Abstract
Objective
To investigate the membrane localization function of the CX26 protein when its 86th amino acid is Thr, Ser or Arg, and its relations to deafness.
Methods
CX26-GFP protein with either Thr, Ser or Arg as the 86th amino acid was expressed in mouse SGN cells via the GFP fusion type lenti-virus expression system. The membrane localization of the fusion protein was observed under a fluorescence microscope.
Results
The mutated protein of CX26 T86S was localized to cell membrane and form gap conjunction structures, showing no difference to the wild type CX26 protein (with Thr as the 86th amino acid). However, the gap conjunction structure disappeared when the mutation was CX26 T86A.
Conclusion
These results indicate that the CX26 T86R mutation may be a cause of hearing loss, but CX26 T86S as a non-pathogenic polymorphism mutation does not affect functions of the CX26 protein. The results are in accordance with the results of clinical screening.
Keywords: Heredity deafness, CX26, SGN
1. Introduction
Hereditary hearing loss is a common human birth defect with an incidence of about 1 in 700–1000 (Li et al., 2015) new births, over half of the which are a result of hereditary hearing disabilities. GJB2 gene mutations are related to 36.9% of sensory neural hearing loss (SNHL) among children (Ouyang et al., 2009), while the CX26 – the GJB2-coded protein – is involved in the transportation of potassium and serves as the connexon, key to maintaining the balance and stability of inner ear ions. By far, more than 150 mutation sites in the GJB2 genes have been discovered (http://davinci.crg.es/deafness) (Wei et al., 2014, Zelante et al., 1997, Petersen and Willems, 2006, Choi, 2009, Van Laer et al., 1999, Welch et al., 2007, Yan et al., 2006).
The 86th amino acid of CX26 displays a certain degree of polymorphism. Many reports consider the 86th amino acid of the wild-type CX26 being Threonine (Thr), whose corresponding pathogenic mutations is CX26 T86R (GJB2c.257C > G) (Wei et al., 2014, Choi, 2009). CX26 T86S (GJB2c.257-258CG > GC), a form of non-pathogenic mutation, has no clinical evidence to establish causal link to hereditary hearing loss (Dai et al., 2009, Hilgert et al., 2009). As a result, there are two CDS sequences of the GJB2 gene (EBIID AAP35378; ID AAD21314) in the EBI database (Fig. 1) (Lee et al., 1992). However, by far there is no systematic study about the biological function of CX26 proteins in which the 86th amino acid is Thr, Ser or Arg (whose codons are ACG, AGC or AGG) respectively. This research used GFP infused lenti virus system to study the biological function of the polymorphism of CX26's 86th amino acid (Thr, Ser or Arg), which to some extent helped explain the mechanism behind the relation between polymorphism of the amino acid and hereditary hearing loss.
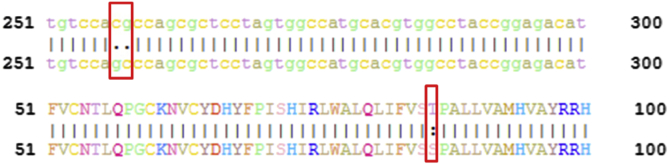
Fig. 1.
Polymorphism analysis of the GJB2 gene and the sequence of their corresponding CX26 proteins. Top: Differences in polymorphism of the 257-258th base pairs in GJB2. Bottom: Polymorphism comparison between the 86th amino acid of CX26 proteins.
2. Results
2.1. Comparison of non-pathogenic polymorphism of CX26's 86th amino acid using Peiking University's online biological information analysis platform (www.abc.pku.cn)
We analyzed the similarity of CDS sequences of the two normal GJB2 genes (EBIID AAP35378; ID AAD21314) submitted by previous studies to the EBI database. We found that the 257-258th base pairs of the two sequences were CG or GC (whose codons are ACG or AGC (256-258)) and the corresponding amino acids are Thr and Ser. This in turn helped decide the polymorphism of CX26's 86th amino acids (Fig. 1).
2.2. Analysis of the biological function of CX26 proteins
Based on previous research that studied the two non-pathogenic forms of the 86th amino acid, namely Thr and Ser, of normal CX26s and the pathogenic mutation of Arg, this study successfully constructed a series of CX26-GFP infused protein lenti-virus. The packaged lenti-virus was used to infect prime SCN cells of mice and sub-cellular localization was examined after 48 h using fluorescent microscopy. The results are shown in Fig. 2.
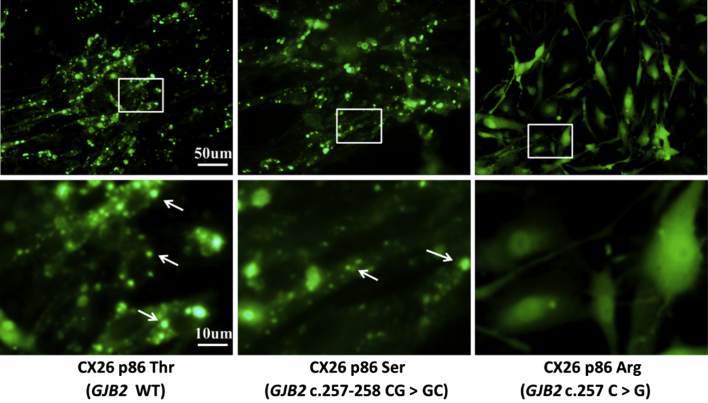
Fig. 2.
Sub-cellular localization of CX26-GFP proteins whose 86th amid acid is Thr, Ser or Arg. The bottom row shows magnified images in the corresponding boxed in the top row. Arrowhead point to conjunctional structures formed by CX26-GFPp86Thr (left) and CX26-GFPp86Ser (middle) in cell membrane. The far right column shows CX26-GFPp86Arg failing to locate in cell membrane and form conjunctional structures.
The far left pictures in Fig. 2 show the foci-pattern of CX 26-GFP in the SGN cells with the 86th amino acid being Thr, indicating that the protein was identified in cell membrane and formed conjunction structures. When the 86th amino acid was Ser (middle pictures in Fig. 2), sub-cellular localization showed a similar pattern. But when the 86th amino acid was Arg (right side pictures in Fig. 2), the mutation resulted in the mutated proteins unable to be identified in cell membrane but diffused evenly in cytoplasm, indicating a high possibility that the mutation may be a potential cause of hereditary hearing loss.
3. Discussion
This study analyzed sub-cellular localization of different CX26-GFP proteins. When the 86th amino acid was Thr or Ser, the CX26 protein was identified in cell membrane and formed gap junction structure. But when the 86th amino acid became Arg, the mutated protein was not identified in cell membrane but found diffused evenly in cytoplasm. Such phenomenon is similar to results of studies on 293T celline (Wei et al., 2014, Choi, 2009). This research for the first time proves this phenomenon in neural cells in the inner ear of mice while providing evidence that has been previously lacked with regard to the biological function of the 86th Ser of CX26 protein, which lends theoretical basis in support of non-pathogenic mutations in clinical screening. An comparative analysis of amino acid structures shows that Thr and Ser are hydrophobic amino acids which share similar structures with side chains of similar lengths and whose function group exhibit no more difference than a single methyl. This leads us to hypothesize that when the 86th Thr is replaced with Ser, the impact on CX26's structure is negligible. As a result, the claim that the 86th Ser is a non-pathogenic mutations can be reasonably explained. On the contrary, if the 86th amino acid of the CX26 protein becomes Arg, the likely result is a change of the protein's structure due to the fact that Arg's polarity is far greater that of Thr and it tends to have longer side chains. One of the major changes is loss of function caused by the formation of an effective protein hexamers, which to some extent helps explain the mechanism of CX26 T86A as a deafness-inducing mutation.
Polymorphism of the 86th amino acid in CX26 has been widely discussed in many studies on the screening of deadness-inducing genes around the world. Polymorphism exists in the form of Thr, Ser and Arg whose corresponding cordons are ACG, AGC and AGG (GJB2 256-258bp) respectively. Research results have demonstrated biological malfunction of the CX26 protein when the 86th amino acid is mutated into Arg. The conclusion coincides with results of many studies about GJB2c.257C > G p.T86R. Another finding of this study is that when the 86th amino acid becomes Ser rather than Thr, the function of CX26 changes little (Fig. 2). Clinical screening data have also proved such a finding, i.e. GJB2c.257-258 GC > CGp. S86T is a polymorphism mutation that does not cause hearing loss. However, it is worth noting that some studies believe that the polymorphism of CX26 is a result of mutation of the 86th Ser into Thr (Liu et al., 2012, Zheng et al., 2000). Despite that these two amino acids do not affect the biological function of the CX26 protein, the statement (GJB2c.257-258 GC > CGp.S86T) contradicts with the other one about pathogenic mutations (the GJB2c.257C > G p.T86R) (Wei et al., 2014, Choi, 2009, Dai et al., 2009), while the latter tacitly regards the 86th amino acid of the CX26 protein as Thr while the former treats it as Ser, leading to confusion about its polymorphism and statistical margins of error. The authors suggest that the 86th amino acid of the wild type CX26 be treated as Thr in general and the statement (GJB2 256-258 CG > GCp.T86S) is preferred in the description of the non-pathogenic mutation of Ser to ensure that a standard wild type sequence is adopted as the basis for data analysis in the screening of the polymorphism of the 86th amino acid of the CX26 protein. This study also offers a novel technique to conduct an effective assessment of the biological function of the GJB2 gene with polymorphism. By providing strong empirical evidence for pathogenic analysis at rare sites of GJB2 mutations, our study offers clear theoretical guidance for explaining the pathogenic mechanism of GJB2 rare-site mutation carriers during clinical screening.
4. Conclusion
To sum up, the study analyzed for the first time the polymorphism of the 86th amino acid in the CX26 protein and tested their cellular function through techniques of molecular biology. The findings show that only when the 86th amino acid mutates into Arg does the CX26 protein lose function, which coincides with empirical results of clinical screening. In addition, the study explored a technical method that effectively identified membrane localization of the mutational CX26 protein, which in turn helped provide diagnostic advice for mutation carriers on the incidence of hereditary deafness.
5. Material and methods
5.1. Sequence similarity analysis
Using Peiking University's online biological information platform (www.abc.pku.cn), the similarity of CDS sequences of two normal GJB2 genes (EBIID AAP35378; ID AAD21314) submitted by previous studies to the EBI database were analyzed.
5.2. Vector construction and viral packing
The pWPXLD lentiviral vectors were used. The GJB2 gene was cloned by RT–PCR from total RNA of the human WI38 cells line. The amplified PCR product was inserted into the PmeI/BamHI or BamHI/EcoRI sites of the pWPXLD vector, and then fused with the EGFP (enhanced green fluorescent protein) gene. Lentiviruses were packed using HEK-293T cells co-transfection with the plasmid of pWPXLD, pPAX and pMD-2G. Lentivirus supernatant was collected together at 48 h, 72 h and 96 h respectively after transfection and concentrated by ultrafiltration with 100 kD membrane. The concentrated viral liquid was aliquoted into 50 ul in each 1.5 ml EP tube for storage at −80 °C for preparation.
5.3. SGN cells infection and detection of GJB2-GFP protein localization
Primary SGN cells were isolated from neonatal mice as previously described. Concentrated virus was then diluted with culture medium and applied to SGN cells for 24 h with change of the culture medium. Images were taken under a confocal laser-scanning microscope (Olympus FV1000) at 72 h after infection.
Conflict of interest
There are no any conflict of interest in this MS.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (973 Program) (#2012CB967900), National Natural Science Foundation of China (31300624, 81470684), Postdoctoral Science Foundation of China (2015M571818), Six Major Categories Talent (2014-WSN-043, 2011-WS-074), Innovation and Entrepreneurship Training Program for College Students in Jiangsu Province (201510313003Z, 201510313003, KYLX14-1455), Clinic Medical Special Foundation of Jiangsu province (b12014032).
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Shiming Yang, Email: yangsm301@263.net.
Yuehua Qiao, Email: oto8558@163.com.
References
- Choi Soo-Young, others Different functional consequences of two Missense mutations in the GJB2 gene associated with nonsyndromic hearing loss. Hum. Mutat. Mutat. Brief. 2009;30:E716–E727. doi: 10.1002/humu.21036. [DOI] [PubMed] [Google Scholar]
- Dai P., Yu F., Han B. GJB2 mutation spectrum in 2,063 Chinese patients with nonsyndromic hearing impairment. J. Transl. Med. 2009;7:26. doi: 10.1186/1479-5876-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgert N., Smith R.J., Van Camp G. Forty- six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics. Mutat. Res. 2009;681(2–3):189–196. doi: 10.1016/j.mrrev.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.W., Tomasetto C., Paul D.L., Keyomarsi K., Sager R. Transcriptional downregulation of gap-junction proteins blocks junctional communication in human mammary tumor cell lines. J. Cell Biol. 1992;118(5):1213–1221. doi: 10.1083/jcb.118.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.X., Chen D.L., Zhao S.B., Guo L.L., Feng H.Q., Zhang X.F., Ping L.L., Yang Z.M., Sun C.X., Yao G.D. Cordblood-based high-throughput screening for deafness gene of 646 newborns in Jinan area of China. Clin. Exp. Otorhinolaryngol. September 2015;8(3):211–217. doi: 10.3342/ceo.2015.8.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Desheng, Wang Jinli, Lin Isaiah, Yan Xinmin, Wang Xiaojie, Shen Tao, Peng Zhengguo. B2 gene analysis Dai Han non-syndromic deafness G. J. Kunming Med. Univ. 2012;10:49–52. [Google Scholar]
- Ouyang X.M., Yan D., Yuan H.J., Pu D., Du L.L., Han D.Y. The genetic bases for non-syndromic hearing loss among Chinese. J. Hum Genet. 2009 Mar;54(3):131–140. doi: 10.1038/jhg.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.B., Willems P.J. Non-syndromic, autosomal-recessive deafness. Clin. Genet. 2006;69(5):371–392. doi: 10.1111/j.1399-0004.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- Van Laer L., McGuirt W.T., Yang T., Smith R.J., Van Camp G. Autosomal dominant nonsyndromic hearing impairment. Am. J. Med. Genet. 1999;89(3):167–174. doi: 10.1002/(sici)1096-8628(19990924)89:3<167::aid-ajmg7>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- Wei Qinjun, Liu Youguo, Wang Shuai, Liu Tingting, Lu Yajie, Xing Guangqian, Cao Xin. A novel compound heterozygous mutation in the GJB2 gene causing non-syndromic hearing loss in a family. Int. J. Mol. Med. 2014;33:310–316. doi: 10.3892/ijmm.2013.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K.O., Marin R.S., Pandya A., Arnos K.S. Compound heterozygosity for dominant and recessive GJB2 mutations: effect on phenotype and review of the literature. Am. J. Med. Genet. A. 2007;143A(14):1567–1573. doi: 10.1002/ajmg.a.31701. [DOI] [PubMed] [Google Scholar]
- Yan D., Ke X., Blanton S.H., Ouyang X.M., Pandya A., Du L.L., Nance W.E., Liu X.Z. A novel locus for autosomal dominant non-syndromic deafness, DFNA53, maps to chromosome 14q11.2-q12. J. Med. Genet. 2006;43(2):170–174. doi: 10.1136/jmg.2005.034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante L., Gasparini P., Estivill X., Melchionda S., D'Agruma L., Govea N., Mila M., Monica M.D., Lutfi J., Shohat M. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol. Genet. 1997;6(9):1605–1609. doi: 10.1093/hmg/6.9.1605. [DOI] [PubMed] [Google Scholar]
- Zheng, Wenbo, Luo, Jianhong, Li, Yun, Yu, Yu. Money should be in force before the Chinese language mutation non-syndromic deafness GJB2 gene of Pediatrics in 2000, 10–38.