor FcγRIIB/RIII, which is expressed on APC, natural killer cells and B cells. Although it has been shown that naive T cells do not express FcγRIIB/RIII, in the setting of viral infection, activated T cells may express FcγRIIB/RIII. Hence, in chronic viral disease, the T‐cell inhibitory effects may be both direct and indirect.23, 24, 25
Engagement of Fcγ receptors have been implicated in the pathogenesis of a number of immunologically mediated diseases26, 27, 28 and regulates the response to parasitic29 and bacterial30, 31 infections. We and others have reported that FGL2 contributes to the pathogenesis of fulminant viral hepatitis induced by mouse hepatitis virus‐3 and an acute self‐limiting viral hepatitis caused by LCMV WE.22, 32, 33
In the current study, we used two complementary but independent approaches to evaluate whether inhibition of FGL2 would restore innate and adaptive immunity leading to clearance of chronic LCMV cl‐13 infection. We first showed that targeted deletion of fgl2 (fgl2 −/−) enhanced maturation of macrophage and dendritic cells (DC), antiviral T‐cell and B‐cell immunity, resulting in clearance of chronic LCMV cl‐13 infection. Second, the use of neutralizing antibodies to FGL2 and the receptor for FGL2, FcγRIIB/RIII, both before infection and during established chronic infection restored CD4+ and CD8+ T‐cell responses, resulting in clearance of LCMV cl‐13. These studies confirm the role of FGL2 in chronic viral persistence and the potential to disrupt the FGL2‐FcγRIIB/RIII pathway as a novel therapeutic strategy to treat humans with chronic infections.
Materials and methods
Mice
The fgl2 −/− mice were generated as described previously.34 Eight‐ to ten‐week‐old weight‐matched C57BL/6 littermate fgl2 +/+ and fgl2 −/− female mice received 2 × 106 plaque‐forming units (PFU) of LCMV cl‐13. Animal protocols were approved by the University Health Network in accordance with guidelines set by the Canadian Council on Animal Care.
LCMV virus and viral titres
LCMV cl‐13 was obtained as a gift from the laboratory of Dr M. Oldstone (The Scripps Research Institute, La Jolla, CA) and was propagated in BHK‐21 cells (ATCC # CCL‐10).15 Mice were infected intravenously with LCMV and at defined time‐points blood samples were collected into heparinized microvettes (Sarstedt, Nümbrecht, Germany) as previously described.33 Blood was centrifuged and plasma was collected. Tissues were harvested and snap‐frozen in liquid nitrogen. Viral titres were determined on MC57 cells (ATCC # CRL‐2295) using focus‐forming assay.35
Total LCMV‐specific IgG detection
An LCMV antibody ELISA was used for the detection of total LCMV‐specific antibodies.36 The absorbance value measured at 450 nm correlated with the captured total LCMV‐specific antibody within plasma samples. The dilution series for each plasma sample was plotted and read where the dilution and observed absorbance values had a linear relationship with one another. Samples were expressed as a fold increase from naive absorbance.
Neutralizing antibody detection
LCMV neutralizing antibody titres were quantified in plasma from LCMV cl‐13 infected mice using a plaque reduction assay.37 Plasma was diluted 1 : 10 in complete α‐modified Eagle's minimum essential medium supplemented with 2% fetal bovine serum and was serially diluted and mixed with 100 PFU of LCMV cl‐13 for 1 hr at 37°. Following incubation, samples were added to MC57 cells and plaques were visualized using a standard focus‐forming assay.35
LCMV ex vivo peptide re‐stimulation
Splenic mononuclear cells were isolated as previously described38 and stimulated with 10 µg/ml of the MHC class I peptide glycoprotein GP33‐41 or nucleoprotein NP396‐404 for 6 hr as previously described.39, 40, 41 The LCMV peptide GP33‐41 H‐2Db (KAVYNFATC) and NP396‐404 H‐2Db (FQPQNGQFI) was synthesized by Anaspec Inc. (Fremont, CA). Brefeldin A (Sigma‐Aldrich, St Louis, MO) was added to cultures after 1 hr of peptide re‐stimulation for 5 hr at a final concentration of 10 µg/ml. Flow cytometry was used to assess the frequency of splenic mononuclear cells producing IFN‐γ following ex vivo peptide re‐stimulation.
Macrophage and DC isolation
Macrophages (CD11b+ NK1.1−) and DC (CD11c+) were isolated as previously described.33, 42 Following incubation for 20 min with 5% mouse serum (Cedarlane Laboratories, Burlington, ON, Canada) in PBS at 4°, splenic mononuclear cells were fixed with 2% paraformaldehyde in PBS solution (Santa Cruz Biotechnology, Dallas, TX) for 20 min and stained with antibodies and gated as shown in the Supplementary material (Fig. S1).
Flow cytometry
Antibodies
Antibodies used for flow cytometry: The following antibodies were purchased from eBioscience (San Diego, CA) and were used to stain cells for FACS analysis as described below: Peridinin chlorophyll protein (PerCP) ‐Cy5.5‐CD3ε (Clone 17A2), fluorescein isothiocyanate (FITC) ‐CD4 (Clone GK1.5), phycoerythrin (PE) ‐CD8α (Clone 53‐6.7), PerCP‐Cy5.5‐CD11b (Clone M1/70), allophycocyanin‐CD80 (Clone 16‐10A1), PE‐MHC‐II (I‐A) (Clone NIMR‐4), FITC‐CD86 (Clone GL‐1), FITC‐IFN‐γ (Clone XMG1.2), PerCP‐Cy5.5‐TNF‐α (Clone MP6‐XT22), CD16/CD32 (Clone 93), PE‐CD11c (Clone N418), FITC‐CD45R (Clone RA3‐6B2), PE‐CD19 [eBio1D3(1D3)] and PE‐NK1.1 (Clone PK136). Fixable viability dye eFluor 450 (eBioscience) was used, diluted 1 : 1000, as the viability dye.
Tetramers
Biotinylated MHC‐I monomers (GP33–41) were provided by the NIH Tetramer Core Facility, Emory University (Atlanta, GA). MHC‐I monomers were tetramerized with streptavidin‐PE according to NIH Tetramer Core Facility instructions. Fixable viability dye eFluor 450 (eBioscience) was used to confirm cell viability. Tetramer staining was performed on freshly isolated and unstimulated cells.
Cell staining
Mononuclear cells were isolated from the spleen, washed and resuspended in FACS buffer (PBS containing 1% fetal calf serum and 1 mM EDTA) at a final concentration of 1 × 107 cells/ml. Cells were treated with CD16/CD32 to block non‐specific binding to Fc‐receptors. Cells were surface stained with antibodies and LCMV‐specific tetramers. Cells were then fixed with 2% paraformaldehyde. FACS analysis was performed using a BD LSRII Flow Cytometer and data were analysed using flowjo software (Tree Star Inc., Ashland, OR). Live cells were discriminated according to forward‐scatter and side‐scatter parameters and a fixable viability dye (eBioscience).
In vivo antibody treatment
The monoclonal antibody 9D8 was generated as previously described.43 The effects of 9D8 on both the innate and adaptive immune systems are shown in the Supplementary material (Fig. S2). Anti‐mouse FcγRIIB/RIII (clone 2.4G2 ATCC HB‐197) was purchased from the American Tissue Culture Centre (Manassas, VA). Studies were undertaken to determine if antibody to FGL2, FcγRIIB/RIII alone or in combination could prevent the development of chronic infection or treat established chronic infection. The fgl2 +/+ mice were divided into five groups (n > 5/group) and treated with 150 µg of anti‐FGL2 monoclonal antibody (9D8) (Group 1); 150 µg of anti‐FcγRIIB/RIII monoclonal antibody (2.4G2) (Group 2); 150 µg of 9D8 and 150 µg 2.4G2 (Group 3) or with 150 µg mouse IgG1 isotype control antibody (clone MOPC‐21) and 150 µg of rat IgG2A isotype control antibody (clone 2A3) (Group 4) or PBS alone (Group 5). For studies to determine if antibody to FGL2 could prevent progression to chronic disease, antibody was initiated 2 days before infection and continued every 2 days until day 20 post infection (p.i.) and then given at days 23, 26, 32 and 45 p.i. until the mice were killed on day 56 p.i. To determine if antibody treatment was effective in established chronic infection, treatment with antibody was initiated at day 25 p.i. and continued every second day until death on day 56 p.i.
FGL2 ELISA
Plasma levels of FGL2 were quantified as previously described.33 Briefly, plates were coated and incubated overnight with 1 ng/ml monoclonal anti‐FGL2 (6H12) (IgG1) as a capture antibody. Plasma samples (50 µl) were added to each well, and following 1 hr incubation at 37° and three washes with Tris‐buffered saline, the wells were incubated with 2 µg/ml polyclonal rabbit anti‐FGL2 antibody for 2 hr at 37°. The plate was washed again and polyclonal anti‐FGL2 binding was detected with secondary horseradish peroxidase‐conjugated anti‐rabbit antibody. Tetrametylbenzidine was then added and absorbance was measured at 450 nm using an ELISA plate reader. Levels of FGL2 were undetectable in the plasma of fgl2 −/− mice both before and after LCMV infection.
Immunohistochemistry and morphometric analysis
The fgl2 +/+ mice and fgl2 −/− mice were infected intravenously with 2 × 106 PFU of LCMV cl‐13. Tissues were frozen in Optimal Cutting Temperature compound (Sakura Finetek, Alphen aan den Rijn, the Netherlands) and 5‐µm thick tissue sections were stained with a rat antibody to the nucleoprotein of LCMV (VL4) provided as a gift from Dr P. Ohashi (Princess Margaret Hospital, Toronto, ON, Canada). The tissues were then incubated with a horseradish peroxidase‐conjugated secondary anti‐rat IgG antibody that allowed for colour development after addition of substrate 3,3′‐diaminobenzidine (Zymed, San Francisco, CA). Morphometric analysis for LCMV nucleoprotein (NP) was performed in liver at days 28 and 56 p.i. as previously described.33 Slides were digitally scanned and the number of LCMV NP+ cells was quantified by morphometric analysis using Spectrum V.10.2.2.2317 (Aperio Technologies Inc., Vista, CA). The number of LCMV NP+ cells was expressed as a percentage of total cells. Control uninfected fgl2 +/+ and fgl2 −/− livers were used as a control and did not stain positive for LCMV NP.
Statistics
Statistical significance was assessed using the analysis of variance or a Student's t‐test. Statistical analysis was performed using prism 5 software (Graphpad Software Inc., San Diego, CA). Differences with P ≤ 0·05 were considered statistically significant.
Results
LCMV cl‐13 infection leads to increased expression of FGL2
Following infection with 2 × 106 PFU of LCMV cl‐13 intravenously, increased plasma levels of FGL2 were detected in fgl2 +/+ mice by day 7 p.i., reaching maximum levels on day 10 p.i. (52‐fold) and remained persistently elevated until day 56 p.i., although differences were only statistically significant until day 42 p.i. (Fig. 1).
Figure 1.
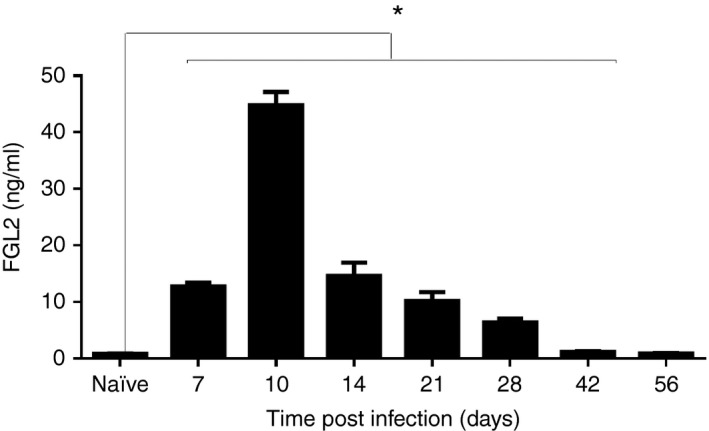
Chronic infection by lymphocytic choriomeningitis virus clone 13 (LCMV cl‐13) induces the expression of fibrinogen‐like protein 2 (FGL2). Fgl2 +/+ mice were infected with 2 × 106 PFU of LCMV cl‐13 intravenously and levels of FGL2 were measured in the plasma by ELISA. Graphs represent the mean ± SEM of five mice per time‐point from two independent experiments *P < 0·05.
Targeted deletion of fgl2 leads to clearance of LCMV cl‐13
To examine the effect of deletion of fgl2 on viral clearance, both fgl2 +/+ and fgl2 −/− mice were infected intravenously with LCMV cl‐13 and viral titres were measured in plasma (Fig. 2a), lung (Fig. 2b) and liver (Fig. 2c) using a focus‐forming assay. On day 28 p.i., the titres of LCMV cl‐13 were significantly lower in the plasma (P < 0·005), lung (P < 0·05) and liver (P < 0·005) of fgl2 −/− mice compared with fgl2 +/+ mice. Furthermore, by day 56 p.i., all fgl2 −/− mice had undetectable virus from the lung, liver and plasma whereas fgl2 +/+ mice remained persistently infected. To confirm that fgl2 −/− mice had cleared LCMV cl‐13, liver tissues were stained for LCMV nucleoprotein and the percentage of LCMV+ cells was analysed by morphometric analysis at days 28 and 56 p.i. Consistent with the focus‐forming assay, morphometric analysis revealed that LCMV antigen was undetectable in the livers of fgl2 −/− mice at day 56 p.i., whereas LCMV NP antigen was detected in fgl2 +/+ mice indicating LCMV cl‐13 viral clearance in fgl2 −/− mice (Fig. 2d,e).
Figure 2.
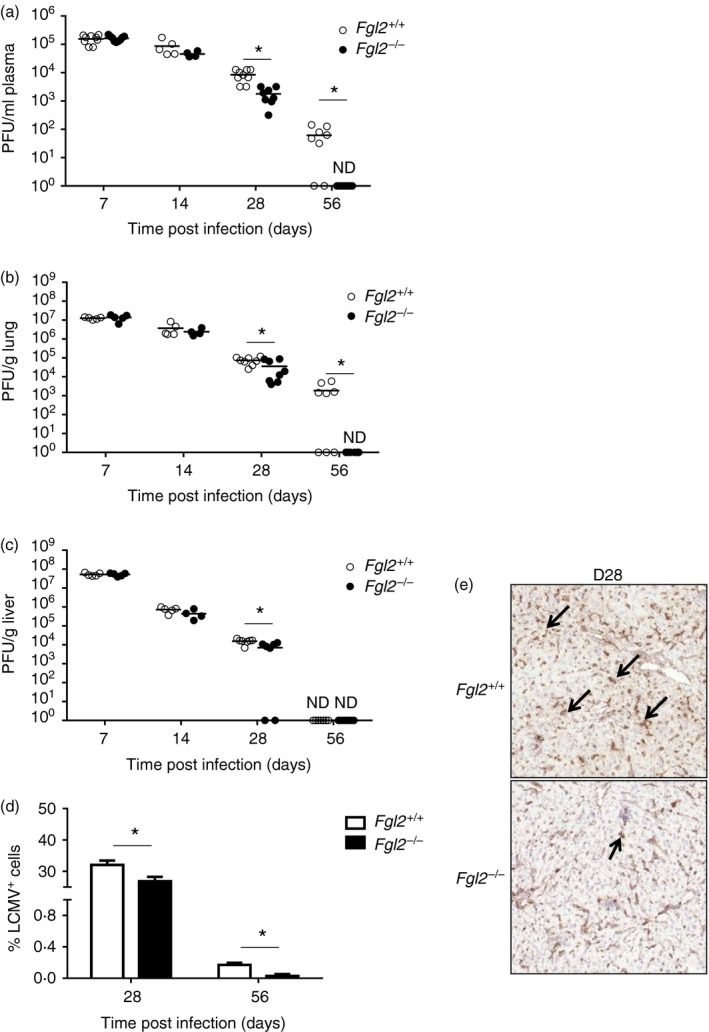
Fgl2 −/− mice clear lymphocytic choriomeningitis virus clone 13 (LCMV cl‐13) in contrast to fgl2 +/+ mice. The fgl2 +/+ and fgl2 −/− mice were infected with 2 × 106 PFU of LCMV cl‐13. Viral titres were measured in plasma (a), lung (b) and liver (c) using an LCMV focus‐forming assay. Data are presented as the mean ± SEM of five mice per group and per time‐point from two independent experiments *P < 0·05. The limit of detection of the LCMV focus‐forming assay is denoted by the dashed line. Morphometric analysis for LCMV nucleoprotein in the liver was performed at day 28 and 56 post infection (p.i.) (d). Graph shows the mean ± SEM of four to eight mice per group from one experiment per time‐point. *P < 0·05. (e) Representative photomicrograph of LCMV‐infected liver from fgl2 +/+ and fgl2 −/− at day 28 p.i. Livers were recovered on day 28 p.i., embedded in OCT and snap frozen. Tissue sections were cut into 5‐µm‐thick sections and stained with a rat anti‐LCMV nucleoprotein (NP) antibody (VL‐4). Tissue was incubated with a secondary anti‐rat antibody conjugated with horseradish peroxidase that stained positive cells brown after addition of the substrate (3,3′‐diaminobenzadine. Marked deposits of LCMV‐NP (black arrow) were seen in the hepatocytes and endothelial cells of fgl2 +/+ in comparison with fgl2 −/− mice (100× magnification). [Colour figure can be viewed at http://wileyonlinelibrary.com]
Targeted deletion of fgl2 leads to increased numbers of macrophages and DC expressing CD80, CD86 and MHC‐II
To study the effects of FGL2 on APC maturation after LCMV cl‐13 infection, DC (CD11c+) and macrophages (CD11b+, NK1.1−) were isolated from the spleen of fgl2 +/+ and fgl2 −/− mice both before infection and at day 2 p.i. Before infection, macrophage (Fig. 3a) and DC (Fig. 3c) expression of CD80, CD86 and MHC‐II were equivalent in DCs and macrophages isolated from both fgl2 +/+ and fgl2 −/− mice. However, on day 2 p.i., the total number of macrophages and DC expressing CD80, CD86 and MHC‐II were significantly increased in fgl2 −/− mice compared with fgl2 +/+ mice (Fig. 3b,d) indicating enhanced maturation of APC isolated from fgl2 −/− mice p.i.
Figure 3.
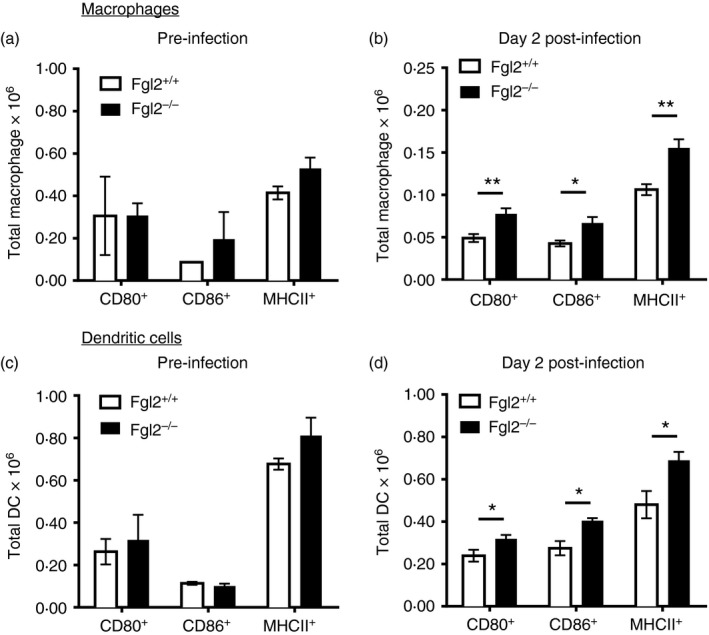
Targeted deletion of fgl2 leads to increased maturation of macrophages and dendritic cells after lymphocytic choriomeningitis virus clone 13 (LCMV cl‐13) infection. Macrophages and dendritic cells were isolated from the spleen of fgl2 +/+ mice and fgl2 −/− before infection and on day 2 p.i. with 2 × 106 PFU of LCMV cl‐13. The number of macrophages (CD11b+ NK1·1− cells) (a, b) and number of dendritic cells (CD11c+) (c, d) expressing CD80, CD86 and MHC‐II were determined both before and on day 2 post infection by flow cytometry. Data are presented as the mean ± SEM of five mice per group and per time‐point from one independent experiment *P < 0·05.
Targeted deletion of fgl2 leads to increased numbers of virus‐specific CD8+ T cells following infection with LCMV cl‐13
To determine whether FGL2 affected the number of LCMV‐specific CD8+ T cells, fgl2 −/− mice and fgl2 +/+ mice were infected with 2 × 106 PFU of LCMV cl‐13 intravenously and on days 7, 28 and 56 p.i., splenic mononuclear cells were isolated and stained with the MHC‐I tetramer glycoprotein GP33–41 to determine the number of LCMV‐specific CD8+ T cells. Before infection, CD8+ TetGP33‐41 + T cells were undetectable in both fgl2 +/+ mice and fgl2 −/− mice (data not shown). On day 7 p.i., increased numbers of CD8+ TetGP33‐41 + T cells were detected in both fgl2 +/+ and fgl2 −/− mice. Thereafter, the number of CD8+ TetGP33‐41 + T cells from fgl2 +/+ mice contracted both on day 28 and 56 compared with day 7. In contrast, numbers of CD8+ TetGP33‐41 + T cells from fgl2 −/− mice persisted at near equivalent levels at days 7 and 28, and were significantly increased at day 56 compared with fgl2 +/+ mice (Fig. 4a,b).
Figure 4.
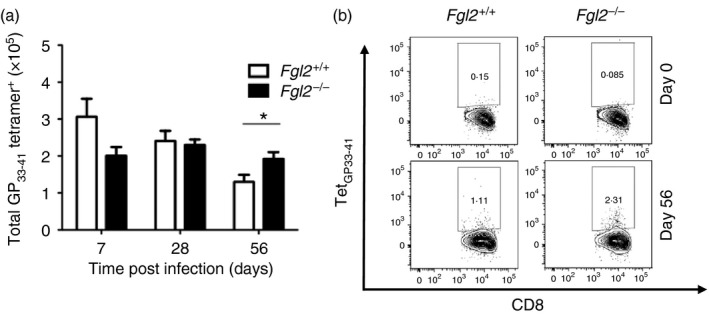
The fgl2 −/− mice have increased numbers of virus‐specific T cells to lymphocytic choriomeningitis virus (LCMV). Both fgl2 +/+ and fgl2 −/− mice were infected with 2 × 106 PFU of LCMV cl‐13 and the number of splenic CD3+ CD8+ TetGP 33‐41 + cells were assessed at days 7, 28 and 56 post infection (p.i.) (a). Representative flow plots showing the frequency of CD8+ TetGP 33‐41 + gated on CD3+ CD8+ T cells isolated from both fgl2 +/+ and fgl2 −/− mice at days 0 and 56 p.i. are shown (b). Data are presented as the mean ± SEM of five mice per group and per time‐point from two independent experiments *P < 0·05.
The effects of targeted deletion of fgl2 on LCMV‐specific CD8+ T‐cell effector function was determined by measurement of IFN‐γ and TNF‐α production in CD8+ T cells following ex vivo peptide re‐stimulation with the MHC‐I‐restricted peptide GP33‐41. The number of CD8+ T cells producing TNF‐α and both TNF‐α and IFN‐γ in response to ex vivo peptide re‐stimulation was maximal at day 7 p.i. but was still detectable on day 28 and day 56 p.i. in both fgl2 +/+ mice and fgl2 −/− mice (data not shown).
Fgl2 −/− mice have reduced frequencies of PD‐1 expressing CD4+ and CD8+ T cells after LCMV cl‐13 infection
We next examined the effect of targeted deletion of fgl2 on expression of PD‐1 after LCMV infection. At baseline, the number of PD‐1+ CD4+ and CD8+ T cells was less than 10% and near equivalent in fgl2 −/− and fgl2 +/+ mice. However, after LCMV infection, the number of CD4+ PD‐1+ T cells was reduced in fgl2 −/− mice compared with fgl2 +/+ mice (Fig. 5a,b). In contrast to CD4+ cells, the number of PD‐1+ CD8+ T cells was only reduced in fgl2 −/− mice compared with fgl2 +/+ mice at day 56 and day 100 p.i. (Fig. 5c,d). Furthermore, the frequency of CD8+ TetGP33‐41 + T cells expressing PD‐1 was reduced in fgl2 −/− mice compared with fgl2 +/+ mice (Fig. 5e,f). Collectively, these data demonstrate that deletion of fgl2 leads to increased numbers of pro‐inflammatory LCMV‐specific CD8+ T cells and a reduced percentage of PD‐1‐expressing CD4+ and CD8+ T cells after LCMV cl‐13 infection.
Figure 5.
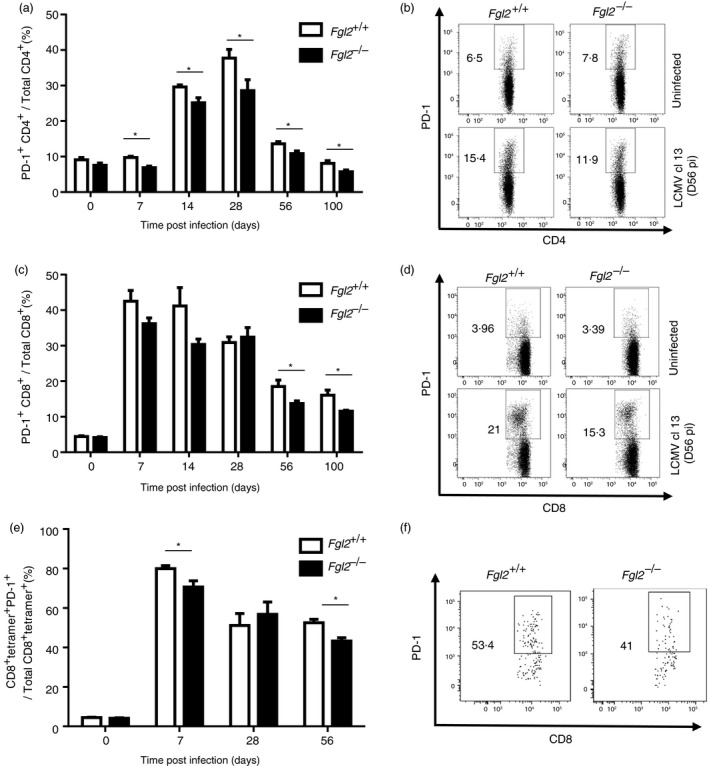
Fgl2 −/− mice have reduced frequency (%) of programmed cell death protein 1 (PD‐1) expressing CD4+ and CD8+ T cells following infection with lymphocytic choriomeningitis virus clone 13 (LCMV cl‐13). Fgl2 +/+ and fgl2 −/− mice were infected with 2 × 106 PFU of LCMV cl‐13 and PD‐1 expression on CD4+ T cells (a, b) and on CD8+ T cells (c, d) were determined by flow cytometry. Representative flow plots for CD4+ (b) and CD8+ cells (d) are shown. The frequency of CD3+ CD8+ TetGP 33‐41 + T cells expressing PD‐1 was determined by flow cytometry (e) and a representative flow plot is shown (f). Data are presented as the mean ± SEM from five mice per group and per time‐point from two independent experiments *P < 0·05.
Fgl2 −/− mice generate increased numbers of T‐cell memory cells and increased neutralizing antibody titres
To assess virus‐specific memory, splenic mononuclear cells were isolated from virus‐infected mice and assessed by flow cytometry according to the gating strategy in the Supplementary material (Fig. S3). The total number of CD8+ effector memory (Fig. 6a), CD4+ effector memory (Fig. 6c), CD8+ central memory (Fig. 6e) and CD4+ central memory (Fig. 6f) cells was near equivalent in both fgl2 −/− and fgl2 +/+ uninfected mice. At day 56 following infection, fgl2 −/− mice had a 1·8‐fold increase in the number of CD8+ effector memory cells (Fig. 6a); a 1·7‐fold increase in CD8+ TetGp33‐41 + effector memory cells (Fig. 6b); a twofold increase in CD4+ effector memory cells (Fig. 6c); a 21‐fold increase in CD4+ TetGP61‐80 + effector memory cells (Fig. 6d); a twofold increase in CD8+ central memory cells (Fig. 6e) and a 2·8‐fold increase in CD4+ central memory cells (Fig. 6f) compared with fgl2 +/+ mice. There were elevated levels of CD4+ TetGP61‐80 + and CD8+ TetGp33‐41 + central memory in fgl2 −/− mice, but these did not reach statistical significance (data not shown).
Figure 6.
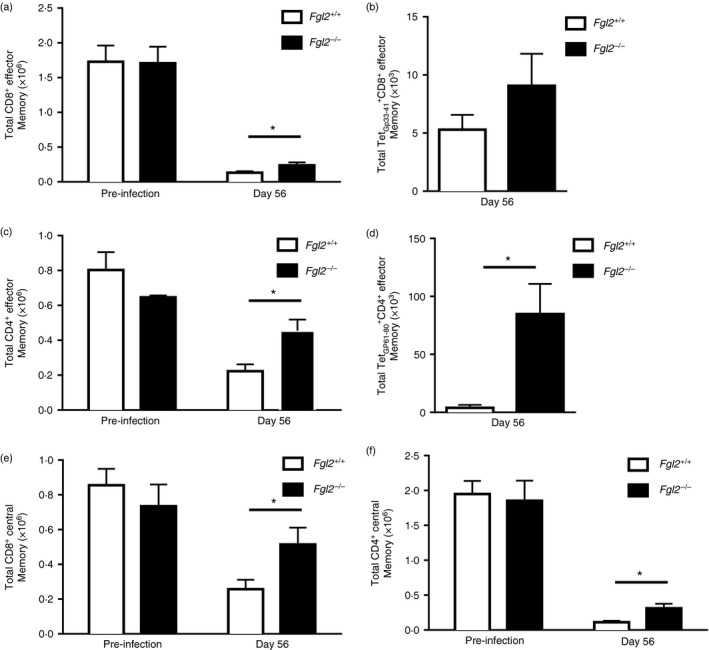
Fgl2 −/− mice have increased central memory, effector memory after lymphocytic choriomeningitis virus clone 13 (LCMV cl‐13) infection. Total CD8+ (CD8+ CD44high CD62Llow) and CD4+ (CD4+ CD44high CD62Llow) effector memory cells in the spleen in both fgl2 +/+ and fgl2 −/− mice pre‐infection and at day 56 post infection (p.i.) (a, c) were analysed. Virus‐specific CD8+ (CD8+ TetGp33‐41 + CD44high CD62Llow) (b) and CD4+ (CD4+ TetGp61‐80 + CD44high CD62Llow) (d) effector memory cells were also determined at day 56 p.i. Total CD8+ (CD8+ CD44high CD62Lhigh) and CD4+ (CD4+ CD44high CD62Lhigh) central memory cells were determined from both fgl2 +/+ and fgl2−/− mice both pre‐infection and at day 56 p.i. (e, f). Data are presented as the means ± SEM of five mice per group and time‐point from three independent experiments *P < 0·05.
Total IgG antibody to LCMV was near equivalent in both fgl2 +/+ and fgl2 −/− mice and was first detected on day 7 p.i. and persisted until day 56 p.i. (Fig. 7a). In contrast, neutralizing antibody to LCMV was detected at day 56 and continued to increase to day 130 p.i. in fgl2 −/− mice (Fig. 7b). On day 70 p.i., neutralizing antibody was detected in both strains of mice, but the titre was significantly higher in fgl2 −/− mice. Neutralizing antibody persisted until 130 days p.i. but at significantly higher titres in fgl2 −/− mice. Hence, while both fgl2 +/+ and fgl2 −/− mice produced antibodies to LCMV, only fgl2 −/− generated neutralizing antibodies until day 56.
Figure 7.
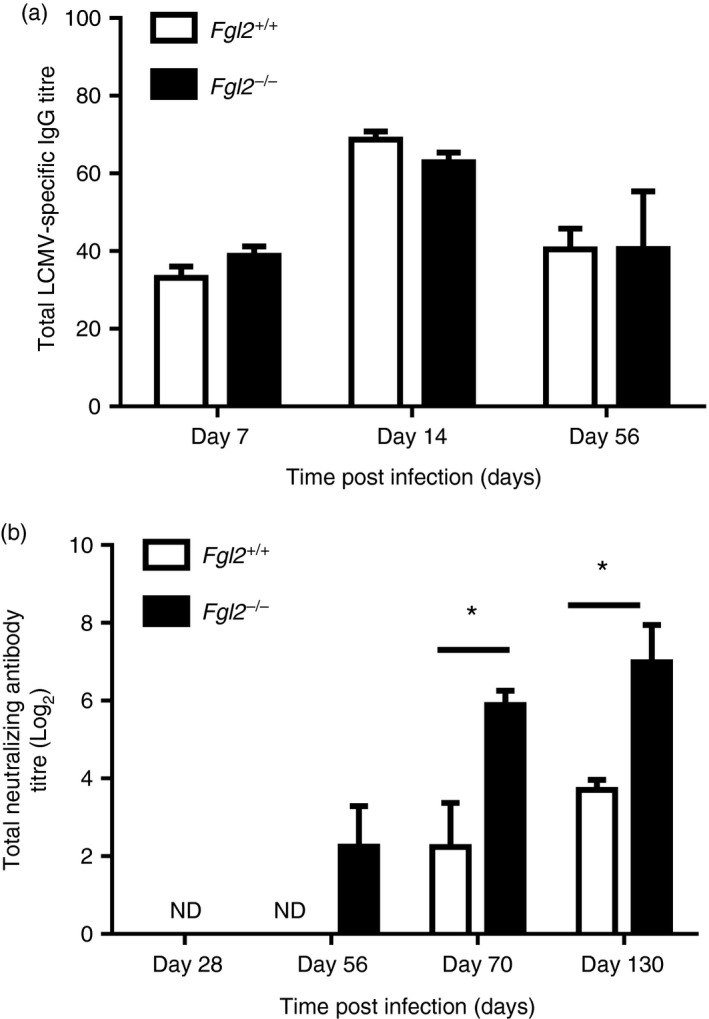
Fgl2 −/− mice have enhanced production of neutralizing antibody after lymphocytic choriomeningitis virus clone 13 (LCMV cl‐13) infection. Total LCMV‐specific IgG was measured using an LCMV IgG specific ELISA (a). Data are presented as the mean absorbance at 450 nm × dilution of plasma ± SEM of five mice per group and per time‐point from one independent experiment *P < 0·05. (b) Total neutralizing antibody titres were measured in the plasma from LCMV‐infected mice. The graph shows the dilution of plasma required for 50% plaque reduction of 200 PFU of LCMV cl‐13 in a standard LCMV focus‐forming assay. Data represent the log2 mean ± SEM of five mice per group and per time‐point from one independent experiment *P < 0·05.
To determine that antibody treatment could prevent the development of chronic LCMV infection, mice were pre‐treated with either PBS, isotype control antibodies, anti‐FGL2 (9D8) or anti‐FcγRIIB /RIII (2.4G2) antibody alone, which had minimal effect on T‐cell antiviral responses and none of these mice cleared LCMV cl‐13 (data not shown). In contrast, pre‐treatment of fgl2 +/+ mice with combination antibodies to FGL2 (9D8) and to FcγRIIB/RIII (2.4G2) led to viral clearance (Fig. 8a). Treated mice had a 3·4‐fold increase in numbers of CD8+ TetGP33‐41 + T cells (Fig. 8b) and a 2·3‐fold and twofold increase in numbers of IFN‐γ + CD8+ T cells to ex vivo peptide re‐stimulation with the MHC‐I‐restricted peptides GP33‐41 and NP396‐404, respectively, in comparison with PBS and isotype control treated mice (Fig. 8c). The number of CD8+ T cells expressing PD‐1 in LCMV‐infected fgl2 +/+ mice treated with anti‐FGL2 and anti‐FcγRIIB/RIII was also reduced compared with PBS and isotype control treated mice (Fig. 8d).
Figure 8.
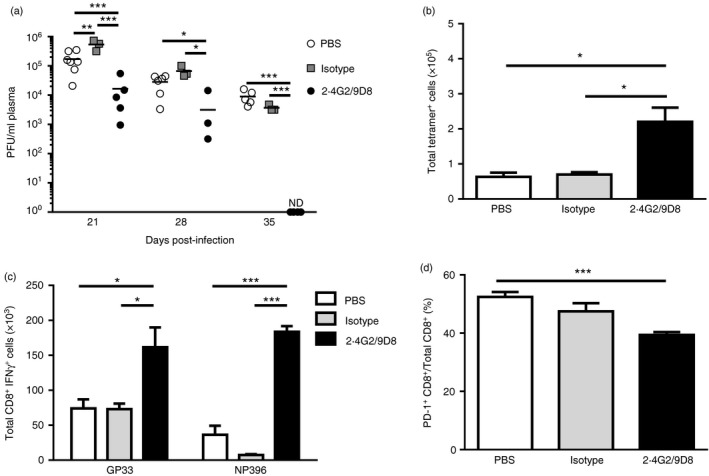
Pre‐treatment with antibodies to fibrinogen‐like protein 2 (FGL2) and Fcγ RIIB/RIII enhances CD4+ and CD8+ antiviral immunity with enhanced viral clearance. Fgl2 +/+ mice were pre‐treated with 150 µg of neutralizing antibodies to FGL2 and 150 µg Fcγ RIIB/RIII (2.4G2/9D8), or 150 µg isotype control antibodies (150 µg of MOPC‐21 and 150 µg 2A3), or 150 µl of PBS 2 days before infection. Mice were infected with 2 × 106 PFU of lymphocytic choriomeningitis virus clone 13 (LCMV cl‐13) and subsequently treated with antibody or PBS every 2 days until day 20 p.i. Treatment with antibody or PBS was continued and given at days 23, 26, 32 and 45 p.i. Viral titres were determined in the plasma (a). The limit of detection of the LCMV focus‐forming assay is denoted by the dashed line. Splenic mononuclear cells (SMNC) were isolated from 2.4G2/9D8, isotype control antibodies and PBS‐treated mice at day 56 p.i. and stimulated with MHC‐I restricted peptides and analysed by flow cytometry. Total numbers of CD3+ CD8+ TetGP 33‐41 + T cells were determined (b). The total numbers of CD3+ CD8+ IFN γ + T cells responding to ex vivo peptide re‐stimulation to GP 33‐41 or NP 396‐404 was determined (c). The number of programmed cell death prtein 1 (PD‐1) ‐expressing CD3+ CD8+ T cells was determined (d). Data are presented as the mean ± SEM of five mice per group from two independent experiments. *P < 0·05 ***P < 0·001, ****P < 0·0001.
To examine whether antibodies to both FGL2 and FcγRIIB/RIII could be an effective treatment for chronic infection, fgl2 +/+ mice, which had been previously infected with 2 × 106 PFU of LCMV cl‐13 and had developed chronic disease, were treated on day 25 p.i. with 150 µg of 9D8 and 2.4G2 or PBS. Treatment was continued every 2 days until death on day 56 p.i.44 At day 56 p.i., the number of CD3+ CD8+ TetGP33‐41 + in antibody‐ or PBS‐treated mice was equivalent (Fig. 9a). Total CD3+ CD8+ TetNP396‐404 + and CD3+ CD4+ TetGP61‐80 + were significantly increased in 2.4G2/9D8 antibody‐treated mice compared with control mice (Fig. 9a). CD4+ and CD8+ T cells from 2.4G2/9D8‐treated mice produced increased amounts of IFN‐γ (Fig. 9b) and TNF‐α in response to ex vivo peptide re‐stimulation with GP33‐41, NP396‐404 or GP61‐80 in contrast to PBS controls (Fig. 9c). The number of CD3+ CD8+ (Fig. 9d) and CD3+ CD4+ T cells (Fig. 9e) expressing PD‐1 was also significantly reduced in 2.4G2/9D8‐treated mice. The frequency (%) of LCMV‐specific (TetGP33‐41 +) CD8+ T cells expressing PD‐1 was also reduced in antibody‐treated mice (Fig. 9f). All mice treated with 2.4G2/9D8 cleared LCMV cl‐13 by day 56 whereas PBS‐treated control mice remained chronically infected (Fig. 9g).
Figure 9.
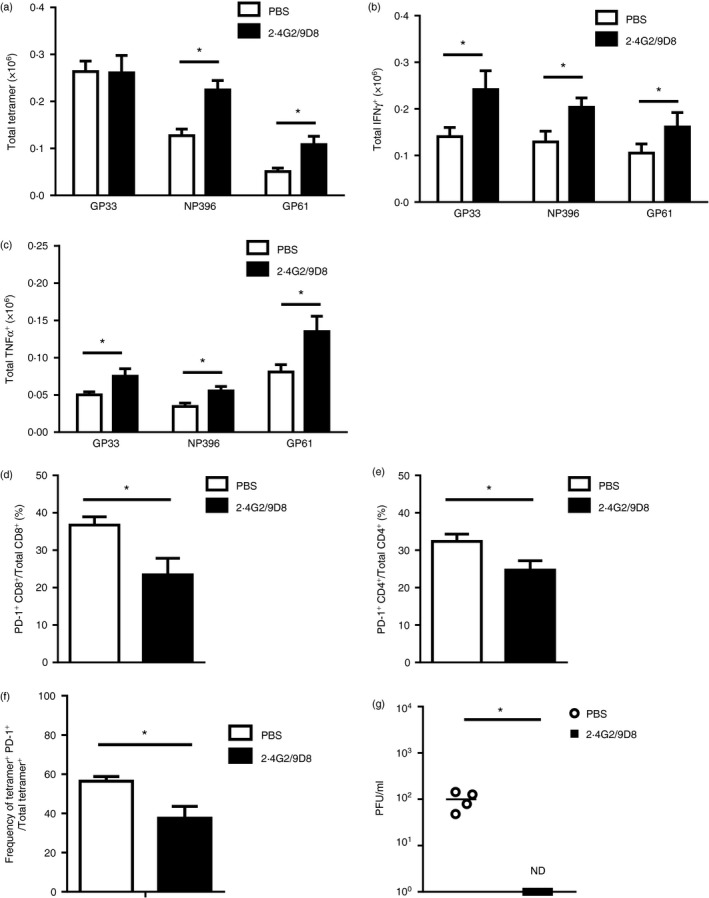
Treatment with antibody to fibrinogen‐like protein 2 (FGL2) and Fcγ RIIB/RIII in mice chronically infected with lymphocytic choriomeningitis virus clone 13 (LCMV cl‐13) enhances T‐cell antiviral immunity and enhances viral clearance. Fgl2 +/+ mice were infected with 2 × 106 PFU of LCMV cl‐13 and on day 25 post infection (p.i.) were treated with 150 µg of neutralizing antibody to FGL2 and 150 µg of neutralizing antibody to Fcγ RIIB/RIII (2.4G2/9D8) or 150 µl of PBS every 2 days until day 56 p.i. The total numbers of splenic CD3+ CD8+ TetGp33‐41 +, CD3+ CD8+ TetNP 396‐404 + and CD3+ CD4+ TetGP 61‐80 + were quantified by flow cytometry (a). Splenic mononuclear cells (SMNC) were isolated from antibody (2.4G2/9D8) and PBS‐treated mice and were stimulated with MHC‐I restricted peptides GP 33‐41, NP 396‐404 or the MHC‐II restricted peptide GP 61‐80 and production of intracellular IFN γ + (b) and TNF + (c) was measured. Frequencies of PD‐1‐expressing CD3+ CD8+ T cells (d),CD3+ CD4+ T cells (e) and TetGP 33‐41 + CD3+ CD8+ T cells (f) were determined by flow cytometry. Plasma viral titres (g) were measured on day 56 p.i. The limit of detection of the LCMV focus‐forming assay is denoted by the dashed line. Data are presented as the mean ± SEM of five mice per group from two independent experiments. *P < 0·05.
Discussion
Effective viral clearance requires a robust innate and adaptive antiviral immune response.15, 44 A common feature of chronic infection is the lack of virus‐specific T‐cell responses, which is characterized by progressive functional T‐cell exhaustion and deletion of virus‐specific CD4+ and CD8+ T cells. Accounting at least in part for T‐cell exhaustion is the overproduction of Treg cells45 with a shift in the ratio of T‐cell sustaining pro‐inflammatory cytokines including interleukin‐2,46, 47 IFN‐γ and TNF‐α 48 and increased production of anti‐inflammatory cytokines including interleukin‐10.15 FGL2 has been identified as an important immunosuppressive effector of Treg cells and central to the suppressive function of a major subset of CD4+ CD25+ FoxP3+ Treg cells expressing TIGIT.32, 34, 49, 50 Ligation of TIGIT to CD155 on APC leads to increased expression of FGL2, resulting in suppression of both Th1 and Th17 responses in vivo.22 We and others have shown that FGL2 exerts its immunosuppressive activity following binding to FcγRIIB/RIII, which is expressed primarily on APC.49 The data generated in Fig. 4 show that the differences between fgl2 +/+ mice and fgl2 −/− mice emerge late in LCMV infection, which supports the contention that enhanced maturation of APC early in infection does not result in early priming of T cells, but rather is involved in the maintenance of virus‐specific T cells late in infection when T‐cell exhaustion has begun. Consistent with this is the finding that expression of programmed death‐ligand 1 on APC isolated from LCMV‐infected fgl2 +/+ and fgl2 −/− mice at days 1 and 2 was not different (data not shown). This suggests that FGL2 could be involved in the contraction of cells rather than their expansion. Although naive T cells do not express FcγRIIB/RIII, virus‐activated T cells have been shown to express FcγRIIB/RIII and hence the immunosuppressive effects of FGL2 on T cells in the setting of LCMV infection may be both direct and indirect.23, 24, 25
In the present study, we examined the contribution of the FGL2‐FcγRIIB/RIII pathway to the establishment of chronic infection caused by LCMV cl‐13. Plasma levels of FGL2 were significantly increased early after LCMV cl‐13 infection and remained elevated at all time‐points p.i. in contrast to infection with LCMV WE, which causes only a short self‐limiting acute infection where FGL2 was only elevated early post infection and to a lesser extent.33 Although we have reported that Treg cells are a major source of FGL2, other cells including macrophages and CD8+ T cells can produce FGL2. Hence at this time we cannot unequivocally state that increased FGL2 in the plasma is solely due to production by Treg.22, 51, 52 We used mice that were genetically deficient in fgl2 to study the effects of FGL2 on both the innate and adaptive antiviral immune response. Post LCMV cl‐13 infection, fgl2 −/− mice had increased numbers of macrophages and DC that expressed CD80, CD86 and MHC‐II, markers of macrophage and DC activation in comparison with fgl2 +/+ mice. Early post LCMV infection at day 7, fgl2 +/+ generated a robust virus‐specific T‐cell response with enhanced numbers of CD3+ CD8+ TetGP33‐41 + T cells. However, by day 56, there was a significant contraction in both numbers of CD3+ CD8+ TetGP33‐41 + cells in fgl2 +/+ mice coupled with increased expression of PD‐1 on both total and LCMV GP33‐specific T cells, which was associated with reduced cellular and humoral antiviral activity and lower central and effector memory. In contrast, by day 56 p.i., fgl2 −/− mice had increased frequencies of LCMV‐specific CD3+ CD8+ TetGP33‐41 + T cells. CD8+ and CD4+ T cells as well as LCMV‐specific CD8+ TetGP33‐41 + T cells from LCMV‐infected fgl2 −/− mice had reduced expression of PD‐1, coincident with enhanced control of viral replication. This result is similar to what has been reported with disruption of interleukin‐10.15
To firmly establish that binding of FGL2 to its receptor FcγRIIB/III accounted for changes in the innate and adaptive immune response to LCMV, mice chronically infected with LCMV were treated with antibody to FGL2 and FcγRIIB/III alone or in combination. Although treatment with either antibody alone partially restored virus‐specific T‐cell responses, these mice failed to clear LCMV. In contrast, treatment with combination of antibodies to both FGL2 and FcγRIIB/RIII led to restoration of CD4+ and CD8+ T‐cell antiviral immunity and viral clearance. That antibody to FGL2 or FcγRIIB/RIII alone did not completely restore antiviral immunity and viral clearance may reflect that the dose and/or frequency of antibody administration might have been insufficient to fully neutralize FGL2. By increasing the dose and timing of administration, viral clearance may be possible, although this was not examined here. Alternatively, it has been reported that antibody administration in the setting of chronic viral infection is less effective, which was thought to be due to the generation of immune complexes.53, 54, 55, 56 The fgl2 −/− mice generated high titred neutralizing antibody at days 70 and 130 p.i. and robust virus‐specific central and effector T‐cell and B‐cell memory responses co‐incident with viral clearance in the plasma, lung and liver by day 56. The changes in antibody concentration seen in this study may reflect differences in follicular T‐cell helper function, which was not examined in this study. Treatment of chronically infected fgl2 +/+ mice with combination antibody to FGL2 and FcγRIIB/RIII resulted in similar results. Interestingly, virus could still be detected in the kidneys of fgl2 −/− mice and fgl2 +/+ mice treated with anti‐FGL2 and anti‐FcγRIIB/RIII. The significance of this finding is not totally clear, although similar findings are seen in patients with chronic HCV infection who clear virus from the plasma. This finding may explain why transplantation of organs from virus‐infected individuals who have cleared the virus from the plasma is known to be associated with a risk of virus transmission.57 This is supportive of the concept that establishment of a sustained virological response in HCV infection leads to immunological control of infection but not total viral eradication.58
Wherry et al. have previously reported an increase in fgl2 mRNA transcripts in effector and exhausted virus‐specific T cells and expression of FcγRIIB on effector T cells.24 In chronic infection, patients with chronic HBV and HCV infection have increased plasma levels of FGL2, as well as increased frequencies of Treg cells expressing FGL2.50, 59 Increased FGL2 during chronic infection could influence T‐cell responsiveness to antigen directly by binding FcγRIIB on activated effector T cells and promoting T‐cell exhaustion or indirectly by binding Fc receptors on APC, preventing DC maturation and antigen presentation. In a study by Starbeck‐Miller et al., expression of FcγRIIB following challenge with LCMV cl‐13 was associated with reduced cytotoxicity and reduced secondary expansion of memory CD8+ T cells.23 It is known that FcγRIIB is an inhibitory receptor widely expressed on DC and B cells and signals through immunoreceptor tyrosine‐based inhibition motif phosphorylation, which causes the recruitment of SHIP leading to reduction in activation of DC and apoptosis of B cells.25 Additionally, FGL2 has been shown to augment immunosuppression by increasing expression levels of PD‐1 and CD39, expanding the frequency of M2 macrophages through FcγRIIB, while increasing the number of myeloid‐derived suppressor cells and Treg cells.60
In summary, the data presented here demonstrate an important role for FGL2 and FcγRIIB/RIII in the development of chronic LCMV cl‐13 infection. Here, we show that targeted deletion of fgl2 and treatment of fgl2 +/+ mice with neutralizing antibodies to FGL2 and FcγRIIB/RIII restores T‐cell and B‐cell antiviral immunity to LCMV cl‐13, resulting in viral clearance. The studies support the concept that targeting the FGL2‐FcγRIIB/RIII pathway might be useful to treat patients with chronic viral infections including HBV, HCV and HIV, which are known to induce expression of FGL2 and FcγRIIB/RIII.
Author contributions
OK, RK, DF, NY, KF, HS, JZ and OA performed the experiments. OK, RK, NS and GL were responsible for design of experiments, interpretation of data and writing the paper.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Fig. S1. Gating strategy for macrophage and dendritic cells.
Fig. S2. Characterization of the anti‐fibrinogen‐like protein 2 antibody 9D8.
Fig. S3. Gating strategy for CD8+ lymphocytic choriomeningitis virus‐specific memory cells.
Acknowledgements
We would like to thank Dr M. Oldstone for LCMV cl‐13 and Dr P. Ohashi for the antibody to LCMV (VL4). Dr Nazia Selzner and Dr Gary Levy are recipients of grants from the Canadian Institute of Health Research (Grant 136781) and the Physician Services Incorporated (Grant 12‐06).
O.K. and R.K. contributed equally to this work.
References
- 1. El‐Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–73.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 2014; 384:258–71. [DOI] [PubMed] [Google Scholar]
- 3. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH et al Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682–7. [DOI] [PubMed] [Google Scholar]
- 4. Chen JH, Perry CJ, Tsui YC, Staron MM, Parish IA, Dominguez CX et al Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat Med 2015; 21:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garidou L, Heydari S, Truong P, Brooks DG, McGavern DB. Therapeutic memory T cells require costimulation for effective clearance of a persistent viral infection. J Virol 2009; 83:8905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K et al Cooperation of Tim‐3 and PD‐1 in CD8 T‐cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 2010; 107:14733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A et al High level of PD‐1 expression on hepatitis C virus (HCV)‐specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol 2008; 82:3154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A et al Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T et al Induction and exhaustion of lymphocytic choriomeningitis virus‐specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I‐peptide complexes. J Exp Med 1998; 187:1383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wherry EJ, Blattman JN, Murali‐Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T‐cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 2003; 77:4911–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J Immunol 2009; 182:6697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Welsh RM. Assessing CD8 T cell number and dysfunction in the presence of antigen. J Exp Med 2001; 193:F19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S et al Resolution of a chronic viral infection after interleukin‐10 receptor blockade. J Exp Med 2006; 203:2461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR et al Blockade of programmed death‐1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003; 170:1257–66. [DOI] [PubMed] [Google Scholar]
- 15. Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin‐10 determines viral clearance or persistence in vivo . Nat Med 2006; 12:1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakthivel P, Gereke M, Bruder D. Therapeutic intervention in cancer and chronic viral infections: antibody mediated manipulation of PD‐1/PD‐L1 interaction. Rev Recent Clin Trials 2012; 7:10–23. [DOI] [PubMed] [Google Scholar]
- 17. Cusick MF, Schiller JJ, Gill JC, Eckels DD. Hepatitis C virus induces regulatory T cells by naturally occurring viral variants to suppress T cell responses. Clin Dev Immunol 2011; 2011:806061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng G, Li S, Wu W, Sun Z, Chen Y, Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology 2008; 123:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Punkosdy GA, Blain M, Glass DD, Lozano MM, O'Mara L, Dudley JP et al Regulatory T‐cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc Natl Acad Sci U S A 2011; 108:3677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG et al Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005; 41:771–8. [DOI] [PubMed] [Google Scholar]
- 21. Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C et al An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 2004; 40:1062–71. [DOI] [PubMed] [Google Scholar]
- 22. Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C et al Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014; 40:569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Starbeck‐Miller GR, Badovinac VP, Barber DL, Harty JT. Cutting edge: expression of FcγRIIB tempers memory CD8 T cell function in vivo. J Immunol 2014; 192:35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V et al Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27:670–84. [DOI] [PubMed] [Google Scholar]
- 25. Huang ZY, Hunter S, Kim MK, Indik ZK, Schreiber AD. The effect of phosphatases SHP‐1 and SHIP‐1 on signaling by the ITIM‐ and ITAM‐containing Fcgγ receptors FcγRIIB and FcγRIIA. J Leukoc Biol 2003; 73:823–9. [DOI] [PubMed] [Google Scholar]
- 26. Bolland S, Ravetch JV. Spontaneous autoimmune disease in FcγRIIB‐deficient mice results from strain‐specific epistasis. Immunity 2000; 13:277–85. [DOI] [PubMed] [Google Scholar]
- 27. Kyogoku C, Dijstelbloem HM, Tsuchiya N, Hatta Y, Kato H, Yamaguchi A et al Fcγ receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum 2002; 46:1242–54. [DOI] [PubMed] [Google Scholar]
- 28. Iruretagoyena MI, Riedel CA, Leiva ED, Gutierrez MA, Jacobelli SH, Kalergis AM. Activating and inhibitory Fcγ receptors can differentially modulate T cell‐mediated autoimmunity. Eur J Immunol 2008; 38:2241–50. [DOI] [PubMed] [Google Scholar]
- 29. Araujo‐Jorge T, Rivera MT, el Bouhdidi A, Daeron M, Carlier Y. An FcγRII‐, FcγRIII‐specific monoclonal antibody (2.4G2) decreases acute Trypanosoma cruzi infection in mice. Infect Immun 1993; 61:4925–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gjertsson I, Kleinau S, Tarkowski A. The impact of Fcγ receptors on Staphylococcus aureus infection. Microb Pathog 2002; 33:145–52. [PubMed] [Google Scholar]
- 31. Clatworthy MR, Smith KG. FcγRIIb balances efficient pathogen clearance and the cytokine‐mediated consequences of sepsis. J Exp Med 2004; 199:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shalev I, Wong KM, Foerster K, Zhu Y, Chan C, Maknojia A et al The novel CD4+CD25+ regulatory T cell effector molecule fibrinogen‐like protein 2 contributes to the outcome of murine fulminant viral hepatitis. Hepatology 2009; 49:387–97. [DOI] [PubMed] [Google Scholar]
- 33. Khattar R, Luft O, Yavorska N, Shalev I, Phillips MJ, Adeyi O et al Targeted deletion of FGL2 leads to increased early viral replication and enhanced adaptive immunity in a murine model of acute viral hepatitis caused by LCMV WE. PLoS ONE 2013; 8:e72309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shalev I, Liu H, Koscik C, Bartczak A, Javadi M, Wong KM et al Targeted deletion of fgl2 leads to impaired regulatory T cell activity and development of autoimmune glomerulonephritis. J Immunol 2008; 180:249–60. [DOI] [PubMed] [Google Scholar]
- 35. Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24‐ or 96‐well plates. J Virol Methods 1991; 33:191–8. [DOI] [PubMed] [Google Scholar]
- 36. Takimoto K, Taharaguchi M, Morikawa S, Ike F, Yamada YK. Detection of the antibody to lymphocytic choriomeningitis virus in sera of laboratory rodents infected with viruses of laboratory and newly isolated strains by ELISA using purified recombinant nucleoprotein. Exp Anim 2008; 57:357–65. [DOI] [PubMed] [Google Scholar]
- 37. Battegay M, Moskophidis D, Waldner H, Brundler MA, Fung‐Leung WP, Mak TW et al Impairment and delay of neutralizing antiviral antibody responses by virus‐specific cytotoxic T cells. J Immunol 1993; 151:5408–15. [PubMed] [Google Scholar]
- 38. Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W (eds) Current Protocols in Immunology. New York: John Wiles & Sons, Inc., 1998. [Google Scholar]
- 39. Jackson HM, Dimopoulos N, Chen Q, Luke T, Yee Tai T, Maraskovsky E, et al A robust human T‐cell culture method suitable for monitoring CD8+ and CD4+ T‐cell responses from cancer clinical trial samples. J Immunol Methods 2004; 291:51–62. [DOI] [PubMed] [Google Scholar]
- 40. Rydyznski C, Daniels KA, Karmele EP, Brooks TR, Mahl SE, Moran MT, et al Generation of cellular immune memory and B‐cell immunity is impaired by natural killer cells. Nat Commun 2015; 6:6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dimopoulos N, Jackson HM, Ebert L, Guillaume P, Luescher IF, Ritter G, et al Combining MHC tetramer and intracellular cytokine staining for CD8+ T cells to reveal antigenic epitopes naturally presented on tumor cells. J Immunol Methods 2009; 340:90–4. [DOI] [PubMed] [Google Scholar]
- 42. Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G‐based strategy to analyze the mouse splenic myeloid compartment. Cytometry A 2012; 81:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chan CW, Kay LS, Khadaroo RG, Chan MW, Lakatoo S, Young KJ, et al Soluble fibrinogen‐like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow‐derived dendritic cells. J Immunol 2003; 170:4036–44. [DOI] [PubMed] [Google Scholar]
- 44. Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL‐10 and PD‐L1 operate through distinct pathways to suppress T‐cell activity during persistent viral infection. Proc Natl Acad Sci U S A 2008; 105:20428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Penaloza‐MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, et al Interplay between regulatory T cells and PD‐1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med 2014; 211:1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. West EE, Jin H‐T, Rasheed A‐U, Penaloza‐MacMaster P, Ha S‐J, Tan WG, et al PD‐L1 blockade synergizes with IL‐2 therapy in reinvigorating exhausted T cells. J Clin Invest 2013; 123:2604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Starbeck‐Miller GR, Xue H‐H, Harty JT. IL‐12 and type I interferon prolong the division of activated CD8 T cells by maintaining high‐affinity IL‐2 signaling in vivo . J Exp Med 2014; 211:105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oldstone MB. Anatomy of viral persistence. PLoS Pathog 2009; 5:e1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu H, Shalev I, Manuel J, He W, Leung E, Crookshank J, et al The FGL2‐FcγRIIB pathway: a novel mechanism leading to immunosuppression. Eur J Immunol 2008; 38:3114–26. [DOI] [PubMed] [Google Scholar]
- 50. Foerster K, Helmy A, Zhu Y, Khattar R, Adeyi OA, Wong KM, et al The novel immunoregulatory molecule FGL2: a potential biomarker for severity of chronic hepatitis C virus infection. J Hepatol 2010; 53:608–15. [DOI] [PubMed] [Google Scholar]
- 51. Urbanellis P, Shyu W, Khattar R, Wang J, Zakharova A, He W, et al The regulatory T cell effector molecule fibrinogen‐like protein 2 is necessary for the development of rapamycin‐induced tolerance to fully MHC‐mismatched murine cardiac allografts. Immunology 2015; 144:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bartczak A, Zhang J, Adeyi O, Amir A, Grant D, Gorczynski R, et al Overexpression of fibrinogen‐like protein 2 protects against T cell‐induced colitis. World J Gastroenterol 2017; 23:2673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamada DH, Elsaesser H, Lux A, Timmerman JM, Morrison SL, de la Torre JC, et al Suppression of Fcγ‐receptor‐mediated antibody effector function during persistent viral infection. Immunity 2015; 42:379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wieland A, Shashidharamurthy R, Kamphorst AO, Han JH, Aubert RD, Choudhury BP, et al Antibody effector functions mediated by Fcγ‐receptors are compromised during persistent viral infection. Immunity 2015; 42:367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trépanier P, Chabot D, Bazin R. Intravenous immunoglobulin modulates the expansion and cytotoxicity of CD8+ T cells. Immunology 2014; 141:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aubin E, Lemieux R, Bazin R. Indirect inhibition of in vivo and in vitro T‐cell responses by intravenous immunoglobulins due to impaired antigen presentation. Blood 2010; 115:1727–34. [DOI] [PubMed] [Google Scholar]
- 57. Levitsky J, Doucette K. Viral hepatitis in solid organ transplantation. Am J Transplant 2013; 4:147–68. [DOI] [PubMed] [Google Scholar]
- 58. Recher M, Lang KS, Navarini A, Hunziker L, Lang PA, Fink K, et al Extralymphatic virus sanctuaries as a consequence of potent T‐cell activation. Nat Med 2007; 13:1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shalev I, Selzner N, Helmy A, Foerster K, Adeyi OA, Grant DR, et al The role of FGL2 in the pathogenesis and treatment of hepatitis C virus infection. Rambam Maimonides Med J 2010; 1:e0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yan J, Kong LY, Hu J, Gabrusiewicz K, Dibra D, Xia X, et al FGL2 as a multimodality regulator of tumor‐mediated immune suppression and therapeutic target in gliomas. J Natl Cancer Inst 2015; 107:djv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Gating strategy for macrophage and dendritic cells.
Fig. S2. Characterization of the anti‐fibrinogen‐like protein 2 antibody 9D8.
Fig. S3. Gating strategy for CD8+ lymphocytic choriomeningitis virus‐specific memory cells.


