Abstract
Over the past two decades considerable progress has been made in understanding the ototoxic effects and mechanisms underlying loop diuretics. As typical representative of loop diuretics ethacrynic acid or furosemide only induces temporary hearing loss, but rarely permanent deafness unless applied in severe acute or chronic renal failure or with other ototoxic drugs. Loop diuretic induce unique pathological changes in the cochlea such as formation of edematous spaces in the epithelium of the stria vascularis, which leads to rapid decrease of the endolymphatic potential and eventual loss of the cochlear microphonic potential, summating potential, and compound action potential. Loop diuretics interfere with strial adenylate cyclase and Na+/K+-ATPase and inhibit the Na-K-2Cl cotransporter in the stria vascularis, however recent reports indicate that one of the earliest effects in vivo is to abolish blood flow in the vessels supplying the lateral wall. Since ethacrynic acid does not damage the stria vascularis in vitro, the changes in Na+/K+-ATPase and Na-K-2Cl seen in vivo may be secondary effects results from strial ischemia and anoxia. Recent observations showing that renin is present in pericytes surrounding stria arterioles suggest that diuretics may induce local vasoconstriction by renin secretion and angiotensin formation. The tight junctions in the blood-cochlea barrier prevent toxic molecules and pathogens from entering cochlea, but when diuretics induce a transient ischemia, the barrier is temporarily disrupted allowing the entry of toxic chemicals or pathogens.
Keywords: Diuretics, Stria vascularis, Ischemia, Pericytes, Renin
1. Introduction
The development of diuretics has a long history. Inorganic mercury was used as a diuretic dating back to the 16th century. The past century has witnessed the development of several new classes of diuretics such as merbaphen, carbonic anhydrase inhibitors, derivatives of sulfanilamide, chlorothiazide, and thiazides (1927, Kruck, 1958, Schultz et al., 1971, Maren and Ellison, 1972, Friedel and Buckley, 1991). Loop diuretics are widely used in the treatment of edematous states, congestive heart failure, and hypertension. However, their use is constrained by a wide range of side effects including hypokalemia, hypocalcemia, hypomagnesemia, hyperuricemia, hypernatremia, dehydration, and ototoxicity. In addition, dizziness, headache, and gastrointestinal upset are major symptoms of diuretics. The ototoxic effects of loop diuretics have attracted the attention of auditory neuroscientists and otologist interested in understanding their mechanism of action alone or in combination with other ototraumatic agents.
2. Classification of diuretics and uretic mechanisms of loop diuretics
Diuretics can be divided into four classes based on the site at which they impair sodium reabsorption. (1) Loop diuretics act on the thick ascending limb of the loop of Henle. (2) Thiazide diuretics act at the distal tubule and cortical connection tubule. (3) Potassium-sparing diuretics act at the aldosterone-sensitive principal cells in the cortical collecting tubule. (4) Acetazolamide and mannitol act at the proximal tubule. Loop diuretics mainly act on the Na+-K+-2Cl− symporter at the thick ascending links of the loop of Henle in the kidney by inhibiting sodium, chloride and potassium reabsorption. Since magnesium and calcium reabsorption is also dependent on the positive lumen voltage of renal outer medullary potassium channel at the thick ascending limb, the reabsorption of magnesium ions and calcium ions can be also suppressed by loop diuretics. The malabsorption of these ions causes less osmotic driving force to liquid that results increased urine production. Ultimately, loop diuretics are highly effective at reducing extracellular volume (Greger and Schlatter, 1983, Bleich and Greger, 1997, Greger, 1999).
3. Reversible diuretics-induced changes in cochlear potentials
Diuretics-induced changes in the standing direct current (DC), endocochlear potential (EP) and sound-evoked alternating current (AC) of cochlear microphonic potential (CM), DC potential of summating potential (SP), and compound action potential (CAP) have been extensively studied (Kusakari et al., 1978a, Kusakari et al., 1978b, Ding et al., 1996, Ding et al., 2002, Ding et al., 2008). Based on experimental studies, the resting EP, normally around +90 mV, can be subdivided into positive and negative EP components. The positive EP is considered to be generated in the stria vascularis which is heavily dependent on aerobic metabolism. In contrast, the negative EP is believed to be a K+ leakage current from outer hair cells that depend on K+ gradient between the cell's cytoplasm and the fluid around the cell body (Tasaki and Spyropoulos, 1959, Bosher, 1979, Ding et al., 1993, Ding et al., 1996, Rybak, 1993, Ding and Jin, 1998). A single intravenous injection of ethacrynic acid (40 mg/kg) induces a rapid and complete abolition of the positive EP within a few minutes (Fig. 1A) (Brown and McElwee, 1972, Kusakari and Thalmann, 1976, Kusakari et al., 1978b, Bosher, 1979, Rybak, 1993, Ding et al., 1996, Ding et al., 2002, Ding et al., 2004, Ding et al., 2010b) which fully recovers after a few hours. This suggests that the stria vascularis is the major target of loop diuretics.
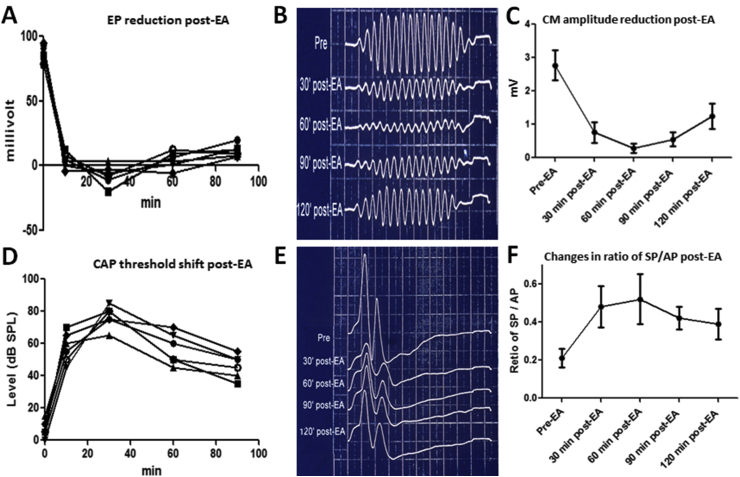
Fig. 1.
Cochlear dysfunction post-EA. (A) Endocochlear potential (EP) measured from six guinea pigs as a function of time after EA injection. (B) Waveform changes of the cochlear microphonic potential (CM) stimulated by 2 kHz tone presented at 80 dB SPL post-EA. (C) Amplitude of CM was greatly reduced post-EA. (D) Threshold shift of click-evoked compound action potentials (CAP) from six guinea pigs as a function of time after EA injection. (E) Waveform changes of summating potentials (SP) and CAP complexes at 90 dB SPL post-EA. (F) Changes in ratio of SP/CAP amplitude post-EA.
The CM is an alternating voltage, predominantly generated by the outer hair cells, that mirrors the waveform of the acoustic stimulus. The CM amplitude largely depends on the flow of positively ions from the +90 mV endolymphatic space across the cuticular plate and into −60 mV interior of the hair cell (Wever and Bray, 1930, Eggermont, 1974). Not surprisingly, ethacrynic acid, which reduces the EP, decrease the amplitude of the CM to a similar degree (Fig. 1B–C) (Syka and Melichar, 1985, Ding et al., 1996, Ding et al., 2002, Ding et al., 2010b). The amplitude of the CM recovers in parallel with the restoration of the EP as long as the hair cells remain intact (Rybak, 1993, Ding et al., 1996, Ding et al., 2002, Ding et al., 2004, Ding et al., 2010b). The SP evoked by a tone burst or noise burst gives rise to a DC voltage shift from baseline that continues over the duration of the stimulus. The SP is thought to arise predominantly from the IHC or its afferent terminal (Goldstein, 1954, Tasaki et al., 1954, Davis et al., 1958, Dallos et al., 1972, Ding et al., 1993, Ding et al., 1996, Ding et al., 2010b, Zheng et al., 1997, Ding and Jin, 1998, Durrant et al., 1998). Diuretics typically induce a high positive SP no matter if the original polarity was positive or negative; this effect recovered slowly in conjunction with the recovery of the EP and CM (Syka and Melichar, 1985, Rybak, 1993, Ding et al., 1996, Ding et al., 2004, Ding et al., 2010b). The CAP arises from the synchronous depolarization of a large number of auditory nerve fibers to the onset of a sound (Nagel, 1974) and therefore depends on the EP and CM (Remond et al., 1982, Ding et al., 1993, Ding et al., 1996, Ding and Jin, 1998). Loop diuretics cause a rapid and large reduction in CAP amplitude that parallel the reduction of the EP and CM (Fig. 1D). The amplitude of the CAP recovers in parallel with the EP and CM. Following diuretic treatment, the SP/CAP amplitude ratio increases due to the increase of the SP and decrease of the CAP (Fig. 1E, F) (Rybak, 1993, Ding et al., 1996, Ding et al., 2010b). High SP/AP ratios are not only seen in case of Meniere's disease (Kanzaki et al., 1982, Gibson, 1991, Ferraro and Tibbils, 1999), but also cochlear ischemia and asphyxia, noise-induced temporary threshold shift, perilymphatic fistula, and autoimmune disease (Ohashi and Takeyama, 1989, Meyerhoff and Yellin, 1990, Ding et al., 1993, Ding and Jin, 1998, Kakigi et al., 2003, Nam and Won, 2004, Ding et al., 2008, Ding et al., 2010b).
4. Diuretics-induced reversible pathological damage in the cochlear stria vascularis
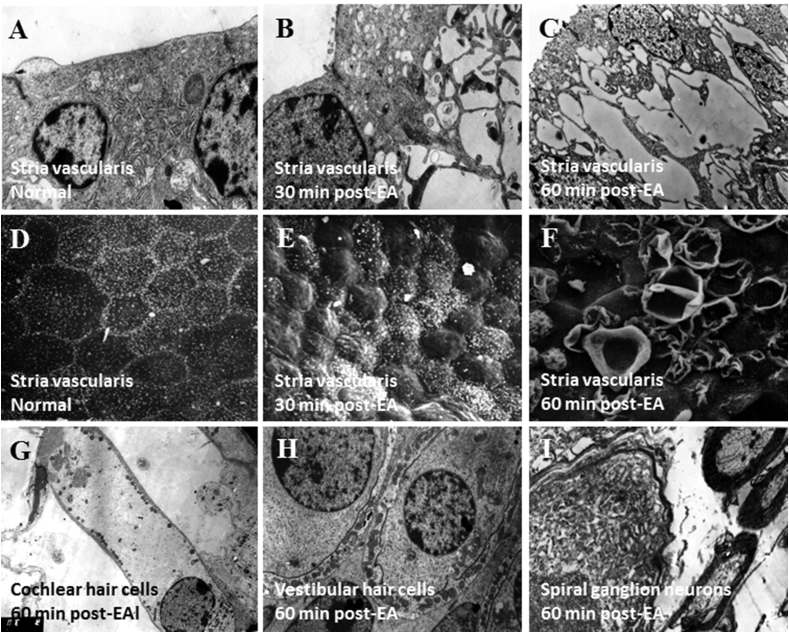
While several studies have reported permanent hearing loss and cochlear hair cell degeneration after a single diuretic treatment possibly due to autolysis and inadequate fixation (Ng et al., 1969, Meriwether et al., 1971, Quick and Hoppe, 1975, Rifkin et al., 1978, Gallagher and Jones, 1979, Akiyoshi, 1981, Forge and Brown, 1982) the vast majority of published articles indicate that a single systemic administration of loop diuretics to experimental animals only induces temporary pathological damage or edema to the stria vascularis and cochlear lateral wall. The characteristic features include damage to the marginal cells and swelling of both the intermediate cells and intrastrial space. Diuretics alone never directly damage the cochlear or vestibular hair cells or the spiral or vestibular ganglion neurons in experimental animals (Fig. 2) (Silverstein and Yules, 1971, Forge, 1976, Kasajima et al., 1978, Santi and Duvall, 1979, Bosher, 1980, Santi and Lakhani, 1983, Rybak, 1993, Ikeda et al., 1997, Ding and Salvi, 2005, Ding et al., 2010b). The pathological changes with edema occur in the stria vascularis approximately 30 min post-treatment; the changes typically completely recover within a day.
Fig. 2.
Ethacrynic acid-induced ultrastructural lesions in the inner ear. (A) Epithelium of stria vascularis in normal control animal. (B) At 30 min post-EA, strial edema present in region of intermediate cells. (C) At 60 min post-EA, cytoplasm of epithelium in stria vascularis disrupted by edema; note splitting and vacuolization. (D) Edge of marginal cells in stria vascularis is smooth and flat in normal control animal. (E) At 30 min post-EA, the surface of stria vascularis was uneven due to cellular swelling. (F) At 60 min post-EA, the marginal cells on the surface of stria vascularis were destroyed. (G, H, I). The cochlear hair cells (G), vestibular hair cells (H), and spiral ganglion neurons (I) exhibited were normal 60 min after EA injection.
5. Possible ototoxic mechanisms of loop diuretics
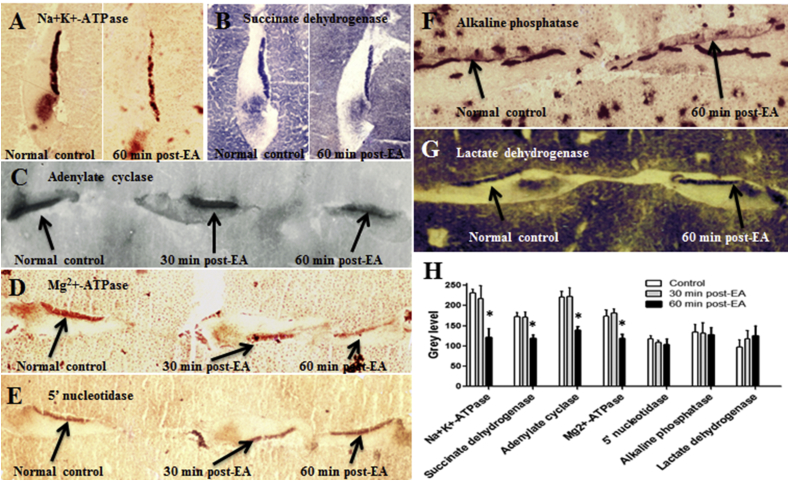
Since diuretics induce edema only in the epithelium of stria vascularis, the stria has long been recognized as the major target of loop diuretics (Matz, 1976, Arnold et al., 1981, Ding et al., 2002, Ding et al., 2010b, Ding and Salvi, 2005). The ototoxic mechanisms of loop diuretics were originally believed to be caused by inhibition of Na+, K+-ATPase and adenylate cyclase (Paloheimo and Thalman, 1977, Kusakari et al., 1978a). However, other reports suggest that the alteration of these enzymes may be a secondary consequence of loop diuretic ototoxicity (Marks and Schacht, 1981, Thalmann et al., 1982, Zhao et al., 1988a). Our early experiments confirmed that loop diuretics inhibit many enzymes in the stria vascularis including Na+, K+-ATPase, Mg++-ATPase, adenylate cyclase and succinate dehydrogenase 1 h after ethacrynic acid injection. However, these enzymes were present at normal levels at earlier stage of hearing loss and did not decline until large vacuoles appeared in cells of the stria 1 h post-ethacrynic acid. Moreover, some enzymes, such as alkaline phosphatase, 5' nucleotidase, and lactate dehydrogenase were unchanged at this time. Since hearing loss precedes the decline in enzymatic activities, enzyme inhibition may be a consequence rather than the immediate cause of the hearing loss (Fig. 3) (Zhao et al., 1988a, Ding et al., 2010b).
Fig. 3.
EA-induced inactivation of enzymes within epithelium of stria vascularis. (A) Frozen section of spiral ligament embedded in the liver tissue showing intense Na+, K+-ATPase labeling in stria vascularis in normal control animal. In contrast, Na+, K+-ATPase expression in stria vascularis was greatly reduced 60 min after EA injection. (B) Frozen section of spiral ligament shows intense succinate dehydrogenase labeling in stria vascularis in normal animal. However, the staining of succinate dehydrogenase was decreased 60 min post-EA. (C) Frozen section of spiral ligament shows intense adenylate cyclase activity in stria vascularis in normal controls and 30 min post-EA treated animals. Labeling of adenylate cyclase was decreased 60 min after EA injection. (D) Frozen section of spiral ligament shows intense Mg++-ATPase in stria vascularis in normal and 30 min post-EA treated animals. However, the staining of Mg++-ATPase was reduced 60 min post-EA. (E) Frozen section of spiral ligament shows intense 5'nucleotidase in the stria vascularis in normal control and 30 min, and 60 min after EA injection. (F) Frozen section of spiral ligament shows moderate expression of alkaline phosphatase in the stria vascularis in normal control and 60 min after EA injection. (G) Frozen section of spiral ligament shows intense lactate dehydrogenase in the stria vascularis in normal control and 60 min after EA injection. (H) Mean gray level of enzyme expression in the region of stria vascularis. In comparison to normal controls. Activities of Na+, K+-ATPase, succinate dehydrogenase, adenylate cyclase, and Mg++-activated ATPase were significantly inhibited 60 min after EA injection. Activity of 5' nucleotidase, alkaline phosphatase and lactate dehydrogenase were not affected by EA injection. *Significantly different from normal control by ANOVA statistical analysis and posthoc test (Tukey) using the GraphPad Prism (P < 0.05).
Diuretic-induced inhibition of the Na-K-2Cl cotransport system in the ascending limb of Henle's loop in the kidney suggested that this cotransporter might play an important role in the stria (Burg et al., 1973, Sellick and Johnstone, 1975, Shindo et al., 1992). Indeed, many studies indicate that diuretics and anoxia affect the Na-K-2Cl cotransporter in strial marginal cells (Bosher, 1979, Bosher, 1980, Ikeda and Morizono, 1989b, Ikeda and Morizono, 1989c, Ikeda and Morizono, 1989a, Shindo et al., 1992, Xu et al., 1994, Wangemann et al., 1995, Ikeda et al., 1997), however, inhibition of this cotransporter occurs much later that the immediate reduction of EP (Bosher, 1980).
6. Novel effect of diuretics
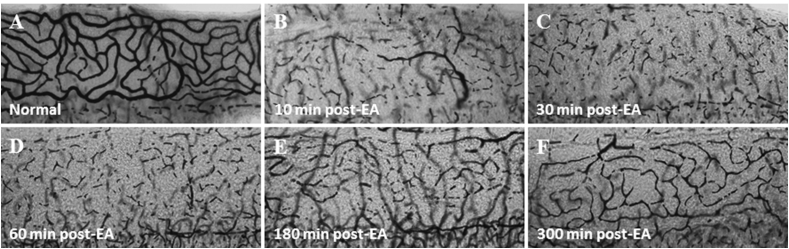
Since fixation and decalcification can disrupt the measurement sodium/potassium ATPase activity in the stria vascularis, we quickly micro-dissected out the stria vascularis together with the spiral ligament and embedded the specimen into liver tissue for freeze substitution followed by frozen sectioning (Ding et al., 1987). In guinea pigs, the stria capillaries filled with red blood cells are clearly visible under a dissection microscope (400X). However, few minutes after intravenous injection of ethacrynic acid, the red blood cells in the capillaries of the stria vascularis were undetectable (Fig. 4). These results suggest that diuretics rapidly eliminated the blood flow through the stria vascularis providing the first evidence of ethacrynic acid-induced ischemia (Zhao et al., 1988b). Using a microscope, we counted the blood cells in the capillaries of stria vascularis in a 0.66 mm2 area located about 3 mm from the apex. The percentage of red blood cells in the stria capillaries decreased by more than 50% two minutes after ethacrynic acid injection, and slowly recovered to normal levels approximately 5 h post-treatment (Ding et al., 1990). We have confirmed that ethacrynic acid and furosemide can also abolish the strial blood flow in the cochlear lateral wall of chinchillas, rats and mice in a same manner to that seen in guinea pigs (Zhao et al., 1988b, Ding et al., 2002, Ding et al., 2003, Ding et al., 2004, McFadden et al., 2002, McFadden et al., 2004, Ding and Salvi, 2005, Li et al., 2011, Liu et al., 2011b). It is noteworthy that ethacrynic acid does not block the blood supply to the vessels beneath the cochlear basilar membrane and the vestibular end-organs (Fig. 5) (Zhao et al., 1988b, Ding et al., 1990, Ding et al., 2002, Ding et al., 2004, Ding et al., 2010b, Ding and Salvi, 2005, Li et al., 2011). Diuretic-induced blockade of blood flow to the cochlear lateral wall were further confirmed by the intravital microscopy, microsphere labeling, and the laser Doppler velocimetry (Chen et al., 1992, Chen, 1993, Liu and Wang, 1994, Shi et al., 1994, Shi et al., 1997, Ding et al., 2010b). Since the diuretic-induced suppression of blood flow in cochlear lateral wall occurs much earlier than enzyme inactivation and inhibition of Na-K-2Cl cotransport in the stria vascularis, the obstruction of microcirculation in the cochlear lateral wall might be the earliest changes induced by ethacrynic acid, consistent with the rapid reduction of EP. The delayed inactivation of metabolic enzymes and inhibition of Na-K-2Cl cotransport is most likely a secondary effect of ischemia and anoxia (Zhao et al., 1988a, Zhao et al., 1988b, Ding et al., 1990, Ding et al., 2002, Ding et al., 2010b).
Fig. 4.
Surface preparations of rat stria vascularis after EA injection. (A) normal control; (B) 10 min post-EA; (C) 30 min post-EA; (D) 60 min post-EA; (E) 180 min post-EA; (F) 300 min post-EA. At 10, 30 and 60 min, the blood supply to stria vascularis was greatly abolished whereas at 180–300 min post-EA the blood supply was in the early stage of recovery.
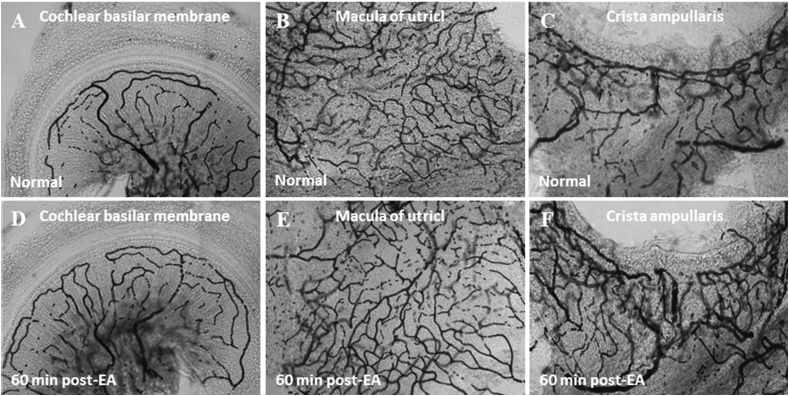
Fig. 5.
Surface preparations of rat inner ear with or without EA treatment. (A) Blood supply to cochlear basilar membrane in normal control. (B) Blood supply to macula of utricle in normal control. (C) Blood supply to crista ampullaris in normal control. (D) At 60 min post-EA, blood supply to cochlear basilar membrane was unaffected. (E) At 60 min post-EA, blood supply to macula of utricle was unaffected. (F) At 60 min post-EA, blood supply to crista ampullaris was unaffected. The appearance of vessels at 60 min post EA was not notably different from the appearance at any earlier or later time point.
The preceding results are consistent with more recent in vitro data from postnatal cochlear lateral wall explants treated with ethacrynic acid. Surprisingly, ethacrynic acid treated explants of the stria vascularis were remarkably intact after ethacrynic acid treatment (Fig. 7) (Liu et al., 2011a) and the organelles looked remarkably normal. Apparently, the postnatal lateral wall explants, which lack blood flow, obtain sufficient nutrients and oxygen from the culture medium in order to maintain normal metabolic function.
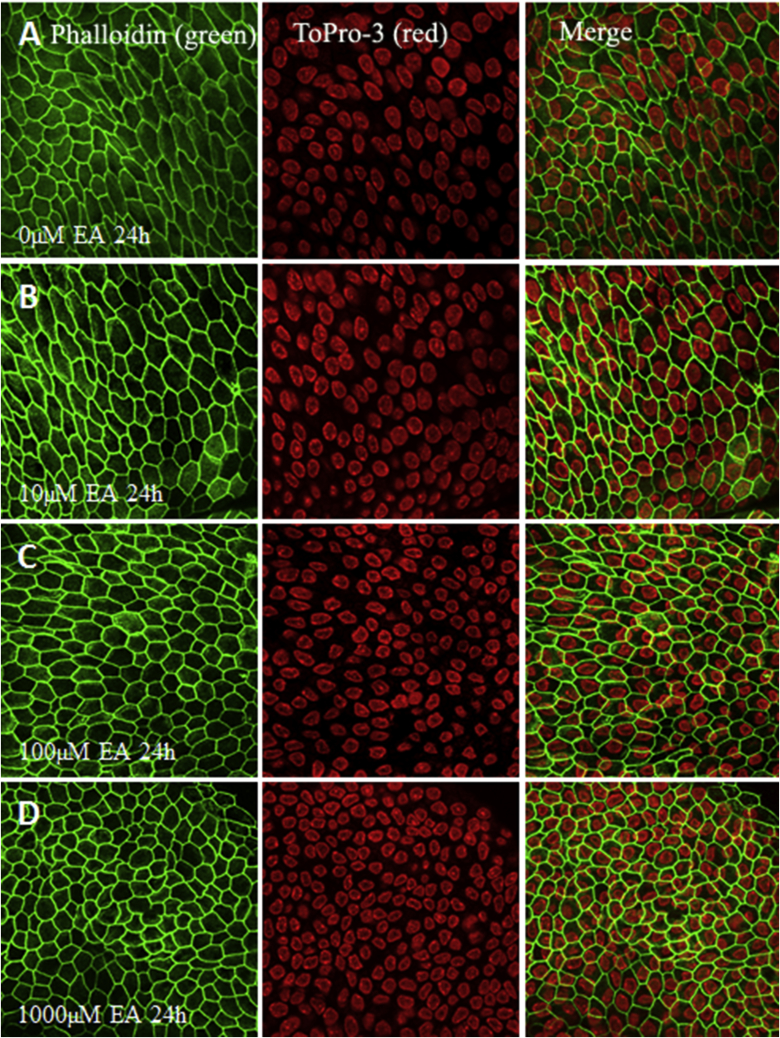
Fig. 7.
Dose-response of EA to epithelium of stria vascularis in vitro. The surface structure of marginal cells of stria vascularis were stained with phalloidin in green, and the nuclei of cells in the epithelium were labeled with ToPro-3 in red. (A) Stria vascularis was cultured in standard serum-free medium without EA for 24 h as normal control. (B) Stria vascularis cultured in 10 μM EA for 24 h. (C) Stria vascularis cultured in 100 μM EA for 24 h. (D) Stria vascularis cultured in 1000 μM EA for 24 h. EA had little or no effect on stria vascularis morphology.
7. Diuretics disrupt the blood-cochlear barrier
Because of their relatively slow uptake into the cochlea, ototoxic drugs such as gentamycin and cisplatin seldom cause hair cell loss or hearing loss after a single treatment. However, when high doses of loop diuretics are administered simultaneously with ototoxic drugs such as cisplatin or gentamycin, significant hair cell loss and permanent hearing loss often develops (Ding et al., 1995, Ding et al., 2004, Ding et al., 2007, Ding et al., 2010a, Ding et al., 2010b, Ding et al., 2011, Ding et al., 2012, McFadden et al., 2002, McFadden et al., 2004, Ding and Salvi, 2005, Li et al., 2011, Liu et al., 2011b). The most likely mechanism responsible for the potentiation of ototoxicity by loop diuretics is damage to the tight cell junctions in the blood vessels in the stria vascularis resulting in temporary disruption of the blood-cochlear barrier which increases the permeability of the lateral wall to ototoxic drugs. In earlier studies, we found that simultaneous injection of ethacrynic acid and gentamicin significantly increased the peak concentration and half-life of gentamicin in the perilymph; this increase was equivalent to that resulting from 20 injections of gentamicin every 12 h (Fig. 6) (Ding et al., 2003, Ding et al., 2004, Ding et al., 2010b, Ding and Salvi, 2005). Similar effects are seen when loop diuretics are administered in combination kanamycin or cisplatin (Ding et al., 2007, Ding et al., 2011, Ding et al., 2012, Li et al., 2011, Liu et al., 2011b). Conversely, if loop diuretics are administered at time when gentamicin concentrations are higher in the cochlea than the blood (∼12–18 h post-gentamicin) then gentamicin flows down its concentration into the blood. Under these conditions, a single injection of ethacrynic acid does not cause permanent damage to the cochlear hair cells (Ding et al., 2003, Ding et al., 2004, Ding et al., 2010b). These results indicate that time at which loop diuretics are administered relative to other ototoxic agents will determine whether the diuretic potentiates or attenuates ototoxicity. Since loop diuretics can break open the blood-cochlea barrier, they might prove useful for promoting cochlear gene transfection or the delivery of otoprotective compound to the cochlea.
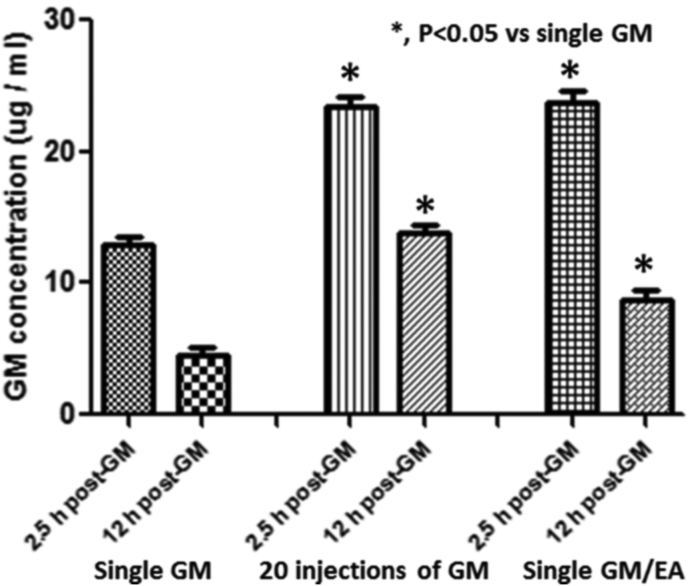
Fig. 6.
Gentamicin concentration (μg/ml; mean ± S.D. for the groups) in perilymph at the peak value of 2.5 h and the half-life of 12 h after (1) a single gentamicin injection, (2) 20 daily gentamicin injections or (3) one treatment of ethacrynic acid gentamicin. The concentration of gentamicin in perilymph after one co-administration of ethacrynic acid/gentamicin was significantly increased (p < 0.05) to a level was equivalent to the gentamicin accumulation in perilymph produced by 20 daily gentamicin treatments.
8. Co-administration of diuretics and gentamicin selectively damage cochlear hair cells, but spares vestibular hair cells
Gentamicin is considered primarily vestibulotoxic (i.e., more toxic to vestibular than cochlear hair cells), therefore, systemic or local administration of gentamicin has been used to treat intractable vertigo in unilateral Meniere's disease (Odkvist, 1997, Odkvist et al., 1997, Silverstein et al., 1999, Li et al., 2004). Paradoxically, a single injection of gentamicin and ethacrynic acid can cause massive damage to cochlear hair cells while vestibular hair cells remain intact (McFadden et al., 2002, Ding and Salvi, 2005, Ding et al., 2010a, Ding et al., 2010b). Cortilymph, fluid similar to perilymph within the tunnel of Corti, is believed to communicate with perilymph through relatively large channels in the basilar membrane located in the lower shelf of the osseous spiral lamina (Engstrom, 1960, Schuknecht and Seifi, 1963, Tanaka et al., 1973). Since the body of cochlear hair cells, particularly outer hair cells, are bathed in Cortilymph, the gentamicin within this fluid has greater access to cochlear hair cells than vestibular hair cells which are tightly embedded and completely surrounded by supporting cells (Schucnect, 1974).
9. Diuretic-induced vascular occlusion to external radiating arterioles
The cochlear spiral artery, a branch of the labyrinthine artery, enters the central canal in the modiolus and then was divided into two sets of radiating arterioles, the external radiating arterioles and the internal radiating arterioles. The external radiating arterioles supply the cochlear lateral wall whereas the internal radiating arterioles supply the medial wall of the cochlea. The external radiating arterioles pass over the scala vestibuli toward the cochlear spiral ligament and divide into four capillary networks, the vestibular crest of the spiral ligament, the capillary network of stria vascularis, the vessels of the spiral prominence and the vessels at basilar crest. The internal radiating arterioles project toward the base within the modiolus and divide into three groups of capillary networks which end in Rosenthal's canal to support the spiral ganglion neurons, cochlear limbus, and vessel of basilar membrane under the tunnel of Corti. The blood flow though the capillary networks enter the venules in the modiolus from the internal circulation or the collecting venules in scala tympani from the external circulation and is relayed to the spiral vein in the modiolus (Smith, 1951, Hawkins, 1967, Schucnect, 1974, Ding and Salvi, 2005). Remarkably, loop diuretics only abolish blood flow through the external radiating arterioles in the lateral wall of the cochlea (Fig. 4), but fail to interrupt blood flow through the internal radiating arterioles supplying the cochlear basilar membrane and spiral ganglion neurons or blood flow to the vestibular membranous labyrinth (Fig. 5). This raises the important question as to why loop diuretics only occlude blood flow through the external radiating arterioles (Zhao et al., 1988b, Ding et al., 1990, Ding et al., 2002, Ding et al., 2004, Ding et al., 2010b, McFadden et al., 2002, Ding and Salvi, 2005, Li et al., 2011).
Capillary vessels are composed of endothelial cells and pericytes. Vascular endothelial cells line the interior surface of blood vessels, while the pericytes, which contain contractile filaments, wrap around the endothelial cells. Pericytes have multiple functions, such as regulating vascular permeability, guiding sprouting vessels, promoting endothelial survival, exhibiting macrophage-like activities and promoting angiogenesis. However, one of the most important function of pericytes is to regulate capillary blood flow by adjusting the vascular diameter (Pallone and Silldorff, 2001, Bergers and Song, 2005, Dore-Duffy et al., 2006, Edelman et al., 2006, Peppiatt et al., 2006, Hall et al., 2014). The specific location of cochlear pericytes has been identified in the capillary network of the cochlear lateral wall including the vessels of stria vascularis and spiral ligament (Shi et al., 2008). Loop diuretics strongly enhance renin secretion (Vaughan et al., 1978, Kurtz et al., 2007). Renin secretion and angiotensin formation can cause widespread vasoconstriction thereby diminishing blood flow and increasing peripheral resistance in arterioles. In the kidney, renin is mainly synthesized by pericytes in mesangial (Castrop et al., 2006, Navar et al., 2012) and arteriolar smooth muscle cells which are confined to the wall of the afferent arteriole at entrance to the glomerulus. Therefore, renin cells in the kidney are actually located at the juxtaglomerular portion of the afferent arterioles and specifically affected by diuretics (Vaughan et al., 1978, Sequeira Lopez et al., 2004, Berg et al., 2013). A minority of renin cells are also present in other organs, such as retinal arterioles and brain (Ganten et al., 1971, Wagner et al., 1996, Okada et al., 2001, Winkler et al., 2016). It is noteworthy that diuretic-induced transient visual impairment has been reported as one of the side effects of loop diuretics (Canon, 1985, Jaanus, 1992). Recently, we observed the fluorescence labeled renin cells in the cochlea either from a renin BAC transgenic mouse model, which was established using GFP cassette into exon one of the renin gene contained within a 240-kb BAC to create a construct that has GFP expression controlled by the renin regulatory region (RenGFP) (Glenn et al., 2014) or from immunolabeling of renin protein. We found that renin cells in the cochlea are specifically located in pericytes only in the arterioles in the stria vascularis and the arterioles to the cochlear lateral wall in the spiral ligament. In contrast, renin cells were completely absent from the pericytes in the internal radiating arterioles (Fig. 8). Taken together with the result presented above, our results strongly suggest that loop diuretic activate the renin-angiotensin system in the cochlear pericytes which are exclusively located in the arterioles in the stria vascularis and cochlear lateral wall spiral ligament. Loop diuretic activate the renin-angiotensin system in pericytes which are exclusively located in the arterioles in the stria vascularis and lateral wall spiral ligaments which lead to vasoconstriction of the vessels within a discrete region of the lateral wall. We hypothesize that loop diuretics, which activate the renin-angiotensin system in the pericytes of the stria vascularis and spiral ligament, is the first step in vasoconstriction-induced cochlear ischemia that lead to an immediate and precipitous drop in the endolymphatic potential.
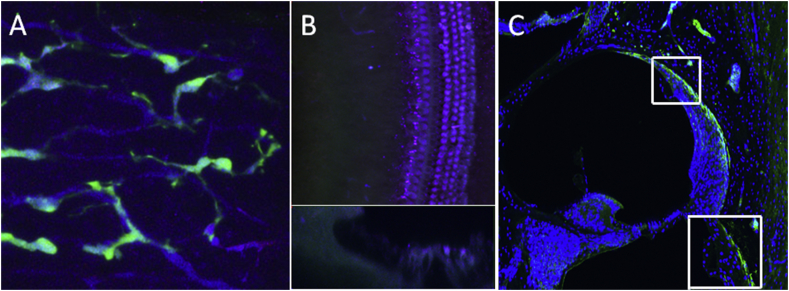
Fig. 8.
Localization of renin cells (green) in the cochlea. (A) Pericytes surrounding capillaries in stria vascularis express green fluorescence in renin-gfp mouse. (B) Capillaries in cochlear basilar membrane do not express renin-gfp. (Top figure is a surface view of cochlear basilar membrane. Bottom figure is Z axis image plane showing the section of cochlear basilar membrane). These results suggest that pericytes around capillaries in the cochlear lateral wall contains the renin-angiotensin aldosterone system whereas pericytes in the vessels in the modiolus and cochlear basilar membrane do not contain renin. (C) Renin immunolabeling in rat was only present in the vessels of the cochlear lateral wall, but in the vessels in the modiolus or cochlear basilar membrane.
Acknowledgments
This research was supported in part by a grant from National Natural Science Foundation of China 81470706 and a grant from Guangdong Natural Science Foundation No 2015A030313180.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Akiyoshi M. Effect of loop-diuretics on hair cells of the cochlea in Guinea pigs. Histological and histochemical study. Scand. Audiol. Suppl. 1981;(14 Suppl):185–199. [PubMed] [Google Scholar]
- Arnold W., Nadol J.B., Jr., Weidauer H. Ultrastructural histopathology in a case of human ototoxicity due to loop diuretics. Acta Oto-Laryngol. 1981;91:399–414. doi: 10.3109/00016488109138521. [DOI] [PubMed] [Google Scholar]
- Berg A.C., Chernavvsky-Sequeira C., Lindsey J., Gomez R.A., Sequeira-Lopez M.L. Pericytes synthesize renin. World J. Nephrol. 2013;2:11–16. doi: 10.5527/wjn.v2.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G., Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich M., Greger R. Mechanism of action of diuretics. Kidney Int. Suppl. 1997;59:S11–S15. [PubMed] [Google Scholar]
- Bosher S.K. The nature of the negative endocochlear potentials produced by anoxia and ethacrynic acid in the rat and Guinea-pig. J. Physiol. 1979;293:329–345. doi: 10.1113/jphysiol.1979.sp012892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher S.K. The nature of the ototoxic actions of ethacrynic acid upon the mammalian endolymph system. II. Structural-functional correlates in the stria vascularis. Acta Oto-Laryngol. 1980;90:40–54. doi: 10.3109/00016488009131696. [DOI] [PubMed] [Google Scholar]
- Brown R.D., McElwee T.W., Jr. Effects of intra-arterially and intravenously administered ethacrynic acid and furosemide on cochlear N 1 in cats. Toxicol. Appl. Pharmacol. 1972;22:589–594. doi: 10.1016/0041-008x(72)90286-4. [DOI] [PubMed] [Google Scholar]
- Burg M., Stoner L., Cardinal J., Green N. Furosemide effect on isolated perfused tubules. Am. J. Physiol. 1973;225:119–124. doi: 10.1152/ajplegacy.1973.225.1.119. [DOI] [PubMed] [Google Scholar]
- Canon S.H. Optometric implications of systemic drug therapy. J. Am. Optom. Assoc. 1985;56:843–844. [PubMed] [Google Scholar]
- Castrop H., Oppermann M., Weiss Y., Huang Y., Mizel D., Lu H., Germain S., Schweda F., Theilig F., Bachmann S., Briggs J., Kurtz A., Schnermann J. Reporter gene recombination in juxtaglomerular granular and collecting duct cells by human renin promoter-Cre recombinase transgene. Physiol. Genom. 2006;25:277–285. doi: 10.1152/physiolgenomics.00302.2005. [DOI] [PubMed] [Google Scholar]
- Chen X.M. Experimental study on the therapeutic value of microwave on hearing loss due to ethacrynic acid ototoxicity. Zhonghua er bi yan hou ke za zhi. 1993;28 225–227, 252. [PubMed] [Google Scholar]
- Chen X.M., Din D.L., Luo D.F., Huangfu M.S., Jin X.M. Deafness, induced by sodium ethacrynate in Guinea pigs, alleviated by microwave treatment. Rev. Laryngol. Otol. Rhinol. 1992;113:133–135. [PubMed] [Google Scholar]
- Dallos P., Schoeny Z.G., Cheatham M.A. Cochlear summating potentials. Descriptive aspects. Acta Oto-Laryngol. Suppl. 1972;302:1–46. [PubMed] [Google Scholar]
- Davis H., Deatherage B.H., Eldredge D.H., Smith C.A. Summating potentials of the cochlea. Am. J. Physiol. 1958;195:251–261. doi: 10.1152/ajplegacy.1958.195.2.251. [DOI] [PubMed] [Google Scholar]
- Ding D., Jin X. The changes of cochlear bioelectric potential in Guinea pigs during brief anoxia. Acta Otorhinolaryngol. 1998;12:4–6. [Google Scholar]
- Ding D., Salvi R. Review of cellular changes in the cochlea due to aminoglycoside antibiotics. Volta Rev. 2005;105:407–438. [Google Scholar]
- Ding D., Li H., Zhao J., Huangfu M. Histochemistry of stria vascularis in the inner ear of Guinea pigs. J. Clin. Otorhinolaryngol. 1987;1:173–174. [Google Scholar]
- Ding D., Zhao J., Luo D., Huiangfu M. The microcirculation static quantitative observation of the stria vascularis. Acta Otolaryngol. 1990;4:1–2. [Google Scholar]
- Ding D., Jin X., Huangfu M. Functional and morphological changes of the cochlea in Guinea pigs during anoxia. Chin. J. Otorhinolaryngol. 1993;28:265–267. [PubMed] [Google Scholar]
- Ding D., Zhang Z., Zhu Q. Experimental study of concurrent ototoxicity between ethacrynic acid and gentamycin. J. Audiol. Speech Disord. 1995;3:76–79. [Google Scholar]
- Ding D., Jin X., Zhao J. The changes of cochlear bioelectric potential on Guinea pigs deafened with ethacrynic acid. J. Clin. Otorhinolaryngol. 1996;10:330–332. [Google Scholar]
- Ding D., McFadden S.L., Woo J.M., Salvi R.J. Ethacrynic acid rapidly and selectively abolishes blood flow in vessels supplying the lateral wall of the cochlea. Hear. Res. 2002;173:1–9. doi: 10.1016/s0378-5955(02)00585-3. [DOI] [PubMed] [Google Scholar]
- Ding D., McFadden S.L., Browne R.W., Salvi R.J. Late dosing with ethacrynic acid can reduce gentamicin concentration in perilymph and protect cochlear hair cells. Hear. Res. 2003;185:90–96. doi: 10.1016/s0378-5955(03)00258-2. [DOI] [PubMed] [Google Scholar]
- Ding D., Jiang H., McFadden S., Salvi R. Ethacrynic acid is the key for opening of the blood-labyrinth barrier. Chin. J. Otol. 2004;2(1):42–47. [Google Scholar]
- Ding D., Jiang H., Wang P., Salvi R. Cell death after co-administration of cisplatin and ethacrynic acid. Hear. Res. 2007;226:129–139. doi: 10.1016/j.heares.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ding D., Qu Y., Qi W., Jiang H., Salvi R. Hypoxia-induced inner ear damage. Chin. J. Otol. 2008;6:468–474. [Google Scholar]
- Ding D., Jiang H., Salvi R.J. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear. Res. 2010;259:16–23. doi: 10.1016/j.heares.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Jiang T., Qu Y., Qi W., Salvi R. Chinese Science and Technology Publishing Company; 2010. Science of the inner ear. [Google Scholar]
- Ding D., Allman A., Yin S., Sun H., Salvi R.J. Nova Science Publishers, Inc; 2011. Cisplatin Ototoxicity; pp. 39–63. (Chapter 2) [Google Scholar]
- Ding D., Allman B.L., Salvi R. Review: ototoxic characteristics of platinum antitumor drugs. Anat. Rec. (Hoboken) 2012;295:1851–1867. doi: 10.1002/ar.22577. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P., Katychev A., Wang X., Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J. Cereb. Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Durrant J.D., Wang J., Ding D.L., Salvi R.J. Are inner or outer hair cells the source of summating potentials recorded from the round window? J. Acoust. Soc. Am. 1998;104:370–377. doi: 10.1121/1.423293. [DOI] [PubMed] [Google Scholar]
- Edelman D.A., Jiang Y., Tyburski J., Wilson R.F., Steffes C. Pericytes and their role in microvasculature homeostasis. J. Surg. Res. 2006;135:305–311. doi: 10.1016/j.jss.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J. Basic principles for electrocochleography. Acta Oto-Laryngol. Suppl. 1974;316:7–16. doi: 10.1080/16512251.1974.11675742. [DOI] [PubMed] [Google Scholar]
- Engstrom H. The cortilymph, the third lymph of the inner ear. Acta Morphol. Neerl. 1960;3:195–204. [PubMed] [Google Scholar]
- Ferraro J.A., Tibbils R.P. SP/AP area ratio in the diagnosis of Meniere's disease. Am. J. Audiol. 1999;8:21–28. doi: 10.1044/1059-0889(1999/001). [DOI] [PubMed] [Google Scholar]
- Forge A. Observations on the stria vascularis of the Guinea pig cochlea and the changes resulting from the administration of the diuretic furosemide. Clin. Otolaryngol. Allied Sci. 1976;1:211–219. doi: 10.1111/j.1365-2273.1976.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Forge A., Brown A.M. Ultrastructural and electrophysiological studies of acute ototoxic effects of furosemide. Br. J. Audiol. 1982;16:109–116. doi: 10.3109/03005368209081455. [DOI] [PubMed] [Google Scholar]
- Friedel H.A., Buckley M.M. Torasemide. A review of its pharmacological properties and therapeutic potential. Drugs. 1991;41:81–103. doi: 10.2165/00003495-199141010-00008. [DOI] [PubMed] [Google Scholar]
- Gallagher K.L., Jones J.K. Furosemide-induced ototoxicity. Ann. Intern. Med. 1979;91:744–745. doi: 10.7326/0003-4819-91-5-744. [DOI] [PubMed] [Google Scholar]
- Ganten D., Minnich J.L., Granger P., Hayduk K., Brecht H.M., Barbeau A., Boucher R., Genest J. Angiotensin-forming enzyme in brain tissue. Science. 1971;173:64–65. doi: 10.1126/science.173.3991.64. [DOI] [PubMed] [Google Scholar]
- Gibson W.P. The use of electrocochleography in the diagnosis of Meniere's disease. Acta Oto-Laryngol. Suppl. 1991;485:46–52. [PubMed] [Google Scholar]
- Glenn S.T., Jones C.A., Sexton S., LeVea C.M., Caraker S.M., Hajduczok G., Gross K.W. Conditional deletion of p53 and Rb in the renin-expressing compartment of the pancreas leads to a highly penetrant metastatic pancreatic neuroendocrine carcinoma. Oncogene. 2014;33:5706–5715. doi: 10.1038/onc.2013.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. Analysis of summating potential in cochlear responses of Guinea pigs. Am. J. Physiol. 1954;178:331–337. doi: 10.1152/ajplegacy.1954.178.2.331. [DOI] [PubMed] [Google Scholar]
- Greger R. New insights into the molecular mechanism of the action of diuretics. Nephrol. Dialysis Transplant Off. Publ. Eur. Dialysis Transpl. Assoc. Eur. Ren. Assoc. 1999;14:536–540. doi: 10.1093/ndt/14.3.536. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Cellular mechanism of the action of loop diuretics on the thick ascending limb of Henle's loop. Klin. Wochenschr. 1983;61:1019–1027. doi: 10.1007/BF01537500. [DOI] [PubMed] [Google Scholar]
- Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A., O'Farrell F.M., Buchan A.M., Lauritzen M., Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J.J. IIIrd Symposium on the Role of the Vestibular Organ in Space Exploration NASA SP-152:241. 1967. Vascular pattern of the membranous labyrinth. [Google Scholar]
- Ikeda K., Morizono T. Effect of albumin-bound furosemide on the endocochlear potential of the chinchilla. Alleviation of furosemide-induced ototoxicity. Arch. Otolaryngol. Head Neck Surg. 1989;115:500–502. doi: 10.1001/archotol.1989.01860280098025. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Morizono T. Electrochemical profile for calcium ions in the stria vascularis: cellular model of calcium transport mechanism. Hear Res. 1989;40:111–116. doi: 10.1016/0378-5955(89)90104-4. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Morizono T. Electrochemical profiles for monovalent ions in the stria vascularis: cellular model of ion transport mechanisms. Hear Res. 1989;39:279–286. doi: 10.1016/0378-5955(89)90047-6. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Oshima T., Hidaka H., Takasaka T. Molecular and clinical implications of loop diuretic ototoxicity. Hear Res. 1997;107:1–8. doi: 10.1016/s0378-5955(97)00009-9. [DOI] [PubMed] [Google Scholar]
- Jaanus S.D. Ocular side effects of selected systemic drugs. Optom. Clin. Off. Publ. Prentice Soc. 1992;2:73–96. [PubMed] [Google Scholar]
- Kakigi A., Sawada S., Takeda T., Takeuchi S., Higashiyama K., Azuma H., Yamakawa K. Electrocochleographic findings in cases of autoimmune disease with sensorineural deafness. Auris Nasus Larynx. 2003;30:349–354. doi: 10.1016/j.anl.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Kanzaki J., Ouchi T., Yokobori H., Ino T. Electrocochleographic study of summating potentials in Meniere's disease. Audiology. 1982;21:409–424. doi: 10.3109/00206098209072755. [DOI] [PubMed] [Google Scholar]
- Kasajima K., Kaneko Y., Ouchi J. Changes of the stria vascularis induced by ethacrynic acid. Auris Nasus Larynx. 1978;5:1–16. doi: 10.1016/s0385-8146(78)80002-9. [DOI] [PubMed] [Google Scholar]
- Kruck F. Mechanism of action & indication for therapeutic use of anionic diuretics; organic mercurial preparations carbonic anhydrase inhibitors, chlorothiacide. Arztliche Wochenschr. 1958;13:1058–1064. [PubMed] [Google Scholar]
- Kurtz L., Schweda F., de Wit C., Kriz W., Witzgall R., Warth R., Sauter A., Kurtz A., Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J. Am. Soc. Nephrol. JASN. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- Kusakari J., Thalmann R. Effects of anoxia and ethacrynic acid upon ampullar endolymphatic potential and upon high energy phosphates in ampullar wall. Laryngoscope. 1976;86:132–147. doi: 10.1288/00005537-197601000-00025. [DOI] [PubMed] [Google Scholar]
- Kusakari J., Ise I., Comegys T.H., Thalmann I., Thalmann R. Effect of ethacrynic acid, furosemide, and ouabain upon the endolymphatic potential and upon high energy phosphates of the stria vascularis. Laryngoscope. 1978;88:12–37. doi: 10.1002/lary.1978.88.1.12. [DOI] [PubMed] [Google Scholar]
- Kusakari J., Kambayashi J., Ise I., Kawamoto K. Reduction of the endocochlear potential by the new “loop” diuretic, bumetanide. Acta Oto-Laryngol. 1978;86:336–341. doi: 10.3109/00016487809107512. [DOI] [PubMed] [Google Scholar]
- Li M., Ding D., Zheng X.Y., Salvi R. Vestibular destruction by slow infusion of gentamicin into semicircular canals. Acta Oto-Laryngol. 2004;552:1–7. doi: 10.1080/03655230410017102. [DOI] [PubMed] [Google Scholar]
- Li Y., Ding D., Jiang H., Fu Y., Salvi R. Co-administration of cisplatin and furosemide causes rapid and massive loss of cochlear hair cells in mice. Neurotox. Res. 2011;20:307–319. doi: 10.1007/s12640-011-9244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Wang J. Determination of prostaglandin F (2α) and prostacyclindiuresis in the cochlear lateral wall after echacrynic acid injection to Guinea pigs. Acta Univ. Medictnae Tangji. 1994;2:105–107. [Google Scholar]
- Liu H., Ding D., Wu X., Jiang H., Salvi R. Ethacrynic acid-induced cochlear lesions in vitro. Abstr. Assoc. Res. Otolaryngol. 2011;34:26. [Google Scholar]
- Liu H., Ding D.L., Jiang H.Y., Wu X.W., Salvi R., Sun H. Ototoxic destruction by co-administration of kanamycin and ethacrynic acid in rats. J. Zhejiang Univ. Sci. B. 2011;12:853–861. doi: 10.1631/jzus.B1100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T.H., Ellison A.C. The teratological effect of certain thiadiazoles related to acetazolamide, with a note on sulfanilamide and thiazide diuretics. Johns Hopkins Med. J. 1972;130:95–104. [PubMed] [Google Scholar]
- Marks S.C., Schacht J. Effects of ototoxic diuretics on cochlear Na+/K+-ATPase and adenylate cyclase. Scand. Audiol. Suppl. 1981;(14 Suppl):131–138. [PubMed] [Google Scholar]
- Matz G.J. The ototoxic effects of ethacrynic acid in man and animals. Laryngoscope. 1976;86:1065–1086. doi: 10.1288/00005537-197608000-00001. [DOI] [PubMed] [Google Scholar]
- McFadden S.L., Ding D., Jiang H., Woo J.M., Salvi R.J. Chinchilla models of selective cochlear hair cell loss. Hear. Res. 2002;174:230–238. doi: 10.1016/s0378-5955(02)00697-4. [DOI] [PubMed] [Google Scholar]
- McFadden S.L., Ding D., Jiang H., Salvi R.J. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997:40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 1927.Merbaphen (Novasurol) as Diuretic in Congestive Heart Failure. Can. Med. Assoc. J. 1927;17:156. [PMC free article] [PubMed] [Google Scholar]
- Meriwether W.D., Mangi R.J., Serpick A.A. Deafness following standard intravenous dose of ethacrynic acid. JAMA. 1971;216:795–798. [PubMed] [Google Scholar]
- Meyerhoff W.L., Yellin M.W. Summating potential/action potential ratio in perilymph fistula. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 1990;102:678–682. doi: 10.1177/019459989010200609. [DOI] [PubMed] [Google Scholar]
- Nagel D. Compound action potential of the cochlear nerve evoked electrically. Electrophysiological study of the acoustic nerve (Guinea pig) Arch. Oto-Rhino-Laryngol. 1974;206:293–298. doi: 10.1007/BF00460282. [DOI] [PubMed] [Google Scholar]
- Nam E.C., Won J.Y. Extratympanic electrocochleographic changes on noise-induced temporary threshold shift. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2004;130:437–442. doi: 10.1016/j.otohns.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Navar L.G., Satou R., Gonzalez-Villalobos R.A. The increasing complexity of the intratubular Renin-Angiotensin system. J. Am. Soc. Nephrol. JASN. 2012;23:1130–1132. doi: 10.1681/ASN.2012050493. [DOI] [PubMed] [Google Scholar]
- Ng P.S., Conley C.E., Ing T.S. Deafness after ethacrynic acid. Lancet. 1969;1:673–674. doi: 10.1016/s0140-6736(69)92032-7. [DOI] [PubMed] [Google Scholar]
- Odkvist L. Gentamicin Cures vertigo, but what Happens to hearing? Int. tinnitus J. 1997;3:133–136. [PubMed] [Google Scholar]
- Odkvist L.M., Bergenius J., Moller C. When and how to use gentamicin in the treatment of Meniere's disease. Acta Oto-Laryngol. Suppl. 1997;526:54–57. doi: 10.3109/00016489709124023. [DOI] [PubMed] [Google Scholar]
- Ohashi T., Takeyama I. Clinical significance of SP/AP ratio in inner ear diseases. ORL J. Oto-Rhino-Laryngol. Relat. Specialties. 1989;51:235–245. doi: 10.1159/000276065. [DOI] [PubMed] [Google Scholar]
- Okada Y., Yamanaka I., Sakamoto T., Hata Y., Sassa Y., Yoshikawa H., Fujisawa K., Ishibashi T., Inomata H. Increased expression of angiotensin-converting enzyme in retinas of diabetic rats. Jpn. J. Ophthalmol. 2001;45:585–591. doi: 10.1016/s0021-5155(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Pallone T.L., Silldorff E.P. Pericyte regulation of renal medullary blood flow. Exp. Nephrol. 2001;9:165–170. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- Paloheimo S., Thalman R. Influence of “loop” diuretics upon Na+K+-ATPase and adenylate cyclase of the stria vascularis. Arch. Oto-Rhino-Laryngol. 1977;217:347–359. doi: 10.1007/BF00465552. [DOI] [PubMed] [Google Scholar]
- Peppiatt C.M., Howarth C., Mobbs P., Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick C.A., Hoppe W. Permanent deafness associated with furosemide administration. Ann. Otol. Rhinol. Laryngol. 1975;84:94–101. doi: 10.1177/000348947508400114. [DOI] [PubMed] [Google Scholar]
- Remond M.C., Harrison R.V., Legouix J.P. A comparison of compound action potential and cochlear microphonic two-tone suppression in the Guinea pig. Hear Res. 1982;8:83–91. doi: 10.1016/0378-5955(82)90037-5. [DOI] [PubMed] [Google Scholar]
- Rifkin S.I., de Quesada A.M., Pickering M.J., Shires D.L., Jr. Deafness associated with oral furosemide. South. Med. J. 1978;71:86–88. doi: 10.1097/00007611-197801000-00029. [DOI] [PubMed] [Google Scholar]
- Rybak L.P. Ototoxicity of loop diuretics. Otolaryngol. Clin. North Am. 1993;26:829–844. [PubMed] [Google Scholar]
- Santi P.A., Duvall A.J., 3rd Morphological alteration of the stria vascularis after administration of the diuretic bumetanide. Acta Oto-Laryngol. 1979;88:1–12. doi: 10.3109/00016487909137133. [DOI] [PubMed] [Google Scholar]
- Santi P.A., Lakhani B.N. The effect of bumetanide on the stria vascularis: a stereological analysis of cell volume density. Hear. Res. 1983;12:151–165. doi: 10.1016/0378-5955(83)90103-x. [DOI] [PubMed] [Google Scholar]
- Schucnect H.F. Harvard University Press; Cambridge, Massachustts: 1974. Pathology of the Ear. [Google Scholar]
- Schuknecht H.F., Seifi A.E. Experimental observations on the fluid Physiology of the inner ear. Ann. Otol. Rhinol Laryngol. 1963;72:687–712. doi: 10.1177/000348946307200308. [DOI] [PubMed] [Google Scholar]
- Schultz E.M., Bicking J.B., DeSolms S.J., Stokker G.E. Structure of the diuretic merbaphen. J. Med. Chem. 1971;14:998–999. doi: 10.1021/jm00292a033. [DOI] [PubMed] [Google Scholar]
- Sellick P.M., Johnstone B.M. Production and role of inner ear fluid. Prog. Neurobiol. 1975;5:337–362. doi: 10.1016/0301-0082(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Sequeira Lopez M.L., Pentz E.S., Nomasa T., Smithies O., Gomez R.A. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev. Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- Shi X., Jin X., Huangfu M., Ding D. Study of the action of 9370 MHz microwave on cochlea microcirculation. Chin. Arch. Otolaryngol. Head Neck Surg. 1994;1:151–154. [Google Scholar]
- Shi X., Niu Y., Wang W. Effect of microwave on cochlear microcirculation. Chin. J. Zool. 1997;32:52–55. [Google Scholar]
- Shi X., Han W., Yamamoto H., Tang W., Lin X., Xiu R., Trune D.R., Nuttall A.L. The cochlear pericytes. Microcirculation. 2008;15:515–529. doi: 10.1080/10739680802047445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo M., Miyamoto M., Abe N., Shida S., Murakami Y., Imai Y. Dependence of endocochlear potential on basolateral Na+ and Cl- concentration: a study using vascular and perilymph perfusion. Jpn. J. Physiol. 1992;42:617–630. doi: 10.2170/jjphysiol.42.617. [DOI] [PubMed] [Google Scholar]
- Silverstein H., Yules R.B. The effect of diuretics on cochlear potentials and inner ear fluids. Laryngoscope. 1971;81:873–888. doi: 10.1288/00005537-197106000-00008. [DOI] [PubMed] [Google Scholar]
- Silverstein H., Arruda J., Rosenberg S.I., Deems D., Hester T.O. Direct round window membrane application of gentamicin in the treatment of Meniere's disease. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 1999;120:649–655. doi: 10.1053/hn.1999.v120.a91763. [DOI] [PubMed] [Google Scholar]
- Smith C.A. Capillary areas of the cochlea in the Guinea pig. Laryngoscope. 1951;61:1073–1095. doi: 10.1288/00005537-195111000-00002. [DOI] [PubMed] [Google Scholar]
- Syka J., Melichar I. The effect of loop diuretics upon summating potentials in the Guinea pig. Hear. Res. 1985;20:267–273. doi: 10.1016/0378-5955(85)90031-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Takiguchi T., Aoki T. A suggestion on the Origin of cortilymph by Scanning Electron microscope. Pract. Oto-Rhino-Laryngol. 1973;66:333–339. [Google Scholar]
- Tasaki I., Spyropoulos C.S. Stria vascularis as source of endocochlear potential. J. Neurophysiol. 1959;22:149–155. doi: 10.1152/jn.1959.22.2.149. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Davis H., Eldredge D.H. Exploration of cochlear potentials in Guinea pig with a microelectrode. Acoust. Soc. Am. 1954;765(26):765–773. [Google Scholar]
- Thalmann I., Kobayashi T., Thalmann R. Arguments against a mediating role of the adenylate cyclase–cyclic AMP system in the ototoxic action of loop diuretics. Laryngoscope. 1982;92:589–593. doi: 10.1288/00005537-198205000-00025. [DOI] [PubMed] [Google Scholar]
- Vaughan E.D., Jr., Carey R.M., Peach M.J., Ackerly J.A., Ayers C.R. The renin response to diuretic therapyl A limitation of antihypertensive potential. Circulation Res. 1978;42:376–381. doi: 10.1161/01.res.42.3.376. [DOI] [PubMed] [Google Scholar]
- Wagner J., Jan Danser A.H., Derkx F.H., de Jong T.V., Paul M., Mullins J.J., Schalekamp M.A., Ganten D. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br. J. Ophthalmol. 1996;80:159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P., Liu J., Marcus D.C. Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hear Res. 1995;84:19–29. doi: 10.1016/0378-5955(95)00009-s. [DOI] [PubMed] [Google Scholar]
- Wever E.G., Bray C.W. Auditory nerve impulses. Science. 1930;71:215. doi: 10.1126/science.71.1834.215. [DOI] [PubMed] [Google Scholar]
- Winkler M., Schuchard J., Stolting I., Vogt F.M., Barkhausen J., Thorns C., Bader M., Raasch W. The brain renin-angiotensin system plays a crucial role in regulating body weight in diet-induced rat obesity. Br. J. Pharmacol. 2016;173:1602–1617. doi: 10.1111/bph.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.C., Lytle C., Zhu T.T., Payne J.A., Benz E., Jr., Forbush B., 3rd Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2201–2205. doi: 10.1073/pnas.91.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Ding D., Huangfu M. The influence of enthacrynic acid on the activity of enzyme in the stria vascularis in Guinea pigs. J. Clin. Otorhinolaryngol. 1988;2:65–67. [Google Scholar]
- Zhao J., Ding D., Wang J., Huangfu M. Influence of ethacrynic acid on microcirculation of stria vascularis of cochlea in Guinea pigs. Acta Univ. Med. Second. Shanghai. 1988;8:34–37. [Google Scholar]
- Zheng X.Y., Ding D., McFadden S.L., Henderson D. Evidence that inner hair cells are the major source of cochlear summating potentials. Hear. Res. 1997;113:76–88. doi: 10.1016/s0378-5955(97)00127-5. [DOI] [PubMed] [Google Scholar]