Abstract
Introduction
Scarring is an unfortunate clinical outcome of acne. Current treatment options for atrophic acne scars are dominated by non-pharmacological, invasive procedures which may not be suitable or affordable to all patients. This phase II, single-center, open-label, exploratory study assessed the efficacy, safety and subject-reported outcomes of adapalene 0.3% gel in the treatment of atrophic acne scars.
Methods
The study included subjects aged 18–50 years with past history of acne and moderate to severe facial atrophic acne scars. Subjects received adapalene 0.3% gel once daily for the first 4 weeks and twice daily for the following 20 weeks. Assessments were performed at baseline, day 10 and weeks 4, 8, 16 and 24, and at post-treatment follow-ups (weeks 36 and 48–72).
Results
At week 24, investigator and subject assessments reported improvement in skin texture/atrophic scars in 50% and > 80% of subjects, respectively. Subjects were satisfied with the treatment and reported improvements in quality of life.
Conclusion
Daily use of adapalene 0.3% gel for the treatment of atrophic acne scars showed promising clinical efficacy, a favorable tolerability profile, and improvement in quality of life.
Funding
Nestlé Skin Health–Galderma R&D.
Trial Registration
ClinicalTrials.gov Identifier NCT01213199.
Keywords: Acne, Adapalene, Atrophic scars, Molecular markers, Scarring, Skin texture, Subject-reported outcome, Topical retinoids
Introduction
Acne vulgaris affects approximately 85% of youths (aged 12–24 years), and its occurrence is not uncommon in adults [1–4]. Although acne can be treated effectively, in many cases scarring is an unfortunate clinical outcome of this condition [5]. Acne scars result from delayed or inadequate treatment and healing of inflammatory acne lesions [6]. Their clinical appearance ranges from hypertrophic and keloidal scars to atrophic scars [7–9]. Atrophic scars are the most common type and may cause serious physical and emotional scarring, impacting patients’ quality of life [10]. The severity of scars is correlated with acne grade and the delay between onset of the disease and initiation of treatment.
Current treatment modalities for atrophic acne scars are dominated by non-pharmacological interventions and require invasive procedures (laser resurfacing, volumizing fillers, dermabrasion, microneedling, radiofrequency, subcision and excisional techniques) to improve appearance [11–13]. However, these invasive procedures may not be suitable or affordable to all patients. Finding effective non-procedural medical treatments has proven challenging, in part due to persistent dermal tissue loss.
Loss of dermal matrix is a contributing factor in atrophic acne scarring and involves the degradation of collagen, especially during the inflammatory phase of acne lesions [14, 15]. Activation of transcription factor AP-1 stimulates the formation of matrix-degrading metalloproteinases (MMPs), which degrade extracellular matrix molecules during physiological and pathological tissue remodeling. Studies have shown that the levels of MMP-1 (interstitial collagenase), MMP-3 (stromelysin-1) and MMP-9 (gelatinase-B) are increased in inflammatory acne lesions [14–17]. Of note, photoaging is also characterized by the loss of dermal matrix, resulting from the same MMPs shown to play a role in inflammatory acne lesions [14, 16–19].
Use of topical retinoids has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of acne and photodamaged skin. In photodamaged skin, levels of both type I and III procollagen (Procol-1 and Procol-3, respectively) are reduced. Several studies have demonstrated that topical retinoid-mediated improvement in fine wrinkles of photoaged skin is accomplished through partial restoration of reduced levels of collagen in sun-exposed skin toward those observed in sun-protected skin [19–21]. The ability of topical retinoids to stimulate dermal fibroblasts to increase the production of procollagen in photoaged skin is well established [16, 18, 19]. Furthermore, topical tretinoin has a protective effect against ultraviolet radiation-induced loss of procollagen by antagonizing transcription factor AP-1, thereby blunting the increase in MMP synthesis [16, 18, 19].
Adapalene 0.3% gel is approved in the USA for the treatment of acne with demonstrated efficacy [22–24] and a favorable tolerability profile compared to other retinoids [22–25]. Adapalene 0.1% and 0.3% gel formulations have been shown to treat actinic keratoses and solar lentigines, and improve the effects of photodamage (fine and coarse wrinkles, mottled hyperpigmentation, and sallow complexion) [26]. As both photodamaged skin and atrophic acne scars share the feature of dermal matrix loss, adapalene 0.3% may potentially exert a beneficial effect in the treatment of atrophic acne scars, similar to its effect on photoaging.
This exploratory study assessed the efficacy and safety of adapalene 0.3% gel in the treatment of atrophic acne scars. For a subgroup of patients, skin biopsy samples were analyzed for molecular markers to ascertain changes induced by the topical retinoid. Subject-reported outcomes were also investigated through subject satisfaction and quality-of-life questionnaires.
Methods
Study Design
This was a phase II, single-center, open-label pilot study including subjects aged 18–50 years. Subjects had a past history of acne (no active acne lesions at time of enrollment), moderate to severe facial atrophic acne scars (grade 3 or 4 according to the Goodman–Baron system [8]) and presence of at least 5 atrophic acne scars of any type within a 3 × 3-cm (9-cm2) area designated as the region of interest (ROI). The atrophic scars were classified as icepick (0.5–1.5 mm), boxcar (1.5–4 mm) or rolling (> 4 mm).
All subjects received topical treatment with adapalene 0.3% gel (Differin® 0.3%; Galderma Laboratories, Fort Worth, TX, USA) once daily for the first 4 weeks and twice daily for the following 20 weeks. Skin cleanser, moisturizing lotion and SPF50 sunscreen were provided to all subjects. The total duration of treatment was 24 weeks, with visits at baseline, day 10 and weeks 4, 8, 16 and 24. Post-treatment follow-up included on-site visits at weeks 36 and 48–72 (i.e. anytime between 6 and 12 months after treatment). Patients with a prior history of dermabrasion or laser resurfacing on the face were excluded. Patients with a treatment history of < 4 weeks for topical retinoid and alpha/beta hydroxy acids, < 3 months for microdermabrasion and < 6 months for oral retinoids were also excluded.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This study was approved by institutional review boards, and all subjects provided written informed consent (ClinicalTrials.gov identifier NCT01213199).
Assessments
Efficacy (Full Face and ROI)
Efficacy was assessed clinically for the full face and the ROI by a single investigator. Full-face assessments included global scarring grade (at baseline and weeks 16 and 24) and investigator and subject global assessment (IGA and SGA, respectively) of improvement in skin texture and atrophic acne scars (at weeks 16 and 24). Post-treatment follow-up visits (at weeks 36 and 48–72) were included to assess the clinical effect of the study drug after treatment termination.
Assessment of efficacy in the ROI included atrophic scar counts (at baseline and weeks 16, 24, 36 and 48–72). In addition, skin biopsies were obtained from study patients to ascertain any molecular alterations that may explain potential clinical improvement.
Punch biopsy specimens (diameter: 3 mm) were obtained at baseline and week 24 from similar atrophic acne scars that were symmetrical and localized on the same anatomical area.
The first ten subjects were designated to have a biopsy for immunohistochemistry (IHC) studies. The remaining ten subjects were designated to have a biopsy for transcriptomics analysis. Standard IHC methods were used to examine the expression levels of dermal matrix molecules, including Procol-1 and Procol-3. Immunostaining was quantified using the Bakos semi-quantitative method [27] for intensity and depth, and an image analysis program to quantify staining intensity (ScanScope®; Aperio, Vista, CA, USA). Semi-quantitative IHC results were examined by two independent observers blinded as to whether the samples were pre- or post-treatment. In order to evaluate the expression of these substrates, the semi-quantitative method suggested by Bakos et al. was used where 0 indicates the absence of expression; +, weak expression; ++, moderate expression; +++, strong expression [27]. For the computer analysis of staining, whole-slide scanning was used to capture high-resolution images by the ScanScope® system. Images were analyzed using color deconvolution and automated algorithms to quantify staining intensity.
Biopsy samples for the microarray analysis were stored in RNA Stabilization Reagent (Qiagen, Hilden, Germany). Total RNA was extracted using miRNeasy extraction kits (Qiagen). RNA quantity was measured using a Nanodrop spectrophotometer ND8000 (Thermo Fisher Scientific, Waltham, MA, USA). RNA quality was monitored using a 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). Probes were synthesized and hybridized on Affymetrix U133 Plus 2.0 chips (Affymetrix, Santa Clara, CA, USA). Expression data from the Affymetrix GeneChips were normalized using the Robust Multiarray Analysis [28, 29].
Safety
Safety assessments were performed at each visit, with a focus on local tolerability parameters (erythema, scaling, dryness, and stinging/burning) and the recording of adverse events (AEs).
Subject-Reported Outcomes
Subject-reported outcomes were assessed using the Dermatology Life Quality Index (DLQI) completed at baseline and last visit. Additionally, a study-specific 7-item subject satisfaction questionnaire was completed at the last visit [30, 31].
Statistical Analysis
Analysis of variance (ANOVA) was performed to examine the differences in grading over time from baseline to week 24 for facial global scarring grade, patient and investigator scar assessment, individual scar counts, and tolerability assessments. In addition, post-treatment data (week 48–72), specific to scarring grades and lesion counts, were collected from 16 subjects and compared to data collected at baseline, week 16, and week 24.
Frequencies for each item on the DLQI and subject satisfaction questionnaire were created, and these were compared at baseline/week 24 using t tests. Four scores were derived and analyzed from the DLQI survey, namely total, symptoms and feelings, personal relationships, and treatment).
For the IHC analysis, computer-assisted color deconvolution and semi-quantitative scoring of immunostaining intensity were performed by two independent observers. For the automated color deconvolution algorithms, a paired Student’s t test was performed to determine any significant difference in positive Procol-1 and collagen-3 (Col-3) staining within a pre-defined ROI at baseline and week 24 (significance p < 0.05). For the Bakos method, intensity scores of 0, +, ++, and +++ were converted to an ordinal scale of 0, 1, 2, and 3, respectively. Scores following 24 weeks of retinoid therapy relative to baseline scores were compared using the Wilcoxon signed-rank test. Calculations were conducted using the Stata version 14.0 software (StataCorp LP, College Station, TX, USA).
For large-scale gene expression, data analysis was performed using the Array Studio software (OmicSoft, Cary, NC, USA). Principal component analysis showed that one sample was an outlier and, therefore, the data from this subject were excluded from subsequent analysis. A two-sided paired t test was applied to compare genes significantly differentially expressed at week 24, and the Benjamini–Hochberg procedure was used for correction of multiple testing [32].
Results
Demographics and Baseline Characteristics
A total of 20 subjects were treated at one study site in the USA. The mean age was 35.7 years and slightly more than half of the subjects were male (55%). Scarring grade at baseline was moderate in 60% of subjects and severe in the remaining subjects. Subjects reported a very long duration of both acne (mean 22.9 years) and facial acne scars (mean 19.3 years) (Table 1).
Table 1.
Demographics and baseline disease characteristics of all subjects in the study (N = 20)
| Demographics and baseline disease characteristics | Values |
|---|---|
| Demographics | |
| Mean age (years) | 35.7 ± 8.7 |
| Sex | |
| Male | 11 (55%) |
| Female | 9 (45%) |
| Race | |
| Caucasian | 4 (20%) |
| Black | 6 (30%) |
| Asian | 5 (25%) |
| Hispanic | 3 (15%) |
| Other | 2 (10%) |
| Fitzpatrick skin type | |
| I | 0 |
| II | 3 (15%) |
| III | 7 (35%) |
| IV | 3 (15%) |
| V | 5 (25%) |
| VI | 2 (10%) |
| Baseline disease characteristics | |
| Facial global scarring grade (1 = macular disease, 4 = severe disease) | |
| 3: Moderate disease | 12 (60%) |
| 4: Severe disease | 8 (40%) |
| Mean ± SD | 3.4 ± 0.50 |
| Mean number of atrophic acne scars | 18.7 ± 10.9 |
| Icepick (0.5–1.5 mm) | 10.2 ± 8.0 |
| Boxcar (1.5–4 mm) | 7.2 ± 5.8 |
| Rolling (> 4 mm) | 1.4 ±1.8 |
| Facial acne vulgaris (duration in years) | |
| 10–20 years | 8 (40.0%) |
| > 20 years | 12 (60.0%) |
| Mean ± SD | 22.86 ± 8.38 |
| Facial acne scars (duration in years) | |
| 10–20 years | 11 (55.0%) |
| > 20 years | 9 (45.0%) |
| Mean ± SD | 19.26 ± 7.33 |
Values in table are presented as the mean ± standard deviation (SD) or as a number with the percentage in parenthesis
Of the 20 subjects enrolled, 18 (90%) completed the treatment (i.e. week 24), and 16 (80%) attended the post-treatment follow-up visits. The four subjects (20%) who discontinued the study were lost to follow-up. No subject discontinued treatment due to an AE. Of the ten subjects selected to have a biopsy for IHC studies and reverse transcription-PCR assessment, two subjects did not agree to undergo the procedure (i.e. N = 8). For the transcriptomics analysis, three of the ten subjects planned to have a biopsy declined to undergo the procedure (i.e. N = 7).
Efficacy
Global Scarring Grade
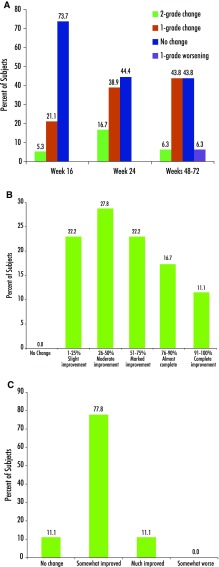
According to full-face global scarring grade, the majority of subjects (60%) had moderate disease at baseline. At week 24, ten of 18 subjects (55.6%) showed improvement of a 1-grade (N = 7; 38.9%) or 2-grade (N = 3; 16.7%) change from baseline (Fig. 1a). Table 2 includes the ANOVA results for those subjects with complete data during the treatment phase (i.e. baseline through to week 24). These ANOVA results indicate that scarring grades at baseline (mean = 3.38) were significantly higher than those at week 24 (mean = 2.67), suggesting greater overall improvement in scarring.
Table 3.
Descriptive statistics of facial scarring grades, lesion counts, pre- and post-treatment in subjects who had complete data through the study period and a post-treatment follow-up (16 subjects)
| Facial scaring grades and lesion counts | Pre-treatment and treatment phase | Post-treatment phase | ANOVA (F, p value) | ||
|---|---|---|---|---|---|
| Baseline | Week 16 | Week 24 | Week 48–72 | ||
| Scarring | |||||
| Scarring grade | 3.43 (0.51) a,b,c | 3.06 (0.92) a,d | 2.62 (0.88)b,d | 2.93 (0.85)b,c | 2.74; p = 0.051 |
| Subject scar assessment | – | 3.06 (0.57) | 3.06 (0.44) | 2.93 (0.68) | 0.25; p = 0.77 |
| Investigator scar assessment | – | 2.81 (1.10) b,c | 3.75 (1.39) a,c | 1.62 (0.80) a,b | 14.28; p < 0.0001 |
| Lesion counts | |||||
| Icepick scars | 9.12 (5.00) | 7.68 (4.04) | 7.56 (4.81) | 5.68 (4.79) | 1.45; p = 0.23 |
| Boxcar scars | 7.06 (6.06) | 4.31 (2.93) | 3.93 (2.46) | 5.50 (4.47) | 1.77; p = 0.16 |
| Rolling scars | 1.56 (1.89) | 0.87 (1.02) | 0.56 (1.15) | 0.75 (1.12) | 1.68; p = 0.18 |
* Treatment average was taken from baseline through week 24. Post hoc tests (Tukey) were performed; subscript letters show where significant differences (p < 0.05) occur between time points (i.e., means for subscript ‘a’ are significantly different from one another, etc.)
Fig. 1.
a Full-face global scarring grade: change from baseline. b Full-face investigator global assessment of improvement in atrophic scars at week 24. c Subject global assessment of improvement in atrophic scars at week 24
Table 2.
Descriptive statistics of facial scaring grades and tolerability assessments of subjects who completed the study from baseline to treatment end at 24 weeks (treatment restricted data*) (N = 18 subjects)
| Facial scaring grades and tolerability assessments | Pre-treatment and treatment phase | ANOVA (F, p value) | ||
|---|---|---|---|---|
| Baseline | Week 16 | Week 24 | ||
| Scarring | ||||
| Scarring grade | 3.38 (0.50) a | 3.05 (0.87) | 2.67 (0.84) a | 4.10, p = 0.02 |
| Patient scar assessment | – | 3.00 (0.59) | 3.00 (0.48) | 0.00, p = 1.00 |
| Investigator scar assessment | – | 2.77 (1.06) a | 3.67 (1.32) a | 4.92, p = 0.03 |
| Lesion counts | ||||
| Icepick scars | 8.22 (5.39) | 7.33 (3.94) | 7.16 (4.68) | 0.26, p = 0.77 |
| Boxcar scars | 7.05 (5.78) | 4.38 (2.95) | 4.05 (2.43) | 3.03, p = 0.06 |
| Rolling scars | 1.44 (1.82) | 1.00 (1.23) | 0.61 (1.14) | 1.52, p = 0.22 |
| Tolerability assessment | ||||
| Erythema | 0.00 (0.00) | 0.00 (0.00) | 0.05 (0.23) | 1.46, p = 0.21 |
| Scaling | 0.05 (0.23) | 0.05 (0.23) | 0.11 (0.32) | 0.50, p = 0.77 |
| Dryness | 0.05 (0.23) | 0.22 (0.42) | 0.28 (0.46) | 1.44, p = 0.21 |
| Stinging/burning | 0.00 (0.00) a | 0.11 (0.32) b | 0.00 (0.00) c | 5.33, p < 0.001 |
* Treatment data restricted to include only those subjects (N = 18) with complete data from baseline through week 24 (i.e., duration of treatment). Post hoc tests (Tukey) were performed; subscript letters show where significant differences (p < 0.05) occur between time points (i.e., means for subscript ‘a’ are significantly different from one another, etc.)
Post-treatment data (weeks 48–72) specific to scarring grades were also collected on 16 subjects. Six to 12 months after treatment, an improvement was still observed in 50% of subjects (8/16), with seven subjects (43.8%) and one subject (6.3%) showing 1-grade or 2-grade improvement, respectively (Fig. 1a). In order to examine potential significant differences between the treatment period and post-treatment follow-up, we further restricted our data analysis to include only those subjects for whom data were available at week 48–72 (Table 3). ANOVA results indicated that scarring grades at baseline (mean = 3.43) were significantly higher (p < 0.05) than those at week 16 (mean = 3.06), week 24 (mean = 2.62) and post-treatment week 48–72 (mean = 2.93).
IGA of Improvement
Full-face IGA of facial skin texture at week 24 reported varying degrees of improvement for all subjects (N = 18) with slight (N = 3; 16.7%), moderate (N = 6; 33.3%), marked (N = 3; 16.7%) or almost complete improvement (N = 6; 33.3%). At the week 48–72 follow-up, 11 subjects (68.9%) continued to show at least a slight improvement compared to baseline. Similarly, IGA of facial atrophic acne scars at week 24 reported improvement for all subjects, with slight (N = 4; 22.2%), moderate (N = 5; 27.8%), marked (N = 4; 22.2%), almost complete improvement (N = 3; 16.7%) or complete improvement (N = 2; 11.1%) (Fig. 1b). IGAs of atrophic scar improvement at week 24 (mean = 3.67) were significantly improved over those at week 16 (mean = 2.77) (p < 0.05), suggesting greater overall improvement in scarring (Table 2).
At the week 48–72 follow-up, a total of seven subjects (43.8%) continued to show improvement. Of these, four subjects (25%) showed slight improvement while three (18.8%) showed moderate improvement. ANOVA showed that post-treatment assessments (weeks 48–72) were significantly lower (mean = 1.62) than those at week 16 (mean = 2.81) and week 24 (mean = 3.75) (p < 0.0001 for both). Moreover, significant differences were also noted between the week 16 and week 24 assessments.
Subject Global Assessment
The SGA for skin texture and atrophic acne scars improved in 83 and 89% of cases, respectively, at the end of treatment. At week 24, 15 of the 18 subjects (83.4%) reported that their facial skin texture had somewhat improved (N = 10; 55.6%) or much improved (N = 5; 27.8%). Such improvement was maintained at the week 48–72 follow-up in 14 of the 16 subjects followed up (87.5%). At week 24, 16 of the 18 subjects (88.9%) reported that their facial atrophic acne scars had somewhat improved (N = 14; 77.8%) or much improved (N = 2; 11.1%) (Fig. 1c). At the week 48–72 follow-up, maintenance of improvement was reported by 12 of these 16 subjects (75.1%).
Lesion Counts
Lesion counts decreased over time for all scar types; however, none of the changes were found to be significant between time points (boxcar scars neared significance, p = 0.06; Table 2). Post-treatment data (weeks 48–72) on lesion counts were also collected on 16 subjects. No significant differences were noted between treatment and post-treatment lesion counts.
Region of Interest
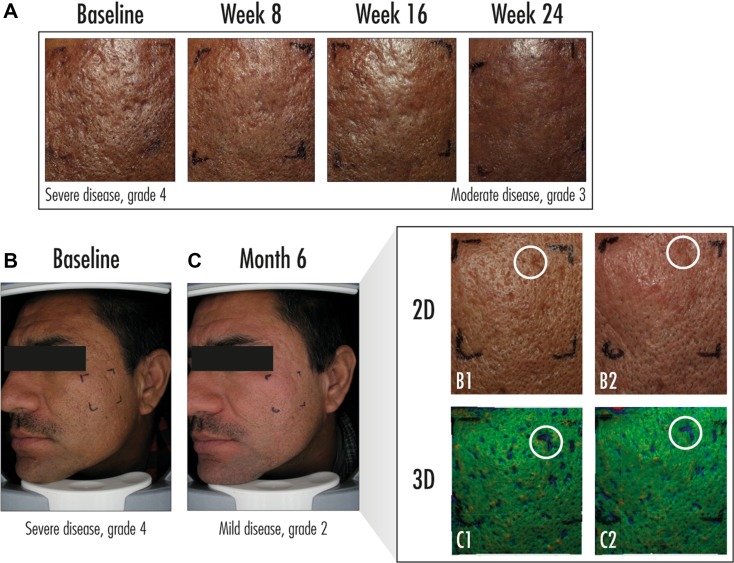
Clinical photographs of lesions prior to and during treatment are shown in Fig. 2. The ROI is marked on the skin and close-up photos illustrate subtle volume and surface changes over time. The efficacy observed on the full face was also observed on the ROI in terms of acne scar counts. At baseline, subjects had an average of 18.7 atrophic acne scars in the ROI; at week 24, the mean count decreased to 11.8 atrophic acne scars. This decrease was maintained at the week 48–72 follow-up with a mean count of 11.9 atrophic scars.
Fig. 2.
Clinical photos (full-face, region of interest [ROI], and close-ups) of lesions before and during treatment in a subject with severe scarring at baseline. a Representative examples of treatment results. b, c Global photographs of a different subject. Lower right panel depicts ROI analyzed digitally. The 3D LifeViz® Micro photography system (Quantificare SA, Valbonne, France) was used to collect two-dimensional (2D; B1, B2) and three-dimensional (3D; C1, C2) images of lesions
Immunohistochemistry
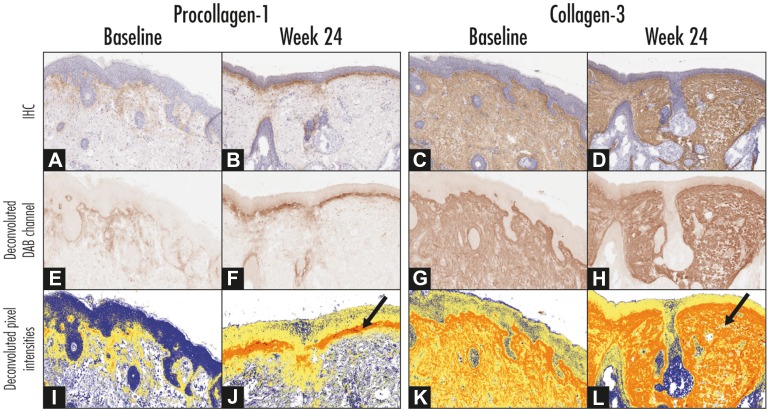
Using the Bakos method [27], immunostaining intensity scores were greater at the end of treatment (week 24) than at baseline for Procol-3 (2.19 vs. 2.00, respectively). In contrast, scores for Procol-1 did not show a similar elevation after 6 months of topical adapalene usage. These findings were not statistically significant.
IHC using color deconvolution image analysis showed a non-statistically significant increase in staining over time for Procol-1 (p = 0.99) and Col-3 (p = 0.96) (Fig. 3).
Fig. 3.
Representation of image processing used for quantitative immunohistochemistry studies of procollagen-1 (left panel) and collagen-3 (right panel). a–d Expression of procollagen-1 and collagen-3 (brown color). e–h The Aperio Color Deconvolution Algorithm (Aperio Technologies, Vista, CA, USA) was used to analyze diaminobenzidine (DAB) substrate staining applied to each slide. Strong positive staining intensity within the dermoepidermal junction was measured. i–l Staining intensity illustrated as a heatmap where blue = no staining, yellow = low intensity, orange = medium intensity, and red = high intensity staining (denoted by arrows)
Transcriptomic Analysis
Evaluation of the gene expression profile of individual subjects from baseline to week 24 revealed a clear change in expression levels of retinoid responsive genes (e.g., CYP26A1, KRT19, KRT4, KRT2, TGM3 and DSC1) in five of seven subjects. Refined statistical analysis was performed on these five subjects. Ninety-five Affymetrix IDs, corresponding to 76 genes, were found to be modulated at week 24 compared to baseline, based on the criteria of a fold-change of > 1.5 and p < 0.05. Among these, change in the expression levels of several genes expressed in differentiated layers of the epidermis, including specific keratins (KRT13, KRT4), cornulin (CRNN), caspase 14 (CASP14) and components of the cornified envelope (SPRR3, IVL, CNFN) was observed.
Furthermore, treatment with adapalene 0.3% gel led to modifications in the expression of serine proteases and serine protease inhibitors. The expression of several serine proteases, including KLK6, KLK9 and KLK13, was upregulated along with that of members of the SERPIN family, suggesting an activation of the desquamation process.
In terms of dermal matrix macromolecules, both type I collagen alpha 1 (COL1A1) and type III collagen alpha 1 (COL3A1) showed higher, although not statistically significant, expression levels after 24 weeks of adapalene treatment compared to baseline.
Safety
A total of five AEs (tooth infection, skin bacterial infection, anxiety, wound dehiscence and hypertrophic scarring) were reported in five subjects (25%). None were deemed related to the study medication. Reported AEs were of mild to moderate severity and resolved without residual effects. Assessment of local tolerability showed a profile that was similar to that previously reported with adapalene treatment. The most commonly observed events were of mild severity and included dryness (N = 11; 55%), stinging/burning (N = 10; 50%), scaling (N = 5; 25%) and erythema (N = 3; 15%). There were no serious AEs, and none of the observed AEs resulted in treatment discontinuation.
Tolerability assessments showed that stinging/burning at week 10 (mean = 0.38) was significantly higher (p < 0.0001) than that in all other weeks. The incidences of erythema and scaling were low over time, whereas dryness was higher and peaked at day 10 (mean = 0.33). However, none of these differences were statistically significant.
Subject-Reported Outcomes
In the subject satisfaction questionnaire, the vast majority of subjects (88.2%) were satisfied with the effectiveness of the treatment and reported that they would consider using this treatment again (83.3%). In addition, almost 90% of subjects (16/18) were satisfied with the rapid onset of efficacy. In terms of local tolerability, 13 of 18 subjects (72.2%) reported that they were not bothered at all by treatment side effects. Furthermore, 81.3% reported that the moisturizing lotion provided during the treatment was effective in reducing any irritation caused by the topical application of adapalene gel.
DLQI assessments were made at baseline and week 24. At baseline, 14 of 20 subjects (70%) reported impairment in quality of life with a mean DLQI score of 4.3. Following 6 months of therapy with topical adapalene, 10 of 18 subjects (55.6%) reported no impact on quality of life, while the remaining eight subjects (44.4%) reported only a small effect on quality of life. Overall, all subjects experienced little or no impairment on quality of life by the end of treatment, with a mean DLQI score of 1.3.
Discussion
This exploratory study investigated the efficacy and safety of adapalene 0.3% gel in the treatment of atrophic acne scars. The findings indicate that topical adapalene 0.3% gel improves skin texture and the appearance of atrophic acne scars. Investigator assessments and subjects’ self-evaluation were consistent. At the end of the study (week 24), the investigators reported a 1- or 2-grade improvement from baseline in more than half of subjects (55.6%) and at least a marked improvement in skin texture and atrophic scars in 50% of subjects. Subjects’ assessments reported improvement in skin texture and atrophic scars in more than 80% of cases. Investigator assessments and subjects’ self-evaluations of improvement in skin texture and scar improvement were maintained after the discontinuation of the treatment.
Similarly, clinical assessment of atrophic lesion counts within the ROI showed improvement. At week 24, all sub-classifications of atrophic scar types showed decreased counts. Although scar sizes decreased and scars became less noticeable, the sub-classification of scar counts did not decrease significantly. This may be due to a re-classification error as scars decreased in diameter. The classification of scar types according to the diameter of the opening on the skin surface meant that as scars improved (shrank in size), they were re-classified according to the pre-set definitions into the small scar category. For example, if a boxcar scar shrank in size due to treatment, it would be re-classified as an icepick scar. This may have affected change in total scar counts over time.
The IHC analysis using two methods showed increases in Col-3 staining (although not statistically significant) over time. For Procol-1, a similar increase from baseline to week 24 was observed with the Bakos method [27] (not statistically significant), but not with the computer-assisted image analysis. Overall, our IHC data supported our clinical observations and gene expression analysis.
Gene expression profile analysis correlated with the clinical efficacy observed. A clear gene signature of retinoid was observable in some subjects, validating the bioactivity of adapalene in the skin. Two subjects did not display the retinoid signature, and we hypothesize less than adequate compliance with twice-daily application. Potential retinoid receptor polymorphisms may account for this finding. Functional analysis of modulated genes between week 24 and baseline for five subjects resulted in a substantial change in the expression of several genes expressed in differentiated epidermal layers and involved in desquamation. This finding may reflect an increased keratinocyte turnover and altered differentiation leading to epidermal thickness.
All-trans retinoic acid has been shown to ameliorate the histological and clinical features of photoaging in numerous controlled clinical studies. Improved skin appearance is associated with reduced levels of collagen-degrading enzymes (MMPs) and increased collagen synthesis [20, 21, 33, 34]. Supporting these previous findings, the mRNA levels of COL1A1 and COL3A1 were slightly increased after 24 weeks of therapy with adapalene 0.3% gel. The clinical improvement of atrophic scars after adapalene 0.3% gel treatment could be concomitantly related to an increase in epidermal thickness and activation of collagen production.
Treatment was well tolerated with only mild dryness and stinging/burning reported by approximately 50% of subjects.
Subject-related outcome assessment indicated that, globally, subjects were satisfied with the study drug, in accordance with full-face clinical outcomes, while DLQI revealed improvements in subject quality of life following treatment.
The limitations of this study include the absence of a control group and the small population size. Since this was an exploratory study, the population size was empirical. However, it was considered to be sufficient to test the initial hypothesis.
Conclusions
In conclusion, daily use of adapalene 0.3% gel for the treatment of atrophic acne scars showed promising clinical efficacy, a favorable tolerability profile and improvement in quality of life. Further clinical investigation involving a larger number of subjects and a control group is warranted to corroborate these findings.
Acknowledgements
Funding
Sponsorship for this study and article processing charges were funded by Nestlé Skin Health–Galderma R&D. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial and Other Assistance
The authors thank the participants of the study, as well as Sharon Ghazarian PhD, Janis Taube MD MSc, Haiying Xu and Jung H Kim for their contribution to this study. Editorial assistance in the preparation of this article was provided by Dr Sotirios Georgantopoulos (SG Medical Writing B.V.). Support for this assistance was funded by Nestlé Skin Health–Galderma R&D.
Disclosures
N Kerrouche is an employee of Nestlé Skin Health–Galderma R&D. S Kang has served as a consultant to Nestlé Skin Health–Galderma R&D and received honoraria for the work. MJ Loss, S Leung, A Chien and AH Fischer have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available as they are proprietary data, but all of the conclusions drawn in the manuscript are based on data included in the publication and supporting literature has been provided.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to 10.6084/m9.figshare.5919436.
References
- 1.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474–485. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- 2.White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34–S37. doi: 10.1016/S0190-9622(98)70442-6. [DOI] [PubMed] [Google Scholar]
- 3.Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577–580. [PubMed] [Google Scholar]
- 4.Canavan TN, Chen E, Elewski BE. Optimizing non-antibiotic treatments for patients with acne: a review. Dermatol Ther (Heidelb). 2016;6:555–578. doi: 10.1007/s13555-016-0138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303–308. doi: 10.1111/j.1365-2230.1994.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 6.Layton AM, Seukeran D, Cunliffe WJ. Scarred for life? Dermatology. 1997;195:15–21. doi: 10.1159/000246015. [DOI] [PubMed] [Google Scholar]
- 7.Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48–52. doi: 10.1111/j.1473-2165.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 8.Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458–1466. doi: 10.1111/j.1524-4725.2006.32354.x. [DOI] [PubMed] [Google Scholar]
- 9.Fabbrocini G, Annunziata MC, D’Arco V, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010:893080. doi: 10.1155/2010/893080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo JY, Smith LL. Psychologic aspects of acne. Pediatr Dermatol. 1991;8:185–188. doi: 10.1111/j.1525-1470.1991.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109–117. doi: 10.1067/mjd.2001.113451. [DOI] [PubMed] [Google Scholar]
- 12.Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000;26:857–871. doi: 10.1046/j.1524-4725.2000.99232.x. [DOI] [PubMed] [Google Scholar]
- 13.Jemec GB, Jemec B. Acne: treatment of scars. Clin Dermatol. 2004;22:434–438. doi: 10.1016/j.clindermatol.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–1699. doi: 10.1016/S0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. 2006;126:1071–1079. doi: 10.1038/sj.jid.5700213. [DOI] [PubMed] [Google Scholar]
- 16.Fisher GJ, Datta S, Wang Z, et al. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106:663–670. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papakonstantinou E, Aletras AJ, Glass E, et al. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J Invest Dermatol. 2005;125:673–684. doi: 10.1111/j.0022-202X.2005.23848.x. [DOI] [PubMed] [Google Scholar]
- 18.Kang S, Fisher GJ, Voorhees JJ. Photoaging and topical tretinoin: therapy, pathogenesis, and prevention. Arch Dermatol. 1997;133:1280–1284. doi: 10.1001/archderm.1997.03890460104012. [DOI] [PubMed] [Google Scholar]
- 19.Kang S, Voorhees JJ. Photoaging therapy with topical tretinoin: an evidence-based analysis. J Am Acad Dermatol. 1998;39:S55–S61. doi: 10.1016/S0190-9622(98)70446-3. [DOI] [PubMed] [Google Scholar]
- 20.Cho S, Lowe L, Hamilton TA, Fisher GJ, Voorhees JJ, Kang S. Long-term treatment of photoaged human skin with topical retinoic acid improves epidermal cell atypia and thickens the collagen band in papillary dermis. J Am Acad Dermatol. 2005;53:769–774. doi: 10.1016/j.jaad.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993;329:530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- 22.Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76:145–151. [PubMed] [Google Scholar]
- 23.Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242–250. doi: 10.1016/j.jaad.2004.10.879. [DOI] [PubMed] [Google Scholar]
- 24.Thiboutot D, Arsonnaud S, Soto P. Efficacy and tolerability of adapalene 0.3% gel compared to tazarotene 0.1% gel in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7:s3–s10. [PubMed] [Google Scholar]
- 25.Weiss JS, Thiboutot DM, Hwa J, Liu Y, Graeber M. Long-term safety and efficacy study of adapalene 0.3% gel. J Drugs Dermatol. 2008;7:s24–s28. [PubMed] [Google Scholar]
- 26.Kang S, Goldfarb MT, Weiss JS, et al. Assessment of adapalene gel for the treatment of actinic keratoses and lentigines: a randomized trial. J Am Acad Dermatol. 2003;49:83–90. doi: 10.1067/mjd.2003.451. [DOI] [PubMed] [Google Scholar]
- 27.Bakos RM, Bakos L, Edelweiss MI, Cartell A, Mariante JC, Masiero NC. Immunohistochemical expression of matrix metalloproteinase-2 and -9 in melanocytic nevi is altered by ultraviolet B. Photodermatol Photoimmunol Photomed. 2007;23:250–254. doi: 10.1111/j.1600-0781.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 28.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 29.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 30.Girman CJ, Hartmaier S, Thiboutot D, et al. Evaluating health-related quality of life in patients with facial acne: development of a self-administered questionnaire for clinical trials. Qual Life Res. 1996;5:481–490. doi: 10.1007/BF00540020. [DOI] [PubMed] [Google Scholar]
- 31.Martin AR, Lookingbill DP, Botek A, Light J, Thiboutot D, Girman CJ. Health-related quality of life among patients with facial acne—assessment of a new acne-specific questionnaire. Clin Exp Dermatol. 2001;26:380–385. doi: 10.1046/j.1365-2230.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodol). 1995;57:289–300. [Google Scholar]
- 33.Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55:1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Bhawan J. Short- and long-term histologic effects of topical tretinoin on photodamaged skin. Int J Dermatol. 1998;37:286–292. doi: 10.1046/j.1365-4362.1998.00433.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available as they are proprietary data, but all of the conclusions drawn in the manuscript are based on data included in the publication and supporting literature has been provided.