Abstract
Purpose
The objective of this study was to assess exposure to anaerobic bacteria released into air from sewage and sludge at workplaces from a wastewater treatment plant (WWTP).
Methods
Samples of both sewage and sludge were collected at six sampling points and bioaerosol samples were additionally collected (with the use of a 6-stage Andersen impactor) at ten workplaces covering different stages of the technological process. Qualitative identification of all isolated strains was performed using the biochemical API 20A test. Additionally, the determination of Clostridium pathogens was carried out using 16S rRNA gene sequence analysis.
Results
The average concentration of anaerobic bacteria in the sewage samples was 5.49 × 104 CFU/mL (GSD = 85.4) and in sludge—1.42 × 106 CFU/g (GSD = 5.1). In turn, the average airborne bacterial concentration was at the level of 50 CFU/m3 (GSD = 5.83) and the highest bacterial contamination (4.06 × 103 CFU/m3) was found in winter at the bar screens. In total, 16 bacterial species were determined, from which the predominant strains belonged to Actinomyces, Bifidobacterium, Clostridium, Propionibacterium and Peptostreptococcus genera. The analysis revealed that mechanical treatment processes were responsible for a substantial emission of anaerobic bacteria into the air. In both the sewage and air samples, Clostridium perfringens pathogen was identified.
Conclusions
Anaerobic bacteria were widely present both in the sewage and in the air at workplaces from the WWTP, especially when the technological process was performed in closed spaces. Anaerobic bacteria formed small aggregates with both wastewater droplets and dust particles of sewage sludge origin and as such may be responsible for adverse health outcomes in exposed workers.
Keywords: Wastewater treatment plant, Anaerobic bacteria, Exposure assessment, PCR, Size distribution
Introduction
Wastewater is always contaminated with different biological agents such as bacteria, viruses, protozoa, fungi, flatworms or roundworms (Sorber and Sagik 1980). Among them, pathogenic bacteria pose the most serious epidemiological risk. Wastewater can carry many opportunistic pathogens (e.g., Enterobacter cloacae, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris or Pseudomonas aeruginosa), which can cause different systemic infections, especially among people with a weakened immune system. In wastewater can be also found obligate pathogens from Salmonella and Shigella genera or enteropathogenic strains of Escherichia coli, which are responsible for salomonellosis, shigellosis or gastroenteritis, respectively (Cyprowski et al. 2005; Gerardi and Zimmerman 2005).
From the oxygen demand standpoint, a majority of bacteria which may occur in this environment are aerobic ones. However, some of them can also survive having only temporary access to the oxygen (facultative anaerobes) or even without it (obligate anaerobes). A substantial part of the anaerobic bacteria is delivered to the wastewater treatment plant (WWTP) by the sewage network. In the recent study of Liu et al. (2015a), 18 species of Longilinea, Georgenia, Desulforhabdus, Thauera, Desulfuromonas and Arcobacter genera were identified in the sewerage system. Furthermore, anaerobic bacteria are an important element in the wastewater treatment processes. They are responsible for methane fermentation of sewage sludge, facilitating decomposition of macromolecular organic matter into simpler compounds. Among the bacterial genera involved in the anaerobic methane fermentation process are Methanosarcina, Methanosaeta (Van Lier et al. 2008; Zinder and Mah 1979) and Clostridium (Lisle et al. 2004; Wang et al. 2003). In treated effluent, there may be faecal bacteria of the genera Bifidobacterium and Bacteroides (Wery et al. 2010) as well as Clostridium perfringens (Ajonina et al. 2015).
During the wastewater treatment processes, bioaerosol is released into the air in the form of nuclei droplets, where the fine particles of water serve as carriers of microorganisms. At WWTPs, bacterial concentrations in the air usually range from 101 to 104 CFU/m3 (Korzeniewska 2011) and can adversely affect the health of sewage workers being responsible for respiratory, digestive tract, eye and skin infections (Cyprowski and Krajewski 2003). Despite the progress in research concerning physiological characteristics of anaerobic bacteria, the knowledge about their presence in working environments is still scarce. Limited data indicate that they may occur at all stages of wastewater treatment and their concentrations may be higher in winter (101–104 CFU/m3) than in summer (101–102 CFU/m3). However, the authors of such study did not usually present the detailed qualitative characteristics of anaerobic bacteria (Fracchia et al. 2006). Another study (Pillai et al. 1996) showed that sludge can be a source of bacteria of the genus Clostridium. These bacteria can be treated as microbial indicators of water pollution. During loading of sewage sludge, they can be released into the air reaching 5 × 102 CFU/m3. However, to date, there is no data on the complex exposure of WWTP workers to anaerobic bacteria. With this in mind, the aim of this preliminary study was to assess such exposure, taking into account the source of the bacteria in sewage and sludge, as well as the air at workplaces. The analysis also included seasonal variations and size distribution of anaerobic bioaerosol particles.
Materials and methods
Sampling sites
The study was carried out in a large (2) wastewater treatment plant in Poland, where the throughput reaches the level of 200,000 m3/day. This is a typical mechanical–biological WWTP where anaerobic conditions are a part of the treatment processes. The biological wastewater treatment stage is carried out using activated sludge, in three phases: anaerobic, hypoxic and aerobic. During the treatment process, sewage sludge is produced at a rate of about 250 tons/day, and screenings of about 6 tons/day. Thickened sludge is stabilized by methane fermentation, which is ultimately neutralized by the combustion process. Screenings and sand, after hygienization with chlorinated lime are stored using lagoons. The studied WWTP employs about 150 people.
Samples of sewage and sludge (S1–S6) were collected during the summer season in 6 sampling points in the WWTP, as described in Table 1. Bioaerosol samples (A1–A11) were collected in single repetition in July 2014 and February 2015, at ten workplaces covering different stages of the technological process (Table 2). Additionally, approximately 300 m outside from the plant, the background samples (to relativize the obtained results) were collected. In total, 22 air samples were collected.
Table 1.
The concentrations of anaerobic bacteria in sewage and sludge samples
| Sampling point symbol | Name of sampling point | Concentration | ||
|---|---|---|---|---|
| GMa | GSDb | |||
| Sewage [× 104 CFU/mL] | ||||
| S1 | Raw sewage (before the bar screens) | 177.5 | 5.49 | 85.4 |
| S2 | Sewage from primary settling tank | 50.7 | ||
| S3 | Leachate from fermented sludge | 0.018 | ||
| ANOVA | p < 0.001 | |||
| Sewage sludge [× 104 CFU/g] | ||||
| S4 | Screens | 425.0 | 142.7 | 5.1 |
| S5 | Sand from grit remover | 390.0 | ||
| S6 | Dry sludge transported into incineration | 17.6 | ||
| ANOVA | p < 0.001 | |||
aGeometric mean
bGeometric standard deviation
Table 2.
The characteristics of sampling point selected for the assessment of airborne anaerobic bacteria in a wastewater treatment plant
| Sampling point symbol | Name of sampling point | Temperature (°C) | Relative humidity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| July | February | July | February | ||||||
| GMa | GSDb | GM | GSD | GM | GSD | GM | GSD | ||
| A1 | Bar screens | 24.8 | 1.01 | 12.7 | 1.04 | 57.5 | 1.01 | 63.9 | 1.09 |
| A2 | Containers with solids in the screens’ hall | 24.2 | 1.01 | 13.2 | 1.01 | 58.0 | 1.02 | 63.9 | 1.09 |
| A3 | Primary settling tank—entrance to the control room | 24.5 | 1.04 | 6.1 | 1.14 | 58.0 | 1.00 | 73.4 | 1.07 |
| A4 | Sewage sludge pumping station | 24.1 | 1.01 | 14.5 | 1.01 | 58.0 | 1.05 | 40.0 | 1.04 |
| A5 | Aeration basins | 30.9 | 1.06 | 6.7 | 1.02 | 39.9 | 1.11 | 67.0 | 1.00 |
| A6 | Incineration plant—sludge chute | 23.0 | 1.00 | 9.3 | 1.01 | 61.0 | 1.02 | 67.0 | 1.00 |
| A7 | Incineration plant—control room | 23.8 | 1.00 | 21.8 | 1.00 | 52.5 | 1.04 | 43.5 | 1.05 |
| A8 | Sludge-thickening building—press and compactors | 24.8 | 1.00 | 14.3 | 1.04 | 67.5 | 1.03 | 59.5 | 1.01 |
| A9 | Sludge-thickening building—conveyor belts | 24.5 | 1.00 | 7.1 | 1.05 | 59.5 | 1.01 | 69.9 | 1.08 |
| A10 | Sludge-thickening building—control room | 24.5 | 1.01 | 21.8 | 1.00 | 56.0 | 1.05 | 40.5 | 1.02 |
| A11 | Background | 29.2 | 1.04 | 7.2 | 1.03 | 40.8 | 1.15 | 59.4 | 1.06 |
aGeometric mean
bGeometric standard deviation
Sampling methods
Sewage and sludge samples were taken directly into 50 mL sterile, screwed-off Falcon tubes and transported to a laboratory for further analysis.
Air samples were stationary collected using 6-stage Andersen impactor (model 10-710, Graseby-Andersen, Inc., Smyrna, USA), which can separate particles of the following aerodynamic diameters: > 7/4.7/3.3/2.1/1.1/0.65 µm. The impactor was set at a height of approx. 0.5 m above the floor or the ground. The sampling time was 5 min, a flow rate of the air was 28.3 L/min and the volume of each collected air sample was 0.1415 m3. Calibration of the flow rate was carried out before and after each measurement using a digital flow meter (model Gilibrator-2, Sensidyne, Inc., Clearwater, USA). Between the sampling sessions, an impactor was subjected to disinfection and cleaning with isopropyl alcohol. For sampling of bacterial aerosols, impactor was loaded with Petri plates containing Schaedler agar with 5% additive of sheep blood (bioMérieux, Marcy l’Etoile, France). The graph including size distribution results was created with Microsoft Excel 2010 software (Microsoft Corp., Redmond, USA).
Simultaneously with bioaerosol measurements, at each sampling point, the temperature and relative humidity were measured with the use of portable thermo-hygrometer (model TFA 30.5024, Conrad Electronic GmbH, Hirschau, Germany).
Laboratory analysis of samples
Sewage and sludge samples were subjected to extraction in saline solution. From these suspensions, three subsequent ten-fold dilutions were made, which were then plated in 1 mL volumes on Schaedler agar with 5% additive of sheep blood (bioMérieux). Plates with sewage and sludge samples together with these from 6-stage Andersen impactor were incubated using AnaeroGen™ system (Oxoid Ltd., Basingstoke, Great Britain) under the following conditions: 2 days (37 °C) + 2 days (30 °C) to allow development of a wide spectrum of bacterial strains with pathogenic properties (Lagier et al. 2015). The final bacterial concentration was expressed in colony-forming units (CFU) present in 1 millilitre of sewage (CFU/mL), 1 gram of sludge (CFU/g) or 1 cubic metre of sampled air (CFU/m3). Limit of detection (LOD) of air sampling was 7 CFU/m3. Concentration values below LOD (n = 3, 13% of samples) were substituted by the lowest determined value divided by the square root of 2.
Microorganisms isolated from plates were identified to genus and/or species level. The analysis of anaerobic bacteria was based on their ability for enzymatic degradation of organic substrates and subsequent detection of the appropriate metabolites generated by these reactions. For this purpose, a biochemical API 20A test (bioMérieux) allowing identification of the clinically important strains was applied.
Molecular confirmation of Clostridium isolates
Taking into account the biochemical imperfections of bacterial identification methods, molecular analysis of Clostridium pathogens was also carried out on the basis of 16S rRNA gene sequence analysis. DNA was isolated from pure bacterial cultures using the Genomic Mini Kit (A&A Biotechnology, Gdynia, Poland). The isolation was preceded by incubation with lysozyme (25 µL, 10 mg/mL) to facilitate digestion of bacterial cell walls. Isolated DNA was used as a template for PCR reaction with primer pair specific to bacteria of the genus Clostridium: Chis150f (5′-AAAGGRAGATTAATACCGCATAA-3′) and ClostIr (5′-TTCTTCCTAATCTCTACGCA-3′) (Hung et al. 2008) which allow amplification of the gene fragment encoding 16S rRNA. The reaction mixture (20 µL) contained 2 µL of 10 × reaction buffer with MgCl2, 0.5 U of RUN-HS Taq Polymerase (A&A Biotechnology), 250 µM of each deoxynucleotide (dNTP), 0.5 µL of each primer (10 pmol/µL) and 0.5 µL of template DNA. Amplification included 35 cycles preceded by initial denaturation (95 °C, 5 min). Each cycle included denaturation (95 °C, 15 s), annealing (58 °C, 60 s) and elongation (72 °C, 60 s) steps. The reaction ended with a final elongation (72 °C, 5 min). The size of the PCR product and the specificity of the primers were checked by performing electrophoretic analysis in 1.5% agarose gel (Certified™ Molecular Biology Agarose, BioRad, Hercules, USA) and comparing the product size to the DNA fragment marker (GeneRuler 1 kb DNA Ladder, Thermo Scientific, Waltham, USA). The photo of PCR products was taken with GelDoc XR+ camera and created in Image Lab Software version 5.2 (BioRad). The reaction product was enzymatically purified using the EPPiC kit (A&A Biotechnology) and sequenced using the Sanger method on the ABI3730 Genetic Analyzer sequencer (Applied Biosystems Inc., Foster City, USA) in the Laboratory of DNA Sequencing and Oligonucleotide Synthesis at Institute of Biochemistry and Biophysics of Polish Academy of Sciences in Warsaw. The resulting sequences were compared to GeneBank nucleotide sequence database (National Center for Biotechnology Information, US National Library of Medicine, USA) using the BLAST (Basic Local Alignment Search Tool) algorithm.
Statistical analysis
The raw data were used to calculate the geometric means (GM) and geometric standard deviations (GSD). To use the Student’s t test, ANOVA and Pearson’s correlation analyses, all data were subsequently log-transformed. To assess taxonomic diversity between the workplace and background samples, the Chi-square (χ2) test was applied. All calculations were performed using the STATISTICA data analysis software package, version 10. (StatSoft, Inc., Tulsa, USA, 2006), assuming p < 0.05 as statistically significant value.
Results
The average concentration of anaerobic bacteria in the wastewater samples was 5.49 × 104 CFU/mL (GSD = 85.4). The highest values were noted in raw sewage flowing into the treatment plant (P1—1.77 × 106 CFU/mL), and the lowest in the leachate from the digested sludge (P3—185 CFU/mL). Analysis of variance (ANOVA) showed significant differences in concentrations between raw sewage and its subsequent treatment stages (p < 0.001). Taking into account anaerobic bacteria in sludge, the average concentration was 1.42 × 106 CFU/g (GSD = 5.1). The most contaminated were the screenings (P4—4.25 × 106 CFU/g), while the least—dry sediment transported to the incinerator (P6—1.76 × 105 CFU/g) and these differences were significant (p < 0.001) (Table 1).
The results of the quantitative analysis of airborne bacterial biota are presented in Table 3. The geometric mean concentration of bacterial aerosol in summer was 34 CFU/m3, ranging from below the limit of detection (at conveyor belts) to 184 CFU/m3 (at containers with the solids in the screens’ hall). The highest average concentration of this aerosol was noted near the aeration basins (127 CFU/m3). During the winter series of measurements, a slightly higher geometric mean concentration (75 CFU/m3) was observed. The highest winter concentrations of airborne bacteria were found near the bar screens (4.06 × 103 CFU/m3) and close to the containers with the solids (1.12 × 103 CFU/m3). As in the summer, there was no growth of anaerobic bacteria at the conveyor belts, as well as in the control room in the building of sludge thickening. Statistical analysis revealed no difference between the studied seasons (t test p > 0.05). On the other hand, the analysis of variance showed significant differences in bacterial concentrations between the studied workplaces, especially between mechanical wastewater treatment processes and sludge thickening (Scheffe test p < 0.05 for S1–S3 v. S4, S8–S10). It was also found that the concentrations of bioaerosol at workplaces did not differ significantly from their background level (Scheffe test p > 0.05) and they were not significantly determined by the microclimate conditions (Pearson correlation p > 0.05).
Table 3.
The concentrations of anaerobic bacteria in the air at workplaces in the wastewater treatment plant
| Technological process (sampling point symbols) | July | February | TOTAL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GMa (CFU/m3) | GSDb | Range (min–max)c | GM (CFU/m3) | GSD | Range (min–max) | GM (CFU/m3) | GSD | Range (min–max) | |
| Mechanical treatment (A1–A3) | 77 | 3.16 | 21–184 | 765 | 6.59 | 99–4057 | 243 | 6.54 | 21–4057 |
| Sewage sludge treatment (A4, A8–A10) | 16 | 3.35 | 0–64 | 16 | 4.79 | 0–134 | 16 | 3.65 | 0–134 |
| Biological treatment (A5) | 127 | – | – | 21 | – | – | 52 | 3.55 | – |
| Sludge incineration (A6, A7) | 22 | 1.91 | 14–35 | 89 | 1.91 | 57–141 | 44 | 2.62 | 14–141 |
| Total | 34 | 3.45 | 0–184 | 75 | 8.69 | 0–4057 | 50 | 5.83 | 0–4057 |
| Background (A11) | 49 | – | – | 7 | – | – | 19 | 3.96 | – |
aGeometric mean
bGeometric standard deviation
cMin. minimal value, max maximal value
The qualitative analysis of sewage and sludge samples showed the presence of 12 bacterial species belonging to 5 genera: Actinomyces, Bifidobacterium, Clostridium, Propionibacterium and Staphylococcus. In the sewage sludge, among isolated species, Clostridium perfringens was identified. Qualitative analysis of bioaerosol showed the presence of 16 bacterial species belonging to 8 genera (Table 4). It was found that all 16 species were solely identified in the air at mechanical wastewater treatment workplaces (bar screens, containers with solids, primary settling tank). Taxonomical diversity within the abovementioned workplaces was significantly higher compared to background samples (χ2 = 11.8, p < 0.001), the biological treatment stage (χ2 = 5.1, p < 0.05), and the incineration of sewage sludge (χ2 = 6.6, p < 0.05). However, no differences in qualitative composition of bacterial biota were found between sewage and sludge and the air at the workplaces (χ2 = 0.96, p > 0.05). Qualitative analysis of air samples also showed that some of the identified species, such as Actinomyces meyeri, Bifidobacterium spp., Clostridium perfringens or Peptostreptococcus spp., occurred across the whole treatment plant. In turn, the species of the genera Propionibacterium, Bacterioides or Fusobacterium were characteristic for the primary treatment stages only.
Table 4.
Qualitative characteristics of anaerobic bacteria present in the wastewater treatment plant samples
| Genus/species | Sewage and sludge | Air | Risk groupa | |||||
|---|---|---|---|---|---|---|---|---|
| Sewage (S1–S3) | Sewage sludge (S4–S6) | Mechanical treatment (A1–A3) | Sewage sludge treatment (A4, A8–A10) | Biological treatment (A5) | Sludge incineration (A6, A7) | Background (A11) | ||
| Actinomyces spp. | + | + | + | + | + | 2 | ||
| Actinomyces israelii | + | + | + | + | + | + | 2 | |
| Actinomyces meyeri | + | + | + | + | 2 | |||
| Actinomyces naeslundii | + | + | + | 2 | ||||
| Bacteroides spp. | + | 1 | ||||||
| Bacteroides distasonis | + | + | 1 | |||||
| Bacteroides ovatus | + | 1 | ||||||
| Bifidobacterium spp. | + | + | + | + | + | + | + | 1 |
| Clostridium spp. | + | + | 2 | |||||
| Clostridium beijerinckii | + | + | + | + | + | 2 | ||
| Clostridium botulinum | + | 2 | ||||||
| Clostridium innocuum | + | + | + | + | + | 2 | ||
| Clostridium perfringens | + | + | + | + | + | + | 2 | |
| Eggerthella lenta | + | + | + | + | 1 | |||
| Fusobacterium mortiferum | + | + | 1 | |||||
| Peptostreptococcus spp. | + | + | + | + | + | 1 | ||
| Propionibacterium acnes | + | 1 | ||||||
| Propionibacterium granulosum | + | 1 | ||||||
| Propionibacterium propionicus | + | + | 1 | |||||
| Propionibacterium spp. | + | + | + | + | + | 1 | ||
| Staphylococcus saccharolyticus | + | 1 | ||||||
aAccording to the Directive 2000/54/EC
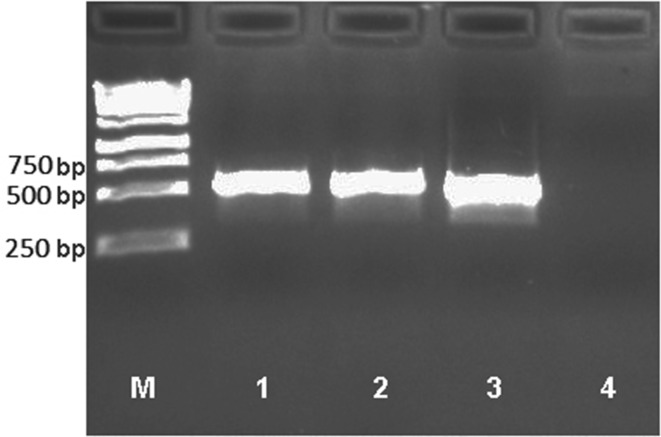
Molecular analysis confirmed the presence of Clostridium strains in the wastewater and in the air (Fig. 1); however, DNA sequencing allowed to determine one species, i.e., Clostridium perfringens only (16S rRNA sequence similarity 99%). This sequence data were submitted to GenBank with MF444962 accession number.
Fig. 1.
Electrophoretic analysis of PCR product using primers specific to the genus Clostridium (Chis150f/ClostIr). As the matrix, DNA isolated from pure cultures of specific strains (identified based on API 20A test) was used 1, Clostridium beijerinckii; 2, Clostridium botulinum; 3, Clostridium perfringens; 4, negative control (instead of DNA, 1 µl of sterile deionised water was added); M, DNA marker
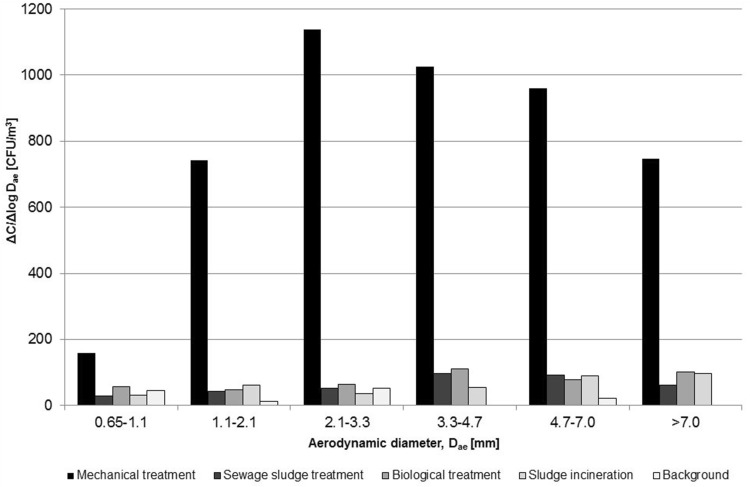
Based on the data obtained using the Andersen impactor, it was possible to analyse the size distribution of anaerobic bacteria (Fig. 2). ANOVA analysis showed statistically significant differences between the technological stages of the plant in the whole range of aerodynamic diameters of bacterial aerosol. The bacterial concentrations at mechanical treatment workplaces were significantly higher than those observed during (other) sewage sludge treatment phases (Scheffe test p < 0.05). Such differences were probably influenced by bacteria from the genus Propionibacterium, which at this sewage treatment stages consisted of about 40% of all the detected microbiota. Analysis of size distribution together with qualitative assessment of isolated species revealed that particles with aerodynamic diameters between 0.65 and 2.1 µm consist of the species of Eggerthella and Bifidobacterium genera, and between 3.3 and 7 µm—the species of Bacteroides and Actinomyces genera. Size distribution analysis showed also that above the aerodynamic diameter of 1.1 µm, the process of aggregation of bacterial cells with dust particles and/or sewage droplets was very pronounced.
Fig. 2.
Size distribution of anaerobic bacteria at workplaces in the wastewater treatment plant
Discussion
The present study confirmed that anaerobic bacteria are commonly present in the wastewater treatment plant and the sewage entering the plant is their main source. The sewer environment creates conditions, which favour the growth of anaerobic bacteria. They are involved in different fermentation processes leading to hydrogen sulfide and methane production as well as the release of volatile organic compounds (odours). Among the various anaerobic microorganisms, the most often present are sulfate-reducing bacteria from, e.g., Desulfovibrio, Desulfotamaculum, Desulfobacter, Desulfuromonas and Desulfococcus genera (Hvitved-Jacobsen 2002; Liu et al. 2015a). It has been also confirmed that bacterial stains from Simplicispira, Comamonas, Azonexus, Thauera and other genera are able to form a biofilm on the walls of the sewers (Satoh et al. 2009; Auguet et al. 2015). Hence, the high dynamics of the processes taking place in WWTP environment as well as the variability of physico-chemical conditions may result in a considerable diversity of the microbial communities in the sewage itself (Liu et al. 2015b). Due to that, it is difficult to directly compare how the results of such research perform under different environmental conditions (e.g., in different climate zones and/or seasons).
As soon as the wastewater flows out from the sewers and subsequently become subject to mechanical treatment processes in WWTP, anaerobic bacteria may be easily released from sewage into the air. Such a situation seems to be natural as the first places of sewage purification (such as bar screens, containers with solids and primary settling tanks) are located at the end of sewerage network. In our study, the phenomenon of such emission was confirmed by the highest bacterial concentrations in the wastewater entering the treatment plant, in screenings, in sand from the grit remover and in the air at workplaces. However, as intensive aeration has negative effect on anaerobic bacteria, they were detected at lower levels in the air and water at subsequent treatment stages.
The qualitative analysis showed a great similarity between the bacteria identified in wastewater and in the air, especially regarding Actinomyces, Bifidobacterium, Clostridium and Propionibacterium genera. In addition, the workplaces where initial phase of wastewater treatment took place were characterized by the largest spectrum of isolated species compared to other sections of the treatment plant. Hence, such a high release rate of bacterial aerosol could be also affected by the location of bar screens and containers with the solids in closed spaces with a limited supply (especially in winter) of the atmospheric air. Similar results were obtained by Fracchia et al. (2006) in the two wastewater treatment plants where the highest microbial concentrations in the air were observed in winter, in the intake chamber (761 CFU/m3) and near the primary settling tank (over 4 × 104 CFU/m3). Furthermore, during summer, they found that significant number of the analysed samples were below the detection level.
At first glance, the search for anaerobic bacteria in the air seems to be a paradox. In such a case, oxygen should normally limit their number in the airborne state. Nevertheless, research has shown that anaerobic bacteria are able to tolerate oxygen in their environment for a relatively long period of time ranging from 45 min (e.g., Peptostreptococcus spp.) up to even 72 h (e.g., C. perfringens) (Rolfe et al. 1978). It was also found that in bioreactors, a special ecosystem can be formed in which both aerobic and anaerobic bacteria may coexist contributing in this way to a more efficient wastewater treatment process (Kato et al. 1997).
The qualitative analysis of anaerobic bacteria in the present study showed moderate taxonomic diversity. The presence of some species from Propionibacterium, Bacteroides, Peptostreptococcus, Bifidobacterium or Clostridium genera seems to have faecal nature and may be directly related to the municipal wastewater. According to some researchers (Ballesté and Blanch 2011; Wéry et al. 2010), these bacteria can be considered as very good indicators of faecal contamination of surface waters. In the present study from among 16 identified species, 7 of them are classified to risk group 2 according to the Directive 2000/54/EC (Directive, 2000) and should be treated as potentially harmful to the health of exposed workers. It should be noted, that the infective dose for most bacteria (including enteric ones) is usually greater than 104 viable cells. However, for some pathogens, even few bacterial cells are needed to cause infection when deposited in the respiratory or digestive systems of a susceptible individual (Crook and Olenchock 1995; Gerardi and Zimmerman 2005).
Among identified bacteria, two species i.e., Actinomyces israelii and Clostridium perfringens deserve special attention due to their isolation frequency and clinical importance. This actinomycete strain may cause suppurative infections of oral as well as thoracic and abdominal cavities (Mabeza and Macfarlane 2003; Smego and Foglia 1998). In turn, Clostridium perfringens is the main etiological agent of myonecreosis of connective tissues (Stevens and Bryant 2002). This bacterium can produce many toxins (including enterotoxin) and enzymes, enhancing in that way its invasiveness. It may be also responsible for food poisoning and diarrhoea (Kądzielska et al. 2012). The growing importance of this species as an indicator of environmental contamination was also confirmed by the fact that in 2008 a draft of the European standard describing the procedure for its determination in sludge, soil and treated bio-waste was prepared (ECN 2008).
Due to the preliminary nature of this study, the qualitative assessment of the isolated strains was mainly based on the biochemical API 20A test, which was preceded with cultivation of bacterial colonies on Schaedler agar. This culture medium guarantees a good growth of anaerobic bacteria, including faecal ones and enables precise selection of colonies for detailed species identification (Murray 1978; Starr et al. 1971). The accuracy of the results obtained using this method are estimated to be 70–85% compared to the other analytical techniques based either on biochemical reactions (Karachewski et al. 1985; Mueller-Spitz et al. 2010) or molecular techniques (Ko et al. 2007). It is clear that molecular techniques utilizing the analysis of 16S rRNA would give more accurate results and it was also shown by our analysis of several samples containing C. perfringens species. It is known that the use of real-time PCR allows for quantitative analysis of pathogens during the sewage treatment process (Shannon et al. 2007). However, the aim of this study was to identify the problem only and the use of API 20A test which is dedicated to clinically relevant strains seemed to be cheaper analytical alternative to molecular methods and fully sufficient for this purpose.
The size distribution analysis of studied bacterial aerosol indicated an ability of bacterial cells to form aggregates with both wastewater droplets and organic dust particles of sewage sludge origin. This process was the most pronounced for the particles of aerodynamic diameters between 2.1 and 3.3 µm at workplaces where the mechanical treatment of wastewater took place. The other studied stages of technological processes did not create such substantial aerosolization of bacterial particles. Although there is no similar data for anaerobic bacteria in the scientific literature, the results for aerobic bacteria in the wastewater treatment plant seem to confirm that, in this range of aerodynamic diameters, the concentration of microorganisms in the air is the highest (Li et al. 2013). As the majority of them can be deposited in the lower parts of the respiratory tract, mainly in secondary bronchi, they may be responsible for adverse health outcomes manifested mainly in the form of allergic alveolitis.
Conclusions
Anaerobic bacteria were widely present both in the sewage and in the air at workplaces in the wastewater treatment plant, reaching the highest concentrations in closed spaces. Some of the identified anaerobic bacteria belonged to the risk group 2 according to the EU Directive 2000/54/EC and should be treated as potentially harmful to the health of exposed workers. Anaerobic bacteria may form aggregates with both wastewater droplets and organic dust particles of sewage sludge origin and as such may be responsible for adverse health outcomes in workers. The control of exposure to anaerobic bacteria should be involved into the risk assessment procedures in wastewater treatment plants and the use of C. perfringens as an indicator of microbial contamination appears to be fully justified.
Acknowledgements
This paper has been based on the results of a research task no. II.P.17 carried out within the scope of the third stage of the National Programme “Improvement of safety and working conditions” partly supported in 2014–2016—within the scope of research and development—by the Ministry of Science and Higher Education/National Centre for Research and Development. The Central Institute for Labour Protection—National Research Institute is the Programme’s main co-ordinator.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ajonina C, Buzie C, Rubiandini RH, Otterpohl R. Microbial pathogens in wastewater treatment plants (WWTP) in Hamburg. J Toxicol Environ Health A. 2015;78:381–387. doi: 10.1080/15287394.2014.989626. [DOI] [PubMed] [Google Scholar]
- Auguet O, Maite P, Guasch-Balcells H, Borrego CM, Gutierrez O. Implications of downstream nitrate dosage in anaerobic sewers to control sulfide and methane emissions. Water Res. 2015;68:522–532. doi: 10.1016/j.watres.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Ballesté E, Blanch AR. Bifidobacterial diversity and the development of new microbial source tracking indicators. Appl Environ Microbiol. 2011;77(10):3518–3525. doi: 10.1128/AEM.02198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook B, Olenchock SA. Industrial workplaces. In: Cox CS, Wathes CM, editors. Bioaerosols handbook. Boca Raton: CRC Press Inc.; 1995. pp. 531–545. [Google Scholar]
- Cyprowski M, Krajewski JA. Harmful agents in municipal waste water treatment plants. Med Pr. 2003;54(1):73–80. [PubMed] [Google Scholar]
- Cyprowski M, Szarapińska-Kwaszewska J, Dudkiewicz B, Krajewski JA, Szadkowska-Stańczyk I. Exposure assessment to harmful agents in workplaces in sewage plant workers. Med Pr. 2005;56(3):213–222. [PubMed] [Google Scholar]
- Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC). OJ L 262, Brussels
- ECN (Energy research Centre of the Netherlands) (2008) European Standard (Project): soils, sludges and treated bio-wastes—Isolation and enumeration of Clostridium perfringens in sludges, soils and treated bio-wastes—part 1: membrane filtration method onto selective agar. https://www.ecn.nl/docs/society/horizontal/D2_2.5_HOR-HYG_Clos_Filtration.pdf. Accessed 28 Mar 2018
- Fracchia L, Pietronave S, Rinaldi M, Martinotti MG. Site-related airborne biological hazard and seasonal variations in two wastewater treatment plants. Water Res. 2006;40:1985–1994. doi: 10.1016/j.watres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Gerardi MH, Zimmerman MC. Wastewater pathogens. New Jersey: Wiley; 2005. [Google Scholar]
- Hung CH, Cheng CH, Cheng LH, Liang CM, Lin CY. Application of Clostridium-specific PCR primers on the analysis of dark fermentation hydrogen-producing bacterial community. Int J Hydrogen Energ. 2008;33:1586–1592. doi: 10.1016/j.ijhydene.2007.09.037. [DOI] [Google Scholar]
- Hvitved-Jacobsen T. Sewer processes—microbial and chemical process engineering of sewer networks. Boca Raton: CRC Press; 2002. [Google Scholar]
- Kądzielska J, Obuch-Woszczatyński P, Pituch H, Młynarczyk G. Clostridium perfringens as the etiological agent of antibiotic associated diarrhoea. Postęp Microbiol. 2012;51(1):17–25. [Google Scholar]
- Karachewski NO, Busch EL, Wells CL. Comparison of PRAS II, RapID ANA, and API 20A systems for identification of anaerobic bacteria. J Clin Microbiol. 1985;21:122–126. doi: 10.1128/jcm.21.1.122-126.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato MT, Field JA, Lettinga G. Anaerobe tolerance to oxygen and the potentials of anaerobic and aerobic cocultures for wastewater treatment. Braz J Chem Eng. 1997;14(4):395–407. doi: 10.1590/S0104-66321997000400015. [DOI] [Google Scholar]
- Ko KS, Kuwahara T, Haehwa L, Yoon YJ, Kim BJ, Lee KH, Ohnishi Y, Kook YH. RNA polymerase beta-subunit gene (rpoB) sequence analysis for the identification of Bacteroides spp. Clin Microbiol Infect. 2007;13:48–54. doi: 10.1111/j.1469-0691.2006.01553.x. [DOI] [PubMed] [Google Scholar]
- Korzeniewska E. Emission of bacteria and fungi in the air from wastewater treatment plants—a review. Front Biosci (Schol Ed) 2011;3:393–407. doi: 10.2741/s159. [DOI] [PubMed] [Google Scholar]
- Lagier LC, Edouard S, Pagnier I, Mediannikov O, Drancourt M, Raoult D. Current and past strategies for bacterial culture in clinical microbiology. Clin Microbiol Rev. 2015;28(1):208–236. doi: 10.1128/CMR.00110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yang L, Meng Q, Qiu X, Feng Y. Emission characteristics of microbial aerosols in a municipal sewage treatment plant in Xi’an, China. Aerosol Air Quality Res. 2013;13:343–349. [Google Scholar]
- Lisle JT, Smith JJ, Edwards DD, McFeters GA. Occurrence of microbial indicators and Clostridium perfringens in wastewater, water column samples, sediments, drinking water, and Weddell seal feces collected at McMurdo Station, Antarctica. Appl Environ Microbiol. 2004;70(12):7269–7276. doi: 10.1128/AEM.70.12.7269-7276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dong Q, Shi H. Distribution and population structure characteristics of microorganisms in urban sewage system. Appl Microbiol Biot. 2015;99(18):7723–7734. doi: 10.1007/s00253-015-6661-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dong Q, Wu C, Zhou X, Shi H. Study of the succession of microbial communities for sulfur cycle response to ecological factors change in sediment of sewage system. Environ Sci Pollut Res. 2015;22:9250–9259. doi: 10.1007/s11356-014-3934-0. [DOI] [PubMed] [Google Scholar]
- Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J. 2003;21:545–551. doi: 10.1183/09031936.03.00089103. [DOI] [PubMed] [Google Scholar]
- Mueller-Spitz SR, Stewart LB, McLellan SL. Reliability of mCP method for identification of Clostridium perfringens from faecal polluted aquatic environments. J Appl Microbiol. 2010;108:1994–2002. doi: 10.1111/j.1365-2672.2009.04605.x. [DOI] [PubMed] [Google Scholar]
- Murray PR. Growth of clinical isolates of anaerobic bacteria on agarmedia: effects of media composition, storage conditions, and reduction under anaerobic conditions. J Clin Microbiol. 1978;8(6):708–714. doi: 10.1128/jcm.8.6.708-714.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai SD, Widmer KW, Dowd SE, Ricke SC. Occurrence of airborne bacteria and pathogen indicators during land application of sewage sludge. Appl Environ Microbiol. 1996;62(1):296–299. doi: 10.1128/aem.62.1.296-299.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe RD, Hentges DJ, Campbell BJ, Barrett JT. Factors related to the oxygen tolerance of anaerobic bacteria. Appl Environ Microbiol. 1978;36(2):306–313. doi: 10.1128/aem.36.2.306-313.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Odagiri M, Ito T, Okabe S. Microbial community structures and in situ sulphate-reducing and sulphur-oxidizing activities in biofilms developed on mortar specimens in a corroded sewer system. Water Res. 2009;43:4729–4739. doi: 10.1016/j.watres.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Lee DY, Trevors JT, Beaudette LA. Application of real-time quantitative PCR for detection of selected bacterial pathogens during municipal wastewater treatment. Sci Total Environ. 2007;382:121–129. doi: 10.1016/j.scitotenv.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Smego RA, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26:1255–1263. doi: 10.1086/516337. [DOI] [PubMed] [Google Scholar]
- Sorber CA, Sagik BP. Indicators and pathogens in wastewater aerosols and factors affecting survivability. In: Pahren H, Jakubowski W, editors. Wastewater aerosols and disease, EPA 600/980-078. Cincinnati, OH: U.S. Environmental Protection Agency, Health Effects Research Laboratory; 1980. pp. 23–35. [Google Scholar]
- Starr SE, Killgore GE, Dowell VR. Comparison of Schaedler agar and Trypticase Soy-yeast extract agar for the cultivation of anaerobic bacteria. Appl Microbiol. 1971;22(4):655–658. doi: 10.1128/am.22.4.655-658.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DL, Bryant AE. The role of clostridial toxins in the pathogenesis of gas gangrene. Clin Infect Dis. 2002;35(Suppl 1):S93–S100. doi: 10.1086/341928. [DOI] [PubMed] [Google Scholar]
- Van Lier JB, Mahmoud N, Zeeman G. Anaerobic wastewater treatment. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D, editors. Biological wastewater treatment, principles, modelling and design. London: IWA Publishing; 2008. pp. 415–456. [Google Scholar]
- Wang CC, Chang CW, Chu CP, Lee DJ, Chang BV, Liao CS. Producing hydrogen from wastewater sludge by Clostridium bifermentans. J Biotechnol. 2003;102:83–92. doi: 10.1016/S0168-1656(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Wéry N, Monteil C, Pourcher AM, Godon JJ. Human-specific fecal bacteria in wastewater treatment plant effluents. Water Res. 2010;44:1873–1883. doi: 10.1016/j.watres.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Zinder SH, Mah RA. Isolation and characterization of a thermophilic strain of Methanosarcina unable to use H2-CO2 for methanogenesis. Appl Environ Microbiol. 1979;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]