Abstract
Ophiocordyceps is a heterogeneous, species-rich genus in the order Hypocreales (Sordariomycetes, Ascomycota) that includes invertebrate-pathogenic taxa. In this study, seven new species in Ophiocordyceps producing superficial perithecia infecting various insect hosts (Lepidoptera, Hemiptera) are described from Thailand – Ophiocordyceps brunneinigra, O. brunneiperitheciata, O. geometridicola, O. multiperitheciata, O. pauciovoperitheciata, O. pseudoacicularis and O. spataforae. Phylogenetic analyses based on multigene loci comprising the large subunit of the ribosomal DNA (LSU), partial sequences of elongation factor 1-alpha (TEF) and the largest and second largest subunit of the RNA polymerase (RPB1, PRB2) strongly support these new species of Ophiocordyceps in the Ophiocordycipitaceae. They differ from species previously described species Ophiocordyceps acicularis, O. atewensis, O. cochlidiicola, and O. crinalis, in the shape and sizes of distinguishing characters such as perithecia, ascospores and conidia. We also report a new record of O. macroacicularis in Thailand.
Key words: Cryptic species, 7 new taxa, Ophiocordyceps, Taxonomy
Taxonomic novelties: New species: Ophiocordyceps brunneinigra Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, O. brunneiperitheciata Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, O. geometridicola Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, O. multiperitheciata Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, O. pauciovoperitheciata Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, O. pseudoacicularis Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, O. spataforae Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard
Introduction
The occurrence of species complexes – morphologically similar but genetically different cryptic closely related species – can underestimate the diversity for a wide range of taxa in Kingdom Fungi. Molecular studies and barcoding efforts have routinely unmasked so many cryptic species and have revealed this as a common phenomenon in the Fungi (Vilgalys and Sun, 1994, Dettman et al., 2003, Kauserud et al., 2007, Pažoutová et al., 2015). Despite this realization, however, the primary step a mycologist often performs when specimens are collected is to study the phenotypic characteristics and initially classify them based on these traits.
Among invertebrate-pathogenic fungi, the phenotypic traits used to describe species are ascospore morphology, size and shape, and the orientation of perithecia, colour and texture of stromata, conidial shapes and sizes, as well as phialide morphology (Petch, 1934, Mains, 1959, Kobayasi and Shimizu, 1980, Evans et al., 2011, Tasanathai et al., 2016, Luangsa-ard et al., 2017a, Luangsa-ard et al., 2017b). Past classifications and current alpha taxonomy place special emphasis on correlations between character states associated between ascospore and perithecium morphology. Ascospores may remain intact (whole) or disarticulate into part-spores after discharge. Perithecia may be immersed, pseudo-immersed or superficial and may be ordinal (vertically attached) or presented at an oblique angle in arrangement along the stroma.
The occurrence of superficial perithecia is a phenotypic trait that can be found in all three hypocrealean families Clavicipitaceae, Cordycipitaceae and Ophiocordycipitaceae with entomogenous nutritional mode and has arisen multiple times. For example, torrubielloid species in the three families have been distinguished from Cordyceps Fr. species by the presence of superficial perithecia on a very thin subiculum. Some species in Cordycipitaceae, such as Akanthomyces coccidioperitheciatus (Kobayasi & Shimizu) Spatafora, Kepler & B. Shrestha, A. tuberculatus (Lebert) Spatafora, Kepler & B. Shrestha and Cordyceps thaxterii Mains also produce superficial perithecia loosely distributed along the stroma. In Clavicipitaceae, genera on scale insects mainly have superficial perithecia (Conoideocrella D. Johnson, G.H. Sung, Hywel-Jones & Spatafora, Orbiocrella D. Johnson, G.H. Sung, Hywel-Jones & Spatafora) but are formed on thin subiculum on or surrounding the scale insect. However, the majority of species with superficial perithecia are found in the Ophiocordycipitaceae.
Ophiocordyceps Petch is a large genus with 223 accepted species names (Spatafora et al. 2015). It was erected originally by Petch (Petch, 1924, Petch, 1931) for species of Cordyceps having asci with conspicuous apical caps and whole ascospores with distinct septation. The type of Ophiocordyceps is Ophiocordyceps blattae (Petch) Petch (Petch 1931) (Fig. 1). The majority of the species possess darkly pigmented stromata or subiculum, especially those with Hirsutella Pat. asexual morphs while some species produce brightly coloured stromata with Hymenostilbe Petch asexual morph. The stromata can be tough, wiry, fibrous or pliant. Perithecia could be superficial to completely immersed, oblique or ordinal in arrangement. Ascospores usually cylindrical, multiseptate, either disarticulating into part-spores or remain whole after discharge. Asexual morphs associated with Ophiocordyceps include Hirsutella, Hymenostilbe, Paraisaria Samson & B.L. Brady and Syngliocladium Petch (Sung et al. 2007). Quandt et al. (2014) proposed to suppress Hymenostilbe, Paraisaria and Syngliocladium because these genera are younger and contain fewer taxa than Hirsutella or Ophiocordyceps. They also proposed to suppress Hirsutella in favour of Ophiocordyceps due to the unavailability of H. entomophila, the type of Hirsutella, the observation of the Hirsutella-like morphology in Harposporium, and Polycephalomyces, as well as in the Clavicipitaceae (Helicocollum Luangsa-ard, Mongkols., Noisrip. & Thanakitp., Aschersonia Mont.), the differential in number of combinations that were made – 178 for Ophiocordyceps vs. 77 for Hirsutella, and the desire to maintain “Cordyceps” in the name O. sinensis, which is an important fungus in traditional Chinese medicine.
Fig. 1.
Ophiocordyceps blattae, the type species of Ophiocordyceps, on forest cockroach (Blattodea, Blattellidae) from Nam Nao National Park, Phetchabun, Thailand. A. Sexual morph. B. Asexual morph. Scale bars: A, B = 2.5 mm.
Species in Ophiocordyceps are found on a wide range of insect hosts and almost exclusively they are entomopathogenic. Insect orders they attack include Coleoptera, Diptera, Hemiptera, Hymenoptera, Isoptera, Lepidoptera, Neuroptera, and Odonata. Collectively, they can infect various stages of the insect life cycle, from larva, pupa to nymph stages, as well as adults (Kobayasi, 1941, Mains, 1958, Shrestha et al., 2016). Examples of Ophiocordyceps species that infect adult insects are O. irangiensis (Moureau) G.H. Sung et al. and O. halabalaensis Luangsa-ard et al. on ants, O. dipterigena (Berk. & Broome) G.H. Sung et al. on flies, O. nutans (Pat.) G.H. Sung et al. on stinkbugs, O. odonatae (Kobayasi) G.H. Sung et al. on dragonflies, and O. sphecocephala (Klotzsch ex Berk.) G.H. Sung et al. on wasps. Those found mainly on larvae and pupae are O. sinensis (Berk.) G.H. Sung et al., O. acicularis (Ravenel) Petch on Lepidoptera larva, O. ravenelii (Berk. & M.A. Curtis) G.H. Sung et al., and O. melolonthae (Tul. & C. Tul.) G.H. Sung et al. on Coleoptera larva. Stromata of these Ophiocordyceps species occur in exposed environments such as in the leaf litter (O. acicularis, O. nutans, O. irangiensis, O. sphecocephala), on the underside of leaves (O. unilateralis (Tul. & C. Tul.) Petch, O. pseudolloydii (H.C. Evans & Samson) G.H. Sung et al.), on stems and or found buried under the ground (O. acicularis (Ravenel) Petch, O. barnesii (Thwaites) G.H. Sung et al. O. communis Hywel-Jones & Samson) and in wood (O. brunneipunctata (Hywel-Jones) G.H. Sung et al.).
Ophiocordyceps has a panglobal distribution, although the tropics and subtropics seem to be where the highest species diversity has been reported (Petch, 1933, Petch, 1937, Kobayasi, 1941, Tzean et al., 1997, Ban et al., 2015,). Ophiocordyceps sinensis could be found in the high altitudes of the Himalayas up to 4 000 m above sea level (Shrestha and Sung, 2005, Negi et al., 2014). In Thailand alone, 35 species in Ophiocordyceps including asexual morphs have been recorded (BIOTEC Culture Collection).
During our study of the diversity of invertebrate-pathogenic fungi, Ophiocordyceps species with superficial perithecia were found in the leaf litter, on the underside of leaves, as well as on stems of plants in national parks in central and northern Thailand. We investigated the differences of the morphological characters of collected specimens and studied the phylogenetic relationships between these sexual morphs and other members of the genus, such as the asexual morph, Hirsutella, using a combined analysis of four loci (TEF, RPB1, RPB2, LSU).
Materials and methods
Fungal materials and isolation
The specimens for this study were collected in various national parks in central and northern Thailand composed of hill and tropical evergreen forests. The stems, undersides and upper sides of leaves and leaf litter in the forest were scanned for fungi growing on dead insects. Collected materials were placed in plastic boxes and returned to the laboratory. The materials were examined under a stereo microscope (Olympus SZ61). For the isolation of the sexual morph: the fertile head or stroma containing mature perithecia was placed over a potato dextrose agar (PDA: potato 200 g/L, dextrose 20 g/L, agar 20 g/L) plate so that the stroma was above and did not touch the agar surface. These were placed in a plastic box with moist tissue paper and examined daily for discharged ascospores. Discharged ascospores were carefully removed with a sterile needle from the agar and transferred to a new PDA plate. Pure cultures were transferred onto two kinds of media: PDA, potato sucrose agar plus (PSA: potato 200 g/L, sucrose 20 g/L, calcium carbonate 5 g/L, agar 20 g/L) and incubated at 20 °C in the dark for 20 d before being deposited in the BIOTEC Culture Collection (BCC) or the Thailand Biological Resource Center (TBRC). Specimens were either air-dried or dried in an electric food dryer (50–55 °C) overnight and stored in plastic boxes for deposit in the BIOTEC Bangkok Herbarium (BBH). For identification of the insect host, specimens were viewed under the stereo microscope.
In the isolation of the asexual morph: a flame-sterilized inoculation needle was used to pick out conidia from sporulating structures, conidia were transferred to PDA plates and these were incubated in a plastic box at room temperature and examined daily using a stereomicroscope (Olympus SZ61) for germinated conidia. These were then treated in the same way as the sexual morph isolations.
Morphological study
Descriptions of the sexual morph are based on host material, while those of the asexual morph are based on sporulating structures on pure culture. Critical fungal structures for characterization, such as synnemata, perithecia, asci, ascospores, phialides and conidia, were mounted in a lactophenol cotton blue solution and measured using a light microscope (Olympus CX31). Twenty to fifty individual length and width measurements were taken, and the amount of variability is provided as average ± standard deviation with absolute minima and maxima in parentheses. For detailed morphological comparisons of conidia, phialides and colony coloration, cultures were grown on PDA and PSA, incubated at 20 °C in darkness in an incubator within 20 d. The colour of freshly collected specimens, and cultures incubated on PDA, PSA and for 20 d at 20 °C, was described according to Kornerup & Wanscher (1963). Nomenclatural novelties and descriptions were deposited in MycoBank. Insect hosts were examined under the stereomicroscope for identification.
Cultivation of fungi for molecular work
For DNA extraction purposes, starter cultures on PDA plates were prepared. After ca. 2 wk, the plates were checked for contaminants and 5–10 of 5 mm2 agar blocks from pure cultures were inoculated into sterile Erlenmeyer flasks containing 50 mL Sabouraud dextrose broth (Difco) (SDB: peptic digest of animal tissue 5 g/L, pancreatic digest of casein 5 g/L, dextrose 40 g/L) and incubated for 1–2 wk at 20 °C, without shaking. Mycelium was then harvested by filtration and washed several times with sterile distilled water. Filtered mycelium was lyophilized to be used for DNA extraction.
DNA extraction, PCR and sequencing
Genomic DNA was extracted from lyophilized mycelium of fungal strains by a modified CTAB method as previously described (Luangsa-ard et al., 2004, Luangsa-ard et al., 2005). Four nuclear gene regions were amplified and sequenced. Regions sequenced were from the large subunit of the ribosomal DNA (nrLSU), translation elongation factor 1-α (TEF), the largest subunit of RNA polymerase II (RPB1) and the second largest subunit of RNA polymerase II (RPB2). The nrLSU was amplified with the primer pairs LROR and LR7 (Rehner & Samuels 1994). The TEF was amplified and sequenced with the primers 983F and 2218R (Rehner & Buckley 2005). The largest subunit of RNA polymerase II (RPB1) was amplified with the primers CRPB1 and RPB1Cr (Rehner & Samuels 1994). The second largest subunit of RNA polymerase II (RPB2) was amplified and sequenced with the primers RPB2-5F2 and RPB2-7Cr (Rehner & Samuels 1994). Amplifications were done in 50 μl volumes consisting of 1× PCR buffer, 200 μM of each of the four dNTPs, 2.5 mM MgCl2, 0.4 M Betaine, 1 U Taq DNA Polymerase, recombinant (Thermo Scientific, US) and 0.5 μM of each primer. Sequencing primers used were the same as the amplification primers. PCR conditions were set as in Sung et al. (2007).
All PCR products were purified using the QIAquick PCR Purification Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instructions. Purified PCR products were sequenced by Macrogen Inc., Korea.
Sequence alignment and phylogenetic analyses
The sequences generated in this study were supplemented with additional sequences from known literature and GenBank blast searches for the construction of the phylogenetic tree. The list is shown in Table 1. Each generated sequence was checked for ambiguous bases and assembled in BioEdit v.7.2.5 (Hall 2004) and multiple alignment was done using MUSCLE 3.6 software (Edgar 2004) and was manually adjusted. To determine the closest matches with the O. superficialis/O. acicularis group, the DNA sequences of our isolates were compared to sequences in the GenBank database by BLAST search. The final alignment of the combined dataset was used to analyse maximum parsimony (MP), Bayesian criteria and maximum likelihood.
Table 1.
List of specimens and their GenBank accession numbers used in this study.
Parsimony analyses were implemented in PAUP 4.0b10 (Swofford 2002) and heuristic searches were performed using the following options: 100 replicates of random sequence addition, tree bisection reconnection (TBR) branch swapping with MulTrees option in effect. Gaps were treated as missing data and uninformative sequences were excluded from the data prior to analysis. Relative support of the resulting trees were determined by 1 000 bootstrap replications on informative characters only with 10 replicates of random sequence addition and TBR branch swapping algorithm. From these separate analyses all members of clades with 70 % bootstrap support were chosen and sequences were assembled for a combined analysis. Bayesian analysis was performed using MrBayes 3.0b4 (Ronquist & Huelsenbeck 2003) using the same methodologies described in Mongkolsamrit et al. (2009). Prior to this test, MrModeltest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model for use in Bayesian analysis. After the best nucleotide substitution model was determined, Bayesian analysis was conducted using Markov chain Monte Carlo (MCMC) using a GTR+I+G model. Four default chains were sampled every 100 generations and run for a total of 3M generations. Bayesian posterior probabilities (PP) were calculated on the posterior distribution of trees excluding the initial set of burn-in trees. Maximum likelihood analysis was performed using RAxML-HPC2 (Stamatakis 2014) under CIPRES Science Gateway (Miller et al. 2010): nodal supports were assessed with 1 000 replicates of rapid bootstrapping (Stamatakis et al. 2008).
Results
Phylogeny
We obtained 62 new sequences from 17 specimens (Table 1). The combined dataset of 66 taxa and four genes consisted of 3 580 bp (TEF 988 bp, LSU 917 bp, RPB1 705 bp, RPB2 920 bp). Sequences of Orbiocrella petchii (Hywel-Jones) D. Johnson et al. in the Clavicipitaceae were used as the outgroup.
The combined dataset included 3 580 characters, 2 020 of which are constant, 189 are variable and parsimony-uninformative, while 1 371 are parsimony-informative. The maximum parsimony analyses resulted in eleven equally most parsimonious trees, of which one is shown in Fig. 2 (tree length, 5 847 steps; CI, 0.416; RI, 0.727; RC, 0.303; HI, 0.584).
Fig. 2.
Phylogenetic relationships of Ophiocordyceps specimens collected in Thailand with superficial perithecia in comparison with other species of Ophiocordyceps inferred from the analyses of a combined data set for the partial genes LSU, TEF, RPB1 and RPB2 based on Maximum Parsimony, Bayesian analysis and RAxML. Numbers above lines at significant nodes present MP bootstrap values, Bayesian posterior probabilities of 100 and Maximum Likelihood bootstrap values.
The result of MrModeltest selected the General Time Reversible (GTR) model with proportion in invariable sites (I) and gamma distribution (G) (GTR+I+G; Lanave et al. 1984) as the best-fit model by AIC in MrModeltest 2.2. The parameters included base frequencies—A = 2.235, C = 3.122, G = 2.740, T = 1.904—and the rate matrix for the substitution model: [AC] = 0.8818, [A–G] = 2.9135, [A–T] = 0.7804, [C–G] = 0.8889, [C–T] = 5.8768, [G–T] = 1.000. For the among-site variation the proportion of invariable sites (I) was 0.4237, and the gamma distribution shape parameter was 0.7730. This model was used in MrBayes v.3.0B4 and RAxML v. 8.2.10. Bayesian analyses resulted in 3 K “burn-in” trees; the consensus of the remaining 10 K trees resulted in identical topology (−lnL 31844.8164) as the Maximum Parsimony tree.
The phylogenetic trees recovered from maximum parsimony, Bayesian inference and maximum likelihood analyses had identical topologies and similar well-supported clades by bootstrap and posterior probabilities (Fig. 2). A clade comprising Ophiocordyceps species with brightly coloured fleshy terminal fertile stromata producing Hymenostilbe asexual morph that breaks into 64 part-spores is formed sister to Ophiocordyceps species with Hirsutella asexual morphs, mainly producing whole ascospores (Fig. 2). The subterranean species O. rhizoidea (Höhn.) Petch and O. communis Hywel-Jones & Samson are sister taxa to the remaining species in the Hirsutella group. Although the inner nodes with the Hirsutella group do not have good support, seven independent terminal clades having 100 % bootstrap support and posterior probability within the Hirsutella group were formed and thus represent new species in Ophiocordyceps.
Taxonomy
As a result of morphological comparisons and phylogenetic analyses of 17 strains along with sequences from 49 taxa obtained from GenBank, seven new Ophiocordyceps species, and one new record for Thailand are recognized in Fig. 2. This section contains new species descriptions, specimen and culture information, with annotations.
Ophiocordyceps brunneinigra Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard sp. nov. MycoBank MB822031. Fig. 3.
Fig. 3.
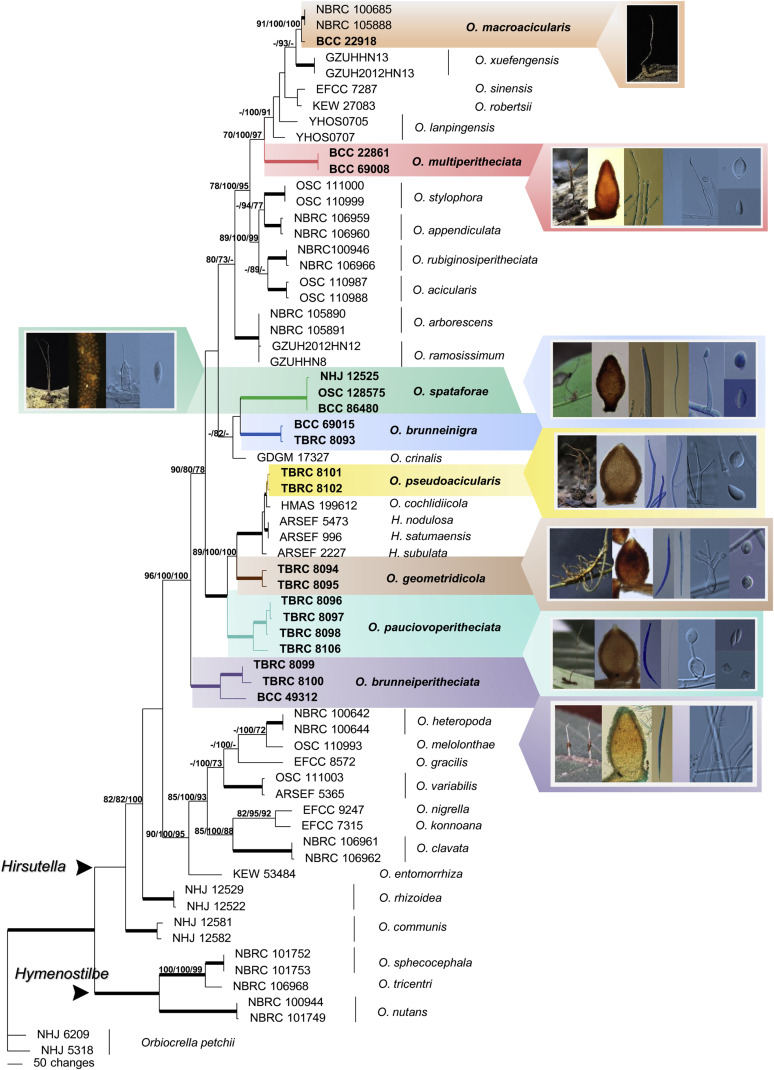
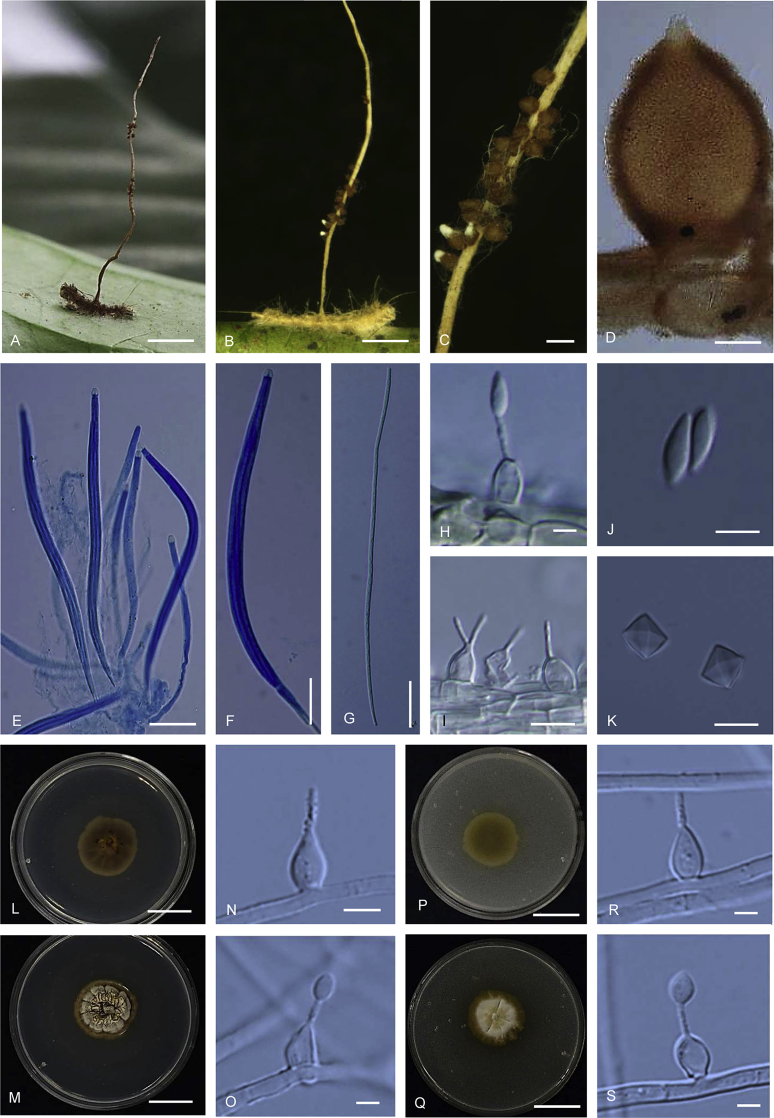
Ophiocordyceps brunneinigra (BBH 38015). A. Stromata arising from host. B. Superficial perithecia. C. Perithecium. D. Asci with ascospores. E. Ascospore. F–H. On PDA, F. Colony reverse. G. Colony obverse. H. Monophialidic conidiogenous cell with conidium. I–L. On PSA, I. Colony reverse. J. Colony obverse. K. Monophialidic conidiogenous cell with conidium. L. Polyphialidic conidiogenous cell. M. Part of stroma showing conidiogenous cells. N. Conidium without mucous sheath showing lemon shaped on PDA. O. Conidia with mucous sheath on PDA. P–Q. Conidia with mucous sheath on PSA. Scale bars: A = 5 mm; B = 10 mm; C = 100 μm; D = 40 μm; E = 30 μm; F, G = 15 mm; H, N, O = 8 μm; I, J = 10 mm; K, L, P, Q = 4 μm; M = 10 μm.
Etymology: Named after the dark brown to black colour of the perithecia
Stroma: Single, cylindrical and flexuous, arising from between the head and the thorax of host, brown-dark brown, 30–40 × 2 mm, unchanged in 3 % KOH. Perithecia superficial, sparse, up to 10, loosely aggregated, arising from middle part of stroma, ovoid with a distinct base, dark brown-black, 560–650 × 200–240 μm. Asci hyaline, cylindrical, 8-spores, 250–320 × 10–15 μm, with thickened apical cap, 6–7 μm in diam. Ascospores hyaline, filiform, 220–300 × 3–5 μm, remain whole after discharge, multiseptate. Conidiogenous structures on the stroma in a discontinuous layer at the terminal end which arise perpendicular to the surface, brown. Conidiogenous cells monophialidic, hyaline, smooth. Phialides (14.5–)15–18(–19) μm, phialide base (8–)8.5–11.5(–13) × 4–5 μm, phialide neck (4.5–)5–7(–9) × 1 μm. Conidia hyaline, smooth, obovoid to falcate, 5–7 × 2–3 μm, embedded in a mucous sheath.
Culture characteristics: Colonies on PDA growing very slowly, funiculose, attaining a diameter of 12–14 mm within 20 d at 20 °C. Colonies orange grey (5A2) to olive (3E8) bearing conidiogenous cells and conidia of the Hirsutella asexual morph. Colony reverse dark brown (6F8) after 30 d. Conidiogenous cells monophialidic, arising from hyphae laterally or terminally, hyaline, smooth, tapering gradually or abruptly into slender neck. Phialides (30–)35.5–43.5(–50) μm, phialide base (16–)23.5–31.5(–35) × 3–3.5(–4) μm, phialide neck (7–)9–14(–18) × 1 μm, rough and warty. Conidia hyaline, 1-celled, smooth-walled, lemon shaped to falcate, (6–)6.5–8(–9) × 3–4 μm, embedded in a mucous sheath. Chlamydospores not observed.
Colonies on PSA growing very slowly, attaining a diameter of 10 mm within 20 d at 20 °C, producing abundant yellowish white (4A2) to greyish yellow (4C7) aerial mycelium on the surface of the medium; reverse yellowish brown (5F8) after 30 d bearing conidiogenous cells and conidia of the Hirsutella asexual morph. Conidiogenous cells monophialidic or polyphialidic, arising from hyphae laterally or terminally, hyaline, smooth. Phialides (18–)22–30(–33) μm, phialide base (13–)16–22(–24) × 4 μm tapering to a thin neck, (5–)5.5–9(–10) × 1 μm, nonseptate or sometimes septate at the base. Conidia hyaline, smooth, ovoid, 6–8 × 2–4 μm surrounded by mucous sheath. Chlamydospores observed on PSA after 20 d.
Habitat: On Hemiptera, Cicadallidae, attached to the underside of living leaves of forest shrubs (Ardisia sanguinolenta) and small trees.
Geographic distribution: Thailand, only from Khao Yai National Park.
Specimens examined: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, at 14°711′N, 101°421′E, on Hemiptera, on the underside of living leaf of forest plant, 27 Nov. 2013, S. Mongkolsamrit, P. Srikitikulchai, A. Khonsanit, W. Noisripoom & D. Thanakitpipattana (holotype BBH 38015; culture ex-type TBRC 8093). Thailand, Nakhon Ratchasima Province, Khao Yai National Park, at 14°711′N, 101°421′E, on Hemiptera, on the underside of living leaf of forest plant, 25 Nov. 2013, S. Mongkolsamrit, P. Srikitikulchai, A. Khonsanit, W. Noisripoom & D. Thanakitpipattana (BBH 37875, BCC 69015).
Note: Ophiocordyceps brunneinigra has a uniquely shaped perithecium. Although it is ovoid, it possesses a prominent, small constriction at the base connecting it to the stroma, like a stand (Fig. 3C). This feature is also present in Cordyceps atewensis Samson, H.C. Evans & Hoekstra. O. brunneinigra resembles C. atewensis in the sparse production of perithecia on a stand, whole filiform ascospores and occur on Hemipteran hosts but differs from C. atewensis in producing small, ovoid perithecia (560–650 × 200–240 μm), containing small cylindrical asci (250–320 × 10–15 μm) and filiform ascospores (220–300 × 3–5 μm). In C. atewensis, the perithecia are also ovoid subglobose but bigger, 600–1100 × 400–600 μm, and the asci are cylindrical to slightly fusiform, 400–600 × 9–12 μm, with whole, multiseptate, filiform ascospores, measuring 5–11 × 3–3.5 μm each cell (Samson et al.1982).
Ophiocordyceps brunneiperitheciata Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, sp. nov. MycoBank MB822099. Fig. 4.
Fig. 4.
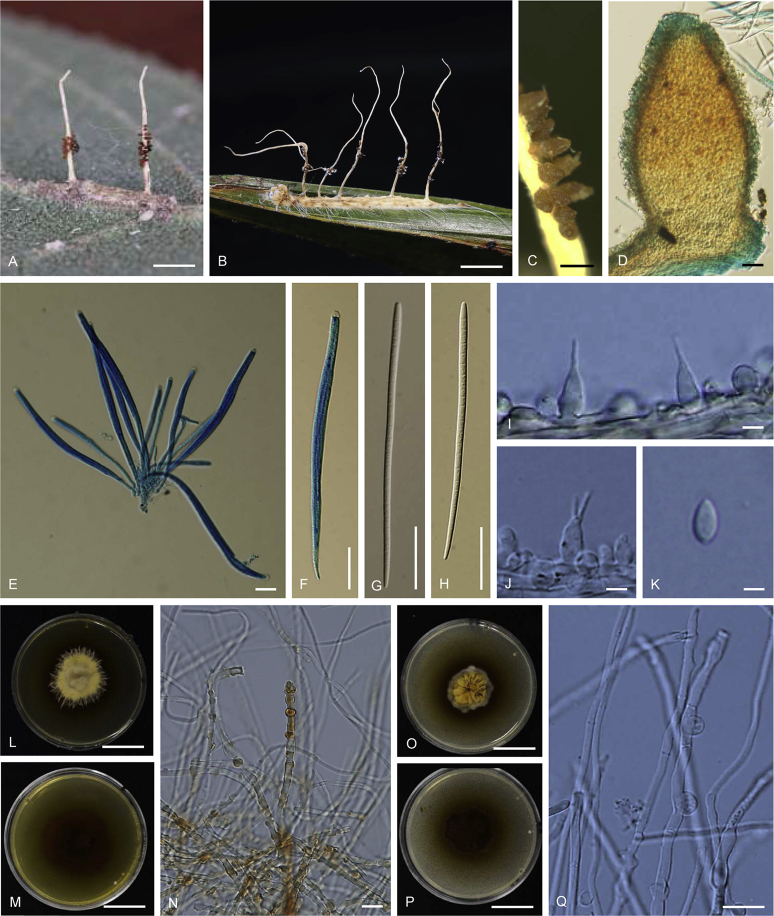
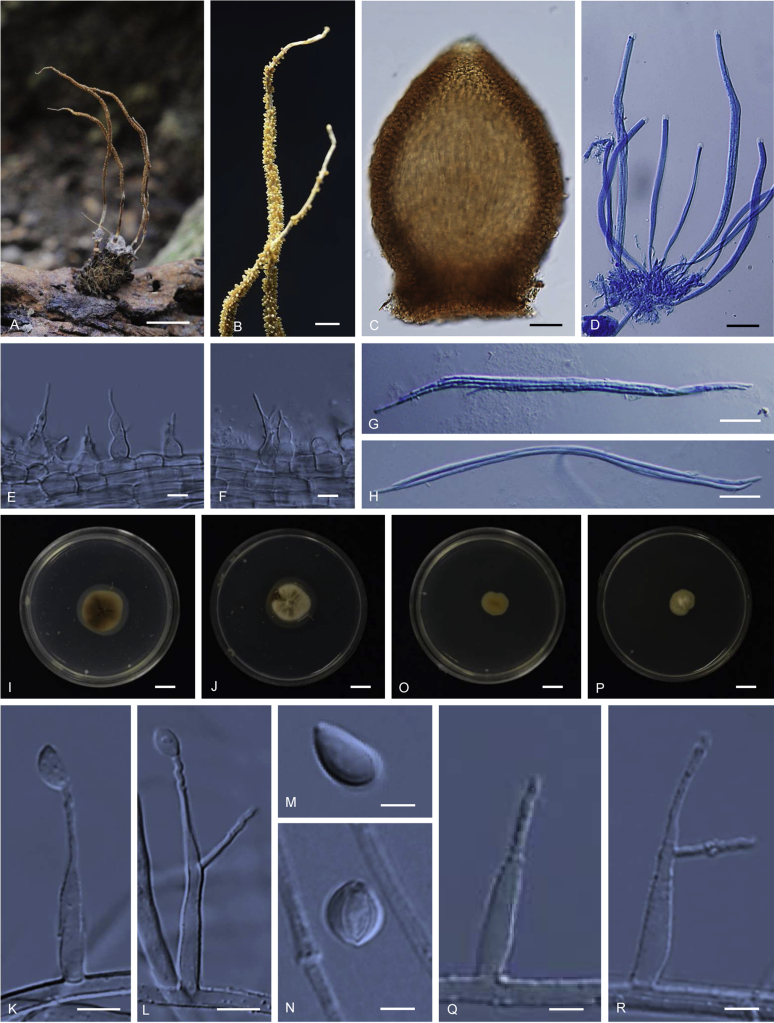
Ophiocordyceps brunneiperitheciata. A–B. Stromata arising from host (A: BBH 36142, B: BBH 38841). C. Superficial perithecia. D. Perithecium. E–F. Asci with ascospores. G–H. Ascospores multiseptate. I–J. Part of stroma showing conidiogenous cells. K. Conidia. L–N. On PDA, L. Colony obverse. M. Colony reverse. N. Chlamydospores. O–Q. On PSA, O. Colony obverse. P. Colony reverse. Q. Chlamydospores. Scale bars: A, B = 2 mm; C = 1 mm; D = 30 μm; E, F = 20 μm; G, H = 30 μm; I = 4 μm; J = 3 μm; K = 2 μm; L, M = 8 mm; N, Q = 10 μm; O, P = 8 mm.
Etymology: Refers to the brown colour of the perithecia on the host.
Stromata: Two to several, simple, wiry to pliant or fibrous, 4–8 mm long, 0.5–1 mm wide on the underside of leaves of forest plants, unchanged in 3 % KOH. Perithecia superficial, sparse, covering the lower middle part of stroma, loosely scattered, ordinal in arrangement, ovoid, brown-dark brown, 350–400 × 180–200 μm. Asci hyaline, cylindrical, 8-spores, 125–175 × 6–8 μm. Ascospores 110–160 × 3–4 μm, filiform, whole, multiseptate.
Asexual morph terminal, pale grey (1B1) to dark grey (1F1). Conidiogenous cells monophialidic, hyaline, smooth, 12–16 μm long, 3–4 μm wide at the base and 0.5–1 μm wide at neck. Conidia hyaline, smooth, fusiform, 4–6 × 2 μm, without a mucous sheath.
Culture characteristics: Colonies on PDA very slow growing attaining a diameter of 8 mm in 20 d at 20 °C. Colonies light yellow (4A4) to reddish yellow (4A7), velvety; colony reverse dark brown (7F8). Colonies on PDA did not produce any conidiogenous structures after 1 mo. Chlamydospores observed on PDA after 1 mo.
Colonies on PSA very slow growing attaining a diameter of 8 mm in 20 d at 20 °C. Similar to colonies on PDA, no asexual morph was seen in culture. Chlamydospores observed on PSA after 1 mo.
Habitat: On small Lepidoptera larvae, 8–10 mm long, 0.5–1 mm wide.
Geographic distribution: Thailand, known from Nam Nao National Park and Hala Bala Wildlife Sanctuary.
Specimens examined: Thailand, Phetchabun Province, Nam Nao National Park, at 16°768′N, 101°671′E, on Lepidoptera larva, 3 Jul. 2013, K. Tasanathai, S. Mongkolsamrit, W. Noisripoom, P. Srikitikulchai & A. Khonsanit (holotype BBH 36142; culture ex-type TBRC 8100). Thailand, Phetchabun Province, Nam Nao National Park, at 16°768′N, 101°671′E, on Lepidoptera larva, 4 Jul. 2013, K. Tasanathai, S. Mongkolsamrit, W. Noisripoom, P. Srikitikulchai & A. Khonsanit (BBH 36150, TBRC 8099), Narathiwat Province, Hala Bala Wildlife Sanctuary, at 5°928′E, 101°883′E, on Lepidoptera larva, 24 Aug. 2011, A. Khonsanit (BBH 38841, BCC 49312).
Note: Ophiocordyceps brunneiperitheciata is similar to O. pauciovoperitheciata in parasitizing small Lepidoptera larvae on the underside of leaves and the production of loosely scattered perithecia on the middle part of the stroma. It differs from O. pauciovoperitheciata in the production of multiple stromata and the colour and shape of the perithecia.
Ophiocordyceps geometridicola Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, sp. nov. MycoBank MB822033. Fig. 5.
Fig. 5.
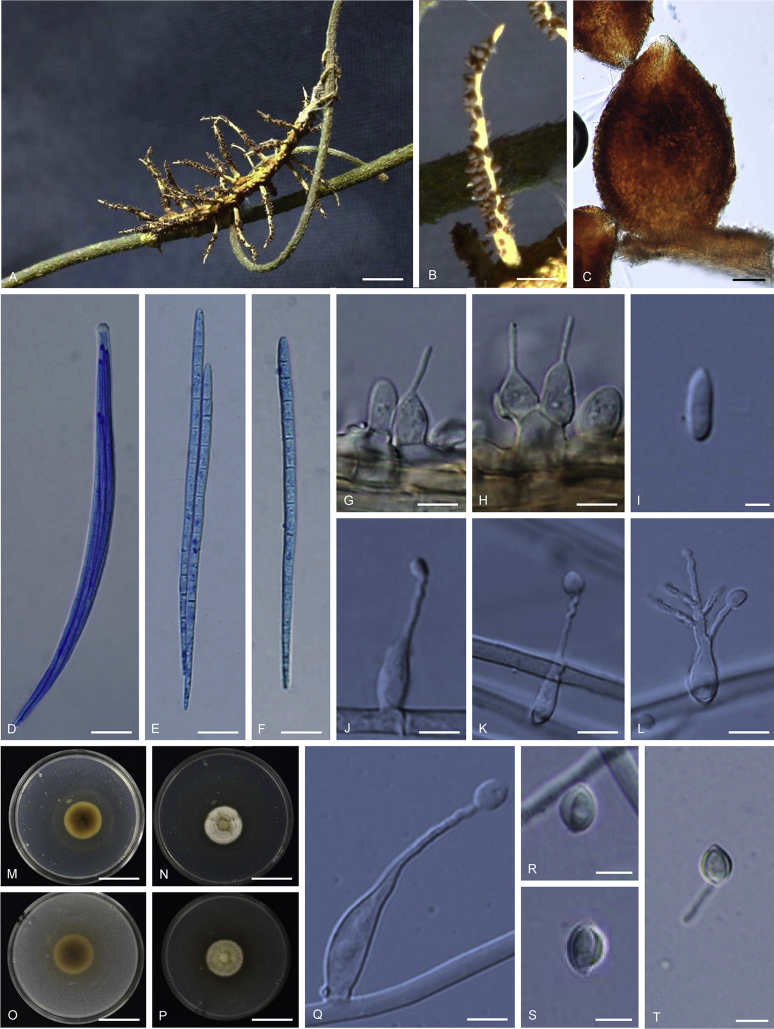
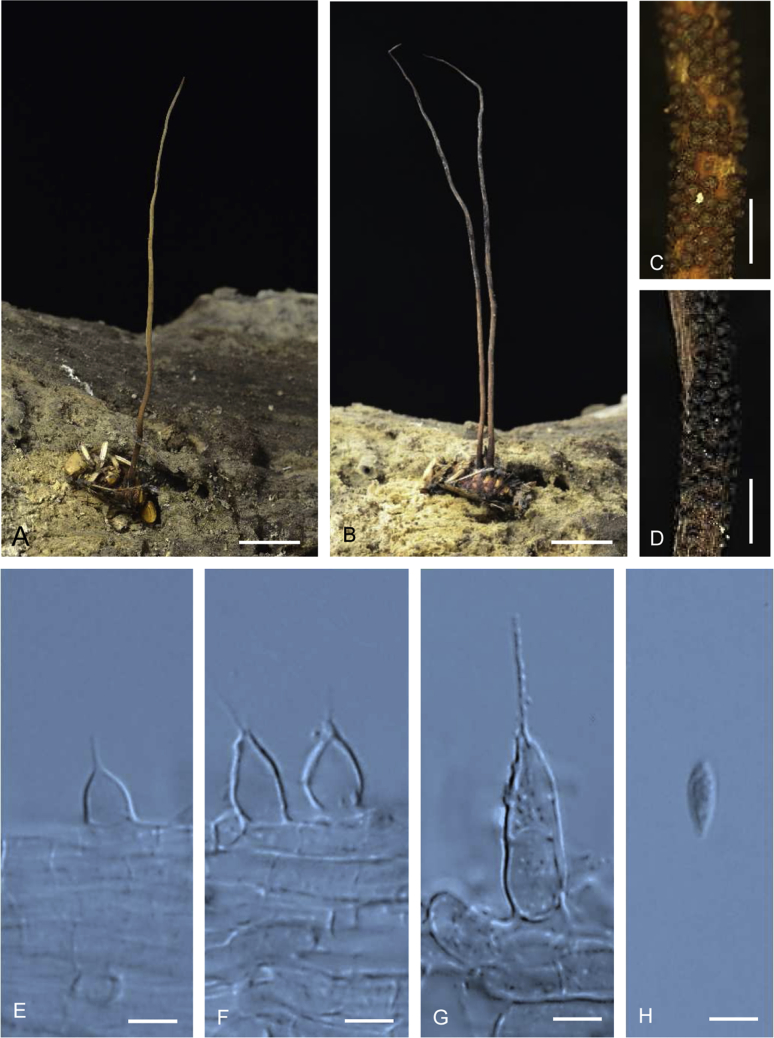
Ophiocordyceps geometridicola (BBH 40665). A. Stromata arising from host. B. Superficial perithecia. C. Perithecium. D. Ascus with ascospores. E–F. Non-disarticulating ascospores. G–H. Part of stroma showing conidiogenous cells. I. Conidium. M–N, J–L. On PDA, M. Colony reverse. N. Colony obverse. J–K. Monophialidic conidiogenous cell with conidia. L. Polyphialidic conidiogenous cell with conidia. O–P, Q–T. On PSA, O. Colony reverse. N. Colony obverse. O. Monophialidic conidiogenous cell with conidia. R–T. Conidia with mucous sheath. Scale bars: A = 5 mm; B = 1 mm; C = 60 μm; D, J, K = 10 μm; E, F = 15 μm; G, H = 5 μm; I = 2 μm; L = 5 μm; M–P = 6 mm; Q = 6 μm; R–T = 4 μm.
Etymology: Refers to the family of Lepidoptera, Geometridae.
Stromata: Numerous, up to 20, simple, wiry to flexuous, 5–8 mm long, 1 mm wide on twig, unchanged in 3 % KOH. Perithecia superficial, loosely aggregated, covering lower part to apex of stroma, ordinal in arrangement, ovoid, brown-dark brown, 280–400 × 240–290 μm. Asci hyaline, cylindrical, 8-spored, 150–190 × 5–8 μm. Ascospores hyaline, cylindrical to vermiform, remaining whole after discharge with distinct septation, 120–150 × 2–2.5 μm.
Asexual morph terminal, pale grey (1B1) to dark grey (1F1), 5–7 mm long, 0.5–1 mm wide, Conidiogenous cells monophialidic, in a continuous layer perpendicular to the stroma, arising from hyphae, hyaline, smooth-walled. Phialides (10–)12–16(–18) μm, phialide base (5–)6.5–9(–12) × 4–5 μm, phialide neck (3–)5–7(–8) × 0.5–1 μm. Conidia hyaline, smooth-walled, fusiform, 6–7(–8) × 2–3 μm, without a mucous sheath.
Culture characteristics: Colonies on PDA, growing very slowly, attaining a diameter of 6 mm within 20 d at 20 °C. Colonies white (2A1) to yellowish white (2A2), velvety to funiculose, colony reverse greyish yellow (1B4) to olive (1E4) bearing conidiogenous cells and conidia of the Hirsutella asexual morph. Conidiogenous cells monophialidic, sometimes polyphialidic, with up to 2 lateral necks arising from hyphae laterally or terminally, hyaline, smooth, tapering gradually or abruptly into a short slender neck. Phialides 30–36(–40) μm, phialide base (16–)17.5–22(–25) × 3–4(–5) μm, phialide neck rough and warty, (10–)11–15.5(–20) × 1 μm. Conidia hyaline, 1-celled, smooth-walled, oval to lemon shaped, 5–6 × 3–4 μm, without a mucous sheath.
Colonies on PSA growing very slowly, attaining a diameter of 6 mm within 20 d at 20 °C. Colonies white (2A1) to yellow white (2A2), funiculose; colony reverse greyish yellow (1B4) to greyish green (1D4). Mycelium hyaline, septate, smooth-walled. Conidiogenous cells monophialidic or polyphialidic, hyaline, tapering gradually or abruptly into a long slender neck. Phialides (22–)24.5–32(–37) μm, phialide base (10–)14–19(–20) × 3–4.5(–6) μm, phialide neck 9–14(–20) × 1 μm, with numerous branching on the neck (Fig. 5L). Conidia hyaline, aseptate, smooth walled, oval to lemon shaped, (3–)4.5–6(–7) × (2–)2.5–4 μm, without a mucous sheath.
Habitat: On Geometridae larva (Lepidoptera) found on twig and stems of forest plants.
Geographic distribution: Thailand, known from Khao Yai National Park and Phlu Kaeng Waterfall, Chiang Rai Province.
Specimens examined: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, at 14°711′N, 101°421′E, on larva of Lepidoptera, on twig of dicotyledonous plant, 8 Sep. 2015, K. Tasanathai, N. Kobmoo, W. Noisripoom & D. Thanakitpipattana, (holotype BBH 40665; culture ex-type TBRC 8095). Thailand, Chiang Rai Province, Phlu Kaeng Waterfall, at 20°26′N, 100°406′E, on larva of Lepidoptera, on twig of dicotyledonous plant, 18 Jan. 2009, K. Tasanathai, S. Mongkolsamrit, T. Chohmee, A. Khonsanit, P. Srikitikulchai & R. Promharn (BBH 31388, TBRC 8094).
Ophiocordyceps multiperitheciata Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard sp. nov. MycoBank MB822030. Fig. 6.
Fig. 6.
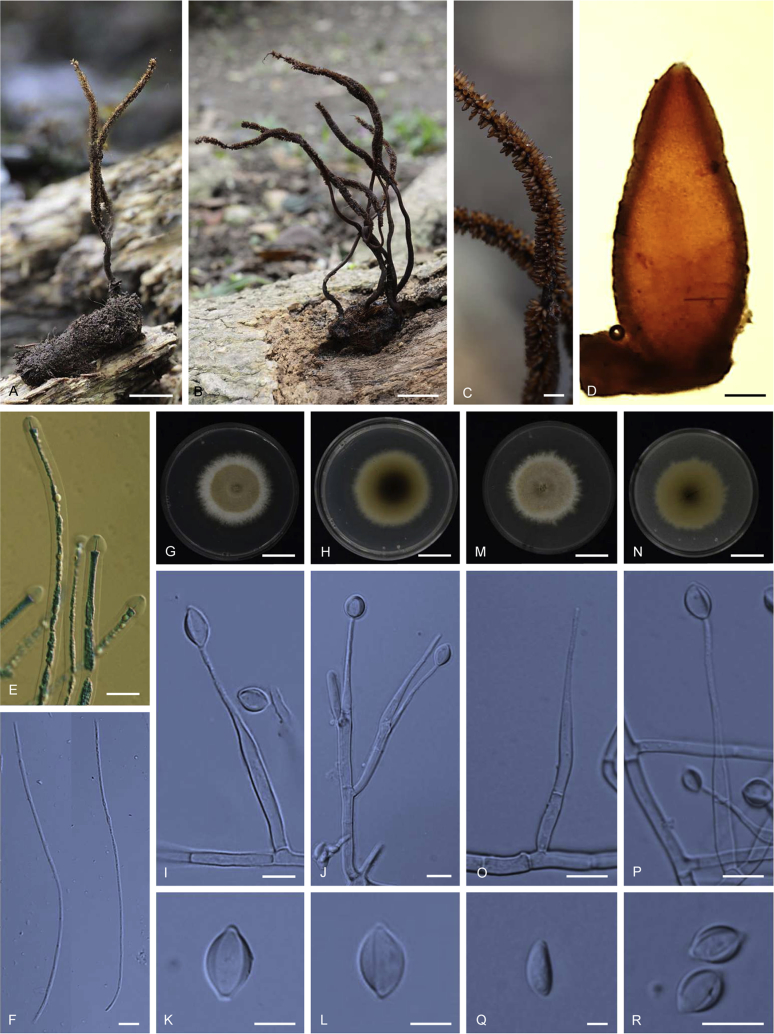
Ophiocordyceps multiperitheciata. A–B. Stromata arising from host (A: BBH 38308, B BBH 18817). C. Superficial perithecia. D. Perithecium. E. Asci. F. Ascospores. G–L. On PDA, G. Colony obverse. H. Colony reverse. I. Monophialidic conidiogenous cell with conidia. J. Polyphialidic conidiogenous cell with conidia. K–L. Conidia with mucous sheath. M–R. On PSA, M. Colony obverse. N. Colony reverse. O–P. Monophialidic conidiogenous cell. Q. Conidium without mucous sheath showing lemon shaped. R. Conidia with mucous sheath. Scale bars: A, B = 15 mm; C = 2 000 μm; D = 150 μm; E = 15 μm; F = 40 μm; G, H, M, N = 10 mm; I, J, O, P, R = 10 μm; K, L = 6 μm; Q = 4 μm.
Etymology: Refers to the multiple perithecia on the stroma.
Stromata: Several, cylindrical, branched dentritic, 75–110 mm long, 1–1.5 mm wide, dark brown (7F8) to black, sometimes with a sterile tip, on larvae of Lepidoptera in the leaf litter, unchanged in 3 % KOH. Perithecia superficial, gregarious, distributing unequally on upper three-fourths of the stroma, ordinal in arrangement, narrowly ovoid, brown (6D6) to dark brown (7F8), 990–1200 × 350–450 μm. Asci hyaline, cylindrical, 8-spores, 400–600 × 6–7.5 μm. Ascospores hyaline, filiform, 470–660 × 1.5–2.5 μm, remaining whole after discharge, multiseptate.
Culture characteristics: Colonies on PDA growing slowly, flat and velvety in the middle, attaining a diameter of 16–22 mm within 20 d at 20 °C, light brown (5D6) in the centre and white (5A1) funiculose growth at the periphery of the colony. Colony reverse olive brown (4F8) in the centre and olive brown (4D7) at the edges. Aerial mycelia dense on the edges of the colony. Conidiogenous cells monophialidic or polyphialidic, arising from hyphae laterally or terminally, hyaline, cylindrical to lanceolate, tapering gradually or abruptly into a long slender neck. Phialides (35–)43–57(–70) μm, phialide base (22–)25–34(–40) × (3–)4–5 μm, phialide neck simple to branched, (12–)16–27(–37) × 1–2 μm, rough to warty, nonseptate or sometimes septate below the phialide base. Conidia hyaline, 1-celled, smooth walled, oval to lemon shaped, (8–)9–11(–14) × (5–)6–7(–8) μm, embedded in a mucous sheath.
Colonies on PSA growing slowly, velvety and flat, attaining a diameter of 20 mm within 2 d at 20 °C, greyish yellow (4C5) to (4C3) with funiculose growth at the edges of the colony. Colony reverse olive brown (4F8) in the center and olive brown (4D7) at the colony edges. Conidiogenous cells monophialidic or polyphialidic, arising from hyphae laterally or terminally, hyaline, cylindrical to lanceolate, tapering gradually or abruptly into a long slender neck. Phialides (41–)50–61.5 (–65) μm, phialide base (25–)27.5–35(–40) × (4–)3.5–4.5(–5) μm, phialide neck rough to warty, (10–)19–28(–31) × 1–1.5 μm. Conidia hyaline, 1-celled, smooth-walled, oval to lemon shaped, (7–)9–10.5(–12) × 4–5 μm, embedded in a mucous sheath, (7–)9–10.5(–12) × (5–)5.5–7.5(–9) μm.
Habitat: On Lepidoptera larva in the leaf litter of forest floor.
Geographic distribution: Thailand, only known from Doi Inthanon National Park.
Specimens examined: Thailand, Chiang Mai Province, Doi Inthanon National Park, at 18°498′N, 98°361′E, on Lepidoptera larva, in the leaf litter, 29 Oct. 2013, K. Tasanathai, P. Srikitikulchai, A. Khonsanit, W. Noisripoom, D. Thanakitpipattana & S. Watcharapayungkit, (holotype BBH 38308; culture ex-type BCC 69008). Thailand, Chiang Mai Province, Doi Inthanon National Park, at 18°498′N, 98°36′E, on Lepidoptera larva, in the leaf litter, 7 Sep. 2006, K. Tasanathai, S. Mongkolsamrit, P. Srikitikulchai, B. Thongnuch, R. Ridkaew & C. Chuaseeharonnachai (BBH 18817, BCC 22861).
Note: Ophiocordyceps multiperitheciata has only been found in Doi Inthanon at 2 000–2 500 m above sea level. It resembles O. macroacicularis S. Ban, T. Sakane & Nakagiri in gross morphology and host but differs in having bigger perithecia and asci (perithecia, 410–760 × 260–420 μm; asci, 235–310 μm). O. multiperitheciata was found in the leaf litter while O. macroacicularis was found on lepidopteran larvae that inhabit the wood or roots of Fallopia japonica Houtt. (Ban et al. 2015).
Ophiocordyceps pauciovoperitheciata Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard, sp. nov. MycoBank MB822034. Fig. 7.
Fig. 7.
Ophiocordyceps pauciovoperitheciata. A–B. Stroma arising from host (A: BBH 29403, B: BBH 32730). C. Superficial perithecia. D. Perithecium. E–F. Asci with ascospores. G. Ascospore. H–I. Part of stroma showing conidiogenous cells with conidia. J. Conidia. K. Crystals on PDA. L–O on PDA, L. Colony reverse. M. Colony obverse. N–O. Monophialidic conidiogenous cell with conidia. P–S on PSA, P. Colony reverse. Q. Colony obverse. R–S. Monophialidic conidiogenous cell with conidia. Scale bars: A, C = 2 mm; B = 4 mm; D = 70 μm; E = 30 μm; F, G = 20 μm; H, I–K, N, O = 5 μm; L, M = 8 mm; P, Q = 10 mm; R, S = 4 μm.
Etymology: Named after the few ovoid shapes of the perithecia along the stroma.
Stroma: Solitary, simple, wiry to pliant or fibrous, arising from any point on the insect, dark grey (1F1)-dark brown (7F7), 14 mm long, 1 mm wide on the underside of leaves of forest plants, unchanged in 3 % KOH. Perithecia superficial, sparse, up to 20, covering middle part to apex of stroma, loosely scattered, vertically placed, ovoid, brown, 300–400 × 210–300 μm. Asci hyaline, cylindrical, 8-spores, 140–220 × 5–7.5 μm. Ascospores 135–215 × 2–3 μm, filiform, remaining whole after discharge, no septation.
Asexual morph, terminal, pale grey (1B1)-dark grey (1F1), 4 mm long, Conidiogenous cell monophialidic, rarely polyphialidic, hyaline, smooth. Phialides (7–)9–10(–11) μm, phialide base (4–)5–6(–7) × (3–)3.5–4(–5) μm, phialide neck (2–)3.5–5 × 0.5–1 μm. Conidia hyaline, smooth-walled, fusiform, 5–7.5(–9) × 2–3 μm, with a mucous sheath.
Culture characteristics: Colonies on PDA growing slowly, attaining a diameter of 10–12 mm within 20 d at 20 °C. Colonies brownish grey (5C2) to yellowish brown (5E8), velvety with depression on the colony, bearing conidiogenous cells and conidia of the Hirsutella asexual morph; colony reverse yellowish brown (5D8-5F8). Conidiogenous cells monophialidic or polyphialidic arising from hyphae laterally or terminally, hyaline, smooth-walled. Phialides (10–)11–15(–16) μm, basal portion globose (5–)6–9(–10) × 4–5.5(–6) μm, phialide neck (2–)4–6.5(–8) × 0.5–1 μm, rough to warty. Conidia hyaline, 1-celled, smooth-walled, oval, 5–6 × 3–4 μm, without a mucous sheath. Formation of crystals observed.
Colonies on PSA growing slowly, attaining a diameter of 12–15 mm within 20 d at 20 °C. Colonies greyish yellow (4C5) to olive brown (4E8), velvety with depression in the colony formation, bearing conidiogenous cells and conidia of the Hirsutella asexual morph; colony reverse dark yellow (4C8) to olive brown (4E8). Conidiogenous cells monophialidic or polyphialidic arising from hyphae laterally or terminally, hyaline, smooth-walled. Phialides (10–)11–15(–17) μm, basal portion globose, 6–9(–10) × (3–)4–5(–6) μm, phialide necks (2–)4–6.5(–9) × 0.5–1 μm, rough and tiny warty. Conidia hyaline, 1-celled, smooth-walled, oval, 5–6 × 3 μm, without a mucous sheath.
Habitat: On small Lepidoptera larva, 7 mm long, 2 mm wide, found on underside of leaves of forest plants.
Geographic distribution: Thailand, only known from Khao Yai National Park.
Specimens examined: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, at 16°203′N, 99°321′E, on Lepidoptera larva on the underside of leaves of dicotyledonous plants, 2 Oct. 2007, K. Tasanathai, S. Mongkolsamrit, P. Srikitikulchai, B. Thongnuch, R. Ridkaew & A. Khonsanit (holotype BBH 29403; culture ex-type TBRC 8106). Thailand, Nakhon Ratchasima Province, Khao Yai National Park, at 14°711′N, 101°421′E, on Lepidoptera larva, 20 June 2012, K. Tasanathai, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, P. Srikitikulchai, K. Sansatchanon & R. Somnuk (BBH 32730, TBRC 8096), Nakhon Ratchasima Province, Khao Yai National Park, at 16°203′N, 99°321′E, on Lepidoptera larva, 7 Oct. 2009, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, T. Chohmee, R. Ridkaew, P. Puyngain & M. Sudhadham (BBH 27112, TBRC 8097), Nakhon Ratchasima Province, Khao Yai National Park, at 14°711′N, 101°421′E, on Lepidoptera larva, 31 Aug. 2010, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, A. Khonsanit, R. Somnuk & K. Sansatchanon (BBH 31409, TBRC 8098).
Note: Ophiocordyceps pauciovoperitheciata shows similarity to O. brunneinigra, O. geometridicola and O. brunneiperitheciata in the production of sparsely distributed perithecia along the stromata. With the exception of O. brunneinigra which occurs on Hemiptera, the three other species are found on the same host (Lepidoptera). O. pauciovoperitheciata differs from the three species in the colour, shape and size of the perithecia.
Ophiocordyceps pseudoacicularis Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard sp. nov. MycoBank MB822032. Fig. 8.
Fig. 8.
Ophiocordyceps pseudoacicularis (BBH 32211). A. Stroma arising from host. B. Superficial perithecia. C. Perithecium. D. Asci with ascospores. E–F. Part of stroma showing conidiogenous cells. G-H. Ascospore. I–N. On PDA, I. Colony reverse. J. Colony obverse. K. Monophialidic conidiogenous cell with conidia. L. Polyphialidic conidiogenous cell with conidium. M. Conidium without mucous sheath. N. Conidium with mucous sheath. O–R. On PSA, O. Colony reverse. P. Colony obverse. Q. Monophialidic conidiogenous cell. R. Polyphialidic conidiogenous cell. Scale bars: A, I, J, O, P = 10 mm; B = 2 mm; C = 50 μm; D = 25 μm; E, L–N, Q, R = 5 μm; F = 7 μm; G, H = 20 μm; K = 6 μm.
Etymology: Named after the similarity to O. acicularis.
Stromata: Solitary to several, cylindrical, 45–70 mm long, 0.5 mm wide, brown to dark brown, on larvae of Lepidoptera in the leaf litter, unchanged in 3 % KOH. Perithecia superficial, densely packed covering lower part and loosely aggregated at the apex of stroma, ordinal in arrangement, ovoid, brown-dark brown, (340–)343.5–386(–420) × (240–)257–292(–310) μm. Asci hyaline, cylindrical, 8-spores, (112.5–)129–187(–225) × 5–7.5 μm. Ascospores hyaline, filiform, 152.5–205 × 2 μm, remain whole after discharge, with septation.
Asexual morph terminal, pale grey (1B1)-dark grey (1F1), 4–5 mm long. Conidiogenous cells monophialidic or polyphialidic, hyaline, smooth. Phialides 12–15(–16) μm, phialide base (6–)6.5–9(–10) × 4–5 μm, phialide neck (2–)4–7(–9) × 0.5–1 μm. Conidia hyaline, smooth, fusiform, 5–6(–7) × 2 μm, surrounded by mucous sheath.
Culture characteristics: Colonies on PDA slow growing, attaining a diameter of 20 mm within 20 d at 20 °C. Colonies pale orange (5A3) to light brown (5E7), velvety, colony reverse pale orange (5A3) to light brown (5E7) after 20 d bearing conidiogenous cells and conidia of the Hirsutella asexual morph. Conidiogenous cells monophialidic or polyphialidic arising from hyphae laterally or terminally, hyaline, smooth. Phialides 22–30 μm, phialide base 12–20 × 3–4 μm, phialide neck, wavy, 8–12 × 1 μm. Conidia hyaline, smooth, obovoid to citriform, 5–7 × 3–4 μm surrounded by mucous sheath. Chlamydospores observed on PDA after 20 d.
Colonies on PSA slow growing, attaining a diameter of 12–15 mm within 20 d at 20 °C. Colonies white (5A1) to pale orange (5A3), colony reverse orange white (5A2) to pale orange (5A3) after 20 d bearing conidiogenous cells and conidia of the Hirsutella asexual morph. Conidiogenous cells monophialidic or polyphialidic arising from hyphae laterally or terminally, hyaline, rough to warty. Phialides (23–)25–34(–40) μm, phialide base (11–)14–21(–25) × 3–3.5 (–4) μm, phialide neck (6–)7.5–15(–20) × 1 μm. Conidia hyaline, oval to lemon shaped, 6–7 × 3–4 μm.
Habitat: On Lepidoptera larva in the leaf litter of forest.
Geographic distribution: Thailand, only known from Khao Yai National Park.
Specimens examined: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, at 14°711′N, 101°421′E, on Lepidoptera larva, on the leaf litter, 9 Jul. 2012, K. Tasanathai, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, P. Srikitikulchai, K. Sansatchanon & R. Somnuk (holotype BBH 32211; culture ex-type TBRC 8102). Thailand, Nakhon Ratchasima Province, Khao Yai National Park, at 14°711′N, 101°421′E, on Lepidoptera larva, on the leaf litter, 3 Aug. 2011, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, A. Khonsanit, K. Sansatchanon & W. Noisripoom (BBH 30689, TBRC 8101).
Note: Ophiocordyceps pseudoacicularis shows similarity to O. acicularis (Ravenel) Petch in the length of the stromata and the size of the perithecia (Petch 1933). It differs from O. acicularis in the presence of slightly smaller ascospores. This species belongs to the Hirsutella nodulosa group while O. acicularis belong to the Hirsutella sinensis group (Simmons et al. 2015). It shares similarities with other species in the same clade by the conidiogenous cells having warty surface and an undulate phialide neck.
Ophiocordyceps spataforae Tasanathai, Thanakitpipattana, Khonsanit & Luangsa-ard sp. nov. MycoBank MB822068. Fig. 9.
Fig. 9.
Ophiocordyceps spataforae. A, B. Stromata arising from host: A. Immature stroma with only Hirsutella asexual state (BBH 43464). B. Stromata containing immature perithecia (BBH 43466). C, D. Part of stroma showing developing superficial perithecia. C. Fresh collection, D. Herbarium material. E–G. Conidiogenous cells. H. Conidium. Scale bars: A, B = 10 mm; C, D = 0.5 mm; E–G = 5 μm; H = 2 μm.
Etymology: In honour of Prof. Joseph W. Spatafora, for his contribution to our knowledge of arthropod-pathogenic fungi.
Habitat: Found on fulgorid planthoppers (Fulgoridae, Hemiptera) and on Coleoptera.
Geographical distribution: Thailand and USA.
The material examined (NHJ 12525, BBH 9271) has immature superficial, brown to dark brown perithecia. Stromata up to two, up to 45 mm long and 0.5 mm wide, cylindrical, cream to pale brown, emerging from the terminal end of the abdomen and between the thorax and abdomen of a fulgorid (Fulgoridae, Hemiptera). Perithecia superficial, ovoid, dark brown. Terminal part of the stroma bearing discontinuous layer of Hirsutella phialides and conidia which are perpendicular to the surface of the stroma. Phialides 9–33 μm, phialide base 5–22 × 4–6 μm, phialide neck 4–11 × 0.5 μm. Conidia hyaline, fusiform to ellipsoidal, herbarium material does not have a mucous sheath due to drying, 5–7 × 1.5–3 μm.
Specimen examined: Thailand, Chanthaburi Province, Khao Soi Dao Wildlife Sanctuary, at 13°102′N, 102°192′E, on fulgorid planthopper, 26 Jul. 2017, W. Himaman, P. Jangsantear & B. Sakolrak (holotype BBH 43466; culture ex-type BCC 86480). Thailand, Chanthaburi Province, Khao Soi Dao Wildlife Sanctuary, on fulgorid planthopper, 20 Jul. 2003, R. Nasit, N.L. Hywel-Jones & JW. Spatafora (BBH 9271).
Notes: Ophiocordyceps spataforae is closely related to O. brunneinigra and O. crinalis (Ellis ex Lloyd) G.H. Sung, J.M. Sung, Hywel-Jones & Spatafora. Both O. spataforae and O. crinalis possess longer stromata compared to O. brunneinigra. However, O. crinalis has numerous stromata compared with both O. spataforae and O. brunneinigra. O. crinalis and O. spataforae were found in the leaf litter while O. brunneinigra was found on the underside of leaves (Wang et al. 2014). In Thailand, O. spataforae was found only on fulgorid plant hoppers while in the USA it was reported on Coleoptera. This is not a common species in Thailand. The first specimen was collected in 2003 while the second sighting and collection was made in 2017.
Discussion
This study advanced our understanding of the genotypic variation among closely related species of Ophiocordyceps with limited morphological differentiation. The degree of morphological variation among various specimens of Ophiocordyceps with superficial perithecia is so low that a continuum seems to exist to accommodate all in a single species. A broader species concept for this group of fungi seem the logical step, an approach advocated by Samson et al. (1982) in the past, but the application of molecular phylogenetics supports that these are distinct evolutionary species, as also shown in the works of Evans et al., 2011, Luangsa-ard et al., 2011, Kobmoo et al., 2012, Araújo et al., 2015 for the O. unilateralis species complex.
To improve species delimitation and resolution in the genus Ophiocordyceps we generated LSU, TEF, RPB1 and RPB2 sequences and added them to published sequences from various taxonomic studies performed for Ophiocordyceps and Hirsutella (Quandt et al., 2014, Ban et al., 2015, Simmons et al., 2015, Spatafora et al., 2015) (Fig. 2, Table 1). The frequency with which cryptic species are unmasked with data from multiple genetic loci (and often subsequently confirmed with morphological and/or ecological data) suggests that multi-gene phylogenies, not only the fungal barcode marker and an additional gene, should be incorporated routinely in taxonomic research. In our analyses using these four combined loci, we recognize seven new species in Ophiocordyceps including one new record of Ophiocordyceps macroacicularis in Thailand. Our study also revealed that O. ramosissimum T.C. Wen, J.C. Kang & K.D. Hyde and O. arborescens S. Ban, Sakane & Nakagiri are conspecific based on analysis of multiple loci. The use of these four loci helped to improve the delimitation of the species, especially in the absence of published data of asexual morphs from the natural specimens. Older records have mostly overlooked or ignored collecting data of the asexual morph, assuming a sterile apex instead of conidia-filled discontinuous layer of conidiogenous cells which arise perpendicular to the surface of a filiform stroma. A significant result of this study is the addition of the number of species in Ophiocordyceps and thus the number of sequenced ex-type cultures and asexual morph records.
Specimens found on the underside of leaves (O. brunneinigra, O. pauciovoperitheciata, O. brunneiperitheciata) produce loosely distributed and sparse perithecia, with up to 30 perithecia developing in the central part of the stromata, while two species found in the leaf litter produce abundant and tightly packed perithecia (O. multiperitheciata, O. pseudoacicularis) starting from the base of the stroma up to the apex. O. spataforae, also found in the leaf litter, was immature and still had very few perithecia along the bottom centre of the stroma. The perithecia of specimens on twigs (O. geometridicola) produce loosely distributed perithecia along the entire stroma.
Ophiocordyceps brunneinigra belong to a well-supported clade with O. crinalis and O. spataforae. It differs from O. spataforae and O. crinalis in the ecology of the host. O. spataforae and O. crinalis are both found in the leaf litter while O. brunneinigra infects insects on the underside of leaves. Three other new species, O. brunneiperitheciata, O. geometridicola and O. pauciovoperitheciata are attached to the underside of leaves like O. brunneinigra. O. brunneinigra and O. pauciovoperitheciata both produce a single filiform stroma with sparsely distributed perithecia. They differ from each other in the host and shape of the perithecia. O. pauciovoperitheciata produces crystals in cultures on PDA while no other newly collected specimen shows this trait. O. brunneiperitheciata produces two or more filiform stromata and does not form conidiogenous cell in culture. It produces chlamydospores on PSA, like O. brunneinigra.
Ophiocordyceps pseudoacicularis grouped with O. cochlidiicola in the Hirsutella nodulosa clade sensu Simmons et al. (2015). It is the second sexual morph to be found in the H. nodulosa group and the macro morphology is similar to O. cochlidiicola. A significant difference in ascospore morphology was revealed following microscopic examination. In our collections O. pseudoacicularis specimens have ascospores that remain intact after discharge whilst in O. cochlidiicola the ascospores readily break into fragments (Kobayasi & Shimizu 1982). They both share the same ecology in that they occur on Lepidoptera larvae in the ground. Two or more morphologically similar and closely related taxa are disjunct in distribution when they are widely separated geographically (Chaverri et al. 2008). This disjunction has been shown as a common event among invertebrate-pathogens, especially in Hypocrella and Moelleriella where and Old World /New World disjunction is sometimes observed (Petch, 1921, Evans, 1982, Evans and Samson, 1984, Evans and Hywel-Jones, 1990, Chaverri et al., 2005a, Chaverri et al., 2005b, Chaverri et al., 2008) or in Nigelia Luangsa-ard, Tasan. & Thanakitp. where a whole ascospore/part-spore disjunction has been reported (Tasanathai et al. 2016).
Ophiocordyceps pseudoacicularis shows similarity with O. acicularis in the length of the cylindrical stromata, the ovoid shape of the perithecia, and the production of whole ascospores. It differs from O. acicularis in the length of the asci and ascospores, and the host. O. pseudoacicularis has shorter asci and ascospores and infect Lepidoptera larvae instead of Coleoptera larvae. O. geometridicola shares similarity with the H. nodulosa group in producing undulate phialides.
One of the most common encountered problems related to Ophiocordyceps taxonomy is the availability of sequence material and cultures from type strains. Out of the 223 species accepted in the genus, only 127 have sequences (Sung et al., 2007, Quandt et al., 2014, Ban et al., 2015, Simmons et al., 2015, Spatafora et al., 2015) and cultures available for comparative studies. The lack of standard media to study growth conditions and macro morphology of the asexual morphs needs to be discussed among the various research groups, and standard criteria established, focussing on invertebrate-pathogens worldwide to facilitate exact comparisons among collections of species deemed to have a cosmopolitan distribution.
Acknowledgements
This work was supported by National Science and Technology Development Agency (Grant no. P15-51424) as well as the European Union’s Horizon 2020 research and innovation programme (RISE) under the Marie Skłodowska-Curie grant agreement No 645701, Project acronym “GoMyTri” (lead beneficiaries JJL and MS). The authors would like to thank Suchada Mongkolsamrit and Wasana Noisripoom for their help in collecting fungi, BIOTEC and CPMO (Mrs. Rungsima Tantalakha) for their support of the biodiversity studies of invertebrate-pathogenic fungi in Thailand. We thank the Department of National Parks for their kind support and permission to collect fungi in the national parks. We are extremely grateful to the two anonymous reviewers for their help and suggestions to improve this manuscript.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Araújo J., Evans H.C., Geiser D.M. Unravelling the diversity behind the Ophiocordyceps unilateralis (Ophiocordycipitaceae) complex: Three new species of zombie-ant fungi from the Brazilian Amazon. Phytotaxa. 2015;220(3):224–238. [Google Scholar]
- Ban S., Sakane T., Nakagiri A. Three new species of Ophiocordyceps and overview of anamorph types in the genus and the family Ophiocordyceps. Mycological Progress. 2015;14:1–12. [Google Scholar]
- Chaverri P., Bischoff J.F., Evans H.C. Regiocrella, a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia. 2005;97:1225–1237. doi: 10.3852/mycologia.97.6.1225. [DOI] [PubMed] [Google Scholar]
- Chaverri P., Bischoff J.F., Hodge K.T. A new species of Hypocrella, H. macrostroma, and its relationship to other species with large stromata. Mycological Research. 2005;109:1268–1275. doi: 10.1017/s0953756205003904. [DOI] [PubMed] [Google Scholar]
- Chaverri P., Liu M., Hodge K.T. A monograph of the entomopathogenic genera Hypocrella, Moelleriella and Samuelsia gen nov. (Ascomycota, Hypocreales, Clavicipitaceae) and their aschersonia-like anamorphs in the Neotropics. Studies in Mycology. 2008;60:1–66. doi: 10.3114/sim.2008.60.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.H., Dai Y.D., Yu H. Systematic analyses of Ophiocordyceps lanpingensis sp. nov., a new species of Ophiocordyceps in China. Microbiological Research. 2013;168:525–532. doi: 10.1016/j.micres.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Dettman J.R., Jacobson D.J., Taylor J.W. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution. 2003;57:2703–2720. doi: 10.1111/j.0014-3820.2003.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H.C. Entomogenous fungi in tropical forest ecosystems: an appraisal. Ecological Entomology. 1982;7:47–60. [Google Scholar]
- Evans H.C., Hywel-Jones N.L. Vth International Colloquium on Invertebrate Pathology and Microbial Control. Society for Invertebrate Pathology; Adelaide, Australia: 1990. Aspects of the genera Hypocrella and Aschersonia as pathogens of coccids and whiteflies; pp. 111–115. [Google Scholar]
- Evans H.C., Samson R.A. Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. II. The Camponotus (Formicinae) complex. Transactions of the British Mycological Society. 1984;82(1):127–150. [Google Scholar]
- Evans H.C., Elliot S.L., Hughes D.P. Hidden diversity behind the Zombie-Ant fungus Ophiocordyceps unilateralis: Four new species described from carpenter ants in Minas Gerais, Brazil. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. Department of Microbiology, North Carolina State University; 2004. BioEdit, version 6.0.7. [Google Scholar]
- Johnson D., Sung G.H., Hywel-Jones N.L. Systematics and evolution of the genus Torrubiella (Hypocreales, Ascomycota) Mycological Research. 2009;113:279–289. doi: 10.1016/j.mycres.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Kauserud H., Svegarden I.B., Decock C. Hybridization among cryptic species of the cellar fungus Coniophora puteana (Basidiomycota) Molecular Ecology. 2007;16:389–399. doi: 10.1111/j.1365-294X.2006.03129.x. [DOI] [PubMed] [Google Scholar]
- Kobayasi Y. The genus Cordyceps and its allies. Science reports of the Tokyo Bunrika Daigaku. 1941;84:53–260. [Google Scholar]
- Kobayasi Y., Shimizu D. Cordyceps species from Japan 2. Bulletin of the National Science Museum, Tokyo. B Botany. 1980;6:77–96. [Google Scholar]
- Kobayasi Y., Shimizu D. Cordyceps species from Japan 4. Bulletin of the National Science Museum, Tokyo Series B. 1982;8:79–91. [Google Scholar]
- Kobmoo N., Mongkolsamrit S., Tasanathai K. Molecular phylogenies reveal host-specific divergence of Ophiocordyceps unilateralis sensu lato following its host ants. Molecular Ecology. 2012;21:3022–3031. doi: 10.1111/j.1365-294X.2012.05574.x. [DOI] [PubMed] [Google Scholar]
- Kornerup A., Wanscher J.H. Methuen & Co. Ltd.; London: 1963. Methuen handbook of color. [Google Scholar]
- Lanave C., Preparata G., Saccone C. A new method for calculating evolutionary substitution rates. Journal of Molecular Evolution. 1984;20:86–93. doi: 10.1007/BF02101990. [DOI] [PubMed] [Google Scholar]
- Luangsa-ard J.J., Hywel-Jones N.L., Samson R.A. The polyphyletic nature of Paecilomyces sensu lato based on 18S-generated rDNA phylogeny. Mycologia. 2004;96:773–780. doi: 10.1080/15572536.2005.11832925. [DOI] [PubMed] [Google Scholar]
- Luangsa-ard J.J., Hywel-Jones N.L., Manoch L. On the relationships of Paecilomyces sect. Isarioidea species. Mycological Research. 2005;109:581–589. doi: 10.1017/s0953756205002741. [DOI] [PubMed] [Google Scholar]
- Luangsa-ard J.J., Houbraken J., Tvan Doorn. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiology Letters. 2011;321:141–149. doi: 10.1111/j.1574-6968.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- Luangsa-ard J.J., Mongkolsamrit S., Thanakitpipattana D. Clavicipitaceous entomopathogens: new species in Metarhizium and a new genus Nigelia. Mycological Progress. 2017;16(4):369–391. [Google Scholar]
- Luangsa-ard J.J., Mongkolsamrit S., Noisripoom W. Helicocollum, a new clavicipitalean genus pathogenic to scale insects (Hemiptera) in Thailand. Mycological Progress. 2017;16(4):419–431. [Google Scholar]
- Mains E.B. North American entomogenous species of Cordyceps. Mycologia. 1958;50:169–222. [Google Scholar]
- Mains E.B. Cordyceps species. Bulletin of the Torrey Botanical Club. 1959;86:46–58. [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. Proceedings of the Gateway Computing Environments Workshop (GCE)New Orleans. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees; pp. 1–8. LA. [Google Scholar]
- Mongkolsamrit S., Luangsa-ard J.J., Spatafora J.W. A combined ITS rDNA and beta-tubulin phylogeny of Thai species of Hypocrella with non-fragmenting ascospores. Mycological Research. 2009;113:684–699. doi: 10.1016/j.mycres.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Negi C.S., Pant M., Joshi P. Yar tsa Gunbu [Ophiocordyceps sinensis (Berk.) G.H. Sung et al.]: the issue of its sustainability. Current Science. 2014;107(5):882–887. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; 2004. MrModeltest 2.2: program distributed by the author. [Google Scholar]
- Pažoutová S., Pešicová K., Chudíčková M. Delimitation of cryptic species inside Claviceps purpurea. Fungal Biology. 2015;119:7–26. doi: 10.1016/j.funbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Petch T. Studies in entomogenous fungi. II. The genera of Hypocrella and Aschersonia. Annals of the Royal Botanic Gardens Peradeniya. 1921;7:167–278. [Google Scholar]
- Petch T. Studies in entomogenous fungus. IV. Some Ceylon Cordyceps. Transactions of the British Mycological Society. 1924;10:28–45. [Google Scholar]
- Petch T. Notes on entomogenous fungi. Transactions of the British Mycological Society. 1931;16:55–75. [Google Scholar]
- Petch T. Notes on entomogenous fungi. Vol XVIII. Part I. Transactions of the British Mycological Society. 1933;16:55–75. [Google Scholar]
- Petch T. Notes on entomogenous fungi. Transactions of the British Mycological Society. 1934;19:160–194. [Google Scholar]
- Petch T. Notes on entomogenous fungi. Vol XXI. Parts I and II. Transactions of the British Mycological Society. 1937;16:55–75. [Google Scholar]
- Quandt C.A., Kepler R.M., Gams W. Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus. 2014;5(1):121–134. doi: 10.5598/imafungus.2014.05.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps. Mycologia. 2005;97(1):84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Rehner S.A., Samuels G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research. 1994;98:625–634. [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Samson R.A., Evans H.C., Hoekstra E.S. Notes on entomogenous fungi from Ghana. Vol VI. The genus Cordyceps. Proceedings of the Koninklijke Nederlandse Akademie Van Wetenschappen. 1982;85:589–605. [Google Scholar]
- Shrestha B., Sung J.M. Notes on Cordyceps species collected from the central region of Nepal. Mycobiology. 2005;33(4):235–239. doi: 10.4489/MYCO.2005.33.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B., Tanaka E., Hyun M.W. Coleopteran and Lepidopteran hosts of the entomopathogenic genus Cordyceps sensu lato. Journal of Mycology. 2016;2016:1–14. [Google Scholar]
- Simmons D.R., Kepler R.M., Rehner S.A. Phylogeny of Hirsutella species (Ophiocordycipitaceae) from the USA: remedying the paucity of Hirsutella sequence data. IMA Fungus. 2015;6(2):345–356. doi: 10.5598/imafungus.2015.06.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatafora J.W., Quandt C.A., Kepler R.M. New 1F1N species combinations in Ophiocordycipitaceae (Hypocreales) IMA Fungus. 2015;6(2):357–362. doi: 10.5598/imafungus.2015.06.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Sung G.H., Hywel-Jones N.L., Sung J.M. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland: 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. [Google Scholar]
- Tasanathai K., Thanakitpipattana D., Noisripoom W. Two new Cordyceps species from a community forest in Thailand. Mycological Progress. 2016;15:28. [Google Scholar]
- Tzean S.S., Hsieh L.S., Wu W.J. Council of Agriculture, Executive Yuan; Taiwan: 1997. Atlas of entomopathogenic fungi from Taiwan. [Google Scholar]
- Vilgalys R., Sun B.L. Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4599–4603. doi: 10.1073/pnas.91.10.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li H.H., Chen Y.Q. Polycephalomyces lianzhouensis sp. nov., a new species, co-occurs with Ophiocordyceps crinalis. Mycological Progress. 2014;13:1089–1096. [Google Scholar]
- Wen T.C., Zhu R.C., Kang J.C. Ophiocordyceps xuefengensis sp. nov. from larvae of Phassus nodus (Hepialidae) in Hunan Province, southern China. Phytotaxa. 2013;123(1):41–50. [Google Scholar]
- Wen T.C., Xiao Y.P., Li W.J. Systematic analyses of Ophiocordyceps ramosissimum sp. nov., a new species from a larvae of Hepialidae in China. Phytotaxa. 2014;161(3):227–234. [Google Scholar]