Abstract
Primary hemifacial spasm (HFS) is a disorder that causes frequent involuntary contractions in the muscles on one side of the face, due to a blood vessel compressing the nerve at its root exit zone (REZ) from the brainstem. Numerous prospective and retrospective case series have confirmed the efficacy of microvascular decompression (MVD) of the facial nerve in patients with HFS. However, while MVD is effective, there are still significant postoperative complications. In this paper, recent technological advances related to MVD (such as lateral spread response, brainstem auditory evokes potential, three dimensional time of flight magnetic resonance angiography, intraoperative neuroendoscopy) are reviewed for the purposes of improving MVD treatment efficacy and reducing postoperative complications.
Keywords: Hemifacial spasm (HFS), Microvascular decompression (MVD), Lateral spread response, Three dimensional time of flight magnetic resonance angiography, Neuroendoscopy
Hemifacial spasm (HFS) is a disorder characterized by involuntary contractions of facial muscles, usually on one side of the face that can be intermittent, rhythmic or sustained. The contraction often starts from orbicularis oculi and spreads to multiple facial expression muscles, and can be triggered or exacerbated by emotional excitability, stress, fatigue or excessive speech. Overseas epidemiology surveys suggest a prevalence of 0.78/100 000 (Auger and Whisnant, 1990), more often seen in women (male:female = 1:2) and rare in children (Titlić et al., 2006). Most cases involve one side of the face, often on left (left:right = 3:2), and bilateral involvement is seen in less than 1% of cases. Primary HFS usually results from microvascular compression of the facial nerve at the root exit zone (REZ). Long term pressure and irritation from the offending vessel cause local demyelination and “shortening” between nerve fibers, leading to ectopic bioelectric transmission (Zhu et al., 2012). About 1–2% of HFS cases are secondary to space occupying lesions in the cerebellopontine angle or posterior fossa. Familial cases have been reported, but in general HFS is not considered hereditary (Lagalla et al., 2010). Many treatments for HFS have been reported, including pharmacological agents, botulinum toxin injection, facial nerve blockage, physical therapy, radiofrequency ablation, acupuncture, as well as facial nerve combing and microvascular decompression (MVD). In 1959, Gardne first reported a case of trigeminal neuralgia caused by vascular compression (Gardner and Miklos, 1959). In 1966, based on Gardne's findings, Jannetta suggested a theory of neural circuitry shortening as a result of demyelination of facial nerve root from vascular compression that could be the cause for more than 95% of HFS cases, and started treating HFS with MVD. Following Jannetta, experiences from others confirmed the efficacy of MVD in treating HFS, which has now become the first choice of treatment for HFS (Jannetta et al., 1977). A review that included 22 reports and 5685 cases of MVD with an average follow up of 2.9 years showed rates of complete symptom resolution as high as 91.1% (Miller and Miller, 2012). Chung et al. reported 1169 cases of MVD for HFS with an average follow up of 28.3 months and overall effective rate of 95% (Chung et al., 2001). At this time, MVD enjoys well established techniques and confirmed efficacy, but certain postoperative complications remain, including hearing loss (0.8–16.2%) and facial paresis (1.2–16.2%) (Jannetta et al., 1977). Many continue to work to improve MVD outcomes and reduce complications. Currently most efforts aim at application of new technologies, such as intraoperative electrophysiological monitoring, neuroimaging three dimensional reconstruction and neuroendoscopy.
1. Intraoperative neuroelectrophysiological monitoring
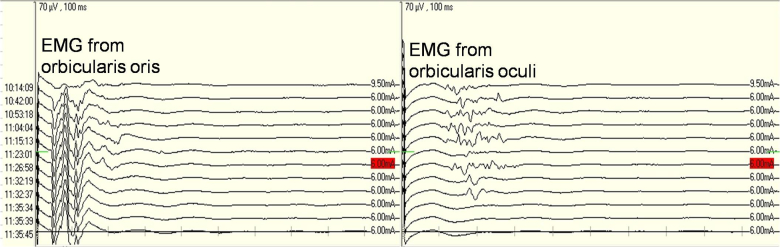
Lateral spread response (LSR), also known as abnormal muscle response, is an abnormal neuromuscular response recorded in HFS patients from muscles supplied by a facial nerve branch different than the one being stimulate (Thirumala et al., 2011). Its mechanisms involve bidirectional conduction of neural impulses. When the mandibular branch of the facial nerve is stimulated in patients with HFS, neural signals can be transmitted not only to the orbicularis oris, causing its contraction, but also in a retrograde fashion toward the facial nucleus in the brainstem, where abnormalities along the proximal facial nerve or inside the facial nucleus can generate abnormal impulses that can return along a different facial nerve branch (zygomatic branch for example) and arrive at orbicularis oculi, resulting a signal with a latency of 8–10 ms (delayed by 4–5 ms compared to the primary orbicularis oris response). This abnormal muscle response indicates abnormal cross connection between different branches of the facial nerve, which is currently believed to be the etiological basis for HFS. Intraoperatively, disappearance of LSR following separation of the offending vessel can be used to confirm the offending vessel and predict postoperative results. Kong et al. (2007) performed MVD in 300 cases of HFS and recorded LSR in 263 cases (87.7%), of which LSR disappeared in 230 cases (87.4%) and persisted in 33 cases (12.5%). At one year follow up, those in whom LSR disappeared during surgery showed significantly better results than those in whom LSR persisted. Neves et al. (2009) studied 32 cases of HFS and concluded that use of intraoperative LRS monitoring could not only predict short term outcomes but also impact long term treatment results. Reports from Kang et al. (2012) and Kim et al. (2010) also support the value of LSR monitoring in MVD. Thirumala et al. (2011) performed electrophysiological monitoring in 293 cases of HFS that underwent surgical treatment and recorded LSR in 259 of these cases (87.7%), of which LSR disappeared following decompression in 207 cases (Group 1) but persisted in 52 cases (Group 2). Group 1 showed a rate of symptom resolution of 94.7% within 24 h following surgery and 93.3% at discharge from hospital, and the rate was 67.3% and 76.9% respectively in Group 2 (P < 0.0001). At 54.5 months following surgery, the rate of symptom resolution was 93.3% in Group 1 and 94.4% in Group 2 (P = 1.000). They therefore concluded that LSR monitoring might predict treatment effects immediately following surgery but not over long term. Joo et al. (2008) also studied the value of LSR in MVD and questioned its value in indicting long term treatment outcomes. In China, extensive studies seem to confirm a positive role of LSR monitoring in MVD (Gao and Zhao, 2013a, Liu et al., 2010, Ying et al., 2011). The authors have used LSR monitoring in relevant surgeries and believe that it is to a certain extent helpful in confirming offending vessels and predicting treatment effects (Fig. 1).
Fig. 1.
Triggered EMG from orbicularis oris (left) and orbicularis oculi (right). Note the disappearance of EMG activities from orbicularis oculi shortly after separation of the offending vessel from the facial nerve (red mark).
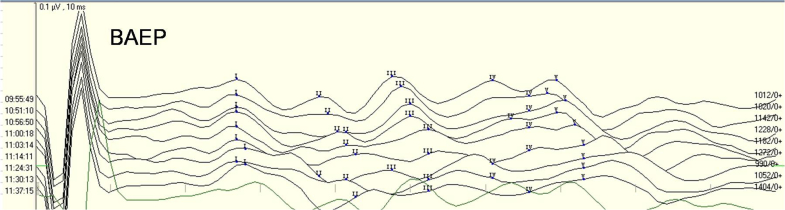
Brainstem auditory evokes potential (BAEP), nerve action potential (NAP) and electrocochleography (EcochG) are the three main techniques for intraoperative monitoring of the auditory nerve. BAEP is non-invasive and capable of assessing function of auditory pathways from the periphery up to the brainstem, and therefore is probably the most commonly used. BAEP is short latency auditory responses representing neuroelectric activities up to the brainstem level in response to acoustic stimulation. It has defined temporal relationship to the stimulus and is not affected by sleep or anesthesia. In an MVD procedure, the surgeon operates in the cerebellopontine angle area where the facial and auditory nerves are located extremely closely to each other and the auditory nerve and neighboring vessels can be impacted by surgical maneuvers (Dou et al., 2009). Rates of hearing impairment at an early time following surgery as high as 14–18% have been reported Jannetta and Kassam, 1999, Murai et al., 1991. Usage of intraoperative electrophysiological monitoring and improvement in microscopic ear surgery have greatly reduced hearing complications. Intraoperative BAEP monitoring helps reduce the rate of postoperative hearing impairment. Delay of BAEP waves I, III or V by ≥10% or a decrease in wave amplitude of 50% or more is considered indicating auditory nerve damage McLaughlin et al., 1999, Akagami et al., 2005] that requires immediate actions to assess and protect the nerve. In 1991, Wilkins (1991) reported that the rate of hearing damage was 7.7–20% before use of BAEP in MVD, which improved to less than 5% after BAEP was routinely used in MVD as shown in numerous reports. Li et al. (2004) reported use of BAEP during MVD in 400 cases of HFS and assessment and correction of factors affecting auditory function as indicated by real time BAEP monitoring. Their results indicated that the rate of postoperative hearing impairment fell from 7.1% before adoption of BAEP monitoring to 2.5% after, proving the value of BAEP in reducing hearing damage during surgical treatment of HFS. Gao and Zhao (2013b) also came to the same conclusion from their studies. Lee et al. (2014) adopted BEAP and neuroendoscopy during MVD in 43 cases of HFS. Follow up (average 3.5 years) results showed a rate of symptom relief of 100% and a rate of hearing impairment at 2%. They concluded that the combination of BEAP monitoring and neuroendoscopy helped improve treatment outcomes and reduce complications. Ying et al. (2014) reviewed 94 cases in which BAEP monitoring was employed and reported correlation of complications to amplitude decrease of wave V and increase of I–V interpeak latency, but not to gender, age, side of surgery or duration of symptoms, concluding that BAEP changes must be closely monitored. Huang et al. (2009) also support that BAEP monitoring during MVD helps reduce surgical complications. The authors use BAEP monitoring during MVD and believe that it is valuable in protecting the auditory nerve and reducing hearing impairment (Fig. 2).
Fig. 2.
Recording of BAEP during a left side MVD procedure.
2. Three dimensional reconstruction of nerve and blood vessels
In primary HFS, due to insufficient partial volume effect, CT and regular MRI do not provide clear information on anatomical relations among nerves and blood vessels in the cebellopontine angle area. In recent years, high resolution MRI and imaging processing software have made assessment of relations between cranial nerves and blood vessels possible. These technologies provide three dimensional reconstruction of the brainstem, nerves and blood vessels, allowing study of their structure and relations from various perspectives and greatly facilitating the planning before a MVD procedure. Three dimensional time of flight magnetic resonance angiography (3D-TOF-MRA), three-dimensional spoiled gradient recalled acquisition in steady state imaging (3D-SPGR) and three-dimensional constructive interference in steady state (3D-CISS) are the three most commonly used technologies for pre-operative assessment.
Niwa et al. (1996) reconstructed cranial nerves and neighboring blood vessels using 3D-TOF-MRA and 3D-TOF-SPGR in 100 cases of trigeminal neuralgia and 53 cases of HFS and found compression at the REZ in 67% and 87% of these cases respectively. MVD in HFS patients confirmed compression in 90% of the cases. A study of the trigeminal (n = 206) and facial (n = 253) nerves in normal subjects showed signs of compression in the REZ in 31.6% and 22.5% of these subjects, respectively. Satoh et al. (2007a) reconstructed nerves and blood vessels using 3D-TOF-MR ventriculography and 3D-TOF-MRA technologies and concluded that they could facilitate diagnosis and treatment planning. Using endoscopy, El Refaee et al. (2013) have demonstrated the diagnostic value of 3D-TOF-MRA in defining the relations between nerves and blood vessels in the posterior fossa. Using 3D-CISS, Tanrikulu et al. (2007) reconstructed nerves and blood vessels in 50 cases with a positive rate of 98%. In 2007, Satoh et al. (2007b) reported 12 cases of HFS treated with MVD, all with preoperative three dimensional MRI reconstruction showing the origin of and location of compression by the offending vessel, which was confirmed during surgery. In a report in 2008, Takao et al. (2008) located 6 compression points in 7 cases via virtual endoscopy, although 8 compression points were identified during MVD surgery, yielding a 75% (6/8) diagnostic sensitivity of MRI 3-D reconstruction in locating HFS compression points. In addition to application in HFS, 3D-TOF-MRA, 3D-TOF-SPGR and 3D-CISS can also play a significant role in the management of trigeminal and hypoglossal neuralgia (Gaul et al., 2011). The authors have used the 3D Slicer (4.3.0) software in combination with diffusion tensor imaging in reconstructing the facial nerve, surrounding blood vessels and brainstem and found it to be useful in identifying offending vessels preoperatively and guiding surgical approaches (Fig. 3). However, MR 3D reconstruction is not perfect, with certain rates of false-positive or false-negative results, poor imaging of veins, difficulties in reconstructing arterioles and incomplete imaging of all vessels when there are multiple offending vessels. Furthermore, vascular compression is not the sole etiology of HFS. Other HFS etiologies including arachnoid adhesion and associated nerve tension may not be visible on 3D-TOF-MRA.
Fig. 3.
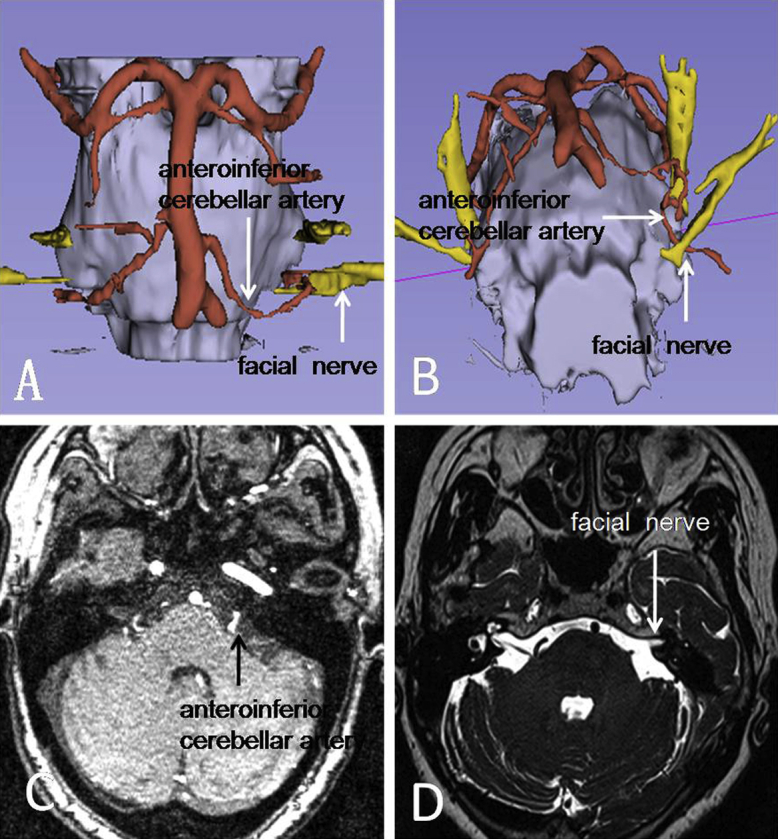
Reconstruction of left facial nerve and blood vessels before MVD using the 3D Slicer 4.3.0 software. A–B: Showing a close relation between the facial nerve REZ and anteroinferior cerebellar artery. C: 3D-TOF-MRA shows a small enhancing artery near the facial nerve root and brainstem. D: T2 imaging showing facial nerve REZ and projection.
3. Neuroendoscopy
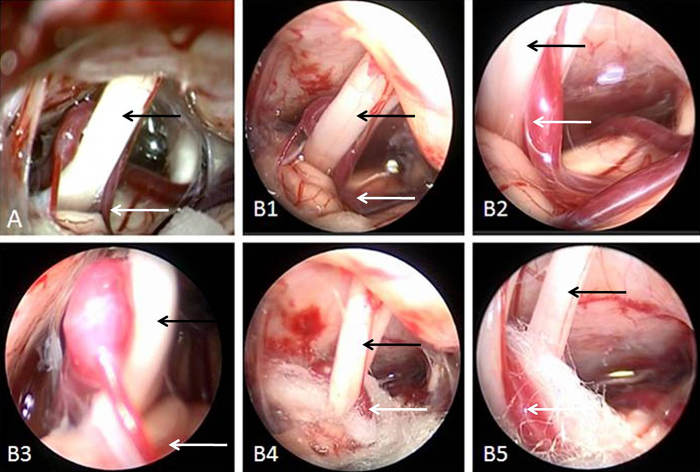
Neuroendcospy has been widely used to treat a variety of intracranial lesions. In recent years, it has gained favor among many scholars for its cold light source, multi-angle capability, magnification and peephole effects. It has been pointed out that, facilitated with neuroendoscopy, a keyhole approach MVD in treating HFS is minimally invasive while yielding satisfactory outcomes with reduced complications. Broggi et al. (2013) performed MVD in 141 patients with use of endoscopy in 40 of these patients. In 12 of these cases, examination under surgical microscope failed to reveal an offending vessel. They concluded that, although most offending vessels can be located under the surgical microscope, endoscopy can be used as an adjunct technology when microscopy fails. In a series of 133 MVD cases by Shimanskiĭ et al. (2012), the offending vessel was identified by endoscopy in 9 cases for HFS where microscopy had failed. They further suggested that endoscopy could be used not only to locate the offending vessel but also to guide placement of the separation graft. There were no postoperative complications in this group of patients. Liang et al. (2009) and Artz et al. (2008) reported their experiences with endoscopy in MVD in comparison to traditional microscopy and confirmed that its use was safe, effective and led to reduced complications and hospital stay time. Yuan et al. (2004) used endoscopy in treating 100 patients with trigeminal neuralgia and HFS and reported no symptoms recurrence at an average follow up of 3.2 years. They also indicated that neuroendoscopy was helpful in identifying the offending vessel, assessing decompression of the facial REZ and guiding placement of the separating graft, resulting in improved treatment outcomes and reduced symptom recurrence as well as complications. The authors have started using endoscopy as an adjunct to microscopy during MVD and felt that it allows examination of areas not accessible by the microscope and can be helpful to complete understanding of nerves and blood vessels in the cerebellopontine angle area and to the success of surgery (Fig. 4).
Fig. 4.
A: Microscopic images showing noticeable tunnel vision disadvantage. B1–3: Neuroendoscopy allows examination of the facial nerve and offending vessel from various angles. B4–5: Endoscopy allows assessment of the location of Teflon separation graft and its relations to the facial nerve (black arrow head) as well as the offending vessel (white arrow head).
However, some have pointed out that, in comparison to the well-established microscopy methodology, neuroendoscopy is a developing technology that does not yet provide depth vision and may lead to lower success rate when used alone (Yuan et al., 2004). Some noticeable shortcomings with neuroendoscopy include blind spots, lack of fixation support, difficult to use when trying to stop bleeding and relatively complex maneuvers, which may limit its broad utility. However, along with technology advances and development of image merging technology and supporting accessories, utility of neuroendoscopy in MVD will continue to expand.
In summary, efficacy of MVD in HFS is well established and has greatly improved with application of new technologies, while surgical complications have significantly decreased. However, use of intraoperative electrophysiological technologies, neuroimaging 3-D reconstruction and neuroendoscopy remains limited, especially in China. It is expected that, as neuroimaging and image processing technologies continue to advance, MVD will serve to relieve suffering in more patients with HFS.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Akagami R., Dong C.C., Westerberg B. Localized transcranial electrical motor evoked potentials for monitoring cranial nerves in cranial base surgery. Neurosurgery. 2005;57(1 Suppl.):78–85. doi: 10.1227/01.neu.0000163486.93702.95. [DOI] [PubMed] [Google Scholar]
- Artz G.J., Hux F.J., Larouere M.J., Bojrab D.I. Endoscopic vascular decompression. Otol. Neurotol. 2008;29(7):995–1000. doi: 10.1097/MAO.0b013e318184601a. [DOI] [PubMed] [Google Scholar]
- Auger R.G., Whisnant J.P. Hemifacial spasm in Rochester and Olmsted county, Minnesota, 1960 to 1984. Arch. Neurol. 1990;47(11):1233–1234. doi: 10.1001/archneur.1990.00530110095023. [DOI] [PubMed] [Google Scholar]
- Broggi M., Acerbi F., Ferroli P. Microvascular decompression for neurovascular conflicts in the cerebello-pontine angle: which role for endoscopy? Acta Neurochir. (Wien) 2013;155(9):1709–1716. doi: 10.1007/s00701-013-1824-8. [DOI] [PubMed] [Google Scholar]
- Chung S.S., Chang J.H., Choi J.Y. Microvascular decompression for hemifacial spasm: a long-term follow-up of 1,169 consecutive cases. Stereotact. Funct. Neurosurg. 2001;77(1–4):190–193. doi: 10.1159/000064620. [DOI] [PubMed] [Google Scholar]
- Dou W.C. People's Medical Publishing House; Beijing: 2009. Intraoperative Neurophysiological Monitoring; pp. 201–203. [Google Scholar]
- El Refaee E., Langner S., Baldauf J. Value of 3-dimensional high-resolution magnetic resonance imaging in detecting the offending vessel in hemifacial spasm: comparison with intraoperative high definition endoscopic visualization. Neurosurgery. 2013;73(1):58–67. doi: 10.1227/01.neu.0000429838.38342.e2. [DOI] [PubMed] [Google Scholar]
- Gao Z.F., Zhao C.S. The value of electrophysiological monitoring in microvascular decompression for primary hemi-facial spasm. Jiangsu Med. 2013;39:1904–1906. [Google Scholar]
- Gao Z.F., Zhao C.S. Role of electrophysiological monitoring during microvascular decompression for primary hemifacial spsam. Jiangsu Med. J. 2013;39:1904–1905. [Google Scholar]
- Gardner W.J., Miklos M.V. Response of trigeminal neuralgia to decompression of sensory root; discussion of cause of trigeminal neuralgia. J. Am. Med. Assoc. 1959;170(15):1773–1776. doi: 10.1001/jama.1959.03010150017004. [DOI] [PubMed] [Google Scholar]
- Gaul C., Hastreiter P., Duncker A. Diagnosis and neurosurgical treatment of glossopharyngeal neuralgia: clinical findings and 3-D visualization of neurovascular compression in 19 consecutive patients. J. Headache Pain. 2011;12(5):527–534. doi: 10.1007/s10194-011-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B.R., Chang C.N., Hsu J.C. Intraoperative electrophysiological monitoring in microvascular decompression for hemifacial spasm. J. Clin. Neurosci. 2009;16(2):209–213. doi: 10.1016/j.jocn.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Jannetta P.J., Kassam A. J. Neurol. Neurosurg. Psychiatry. 1999;66(2):255–256. doi: 10.1136/jnnp.66.2.255a. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannetta P.J., Abbasy M., Maroon J.C. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J. Neurosurg. 1977;47(3):321–328. doi: 10.3171/jns.1977.47.3.0321. [DOI] [PubMed] [Google Scholar]
- Joo W.I., Lee K.J., Park H.K. Prognostic value of intra-operative lateral spread response monitoring during microvascular decompression in patients with hemifacial spasm. J. Clin. Neurosci. 2008;15(12):1335–1339. doi: 10.1016/j.jocn.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kang M.C., Choi Y.S., Choi H.K. Efficacy of the disappearance of lateral spread response before and after microvascular decompression for predicting the long-term results of hemifacial spasm over two years. J. Korean Neurosurg. Soc. 2012;52(4):372–376. doi: 10.3340/jkns.2012.52.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Kong D.S., Lee J.A. The potential value of the disappearance of the lateral spread response during microvascular decompression for predicting the clinical outcome of hemifacial spasms: a prospective study. Neurosurgery. 2010;67(6):1581–1587. doi: 10.1227/NEU.0b013e3181f74120. [DOI] [PubMed] [Google Scholar]
- Kong D.S., Park K., Shin B.G. Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J. Neurosurg. 2007;106(3):384–387. doi: 10.3171/jns.2007.106.3.384. [DOI] [PubMed] [Google Scholar]
- Lagalla G., Logullo F., Di Bella P. Familial hemifacial spasm and determinants of late onset. Neurol. Sci. 2010;31(1):17–22. doi: 10.1007/s10072-009-0153-4. [DOI] [PubMed] [Google Scholar]
- Lee C.C., Liao C.H., Lin C.F. Brainstem auditory evoked potential monitoring and neuro-endoscopy: two tools to ensure hearing preservation and surgical success during microvascular decompression. J. Chin. Med. Assoc. 2014;77(6):308–316. doi: 10.1016/j.jcma.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Li R., Chen G.Q., Guo J. Use of auditory evoked potentials monitoring during microvascular decompression for hemi-facial spasm. Chin. J. Microinvasive Neurosurg. 2004;9:199–201. [Google Scholar]
- Liang J., Li G., Shen Y. Microvascular decompression for the hemifacial spasm with endoscopy. Lin. Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;23(4):145–148. [PubMed] [Google Scholar]
- Liu Q.J., Zhang Y.P., Zhu J. Monitoring of abnormal muscle response during microvascular decompression for hemi-facial spasm. J. Tianjin Med. Univ. 2010;16:264–266. [Google Scholar]
- McLaughlin M.R., Jannetta P.J., Clyde B.L. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J. Neurosurg. 1999;90(1):1–8. doi: 10.3171/jns.1999.90.1.0001. [DOI] [PubMed] [Google Scholar]
- Miller L.E., Miller V.M. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: a systematic review. Br. J. Neurosurg. 2012;26(4):438–444. doi: 10.3109/02688697.2011.641613. [DOI] [PubMed] [Google Scholar]
- Murai K., Kon Y., Obara Y. A study on auditory disturbances after microvascular decompression for hemifacial spasm. Nihon Jibiinkoka Gakkai Kaiho. 1991;94(5):657–666. doi: 10.3950/jibiinkoka.94.657. [DOI] [PubMed] [Google Scholar]
- Neves D.O., Lefaucheur J.P., de Andrade D.C. A reappraisal of the value of lateral spread response monitoring in the treatment of hemifacial spasm by microvascular decompression. J. Neurol. Neurosurg. Psychiatry. 2009;80(12):1375–1380. doi: 10.1136/jnnp.2009.172197. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Shiotani M., Karasawa H. Identification of offending vessels in trigeminal neuralgia and hemifacial spasm using SPGR-MRI and 3D-TOF-MRA. Rinsho Shinkeigaku. 1996;36(4):544–550. [PubMed] [Google Scholar]
- Satoh T., Onoda K., Date I. Preoperative simulation for microvascular decompression in patients with idiopathic trigeminal neuralgia: visualization with three-dimensional magnetic resonance cisternogram and angiogram fusion imaging. Neurosurgery. 2007;60(1):104–113. doi: 10.1227/01.NEU.0000249213.34838.C9. [DOI] [PubMed] [Google Scholar]
- Satoh T., Onoda K., Date I. Fusion imaging of three-dimensional magnetic resonance cisternograms and angiograms for the assessment of microvascular decompression in patients with hemifacial spasms. J. Neurosurg. 2007;106(1):82–90. doi: 10.3171/jns.2007.106.1.82. [DOI] [PubMed] [Google Scholar]
- Shimanskiĭ V.N., Karnaukhov V.V., Sergienko T.A. Endoscopic assistance in microvascular decompression of cranial nerves. Zh Vopr Neirokhir Im N N Burdenko. 2012;76(2):3–10. [PubMed] [Google Scholar]
- Takao T., Oishi M., Fukuda M. Three-dimensional visualization of neurovascular compression: presurgical use of virtual endoscopy created from magnetic resonance imaging. Neurosurgery. 2008;63.1(Suppl. 1):ONS139–145. doi: 10.1227/01.neu.0000335028.77779.7c. [DOI] [PubMed] [Google Scholar]
- Tanrikulu L., Hastreiter P., Troescher-Weber R. Intraoperative three-dimensional visualization in microvascular decompression. J. Neurosurg. 2007;107(6):1137–1143. doi: 10.3171/JNS-07/12/1137. [DOI] [PubMed] [Google Scholar]
- Thirumala P.D., Shah A.C., Nikonow T.N. Microvascular decompression for hemifacial spasm: evaluating outcome prognosticators including the value of intraoperative lateral spread response monitoring and clinical characteristics in 293 patients. J. Clin. Neurophysiol. 2011;28(1):56–66. doi: 10.1097/WNP.0b013e3182051300. [DOI] [PubMed] [Google Scholar]
- Titlić M., Vrebalov-Cindro V., Lahman-Dorić M. Hemifacial spasm in vertebrobasilar dolichoectasia. Acta Neurol. Belg. 2006;106(1):23–25. [PubMed] [Google Scholar]
- Wilkins R.H. Hemifacial spasm: a review. Surg. Neurol. 1991;36(4):251–277. doi: 10.1016/0090-3019(91)90087-p. [DOI] [PubMed] [Google Scholar]
- Ying T.T., Li X.Y., Li S.T. Application of abnormal muscle response tests during microvascular decompression of facial nerve. Chin. J. Neurosur. 2011;27(5):444–448. [Google Scholar]
- Ying T., Thirumala P., Chang Y. Emprical factors associated with brainstem auditory evoked potential monitoring during microvascular decompression for hemifacial spasm and its correlation to hearing loss. Acta Neurochir. (Wien) 2014;156(3):571–575. doi: 10.1007/s00701-013-1957-9. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang L., Li R. Application of neuroendoscopy in surgical treatment of trigeminal neuralgia and hemi-facial spasm. J. Sterotactic Funct. Neurosurg. 2004;17:99–101. [Google Scholar]
- Zhu J., Li S.T., Zhong J. Role of arterioles in management of microvascular decompression in patients with hemifacial spasm. J. Clin. Neurosci. 2012;19(3):375–379. doi: 10.1016/j.jocn.2011.04.046. [DOI] [PubMed] [Google Scholar]