Abstract
Lung cancer is notorious for high morbidity and mortality around the world. Interleukin (IL)-8, a proinflammatory chemokine with tumorigenic and proangiogenic effects, promotes lung cancer cells growth and migration and contributes to cell aggressive phenotypes. Integrin αvβ6 is a receptor of transmembrane heterodimeric cell surface adhesion, and its overexpression correlates with poor survival from non–small cell lung cancer. However, the cross talk between αvβ6 and IL-8 in lung cancer has not been characterized so far. Herein, human lung cancer samples were analyzed, and it revealed that the immunohistochemical and mRNA expression of integrin αvβ6 was significantly correlated with the expression of IL-8. Furthermore, in vitro, integrin αvβ6 increased cell proliferation, migration, and invasion by impairing the expressions of MMP-2 and MMP-9 and inhibited cell apoptosis in human lung cancer cells A549 and H460. In addition, integrin αvβ6 upregulated IL-8 expression through activating MAPK/ERK signaling. The in vivo experiment showed that integrin αvβ6 promoted tumor growth in xenograft model mice by accelerating tumor volume and reducing apoptosis. Meanwhile, lung metastasis model experiment suggested that integrin αvβ6 stimulated tumor metastasis with the increase of lung/total weight and tumor nodules. Simultaneously, integrin αvβ6 upregulated IL-8 expression detected by both Western blots and immunohistochemistry, along with the activation of MAPK/ERK signaling. Overall, these data suggested that, in vitro and in vivo, integrin αvβ6 promoted lung cancer proliferation and metastasis, at least in part, through upregulation of IL-8–mediated MAPK/ERK signaling. Thus, the inhibition of integrin αvβ6 and IL-8 may be the key for the treatment of lung cancer.
Introduction
Lung cancer is one of the most common types of malignancies worldwide, and the two major types consist of small cell lung cancer and non–small cell lung cancer (NSCLC), the later accounting for approximately 85% of lung cancers [1]. At present, the most effective therapy to lung cancer is complete lung resection plus appropriate chemotherapeutic strategy [2]. Nevertheless, lung cancer frequently metastasizes to bone, brain, lung, and liver, causing a shorter survival and deaths [3].
Interleukin (IL)-8, a proinflammatory chemokine with tumorigenic and proangiogenic effects, can be detected in many types of malignant tumors, including lung cancer [4]. Studies have revealed that IL-8 increased cancer stem cell populations and promoted the adhesion, migration, and invasion of gastric and breast cancer cells [5], [6], [7], [8]. Besides, IL-8 overexpression promoted NCI-H1792 NSCLC cell growth and migration and contributed to cell aggressive phenotypes [9]. However, the mechanism involved in IL-8 resulting from lung cancer is still unclear.
Integrins, a family of transmembrane heterodimeric cell surface adhesion receptors, are involved in many cellular functions [10]. Integrins modulate cellular functions depending on the cellular and microenvironmental context, both in physiological and in pathological conditions including cancer [11]. The αvβ6, expressed only by epithelial cells, is usually only detectable on cells undergoing tissue remodeling, including wound healing and cancer [12]. Integrin αvβ6 promotes invasion of carcinoma cells, and its overexpression correlates with poor survival from colon, cervix, and NSCLC [13], [14], [15]. Meanwhile, IL-8 could enhance the migration of colorectal cancer cells by increasing αvβ6 integrin expression through the ERK/Ets-1 pathway [16]. However, the association of integrin αvβ6 with IL-8 in lung cancer has not been characterized so far. Herein, this study was to investigate the association and the mechanism between integrin αvβ6 and IL-8 in lung cancer.
Material and Methods
Chemicals and Reagents
Fetal bovine serum, RPMI-1640 medium, and trypsin were from the United States GIBCO company. Matrigel was purchased from BD Transduction Laboratories (Lexington, KY). Becton Dickinson (Biosciences, San Jose, California). Antibodies against MMP-2, MMP-9, αvβ6, IL-8, ERK, phospho (p)-ERK, JNK, p-JNK, p-38, p-p38, and GAPDH were purchased from Cell Signaling Technology (Beverly, MA, USA). Secondary antibodies for goat-anti-rabbit immunoglobulin G and donkey anti-rabbit IgG-labeled were from Abcam (Cambridge, USA).
Tissue Specimens
Cancer tissues were collected from 60 patients (early stage, n = 20, middle stage, n = 20 and late stage, n = 20) with lung cancer who underwent surgery at the Department of Jiangsu Cancer Hospital (Nanjing Medical University, China). The lung tissue was determined by immunohistochemical analysis. Written informed consents were obtained from all patients, and the study protocol was approved by the Institutional Ethics Committee of Nanjing Medical University.
Immunohistochemistry Assay
Lung samples were freshly isolated and fixed in 10% neutral buffered formalin and then embedded in paraffin wax. Lung sections with a thickness of 4 μm were mounted onto slides. Slides were deparaffinized with xylene, rehydrated with ethanol, and incubated with H2O2 at 37°C for 10 minutes. Following blocking using 1.5% normal goat serum (Shanghai Yeasen Biotechnology Co., Ltd.) at 37°C for 20 minutes, sections were incubated overnight with αvβ6 or IL-8 monoclonal antibody (1:1000 dilutions). The sections were incubated with biotin-conjugated goat-anti-rabbit immunoglobulin G secondary antibody (diluted with 3% bovine serum albumin/PBS) at 37°C for 30 minutes and then incubated with horseradish peroxidase–conjugated streptavidin at 37°C for 30 minutes. 3,3’-Diaminobenzidine (DAB) was used as chromogenic agent. Images were obtained using a fluorescence microscope (FSX100; Olympus, Southend-on-Sea, UK).
RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated by TRIzol according to the manufacturer's protocol. Equal amounts of RNA were transcribed into cDNA using RNeasy plus micro kit. The total cDNA was used as starting material for real-time PCR with FastStart Universal SYBR Green Master (Roche Applied Science, Mannheim, Germany) on the StepOne real-time PCR System (Life Technologies Corp.). The Primer Premier software (PREMIER Biosoft International, USA) was used to design specific primers for integrin αvβ6, IL-8, and GAPDH based on known sequences. The primers for integrin αvβ6 were 5′-TTCCTAATGACGGGCTCTG-3′ (forward) and 5′-TTGGGTTACAGCGAAGATCA-3′ (reverse). The primers for IL-8 were 5′-CAATCCTAGTTTGATA CTCCC-3′ (forward) and 5′-AATTACTAATATTGACTGTGGAG-3′ (reverse). The expression levels of each target gene were normalized to corresponding GAPDH threshold cycle (CT) values using the 2−ΔΔCT comparative method.
Cell Culture
The human lung cancer cell lines A549 and H460 were purchased from the American Type Tissue Culture Collection (Manassas, VA). The cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and streptomycin (Sigma-Aldrich, St. Louis, MO), in a humidified atmosphere containing 5% CO2 at 37°C.
siRNA Transfection
Integrin αvβ6 siRNA and nonspecific control siRNA duplexes were designed and purchased from Dharmacon RNA Technologies (Chicago, IL). A549 (1 × 105 cells) and H460 (3 × 105 cells) were seeded in six-well plates, incubated overnight, and transfected with siRNAs using Lipofectamine 2000 (Life Technologies).
MTT Assay
To the transfected A549 and H460 cells was added 20 μl of a 5-mg/ml MTT solution to each well, and the plate was further incubated at 37°C for 4 hours. Thereafter, the medium was aspirated and the wells washed with PBS; 150 μl of DMSO was added to each well. The microtiter plate was placed on a shaker in order to dissolve the dye. The absorbance was determined spectrophotometrically at 490 nm on an ELX800 UV universal microplate reader after the formazan crystals had dissolved.
Apoptosis Analysis
The effect of integrin αvβ6 on the apoptosis of A549 and H460 cells was evaluated by flow cytometry using the Annexin V PE Apoptosis kit (BD Pharmingen, USA). Firstly, the transfected cells were washed by 1× PBS (4°C) followed by resuspending the cell pellet with 300 μl of 1× Binding Buffer. Next, 5 μl of Annexin V-PE was added to the cell suspension for 15 minutes in the dark at room temperature, according to the manufacturer's instructions. Five microliters of 7-AAD solution was added in the cell suspension 5 minutes before flow cytometry analysis, and then 200 μl of 1× Binding Buffer was added for flow cytometry analysis. The percentage of apoptotic cells was evaluated by FACS Calibur (BD Biosciences, USA).
Cell Migration Assay
The cell migration assay was carried out using Transwell chambers (8-μm pore size, Corning Costar, Cambridge, MA) without Matrigel. The transfected cells (1.0 × 105 cells/chamber) were seeded in the upper chamber and incubated for 24 hours at 37°C, 5% CO2. FBS (20%), acting as a chemoattractant, was placed in the lower chambers. After incubation, all of the noninvaded cells on the upper surface were removed with a cotton swab; the invaded cells on the lower surface were fixed with 100% methanol and then stained with 1% crystal violet. The migrated cells were counted with a microscope, and six randomly chosen fields were counted for each assay.
Cell Invasion Assay
The cell invasion assay was carried out using Transwell chambers coated with Matrigel. The transfected cells (1.0 × 105 cells/chamber) were seeded in the upper chamber and incubated for 24 hours at 37°C, 5% CO2. FBS (20%), acting as a chemoattractant, was placed in the lower chambers. After incubation, all of the noninvaded cells on the upper surface were removed with a cotton swab; the invaded cells on the lower surface were fixed with 100% methanol and then stained with 1% crystal violet. The invaded cells were counted with a microscope, and six randomly chosen fields were counted for each assay.
Western Blot Analysis
The total proteins of cells and lung tissue were extracted according to the manufacturer's recommended protocol (Vazyme, USA). The protein concentrations were determined using the BCA Protein Assay Kit (Vazyme, USA). Samples with equal amounts of protein (25 μg) were fractionated on 10% SDS polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and blocked in 5% skim milk in TBST for 1.5 hour at 25°C ± 1°C. The membranes were then incubated at 4°C overnight with 1:1000 dilutions (v/v) of the primary antibodies. After washing the membranes with TBST, incubations with 1:1000 dilutions (v/v) of the secondary antibodies were conducted for 2 hours at 25°C ± 1°C. Protein expression was detected using an Enhanced Chemiluminescence Detection System. GAPDH was used as a loading control.
Luciferase Assay
The 1796-bp fragment of human integrin β3 promoter was generated by PCR amplification from genomic DNA of MDA-MB-231 cells and subcloned into pGL3-basic luciferase reporter plasmid (Promega). MAPK/ERK siRNA and control siRNA were purchased from Cell Signaling (Beverly, MA). pRL-TK vector was used as internal control. Transfection of siRNA was performed using Lipofectamine 2000 (Life Technologies) regent according to the manufacturer’s instructions. Six hours later, the cells were washed and incubated in complete medium for 36 hours; then the cells were lysed, and lysates were assayed for luciferase activity using Dual-Glo Luciferase assay system (Promega) on an Ultra Multifunctional Microplate Reader (Tecan).
Animal Model
Female nude mice (6 weeks old, 18-22 g) were obtained from Shanghai Jiesijie Experimental Animal Company. Mice were given free access to water and standard rodent chow and were housed in pathogen-free cages. The animals were acclimated for a week before use. Animal welfare and experimental procedures complied with national guidelines and were approved by the Animal Experimental Ethical Committee of Nanjing Medical University.
Mice were randomly divided into three groups: Control (PBS), Control-siRNA (nonspecific control siRNA), and Integrin αvβ6-siRNA (integrin αvβ6-siRNA).
Xenograft model: After being anesthetized by inhalation, mice were inoculated with cells (100 μL of 1 × 106 cells) into the right axilla of the mice. And then mice were sacrificed on day 28, the subcutaneous tumors were removed, and the tumor volume was calculated.
Lung metastasis model: Mice were injected 100 μL of 1 × 105 cells into the median tail vein. Then mice were sacrificed on day 21, and the lungs were removed and weighed.
TUNEL Staining
The apoptosis of paraffin-embedded tumor sections was detected using a TUNEL assay kit according to the manufacturer’s manual (Roche, USA). In brief, fixed and paraffin-embedded sections were dewaxed then permeabilized with proteinase K for 15 minutes at room temperature. Sections were treated with 3% H2O2 to block endogenous peroxidases and incubated with equilibration buffer and terminal deoxynucleotidyl transferase enzyme. Finally, sections were incubated with antidigoxigenin-peroxidase conjugate. Tissue peroxidase activity was evaluated through DAB application. Sections were examined under a light microscope.
Histological Assay
The lung tissues were obtained, fixed in 10% formalin, and then stained by hematoxylin-eosin staining. Ten random areas of interest were examined in each section and were identified by computer-generated field identification. At least six different sections of lung tissues were examined for each animal in groups. Images were obtained using a fluorescence microscope.
Statistical Analysis
GraphPad Prism 5 software was used to carry out all statistical analysis. One-way analysis of variance was used for multiple group comparison; when only two groups were compared, Student’s t test was performed. P values of < .05 were considered statistically significant.
Results
Integrin αvβ6 is Positively Correlated with IL-8 in Lung Cancer Tissues
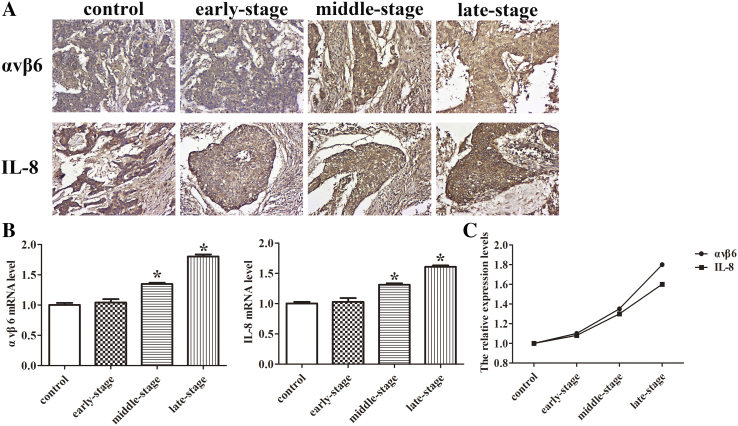
To evaluate whether integrin αvβ6 expression was correlated to IL-8 expression, the early-stage, middle-stage, and late-stage lung tumor samples were detected. The immunohistochemistry assay and qRT-PCR analysis both showed that the expressions of integrin αvβ6 and IL-8 were elevated with the severity of lung cancer (Figure 1, A-B). The relative expression levels showed the positive correlation between integrin αvβ6 and IL-8 expression in lung cancer tissues (Figure 1C).
Figure 1.
Integrin αvβ6 expression is positively correlated with IL-8 expression in lung cancer tissues. (A) The immunohistochemistry assay was used to determine the expressions of integrin αvβ6 and IL-8 in lung cancer tissues. (B) The mRNA levels of integrin αvβ6 and IL-8 in lung cancer tissues were detected by qRT-PCR analysis. GAPDH was used as a control. (C) The relative expression levels between integrin αvβ6 and IL-8 expression. Bars indicate the mean ± SEM, *P <.05 vs. control group.
Integrin αvβ6 Increases IL-8 Expression in Lung Cancer Cells
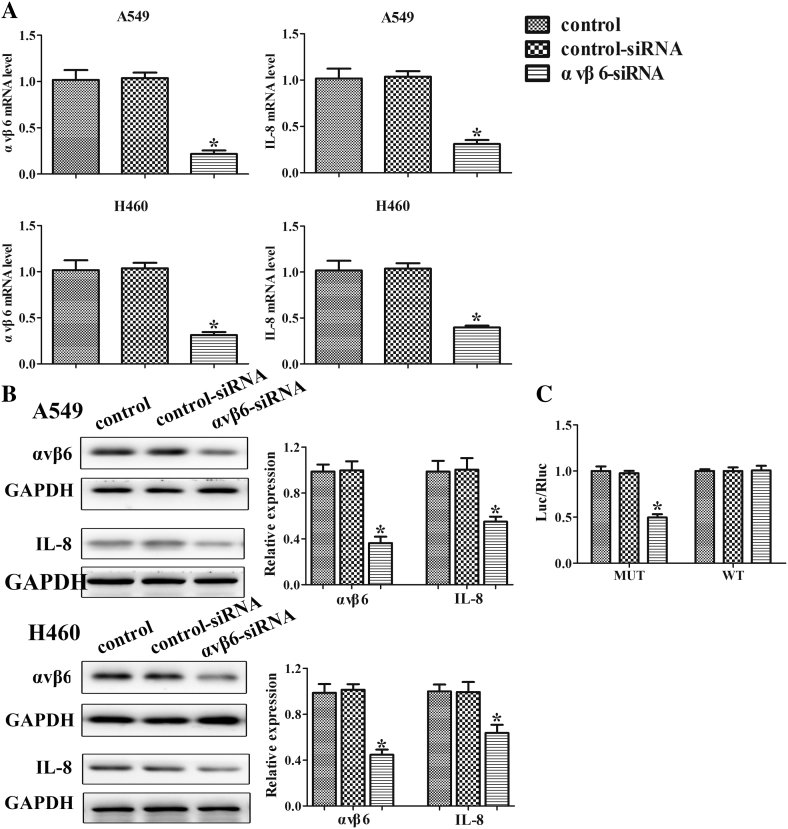
To examine whether integrin αvβ6 regulates IL-8 expression in lung cancer cells, we transfected A549 and H460 cells with integrin αvβ6 siRNA or nonspecific control siRNA. Integrin αvβ6 siRNA led to 79% and 65% reduction in αvβ6 mRNA level in A549 and H460 cells, respectively (Figure 2A). The data demonstrate that integrin αvβ6 siRNA was efficient and specific to knockdown αvβ6 in lung cancer cells. Notably, we found that knockdown of αvβ6 suppressed IL-8 expression at mRNA level in A549 and H460 cells (60% and 55% decrease compared to the controls) (Figure 2A). Knockdown of αvβ6 also reduced IL-8 expression at protein level in A549 and H460 cells (Figure 2B). Furthermore, the luciferase assay showed that the luciferase activity driven by IL-8 promoter was reduced in integrin αvβ6 siRNA transfected cells (Figure 2C). Taken together, these data provide evidence that integrin αvβ6 upregulates IL-8 expression in lung cancer cells specifically.
Figure 2.
Integrin αvβ6 increases IL-8 expression in lung cancer cells. A549 and H460 cells were transfected with integrin αvβ6 siRNA or nonspecific control siRNA. (A) The mRNA levels of integrin αvβ6 and IL-8 in lung cancer cells A549 and H460 were detected by qRT-PCR analysis. GAPDH was used as a control. (B) Western blots were performed to detect protein levels of MAPK along with their phosphorylation. GAPDH was used as a control. (C) Luciferase activity was detected in A549 cells. Bars indicate the mean ± SEM, *P < .05 vs. control group.
Integrin αvβ6 Promotes the Proliferation and Inhibits Apoptosis of Lung Cancer Cells Partially Through the Upregulation of IL-8
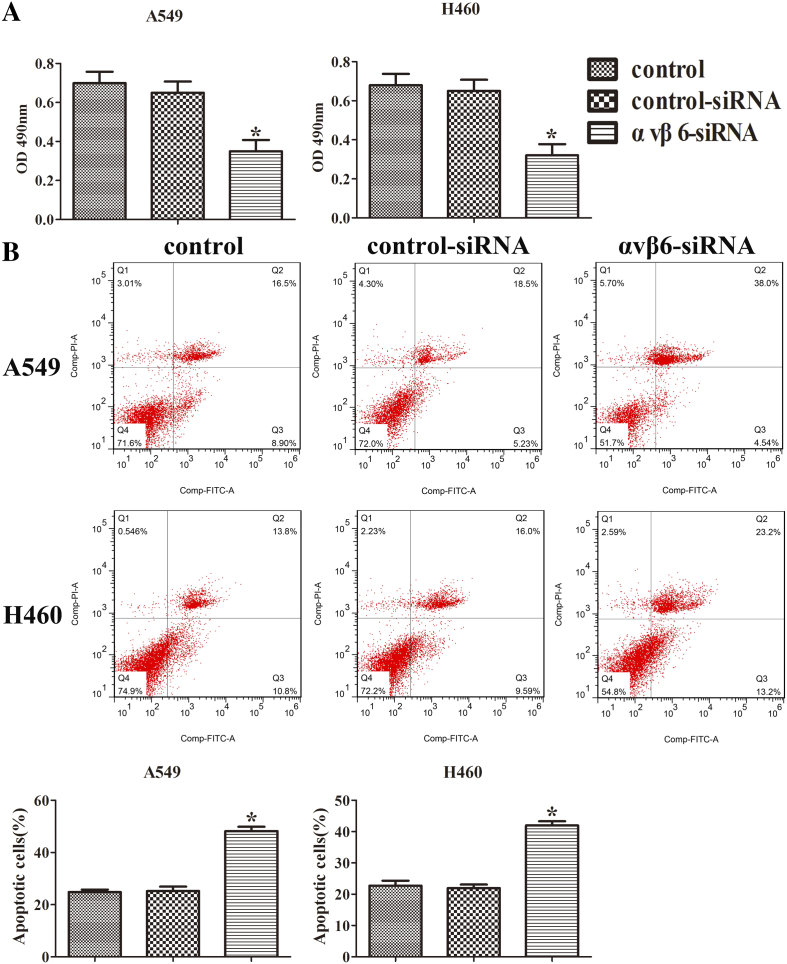
To evaluate the effect of integrin αvβ6 on cell proliferation, the MTT assay was used on A549 and H460 cells. After the transfection with integrin αvβ6 siRNA, the cell viabilities were significantly suppressed both in A549 and H460 cells (Figure 3A).
Figure 3.
Integrin αvβ6 promotes the proliferation and inhibits apoptosis of lung cancer cells partially through the upregulation of IL-8. A549 and H460 cells were transfected with integrin αvβ6 siRNA or nonspecific control siRNA. (A) A549 and H460 cells were transfected and then assayed by MTT. (B) A549 and H460 cells apoptosis was determined by flow cytometry analysis. Quantitative results for apoptosis cell rates have been shown. Bars indicate the mean ± SEM, *P < .05 vs. control group.
Furthermore, flow cytometry analysis was performed to determine the cell apoptosis. The cells in the upper-right (UR, Q2) and lower-right (LR, Q3) quadrants of the FACS histogram represent apoptotic cells. As shown in Figure 3B, after the transfection with integrin αvβ6 siRNA, the apoptosis cell rates of A549 and H460 cells were enhanced compared with the controls. These results suggest that integrin αvβ6 promotes proliferation and inhibits apoptosis in lung cancer partially via the upregulation of IL-8 expression.
Integrin αvβ6 Promotes Migration and Invasion of Lung Cancer Cells Partially through the Upregulation of IL-8
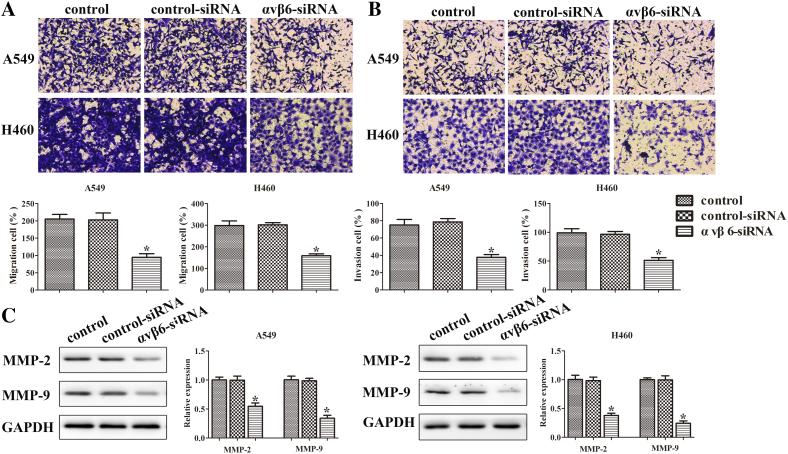
The migration and invasion assays were performed in A549 and H460 cells to detect the cell migration and invasion, respectively. The migration cells were obviously reduced by the transfection with integrin αvβ6 siRNA in both A549 and H460 cells, similar to the result of the invasion cells (Figure 4, A-B).
Figure 4.
Integrin αvβ6 promotes migration and invasion of lung cancer cells partially through the upregulation of IL-8. A549 and H460 cells were transfected with integrin αvβ6 siRNA or nonspecific control siRNA. (A) The migration ability of cells was quantified by counting the number of cells that migrated to the underside of the porous polycarbonate membrane. The photographs were taken at the magnification of ×200. Quantitative results for invasion data have been shown. (B) The invasion ability of cells was quantified by counting the number of cells that invaded the underside of the porous polycarbonate membrane. The photographs were taken at the magnification of ×200. Quantitative results for invasion data have been shown. (C) Western blots were performed to detect protein levels of MMP-2 and MMP-9. GAPDH was used as a control. Bars indicate the mean ± SEM, *P < .05 vs. control group.
Matrix metalloproteinases (MMPs) are zinc-dependent proteolytic enzymes of the extracellular matrix widely used by cells during invasion and migration [17], [18]. MMP2 and MMP9 have been strongly correlated with the invasiveness of many types of cancer cells [19], [20]. Thus, the protein expressions of MMP-2 and MMP-9 were detected to evaluate the effect of integrin αvβ6 on the invasiveness of lung cancer cells. It showed that integrin αvβ6 knockdown significantly decreased the protein expression of MMP-2 and MMP-9 (Figure 4C). These data revealed that integrin αvβ6 promotes migration and invasion in lung cancer partially via the upregulation of IL-8.
Integrin αvβ6 Activates MAPK/ERK Signaling Pathway Partially through the Upregulation of IL-8
Mitogen-activated protein kinases (MAPKs), including ERK, JNK, and p38, control the induction and regulation of inflammatory response, leading to the expression of inflammatory cytokines and chemokines upon pathogen challenge [21]. MAPK pathways are known to be evolutionarily conserved kinase modules which link extracellular signals to the machinery that controls fundamental cell processes such as growth, migration, and apoptosis [22].
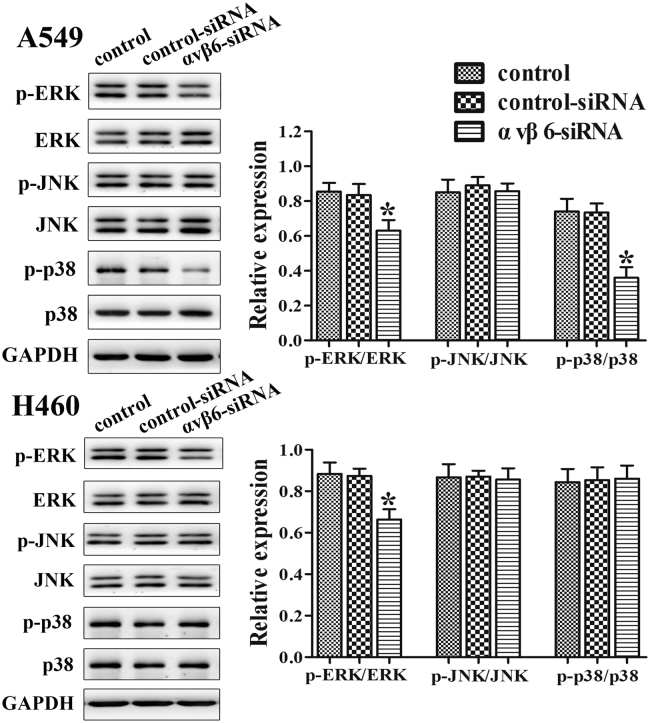
To further investigate the mechanism of integrin αvβ6 with IL-8 in lung cancer, the expressions of MAPK, including ERK, JNK, and p38, were examined. The results showed that the integrin αvβ6 siRNA group significantly inhibited the phosphorylation expression of ERK in both A549 and H460 cells and the phosphorylation expression of p38 in A549 cells but had no significant effect on the phosphorylation expression of JNK (Figure 5). It suggested that integrin αvβ6 could activate MAPK/ERK signaling pathway partially through the upregulation of IL-8.
Figure 5.
Integrin αvβ6 activates MAPK/ERK signaling pathway partially through the upregulation of IL-8. A549 and H460 cells were transfected with integrin αvβ6 siRNA or nonspecific control siRNA. Western blots were performed to detect protein levels of MAPK along with their phosphorylation. GAPDH was used as a control. GAPDH was used as a control. Bars indicate the mean ± SEM, *P < .05 vs. control group.
Integrin αvβ6 Increases Tumor Proliferation and Inhibits Cell Apoptosis in Mice Partially through the Upregulation of IL-8
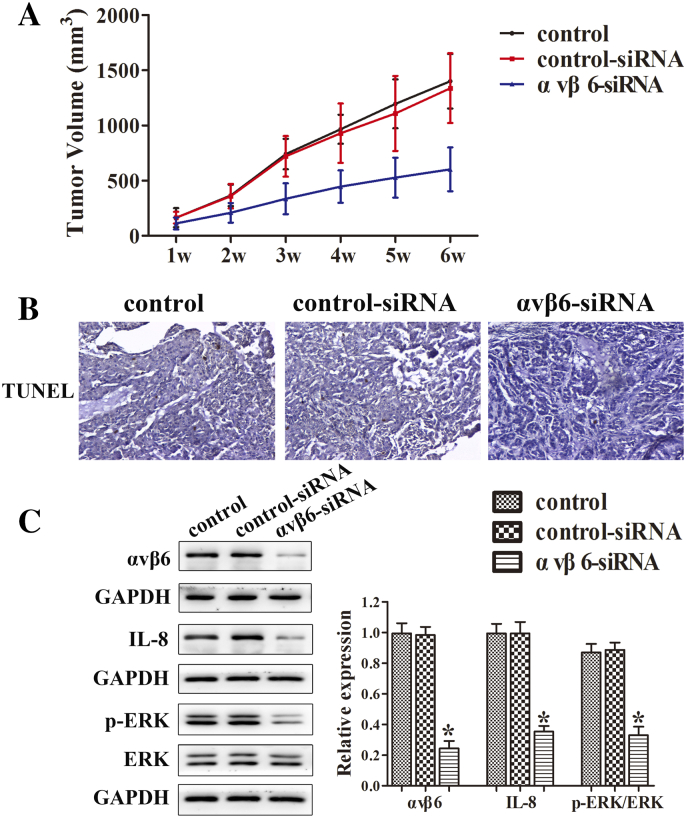
To further confirm the result between integrin αvβ6 and IL-8, different groups of A549 cells were inoculated subcutaneously into the right axilla of nude mice. All mice were sacrificed on day 28, and the tumors were obtained and tumor volume was measure. The result revealed that integrin αvβ6 siRNA group significantly inhibited the tumor volume compared with the controls (Figure 6A). The TUNEL assay indicated that, compared with the controls, the integrin αvβ6 siRNA group clearly decreased tumor cell apoptosis (Figure 6B). As shown in Figure 6C, integrin αvβ6 siRNA group exhibited obviously lower expressions of integrin αvβ6 and IL-8 compared with the controls. Similarly, the phosphorylation expression of ERK was significantly repressed in integrin αvβ6 siRNA group (Figure 6C).
Figure 6.
Integrin αvβ6 increases tumor proliferation and inhibits cell apoptosis in mice partially through the upregulation of IL-8. After the transfection with integrin αvβ6 siRNA, different groups of a549 cells were inoculated subcutaneously into the right axilla of nude mice. All mice were sacrificed on day 28, and the tumors were obtained. (A) The tumor volume was calculated. (B) The TUNEL assay in tumor issue was performed. The photographs were taken at the magnification of ×200. (C) Western blots were performed to detect protein levels of integrin αvβ6, ERK, and p-ERK. GAPDH was used as a control. Bars indicate the mean ± SEM, *P < .05 vs. control group.
Integrin αvβ6 Increases Tumor Metastasis in Mice Partially through the Upregulation of IL-8
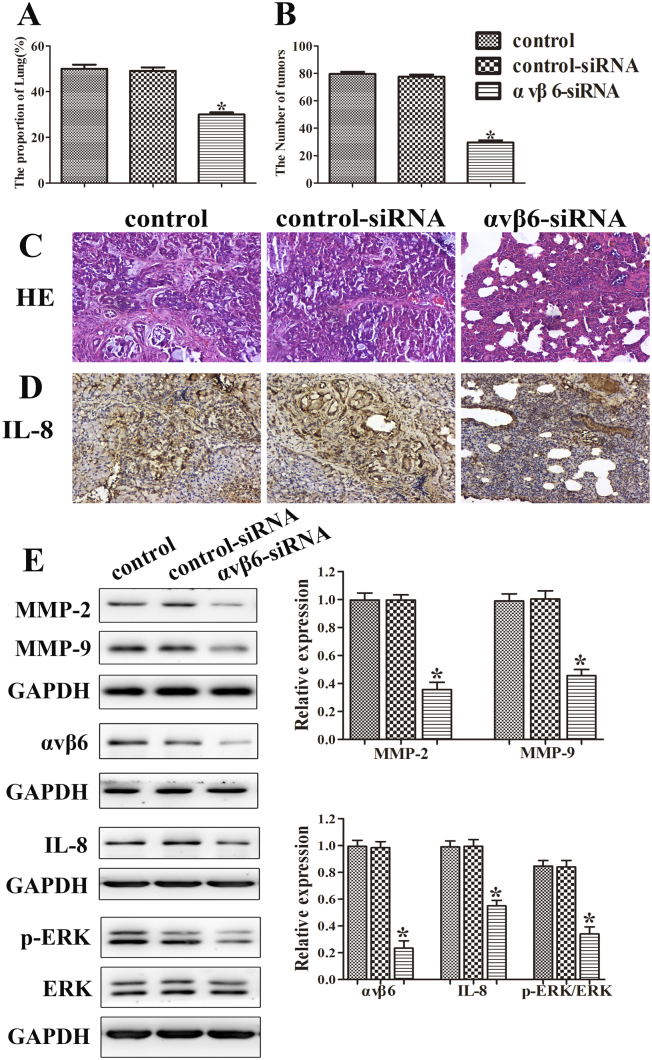
The lung metastasis model was performed to confirm the relationship of integrin αvβ6 and IL-8. Cells were injected from tail vein, and mice were sacrificed on day 21; the lungs were removed and weighed. The tumor nodules were counted, and histological assays were performed on the lung tissues. Afterwards, the proteins were collected in lung tissues, and an immunohistochemistry assay was used to detect the expression of IL-8. The lung/total weight and tumor nodules were reduced in integrin αvβ6 siRNA group compared with the controls (Figure 7, A-B). Additionally, the lung metastases lesions were visualized by H&E staining (Figure 7C). Meanwhile, the immunohistochemistry assay showed that integrin αvβ6 siRNA group exhibited the lowest IL-8 expression between these groups (Figure 7D). Furthermore, integrin αvβ6 siRNA group exhibited obviously lower expressions of integrin αvβ6 and IL-8 compared with the controls. Similarly, the expressions of MMP-2 and MMP-9 and the phosphorylation expression of ERK were significantly repressed in integrin αvβ6 siRNA group (Figure 7E). Collectively, these results provide the direct evidence to support our hypothesis that integrin αvβ6 increased tumor metastasis in mice partially through the upregulation of IL-8.
Figure 7.
Integrin αvβ6 increases tumor metastasis in mice partially through the upregulation of IL-8. After the transfection with integrin αvβ6 siRNA, different groups of a549 cells were injected from tail vein, and mice were sacrificed on day 21; the lung tissues were removed and weighed. (A) The lung/total weight was detected. (B) The number of tumor nodules was detected. (C) A representative histological view of the liver sections was photographed. The photographs were taken at the magnification of ×200. (D) The expression of IL-8 was determined by immunohistochemistry assay. The photographs were taken at the magnification of ×200. (E) Western blots were performed to detect protein levels of MMP-2, MMP-9, integrin αvβ6, ERK, and p-ERK. GAPDH was used as a control. Bars indicate the mean ± SEM, *P < .05 vs. control group.
Discussion
Lung cancer is one of the most common types of malignancies worldwide, and the main difficulties in the treatment of this tumor are the occurrence of invasion and metastasis of lung cancer cells [23]. Cancer cells that disperse from the primary tumor undergo a cascade of events, including localized invasion, intravasation into the blood or lymphatic system, and extravasation from the blood or lymphatic vessel where they colonize and form new tumors [24].
IL-8, a chemokine produced by macrophages and other cell types such as epithelial cells, is a neutrophil chemotactic factor [25]. Considerable data have demonstrated that tumor cells expressing abnormal levels of IL-8 facilitate tumor progression [16]. IL-8 plays an important role in cell proliferation, angiogenesis, migration, and invasion, and thereby is involved in the metastatic process of various cancers including lung cancer [26], [27]. It has been reported that IL-8 could potentiate tumor cell migration or invasion through ERK, NF-κB, and PI3K/Akt signaling [28], [29].
Integrins are the transmembrane receptors that are composed of an α-subunit and a β-subunit involved in regulating a variety of cellular processes, including adhesion, migration, proliferation, and differentiation [30]. Overexpression of αvβ6, an epithelium-specific integrin, has been reported to correlate with malignant progression and poor clinical prognosis in a variety of carcinomas and to promote metastasis [31].
This study showed conclusively that the expression of αvβ6 was significantly correlated with expression of IL-8 in human lung cancer samples. Furthermore, integrin αvβ6 increased cell proliferation, migration, and invasion by impairing the expressions of MMP-2 and MMP-9 and inhibited cell apoptosis in human lung cancer cells A549 and H460. In addition, integrin αvβ6 upregulated IL-8 expression through activation of MAPK/ERK signaling pathway. The in vivo experiment showed that integrin αvβ6 promoted tumor growth in xenograft models by accelerating tumor volume and reducing apoptosis. Meanwhile, lung metastasis model experiment suggested that integrin αvβ6 stimulated tumor metastasis with the increase of lung/total weight and tumor nodules. Simultaneously, integrin αvβ6 upregulated IL-8 expression detected by both Western blots and immunohistochemistry, while the MAPK/ERK signaling pathway was activated.
In conclusion, this study indicated that integrin αvβ6 promoted lung cancer cell proliferation and metastasis, at least in part, through upregulation of IL-8–mediated MAPK/ERK signaling. Thus, the inhibition of integrin αvβ6 and IL-8 may be the key for the treatment of lung cancer.
Acknowledgments
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of Interests: The authors declare no conflict of interests.
References
- 1.Zhang Y, Yang Q, Wang S. MicroRNAs: a new key in lung cancer. Cancer Chemother Pharmacol. 2014;74(6):1105–1111. doi: 10.1007/s00280-014-2559-9. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y., Li H., Hou S., Hu B., Liu J., Wang J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0065309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hess K.R., Varadhachary G.R., Taylor S.H., Wei W., Raber M.N., Lenzi R., Abbruzzese J.L. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 4.Zhang E., Feng X., Liu F., Zhang P., Liang J., Tang X. Roles of PI3K/Akt and c-Jun signaling pathways in human papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression and in vitro angiogenesis in non-small cell lung cancer cells. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Infanger D.W., Cho Y., Lopez B.S., Mohanan S., Liu S.C., Gursel D., Boockvar J.A., Fischbach C. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013;73(23):7079–7089. doi: 10.1158/0008-5472.CAN-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W., Lin S., Li W., Wang W., Li X., Xu D. IL-8 interacts with metadherin promoting proliferation and migration in gastric cancer. Biochem Biophys Res Commun. 2016;478(3):1330–1337. doi: 10.1016/j.bbrc.2016.08.123. [DOI] [PubMed] [Google Scholar]
- 7.Yin J., Zeng F., Wu N., Kang K., Yang Z., Yang H. Interleukin-8 promotes human ovarian cancer cell migration by epithelial-mesenchymal transition induction in vitro. Clin Transl Oncol. 2015;17(5):365–370. doi: 10.1007/s12094-014-1240-4. [DOI] [PubMed] [Google Scholar]
- 8.Kuai W.X., Wang Q., Yang X.Z., Zhao Y., Yu R., Tang X.J. Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J Gastroenterol. 2012;18(9):979–985. doi: 10.3748/wjg.v18.i9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunaga N., Imai H., Shimizu K., Shames D.S., Kakegawa S., Girard L., Sato M., Kaira K., Ishizuka T., Gazdar A.F. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int J Cancer. 2012;130(8):1733–1744. doi: 10.1002/ijc.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 11.Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33(7):405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Breuss J.M., Gallo J., DeLisser H.M., Klimanskaya I.V., Folkesson H.G., Pittet J.F., Nishimura S.L., Aldape K., Landers D.V., Carpenter W. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108(Pt 6):2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 13.Yang G.Y., Guo S., Dong C.Y., Wang X.Q., Hu B.Y., Liu Y.F., Chen Y.W., Niu J., Dong J.H. Integrin αvβ6 sustains and promotes tumor invasive growth in colon cancer progression. World J Gastroenterol. 2015;21(24):7457–7467. doi: 10.3748/wjg.v21.i24.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazelbag S., Kenter G.G., Gorter A., Dreef E.J., Koopman L.A., Violette S.M., Weinreb P.H., Fleuren G.J. Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol. 2007;212(3):316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 15.Elayadi A.N., Samli K.N., Prudkin L., Liu Y.H., Bian A., Xie X.J., Wistuba I.I., Roth J.A., McGuire M.J., Brown K.C. A peptide selected by biopanning identifies the integrin alphavbeta6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007;67(12):5889–5895. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q., Sun F., Wang B., Liu S., Niu W., Liu E., Peng C., Wang J., Gao H., Liang B. Interleukin-8 promotes cell migration through integrin αvβ6 upregulation in colorectal cancer. Cancer Lett. 2014;354(2):245–253. doi: 10.1016/j.canlet.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237(3):273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 18.Folgueras A.R., Pendás A.M., Sánchez L.M., López-Otín C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. 2004;48(5-6):411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q., Ji M., Guan H., Shi B., Hou P. Shikonin inhibits thyroid cancer cell growth and invasiveness through targeting major signaling pathways. J Clin Endocrinol Metab. 2013;98(12):E1909–1917. doi: 10.1210/jc.2013-2583. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X.G., Lu X.F., Jiao X.M., Chen B., Wu J.X. PLK1 gene suppresses cell invasion of undifferentiated thyroid carcinoma through the inhibition of CD44v6, MMP-2 and MMP-9. Exp Ther Med. 2012;4(6):1005–1009. doi: 10.3892/etm.2012.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gómez M.I., Lee A., Reddy B., Muir A., Soong G., Pitt A., Cheung A., Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses byactivating TNFR1. Nat Med. 2004;10(8):842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 22.Dhillon A.S., Hagan S., Rath O., Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 23.Fan C., Miao Y., Zhang X., Liu D., Jiang G., Lin X., Han Q., Luan L., Xu Z., Wang E. Btbd7 contributes to reduced E-cadherin expression and predicts poor prognosis in non-small cell lung cancer. BMC Cancer. 2014;14:704. doi: 10.1186/1471-2407-14-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 25.Hedges JC, Singer CA, Gerthoffer WT. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol. 2000;23(1):86–94. doi: 10.1165/ajrcmb.23.1.4014. [DOI] [PubMed] [Google Scholar]
- 26.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 27.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12(4):375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 28.Park S.H., Kim J.H., Lee D.H., Kang J.W., Song H.H., Oh S.R., Yoon D.Y. Luteolin 8-C-β-fucopyranoside inhibits invasion and suppresses TPA-induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-κB signaling in MCF-7 breast cancer cells. Biochimie. 2013;95(11):2082–2090. doi: 10.1016/j.biochi.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Wang L., Tang C., Cao H., Li K., Pang X., Zhong L., Dang W., Tang H., Huang Y., Wei L. Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther. 2015;16(8):1220–1230. doi: 10.1080/15384047.2015.1056409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel H.L., Li J., Kogan S., Languino L.R. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15(3):657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecht J.L., Dolinski B.M., Gardner H.A., Violette S.M., Weinreb P.H. Overexpression of the alphavbeta6 integrin in endometrial cancer. Appl Immunohistochem Mol Morphol. 2008;16(6):543–547. doi: 10.1097/PAI.0b013e31816bc5ee. [DOI] [PubMed] [Google Scholar]