Abstract
Introduction
The aim of this study was to compare the public health impact of introducing two herpes zoster (HZ) vaccines into the vaccination programs for the Japanese population aged ≥ 50 years: a single-dose Varicella Vaccine Live (VVL) or a two-dose adjuvanted Recombinant Zoster Vaccine (RZV).
Methods
A multi-cohort static Markov model was developed to follow age cohorts (50–59, 60–69, 70–79 and ≥ 80 years) over their remaining lifetime. Japan-specific data inputs for the model were obtained from Japanese data sources. Age-stratified vaccine efficacy and waning rates were based on published clinical trial data. In the base-case analysis, vaccine coverage was assumed to be 40% for both vaccines, and compliance with second-dose of the RZV vaccine was set to 95%.
Results
Vaccination with RZV was projected to prevent approximately 3.3 million HZ cases, 692,000 cases of postherpetic neuralgia (PHN), and 281,000 cases of other complications, compared with the prevention of 0.8 million HZ cases, 216,000 PHN cases, and 57,000 other complications with vaccination with VVL. The number of individuals needed to vaccinate in order to prevent one HZ case ranged from 6 to 14 using RZV (depending on age and assumed second-dose compliance) and from 21 to 138 depending on age using VVL. By preventing a higher number of HZ cases and its complications, RZV vaccination led to fewer outpatient visits and hospitalizations than vaccination with VVL.
Conclusion
Both vaccines had a positive public health impact compared to no vaccination, but due to its higher vaccine efficacy, RZV demonstrated a superior public health impact compared with VVL.
Funding
GlaxoSmithKline Biologicals SA.
Electronic supplementary material
The online version of this article (10.1007/s13555-018-0236-3) contains supplementary material, which is available to authorized users.
Keywords: Herpes zoster (HZ), HZ complications, Live attenuated vaccine, Markov model, Postherpetic neuralgia, Public health impact, Recombinant zoster vaccine, Varicella zoster virus
Introduction
Herpes zoster (HZ or shingles) is caused by reactivation of latent varicella-zoster virus (VZV) residing within the dorsal root ganglia of people who have had a primary varicella infection. The actual cause of reactivation of VZV has not been identified, but it is associated with a decline in VZV-specific cell-mediated immunity either due to aging or immunosuppression caused by disease or induced by medical treatments [1].
The vast majority of adults have been infected with wild-type VZV (chickenpox) and are thus at risk for developing HZ; for example, in Japan, it is reported that > 90% of individuals aged ≥ 20 years have anti-VZV antibodies [2]. The lifetime risk of developing HZ is generally stated to be approximately 30%, and the incidence rises steeply after the age of 50 years [3].
In the acute phase, HZ typically presents as a painful unilateral vesicular rash in the affected dermatome. Pain often precedes the rash by some days, and this pain is referred to as prodromal pain. Most cases of HZ are uncomplicated and resolve within 4 weeks of rash onset, but some patients experience various complications. The most frequent complications during the acute phase are ocular, cutaneous or neurologic, and these may lead to long-term physical impairment [1, 4]. Combined, these complications occur in approximately 10% of HZ patients [5]. The most common complication of HZ, however, is postherpetic neuralgia (PHN). Reports on the proportion of HZ patients developing PHN vary greatly because of differences in the definition used and the populations examined [6]. Approximately 15% of HZ patients experience PHN, defined as at least 90 days of persistent pain after rash onset [6]. Furthermore, increased risks of cardiovascular or cerebrovascular events following HZ have been shown [7]. All HZ-related complications increase in frequency and severity with advancing age [1]. HZ-related deaths, albeit rare, have also been reported [8–10].
HZ and PHN pose a substantial disease burden in Japan in terms of loss of quality of life [11] and health care costs [12]. Studies have found HZ incidence rates of 10–11 per 1000 person-years (PY) in people aged ≥ 50 years [13] or ≥ 60 years [14], with 19% of HZ patients aged ≥ 50 years developing PHN [13]. Another study found that 12.4% of HZ patients had HZ-related pain rated ≥ 3 on a 0- to 10-point scale 3 months after HZ onset [15]. A substantial economic burden on the healthcare system was reported as a result of high medical costs relating to medications, outpatient visits, and hospitalizations. The medical costs were higher for HZ cases with complications than for uncomplicated HZ: 2.7 times higher for PHN and 1.9 times higher for complications other than PHN [12].
Although HZ can be managed effectively if treatment with herpesvirus-specific antiviral oral drugs or injections is initiated within a few days of rash onset, this treatment has no impact on the development of PHN, which is difficult to treat satisfactorily [16]. The first HZ vaccine was licensed in the USA in 2006 for prevention of HZ in people aged ≥ 60 years. This is a live attenuated VZV vaccine (Zoster Vaccine Live [ZVL], Zostavax) using the same Oka strain of VZV as the varicella vaccine developed in 1974 by Takahashi et al. [17], but at a dose of about 14-fold greater than that used for the prevention of varicella [18]. ZVL is currently licensed for HZ prevention in individuals aged ≥ 50 years in more than 60 countries around the world, but not in Japan [16].
Japan was one of the first countries in the world to introduce varicella vaccination, but universal varicella vaccination with the Varicella Vaccine Live (VVL) was not recommended until October 2014. In March 2016, VVL was approved in Japan for the prevention of HZ in individuals aged ≥ 50 years as an extended use [19]. The titer of the VVL used for HZ prevention in Japan is similar to that of the ZVL licensed for HZ prevention elsewhere in the world.
An alternative two-dose adjuvanted Recombinant Zoster Vaccine (RZV, Shingrix) was recently licensed in the USA and Canada for prevention of HZ in individuals aged ≥ 50 years and is now approved in Japan as well. This vaccine is referred to as RZV and combines VZV glycoprotein E, an important and highly abundant protein found on the VZV, with the proprietary adjuvant system AS01B. RZV is to be administered in a two-dose schedule 2 to 6 months apart, contrary to the VVL which is administered as a single dose. On 25 October 2017, the Advisory Committee on Immunization Practices (ACIP) in the USA voted that RZV is: (1) recommended for healthy adults aged ≥ 50 years to prevent shingles and related complications; (2) recommended for adults who previously received the current shingles vaccine (ZVL) to prevent shingles and related complications; (3) the preferred vaccine for preventing shingles and related complications [20].
The objective of the present study was to perform an analysis of the potential public health impact of vaccinating individuals in Japan aged ≥ 50 years with RZV compared to no vaccination and to vaccination with VVL, respectively.
Methods
Markov Model
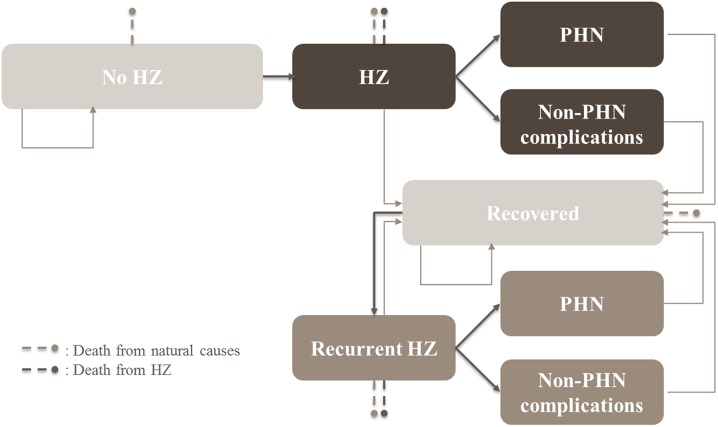
The ZOster ecoNomic Analysis (ZONA) model [21] is a static multi-cohort Markov model with a cycle length of 1 year, which follows the cohorts over their remaining lifetime. An overview of the structure of the model is presented in Fig. 1. Transitions between the health states (no HZ, HZ, PHN, HZ-related complications other than PHN [including ocular, neurologic, and cutaneous complications], recurrent HZ, HZ-related death, death due to natural causes) occur in annual time steps. PHN and HZ complications other than PHN occur during an HZ episode and therefore occur within the same annual time step. Probabilities of moving between the health states are age-specific and, to the extent possible in this study, based on Japanese data.
Fig. 1.
Model structure. HZ herpes zoster, PHN postherpetic neuralgia
Adapted from Curran et al. [21]
The Japanese adaptation of the model considers individuals aged ≥ 50 years partitioned into four age groups (50–59, 60–69, 70–79, and ≥ 80 years) and follows all individuals within a cohort for their remaining lifetime from the year of vaccination. As such, all individuals remain in their initial cohort, and all subsequent events are counted in that cohort only. For the population of individuals aged ≥ 50 years, the results for all age groups are combined.
Three different vaccination strategies are compared: no vaccination (control), vaccination with VVL, and vaccination with RZV. Individuals can be fully or partially compliant with the vaccine dosing schedule or not vaccinated at all, depending on the vaccination coverage and compliance rates assumed for the two-dose RZV.
Model Inputs
The model parameters are divided into three distinct sections: demographics, epidemiology, and natural history of HZ and vaccine efficacy (VE).
Age-stratified population figures and all-cause mortality rates in 5-year age groups starting at age 50 years were retrieved from official Japanese sources, namely, the “Estimation of population (2015)” and “Abridged life tables (2015)”, respectively [22].
The epidemiological inputs to the model are summarized in Table 1. Among some recent studies presenting data on the incidence of HZ in Japan [13, 14, 23], the data from Takao et al. [13] were selected for this analysis because the data collection method was considered to be satisfactory and the study covered the age group of 50-year-olds or older. Data from the other studies mentioned were applied in sensitivity analyses. The SHEZ study [13] was a prospective community-based cohort study following approximately 12,500 individuals over a 3-year period to estimate the incidence of HZ and PHN. Data for HZ complications other than PHN were derived from a study by Nakamura et al. [12]. Although there are various reports on recurrent HZ, due to differences in study design, such as follow-up period and patient populations [23–25], we assumed that the incidence of recurrent HZ was the same as that for the first occurrence, as also assumed in other models [26, 27]. Mortality rates for deaths caused by HZ were retrieved from “Vital statistics (2015)” [22].
Table 1.
Epidemiological input values and data sources
| Age group (years) | Base value | Rangec | Data sources | |||
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | Base value | Range | |||
| HZ incidence (per 1000) | 50–59 | 9.2 | 6.2 | 11.0 | [13]d | [23]; assumedd |
| 60–69 | 9.6 | 6.4 | 11.5 | [13]d | [23]; assumedd | |
| 70–79 | 12.9 | 8.6 | 15.5 | [13]d | [23]; assumedd | |
| ≥ 80 | 12.6 | 8.4 | 15.1 | [13]d | [23]; assumedd | |
| PHNa (%) | 50–59 | 15.7 | – | – | [13]e | N/A |
| 60–69 | 13.6 | – | – | [13]e | N/A | |
| 70–79 | 20.2 | – | – | [13]e | N/A | |
| ≥ 80 | 32.9 | – | – | [13]e | N/A | |
| Complications other than PHNa (%) | 50–69 | 5.1 | – | – | [12]f | N/A |
| ≥ 70 | 10.6 | – | – | [12]f | N/A | |
| HZ mortality ratesa (%) | 50–54 | 0.0000 | – | – | [22] | N/A |
| 55–59 | 0.0000 | – | – | [22] | N/A | |
| 60–64 | 0.0012 | – | – | [22] | N/A | |
| 65–69 | 0.0011 | – | – | [22] | N/A | |
| 70–74 | 0.0020 | – | – | [22] | N/A | |
| 75–79 | 0.0037 | – | – | [22] | N/A | |
| 80–84 | 0.0158 | – | – | [22] | N/A | |
| 85–89 | 0.0277 | – | – | [22] | N/A | |
| 90–94 | 0.0641 | – | – | [22] | N/A | |
| 95–99 | 0.2858 | – | – | [22] | N/A | |
| ≥ 100 | 0.5120 | – | – | [22] | N/A | |
| Hospitalizationb | 50–69 | 0.020 | – | – | [12, 14]g | N/A |
| ≥ 70 | 0.044 | – | – | [12, 14]g | N/A | |
| Number of outpatient visitsb | 50–69 | 4.8 | – | – | [12]h | N/A |
| ≥ 70 | 6.4 | – | – | [12]h | N/A | |
HZ Herpes zoster, n number, PHN postherpetic neuralgia, N/A not applicable
a % of HZ cases
bMean number per HZ case
c–, Not varied in the sensitivity analysis
dTakao et al. [13] reported the incidence rate of HZ in a community-based prospective cohort study. The lower bound estimates were obtained from Shiraki et al. [23], with –33% set based on the ratio of incidence at ages 70–79 years (0.00869/0.01290); assumed was + 20% of base case for the upper bound. The recurrent HZ incidence was assumed to be the same as the initial HZ incidence
eThe percentage of HZ with PHN was obtained from the same data source as the HZ incidence [13]. The PHN proportion among HZ was assumed to be the same for both initial and recurrent cases
fNakamura et al. [12] reported the overall percentage of non-pain complications including ocular, neurological, and cutaneous ones in a prospective, observational cohort study of Japanese adults aged ≥ 60 years. The proportion at ages 50–59 years was assumed to be the same as that at ages 60–69 years
gHospitalization rate reported by Nakamura et al. [12] was adjusted by the rate reported by Sato et al. [14]. The data at ages 50–59 years were assumed to be the same as those at ages 60–69 years
hThe data at ages 50–59 years were assumed to be the same as those at ages 60–69 years
As no data are available for the use of VVL for prevention of HZ, it is assumed that VE and waning data for the ZVL can be applied to VVL without any modifications. A similar assumption was made in a recent cost-effectiveness analysis of HZ vaccination in Japan [19]. The efficacy of ZVL was assessed in two phase III clinical trials that included > 38,000 individuals aged ≥ 60 years (the Shingles prevention study [SPS] [28]) and 22,000 individuals aged 50–59 years (the Zoster Efficacy and Safety Trial [ZEST] [29]). The VEs against PHN were calculated by adding the efficacy against PHN to that against HZ. The assumed overall VEs of VVL against HZ and PHN, respectively, are presented in Table 2. The duration of protection for VVL was modeled based on data from the SPS [28] and a long-time protection study [30]. The model suggested a waning of VE of approximately 5.4% during each of the first 4 years and 5.1% thereafter. The overall VE against PHN is assumed to wane at the same rate as the VE against HZ. In the ZONA model, the VE values used (i.e., age-specific VE at time 0 for the VVL) are automatically adjusted each year to take waning into account.
Table 2.
Values applied for vaccine-specific inputs for base-case and sensitivity analyses and data sources
| Age group (years) | Base value (%) | Rangea (bounds-%) | Data sources | |||
|---|---|---|---|---|---|---|
| Lower | Upper | Base value | Range | |||
| Initial HZ efficacy: RZV (one dose) | 50–69 | 90.00 | 58.90 | 98.90 | [21] | [21] |
| ≥ 70 | 69.50 | 24.90 | 89.10 | [21] | [21] | |
| Initial HZ efficacy: RZV (two doses) | 50–69 | 98.40 | 95.00 | 100.00 | [21] | [21] |
| ≥ 70 | 97.84 | 94.10 | 100.00 | [21] | [21] | |
| Initial HZ efficacy: VVL | 50–59 | 69.80 | 54.10 | 80.60 | [29] | [29] |
| 60–69 | 63.89 | 56.00 | 71.00 | [28, 46] | [21, 46] | |
| 70–79 | 40.85 | 28.00 | 52.00 | [28, 46] | [21, 46] | |
| ≥ 80 | 18.25 | 0.00 | 48.00 | [28, 46] | [21, 46] | |
| Initial PHN efficacy: RZV (one dose) | 50–69 | 90.00 | – | – | [21] | N/A |
| ≥ 70 | 69.50 | – | – | [21] | N/A | |
| Initial PHN efficacy: RZV (two doses) | 50–59 | 98.40 | – | – | [21] | N/A |
| ≥ 70 | 97.84 | – | – | [21] | N/A | |
| Initial PHN efficacy: VVL | 50–59 | 69.80 | – | – | Assumedb | N/A |
| 60–69 | 65.69 | – | – | [28, 46] | N/A | |
| 70–79 | 73.38 | – | – | [28, 46] | N/A | |
| ≥ 80 | 39.51 | – | – | [28, 46] | N/A | |
| Annual waning of efficacy: RZV (one dose) | All for years 1–4 | 5.40 | 1.00 | 7.40 | Assumedc | [21] |
| All for years ≥ 5 | 5.10 | 3.60 | 6.90 | Assumedc | [21] | |
| Annual waning of efficacy: RZV (two doses) | 50–69 for years 1–4 | 1.00 | 0.00 | 2.60 | [21] | [21] |
| 50–69 for years ≥ 5 | 2.30 | 0.70 | 4.60 | [21] | [21] | |
| ≥ 70 | 3.60 | 1.40 | 6.60 | [21] | [21] | |
| Annual waning of efficacy: VVL | All for years 1–4 | 5.40 | 4.50 | 6.40 | [21] | [21] |
| All for years ≥ 5 | 5.10 | 4.10 | 6.00 | [21] | [21] | |
| Coverage | All | 40.00 | 20.00 | 60.00 | Assumedd | Assumedd |
| Second-dose compliance for RZV | All | 95.00 | 70.00 | 100.00 | [31, 32]e | Assumede |
RZV Recombinant Zoster Vaccine, VVL Varicella Vaccine Live, HZ Herpes Zoster, PHN Postherpetic Neuralgia, N/A Not Applicable
a–, Not varied in the sensitivity analysis
bAssumed to be the same as the initial efficacy in preventing HZ
cAssumed to be the same as the waning rate of VVL efficacy
dAssumed to be the same as that in a recently introduced vaccine for elderly, pneumococcal polysaccharide vaccine-23 [34]. Assumed to be ± 20% of base case for the upper/lower bound, respectively
eAs there were no available data in Japan, second-dose compliance in this analysis was assumed to be the same as that obtained from two clinical trials [31, 32]. Assumed to be 70 and 100% for the lower/upper bound, respectively
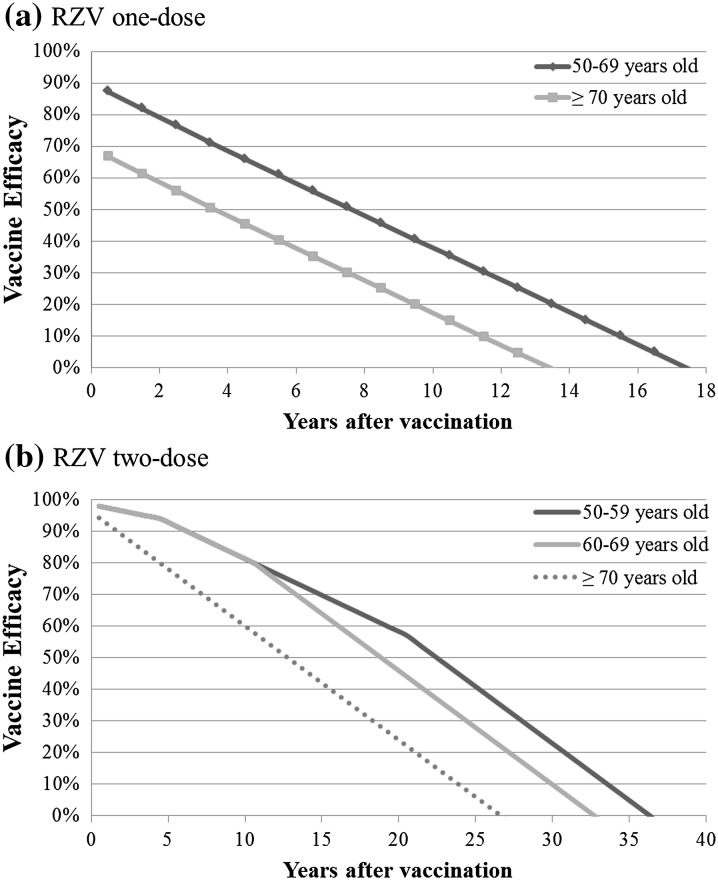
The VE of RZV was assessed in two phase III clinical trials in 16,161 individuals aged ≥ 50 years (ZOE-50) and in 14,816 individuals aged ≥ 70 years (ZOE-70) [31, 32]. Japanese subgroup analyses in these studies revealed that RZV demonstrated a high efficacy in the Japanese population as well [33]. However, the results of the global trials were selected for this model analysis because of their larger sample sizes. Participants aged ≥ 70 years were randomly assigned to one of the two trials to allow for a pre-planned combined analysis of all participants aged ≥ 70 years. In both the ZOE-50 study and the pooled analysis of participants aged ≥ 70 years (referred to as ZOE-70+), the VE of RZV remained consistent across the age groups (Table 2). The waning over time of VE for RZV was derived by a linear approximation to the VE estimates per year obtained in the ZOE-50 and ZOE-70 studies. The estimated waning of VE is illustrated in Fig. 2 and was approximately 1.0% per year in the ZOE-50 study and 3.6% in the ZOE-70+ analyses. Based on these data, it was assumed that for individuals aged 50–69 years, the VE for RZV wanes at 1.0% per year until year 4 after vaccination, at 2.3% per year (the average of the two values observed for the two clinical trials mentioned earlier) during the subsequent years until age 69 years, and at 3.6% per year for individuals aged ≥ 70 years. There were very few breakthrough HZ cases in both studies, and altogether there were only four cases of PHN. Thus, the overall VEs against PHN were assumed to be the same as the VEs against HZ.
Fig. 2.
Waning over time of efficacy of Recombinant Zoster Vaccine (RZV) in preventing HZ.
Adapted from Curran et al. [21]
RZV was developed as a two-dose schedule, but to assess its public health impact the waning after just one dose must also be estimated because some individuals will not be compliant and receive the second-dose. In both the ZOE-50 and ZOE-70 trials, the compliance was high, and the mean follow-up period for individuals who received only the first dose was less than 3 months, so the possibility of obtaining robust VE estimates with one dose of RZV is limited. The observed VE after one dose was 90.1% (95% confidence interval [CI] 58.9–98.8%) in ZOE-50 and 69.5% (95% CI 24.9–89.1%) in the pooled analysis. With no data available on the long-term VE of one dose of RZV, it was assumed that it wanes at the same rate per year as the VE of VVL (Fig. 2).
The vaccination coverage was assumed to be the same as that for a pneumococcal polysaccharide vaccine for older adults recently introduced in Japan [34], i.e., 40%. The compliance for the second-dose of RZV was assumed to be as high as that observed in both clinical trials, i.e., 95% in the base-case analysis because > 95% compliance for the second- or third-dose of pediatric vaccines has been reported in Japan [34]. Two scenario analyses were performed to assess the impact of varying the compliance rates, assuming 70 and 100%, respectively.
Model Outputs
The health outcomes calculated in the model were the numbers of HZ cases, PHN cases, cases of HZ complications other than PHN, and deaths from HZ accumulated over the entire time horizon under each vaccination strategy. For each vaccine, the number of cases with vaccination was compared with the number of cases without vaccination, and the numbers of individuals needed to vaccinate (NNV) to avoid one HZ and one PHN case, respectively, were calculated.
Deterministic one-way sensitivity analyses were performed by varying each of the model’s input variables one at a time over the ranges presented in Tables 1 and 2. The variables examined were HZ incidence rates, vaccination coverage, compliance with the second-dose of RZV, VE against HZ, and waning of VE for both vaccines. The results of the deterministic sensitivity analyses are summarized in a tornado diagram that indicates the parameters’ relative impact on the number of HZ cases avoided.
Scenario analyses were performed to explore the uncertainty about the model’s outcomes under varying combinations of the extreme values for the variables (Table 2). The variables included in the scenario analyses were compliance with the second-dose, VE against HZ and waning of VE for RZV with both one and two doses.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Results
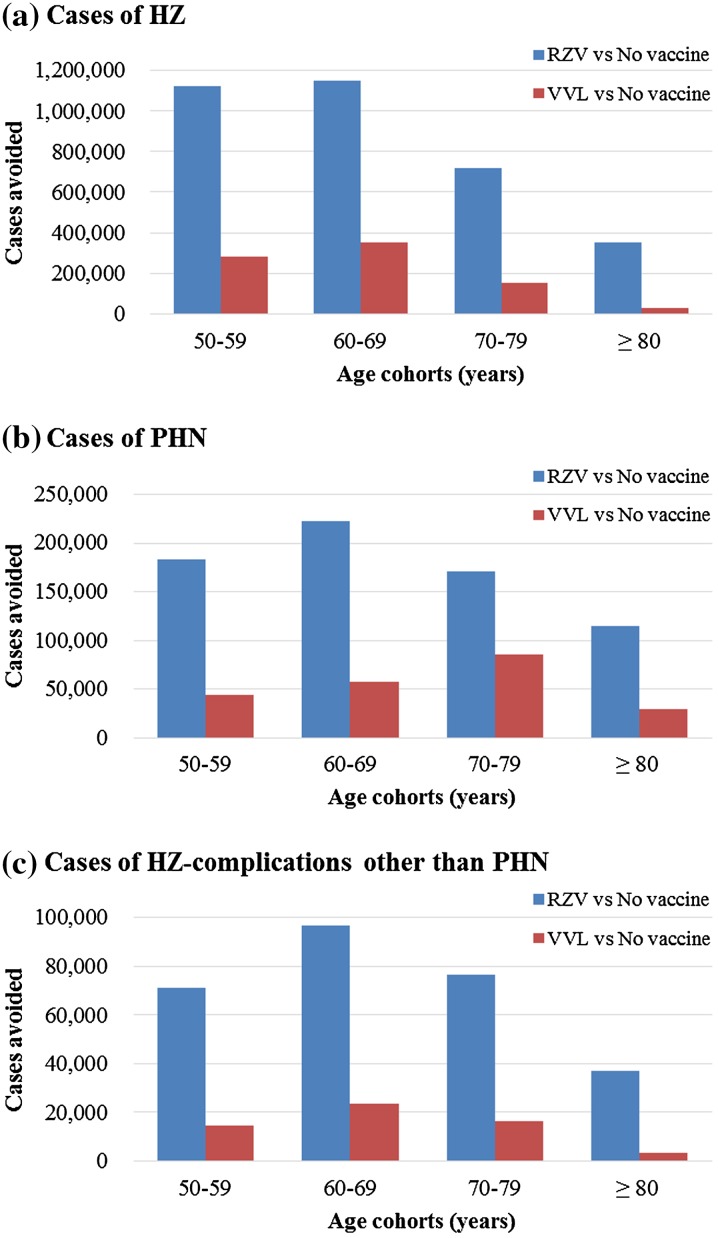
The Japanese general population comprised approximately 58 million people aged ≥ 50 years. The base-case public health impact results of the three vaccination strategies (no vaccination, vaccination with VVL, vaccination with RZV) over the remaining lifetime are presented in Table 3 (overall) and Fig. 3 (stratified across age groups).
Table 3.
Public health impact of Recombinant Zoster Vaccine and Varicella Vaccine Live over a lifetime horizon from the age of vaccination
| Number of cases if no vaccination | Cases avoideda | ||||
|---|---|---|---|---|---|
| RZV | VVL | ||||
| Low second-dose complianceb | Base casec | High second-dose complianceb | |||
| Number of persons vaccinatedd | – | 23,217,200 | 23,217,200 | 23,217,200 | 23,217,200 |
| HZ | 15,233,397 | 2,847,289 | 3,338,693 | 3,436,974 | 815,006 |
| PHN | 3,580,971 | 583,163 | 692,036 | 713,811 | 216,421 |
| Complications | 1,391,444 | 237,058 | 281,223 | 290,056 | 57,242 |
| Deaths | 1489 | 158 | 195 | 202 | 14 |
| Hospitalizations | 572,831 | 97,024 | 115,188 | 118,821 | 23,141 |
| Outpatient visits | 90,997,868 | 16,338,880 | 19,253,353 | 19,836,248 | 4,368,082 |
RZV Recombinant Zoster Vaccine, VVL Varicella Vaccine Live, HZ Herpes Zoste, PHN Postherpetic Neuralgia
aIn vaccinated persons compared with those not vaccinated over the lifetime of the respective cohorts
bLow, 70%; high, 100%
cBase-case assumptions: coverage, 40%; compliance with second-dose for RZV, 95%
dTotal cohort is 58,043,000
Fig. 3.
Cases avoided with vaccination versus no vaccination by age cohort. RZV Recombinant Zoster Vaccine, VVL Varicella Vaccine Live, HZ Herpes Zoste, PHN Postherpetic Neuralgia
The expected number of HZ cases and its complications (including PHN and complications other than PHN) in the no-vaccination strategy are higher in the age groups 50–59 and 60–69 years than in the older age groups as individuals younger than 70 years are assumed to live and be at risk of HZ for more years than individuals in the oldest groups. Overall, compared to VVL vaccination, RZV vaccination (with a compliance with second-dose of 95%) increased the numbers avoided by 310, 220, 391, and 1336% for cases of HZ, PHN, other complications, and deaths, respectively. In addition, the numbers of hospitalizations and outpatient visits avoided were increased by 398 and 341%, respectively.
Comparing the results assuming the compliance rate with the second-dose of RZV of 70% with the base-case results, about 15% reductions in the number of cases of HZ and its complications prevented were observed as well as healthcare resource utilization avoided. A similar reduction in the number of HZ-related deaths avoided (19%) was observed. Despite this decrease, vaccination with RZV prevented a much higher number of cases of HZ and its complications than did vaccination with VVL (Table 3), irrespective of the second-dose compliance scenario used.
The NNV to avoid a case of HZ and a case of PHN with both vaccines under varying assumptions around the second-dose compliance rate for RZV are presented in Table 4. Even with a lower compliance rate for the second-dose, a RZV vaccination strategy requires a lower number of people to be vaccinated to prevent one HZ case and one of PHN than does a VVL vaccination strategy. The NNV to avoid one HZ case increases with age for both vaccines, but at different rates (six individuals aged 50–59 years and 12 individuals aged ≥ 80 years for RZV vs. 23 and 138, respectively, for VVL). For avoiding a case of PHN, there is no similar association between age and the NNV for either vaccine.
Table 4.
Number of people needed to be vaccinated to prevent one herpes zoster case and one postherpetic neuralgia case
| Age group (years) | RZV second-dose compliance | VVL | |||
|---|---|---|---|---|---|
| Lowa | Base caseb | Higha | |||
| NNV for HZ | 50–59 | 7 | 6 | 6 | 23 |
| 60–69 | 8 | 7 | 7 | 21 | |
| 70–79 | 10 | 8 | 8 | 37 | |
| ≥ 80 | 14 | 12 | 12 | 138 | |
| NNV for PHN | 50–59 | 41 | 35 | 34 | 144 |
| 60–69 | 39 | 33 | 32 | 127 | |
| 70–79 | 40 | 34 | 33 | 66 | |
| ≥ 80 | 40 | 35 | 34 | 137 | |
NNV Number Needed to Vaccinate
aBase-case assumptions: coverage, 40%; second-dose compliance for RZV Recombinant Zoster Vaccine, VVL Varicella Vaccine Live, HZ Herpes Zoste, PHN Postherpetic Neuralgia, 95%
bLow compliance, 70%; high compliance, 100%
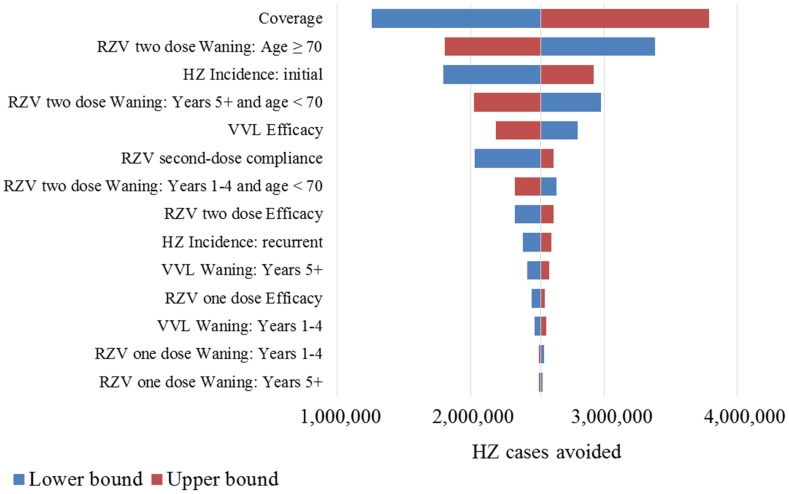
The results of the deterministic sensitivity analyses are presented in a tornado diagram in Fig. 4. In the base-case analysis, vaccination with RZV resulted in approximately 2.5 million more HZ cases avoided over the remaining lifetime of individuals aged ≥ 50 years compared with vaccination with VVL. The bars in Fig. 4 are ordered vertically according to the size of their impact on the outcome, with the variable with the greatest impact on top. The lower (upper) bound end of the bar indicates the impact of the variable when its value is equal to the lower (upper) value of its range. For example, if the coverage for both vaccines was assumed to be 20%, vaccination with RZV resulted in approximately 1.3 million more HZ cases avoided than vaccination with VVL; with a coverage of 60%, about 3.8 million more cases were avoided with vaccination with RZV. In all scenarios considered, vaccination with RZV resulted in more cases of HZ avoided than did vaccination with VVL. The outcome was most sensitive to assumptions regarding the vaccination coverage, the two doses waning, and the incidence of initial HZ (Fig. 4).
Fig. 4.
Tornado diagram for one-way sensitivity analyses results for HZ cases avoided by vaccination of Japanese people aged ≥ 50 years with RZV versus vaccination with VVL. HZ Herpes Zoster, VVL Varicella Vaccine Live, RZV Recombinant Zoster Vaccine
The results of the scenario analyses are presented in Table 5. The number of additional HZ cases avoided with vaccination with RZV compared to vaccination with VVL, assuming the combination of efficacy and waning most favorable for RZV (Scenario 1) and least favorable for RZV (Scenario 4) amounted to approximately 4.3 and 0.7 million, respectively.
Table 5.
Scenario analyses inputs and results for herpes zoster cases avoided comparing vaccination with Recombinant Zoster Vaccine and Varicella Vaccine Live
| Scenario | Second-dose compliance with RZV vaccination | Initial efficacy of RZV (one dose) against HZ | Initial efficacy of RZV (two doses) against HZ | Waning of RZV efficacy (one dose) | Waning of RZV efficacy (two doses) | Number of HZ cases avoided with RZV compared to VVL |
|---|---|---|---|---|---|---|
| Scenario 1 | Upper bound | Upper bound | Upper bound | Lower bound | Lower bound | 4,342,890 |
| Scenario 2 | Base | Base | Upper bound | Base | Lower bound | 4,151,864 |
| Base case | Base | Base | Base | Base | Base | 2,523,687 |
| Scenario 3 | Base | Base | Lower bound | Base | Upper bound | 1,180,198 |
| Scenario 4 | Lower bound | Lower bound | Lower bound | Upper bound | Upper bound | 694,132 |
HZ herpes zoster, RZV Recombinant Zoster Vaccine, VVL Varicella Vaccine Live
Discussion
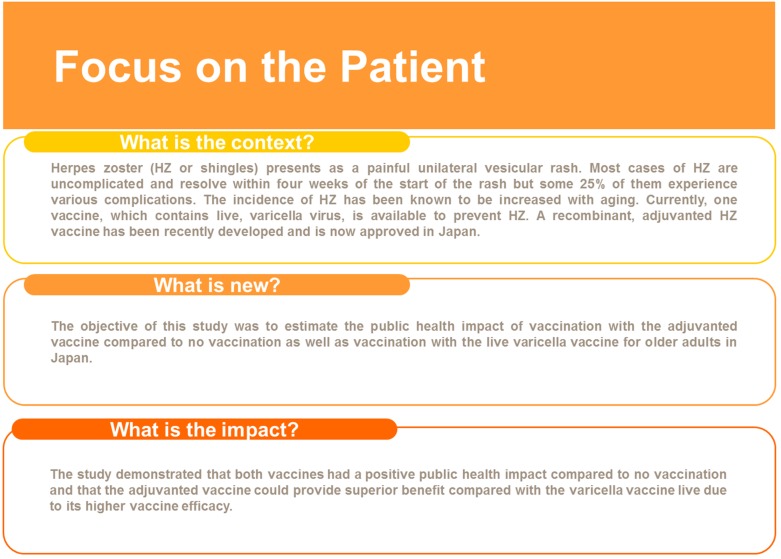
In this study we modeled the potential public health impact of HZ vaccination in the Japanese population aged ≥ 50 years and showed that the RZV vaccine would reduce the number of HZ cases by approximately 3.3 million compared a reduction of 0.8 million using the VVL. The NNV to avoid a case of HZ ranged from 6 to 14 using the RZV vaccine and from 21 to 138 using VVL. Figure 5 presents a summary of the context, outcomes and impact of this study for healthcare providers.
Fig. 5.
Focus on the Patient
RZV has only recently been licensed in the USA, Canada, and Japan and, consequently, no effectiveness data are yet available. For consistency, we used VE data from phase III clinical trials for both vaccines to derive parameter estimates for the impact in preventing HZ and its complications. Effectiveness data for ZVL indicated that the observed effectiveness of the VVL against HZ waned from 68.7% (95% CI 63.3–70.9%) in the first year after vaccination to 4.2% (− 24.0–5.9%) in the eighth year, with a similar reduction in both age groups considered (60–69 and ≥ 70 years) [35]. Another study estimated a much slower waning, however, with an effectiveness through year 8 of 31.8% (95% CI 15.1–45.2%) [36]. Estimating the waning of the effectiveness of the VVL on the basis of the observations of Tseng et al. [35] indicated a linear reduction of the effectiveness by about 7.5% per year, contrary to the yearly waning of the VE by about 5.0% assumed in this model exercise.
The vaccination coverage was assumed to be 40%, based on the observed coverage for a pneumococcal polysaccharide vaccine for older adults recently licensed in Japan. Coverage rates for ZVL observed in the USA and UK have ranged between 14.5% in individuals aged ≥ 65 years in the USA [37] and 61.8% in individuals aged ≥ 70 years in the UK [38]. Varying the assumed vaccination coverage has a large impact on the estimate of the differences in the number of HZ cases avoided by using RZV rather than VVL, ranging from approximately 1.3 million cases avoided with a 20% coverage rate to 3.8 million with a 60% coverage rate.
The base-case incidence rates of initial HZ were taken from Takao et al. and ranged from 9.2/1000 PY for individuals aged 50–59 years to 12.9 for the age group 70–79 years old [13]. Using alternative rates from the study by Shiraki et al. [23] with about 33% lower incidence rates in the deterministic sensitivity analyses suggested that the total number of HZ cases avoided with RZV would range between 1.8 and 2.9 million more than with VVL, depending on the boundary of the uncertainty interval used.
In the base-case analysis, the compliance rate with the second-dose of RZV was assumed to be 95%, corresponding to compliance rates observed in the two pivotal clinical trials ZOE-50 and ZOE-70; these studies provide the only data currently available on second-dose compliance [31, 32]. Curran et al. [21] assumed a second-dose compliance rate of 70% in their deterministic sensitivity analysis. Considering that a compliance rate of > 95% for the second- or third-dose of pediatric vaccines in 2014 has been reported in Japan [34], the 70% compliance rate of Curran et al. [21] may be too low a compliance rate for Japanese people willing to be vaccinated against HZ. Nevertheless, we assessed the impact of a second-dose compliance rate of 70% and found that at this compliance rate the RZV vaccine would ensure avoidance of approximately 2.0 million HZ cases more than vaccination with the VVL.
The results of previous studies estimating the public health impact of using the RZV vaccine for prevention of HZ in older adults compared to the ‘no vaccine’ alternative are consistent with our observations [21, 39–43]. In our study we used VE inputs and assumptions in line with those of Curran et al. [21] who, compared to the previous studies, used more robust methods that were validated during an advisory board meeting with international experts. The Curran study also included a comparison between RZV and ZVL for the prevention of HZ in individuals aged ≥ 50 years. Despite differences in the details of model inputs and assumptions, all studies indicate that RZV will provide public health benefits for the prevention of HZ that are superior to those of the VVL. The same results were obtained in most of the scenarios examined in another study performed in Canada, which applied a different model and alternative assumptions—for example, regarding VE and waning [44].
A key limitation of the present study is the uncertainty about the waning rate and the current lack of data on the effectiveness of RZV outside the realm of controlled clinical trials and over the longer term in a clinical practice setting. The projected waning rates used in the model are based on clinical trial data with a follow-up of < 4 years, but we accounted for the inherent uncertainty in using such data as a source for waning estimates by performing sensitivity analyses with maximum waning rates assumed to be twice as high as those assumed in the base case. Even under the most unfavorable assumptions for RZV regarding VE and waning, we estimated that RZV would result in the avoidance of considerably more HZ cases than the VVL and thus have a positive public health impact. Future surveillance studies will be performed when RZV has been licensed and widely adopted in clinical practice, resulting in the generation of data on its effectiveness in real-world practice.
Conclusions
The evidence provided by this study may help policy-makers, clinicians, and health insurers to assess the potential value of RZV in preventing HZ in older adults not only in Japan but in all settings with an unmet need for the prevention of HZ. With the aging of populations around the world and the age-related steep increase in the incidence of HZ, this need is expected to increase in the future [45].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
GlaxoSmithKline Biologicals SA (Rixensart, Belgium) was the funding source and was involved in all study activities and overall data management (collection, analysis, and interpretation). GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical writing and/or editorial assistance
The authors would like to thank Business & Decision Life Sciences platform (on behalf of GSK) for editorial assistance and manuscript coordination. Gregory Collet coordinated manuscript development and provided editorial support and Niels Neymark provided medical writing services.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Conflict of interest
Daisuke Watanabe reports personal fees from the GSK group of companies. Makoto Shiragami reports personal fees from the GSK group of companies. Katsiaryna Holl is an employee of the GSK group of companies and holds stock option or restricted shares. Desmond Curran is an employee of the GSK group of companies and holds stock options or restricted shares. Akiko Mizukami is an employee of the GSK group of companies. Lijoy Varghese is an employee of the GSK group of companies. Désiree Van Oorschot is an employee of the GSK group of companies.
Author’s contribution
Daisuke Watanabe, Akiko Mizukami, Katsiaryna Holl, Desmond Curran, Désiree Van Oorschot, and Makoto Shiragami participated in the conception and design of the study. Daisuke Watanabe, Akiko Mizukami, Katsiaryna Holl, Desmond Curran, Désirée Van Oorschot, and Makoto Shiragami participated in the collection or generation of the study data. Akiko Mizukami, Désirée Van Oorschot, and Lijoy Varghese performed the analysis. Daisuke Watanabe, Akiko Mizukami, Katsiaryna Holl, Desmond Curran, Désirée Van Oorschot, Lijoy Varghese, and Makoto Shiragami were involved in the interpretation of the data. All authors had full access to the data and the corresponding author was responsible for submission of the publication. GSK takes a commitment to convey a message in a way that would be easily understandable by HCPs.
Compliance with ethics guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. If any request for data sharing comes, the requester should be directed to the website https://www.clinicalstudydatarequest.com which is a secured portal through which data can be made available.
Trademark
Shingrix is a trade mark of the GSK group of companies. Zostavax is a trademark from Merck Sharp & Dohme Corp.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced digital features for this article go to 10.6084/m9.figshare.6061838.
References
- 1.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–S7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NIID (National Institute of Infectious Diseases). Infectious Agents Surveillance Report (IASR). 2016. http://www.niid.go.jp/niid/ja/iasr.html. Accessed 30 Nov 2017.
- 3.Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81:928–930. doi: 10.1212/WNL.0b013e3182a3516e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpi A. Severe complications of herpes zoster. Herpes. 2007;14(Suppl. 2):35–39. [PubMed] [Google Scholar]
- 5.Yawn BP, Itzler RF, Wollan PC, Pellisier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc. 2009;84(9):787–794. doi: 10.4065/84.9.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erskine N, Tran H, Levin L, et al. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS One. 2017;12:e0181565. doi: 10.1371/journal.pone.0181565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisson M, Edmunds WJ. Epidemiology of Varicella-Zoster Virus in England and Wales. J Med Virol. 2003;70(Suppl 1):S9–S14. doi: 10.1002/jmv.10313. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez Chiappe S, Sarazin M, Turbelin C, et al. Herpes zoster: burden of disease in France. Vaccine. 2010;28:7933–7938. doi: 10.1016/j.vaccine.2010.09.074. [DOI] [PubMed] [Google Scholar]
- 10.Mahamud A, Marin M, Nickell SP, Shoemaker T, Zhang JX, Bialek SR. Herpes zoster-related deaths in the United States: validity of death certificates and mortality rates, 1979–2007. Clin Infect Dis. 2012;55:960–966. doi: 10.1093/cid/cis575. [DOI] [PubMed] [Google Scholar]
- 11.Mizukami A, Sato K, Adachi K, et al. Impact of herpes zoster and post-herpetic neuralgia on health-related quality of life in Japanese adults aged 60 years or older: Results from a prospective, observational cohort study. Clin Drug Invest. 2018;38:29–37. doi: 10.1007/s40261-017-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura H, Mizukami A, Adachi K, et al. Economic burden of herpes zoster and post-herpetic neuralgia in adults 60 years of age or older: results from a prospective, physician practice-based cohort study in Kushiro, Japan. Drugs Real World Outcomes. 2017;4:187–198. doi: 10.1007/s40801-017-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takao Y, Miyazaki Y, Okeda M, et al. Incidences of herpes zoster and postherpetic neuralgia in Japanese adults aged 50 years and older from a community-based prospective cohort study: the SHEZ study. J Epidemiol. 2015;25:617–625. doi: 10.2188/jea.JE20140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Adachi K, Nakamura H, et al. Burden of herpes zoster and post-herpetic neuralgia in Japanese adults 60 years of age or older: results from an observational, prospective, physician practice-based cohort study. J Dermatol. 2017;44:414–422. doi: 10.1111/1346-8138.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imafuku S, Nakayama J, Higa K, et al. One-year follow-up of zoster-associated pain in 764 immunocompetent patients with acute herpes zoster treated with famciclovir (FAMILIAR study) J Eur Acad Dermatol Venereol. 2014;28:1716–1722. doi: 10.1111/jdv.12379. [DOI] [PubMed] [Google Scholar]
- 16.Ansaldi F, Trucchi C, Alicino C, Paganino C, Orsi A, Icardi G. Real-world effectiveness and safety of a live-attenuated herpes zoster vaccine: a comprehensive review. Adv Ther. 2016;33:1094–1104. doi: 10.1007/s12325-016-0355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T, Isomura S. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–1290. doi: 10.1016/S0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- 18.Gershon AA. Varicella zoster vaccines and their implications for development of HSV vaccines. Virology. 2013;435:29–36. doi: 10.1016/j.virol.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshi SL, Kondo M, Okubo I. Cost-effectiveness of varicella vaccine against herpes zoster and post-herpetic neuralgia for elderly in Japan. Vaccine. 2017;35:3264–3271. doi: 10.1016/j.vaccine.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 20.ACIP (Advisory Committee on Immunization Practices). 2017. https://www.cdc.gov/shingles/vaccination.html. Accessed 6 Feb 2018.
- 21.Curran D, Van Oorschot D, Varghese L, et al. Assessment of the potential public health impact of herpes zoster vaccination. Hum Vacc Immunother. 2017;13:2213–2221. doi: 10.1080/21645515.2017.1345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.e-STAT (The portal site of Official Statistics in Japan) . https://www.e-stat.go.jp/SG1/estat/eStatTopPortal.do. Accessed 6 Feb 2018.
- 23.Shiraki K, Toyama N, Daikoku T, Yajima M, For the Miyazaki Dermatologist Society Herpes zoster and recurrent herpes zoster. Open Forum Inf Dis. 2017;4:ofx7. doi: 10.1093/ofid/ofx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng HF, Chi M, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis. 2012;206:190–196. doi: 10.1093/infdis/jis334. [DOI] [PubMed] [Google Scholar]
- 26.Ultsch B, Weidemann F, Reinhold T, Sieler A, Krause G, Wichmann O. Health economic evaluation of vaccination strategies for the prevention of herpes zoster and postherpetic neuralgia in Germany. BMC Health Serv Res. 2013;13:359. doi: 10.1186/1472-6963-13-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le P, Rothberg M. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med. 2015;163:489–497. doi: 10.7326/M15-0093. [DOI] [PubMed] [Google Scholar]
- 28.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Eng J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 29.Schmader KE, Levin MJ, Gnann JW, Jr, et al. Efficacy, safety and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54:922–928. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60:900–909. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Eng J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Eng J Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 33.Ikematsu H, Yamashita N, Ogawa M, Hirano M, Kovac M, Watanabe D. Efficacy, safety and immunogenicity of a novel adjuvanted subunit herpes zoster vaccine in Japanese aged 50 years and 70 years and older. Kansenshogaku Zasshi. 2018;92:103–114. [Google Scholar]
- 34.MHLW (Ministry of Health, Labour and Welfare). MHLW vaccination information. http://www.mhlw.go.jp/topics/bcg/other/5.html. Accessed 6 Feb 2018.
- 35.Tseng HF, Harpaz R, Luo Y, et al. Declining effectiveness of herpes zoster vaccine in adults aged ≥ 60 years. J Infect Dis. 2016;213:1872–1875. doi: 10.1093/infdis/jiw047. [DOI] [PubMed] [Google Scholar]
- 36.Baxter R, Bartlett J, Fireman B, et al. Long-term effectiveness of the live zoster vaccine in preventing shingles: a cohort study. Am J Epidemiol. 2017;187:161–169. doi: 10.1093/aje/kwx245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang D, Johnson K, Newransky C, Acosta CJ. Herpes zoster coverage in older adults in the US, 2007–2013. AJPM. 2017;52:e17–23. doi: 10.1016/j.amepre.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 38.Public Health England. Herpes zoster (shingles) immunization programme September 2015 to August 2016. Report for England 2016. https://www.gov.uk/government/publications/herpes-zoster-shingles-immunisation-programme-2015-to-2016-evaluation-report. Accessed 30 Nov 2017.
- 39.Varghese L, Curran D, Yan S, Krishnarajah S, Olivieri A. Estimating the potential public impact of introducing the HZ/su vaccine in the US population aged ≥ 50 years. ID Week. 2015. https://idsa.confex.com/idsa/2015/webprogram/Paper52887.html. Accessed 30 Nov 2017.
- 40.Varghese L, Nissen M, Olivieri A, Curran D. Public health perspective of phase III results of an investigational herpes zoster vaccine. In: Public Health Association of Australia—Communicable Disease Control Conference 2015, Brisbane. 2015. p 42. https://www.phaa.net.au/eventsinfo/communicable-disease-control-conference-2. Accessed 15 June 2017.
- 41.Van Oorschot D, Anastassopoulou A, Schlegel K, Varghese L, von Krempelhuber A, Curran D. The public health impact of a new herpes zoster vaccine to the German population. Value Health. 2016;19:A400–A401. [Google Scholar]
- 42.Van Oorschot D, Hunjan M, Varghese L, Canavan C, Curran D. The public health perspective of an investigational herpes zoster vaccine in the United Kingdom (UK) Value Health. 2016;19:400. [Google Scholar]
- 43.Van Oorschot D, Tavares R, Widenmaier R, Varghese L, Curran D. Forecasting the potential public health impact of introducing a new herpes zoster vaccine to the Canadian population. In: Canadian Immunization Conference, Ottawa, 2016, p 12. http://cic-cci.ca/wp-content/uploads/2017/01/CIC16_posterabstracts-1.pdf. Accessed 30 Nov 2017.
- 44.Johnson KD, Jiang Y, Goyette A et al. Comparing the estimated potential health impact of two herpes zoster vaccines in Ontario. In: Canadian Immunization Conference 2016, Ottawa, 2016, p 13. http://cic-cci.ca/wp-content/uploads/2017/01/CIC16_posterabstracts-1.pdf. Accessed 30 Nov 2017.
- 45.Varghese L, Standaert B, Olivieri A, Curran D. The temporal impact of aging on the burden of herpes zoster. BMC Geriatrics. 2017;17:30. doi: 10.1186/s12877-017-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merck Sharp & Dohme Corp. Prescribing information for ZVL. http://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf. Accessed 6 Feb 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. If any request for data sharing comes, the requester should be directed to the website https://www.clinicalstudydatarequest.com which is a secured portal through which data can be made available.