Abstract
In cancer genetics, technological advances (next generation sequencing) and the expansion of genetic test options have resulted in lowered costs and increased access to genetic testing. Despite this, the majority of patients utilizing cancer genetics services lack diversity of gender, ethnicity, and socioeconomic status. Through retrospective chart review, we compared outcomes of cancer genetics consultations at a tertiary cancer center and a Federally Qualified Health Center (FQHC) (58 tertiary and 23 FQHC patients) from 2013 to 2015. The two groups differed in race, ethnicity, use of translator services, and type of insurance coverage. There were also significant differences in completeness of family history information, with more missing information about relatives in the FQHC group. In spite of these differences, genetic testing rates among those offered testing were comparable across the two groups with 74% of tertiary patients and 60% of FQHC patients completing testing. Implementation of community-based cancer genetics outreach clinics represents an opportunity to improve access to genetic counseling services, but more research is needed to develop effective counseling models for diverse patient populations.
Keywords: Cancer genetics, Disparities, Genetic counseling, FQHC, Underserved, Interpreter, BRCA
Introduction
Cancer genetics is a field in which access to care has been disparate among different racial, ethnic, economic, and geographical cohorts (Allford et al. 2014; Anderson et al. 2012). In the USA, patients presenting to cancer genetics clinics have been predominantly European-American women from middle- and upper-class families (Gao et al. 1997; Gao et al. 2000). Financial, cultural, psychological, patient access, communication, and educational factors underlie some of the disparities observed in access to and utilization of cancer genetics services (Joseph and Guerra 2015; Kinney et al. 2010; Komenaka et al. 2016; McCarthy et al. 2016; Sherman et al. 2014).
In studies that have evaluated barriers experienced by Latina, African-American, and European-American women undergoing genetic counseling and testing, patients from minority groups cited limited knowledge of genetic testing, lack of physician referral, lack of time, low priority due to good current health, fear of test results, and concerns about risks to children as deterrents to undergoing genetic testing (McCarthy et al. 2016; Thompson et al. 2002; Thompson et al. 2003; Vadaparampil et al. 2010). Historically, lack of insurance coverage has been a major barrier to genetic testing (Komenaka et al. 2016; Olaya et al. 2009; Wideroff et al. 2003). In one study of utilization of cancer genetics services by African-American young breast cancer survivors, access to this service was the major barrier associated with lack of genetic testing (Jones et al. 2016). Next-generation sequencing (NGS) technologies as well as increased competition among genetic testing laboratories have driven down genetic testing costs leading to lower patient-pay options and increased insurance coverage including the establishment of contracts with Medicare and Medicaid. Patients who would previously have been turned away due to a lack of coverage and pricing may now have the option to pursue genetic testing. However, patients presenting for cancer genetics care continue to be largely from middle- and upper-socioeconomic groups, suggesting that other barriers persist for lower socioeconomic groups (Armstrong et al. 2015).
The relative paucity of genetic data in non-European populations resulting from research and health care disparities has in parallel limited the clinical utility of genetic testing for minority patients (Manrai et al. 2016; Ricker et al. 2016). Interpretation of test results from ethnically diverse patients has been notably difficult due to a disproportionately high number of uncharacterized genetic variants (Eccles et al. 2015). Increasing uptake of genetic testing through multi-gene panels in minority patients is helping to fill the data and interpretation gaps so that the promise of genetic medicine can be more fully realized in these communities (Forman and Hall 2009; Hall et al. 2009; Hermel et al. 2017; Ricker et al. 2006). Owing in part to existing barriers to genetic services, few studies have sought to characterize the process of genetic testing and outcomes of patients from diverse socioeconomic and ethnic backgrounds. Better understanding of the cultural perceptions, beliefs, and taboos can help in the development of targeted interventions and instruments aimed at improving uptake of testing by underserved women (Lee et al. 2005; Sherman et al. 2014). Some researchers have suggested provision of genetic services in local community clinics as a strategy to reduce existing barriers to access, (Komenaka et al. 2016; Lee et al. 2005; Sussner et al. 2015) an approach that informs the current study.
In 2012, Dana-Farber Cancer Institute (DFCI), a National Cancer Institute designated cancer center, initiated a clinical outreach program called Community Cancer Program (CCP) within a Federally Qualified Health Center (FQHC) as part of a comprehensive initiative to address cancer disparities. The FQHC site is the Whittier Street Health Center, a primary care center in Roxbury, Massachusetts, with a long-standing mission to deliver high-quality care to vulnerable patient populations. Minority groups constitute nearly 95% of the FQHC population; 90% of patients are at or below 200% of the national poverty level; 100% live in public housing; and 43% of patients are best served in a language other than English (HRSA 2014). Since the services provided through the clinical outreach initiative are intended to comprehensively cover the spectrum of cancer-related diseases from prevention through survivorship, patients are not required to have a cancer diagnosis in order to obtain an appointment for consultation. Patients at the FQHC site are referred by their primary care providers for possible hereditary cancer susceptibility. By contrast, patients seen at the academic tertiary care center at DFCI for genetic evaluations are typically referred by a variety of health care providers (e.g., oncologist, surgeon, primary care, gynecologist) or are self-referred. A geneticist and a genetic counselor from DFCI’s Center for Cancer Genetics and Prevention provide cancer genetics services at both sites.
While determinants of access and utilization of genetic services are exceedingly complex, this study aims to better characterize these factors to elucidate continuing obstacles to care, while exploring methods of improving access to cancer genetics services. To this end, we outline demographics, indications for referral, insurance coverage, risk assessment, and genetic testing outcomes of patients who received similar genetic counseling but who present from two distinct settings—patients seen at an academic tertiary cancer care center (hereafter referred to as “tertiary”) and patients seen at an outreach FQHC.
Methods
Participants
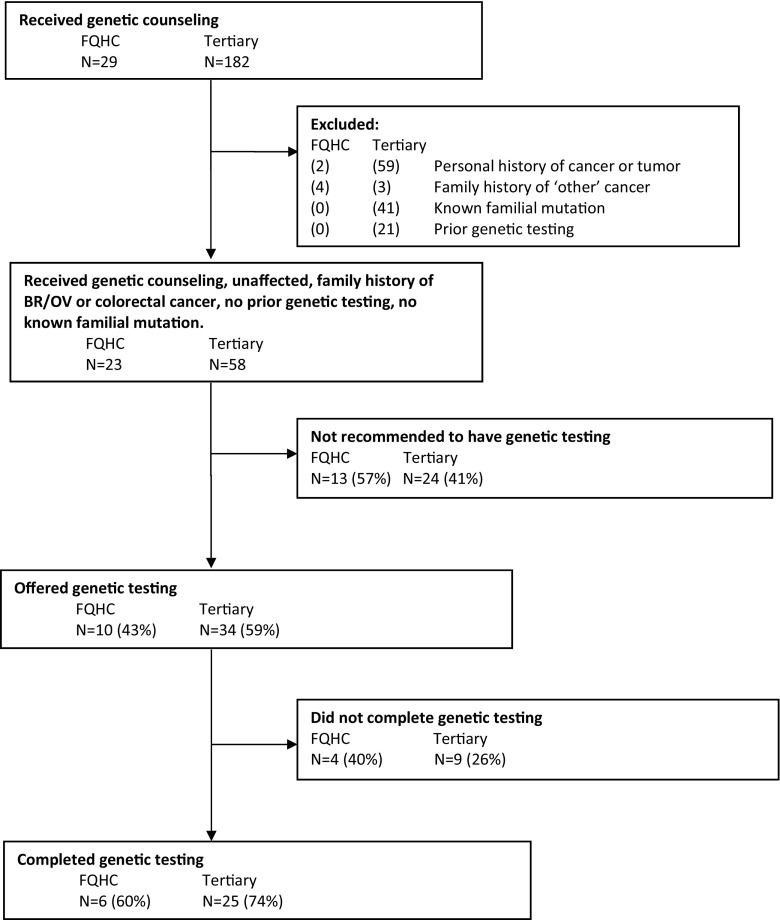
This is a retrospective study of patients from the Dana-Farber Community Cancer Care Program collaborative with a FQHC and patients from DFCI’s Center for Cancer Genetics and Prevention. The sample includes patients who were seen for genetic counseling and risk assessment between August 2013 and May 2015 by the same genetics team at the two centers. Inclusion criteria are outlined in Fig. 1.
Fig. 1.
Summary of patients seen for genetic counseling, offered genetic testing, and completion of genetic testing
Procedures
Following approval by the DFCI Institutional Review Board, clinical data from all initial and follow-up visits to the two clinics were collected retrospectively from electronic medical records. Overall, 29 records from the FQHC group and 182 records from the tertiary group were reviewed. All patients at both sites met with a certified genetic counselor followed by a cancer geneticist, in two back-to-back visits. A three-generation pedigree was captured in-person, during the visit with the genetic counselor. The genetic counselor’s visit focused on counseling the patient about the benefits and limitations of genetic testing, psychosocial concerns surrounding genetic testing, and consenting to the appropriate genetic test. The geneticist’s visit focused on recommendations for screening, prevention, and cancer risk reduction. The hand-drawn pedigree was later entered into Progeny. All patients at both sites had access to interpreter services. An in-person interpreter was used when English was not the patient’s preferred language. 2013/2014 National Comprehensive Cancer Network (NCCN) guidelines and insurance coverage were used to determine which of the patients met criteria for genetic testing. Specific NCCN guidelines for hereditary breast and ovarian cancer (BRCA1/BRCA2), Lynch syndrome, and familial polyposis were used (Burt et al. 2013; Daly et al. 2014; NCCN 2013). Patients not meeting NCCN guidelines were categorized as “low-risk” by the genetics team.
In both groups, as per standard practice at the time of testing, patients with living relatives in the USA who would be more informative genetic testing candidates were asked to encourage their relatives to pursue genetic testing. Therefore, some patients who met NCCN or other guidelines for testing were not recommended to have testing. Likewise, patients who provided vague family histories of cancer were asked to clarify the cancer history if the relative was living, regardless of that relative’s country of residence. For example, if “female cancer” was reported in a living relative, the patient was asked to clarify with their relative whether the cancer was cervical, endometrial, or ovarian in origin. All patients were provided with a business card including the phone number and e-mail address of their genetic counselor, whom they were asked to contact with updated information.
Information including patient demographics, three generation pedigree, personal and family cancer history, hormonal risk factors, risk assessment, genetic testing uptake, and results was systematically extracted through serial review by a genetics clinical research coordinator at the time of the analysis. Lifetime risks of breast cancer and colorectal cancer, as well as probability of carrying a predisposing genetic mutation, were calculated using the Tyrer-Cuzick, BRCAPro, and MMRPro models (Bondy and Newman 2003; Huo et al. 2009; Mercado et al. 2012; Parmigiani et al. 2007; Tyrer et al. 2004; Vogel et al. 2007). Questions about data extraction were answered by genetic counselors and the geneticist. Incomplete information about first-degree relatives had two components: “Missing Information” was noted when no information was known about a first-degree relative. “Unknown cancer” was noted when a patient either did not know whether their relative had cancer or when the patient did not know the relatives’ cancer type.
Data analysis
First, we descriptively summarized cancer and genetic testing histories for all patients who received genetic counseling. Due to substantial imbalance between the two cohorts with respect to these histories that would impact genetic testing outcomes, comparative analyses between the two cohorts were limited to patients who met the following criteria: they had no current or prior history of cancer, had family history of breast, ovarian, and/or colorectal cancer, had not had prior genetic testing, and did not have a previously identified mutation in a cancer predisposition gene in family members (see Fig. 1).
For the comparison cohorts, patient demographics and results of risk assessment were summarized descriptively. Baseline demographics, referral indications, and genetics evaluation outcomes (risk assessment, uptake of genetic testing, completeness of family history, and test results) were compared between the FQHC and tertiary patient cohorts by Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. For the risk assessment and genetic testing outcomes, we calculated absolute differences between groups with 95% confidence intervals. Statistical analyses were conducted using Stata 13.1 (StataCorp, College Station, TX).
Results
Demographics
We initially identified 182 patients at the tertiary center and 29 at FQHC who received genetic counseling. While 41 patients (23%) at the tertiary center were being seen for a known familial mutation, none of the FQHC patients reported this history. Additionally, 21 patients (12%) at the tertiary center had undergone prior limited genetic testing vs. none of the FQHC patients. The subsequent analyses focused on genetics risk assessment outcomes of 58 tertiary and 23 FQHC patients without prior personal history of cancer or known familial cancer syndrome. The cohorts were mostly female (87% FQHC, 90% tertiary) with similar median ages (43 years, FQHC; 41 years, tertiary). Their indications for referral to genetics were also similar (Table 1). It is important to note that the breast/ovary cancer family history and colorectal cancer family history are not mutually exclusive categories.
Table 1.
Characteristics of unaffected patients seen for genetic counseling
| FQHC | Tertiary | P** | |
|---|---|---|---|
| Number of patients | 23 | 58 | |
| Age, median (range) | 43 (25–63) | 41 (20–65) | 0.71 |
| Female gender, n (%) | 20 (87) | 52 (90) | 0.71 |
| Race/ethnicity, n (%) | < 0.001 | ||
| White | 2 (9) | 43 (74) | |
| Black | 6 (26) | 2 (3) | |
| Hispanic | 13 (57) | 6 (10) | |
| Asian | 0 (0) | 2 (3) | |
| Multiracial | 2 (9) | 5 (9) | |
| Ashkenazi Jewish, n (%) | 0 (0) | 6 (10) | 0.18 |
| Health insurance, n (%) | < 0.001 | ||
| Private | 3 (13) | 47 (81) | |
| Medicaid only, safety net only, or none | 18 (78) | 9 (16) | |
| Medicare only | 2 (9) | 2 (3) | |
| Required medical interpreter, n (%) | 5 (22) | 1 (2) | 0.006 |
| Indication*, n (%) | |||
| Breast/ovary | 21 (91) | 49 (84) | 0.72 |
| Colorectal | 7 (30) | 21 (36) | 0.78 |
*Not mutually exclusive
**P values are calculated by the Wilcoxon rank-sum test for age and by Fisher’s exact test for all other variables
The two cohorts differed markedly in race/ethnicity, type of insurance coverage, and use of interpreter services. The FQHC cohort was mostly Hispanic (57%) and Black (26%) with no known Ashkenazi Jewish (AJ) ancestry. The tertiary cohort was 74% non-Hispanic White (p < 0.001), and 14% reported AJ descent. Thirteen (13) percent of the FQHC cohort had private insurance (non-Medicaid/Medicare) while 81% of the tertiary cohort had private insurance (p < 0.001). More FQHC patients required the services of a medical interpreter than did tertiary patients (22 vs. 2%, p = 0.006).
Family history completeness and risk calculations
The tertiary and FQHC groups differed in completeness of family history (Table 2). In the FQHC cohort, 30% (7/23) were missing information about first-degree relatives compared to 3% (2/58) of the tertiary group (p = 0.002). Seventeen (17) percent of the FQHC patients did not have any information about their paternal family history. Thirteen (13) percent of the FQHC patients did not know the type of cancer diagnosed in a first-degree relative. Completeness of family history was independent of interpreter use (see below).
Table 2.
Risk assessment outcomes by cohort
| FQHC | Tertiary | P**** | |
|---|---|---|---|
| Number of patients | 23 | 58 | |
| Incomplete information, first degree, n (%) | 7 (30) | 2 (3) | 0.002 |
| Missing family history, first degree* | 4 (17) | 2 (3) | |
| Unknown cancer, first degree* | 3 (13) | 0 (0) | |
| NCCN criteria met, n (%)** | 17 (74) | 47 (81) | 0.55 |
| Risk assessment***, median (range) | |||
| Tyrer-Cuzick, % lifetime risk | 15.0 (2.8–33.2) | 24.6 (6.0–44.3) | 0.005 |
| BRCAPro, % lifetime risk | 8.2 (0.1–12.3) | 11.3 (0.1–22.3) | < 0.001 |
| BRCAPro, % risk for mutation | 0.1 (0.0–47.5) | 0.2 (0.0–22.6) | 0.96 |
| MMRPro, % lifetime risk | 3.4 (2.9–10.3) | 3.3 (2.6–9.8) | 0.98 |
| MMRPro, % risk for mutation | 0.2 (0.1–24.8) | 0.3 (0.0–21.9) | 0.75 |
| Genetic testing offered to the patient, n (%) | 10 (43) | 34 (59) | 0.16 |
| Reason patient not offered genetic testing, n (%) | 0.26 | ||
| Testing recommended for relative instead | 9 (69) | 20 (83) | |
| Patient at low risk | 2 (15) | 4 (17) | |
| Patient asked to clarify family history | 2 (15) | 0 (0) |
*Not mutually exclusive
**Some patients who met NCCN criteria for testing were not recommended to have testing due to insurance coverage or due to not being the most informative relative available for testing
***Tyrer-Cuzick (N = 18 FQHC and N = 48 tertiary females) and BRCAPro (N = 21 FQHC and N = 49 tertiary patients) risk estimates are calculated among patients with a breast/ovarian cancer indication, and MMRPro estimates are calculated among patients with a colorectal cancer indication (N = 7 FQHC and N = 21 tertiary patients)
****P values are calculated by the Wilcoxon rank-sum test for the risk assessments and by Fisher’s exact test for all other variables
In the tertiary group, 81% of patients (47/58) met NCCN guidelines for genetic testing, while 74% (17/23) of patients in the FQHC group met these guidelines. The FQHC group had lower calculated risk of carrying a mutation in a cancer predisposition gene and concomitantly lower calculated lifetime risk of breast cancer than the tertiary group based on Tyrer-Cuzick (median breast cancer risk was 15.0 vs. 24.6% p = 0.005) and BRCAPro (median risk 8.2 vs. 11.3%, p < 0.001). There were no significant differences in MMRPro scores (for mutation likelihood or colorectal cancer risk) between the groups.
Genetic testing process and results
Genetic testing was recommended less often among patients in the FQHC setting (10/23, 43%) than those in the tertiary setting (34/58, 59%), although this difference was not statistically significant (absolute difference between groups = 16%, 95% CI -39, 9%). For both cohorts, the most common reason for not recommending genetic testing to the patient was that there was better candidate for testing in the family. Of the 20 patients in the tertiary group who did not have testing because their relative was a more informative genetic testing candidate, 30% (n = 6) of these patients had their relative complete genetic testing with our group. None of the relatives of patients in the FQHC group who were deemed to be better candidates for genetic testing completed genetic testing with our group. It is not known whether they pursued testing elsewhere. Two FQHC patients (15%) were asked to clarify family history prior to testing; neither of these patients returned to clinic for genetic testing or provided additional details about their family history.
A similar percentage of patients in each group were not offered testing due to the patients being categorized as “low-risk” (not meeting NCCN guidelines for genetic testing). None of the two low-risk FQHC patients and the four tertiary low-risk patients were missing family history information on first-degree relatives. Nor did any of the low-risk patients across both groups require an interpreter.
In both groups, genetic testing was completed for the majority of patients for whom it was recommended (60% FQHC (6/10) and 74% tertiary (25/34), p = 0.45). Among those who did not complete testing, four of the ten patients in whom we recommended testing in the FQHC cohort were unable to do so because of insurance or cost considerations only. Of the nine tertiary patients who did not undergo testing, four were due to insurance or cost considerations only and five wanted to take more time to consider testing. None of the four patients in the FQHC cohort and none of the four patients in the tertiary group who declined genetic testing because of insurance or cost considerations ended up pursuing genetic testing through our clinic. Of the five tertiary patients who wanted more time to consider testing, none ended up pursuing genetic testing through our center; however, two of the patients did make follow-up appointments with their genetic counselor for further discussion.
While none of the six FQHC patients were found to carry a pathogenic or likely pathogenic mutation, 20% (5/25) of patients from the tertiary group tested positive (mutations were identified in BRCA1, CHEK2, MLH1, and PMS2). Two (2) individuals in the FQHC cohort had variants of uncertain significance, both self-identified as Black. Six (6) individuals in the tertiary cohort had variants of uncertain significance. Among these, five (5) self-identified as non-Hispanic White while one described herself as multiracial (Table 3).
Table 3.
Genetic testing outcomes by cohort
| FQHC | Tertiary | P** | |
|---|---|---|---|
| Number of patients | 10 | 34 | |
| Completed genetic testing, n (%) | 6 (60%) | 25 (74%) | 0.45 |
| Reason genetic testing not completed, n (%) | 0.11 | ||
| Insurance or cost considerations* | 4 (100) | 4 (44) | |
| Patient wanted time to think about it | 0 (0) | 5 (56) | |
| Type of genetic testing, n (%) | 0.36 | ||
| Multi-gene panel testing | 3 (50) | 18 (72) | |
| Single gene testing | 3 (50) | 7 (28) | |
| Type of single gene test, n (%) | |||
| BRCA1 and BRCA2 | 1 (33) | 2 (29) | |
| Lynch syndrome | 2 (67) | 0 (0) | |
| Polyposis (APC, MUTYH) | 0 (0) | 2 (29) | |
| Ashkenazi Jewish founder mutations | 0 (0) | 1 (14) | |
| Other*** | 0 (0) | 2 (29) | |
| Mutation identified, n (%) | 0 (0) | 5 (20) | 0.55 |
| Gene, n | |||
| BRCA1 | 0 | 1 | |
| Lynch (MLH1 (2), PMS2 (1)) | 0 | 3 | |
| CHEK2 | 0 | 1 | |
| Variant of unknown significance identified, n (%) | 2 (33) | 6 (24) | 0.63 |
| Gene, n | |||
| BRCA1 | 0 | 1 | |
| BRCA2 | 1 | 0 | |
| BRIP1 | 1 | 0 | |
| MLH1 | 0 | 1 | |
| MSH2 | 0 | 1 | |
| NBN | 0 | 1 | |
| NF1 | 0 | 2 | |
*FQHC patients: Medicaid (N = 2), Medicare (N = 1), private (N = 1); DFCI patients: Medicare (N = 2), private (N = 2)
**P values are calculated by Fisher’s exact test
***Other is noted for patients who had two single gene tests (i.e., polyposis and Lynch syndrome)
Discussion
This analysis provides insight into the genetics risk assessment and testing outcomes between two distinct cohorts. There were important differences in indications for genetics referral among the unaffected patients referred for evaluation. Patients from the tertiary group were more likely to have already had some prior contact with cancer genetics services than those from the FQHC cohort, as indicated by having had prior genetic testing for themselves or a family member. These differences, evident at the outset, necessitated limitation of the analysis to a smaller subset that was comparable across both practice settings. While the two groups differed with respect to race, ethnicity, insurance coverage, and use of interpreter services, for patients in both groups, genetic testing was recommended at similar rates. The majority of patients in both groups who were recommended testing and desired it were able to complete it, perhaps indicating a shift towards improved access to genetic testing.
Patients in the FQHC group were statistically less likely to have complete family history information than those in the tertiary group, with the most pronounced differences in information about the paternal lineage and the types of cancer diagnosed in first-degree relatives. This is consistent with previous data indicating that sensitivity of family cancer history is lower in Latinas and Spanish-speaking persons than other groups (Mitchell et al. 2004; Murff et al. 2004; Vadaparampil et al. 2006). While community outreach efforts may help to inform patients about the value of accurate family history information, providers must recognize the obstacles that patients face in attempting to obtain this information. Leaving cultural factors aside, logistical challenges for these patients are daunting. For example, in the FQHC group, many patients had relatives living outside the country, often precluding them from gathering medical records about family cancer diagnoses. Although in many situations it would be advisable for a patient’s affected relative to undergo genetic testing first, for relatives living abroad, this was generally not possible due to limitations in international access to cancer genetics services. Likewise, when one of the patient’s parents had not been a part of the family unit or was not known to the patient, there was no reasonable way to obtain health information from that side of the family for inclusion in cancer risk assessment. This was a substantial problem as 30% of the FQHC patients were missing information about first-degree relatives.
Half of the patients in the study were offered genetic testing. The most common reason for not recommending genetic testing was the existence of a “more informative” relative in the family who was available to test (i.e., an individual living nearby with a young diagnosis of cancer). Another frequent reason was that the patient was considered low-risk based on the risk models used and on the NCCN genetic testing guidelines. While not observed in these data, missing information about family history of cancer can certainly contribute to why some individuals do not meet testing criteria. We acknowledge that genetic testing practices across the country have changed since 2013 when many of these patients were seen. A recent and widespread trend towards testing the patient who presents to clinic, rather than waiting for the more informative relative who may never come in for evaluation, has subsequently emerged. Given that this analysis focused mainly on patients without personal diagnoses of cancer, fewer patients were considered high-risk, which meant that fewer patients were recommended to undergo genetic testing at that historical time.
While no disease-causing mutations were identified in the FQHC group, two patients in this group were found to have variants of uncertain significance. More genetic data is needed on minority patients to fill the interpretation gaps so that the genetic testing can be utilized more effectively in these communities (Hall et al. 2009; Hermel et al. 2017; Manrai et al. 2016; Ricker et al. 2016). We found that setting expectations regarding the likelihood of indeterminate or uninformative results in the pretest setting was useful as the interpretation of test results from non-European patients can be difficult due to a high number of uncharacterized genetic variants.
These data must be interpreted in the context of the study design. The small sample size of patients in the FQHC cohort limits the generalizability of this study. More data are needed on this population and other underserved populations. A complication of quantitative risk assessment for FQHC patients is that these models generally have not been fully validated in non-European populations. The lack of information about family history in the FQHC group may further represent a differential misclassification of these patients resulting in the lower cancer risk estimates generated by some models for FQHC patients than tertiary patients. There is a sampling bias in our study given that we were only able to compare the patients that presented to genetics. However, this applies to both the FQHC and tertiary cohorts, as we did not capture the patients who may have been referred but did not present to clinic. Another limitation was that patients in the FQHC group were referred by their primary care provider and thus, in most cases, did not have personal histories of cancer. This necessitated limiting the analysis to patients without personal histories of cancer in the tertiary cohort.
We have outlined a number of challenges in the provision of cancer genetics care in the community setting. Our experience suggests that some modifications in the model of care may be necessary to best meet the needs of this patient population. Some considerations, such as availability of medical interpreters and provision of culturally sensitive care, are self-evident and well-studied (Joseph and Guerra 2015; Wiggins and Middleton 2013). Others may be less so. For example, direct genetic testing for an unaffected individual may be appropriate more often in the community setting than in the tertiary setting due to lack of access to relatives. Providers may also consider whether to offer genetic testing at a lower risk threshold for community patients in recognition of the high frequency of incomplete family histories and concomitant possibility of underestimation of risk by widely used models (Huo et al. 2009). In offering genetic testing in the community setting and perhaps in all settings, it may be reasonable to rely less on quantitative risk models and more on traditional pedigree assessment, diagnostic criteria, consideration of social and familial circumstances, and clinical judgment.
It is important to note that in a clinic where all patients are referred by their primary care provider, that provider significantly influences which patients present to the specialty clinic. This highlights a need for provider education about when a referral to genetics may be appropriate. Increased education will broaden the types of patients seen in genetics clinic. Additionally, in community clinics where a great amount of trust is built between a patient and their primary care provider, having a referral from that doctor to a specialty clinic is very likely to increase the patient’s comfort in going through with the appointment (Delikurt et al. 2015; Jones et al. 2016). Also of note is that no patients in the FQHC group were referred due to a known mutation in their family. However, 41 patients in the tertiary group were referred for this reason. This disparity suggests that there may be differences in family information sharing between these two groups, or that the patients in the FQHC group may not have thought to mention this to their doctors. Future studies could investigate the factors behind this difference and whether physicians are less likely to refer patients to genetics for this indication. Another possibility is that the affected family members are part of health care systems with limited access to genetic testing and have not been tested or are in countries where access to testing is even more limited.
Given the many socioeconomic barriers faced by patients in community clinics, a more traditional, multi-visit approach, in which the more informative relatives are contacted, is unlikely to result in the completion of genetic testing in these families. In this study, several of the family members of the tertiary patients who were recommended to have genetic testing ended up following through with genetic testing. However, none of the relatives of the FQHC patients pursed genetic testing through our clinic. This difference further highlights differences in access between these two cohorts. Therefore, we propose that testing of the patient presenting to clinic may be appropriate particularly in community clinics, where loss to follow-up may be more common. This, of course, requires extensive counseling on the lack of utility of negative genetic test results in a person not affected by cancer. Our experience also indicates that more intensive follow-through may be necessary with insurers to assure coverage for genetic testing under “non-traditional” circumstances such as incomplete family history.
Further study is needed to determine how access to genetics services changes, and what barriers remain, as genetic testing costs continue to decrease and insurance coverage improves. Larger studies and longer follow-up are needed to evaluate the impact of community-based programs in providing equitable access to genetic counseling and testing. Only then can the downstream effects of genetic counseling and testing (including family member/cascade testing and access to personalized cancer prevention and early detection strategies) be fully evaluated. In a study investigating non-inferiority of telephone genetic counseling vs. in-person genetic counseling, minority women who underwent telephone counseling were less likely than their White counterparts to undergo genetic testing (Butrick et al. 2015). This suggests that one approach may not be appropriate for all populations and genetics care needs to be delivered with thoughtful consideration of the target population. While not an issue in these data due to the small number of visits requiring translator services, there is increasing interest in the study of optimal use of interpreter services in patient-genetics interactions (Joseph and Guerra 2015; Wiggins and Middleton 2013). Exploration of novel counseling, risk assessment and genetic testing models based in the community and in partnership with community providers is needed.
Acknowledgements
The authors would like to thank Carleen Gentry for her assistance with data collection, the management and staff of Whittier Street Health Center as well as the study participants for making this research possible.
Funding information
The Dana-Farber Community Cancer Care Program is funded by a grant from the Kraft Family Research Fund.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human studies and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Institutional Review Board approval was obtained and the study was granted a waiver of informed consent for retrospective review of medical records.
Animal studies
No animal studies were carried out by the authors for this article.
References
- Allford A, Qureshi N, Barwell J, Lewis C, Kai J. What hinders minority ethnic access to cancer genetics services and what may help[quest] Eur J Human Genet EJHG. 2014;22(7):866–874. doi: 10.1038/ejhg.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, McLosky J, Wasilevich E, Lyon-Callo S, Duquette D, Copeland G. Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol. 2012;2012:11. doi: 10.1155/2012/298745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J, Toscano M, Kotchko N, Friedman S, Schwartz MD, Virgo KS, Lynch K, Andrews JE, Aguado Loi CX, Bauer JE, Casares C, Bourquardez Clark E, Kondoff MR, Molina AD, Abdollahian M, Walker G, Sutphen R. Utilization and outcomes of BRCA genetic testing and counseling in a national commercially insured population: the ABOUT study. JAMA Oncol. 2015;1(9):1–10. doi: 10.1001/jamaoncol.2015.3048. [DOI] [PubMed] [Google Scholar]
- Bondy ML, Newman LA. Breast cancer risk assessment models. Cancer. 2003;97(S1):230–235. doi: 10.1002/cncr.11018. [DOI] [PubMed] [Google Scholar]
- Burt RW, Cannon JA, David DS, Early DS, Ford JM, Giardiello FM, Halverson AL, Hamilton SR, Hampel H, Ismail MK, Jasperson K, Klapman JB, Lazenby AJ, Lynch PM, Mayer RJ, Ness RM, Provenzale D, Rao MS, Shike M, Steinbach G, Terdiman JP, Weinberg D, Dwyer M, Freedman-Cass D, National Comprehensive Cancer Network Colorectal cancer screening. J Natl Compr Cancer Netw. 2013;11(12):1538–1575. doi: 10.6004/jnccn.2013.0180. [DOI] [PubMed] [Google Scholar]
- Butrick M, Kelly S, Peshkin BN, Luta G, Nusbaum R, Hooker GW, Graves K, Feeley L, Isaacs C, Valdimarsdottir HB, Jandorf L, DeMarco T, Wood M, McKinnon W, Garber J, McCormick SR, Schwartz MD. Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genet Med. 2015;17(6):467–475. doi: 10.1038/gim.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MB, Pilarski R, Axilbund JE, Buys SS, Crawford B, Friedman S, Garber JE, Horton C, Kaklamani V, Klein C, Kohlmann W, Kurian A, Litton J, Madlensky L, Marcom PK, Merajver SD, Offit K, Pal T, Pasche B, Reiser G, Shannon KM, Swisher E, Voian NC, Weitzel JN, Whelan A, Wiesner GL, Dwyer MA, Kumar R, National Comprehensive Cancer Network Genetic/familial high-risk assessment: breast and ovarian, version 1.2014. J Nat Compr Cancer Netw: JNCCN. 2014;12(9):1326–1338. doi: 10.6004/jnccn.2014.0127. [DOI] [PubMed] [Google Scholar]
- Delikurt T, Williamson GR, Anastasiadou V, Skirton H. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet. 2015;23(6):739–745. doi: 10.1038/ejhg.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles DM, Mitchell G, Monteiro AN, Schmutzler R, Couch FJ, Spurdle AB, Gomez-Garcia EB. BRCA1 and BRCA2 genetic testing—pitfalls and recommendations for managing variants of uncertain clinical significance. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2015;26(10):2057–2065. doi: 10.1093/annonc/mdv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman AD, Hall MJ. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J. 2009;15(Suppl 1):S56–S62. doi: 10.1111/j.1524-4741.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- Gao Q, Neuhausen S, Cummings S, Luce M, Olopade OI. Recurrent germ-line BRCA1 mutations in extended African American families with early-onset breast cancer. Am J Hum Genet. 1997;60(5):1233–1236. [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Tomlinson G, Das S, Cummings S, Sveen L, Fackenthal J, Schumm P, Olopade OI. Prevalence of BRCA1 and BRCA2 mutations among clinic-based African American families with breast cancer. Hum Genet. 2000;107(2):186–191. doi: 10.1007/s004390000290. [DOI] [PubMed] [Google Scholar]
- Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, Wenstrup RJ, Ward BE, Scholl TA, Noll WW. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115(10):2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermel DJ, McKinnon WC, Wood ME, Greenblatt MS. Multi-gene panel testing for hereditary cancer susceptibility in a rural Familial Cancer Program. Familial Cancer. 2017;16(1):159–166. doi: 10.1007/s10689-016-9913-5. [DOI] [PubMed] [Google Scholar]
- HRSA. (2014) 2014 Health Center Profile: Whittier Street Health Committee, INC. Roxbury, Massachusetts. Health Resources and Services Administration, Rockville, MD. http://bphc.hrsa.gov/uds/datacenter.aspx?q=d&bid=012070&state=MA&year=2014. Accessed 12 February 2016
- Huo D, Senie RT, Daly M, Buys SS, Cummings S, Ogutha J, Hope K, Olopade OI. Prediction of BRCA mutations using the BRCAPRO model in clinic-based African American, Hispanic, and other minority families in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(8):1184–1190. doi: 10.1200/JCO.2008.17.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, Lockhart JS, Mendelsohn-Victor KE, Duquette D, Northouse LL, Duffy SA, Donley R, Merajver SD, Milliron KJ, Roberts JS, Katapodi MC. Use of cancer genetics services in African-American young breast cancer survivors. Am J Prev Med. 2016;51(4):427–436. doi: 10.1016/j.amepre.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Joseph G, Guerra C. To worry or not to worry: breast cancer genetic counseling communication with low-income Latina immigrants. J Commun Genet. 2015;6(1):63–76. doi: 10.1007/s12687-014-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney AY, Gammon A, Coxworth J, Simonsen SE, Arce-Laretta M. Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 mutation testing and preventive strategies. Genet Med. 2010;12(2):105–115. doi: 10.1097/GIM.0b013e3181c9af2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komenaka IK, Nodora JN, Madlensky L, Winton LM, Heberer MA, Schwab RB, Weitzel JN, Martinez ME. Participation of low-income women in genetic cancer risk assessment and BRCA 1/2 testing: the experience of a safety-net institution. J Commun Genet. 2016;7(3):177–183. doi: 10.1007/s12687-015-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Beattie M, Crawford B, Mak J, Stewart N, Komaromy M, Esserman L, Shaw L, McLennan J, Strachowski L, Luce J, Ziegler J. Recruitment, genetic counseling, and BRCA testing for underserved women at a public hospital. Genet Test. 2005;9(4):306–312. doi: 10.1089/gte.2005.9.306. [DOI] [PubMed] [Google Scholar]
- Manrai AK, Funke BH, Rehm HL, Olesen MS, Maron BA, Szolovits P, Margulies DM, Loscalzo J, Kohane IS. Genetic misdiagnoses and the potential for health disparities. N Engl J Med. 2016;375(7):655–665. doi: 10.1056/NEJMsa1507092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, Bristol M, Domchek SM, Groeneveld PW, Kim Y, Motanya UN, Shea JA, Armstrong K. Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(22):2610–2618. doi: 10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado RC, Hampel H, Kastrinos F, Steyerberg E, Balmana J, Stoffel E, Cohn DE, Backes FJ, Hopper JL, Jenkins MA, Lindor NM, Casey G, Haile R, Madhavan S, de la Chapelle A, Syngal S, Colon Cancer Family Registry Performance of PREMM(1,2,6), MMRpredict, and MMRpro in detecting Lynch syndrome among endometrial cancer cases. Genet Med. 2012;14(7):670–680. doi: 10.1038/gim.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RJ, Brewster D, Campbell H, Porteous ME, Wyllie AH, Bird CC, Dunlop MG. Accuracy of reporting of family history of colorectal cancer. Gut. 2004;53(2):291–295. doi: 10.1136/gut.2003.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murff HJ, Byrne D, Syngal S. Cancer risk assessment: quality and impact of the family history interview. Am J Prev Med. 2004;27(3):239–245. doi: 10.1016/j.amepre.2004.05.003. [DOI] [PubMed] [Google Scholar]
- NCCN (2013) NCCN clinical practice guidelines in oncology: breast cancer risk reduction version 1.2013. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 19 February 2014
- Olaya W, Esquivel P, Wong JH, Morgan JW, Freeberg A, Roy-Chowdhury S, Lum SS. Disparities in BRCA testing: when insurance coverage is not a barrier. Am J Surg. 2009;198:562–565. doi: 10.1016/j.amjsurg.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Parmigiani G, Chen S, Iversen ES Jr, Friebel TM, Finkelstein DM, Anton-Culver H, Ziogas A, Weber BL, Eisen A, Malone KE, Daling JR, Hsu L, Ostrander EA, Peterson LE, Schildkraut JM, Isaacs C, Corio C, Leondaridis L, Tomlinson G, Amos CI, Strong LC, Berry DA, Weitzel JN, Sand S, Dutson D, Kerber R, Peshkin BN, Euhus DM. Validity of models for predicting BRCA1 and BRCA2 mutations. Ann Intern Med. 2007;147(7):441–450. doi: 10.7326/0003-4819-147-7-200710020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker C, Culver JO, Lowstuter K, Sturgeon D, Sturgeon JD, Chanock CR, Gauderman WJ, McDonnell KJ, Idos GE, Gruber SB. Increased yield of actionable mutations using multi-gene panels to assess hereditary cancer susceptibility in an ethnically diverse clinical cohort. Cancer Genet. 2016;209(4):130–137. doi: 10.1016/j.cancergen.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker C, Lagos V, Feldman N, Hiyama S, Fuentes S, Kumar V, Gonzalez K, Palomares M, Blazer K, Lowstuter K, MacDonald D, Weitzel J. If we build it ... will they come?—establishing a cancer genetics services clinic for an underserved predominantly Latina cohort. J Genet Couns. 2006;15(6):505–514. doi: 10.1007/s10897-006-9052-5. [DOI] [PubMed] [Google Scholar]
- Sherman KA, Miller SM, Shaw L-K, Cavanagh K, Sheinfeld Gorin S. Psychosocial approaches to participation in BRCA1/2 genetic risk assessment among African American women: a systematic review. J Commun Genet. 2014;5(2):89–98. doi: 10.1007/s12687-013-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner KM, Edwards T, Villagra C, Rodriguez MC, Thompson HS, Jandorf L, Valdimarsdottir HB. BRCA genetic counseling among at-risk Latinas in New York City: new beliefs shape new generation. J Genet Couns. 2015;24(1):134–148. doi: 10.1007/s10897-014-9746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HS, et al. Psychosocial predictors of BRCA counseling and testing decisions among urban African-American women. Cancer Epidemiol Biomarkers Prev. 2002;11:1579–1585. [PubMed] [Google Scholar]
- Thompson HS, Valdimarsdottir HB, Jandorf L, Redd W. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Educ Couns. 2003;51(3):217–227. doi: 10.1016/S0738-3991(02)00219-7. [DOI] [PubMed] [Google Scholar]
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- Vadaparampil ST, McIntyre J, Quinn GP. Awareness, perceptions, and provider recommendation related to genetic testing for hereditary breast cancer risk among at-risk Hispanic women: similarities and variations by sub-ethnicity. J Genet Couns. 2010;19(6):618–629. doi: 10.1007/s10897-010-9316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadaparampil ST, Wideroff L, Breen N, Trapido E. The impact of acculturation on awareness of genetic testing for increased cancer risk among Hispanics in the year 2000 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2006;15(4):618–623. doi: 10.1158/1055-9965.EPI-05-0378. [DOI] [PubMed] [Google Scholar]
- Vogel KJ, Atchley DP, Erlichman J, Broglio KR, Ready KJ, Valero V, Amos CI, Hortobagyi GN, Lu KH, Arun B. BRCA1 and BRCA2 genetic testing in Hispanic patients: mutation prevalence and evaluation of the BRCAPRO risk assessment model. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(29):4635–4641. doi: 10.1200/JCO.2006.10.4703. [DOI] [PubMed] [Google Scholar]
- Wideroff L, Thomas Vadaparampil S, Breen N, Croyle RT, Freedman AN. Awareness of genetic testing for increased cancer risk in the year 2000 National Health Interview Survey. Public Health Genomics. 2003;6:147–156. doi: 10.1159/000078162. [DOI] [PubMed] [Google Scholar]
- Wiggins J, Middleton A (2013) Getting the message across: communication with diverse populations in clinical genetics. OUP USA