Abstract
Background and Objectives:
Paraesophageal hiatal hernia repair can be performed with or without mesh reinforcement. The use, technique, and mesh type remain controversial because of mixed reports on mesh-related complications. Short-term outcomes have become important in all forms of surgery.
Methods:
From January 2012 through April 2017, all patients who underwent isolated hiatal hernia repair in our center were reviewed. Concomitant bariatric surgery cases were excluded. Repairs reinforced by porcine urinary bladder matrix (UBM) graft were compared to non-UBM repairs. Statistical comparison was based on a Wilcoxon 2-sample test or Fisher's exact test.
Results:
We reviewed 239 charts; 110 bariatric cases and 8 cases with non-UBM reinforcement were excluded. We identified 121 patients: 56 UBM-reinforced (46.3%) versus 65 non-UBM (53.7%). Sixteen (28.6%) UBM cases were male versus 23 (35.4%) non-UBM cases. The UBM patients were significantly older (63.9 versus 54.3; P = .001). There was no difference in mean BMI (29.6 vs 28.5; P = .28). Cases were performed laparoscopically (60.7% vs 67.7%; P = .45) or robotically (39.3% vs 32.3%; P = .45), with no conversions to open. The UBM group had a longer mean operative time (183 minutes vs 139 minutes; P = .001).There was no difference in median length of stay (2 days vs 2 days; P = .09) or 30-day readmission rate (7.1% vs 7.5%; P =.99). Postoperative complications were graded according to the Clavien-Dindo classification, and there was no difference (19.6% vs 9.2%; P = .12).
Conclusions:
Hiatal hernia repair with UBM reinforcement can be performed safely with no increase in postoperative complications.
Keywords: Cruroplasty, Fundoplication, Mesh, Paraesophageal hiatal hernia, Urinary bladder matrix
INTRODUCTION
Hiatal hernia (HH) is a common radiographic finding and a frequent contributor to the pathophysiology of gastroesophageal reflux disease (GERD). The prevalence of HH is unknown because of the subjective nature of diagnostic criteria, but has been estimated at 10 to 80%, and increases with age and body mass index (BMI).1,2 There are 4 classifications of HH: type I, >95% of hernias, referred to as sliding hernias; types II–IV, the remaining 5%, collectively known as paraesophageal hernias, the most common of which is type III, accounting for >90% of that classification.3 Indications for surgical HH repair are largely based on hernia type and presence of symptoms. Symptomatic presentations can include persistent or worsening reflux, regurgitation, dysphagia, nausea, vomiting, and epigastric pain.
Modality and method of HH repair has been widely investigated. The laparoscopic approach has been shown to be superior to open thoracotomy and laparotomy in morbidity, mortality, and recurrence,4–6 making it the standard for HH repair.3 In other areas of hernia repair, such as ventral hernia, mesh reinforcement has been shown to be superior to suture repair alone.7 One study reported a 10-year cumulative recurrence rate of 32% in the polypropylene-reinforced group compared to 63% in the suture group.8 The use of biologic mesh to reinforce ventral hernias has also been reported.9 Similarly, the use and outcomes of both synthetic and biologic mesh to reinforce HH cruroplasty has been the focus of many recent studies, with controversy regarding the risk of complications. Lower recurrence rates have been reported with the use of mesh compared to suture cruroplasty alone in both short-term3,10,11 and long-term.12,13 studies. However, studies have also shown no association with improved outcomes when comparing mesh to nonmesh repair.14,15 Also, mesh-related complications have been reported, including intraluminal mesh erosion, dysphagia, esophageal stenosis, stricture formation, mesh migration, and dense fibrosis.16–18
In light of these findings, there is a continued concern regarding the use of mesh for crural reinforcement during HH repair. Several biomaterials have been developed as a temporary matrix to strengthen the crural repair.7,19,20 One such mesh is an acellular, extracellular matrix scaffold derived from porcine urinary bladder matrix (UBM; Gentrix; Acell, Inc., Columbia, Maryland, USA). Having used this material in several of our cases, we wanted to review its use at our institution. Our goal was to examine the short-term outcomes of patients who underwent isolated HH repair in our practice with and without the use of UBM. Although in prior studies emphasis has been placed on long-term outcomes, particularly recurrence, in this era of pay-for-performance there is a desire to reduce readmission rates. As such, a focus on short-term outcomes is essential.
METHODS
We performed a retrospective chart review of all patients who underwent isolated paraesophageal hiatal hernia (PHH) repair by 3 surgeons on our foregut surgery service from January 2012 through April 2017. Patients who underwent concomitant bariatric surgery were excluded. Fundoplication and cruroplasty reinforced with the use of porcine UBM were compared to fundoplication and suture cruroplasty alone. Figure 1 illustrates the criteria for patients to be included in the final comparison. The use and shape (eg, U- vs keyhole-shaped) of crural reinforcement was at the discretion of the surgeon. In general, reinforcement tended to be placed when large defects were encountered or when the crural fibers appeared attenuated. The type of procedure performed (eg, Nissen vs Toupet fundoplication) was the choice of the surgeon. However, in cases where esophageal dysfunction was present or patients refused esophageal manometry, a partial fundoplication was performed. Summary statistics such as mean (±SD), median (interquartile range [IQR]), and frequency (%) were generated. Statistical comparison was based on t tests for normally distributed, continuous variables, Wilcoxon 2-sample tests for nonnormally distributed variables, or Fisher's exact test for association between categorical variables. Two-sided P < .05 was considered statistically significant. All statistical analyses were performed in SAS, ver. 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Figure 1.
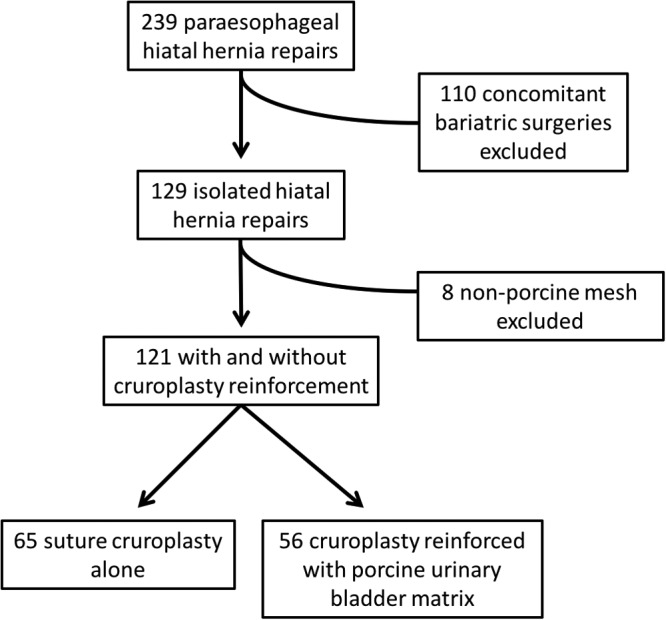
Patient inclusion flow chart.
RESULTS
We reviewed 239 charts initially. One hundred ten concomitant bariatric cases and 8 cases with crural reinforcement with materials other than UBM were excluded. We identified 121 patients: 56 UBM-reinforced (46.3%) repairs and 65 non-UBM (53.7%) repairs. Sixteen (28.6%) of the UBM patients were male versus 23 (35.4%) of the non-UBM patients. The mean age of the UBM group was significantly higher than that of the non-UBM group (63.9 years vs 54.3 years; P = .001). The mean BMI of the UBM group was 29.6 compared with 28.5 in the non-UBM group (P = 0.28). Laparoscopic repair was performed in 34 (60.7%) UBM cases and 44 (67.7%) non-UBM cases. Robot-assisted repair was performed in 22 (39.3%) UBM cases and 21 (32.3%) non-UBM cases, with no conversion to open in either group. UBM use was associated with a significantly longer mean operative time (183 min vs 139 min; P = .001). Table 1 illustrates the patient demographics.
Table 1.
Patient Demographics
| UBM (n = 56) | No UBM (n = 65) | P* | |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 40 (71) | 42 (65) | .44 |
| Male | 16 (29) | 23 (35) | |
| Age (years) | 63.9 (13.8) | 54.3 (17.2) | .001 |
| Range | 29–91 | 20–88 | |
| BMI | 29.6 (5.5) | 28.5 (5.4) | .28 |
| Range | 19–42 | 17.7–45 | |
| Modality, n (%) | |||
| Laparoscopic | 34 (61) | 44 (68) | .45 |
| Robotic | 22 (39) | 21 (32) | |
| Surgery time (min) | |||
| Overall | 182.9 (61.6) | 139.4 (61.4) | .001 |
| Laparoscopic, mean | 162 | 120.6 | .001 |
| Robotic, mean | 214.1 | 178.0 | .05 |
Data are means (SD) unless otherwise specified.
*Based on t test (continuous) or Fisher's exact test (categorical).
Tables 2 and 3 demonstrate operative details regarding the use of mesh, hiatal closure, and anchoring of the repair, if performed. There was no significant difference in overal length of stay (median 2 days vs 2 days; P = .09) or 30-day readmission rate (7.1% vs 7.5%; P = 1.00). One patient underwent 30-day reoperation in each group. Table 4 shows the outcomes of the UBM vs non-UMB groups.
Table 2.
Mesh Details
| Details | n |
|---|---|
| Shape | |
| U | 14 |
| Keyhole | 23 |
| Not specified | 19 |
| Securing method | |
| Tacks | 13 |
| Suture | 19 |
| Tacks + suture | 23 |
| Not specified | 1 |
Table 3.
Surgical Details
| Procedure | UBM (n = 56) | No UBM (n = 65) |
|---|---|---|
| Anchoring | ||
| Wrap anchored to crura | 39 | 51 |
| No anchor | 13 | 9 |
| Not specified | 3 | 3 |
| Stomach to abdominal wall* | 1 | 2 |
| Hiatal closure suture | ||
| Not specified | 6 | 8 |
| V-loc | 40 | 42 |
| 2-0 silk | 5 | 11 |
| Other (combination; PDS; Vicryl) | 5 | 4 |
Anchoring refers to whether the method of repair was to anchor to nearby structures (eg, diaphragmatic crura).
*Suturing of the gastric body with fundoplication and without mesh was performed once to the anterior and once to the posterior abdominal wall. Gastropexy to the anterior abdominal wall without fundoplication was performed in 1 mesh-reinforced repair. V-loc: barbed suture (Medtronic, Minneapolis, Minnesota, USA); PDS: polydioxanone suture; Vicryl: polyglactin 910 (Ethicon, Cincinnati, Ohio, USA).
Table 4.
Patient Outcomes
| Outcome | UBM (n = 56) | No UBM (n = 65) | P* |
|---|---|---|---|
| Length of stay, days (IQR) | |||
| Overall | 2 (0–10) | 2 (0–17) | 0.09 |
| Laparoscopic | 2 (0–10) | 1 (0–17) | 0.04 |
| Robotic | 2 (1–9) | 2 (1–9) | 0.87 |
| Method, n (%) | |||
| Toupet | 33 (58.9) | 31 (47.7) | |
| Dor | 1 (1.8) | 4 (6.2) | |
| Suture | 9 (16.1) | 8 (12.3) | 0.38 |
| Nissen | 13 (23.2) | 22 (33.8) | |
| Concomitant Collis | 3 (5.4) | 2 (3.0) | |
| Postoperative outcome | |||
| Thirty-day readmission, n (%) | 4 (7.1) | 5 (7.5) | 1.00 |
| Post-op complication, n (%) | 11 (19.6) | 6 (9.2) | 0.12 |
| Thirty-day reoperation, n (%) | 1 (1.8) | 1 (1.5) | 1.00 |
Data are presented as median (IQR) or counts (%).
*Based on a Wilcoxon Mann-Whitney test (continuous) or Fisher's exact test (categorical).
Complications were graded according to the Clavien-Dindo classification. Table 5 shows the number of complications that occurred in the UBM and non-UBM groups. There was no statistically significant difference in the rate of postoperative complications between the 2 groups (19.6% vs 9.2%; P = .12).
Table 5.
Postoperative Complications According to Clavien-Dindo Classification
| Clavien-Dindo Classification | UBM (n = 56) | No UBM (n = 65) | P |
|---|---|---|---|
| I | 6 | 2 | .99 |
| II | 1 | 1 | |
| IIIa | 3 | 2 | |
| IIIb | 1 | 1 | |
| IV | 0 | 0 | |
| V | 0 | 0 |
Table 5 demonstrates the postoperative complications according to Clavien-Dindo classification in the UBM versus non-UBM groups. Grade I complications included postoperative hypoxemia requiring new home oxygen, narcotic-induced lethargy, delayed gastric emptying that resolved with bowel rest, 2 self-limiting small pneumothoraces, and 3 failed trial of urinary voiding requiring Foley catheter reinsertion. Grade II complications included 1 new-onset atrial fibrillation and 1 deep vein thrombosis, both requiring anticoagulation. Grade IIIa complications included 1 case of bilateral pleural effusion requiring thoracentesis and 4 cases of dysphagia requiring esophagogastroduodenoscopy (EGD). Details of the patients' histories are given below.
Case 1
A 46-year-old woman with a history of hiatal hernia repairs at the ages of 8 and 10 years, both of which were open procedures with known postoperative recurrence and subsequent severe GERD for over 30 years. Surgical history also included appendectomy, cholecystectomy, and 3 caesarian deliveries. She underwent a robot-assisted PHH repair with UBM reinforcement and was noted to have significant adhesions surrounding the liver bed and spleen that required extensive adhesiolysis. The patient tolerated the procedure well and was discharged on postoperative day (POD) 2. She returned to the emergency department (ED) on POD 7 with worsening nausea and vomiting and was found to have bilateral pleural effusions that were drained successfully.
Case 2
A 46-year-old man underwent uncomplicated, robot-assisted PHH repair and was discharged on POD 3. He returned to the ED on POD 5 complaining of chest pain and dysphagia to liquids. EGD performed on POD 8 revealed Candida esophagitis, for which the patient was treated with fluconazole.
Case 3
A 49-year-old woman underwent uncomplicated, laparoscopic PHH repair with UBM reinforcement and was discharged on POD 1. She returned to the ED on POD 7 complaining of dysphagia. EGD revealed moderate resistance at the gastroesophageal junction (GEJ), which was resolved with dilation, and the patient was discharged on the same day.
Case 4
A 71-year-old woman underwent uncomplicated, laparoscopic PHH repair and was discharged on POD 2. She returned to the ED on POD 25 with nausea, vomiting, and heart palpitations. EGD revealed decreased esophageal motility that was managed expectantly.
Case 5
A 61-year-old woman with a surgical history significant for duodenal switch procedure underwent uncomplicated laparoscopic HH repair with UBM reinforcement and was discharged on POD 1. She returned to the ED on POD 16 complaining of dysphagia to solids after eating a meatball 1 week after the HH repair. In our practice, patients are placed on a full liquid diet for 2 weeks after surgery, and this patient was noted to have eaten solid food during this time. EGD revealed a large quantity of retained food in the middle third of the esophagus. The scope was noted to move freely across the GEJ with an area of luminal narrowing just proximal to the Z line, which was dilated, and the patient was discharged on the same day.
Grade IIIb complications included 1 reoperation for incarcerated ventral and incisional hernias in a patient who underwent laparoscopic PHH repair with UBM reinforcement and 1 reoperation for evacuation of abdominal hematoma in a patient who underwent robot-assisted repair of PHH.
DISCUSSION
Recent guidelines released by the Society of American Gastrointestinal and Endoscopic Surgeons reviewed the management of hiatal hernias, including the use of mesh reinforcement. Strong evidence showed decreased short-term recurrence rates with the use of mesh in large hiatal hernia repairs; however, inadequate long-term data were available to arrive at recommendations either for or against the use of mesh.3 Short-term complications were evaluated in 3 prospective randomized controlled trials. The first trial included 72 patients with a >8 cm hiatal defect who underwent Nissen fundoplication and posterior cruroplasty, with or without onlay polytetrafluoroethylene (PTFE) mesh.21 Main outcomes included recurrence, hospital stay, operative time, cost, and complications over a mean follow-up of 3.3 years. Recurrence occurred in 22% of the non-PTFE group and 0% in the PTFE group. No mesh-related complications were reported. The second trial included 100 consecutive patients undergoing Nissen fundoplication, with or without polypropylene mesh onlay and an up to 1-year follow-up.22 The mesh group had a higher postoperative rate of dysphagia, but a lower rate of wrap migration than the nonmesh group. The third trial included both the short-23 and long-term24 results of 108 patients who had repair of a paraesophageal HH, with or without a small-intestine submucosal biologic mesh buttressed in place. Six-month follow-up showed a reduction in recurrence rates; however, this difference equalized at the 4-year follow-up with a >50% recurrence rate in both groups.
Mesh-related complications have been reported in the literature and vary according to the type of mesh used. Porcine survival models showed that the use of UBM for HH reinforcement resulted in full histological integration of the scaffold into the native tissue and higher tensile strength versus controls.25 These results led to the hypothesis that crural reinforcement could reduce human HH recurrence rates. Clinical studies have shown a decreased recurrence rate; however, reinforcement with synthetic mesh has been linked to intraluminal mesh erosion.16,26 Suture repair alone was linked to better improvement of dysphagia compared to biologic mesh augmentation.27 One small, retrospective study evaluating short- and long-term outcomes of patients who underwent HH repair with various biologic graft reinforcement showed no mesh-related complications.28 In the case summaries, we report 17 postoperative complications that occurred within 30 days of PHH repair: 10 in the UBM group and 7 in the non-UBM group. We believe that only 2 of the complications in the UBM group (dysphagia requiring EGD and dilation) could be deemed as mesh-related. In a study by Sasse and colleagues,13 15 patients were observed for 24 to 56 months after undergoing laparoscopic HH repair with UBM reinforcement.13 Of those, only 1 required EGD and dilation 4 weeks after surgery for dysphagia, and no other complications occurred. We believe that the technique of completely encircling the esophagus is a contributor to stenosis and have replaced that practice with the use of U-shaped reinforcement.
In this retrospective study, the use of UBM was not associated with an increased rate of complications, hospital length of stay, or 30-day readmissions—known benchmarks of quality of care. The decision of whether to use UBM was left to the surgeon. In general, a cruroplasty is reinforced when attenuated or compromised or when large defects are encountered. The older patient population may also have relatively weakened crura, necessitating the addition of UBM, as evidenced by the significant age difference found between the UBM and non-UBM groups. The retrospective, observational nature of this study in addition to the lack of a control group limits the conclusions that can be made. In addition, surgeon-specific technical factors play a role in the rate of complications and other short-term outcome parameters. In this report, our goal was to focus on the short-term outcomes because of their importance in physician profiling and the current pay-for-performance reimbursement initiatives. Future, prospective randomized controlled studies are being designed to further delineate the benefits and short- and long-term outcomes of mesh use.
CONCLUSION
The use of UBM to reinforce HH cruroplasty repair has been associated with a lower short-term recurrence rate and no difference in long-term outcomes. In our institution, the use of porcine UBM was not associated with an increase in postoperative complications. Long-term prospective studies are needed to further delineate the effectiveness of this useful modality.
Contributor Information
Raelina S. Howell, Departments of Surgery.
Melissa Fazzari, Biostatics, NYU Winthrop Hospital, Mineola, New York..
Patrizio Petrone, Departments of Surgery.
Alexander Barkan, Departments of Surgery.
Keneth Hall, Departments of Surgery.
María José Servide, Departments of Surgery.
María Fernanda Anduaga, Departments of Surgery.
Collin E. M. Brathwaite, Departments of Surgery.
References:
- 1. Kahrilas PJ, Hirano I. Diseases of the Esophagus. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrison's Principles of Internal Medicine. 19th ed McGraw-Hill Medical; Chapter 347, 2014. http://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79747483#1120810463 Accessed September 28, 2017.
- 2. Roman S, Kahrilas PJ. The diagnosis and management of hiatus hernia. BMJ. 2014;349:g6154. [DOI] [PubMed] [Google Scholar]
- 3. Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc. 2013;27:4409–4428. [DOI] [PubMed] [Google Scholar]
- 4. Zehetner J, DeMeester SR, Ayazi S, et al. Laparoscopic versus open repair of paraesophageal hernia: the second decade. J Am Coll Surg. 2011;212:813–820. [DOI] [PubMed] [Google Scholar]
- 5. Fullum TM, Oyetunji TA, Ortega G, et al. Open versus laparoscopic hiatal hernia repair. JSLS. 2013;17(1):23–29. DOI: 10.4293/108680812X13517013316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen NT, Christie C, Masoomi H, Matin T, Laugenour K, Hohmann S. Utilization and outcomes of laparoscopic versus open paraesophageal hernia repair. Am Surg. 2011;77:1353–1357. [PubMed] [Google Scholar]
- 7. El-Hayek KM, Chand B. Biologic prosthetic materials for hernia repairs. J Long Term Eff Med Implants. 2010;20:159–169. [DOI] [PubMed] [Google Scholar]
- 8. Burger JWA, Luijendijk RW, Hop WCJ, Halm JA, Verdaasdonk EGG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roth JS, Brathwaite C, Hacker K, Fisher K, King J. Complex ventral hernia repair with a human acellular dermal matrix. Hernia. 2015;19:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Priego Jiménez P, Salvador Sanchís JL, Angel V, Escrig-Sos J. Short-term results for laparoscopic repair of large paraesophageal hiatal hernias with Gore Bio A® mesh. Int J Surg. 2014;12:794–797. [DOI] [PubMed] [Google Scholar]
- 11. Ruscio S, Abdelgawad M, Badiali D, et al. Simple versus reinforced cruroplasty in patients submitted to concomitant laparoscopic sleeve gastrectomy: prospective evaluation in a bariatric center of excellence. Surg Endosc. 2016;30:2374–2381. [DOI] [PubMed] [Google Scholar]
- 12. Priego P, Ruiz-Tovar J, Pérez de Oteyza J. Long-term results of giant hiatal hernia mesh repair and antireflux laparoscopic surgery for gastroesophageal reflux disease. J Laparoendosc Adv Surg Tech. 2012;22:139–141. [DOI] [PubMed] [Google Scholar]
- 13. Sasse KC, Warner DL, Ackerman E, Brandt J. Hiatal hernia repair with novel biological graft reinforcement. JSLS. 2016;20(2):e2016.00016 DOI: 10.4293/JSLS.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abu Saleh WK, Morris LM, Tariq N, et al. Routine use of mesh during hiatal closure is safe with no increase in adverse sequelae. Surg Endosc. 2018;332:879–888. [DOI] [PubMed] [Google Scholar]
- 15. Tam V, Luketich JD, Levy RM, et al. Mesh cruroplasty in laparoscopic repair of paraesophageal hernias is not associated with better long-term outcomes compared to primary repair. Am J Surg. 2017;214:651–656. [DOI] [PubMed] [Google Scholar]
- 16. Stadlhuber RJ, Sherif A El, Mittal SK, et al. Mesh complications after prosthetic reinforcement of hiatal closure: a 28-case series. Surg Endosc. 2009;23:1219–1226. [DOI] [PubMed] [Google Scholar]
- 17. Antonakis F, Köckerling F, Kallinowski F. Functional results after repair of large hiatal hernia by use of a biologic mesh. Front Surg. 2016;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Priego P, Perez de Oteyza J, Galindo J, et al. Long-term results and complications related to Crurasoft(®) mesh repair for paraesophageal hiatal hernias. Hernia. 2017;21:291–298. [DOI] [PubMed] [Google Scholar]
- 19. Alicuben ET, Worrell SG, DeMeester SR. Resorbable biosynthetic mesh for crural reinforcement during hiatal hernia repair. Am Surg. 2014;80:1030–1033. [PubMed] [Google Scholar]
- 20. Antoniou SA, Pointner R, Granderath FA. Hiatal hernia repair with the use of biologic meshes. Surg Laparosc Endosc Percutan Tech. 2011;21:1–9. [DOI] [PubMed] [Google Scholar]
- 21. Frantzides CT, Madan AK, Carlson MA, Stavropoulos GP. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg. 2002;137:649–652. [DOI] [PubMed] [Google Scholar]
- 22. Granderath FA. Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation. Arch Surg. 2005;140:40. [DOI] [PubMed] [Google Scholar]
- 23. Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg. 2006;244:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg. 2011;213:461–468. [DOI] [PubMed] [Google Scholar]
- 25. Riganti JM, Ciotola F, Amenabar A, et al. Urinary bladder matrix scaffolds strengthen esophageal hiatus repair. J Surg Res. 2016;204:344–350. [DOI] [PubMed] [Google Scholar]
- 26. Frantzides CT, Carlson MA, Loizides S, et al. Hiatal hernia repair with mesh: a survey of SAGES members. Surg Endosc. 2010;24:1017–1024. [DOI] [PubMed] [Google Scholar]
- 27. Zhang C, Liu D, Li F, et al. Systematic review and meta-analysis of laparoscopic mesh versus suture repair of hiatus hernia: objective and subjective outcomes. Surg Endosc. 2017;31:4913–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reznichenko AA. Different biologic grafts for diaphragmatic crura reinforcement during laparoscopic repair of large hiatal hernia: a six-year single surgeon experience. J Curr Surg. 2016;6:6–13. [Google Scholar]


