Abstract
Radiation esophagitis (RE) is a common adverse event associated with radiotherapy for non–small cell lung cancer (NSCLC). While plasma cytokine levels have been correlated with other forms of radiation-induced toxicity, their association with RE has been less well studied. We analyzed data from 126 patients treated on 4 prospective clinical trials. Logistic regression models based on combinations of dosimetric factors [maximum dose to 2 cubic cm (D2cc) and generalized equivalent uniform dose (gEUD)], clinical variables, and pretreatment plasma levels of 30 cytokines were developed. Cross-validated estimates of area under the receiver operating characteristic curve (AUC) and log likelihood were used to assess prediction accuracy. Dose-only models predicted grade 3 RE with AUC values of 0.750 (D2cc) and 0.727 (gEUD). Combining clinical factors with D2cc increased the AUC to 0.779. Incorporating pretreatment cytokine measurements, modeled as direct associations with RE and as potential interactions with the dose-esophagitis association, produced AUC values of 0.758 and 0.773, respectively. D2cc and gEUD correlated with grade 3 RE with odds ratios (ORs) of 1.094/Gy and 1.096/Gy, respectively. Female gender was associated with a higher risk of RE, with ORs of 1.09 and 1.112 in the D2cc and gEUD models, respectively. Older age was associated with decreased risk of RE, with ORs of 0.992/year and 0.991/year in the D2cc and gEUD models, respectively. Combining clinical with dosimetric factors but not pretreatment cytokine levels yielded improved prediction of grade 3 RE compared to prediction by dose alone. Such multifactorial modeling may prove useful in directing radiation treatment planning.
Introduction
Locally advanced non–small cell lung cancer (NSCLC) is often treated with concurrent chemotherapy and radiation [1], [2]. Radiation esophagitis (RE) is a common complication of this treatment, with the incidence of grade 3 or greater symptoms having been reported to be as high as 25% in prospective trials [3]. Grade 3 esophagitis, per Common Terminology Criteria for Adverse Events, indicates severe symptoms requiring intervention such as tube feeding or parenteral nutrition [4]. Acute RE usually develops between 2 and 4 weeks of treatment and, in addition to affecting quality of life, can necessitate treatment break, which is associated with inferior outcomes [5], [6], [7]. With improved understanding of the clinical, dosimetric, and biologic risk factors for RE, it may be possible to identify patients for whom stricter esophageal dose constraints, prophylactic nutritional optimization, or other action may be beneficial for reducing toxicity and increasing chances for completing treatment.
Clinical factors associated with RE include concurrent chemotherapy, gender, age, body mass index, pretreatment dysphagia, and nodal stage [8], [9], [10]. Dose-escalated, twice-daily, and hyopfractionated radiotherapy courses increase risk [2], [9], [11], [12], [13]. Improved esophageal sparing with intensity-modulated radiotherapy (IMRT) has shown promise for reducing rates of grade 3+ RE compared to 3D-conformal (3DCRT), although this has not been consistent in all studies [14], [15], [16], [17], [18], [19], [20], [21], [22]. A multitude of dosimetric factors predictive of RE have been described, including mean esophageal dose, maximum esophageal dose, and various doses to esophageal surface area, length, and volumes (including total, infield, and relative volumes) [8], [9], [10], [14], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]. More advanced approaches, including normal tissue complication probability modeling and anatomic correction, have shown unclear benefit [27], [38], [40]. More recently, a multi-institutional study evaluating multiple dose-volume metrics identified the maximum dose to 2 cubic cm (D2cc) and generalized equivalent uniform dose (gEUD) as superior parameters [41].
Cytokines represent a large, heterogeneous group of proteins involved in regulating inflammatory and fibrotic responses to injury [42]. Multiple cytokines have been linked with reflux-associated and eosinophilic esophagitis [43], [44], [45]. Single nucleotide polymorphisms (SNPs) in several cytokines and cytokine receptors have been associated with an increased risk of RE [46], [47], [48]. In mouse models, esophageal radiation has been found to induce transcription of multiple inflammatory cytokines [49], [50].
While plasma cytokine levels have been extensively investigated as potential biomarkers for radiation-induced lung toxicity (RILT) (reviewed in [51]), their potential role in RE has been less well studied. In predicting RILT, differences in both pretreatment and radiation-induced plasma cytokine levels have been shown to correlate with risk [51], [52], [53]. However, as RE, compared to radiation pneumonitis and pulmonary fibrosis, often develops relatively early in radiation course, biomarkers evaluable prior to initiation of radiotherapy would be of greatest utility for RE, as they could direct intervention prior to the onset of toxicity. We hypothesized that variations in pretreatment plasma cytokine levels may correlate with increased or decreased risk of RE. We investigated combining pretreatment plasma cytokine data with dosimetric and clinical factors in an effort to improve prediction of RE in patients undergoing definitive radiotherapy for NSCLC. Dosimetric factors of D2cc and gEUD were selected due to recently published favorable results using these parameters [41].
Materials and Methods
Study Population
This work analyzed data from 4 prospective Institutional Review Board–approved lung-cancer studies: 1) a phase 1/2 study of radiation dose escalation with concurrent chemotherapy, 2-3) two consecutive studies using functional imaging and biomarkers to assess patient outcome, and 4) a study using midtreatment positron emission tomography (PET) to guide individualized dose escalation. Included in this analysis were patients with stage I to III NSCLC treated with standard fractionation, i.e., not stereotactic body radiotherapy. All clinical data were prospectively collected. Smoking status was missing for 10 patients, which was handled via single imputation.
Treatment Regimen
All patients were treated with definitive radiotherapy with or without sequential or concurrent chemotherapy. In cases of sequential treatment, chemotherapy was administered following radiotherapy. In most cases, radiation was delivered using 3DCRT as previously described [54], whereas IMRT was used for a minority of patients. Gross tumor volume included the primary tumor and any involved hilar or mediastinal lymph nodes, as determined by tissue diagnosis and/or PET–computed tomography (CT). Uninvolved lymph node regions were not included in the clinical target volume. The esophagus was contoured per Radiation Therapy Oncology Group guidelines on each patient's CT simulation scan. Tissue inhomogeneity corrections were applied for all plans.
As dose and fractionation varied among patients, we standardized values to biologic effective dose (BED), which normalizes doses of various fractionations by supposing a hypothetical condition of an infinite number of fractions. Tumor and esophageal BEDs were calculated using the linear-quadratic formula using an alpha/beta ratio of 10 Gy. Patients were evaluated weekly during radiotherapy and at regular intervals following completion of treatment. Radiation-induced esophageal toxicity was graded by physicians during on-treatment and follow-up visits per Common Terminology Criteria for Adverse Events v3.0 [4].
Cytokine Analysis
Plasma concentrations of 30 cytokines were measured: epidermal growth factor, eotaxin, fractalkine, granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, interferon α, interleukin (IL)1α, IL1β, IL2, IL4, IL5, IL6, IL7, IL8, IL10, IL12 subunit 40 (IL12p40), IL12 p35 and p40 heterodimer (IL12p70), IL13, IL15, IL17, IL1 receptor antagonist, monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, macrophage inflammatory protein 1β, soluble CD40 ligand, TGFα, TGFβ1, TNFα, and vascular endothelial growth factor. This panel was selected to represent a diverse collection of cytokines implicated in many inflammatory processes including RILT and esophagitis of various etiologies. Cytokine measurements were performed in platelet-poor plasma samples within 2 weeks prior to the start of RT. Plasma samples were collected and prepared as previously described [55]. Briefly, blood samples were collected in the presence of the anticoagulant K2EDTA (dikalium salt of ethylenediaminetetraacetic acid) and placed on ice immediately after collection. Samples were centrifuged within 2 hours of collection, after which the upper one-third of the supernatants was collected and stored at −80°C. Prior to cytokine measurements, samples were recentifuged in order to generate platelet-poor samples for analysis. TGFβ1 levels were measured using enzyme-linked immunosorbent assay as previously described [53], while the other 29 cytokines were measured using luminex multiplex assay (xMAP plasma assay; Luminx, St. Charles, MO). All sample tests were run in duplicate. Some cytokine measurements fell below a lower limit of detection. We used an ad hoc methodology to detect and account for these censored measurements, which is described in the Supplement.
Statistical Modeling
We modeled the incidence of grade 3 esophagitis after initiation of radiotherapy as a function of associations with dose, selected clinical and dosimetric factors, and pretreatment cytokine levels using multivariable logistic regression. To account for the low ratio of number of events to number of covariates, which can negatively impact precision, we applied “elastic net” penalization to the regression [56]. The elastic net is equipped to automatically exclude unimportant variables from the model and shrink the estimated associations of those that remain. This first characteristic encourages simplicity and interpretability in the selected model, whereas the second helps improve the external validity of the model in subsequent assessments. The number of variables excluded and the degree of shrinkage are automatically tuned based upon optimizing performance in a cross-validated framework, whereby proportions of the data are sequentially held apart and treated as small validation sets. Specifically, we maximized the cross-validated log-likelihood, a statistical measure of the agreement between each patient's model-predicted risk of grade 3 esophagitis and whether she/he developed it. We also measured cross-validated area under the receiver-operating characteristic curve (AUC), a measure of discriminatory ability. We note that standard measures of “statistical significance” such as P values do not account for the variable selection or shrinkage process and thus would be potentially misleading in our analysis. Rather, by definition of being included in the final model, a covariate is considered to have a meaningful association with the outcome (grade 3 esophagitis).
To focus on and distinguish between the impacts of associations corresponding to the set of clinical factors and those corresponding to the set of pretreatment cytokines, we compared four families of prediction models for esophagitis, differing in terms of the maximum possible level of complexity. The simplest model was a baseline comparison model for predicting grade 3 esophagitis using dosimetric factors only. We applied our model building approach separately for two dosimetrics: D2cc and gEUD to the esophagus, with a = 5. Generalized equivalent uniform dose is a numerical representation of the estimated biologic effect of dose distributed heterogeneously throughout a given structure, represented as a single dose distributed uniformly, and is expressed in units of Gy [57]. The relative contribution of maximum dose can be differentially weighted by adjusting the “a” factor. When calculated with an “a” of 1, gEUD is equivalent to mean dose. Increasing the “a” value leads to greater weighting of maximum dose even if the volume receiving this dose is small. High “a” values are appropriate for serial organs, such as the esophagus and bowel, as the function of such organs can be impaired by damage to a small volume. Conversely, low “a” values are more appropriate for parallel organs, such as the liver, as functional impairment requires damage to a relatively larger volume.
We then added a set of prespecified clinical factors: concurrent chemotherapy, smoking status (current, former, never), age, sex, simple stage, and Karnofsky performance status (KPS). Finally, we also added pretreatment cytokine levels, modeled either as having direct associations with toxicity or as indirectly modifying the dose-esophagitis association. To ensure valid comparability, all models were built and cross-validated against an identical subcohort of 126 patients with observed pretreatment cytokine levels but potentially missing dose levels. These missing values were accounted for using multiple imputation. Given the number of cytokines, we did not consider an approach for imputing the missing cytokine values.
The statistical shrinkage of the elastic net approach was applied to each family of models except for the dose-only model. However, in none of the models was this shrinkage applied to the association parameter corresponding to dose, as doing so would contradict the clear underlying biological rationale for a strong association between dose and esophagitis. All analyses were conducted in R [58], [59]. Additional details are given in the Supplement.
Results
We identified 147 patients treated with definitive, fractionated radiotherapy on the above-described protocols from 2004 to 2013. At a median follow-up of 3.5 years (range 1.3-10.1), the incidence of grade 3 esophagitis in this cohort was 10.2% (15/147). There were no cases of grade 4 or 5 esophagitis. Of these 147 patients, 22 had missing or corrupted dose information and 21 did not have any cytokines measured. As described above, patients without cytokine data were excluded from our model-building cohort, while the patients with missing dose values were accounted for using multiple imputation. The incidence of grade 3 esophagitis in the 126 analyzed patients was 10.3% (13/126), which was similar to that observed in the full cohort. Characteristics of the full cohort (n = 147) and the reduced cohort with pretreatment cytokines measured (n = 126) are reported in Table 1.
Table 1.
Patient Characteristics
| Cohort with Pretreatment Cytokine Data (n = 126) | Full Cohort (n = 147) | |
|---|---|---|
| Grade 3 esophagitis, # (%) | 13 (10.3%) | 15 (10.5%) |
| Esophageal dose, D2cc | ||
| Median (IQR) | 62.7 Gy (56.1, 69.6) | 62.1 Gy (56.0, 68.5) |
| # Missing | n = 17 | n = 22 |
| Esophageal dose, gEUD | ||
| Median (IQR) | 46.9 Gy (39.0, 51.8) | 46.8 Gy (39.0, 51.7) |
| # Missing | n = 17 | n = 22 |
| Target dose, BED | ||
| Median (IQR) | 84.0 Gy (78.0, 93.6) | 84.0 Gy (78.0, 94.4) |
| # Missing | n = 3 | n = 6 |
| Sex, # (%) | ||
| Female | 28 (22.2%) | 33 (22.4%) |
| Age, year | ||
| Median (IQR) | 65.8 (59.3, 73.4) | 65.6 (59.3, 73.2) |
| Race/ethnicity, # (%) | ||
| Caucasian | 121 (97.6%) | 140 (97.2%) |
| African American | 2 (1.6%) | 3 (2.1%) |
| Asian | 1 (0.8%) | 1 (0.7%) |
| Not reported | 2 | 3 |
| KPS | ||
| Median (IQR) | 87.5 (80, 90) | 90 (80, 90) |
| Smoking status, # (%) | ||
| Former | 57 (48.8%) | 71 (51.8%) |
| Current | 57 (48.8%) | 62 (45.3%) |
| Never smoker | 3 (2.6%) | 4 (2.9%) |
| Not reported | 9 | 10 |
| Simple stage, # (%) | ||
| I | 14 (11.1%) | 15 (10.2%) |
| II | 14 (11.1%) | 17 (11.6%) |
| III | 98 (77.8%) | 114 (77.6%) |
| IV | 0 | 1 (0.7%) |
| Concurrent chemotherapy, # (%) | ||
| Yes | 103 (81.7%) | 121 (82.3%) |
| Radiation technique, # (%) | ||
| 3DCRT | 122 (96.8%) | 143 (97.3%) |
| IMRT | 4 (3.2%) | 4 (2.7%) |
IQR, interquartile range.
Table 2 gives the results from the model fitting procedure against the 126 patients for both analyzed dosimetrics, namely, D2cc and gEUD. In these “dose-only” models, the cross-validated log-likelihood and AUC were, respectively, −37.7 and 0.750 for D2cc and −37.7 and 0.727 for gEUD. These increased to −37.1 and 0.779, respectively, when the set of clinical covariates was added to D2cc (dose + clinical). Clinical factors analyzed included concurrent chemotherapy, former smoking status, current smoking status, age, sex, simple TNM stage, and KPS. There was a similar improvement in model fit when using gEUD, with log-likelihood increasing to −37.2 and the AUC to 0.768. We emphasize that these metrics are cross-validated and thus adjusted for overfitting. As such, numerical increases reflect an improvement in model fit and predictive performance.
Table 2.
Prediction of Grade 3 Esophagitis Based upon 126 Patients with Measured Cytokines from Four Models Using Increasing Amounts of Clinical and Cytokine Data
| Dose = D2cc |
Dose = gEUD |
|||
|---|---|---|---|---|
| AUC | Log Likelihood | AUC | Log Likelihood | |
| Dose only | 0.750 | −37.7 | 0.727 | −37.7 |
| Dose + clinical | 0.779 | −37.1 | 0.768 | −37.2 |
| Dose + clinical + Cyt0 | 0.758 | −37.8 | 0.735 | −37.6 |
| Dose + clinical + Dose*Cyt0 | 0.773 | −37.4 | 0.749 | −37.5 |
The model with the largest log-likelihood (in bold) was selected.
Cyt0, pretreatment cytokine levels.
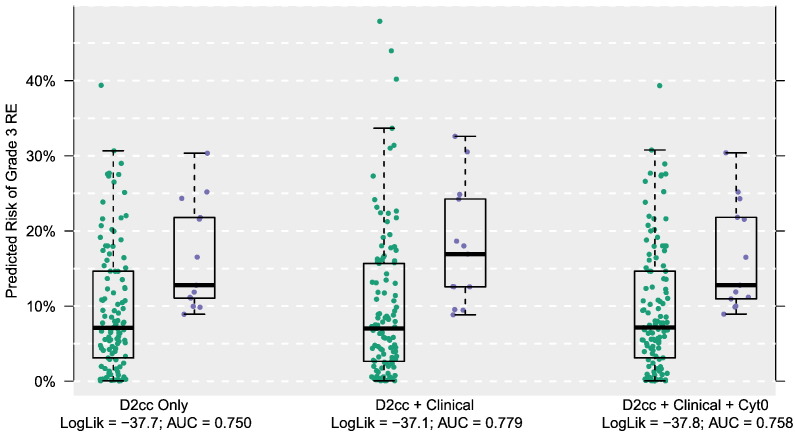
Upon adding pretreatment cytokine measurements, either as having associations with esophagitis directly or as modifying the dose-esophagitis relationship, predictive performance degraded. For D2cc, the log-likelihood values from the cytokine models were −37.8 and −37.4, respectively, and the AUCs were 0.758 and 0.773. For gEUD, the log-likelihood values from the cytokine models were −37.6 and −37.5, and the AUCs were 0.749 and 0.729. Comparing the two approaches to modeling cytokine levels, the better fitting set allowed a cytokine to adjust the dose-esophagitis association up or down (a statistical interaction) rather than being itself directly associated with esophagitis. However, in an overall comparison, none of the cytokine models could further improve upon a simpler model using only dose and clinical covariates. Comparing performance across dosimetrics, D2cc had marginally better performance than gEUD in the dose + clinical model. Figure 1 plots the model-based (cross-validated) risks of grade 3 RE using the D2cc dosimetric.
Figure 1.
Predicted risk of grade 3 RE by various D2cc-based prediction models. Green and purple dots represent patients who did not and did develop grade 3 RE, respectively.
Focusing on the dose + clinical models, which had the best operating characteristics, we next included data from the 21 patients who were excluded from the model-building process due to missing pretreatment cytokine data. We report the individual estimated associations from refitting the selected dose + clinical model to all 147 patients (Table 3). As expected, increasing dose was strongly associated with increased risk of esophagitis, with odds ratios (ORs) of 1.094/Gy (D2cc) and 1.096/Gy (gEUD). In addition to dose, two clinical covariates were selected for the models. First, women had slightly higher risk than men, with ORs of 1.090 (D2cc) and 1.112 (gEUD). Additionally, with each dosimetric, there was an incremental decline in the risk of RE with increasing age, with ORs of 0.992/year (D2cc) and 0.991/year (gEUD).
Table 3.
ORs for Dosimetric and Clinical Factors from Selected “Dose + Clinical” Multivariable Models of Grade 3 RE in Cohort of 147 Patients (21 Additional Patients Who Were Missing Cytokine Measurements Were Readded into the Analysis Cohort after Model Selection)
| Dose = D2cc | Dose = gEUD | |
|---|---|---|
| Dose (OR per Gy) | 1.094 | 1.096 |
| Female sex | 1.090 | 1.112 |
| Age (OR per year) | 0.992 | 0.991 |
Discussion
In the work described here, RE models combining dosimetric and clinical factors showed improved prediction accuracy compared to that obtained by dose alone, as reflected in higher AUC and log likelihood values. Incorporating pretreatment plasma levels of 30 cytokines, however, did not improve results. This was true whether cytokine levels were modeled as direct interactions with RE or as potential modifiers of the dose-RE association. Of the various models tested, we found the D2cc + clinical model to yield the highest prediction accuracy, with an AUC of 0.779 and a log likelihood of −37.1. We found female gender to be associated with a mild increase in the risk of RE, while increasing age was protective. While these results do not support the use of pretreatment cytokines to predict grade 3 RE, they do demonstrate the superiority of a multifactorial approach. While many physicians already consider patient-specific factors when generating treatment plans, standardized methods for quantifying risk could allow for more robust individualization of treatment planning, nutritional optimization, or other prophylactic intervention.
The incidence of grade 3 RE in this cohort was 10.2%, which is similar to other recent studies [11]. The majority of our patients received concurrent chemotherapy, which is both consistent with the standard of care for locally advanced NSCLC and a previously described risk factor for RE. The majority of patients analyzed in this work were treated with 3DCRT. Multiple studies have shown that the use of IMRT is associated with reduced rates of RE, although others have shown no improvement [14], [15], [16], [17], [18], [19], [20], [21], [22]. With increasing utilization of IMRT for NSCLC, it is possible that the generalizability of our results may be reduced.
Despite the abundance of dosimetric factors that have been correlated with RE, no clearly superior parameter has been identified for standardized implementation. In 2010, a Quantitative Analysis of Normal Tissue Effects in the Clinic report identified limiting mean esophageal dose to <34 Gy as the most significant factor for reducing the risk of grade 3+ RE, a planning goal also recommended in Radiation Therapy Oncology Group 0617 [11], [60]. The dosimetric variables analyzed here, D2cc and gEUD, were selected due to recent promising results regarding their use in predicting RE [41]. D2cc is a parameter often used as surrogate for maximum point dose and is commonly used in other settings [61], [62]. The importance of volume of esophagus receiving high radiation dose is supported by a large meta-analysis that identified the volume of esophagus receiving 60 Gy (V60) as the strongest correlate of RE [30]. As discussed in the methods section above, gEUD calculated with an “a” value >1 incorporates maximum dose while also accounting for dose to the entire organ.
We note that the finding that increasing age correlated with lower risk of RE is consistent with our previous report focusing specifically on this phenomenon [63] but that the magnitude of the association is smaller here. This was likely due to multiple factors, including the use of statistical shrinkage via the elastic net, which was employed in this study to reduce false-positive findings given the high number of model-building parameters and which forces all estimated associations to be smaller; the inclusion of the additional clinical covariate of sex; and the inclusion of an additional 22 patients in this study with missing dose information. That the association between age and RE was similar despite these differences supports the findings in the previous study.
There are several potential reasons why our analysis failed to show a correlation between pretreatment plasma cytokine levels and grade 3 RE. One possibility is that an inadequate number of cytokines was investigated and that some cytokine that was not measured could correlate with RE. However, this is unlikely given the volume and diversity of the panel, as well as the interrelatedness of cytokine expression patterns and functions. We investigated a broad panel of cytokines, including many of those linked to esophagitis of other etiologies, those associated with other radiation-induced toxicities, those in which SNPs have been shown to correlate with RE, and those induced by radiation in mouse models [43], [44], [46], [47], [48], [49], [51]. Another possibility is that cytokines may have been lost or degraded during isolation or storage, as they are labile in plasma. However, our group has successfully collected and analyzed plasma cytokine levels in multiple previous studies [64], [65], [66], with these samples having been handled similarly.
While we analyzed pretreatment cytokine levels in this work, it is possible that cytokine data obtained mid- or following treatment could distinguish patients with and without RE. Such a phenomenon has been observed in lung toxicity, with both pretreatment and treatment-induced cytokine levels having been shown to correlate with risk of RILT (reviewed in [51]). However, as RE often develops within the first several weeks of radiotherapy, as opposed to radiation pneumonitis and pulmonary fibrosis, which generally develop months after treatment, such information would be inherently less able to direct meaningful adaptation of radiotherapy. While our analysis of plasma cytokines was negative, this alone is a significant finding, as little information regarding cytokines and RE exists in the published literature.
While the work presented here represents progress in RE prediction, continued efforts to improve accuracy are warranted. Several areas are promising, including incorporation of cross-sectional and functional imaging. For example, midtreatment esophageal expansion on CT and increased esophageal fludeoxyglucose avidity on PET have been correlated with RE [67], [68], [69]. However, as these are midtreatment findings, their ability to guide treatment optimization may be limited. In addition, other, noncytokine biologic factors have shown potential for predicting RE, including micro-RNAs (miRNAs). In one study, Xu et al. showed elevation of midtreatment serum levels of certain miRNA species to be associated with RE [70]. However, that study failed to show a correlation between pretreatment miRNA levels and RE, which, again, could limit applicability. Certain miRNA-related SNPs have also been linked with an increased risk of RE [71] and could represent an avenue to predict risk prior to initiation of treatment.
Conclusions
In this analysis, prediction of grade 3 RE based on dosimetric and clinical factors was superior to prediction by dosimetry alone. We did not find evidence that variation in pretreatment plasma cytokine levels further improved performance, which represents a novel finding. These results demonstrate the utility of multivariable modeling for predicting RE. Identification of additional clinical, biologic, imaging, or other factors could further improve prediction, possibly leading to more personalized treatment planning with associated reduced toxicity and improved outcomes.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Funding: This work was supported in part by R01 CA142840 (Kong) and P01 CA059827 (Ten Haken and Lawrence).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2017.11.005.
Appendix A. Supplementary data
Supplementary Material.
References
- 1.Auperin A, Le Pechoux C, Rolland E. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non–small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 2.Curran WJ, Jr., Paulus R, Langer CJ. Sequential vs. concurrent chemoradiation for stage III non–small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werner-Wasik M, Paulus R, Curran WJ., Jr. Acute esophagitis and late lung toxicity in concurrent chemoradiotherapy trials in patients with locally advanced non–small-cell lung cancer: analysis of the Radiation Therapy Oncology Group (RTOG) database. Clin Lung Cancer. 2011;12:245–251. doi: 10.1016/j.cllc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Trotti A, Colevas AD, Setser A. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 5.Cox JD, Pajak TF, Asbell S. Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non–small cell carcinoma of the lung: analysis of 1244 cases from 3 Radiation Therapy Oncology Group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1993;27:493–498. doi: 10.1016/0360-3016(93)90371-2. [DOI] [PubMed] [Google Scholar]
- 6.Choy H, LaPorte K, Knill-Selby E. Esophagitis in combined modality therapy for locally advanced non–small cell lung cancer. Semin Radiat Oncol. 1999;9:90–96. [PubMed] [Google Scholar]
- 7.Antonadou D, Coliarakis N, Synodinou M. Randomized phase III trial of radiation treatment +/− amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:915–922. doi: 10.1016/s0360-3016(01)01713-8. [DOI] [PubMed] [Google Scholar]
- 8.Maguire PD, Sibley GS, Zhou SM. Clinical and dosimetric predictors of radiation-induced esophageal toxicity. Int J Radiat Oncol Biol Phys. 1999;45:97–103. doi: 10.1016/s0360-3016(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 9.Ahn SJ, Kahn D, Zhou S. Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys. 2005;61:335–347. doi: 10.1016/j.ijrobp.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Patel AB, Edelman MJ, Kwok Y. Predictors of acute esophagitis in patients with non–small-cell lung carcinoma treated with concurrent chemotherapy and hyperfractionated radiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2004;60:1106–1112. doi: 10.1016/j.ijrobp.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Bradley JD, Paulus R, Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non–small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren XC, Wang QY, Zhang R. Accelerated hypofractionated three-dimensional conformal radiation therapy (3 Gy/fraction) combined with concurrent chemotherapy for patients with unresectable stage III non–small cell lung cancer: preliminary results of an early terminated phase II trial. BMC Cancer. 2016;16:288. doi: 10.1186/s12885-016-2314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zehentmayr F, Sohn M, Exeli AK. Normal tissue complication models for clinically relevant acute esophagitis (>/= grade 2) in patients treated with dose differentiated accelerated radiotherapy (DART-bid) Radiat Oncol. 2015;10:121. doi: 10.1186/s13014-015-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez DR, Tucker SL, Martel MK. Predictors of high-grade esophagitis after definitive three-dimensional conformal therapy, intensity-modulated radiation therapy, or proton beam therapy for non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:1010–1016. doi: 10.1016/j.ijrobp.2012.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun SG, Hu C, Choy H. Impact of intensity-modulated radiation therapy technique for locally advanced non–small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grills IS, Yan D, Martinez AA. Potential for reduced toxicity and dose escalation in the treatment of inoperable non–small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57:875–890. doi: 10.1016/s0360-3016(03)00743-0. [DOI] [PubMed] [Google Scholar]
- 17.Sura S, Gupta V, Yorke E. Intensity-modulated radiation therapy (IMRT) for inoperable non–small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol. 2008;87:17–23. doi: 10.1016/j.radonc.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirvani SM, Jiang J, Gomez DR. Intensity modulated radiotherapy for stage III non–small cell lung cancer in the United States: predictors of use and association with toxicities. Lung Cancer. 2013;82:252–259. doi: 10.1016/j.lungcan.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling DC, Hess CB, Chen AM. Comparison of toxicity between intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy for locally advanced non–small-cell lung cancer. Clin Lung Cancer. 2016;17:18–23. doi: 10.1016/j.cllc.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murshed H, Liu HH, Liao Z. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1258–1267. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 21.Al-Halabi H, Paetzold P, Sharp GC. A contralateral esophagus-sparing technique to limit severe esophagitis associated with concurrent high-dose radiation and chemotherapy in patients with thoracic malignancies. Int J Radiat Oncol Biol Phys. 2015;92:803–810. doi: 10.1016/j.ijrobp.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Niedzielski J, Bluett JB, Williamson RT. Analysis of esophageal-sparing treatment plans for patients with high-grade esophagitis. J Appl Clin Med Phys. 2013;14:4248. doi: 10.1120/jacmp.v14i4.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner-Wasik M, Pequignot E, Leeper D. Predictors of severe esophagitis include use of concurrent chemotherapy, but not the length of irradiated esophagus: a multivariate analysis of patients with lung cancer treated with nonoperative therapy. Int J Radiat Oncol Biol Phys. 2000;48:689–696. doi: 10.1016/s0360-3016(00)00699-4. [DOI] [PubMed] [Google Scholar]
- 24.Singh AK, Lockett MA, Bradley JD. Predictors of radiation-induced esophageal toxicity in patients with non–small-cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:337–341. doi: 10.1016/s0360-3016(02)03937-8. [DOI] [PubMed] [Google Scholar]
- 25.Bradley J, Deasy JO, Bentzen S. Dosimetric correlates for acute esophagitis in patients treated with radiotherapy for lung carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:1106–1113. doi: 10.1016/j.ijrobp.2003.09.080. [DOI] [PubMed] [Google Scholar]
- 26.Belderbos J, Heemsbergen W, Hoogeman M. Acute esophageal toxicity in non–small cell lung cancer patients after high dose conformal radiotherapy. Radiother Oncol. 2005;75:157–164. doi: 10.1016/j.radonc.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Chapet O, Kong FM, Lee JS. Normal tissue complication probability modeling for acute esophagitis in patients treated with conformal radiation therapy for non–small cell lung cancer. Radiother Oncol. 2005;77:176–181. doi: 10.1016/j.radonc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Qiao WB, Zhao YH, Zhao YB. Clinical and dosimetric factors of radiation-induced esophageal injury: radiation-induced esophageal toxicity. World J Gastroenterol. 2005;11:2626–2629. doi: 10.3748/wjg.v11.i17.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez N, Algara M, Foro P. Predictors of acute esophagitis in lung cancer patients treated with concurrent three-dimensional conformal radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2009;73:810–817. doi: 10.1016/j.ijrobp.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 30.Palma DA, Senan S, Oberije C. Predicting esophagitis after chemoradiation therapy for non–small cell lung cancer: an individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;87:690–696. doi: 10.1016/j.ijrobp.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Wei X, Liu HH, Tucker SL. Risk factors for acute esophagitis in non–small-cell lung cancer patients treated with concurrent chemotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:100–107. doi: 10.1016/j.ijrobp.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Kim TH, Cho KH, Pyo HR. Dose-volumetric parameters of acute esophageal toxicity in patients with lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:995–1002. doi: 10.1016/j.ijrobp.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Rosenman JG, Halle JS, Socinski MA. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non–small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2002;54:348–356. doi: 10.1016/s0360-3016(02)02958-9. [DOI] [PubMed] [Google Scholar]
- 34.Takeda K, Nemoto K, Saito H. Dosimetric correlations of acute esophagitis in lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:626–629. doi: 10.1016/j.ijrobp.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Nemoto K, Saito H. Predictive factors for acute esophageal toxicity in thoracic radiotherapy. Tohoku J Exp Med. 2006;208:299–306. doi: 10.1620/tjem.208.299. [DOI] [PubMed] [Google Scholar]
- 36.Hirota S, Tsujino K, Endo M. Dosimetric predictors of radiation esophagitis in patients treated for non–small-cell lung cancer with carboplatin/paclitaxel/radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:291–295. doi: 10.1016/s0360-3016(01)01648-0. [DOI] [PubMed] [Google Scholar]
- 37.Langer CJ, Movsas B, Hudes R. Induction paclitaxel and carboplatin followed by concurrent chemoradiotherapy in patients with unresectable, locally advanced non–small cell lung carcinoma: report of Fox Chase Cancer Center study 94–001. Semin Oncol. 1997;24 [S12-89-S12-95] [PubMed] [Google Scholar]
- 38.Huang EX, Bradley JD, El Naqa I. Modeling the risk of radiation-induced acute esophagitis for combined Washington University and RTOG trial 93-11 lung cancer patients. Int J Radiat Oncol Biol Phys. 2012;82:1674–1679. doi: 10.1016/j.ijrobp.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landau DB, Hughes L, Baker A. IDEAL-CRT: a phase 1/2 trial of isotoxic dose-escalated radiation therapy and concurrent chemotherapy in patients with stage II/III non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;95:1367–1377. doi: 10.1016/j.ijrobp.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahn D, Zhou S, Ahn SJ. "Anatomically-correct" dosimetric parameters may be better predictors for esophageal toxicity than are traditional CT-based metrics. Int J Radiat Oncol Biol Phys. 2005;62:645–651. doi: 10.1016/j.ijrobp.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 41.Paximadis P., Schipper M., Matuszak M. Dosimetric predictors for acute esophagitis during radiation therapy for lung cancer — results of a large statewide observational study. Pract Radiat Oncol. 2017 doi: 10.1016/j.prro.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lackie JM. Oxford University Press; Oxford: 2010. A dictionary of biomedicine. [Google Scholar]
- 43.Souza RF, Huo X, Mittal V. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776–1784. doi: 10.1053/j.gastro.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 44.Blanchard C, Stucke EM, Rodriguez-Jimenez B. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208–217. doi: 10.1016/j.jaci.2010.10.039. [217 e201–207] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunbar KB, Agoston AT, Odze RD. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA. 2016;315:2104–2112. doi: 10.1001/jama.2016.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hildebrandt MA, Komaki R, Liao Z. Genetic variants in inflammation-related genes are associated with radiation-induced toxicity following treatment for non–small cell lung cancer. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guerra JL, Gomez D, Wei Q. Association between single nucleotide polymorphisms of the transforming growth factor beta1 gene and the risk of severe radiation esophagitis in patients with lung cancer. Radiother Oncol. 2012;105:299–304. doi: 10.1016/j.radonc.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Yang M, Bi N. Association of TGF-beta1 and XPD polymorphisms with severe acute radiation-induced esophageal toxicity in locally advanced lung cancer patients treated with radiotherapy. Radiother Oncol. 2010;97:19–25. doi: 10.1016/j.radonc.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Epperly MW, Gretton JA, DeFilippi SJ. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Epperly MW, Guo HL, Jefferson M. Cell phenotype specific kinetics of expression of intratracheally injected manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) during lung radioprotective gene therapy. Gene Ther. 2003;10:163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 51.Kong FM, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol. 2015;25:100–109. doi: 10.1016/j.semradonc.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hart JP, Broadwater G, Rabbani Z. Cytokine profiling for prediction of symptomatic radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2005;63:1448–1454. doi: 10.1016/j.ijrobp.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 53.Stenmark MH, Cai XW, Shedden K. Combining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non–small-cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:e217–222. doi: 10.1016/j.ijrobp.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong FM, Ten Haken RK, Schipper MJ. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non–small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L, Wang L, Ji W. Elevation of plasma TGF-beta1 during radiation therapy predicts radiation-induced lung toxicity in patients with non–small-cell lung cancer: a combined analysis from Beijing and Michigan. Int J Radiat Oncol Biol Phys. 2009;74:1385–1390. doi: 10.1016/j.ijrobp.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 56.Zou H, Hastie T. Regularization and variable selection via the elastic net (vol B 67, pg 301, 2005) J Roy Stat Soc B. 2005;67:768. [Google Scholar]
- 57.Liu F, Yorke ED, Belderbos JS. Using generalized equivalent uniform dose atlases to combine and analyze prospective dosimetric and radiation pneumonitis data from 2 non–small cell lung cancer dose escalation protocols. Int J Radiat Oncol Biol Phys. 2013;85:182–189. doi: 10.1016/j.ijrobp.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wickham H. Springer; New York: 2009. Ggplot2 : elegant graphics for data analysis. [Google Scholar]
- 59.Team RC . R Foundation for Statistical Computing; Vienna, Austria: 2016. R: a language and environment for statistical computing.https://www.R-project.org Available at. [Google Scholar]
- 60.Werner-Wasik M, Yorke E, Deasy J. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76:S86–93. doi: 10.1016/j.ijrobp.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Georg P, Lang S, Dimopoulos JC. Dose-volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:356–362. doi: 10.1016/j.ijrobp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Potter R, Haie-Meder C, Van Limbergen E. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Soni PD, Boonstra PS, Schipper MJ. Lower incidence of esophagitis in the elderly undergoing definitive radiation therapy for lung cancer. J Thorac Oncol. 2017;12:539–546. doi: 10.1016/j.jtho.2016.11.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong F, Jirtle RL, Huang DH. Plasma transforming growth factor-beta1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer. 1999;86:1712–1719. [PubMed] [Google Scholar]
- 65.Anscher MS, Kong FM, Andrews K. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998;41:1029–1035. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 66.Anscher MS, Kong FM, Marks LB. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 1997;37:253–258. doi: 10.1016/s0360-3016(96)00529-9. [DOI] [PubMed] [Google Scholar]
- 67.Mehmood Q, Sun A, Becker N. Predicting radiation esophagitis using 18F-FDG PET during chemoradiotherapy for locally advanced non–small cell lung cancer. J Thorac Oncol. 2016;11:213–221. doi: 10.1016/j.jtho.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Niedzielski JS, Yang J, Liao Z. (18)F-Fluorodeoxyglucose positron emission tomography can quantify and predict esophageal injury during radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96:670–678. doi: 10.1016/j.ijrobp.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niedzielski JS, Yang J, Stingo F. Objectively quantifying radiation esophagitis with novel computed tomography-based metrics. Int J Radiat Oncol Biol Phys. 2016;94:385–393. doi: 10.1016/j.ijrobp.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu T, Liao Z, O'Reilly MS. Serum inflammatory miRNAs predict radiation esophagitis in patients receiving definitive radiochemotherapy for non–small cell lung cancer. Radiother Oncol. 2014;113:379–384. doi: 10.1016/j.radonc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Li R, Pu X, Chang JY. MiRNA-related genetic variations associated with radiotherapy-induced toxicities in patients with locally advanced non–small cell lung cancer. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material.