Abstract
Apoptosis, or controlled cell death, is a normal part of cellular lifespan. Cell death of cochlear hair cells causes deafness; an apoptotic process that is not well understood. Worldwide, 1.3 billion humans suffer some form of hearing loss, while 360 million suffer debilitating hearing loss as a direct result of the absence of these cochlear hair cells (Worldwide Hearing, 2014). Much is known about apoptosis in other systems and in other cell types thanks to studies done since the mid-20th century. Here we review current literature on apoptosis in general, and causes of deafness and cochlear hair cells loss as a result of apoptosis. The family of B-cell lymphoma (Bcl) proteins are among the most studied and characterized. We will review current literature on the Bcl2 and Bcl6 protein interactions in relation to apoptosis and their possible roles in vulnerability and survival of cochlear hair cells.
Keywords: Apoptosis, Hair cell, Bcl2, Bcl6
1. Introduction
In normal tissues, there is a balance between the generation of new cells via cell division and the loss of cells via cell death. Old cells become damaged over time and are eliminated. Programmed cell death, or apoptosis, is an orderly process during which the genome of the cell is broken down, the cell is fragmented into smaller pieces and the debris is consumed by nearby cells (phagocytes) that clean up the cell fragments. Apoptosis is crucial for embryological development, renewal and aging, as well as for maintaining hemostasis. In the inner ear, loss of the sensorineural cells, known as inner hair cells (IHC) and outer hair cells (OHC), is a key factor contributing to hearing loss. Apoptosis appears to be the most common mechanism of hair cell loss. Cell death can also occur in a chaotic manner, necrosis. Although either mechanism can cause hair cell death, we will first review apoptosis, and its known pathways. We will then discuss possible mechanisms of hair cell apoptosis and how the B-Cell Lymphoma (Bcl) family of proteins is involved and possible mechanisms in the second part of this review.
2. Apoptosis
Apoptosis is a genetically controlled mechanism involving an enormous collection of signaling pathways, triggers, genes, proteins, and molecules. There are two distinct phases in apoptosis, the initiation phase and the execution phase. The initiation phase involves many different proteins and is quite complex. It is started by various “stresses” from either outside the cell (extracellular) or inside the cell (intracellular). Some examples of extracellular signals that trigger apoptosis include loss of growth factors, low oxygen levels (hypoxia), and radiation. Intracellular signals include DNA damage, the damage caused by chemotherapy drugs, telomere malfunction, and infection with viruses. The initiation phase triggers the execution phase. The execution phase involves the activation of specialized enzymes (caspases and others) that directly result in cell death.
2.1. Apoptotic pathways
There are two main pathways that apoptosis can take: intrinsic and extrinsic pathways. These pathways, although initiating at different places and with different mechanisms, both meet up with caspase activation leading to protein cleavage. See Fig. 1.
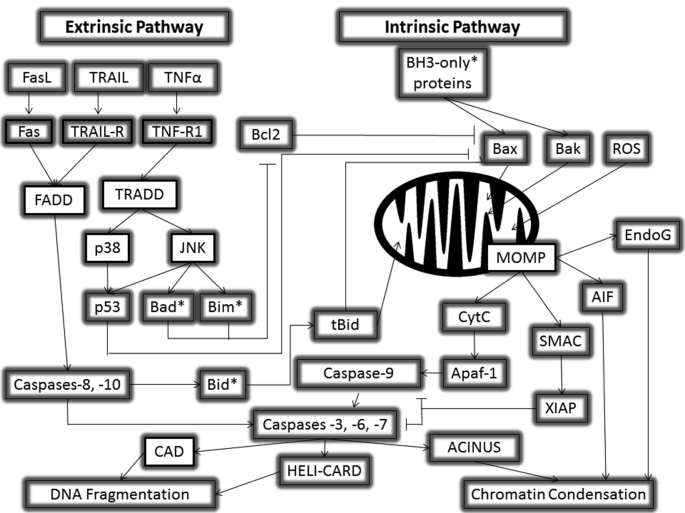
Fig. 1.
Extrinsic and Intrinsic Apoptotic Pathways. The extrinsic and intrinsic pathways of apoptosis are graphically shown with their associated receptors, proteins, molecules, and cross talk.
2.1.1. Extrinsic pathway
The extrinsic, or death receptor, pathway is principally facilitated through signaling of the tumor necrosis factor (TNF) super family (or membrane bound death) receptors. Several examples of types of receptors found in the TNF superfamily are Fas receptor, TNF R1, TNF-related apoptosis inducing ligand receptor 1 (TRAIL R1), and receptor activator of NFκB (RANK). Each of these receptors is associated with a ligand to activate them; Fas ligand (FasL), TNF, TRAIL, and RANK ligand (RANKL) (Locksley et al., 2001). Each of these receptors initiates a cascade of activity that can lead to apoptosis via the death inducing signaling complex (DISC) (Ashkenazi and Dixit, 1998; Bharwaj and Aggarwal, 2003, Kischkel et al., 1995). FasL binding to the Fas receptor generates the Fas-associated Death Domain (FADD), while TNFR binding of TNF induces the TNFR-associated death domain (TRADD) (Hsu et al., 1995). These both activate caspase-8 and -10. Curiously, caspase-10 is not activated in mice (Reed et al., 2003). Caspase-8 can activate tBid to recruit the help of the intrinsic pathway (more on this later). Caspase-8 and -10 can also activate caspases-3, -6, and -7. Paradoxically, TNFα can also activate the TNFR-1 receptor, which can activate a cascade leading to NFκB stimulation and pro-survival gene activation (Marques Fernandez et al., 2013). The extrinsic pathways are depicted in Fig. 1.
2.1.2. Intrinsic pathway
The intrinsic pathway is initiated via intracellular mechanisms, including reactive oxygen species (ROS) and activation from the extrinsic pathway via Bid (Wu and Bratton, 2013). DNA damage is a major inducer of apoptosis as a mechanism to prevent the cell from undergoing mitosis with imperfect DNA (Pistritto et al., 2016). The intrinsic pathway of apoptosis is heavily regulated by the Bcl2 family of proteins. Apoptotic stimuli signal Bcl2 family proteins Bax and Bak to oligamerize and insert into the mitochondrial outer membrane (Antonsson et al., 1997, Eskes et al., 2000, Fesik, 2000, Green and Kroemer, 2004, Kuwana et al., 2002, Minn et al., 1997, Muchmore et al., 1996, Schendel et al., 1997, Wei et al., 2000). These proteins form a pore causing mitochondrial outer membrane permeabilization (MOMP) (Green and Kroemer, 2004) that will allow for release of cytochrome C (CytC) from the mitochondria. CytC released will bind to Apaf-1, creating the “apoptosome” complex in the cytosol. The apoptosome will activate caspase-9, which will activate caspases-3, -6, and -7, aligning with the extrinsic pathway. Caspase-3 moves apoptosis into the nucleus to initiate caspase-activated DNase (CAD), DNA condensation, chromatin condensation via apoptotic chromatin condensation inducer in the nucleus (ACINUS), and acceleration of DNA degradation by cutting cytosolic helicase with an N-terminal caspase-recruitment domain (HELI-CARD) (Enari et al., 1998, Kovacsovics et al., 2002, Liu et al., 1997, Sahara et al., 1999).
The SMAC protein, which is encoded from the DIABLO gene, is also released from the mitochondria following MOMP. SMAC will inhibit several apoptosis inhibitors, like XIAP (Galluzzi et al., 2008). Another protein that acts in conjunction with SMAC is mammalian homolog of bacterial high temperature requirement protein A2 (Omi/HtrA2) (Deveraux et al., 1999). XIAP, when not inhibited, will inhibit caspase-9 activity, preventing the initiator caspase from activating the executioner caspases. XIAP can also inhibit the executioner caspases (Jost et al., 2009). The intrinsic pathways are summarized in Fig. 1.
2.2. Phases of apoptosis
2.2.1. Initiation phase
2.2.1.1. Extrinsic or receptor-mediated pathway
Members of the tumor necrosis factor (TNF) receptor superfamily of transmembrane proteins control the extrinsic pathway. All TNF receptors, also known as death receptors, share a region of 80 amino acids called the “death domain”. This region plays a critical role in transmitting death signals across the cell membrane. Inside the cell a cascade of proteins is turned on. At the end of these pathways, initiator caspase-8 is activated and the execution phase of apoptosis is triggered.
2.2.1.2. Intrinsic or mitochondrial pathway
The Bcl-2 family of proteins controls the intrinsic pathway. There are 25 known proteins in the Bcl-2 family. The different members function to either stimulate apoptosis (pro-apoptotic) or block apoptosis (anti-apoptotic). There is a delicate balance between pro-apoptotic and anti-apoptotic proteins within a cell. BH3-only proteins sense intrinsic signals to undergo apoptosis, such as DNA damage. They travel to the mitochondrial membrane and activate the pro-apoptotic proteins Bax or Bak, or inhibit anti-apoptotic proteins. When activated, Bax and Bak bind, and cause mitochondrial outer membrane permeabilization (MOMP). This perforates the mitochondrial membrane, and induces the release of a crucial pro-apoptotic factor, cytochrome c, into the cytosol. Cytochrome c joins another pro-apoptotic factor, APAF1, to form the “apoptosome” complex, which in turn activates a series of caspases, leading to cell destruction. The cell death proteins are closely regulated by the tumor suppressor protein p53.
2.2.1.3. Perforin/granzyme
In some cases, immune cells called cytotoxic T lymphocytes can start apoptosis. This happens when the lymphocytes secrete a protein called perforin and small particles containing specialized enzymes. Perforin creates holes in plasma membrane of the target cell. The additional particles use the holes to enter the cell. After entering the cell, they release their enzymes (granzymes A and B) that start the execution pathway and wreak havoc on cell structure and function.
2.2.2. Execution phase
The extrinsic and intrinsic pathways both stimulate the execution phase. During this phase a group of protein-cutting enzymes called caspases lead directly to cell death. The main execution caspases are caspases. Caspases are present in lethal doses within each cell, but they only become active via the initiation process. Caspase-3 is considered the most important of all the caspases. It can cause DNA and chromatin damage, re-arrange the cytoskeleton, and disrupt intracellular transport, cell division, and signal transduction. Once activated the execution caspases cannot be stopped, cell death is certain. Cell fragments produced during the final stage of apoptosis are quickly recognized, engulfed, and digested by macrophages or surrounding epithelial cells.
2.3. Caspases
One thing relating all the pathways, triggers, and proteins is that the grunt work of cutting up the cellular proteins for apoptosis are a class of enzymes termed cysteine-aspartic protease (caspase) enzymes. Within the caspase class, there are two types: Initiator (or apical) caspases and effector (or executioner) caspases (Riedl and Salvesen, 2007, Timmer and Salvesen, 2007). Initiator caspases include caspases -8 and -9. Following an initial signal for apoptosis, these enzymes target scaffold proteins. Additionally, initiator caspases cleave executioner caspases into 10 and 20 kDa sections that can bind into a tetrameric protease. Executioner caspases include caspases -3, -6, and -7. The cleavage of executioner caspases begins a proteolytic cascade that will lead to the lysis of nearly all parts of the cell. As part of this cascade, executioner caspases will also activate caspase activated DNases.
2.4. p53 in apoptosis
The gene encoding p53 is encoded on the short arm of chromosome 17, and the corresponding p53 protein has a molecular mass of 43.7 kDa (Levine and Oren, 2009). It has 3 main domains; a DNA binding domain for binding to other genes, an N-terminal trans-activational domain that forms binding sites for positive or negative regulators, and a C-terminal oligomerization domain that undergoes post-translational modifications and alternative splicing (Mollereau and Ma, 2014, Pflaum et al., 2014). In normal, healthy cells, levels of p53 are low because turnover is high. However, when damage occurs, p53 stabilizes and the levels rise (Haupt et al., 2003). Stability is conferred to p53 by the mouse double minute 2 (MDM2) protein (Perry, 2010), a protein that is also overexpressed in many cancers (Jansson et al., 2014, Riley et al., 2016).
3. Bcl2 and Bcl6
3.1. Bcl2 family of proteins
The Bcl2 family of proteins can be broadly categorized into 2 groups: pro-apoptotic and anti-apoptotic. All Bcl2 family members have certain sequence homology domains, termed Bcl2 homology (BH) domains. There are currently 4 known BH domains. The anti-apoptotic proteins generally contain 3-4 BH domains and include Bcl-2, Bcl-xL, Bcl-W, Mcl-1, A1, and Bcl-B. The pro-apoptotic groups can be further sub divided based upon these BH domains; a BH3-only group, including Bid, Bim, Bad, Noxa, and Puma, and the multi-domain group, comprising Bax, Bak, and Bok.
3.1.1. BH domains
The functions of the various domains have been elucidated (Danial, 2007, Lomonosova and Chinnadurai, 2009). The BH1 and BH2 domains are critical for dimerization with other pro-apoptotic members, then subsequent pore formation and insertion into the mitochondrial membrane for MOMP. The BH3 domain is contained by all Bcl-2 family proteins and is crucial for homo- and hetero-dimerization among Bcl-2 family member interactions. The BH4 domain is essential for interaction and regulation of several proteins involved in apoptosis.
3.1.2. Interactions of Bcl2 family of proteins
Many members of the Bcl2 family of proteins interact with each other to either drive apoptosis, or to prevent apoptosis (see Fig. 2). The most notable of these interactions is homo- and hetero-dimerization between Bax and Bak proteins to form proteinaceous or lipidic pores. Upon oligamerization, these pores will insert into the mitochondrial membrane, allowing for the escape of CytC, and other proteins, from the mitochondria (Eskes et al., 2000, Reed, 2006, Wei et al., 2000, Wei et al., 2001). MOMP requires only 1 of the multi domain pro-apoptotic proteins in order for apoptosis to occur (Wei et al., 2001). The Bcl2 family protein Bid serves as an agonist for Bax oligamerization and insertion into membranes (Kuwana et al., 2002, Wei et al., 2000). Bcl2 protein itself is an integral membrane protein (Chen-Levy et al., 1989, Tsujimoto et al., 1987), but it can also serve as in inhibitor of CytC release from the mitochondria (Yang et al., 1997) as it binds to and inhibits Bax oligamerization (Eskes et al., 2000, Nechushtan et al., 1999, Wei et al., 2000, Wolter et al., 1997). In addition to Bid serving as an agonist for Bax oligamerization, both Bid and Bim are direct activators of Bax and Bak. Additionally, all the BH3-only proteins can bind and deactivate the Anti-apoptotic Bcl2 family members (Du et al., 2011, Green and Kroemer, 2004). In recent years, the Bcl2 protein has been referred to as the apoptotic switch (Adams and Cory, 2007) in reference to the regulation of MOMP by Bcl2 as it can bind to MOMP effectors Bax and Bak.
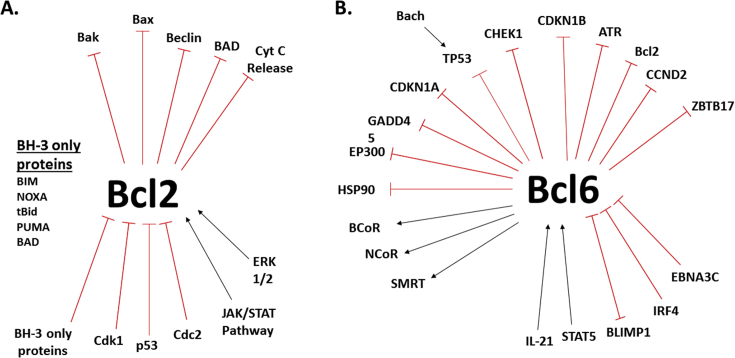
Fig. 2.
Interactions of Bcl2 and Bcl6. Shown are some of the known interactions of Bcl2 (panel A) and Bcl6 (panel B). Shown are protein interactions relating to cell cycle and cell death.
3.1.2.1. p53 interactions with Bcl proteins
There are many interactions between p53 and the Bcl2 family of proteins. Among those being transcriptionally activated by p53 in the nucleus are PUMA, Bid, Bax, TRAILR2, CD95 (Beckerman and Prives, 2010, Nakano and Vousden, 2001, Oda et al., 2000, Sax et al., 2002, Thornborrow et al., 2002, Yu et al., 2003). Because Bax is a direct target of p53, in p53 mutant cancers, the Bax levels can drop and MOMP can be abolished, stopping apoptosis from occurring. When p53 is constitutively active, Bax levels rise and MOMP will be up regulated and apoptosis will occur. Bid can be induced by p53 following irradiation by gamma radiation and aids in sensitizing cells to chemotherapeutic agents (Sax et al., 2002). Other agents are not able to induce apoptosis, but sensitize cells to chemotherapeutic agents, improving apoptotic results, include Apaf-1 and caspase-6 (MacLachlan and El-Deiry, 2002, Moroni et al., 2001, Sax and El-Deiry, 2003). p53 can also aid in apoptosis in a transcriptionally independent manner. It can act directly in the mitochondria by binding Bcl-xL and Bcl2, freeing up Bax and Bak proteins to activate MOMP, eliciting CytC release (Tomita et al., 2006).
In addition to the Bcl2 family of proteins, p53 interacts with Bcl6 proteins (see Fig. 2). In chronic myeloid leukemia, Bcl6 represses p53 in the initiation process of colony formation (Hurtz et al., 2011). Additionally, Bcl6 and BACH2 are binding competitors, with BACH2 reversing Bcl6 repression of p53 in normal healthy cells (Swaminathan et al., 2013, Swaminathan et al., 2014). The Bcl6 and BACH axis expression are implicated in controlling p53 expression in acute lymphoblastic leukemia (Ge et al., 2017).
4. Possible Bcl6 mechanisms
The exact mechanism of Bcl6 action is unknown. This may be due to the possibility of Bcl6 having various mechanisms in different cell types. For example, IL-21 mediates Bcl6 regulation in follicular helper T-cells as well as in germinal center B-cells to turn on Bcl6 (Linterman et al., 2010, Ozaki et al., 2004, Spolski and Leonard, 2010, Zotos et al., 2010). Alternatively, STAT5 is responsible for induction of Bcl6 expression in tonsillar B-cells (Scheeren et al., 2005). In development, it is known that Bcl6 is necessary for embryological development (Sakano et al., 2010). Mouse models deficient in Bcl6 have no germinal centers and are unable to produce high affinity antibodies (Toney et al., 2000). Subpopulations of CD4+ T-cells require Bcl6 for development (Mondal et al., 2010), as well as do Follicular T-helper cells (Johnston et al., 2009, Nurieva et al., 2009, Yu et al., 2009).
There are several factors that repress Bcl6 expression. Acetylation of Bcl6 can deactivate it (Bereshchenko et al., 2002). In breast epithelium, STAT5a represses Bcl6 expression (Tran et al., 2010). In bone marrow antibody production, STAT5 represses Bcl6 (Duy et al., 2010). This interaction also leads to a non-normal repertoire of primary antibodies in bone marrow. B-cell crosslinking reduces Bcl6 expression via ubiquitylation by extracellular signal-related kinases 1 and 2 (ERK 1/2; MAPK1) activation (Niu et al., 1998). Additionally, CD40 signaling triggers reduction of Bcl6 expression due to induction of transcription factor interferon regulatory factor 4 (IRF4) via NFκB pathway (Saito et al., 2007).
Bcl6 is transcriptional repressor that works by reducing mRNA expression of target genes (Deweindt et al., 1995, Seyfert et al., 1996). It binds to target genes as a homodimer and then recruits co-repressors molecules such as SMRT (silencing mediator of retinoid and thyroid receptor), nuclear receptor co-repressor (NCoR), and Bcl6 co-repressor (BCoR) (Dhordain et al., 1997, Dhordain et al., 1998, Huynh et al., 2000). The N-terminal POZ virus and zinc-finger protein Bric-a-brac, Tramtrack, broad complex protein (POZ/BTB) domain is where homo-dimerization occurs as well as co-repressor recruitment (Dhordain et al., 1995). Co-repressors recruit de-acetylases 1 and 2 (HDAC1 & HDAC2). The deacetylation of histones leads to transcriptional repression of Bcl6 target genes (Miles et al., 2005).
Bcl6 can bind to and repress several oncogenes and tumor suppressors: CDKN1A (Phan et al., 2005), CDKN1B (Shaffer et al., 2000), CCND1 (Shvarts et al., 2002), CCND2 (Shaffer et al., 2000), TP53 (Phan and Dalla-Favera, 2004), and Bcl2 (Saito et al., 2009). When Bcl6 binds to the Bcl2 locus, it disrupts the transcriptional activator ZBTB17, and by this means, repressing Bcl2. It is possible that by disrupting ZBTB17, or mutation of the Bcl6 binding site in the Bcl2 locus, that constitutive expression of Bcl2 will occur, resulting in clinically aggressive diseases that are resistant to apoptosis. There is a mechanism by which Bcl6 aids in transcriptional repression, even though a Bcl6 binding site is not present. Two separate reports show that Bcl6 can bind to Miz-1 (Phan et al., 2005, Wu and Jelinek, 2005). Binding of Bcl6 to Miz-1 can promote cell proliferation by repressing cyclin-dependent kinase inhibitor 1A (CDKN1A). At the very least, this is a way for Bcl6 to influence the cell cycle, in addition to inhibiting other DNA repair pathways (Ranuncolo et al., 2007, Ranuncolo et al., 2008).
5. Bcl2 and Bcl6 protein deregulation and disease
Deregulation of Bcl6 and Bcl2 family of proteins can lead to many types of cancers. These deregulations can give an insight into possible therapeutics. I will briefly describe some of the diseases and refer the reader to several reviews written to treat the subject of possible therapeutics.
Malignant gliomas have been shown to use Bcl3 to positively regulate Bcl2 downstream. Specifically, astrocytic glioma cells proliferate and apoptotic inhibition through the STAT3 pathway occurs (Wu et al., 2016). Also notable is the fact that Bcl2-xL has been linked to the STAT pathway (Grad et al., 2000, Zaanan et al., 2015). Cells of the hippocampus are vulnerable to ischemia-reperfusion (I-R) injury. Overexpression of Bcl2 in hippocampal tissues resulted in Granule cells resisting apoptosis, while CA1 cells only showed a delay in apoptosis (Wang et al., 1999). What could be regulating Bcl2 in I-R injury? A 7-mer peptide that acts as a glutathione peroxidase (GPX) mimetic that was given in I-R injury. The result was an increase in both mRNA and protein levels of Bcl2 while Bax was down regulated (Jiang et al., 2016). This suggests a possibility for GPX regulation of Bcl2 and Bax in liver tissue, as well as suppression of reactive oxygen species. Integrin-linked kinase (ILK) overexpression with treatment of temozolomide (TMZ) in glioma cells resulted in an increase of Bcl2 (anti-apoptotic) expression and a decrease of Bax (pro-apoptotic) expression (Liang et al., 2017). This suggests that the overexpression of ILK reduces sensitivity of the tissues to the anti-cancer drug, though no hypotheses as to a mechanism have been given.
5.1. Bcl2 in disease
The Bcl2 family of proteins, as discussed previously, is heavily involved in the intrinsic pathway of apoptosis. As such, they are not only involved in mechanisms related to lymphomas, but also to many other disease states, such as colorectal cancers, ischemia, diffuse large B-Cell lymphoma, malignant gliomas, and more. Many of these disease states have mechanisms that are still unknown. Such an example is in ovarian serous carcinoma where high expression of TRAIL-receptor 2 (TRAIL-R2) has been associated with the disease state (Braga et al., 2014). This study also showed that higher levels of Bcl2 were associated with TRAIL-R2 and the disease state. In other correlative studies, high expression of Bcl2, concurrently with Myc, has been correlated with pathogenesis of a family of diffuse large B-cell lymphoma (Bogusz et al., 2017).
In colorectal cancers (CRC), the effects of the Bcl2 and Bax were correlated to weight and size of xenograph tumors in a murine model (Ye et al., 2016). As a result of many studies like these, many studies looking into therapeutics that modulate Bcl2 and Bax expression in hopes of viable treatments. Both in vitro and in vivo models have shown promise for possible drug therapies (Kim et al., 2014, Li et al., 2017, Moghtaderi et al., 2017).
5.2. Bcl6 in disease
In attempting to elucidate the pathway of Bcl6 in Follicular Lymphoma, it was determined that Bcl6 represses the NOTCH2 signaling pathway (Valls et al., 2017). This repression signals for cell survival and growth, suggesting that suppression of Bcl6 will allow for normal apoptosis to occur, preventing lymphoma growth. Additionally, in follicular lymphoma, a knock down of endogenous Bcl6 resulted in a 6.8-fold increase of RUVBL1 (aka pontin, Tip49, NMP238) protein expression (Baron et al., 2016). RUVBL1 is involved in cell activities like DNA repair, gene transcription regulation, and chromatin remodeling. Disruption of this relationship may be enough to cause lymphoma growth, though further studies into these effects and pathway are needed for support.
Another question is how much dysregulation is needed in the Bcl6 system to cause oncogenesis? In a murine model of transient expression of Bcl6, it was noted that the experimental mice lived much shorter lives than their wild type littermates. Accompanying these shorter lives was the presence of splenomegaly and lymphomas in the form of enlarged white pulp nodules with pleomorphic large B-cells (Green et al., 2014). In the realm of prognostics, it was revealed that Bcl2 positive expression and negative Bcl6 expression are associated with poor prognostics in DLBCL (Fang et al., 2017).
Bcl6 is not only involved in B-Cell lymphoma, but also in other cancers such as breast cancer. It was recently observed that expression of Bcl6 mRNA can serve as a marker for breast cancer (Badr et al., 2016). In breast cancer cell lines, miRNA-127 repression of Bcl6 showed significant reduction is cell proliferation (Chen et al., 2013). Although the exact mechanism of Bcl6 function in breast cancer is unknown, it is known that Bcl6 can activate ZEB1, a transcriptional repressor. And when ZEB1 binds to the E-cadherin promoter, E-cadherin expression is repressed. Activation of Bcl6 and ZEB1, along with the subsequent repression of E-cadherin transcription results in activation of epithelial to mesenchymal transition (EMT), and induction of EMT lead to growth, invasion, and migration of breast cancer cells (Yu et al., 2015). Another mechanism of Bcl6 action in breast cancer is through STAT3 and STAT5 proteins as they have opposing effects in regulating Bcl6 (Nelson et al., 2009, Walker and Frank, 2015). Bcl6 is also known to be involved heavily with the STAT family of proteins in primary mediastinal B-Cell lymphoma, bone marrow B-cells, and tonsillar B-cells (Ritz et al., 2013, Wagner et al., 2011).
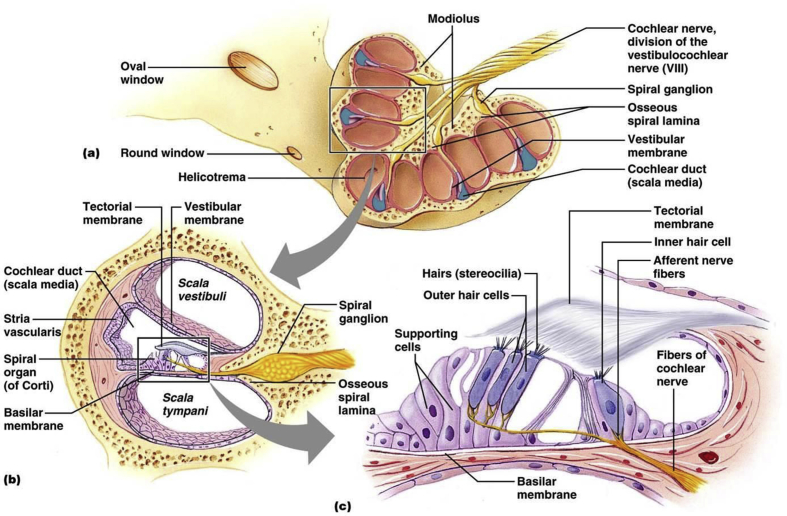
In mammals, the ear is separated into three sections: the outer, middle, and inner ear. The outer ear is lined on the medial side by the tympanic membrane. The middle ear is limited by the tympanic membrane laterally, and the oval window medially. The inner ear consists of the membranous labyrinths; vestibule, semi-circular canals, and the cochlea. Within the cochlea, there are three compartments: scala tympani, scala vestibuli, and the cochlear duct (see Fig. 3). The scala tympani and the scala vestibuli both contain an extracellular fluid with a high sodium (Na+) and low potassium (K+) ion content called perilymph. The cochlear duct is limited by Reissner membrane medially and the stria vascularis and spiral ligament laterally. The cochlear duct contains endolymph, which holds a higher concentration of K+. Accordingly, the transduction current in the hair cells is carried by K+ ions. The K+ concentration is generated and maintained by the stria vascularis. Within the cochlear duct lies the basilar membrane on which the sensory epithelium, the organ of Corti is located. The organ of Corti contains sensory hair cells and supporting cells. Hair cells transduce mechanical stimuli into electrical activity (Hudspeth and Corey, 1977, Hudspeth, 1989). Mechanoelectrical transduction is mediated by the hair bundle, an array of modified microvilli or stereocilia arranged in a staircase on the apical surface of the hair cell (Fettiplace and Hackney, 2006). In addition to the stereocilia specialization critical for mechanoelectrical transduction in the apical membrane, all hair cells also have specializations in the basolateral and synaptic membranes that are responsible for electrical activities and synaptic transmission. The supporting cells, which include pillar cells, Deiters cells, and inner phalangeal cells, contribute to the stiffness of the cochlear partition and the homeostasis of the ionic and chemical environment (Raphael and Altschuler, 2003, Slepecky, 1996).
Fig. 3.
Anatomy of the Cochlea. Cartoon illustration of the cochlea. Panel a. A split cochlea showing the various turns, base, and helicotrema. Panel b. Cartoon illustration of the 3 compartments of the cochlea. Panel c. Relation of the cochlear hair cells, basilar membrane, and tectorial membrane. (Anatomybody-charts, 2016).
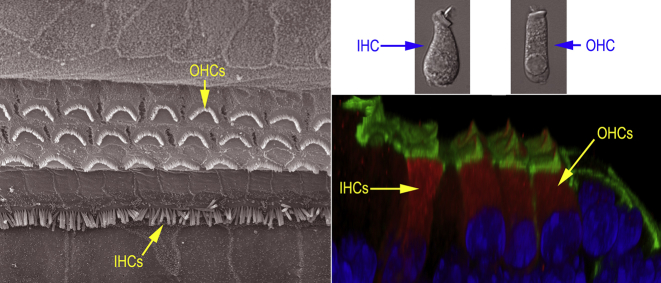
There are two hair cell types in the organ of Corti (see Fig. 3, Fig. 4); inner hair cells (IHCs) and outer hair cells (OHCs). One row of IHCs and three rows of OHCs are tonotopically arranged along the entire basilar membrane in the cochlea. IHCs and OHCs are distinct morphologically (see Fig. 3, Fig. 4) and functionally (Dallos, 1992). IHCs predominantly possess afferent innervation (Spoendlin, 1970), and are considered to be the major sensory receptor as they transmit information to the brain. OHCs predominantly possess efferent fibers (Spoendlin, 1970), serving as an effector cell that amplifies input to IHCs by a receptor potential-driven somatic motility (Brownell et al., 1985, Dallos et al., 2008, He et al., 2003, Liberman et al., 2002, Zheng et al., 2000). The human cochlea contains on the order of 3,500 IHCs and 12,000 OHCs at birth. The mechanosensitive HCs are lost with age and are highly susceptible to damage from noise and ototoxic drugs. Hair cell loss is the most common cause of hearing loss. Mammalian hair cells are no longer able to regenerate once lost. Lower vertebrates such as bird and fish can spontaneously regenerate lost hair cells through proliferation and trans differentiation of supporting cells.
Fig. 4.
Anatomy of the Cochlear Hair Cells. Left panel: organization of the inner and outer hair cells that line the tonotopic axis of the cochlea. Top right: morphological differences of the IHC and OHC. IHC are more pear-shaped with a central nucleus and apical stereocilia. OHC are more cylindrical with a basal-located nucleus and apical stereocilia. Bottom right: image showing grouping of outer hair cells versus inner hair cells.
6. Hair cell loss
Sensory hearing loss is the result of deficient, damaged, or inadequate cochlear hair cell function, and affects 1.3 billion humans worldwide (Worldwide Hearing, 2014). There are 4 main causes of hair cell loss: aging, ototoxicity, noise insult, and genetic defect.
6.1. Genetic disposition in hearing loss
One of the most common birth defects (or congenital defects) is hearing loss or deafness. This congenital abnormality can affect as many as three of every 1000 babies born. Inherited genetic defects contribute to about 60% of deafness/hearing loss occurring in infants. So these genes play an important role in congenital hearing loss. The two most common classifications of hearing impairment are syndromic and non-syndromic.
6.1.1. Non-syndromic hearing impairment
Non-syndromic hearing impairment is responsible for a considerable majority of inherited hearing loss, approximately 70%. Autosomal-recessive inheritance consists of about 80% of cases of non-syndromic hearing impairment, while autosomal-dominant genes make up 20%. Less than two percent of cases are the result of X-linked and mitochondrial genetic malfunctions.
One example of non-syndromic hearing impairment, DFNA3, is found in the autosomal dominant mutations of GJB2 or GJB6. These genes encode for connexins, specifically for connexin 26 and connexin 30 respectively (Smith and Ranum, 1998). Within the mitochondria, two genes are specific to non-syndromic hearing loss, MTTS1 and MTRNR1. MTRNR1 translates to the mitochondrial ribosomes (Ballana et al., 2006) and MTTS1 correlates to mitochondrial tRNA for serine (Reid et al., 1994).
6.1.2. Syndromic hearing impairment
Syndromic hearing impairment means that the hearing impairment is correlated with additional clinical abnormalities. Approximately 15–30% of hereditary hearing impairments are syndromic. Over 400 syndromes are known to include hearing impairment and can be classified as: syndromes due to cytogenetic or chromosomal anomalies, syndromes transmitted in classical monogenic or Mendelian inheritance, or syndromes due to multi-factorial influences, and finally, syndromes due to a combination of genetic and environmental factors.
SLC26A4 is the gene that is often mutated in Pendred Syndrome. It is characterized by congenital deafness and the development of a goiter during puberty. Waardenburg Syndrome is an autosomal dominant disease that includes hearing loss. It can be accompanied by pigment abnormalities of the skin and/or irises. In the 4 identified types of Waardenburg Syndrome, some of the mutated genes include Pax3, MITF, EDNRB, EDN3, and Sox10. Mutation of the NF2 gene can cause neurofibromatosis; a disease that, if caught early enough, is treatable. Mohr-Tranebjaerg syndrome is caused by a mutation in the TIMM8A gene and is characterized by hearing loss as well as movement of cytosolic proteins into the mitochondrial matrix across the inner mitochondrial membrane.
6.2. Age-related hearing loss
Many physiological changes occur in the aging process. Age-related hearing loss (ARHL), or presbycusis, occurs is one of the most common conditions affecting older and elderly adults. In the United States population between the ages of 65 and 74, roughly one in three people has hearing loss. Of those older than 75, nearly half have difficulty hearing.
Presbycusis is characterized by decreased hearing sensitivity, decreased processing of acoustic impulses, and reduced speech recognition (especially in noisy environments). The earliest signs of presbycusis are tinnitus and high frequency hearing loss. Over time, the hearing threshold can progress to lower frequencies. Presbycusis is also bilateral and symmetrical. There is no known single cause for age-related hearing loss. Most commonly, it is caused by loss of mechanosensitive hair cells in the inner ear as one grows older. However, genetic deficits and repeated exposure to loud noises may play a major role (Fransen et al., 2003). Smoking and certain medical conditions and medications can aggravate presbycusis (Gates et al., 1993, Agrawal et al., 2009, Sha et al., 2008). Recent studies have shown that microRNAs (miRNA) can also play a role in age-related hearing loss (Zhang et al., 2013). Several miRNAs (such as miR-34 and miR-29 families) that are pro-apoptotic are up regulated, while anti-apoptotic microRNAs (such as miR-181 and miR-183 families) are downregulated during aging.
As just noted, there are numerous factors that can contribute to presbycusis. As a result, a single mechanism is difficult to isolate. However, multiple studies using various model organisms have indicated that increases in caspases-3, -7, and -9 accompany this ailment. Additionally, an increase in Bax expression and a decrease in Bcl-2 expression have both been found to accompany ARHL (Alam et al., 2001, Nevado et al., 2006, Someya et al., 2009). For further treatment of the subject, the reader is referred to a recent review that weaves epidemiological data with mechanistic information to paint a broader story of the causes of ARHL (Yamasoba et al., 2013).
With aging, it is possible that loss of Bcl2 expression can lead to increased Bax expression. With less Bcl2 binding to Bax, Bax can then oligamerize and insert into the mitochondrial membrane leading to MOMP, shifting the hair cell to apoptosis. As p53 also binds to Bcl2 in the mitochondria, loss of Bcl2 expression can free p53 to act as a transcriptional activator of Bax and Bid, pushing the hair cell towards apoptosis and presbycusis.
6.3. Ototoxicity
Ototoxicity has long been known to be a cause of hair cell and hearing loss. Some examples of drugs that cause ototoxicity are cisplatin, carboplatin, bumetanide, and aminoglycosides (i.e. gentamicin, neomycin, kanamycin). Some studies show that kanamycin-induced hair cell loss is accompanied by features of both classical apoptosis and necrosis, but also lacked critical features of the apoptotic pathway (Jiang et al., 2006). A subsequent study revealed the presence of some apoptotic features, TUNEL labeling and caspase-3 activation, as well as some necrosis features, large amounts of cell debris, following injection of kanamycin and a loop diuretic, bumetanide (Taylor et al., 2008). A common class of antibiotics, aminoglycosides, which also induce hearing loss, have been recently described being transported into cochlear hair cells via mechanotransducer channels and endocytosis (Alharazneh et al., 2011, Hailey et al., 2017). The transient receptor potential vanilloid 1 and 4 (TRPV 1 and 4) participates in transport of the antibiotic, and ototoxic, drug gentamicin into the basal hair cells (Lee et al., 2013). Finally, basal hair cells show an increased vulnerability to free-radical damage, as described by lower expression of the anti-oxidant glutathione (Sha et al., 2001). Ototoxicity-induced hair cell loss only shows a general trend, that is OHCs are more vulnerable than IHCs and basal turn hair cells are more vulnerable than apical turn hair cells. The mechanism of this differential vulnerability is unknown. Currently, models are being created to study for therapeutics for cisplatin-induced hearing loss (Callejo et al., 2017, Wu et al., 2017). Many studies target possible therapeutic drugs for platinum-induced hearing loss (Theunissen et al., 2015, van As et al., 2016a, van As et al., 2016b).
The mechanism of ototoxicity, though not perfectly understood, is very similar to apoptosis in many other cells. Specifically, aminoglycosides induce Bax activation with subsequent MOMP and release of CytC. Caspase-3 activation will result in DNA degeneration (Mangiardi et al., 2004, Matsui et al., 2004, Coffin et al., 2013). In cisplatin induced hair cell and hearing loss, cisplatin triggers free radical production in the cochlea, Bax activation, and subsequent intrinsic pathway apoptosis (Rybak et al., 2007). Additionally, the increase of Bax expression could possibly bind all possible Bcl2 proteins, leaving p53 to activate further Bax and Bid. This could also affect Bcl6 binding of p53, leaving Bcl6 to further repress Bcl2 and push the hair cell towards cell death via apoptosis.
6.4. Acoustic trauma
Noise induced hair cell loss shares many of the same features that ototoxicity induced hair cell loss contains. It is unclear what proportion of hair cells die via apoptotic intrinsic pathway versus apoptotic extrinsic pathway versus the necrotic pathway, although it is clear that all are involved. Some studies show that the inhibiting caspases increases the necrosis proteins RIP1 and RIP3 and number of necrotic nuclei. The opposite holds true as well, inhibiting necrosis proteins RIP1 and RIP3 increases the number of apoptotic nuclei with an up regulation of caspase-9 and no increase in caspase-8 activity (Zheng et al., 2014).
Loud noises initiate large displacements of the tympanic membrane, creating waves of mechanical energy into the inner ear. These waves cause shearing forces to cause possible damage to the basilar membrane, organ of Corti, hair cells, and alteration of the endocochlear potential. Intrinsic pathway activation can appear via ROS activity (Fetoni et al., 2013), caspase independent activation via EndoG and AIF action (Yamashita et al., 2004), and necrosis (Bohne et al., 2007). In extrinsic pathway, activation can transpire via activation of the TNFα and Fas receptors, TNFR1 and FasR (Li et al., 1998, Nicotera et al., 2003, Jamesdaniel et al., 2011). TNFα-mediated p38 and JNK signaling can be up regulated following loud noise exposure. This signaling occurs in the sensory epithelium of the inner ear and propagates Bax-induced mitochondrial release of CytC, and other apoptotic proteins, as well as pro-death gene transcription (Wang et al., 2007a, Wang et al., 2007b, Dinh et al., 2008a, Dinh et al., 2008b, Jamesdaniel et al., 2011).
Most likely, ROS released from shearing forces of loud noise lead to an up regulation of p53 in cochlear cells, leading to an up regulation of Bid and Bax that will lead to MOMP and hair cell death. Additionally, anti-apoptotic molecules such as Bcl2 and Bcl-xL are down regulated in response to oxidative stress, possibly allowing for more pro-apoptotic molecules to push a hair cell towards cell death via apoptosis.
7. Bcl6 and Bcl2 family of proteins in cochlear hair cells
Recently, transcriptome and transcription factor studies have shown differential expression of both transcription factors and genes in inner versus outer hair cells corresponding to both bcl2 and bcl6 in IHC (Liu et al., 2014, Li et al., 2016). The difference of these molecules in between inner and outer hair cells is a possible explanation to OHC susceptibility to early cell death. It is also possibly the key to unlocking the exact mechanisms of Bcl2 and Bcl6 involvement in hair cell loss. The current hypothesis is that IHC are more resilient against cell death due to their increased expression of Bcl2 and Bcl6. We have generated a conditional knock out of Bcl6 and plan on examining the effects the knockout has on the cochlear hair cells. We hope to elucidate the role of Bcl6, and its up- and down-stream effects on other molecular signals.
8. Concluding comments
Recently, Bcl6 has become of interest as it is an oncoprotein that can be targeted as a cancer therapeutic (Cardenas et al., 2017). In this article, the common causes of sensorineural hair cell loss and their relation to the apoptotic pathway have been reviewed. Other cell types within the inner ear, such as the cells of the stria vascularis, spiral ganglion cells, Deiters' cells, and other supporting cells, may also contribute to cell death and hearing loss. Routes leading to cell death include necrosis and apoptosis, including the various pathways within apoptosis and necrosis. Many potential targets for clinical treatment, including the Bcl2 and Bcl6 families of proteins, have been noted. Further studies will lead to more advanced therapeutic treatments to mitigate hearing loss.
Acknowledgments
The study was supported by in part by a grant from Bellucci Foundation of Creighton University. Conflicts of interest: none.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Seth Morrill, Email: sethmorrill@creighton.edu.
David Z.Z. He, Email: davidhe@creighton.edu.
References
- Alharazneh A., Luk L., Huth M., Monfared A., Steyger P.S., Cheng A.G., Ricci A.J. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One. 2011;6:e22347. doi: 10.1371/journal.pone.0022347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.M., Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal Y., Platz E.A., Niparko J.K. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol. Neurotol. 2009;30:139–145. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- Alam S.A., Oshima T., Suzuki M., Kawase T., Takasaka T., Ikeda K. The expression of apoptosis-related proteins in the aged cochlea of Mongolian gerbils. Laryngoscope. 2001;111:528–534. doi: 10.1097/00005537-200103000-00026. [DOI] [PubMed] [Google Scholar]
- Anatomybody-charts . 2016. Anatomy Body Charts.http://anatomybody-charts.us/inner-ear-anatomy/inner-ear-anatomy-2/ [Google Scholar]
- Antonsson B., Conti F., Ciavatta A., Montessuit S., Lewis S., Martinou I., Bernasconi L., Bernard A., Mermod J.J., Mazzei G., Maundrell K., Gambale F., Sadoul R., Martinou J.C. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Dixit V.M. Death receptors: signaling and modulation. Sciences. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Badr E., Masoud E., Abdou A.G., Eldien M.S. BCL6 mRNA expression level in invasive duct carcinoma not otherwise specified. J. Clin. Diagn. Res. 2016;10:XC01–XC04. doi: 10.7860/JCDR/2016/22796.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballana E., Morales E., Rabionet R., Montserrat B., Ventayol M., Bravo O., Gasparini P., Estivill X. Mitochondrial 12S rRNA gene mutations affect RNA secondary structure and lead to variable penetrance in hearing impairment. Biochem. Biophys. Res. Commun. 2006;341:950–957. doi: 10.1016/j.bbrc.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Baron B.W., Baron R.M., Baron J.M. The relationship between RUVBL1 (Pontin, Tip49, NMP238) and BCL6 in benign and malignant lymphoid tissues. Biochem. Biophys. Rep. 2016;6:1–8. doi: 10.1016/j.bbrep.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman R., Prives C. Transcriptional regulation by p53. Cold Spring Harb. Perspect. Biol. 2010;2:a000935. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereshchenko O.R., Gu W., Dalla-Favera R. Acetylation inactivates the transcriptional repressor BCL6. Nat. Genet. 2002;32:606–613. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- Bharwaj A., Aggarwal B.B. Receptor-mediated choreography of life and death. J. Clin. Immunol. 2003;23:317–332. doi: 10.1023/a:1025319031417. [DOI] [PubMed] [Google Scholar]
- Bogusz A.M., Kovach A.E., Le L.P., Feng D., Baxter R.H.G., Sohani A.R. Diffuse large B-cell lymphoma with concurrent high MYC and BCL2 expression shows evidence of active B-cell receptor signaling by quantitative immunofluorescence. PLoS One. 2017;12(2):e0172364. doi: 10.1371/journal.pone.0172364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne B.A., Harding G.W., Lee S.C. Death pathways in noise-damaged outer hair cells. Hear Res. 2007;223:61–70. doi: 10.1016/j.heares.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Braga L.C., Silva L.M., Piedade J.B., Traiman P., da Silva Filha A.L. Epigenetic and expression analysis of TRAIL-R2 and BCL2: on the TRAIL of knowledge of apoptosis in ovarian tumors. Arch. Gynecol. Obstet. 2014;289(5):1061–1069. doi: 10.1007/s00404-013-3060-0. [DOI] [PubMed] [Google Scholar]
- Brownell W.E., Bader C.R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Callejo A., Durochat A., Bressieux S., Saleur A., Chabbert C., Juan I.D., Llorens J., Gaboyard-Niay S. Dose-dependent cochlear and vestibular toxicity of trans-tympanic cisplatin in the rat. Neurotoxicology. 2017;60:1–9. doi: 10.1016/j.neuro.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Cardenas M.G., Oswald E., Yu W., Xue F., MacKerell A.D., Jr., Melnick A.M. The expanding role of the BCL6 oncoprotein as a cancer therapeutic target. Clin. Cancer Res. 2017;23:885–893. doi: 10.1158/1078-0432.CCR-16-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang M., Guo M., Xie Y., Cong Y.S. miR-127 regulates cell proliferation and senescence by targeting BCL6. PLoS One. 2013;8:e80266. doi: 10.1371/journal.pone.0080266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Levy S., Nourse J., Cleary M.Z. The Bcl-2 candidate proto-oncogene product is a 24-KD integral membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol. Cell. Biol. 1989;9:701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin A.B., Rubel E.W., Raible D.W. Bax, Bcl2 and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebra fish lateral line. J. Assoc. Res. Otolaryngol. 2013;14:645–659. doi: 10.1007/s10162-013-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J. Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P., Wu X., Cheatham M.A., Gao J., Zheng J., Anderson C.T., Jia S. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58(3):333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial N.N. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin. Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L., Leo E., Stennicke H.R., Welsh K., Salvesen G.S., Reed J.C. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO. 1999;18:5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweindt C., Albagli O., Bernardin F., Dhordain P., Quief S., Lantoine D., Kerckaert J.P., Leprince D. The LAZ3/BCL6 oncogene encodes a sequence-specific transcriptional inhibitor: a novel function for the BTB/POZ domain as an autonomous repressing domain. Cell Growth Diff. 1995;6:1495–1503. [PubMed] [Google Scholar]
- Dhordain P., Albagli O., Ansieau S., Koken M.H., Deweindt C., Quief S., Lantoine D., Leutz A., Kerckaert J.P., Leprince D. The BTB/POZ domain targets the LAZ3/BCL6 oncoprotein to nuclear dots and mediates homomerisation in vivo. Oncogene. 1995;11:2689–2697. [PubMed] [Google Scholar]
- Dhordain P., Albagli O., Lin R.J., Ansieau S., Quief S., Leutz A., Kerckaert J.P., Evans R.M., Leprince D. Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10762–10767. doi: 10.1073/pnas.94.20.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhordain P., Lin R.J., Quief S., Lantoine D., Kerckaert J.P., Evans R.M., Albagli O. The LAZ3(BCL6) oncoprotein recruits a SMRT/mSIN3A/histone deacetylase containing complex to mediate transcriptional repression. Nucleic Acids Res. 1998;26:4645–4651. doi: 10.1093/nar/26.20.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh C., Hoang K., Haake S., Chen S., Angeli S., Nong E., Eshragi A.A., Balkany T.J., Can De Water T.R. Biopolymer-released dexamethasone prevents tumor necrosis factor alpha-induced loss of auditory hair cells in vitro: implications towards the development of a drug-eluting cochlear implant electrode array. Otol. Neurotol. 2008;29:1012–1019. doi: 10.1097/MAO.0b013e3181859a1f. [DOI] [PubMed] [Google Scholar]
- Dinh C.T., Haake S., Chen S., Hoang K., Nong E., Eshraghi A.A., Balkany T.J., Van De Water T.R. Dexamethasone protects organ of Corti explants against tumor necrosis factor alpha-induced loss of auditory hair cells and alters the expression levels of apoptosis-related genes. Neuroscience. 2008;157:405–413. doi: 10.1016/j.neuroscience.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Du H., Wolf J., Schafer B., Moldoveanu T., Chipuk J.E., Kuwana T. BH3 domains other than Bim and Bid can directly activate Bax/Bak. J. Biol. Chem. 2011;286:491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy C., Yu J.J., Nahar R., Swaminathan S., Kweon S.M., Polo J.M., Valls E., Klemm L., Shojaee S., Cerchietti L., Schuh W., Jack H.M., Hurtz C., Ramezani-Rad P., Herzog S., Jumaa H., Koeffler H.P., de Alboran I.M., Melnick A.M., Ye B.H., Muschen M. BCL6 is critical for the development of a diverse primary B cell repertoire. J. Exp. Med. 2010;207:109–1221. doi: 10.1084/jem.20091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S. A caspase-activated DNase that degrades DNA during apoptosis and its inhibitors ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Eskes R., Desagher S., Antonsson B., Martinou J.C. Bid induces the oligamerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P., Liang G., Wei-Kai Y., Yan Z., Ye L., Xiu-Mei D., Yin-Ping W. Identification of prognostic factors in patients with diffuse large B-cell lymphoma. Indian J. Pathol. Microbiol. 2017;60:87–91. doi: 10.4103/0377-4929.200056. [DOI] [PubMed] [Google Scholar]
- Fesik S.W. Insights into programmed cell death through structural biology. Cell. 2000;103:273–282. doi: 10.1016/s0092-8674(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Fetoni A.R., De Bartolo P., Eramo S.L., Rolesi R., Paciello F., Bergamini C., Fato R., Paludetti G., Petrosini L., Troiana D. Noise induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J. Neurosci. 2013;33:4011–4023. doi: 10.1523/JNEUROSCI.2282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R., Hackney C.M. The sensory and motor roles of auditory hair cells. Nat. Rev. Neurosci. 2006;7:19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- Fransen E., Lemkens N., Van Laer L., Van Camp G. Age-related hearing impairment (ARHI): environmental risk factors and genetic prospects. Exp. Gerontol. 2003;38:353–359. doi: 10.1016/s0531-5565(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Joza N., Tasdemir E., Maiuri M., Hengartner M., Abrams J., Tavernarakis N., Penninger J., Madeo F., Kroemer G. No death without life: vital functions of apoptotic effectors. Cell Death Diff. 2008;15:1113–1123. doi: 10.1038/cdd.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates G.A., Cobb J.L., D'Agostino R.B., Wolf P.A. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch. Otolaryngol. Head. Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- Ge Z., Zhou X., Gu Y., Han Q., Li J., Chen B., Ge Q., Dovat E., Payne J.L., Sun T., Song C., Dovat S. Ikaros regulation of the BCL6BACH2 axis and its clinical relevance in acute lymphoblastic leukemia. Oncotarget. 2017;8:8022–8034. doi: 10.18632/oncotarget.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad J.M., Zeng X.R., Boise L.H. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr. Opin. Oncol. 2000;12(6):543–549. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Green D.R., Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Green M.R., Vicente-Duenas C., Romero-Camarero I., Liu C.L., Dai B., Gonzalez-Herrero I., Garcia-Ramirez I., Alonso-Escudero E., Igbal J., Chan W.C., Campos-Sanchez E., Orfao A., Pintado B., Flores T., Blanco O., Jimenez R., Martinez-Climent J.A., Criado F.J., Cenador M.B., Zhao S., Natkunam Y., Lossos I.S., Majeti R., Melnick A., Cobaleda C., Alizadeh A.A., Sanchez-Garcia I. Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nat. Commun. 2014;5:3904. doi: 10.1038/ncomms4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey D.W., Esterberg R., Linbo T.H., Rubel E.W., Raible D.W. Fluorescent aminoglycosides reveal intracellular trafficking routes in mechanosensory hair cells. J. Clin. Invest. 2017;127(2):472–486. doi: 10.1172/JCI85052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt S., Berger M., Goldberg Z., Haupt Y. Apoptosis – the p53 network. J. Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- He D.Z., Jia S., Dallos P. Prestin and the dynamic stiffness of cochlear outer hair cells. J. Neurosci. 2003;23(27):9089–9096. doi: 10.1523/JNEUROSCI.23-27-09089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H., Xiong J., Goeddel D.V. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–500. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Hudspeth A.J. How the ear's works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Hudspeth A.J., Corey D.P. Sensitivity, polarity and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl. Acad. Sci. U. S. A. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtz C., Hatzi K., Cerchietti L., Braig M., Park E., Kim Y.M., Herzog S., Ramezani-Rad P., Jumaa H., Muller M.C., Hofmann W.K., Hochhaus A., Ye B.H., Agarwal A., Druker B.J., Shah N.P., Melnick A.M., Muschen M. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J. Exp. Med. 2011;208:2163–2174. doi: 10.1084/jem.20110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh K.D., Fischle W., Verdin E., Bardwell V.J. BCoR, a novel corepressor involved in BCL-6 repression. Genes Dev. 2000;14:1810–1823. [PMC free article] [PubMed] [Google Scholar]
- Jamesdaniel S., Hu B., Kermany M.H., Jiang H., Ding D., Coling D., Salvi R. Noise induced changes in the expression of p38/MAPK signaling proteins in the sensory epithelium of the inner ear. J. Proteomics. 2011;75:410–424. doi: 10.1016/j.jprot.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson M.D., Damas N.D., Lees M., Jacobsen A., Lund A.H. miR-339-5p regulates the p53 tumor-suppressor pathway by targeting MDM2. Oncogene. 2014;34:1908–1918. doi: 10.1038/onc.2014.130. [DOI] [PubMed] [Google Scholar]
- Jiang H., Sha S.H., Forge A., Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Diff. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Pan Y., Cheng Y., Li H., Li H. Protection of rat liver against hepatic ischemia-reperfusion injury by a novel selenocysteine-containing 7-mer peptide. Mol. Med. Rep. 2016;14(3):2007–2015. doi: 10.3892/mmr.2016.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P.J., Grabow S., Gray D., McKenzie M.D., Nachbur U., Huang D.C., Bouillet P., Thomas H.E., Borner C., Silke J., Strasser A., Kaufmann T. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.E., Ha T.K., Yoon J.H., Kee J.S. Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res. 2014;34(2):701–706. [PubMed] [Google Scholar]
- Kischkel F.C., Hellbardt S., Behrman I., Germer M., Pawlita M., Krammer P.H., Peter M.E. Cytotoxicity-dependent APO-1 (Fas/CD95) – associated proteins form a death inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics M., Martinon F., Micheau O., Bodmer J.L., Hofmann K., Tschopp J. Overexpression of Helicard, a CARD-containing helicase cleaved during apoptosis, accelerates DNA degradation. Curr. Biol. 2002;12:838–843. doi: 10.1016/s0960-9822(02)00842-4. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Mackey M.R., Perkins G., Ellisman M.H., Latterich M., Schneiter R., Green D.R., Newmeyer D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Park C., Kim S.J., Kim H.J., Oh G.S., Shen A., So H.S., Park R. Different uptake of gentamicin through TRPV1 and TRPV4 channels determines cochlear hair cell vulnerability. Exp. Mol. Med. 2013;45:e12. doi: 10.1038/emm.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A.J., Oren M. The first 30 years of p53: growing ever more complex. Nat. Rev. Cancer. 2009:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhu H., Xu C.J., Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu H., Barta C.L., Judge P.D., Zhao L., Zhang W.J., Gong S., Beisel K.W., He D.Z. Transcription factors expressed in mouse cochlear inner and outer hair cells. PLoS One. 2016;11:e0151291. doi: 10.1371/journal.pone.0151291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lu S., Wang Y., Guo S., Zhao T., Wang X., Song B. Influence of microRNA-34a on proliferation, invasion and metastasis of HCT116 cells. Mol. Med. Rep. 2017;15(2):833–838. doi: 10.3892/mmr.2016.6088. [DOI] [PubMed] [Google Scholar]
- Liang F., Wang B., Bao L., Zhao Y., Zhang S., Zhang S. Overexpression of ILK promotes temozolomide resistance in glioma cells. Mol. Med. Rep. 2017;15(3):1297–1304. doi: 10.3892/mmr.2017.6157. [DOI] [PubMed] [Google Scholar]
- Liberman M.C., Gao J., He D.Z.Z., Wu X., Jia S., Zuo J. Prestin is required for outer hair cell motility and the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Linterman M.A., Beaton L., Yu D., Ramiscal R.R., Srivastava M., Hogan J.J., Verma N.K., Smyth M.J., Rigby R.J., Vinuesa C.G. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zou H., Slaughter C., Wang X. DFF, a heteromeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Liu H., Pecka J.L., Zhang Q., Soukup G.A., Beisel K.W., He D.Z. Characterization of transcriptomes of cochlear inner and outer hair cells. J. Neurosci. 2014;34:11085–11095. doi: 10.1523/JNEUROSCI.1690-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Lomonosova E., Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2009;27:S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan T.K., El-Deiry W.S. Apoptotic threshold is lowered by p53 transactivation of caspase-6. Proc. Natl. Acad. Sci. 2002;99:9492–9497. doi: 10.1073/pnas.132241599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiardi D.A., McLaighlin-Williamson K., May K.E., Messana E.P., Mountain D.C., Cotanche D.A. Progression of Hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J. Comp. Neurol. 2004;475:1–8. doi: 10.1002/cne.20129. [DOI] [PubMed] [Google Scholar]
- Marques Fernandez F., Planells-Ferrer L., Gozzelino R., Gallenkamp K.M.O., Reix S., Llecha-Cano N., Lopez-Soriano J., Yuste V.J., Moubarak R.S., Comella J.X. TNFα induces survival through the FLIP-L-dependent activation of the MAPK/ERK pathway. Cell Death Dis. 2013;4:e493. doi: 10.1038/cddis.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui J.I., Gale J.E., Warchol M.E. Critical Signaling events during the aminoglycoside-induced death of sensory hair cells in vitro. J. Neurobiol. 2004;61:250–266. doi: 10.1002/neu.20054. [DOI] [PubMed] [Google Scholar]
- Miles R.R., Crockett D.K., Lim M.S., Elenitoba-Johnson K.S. Analysis of BCL6-interacting proteins by tandem mass spectrometry. Mol. Cell. Proteomics. 2005;4:1898–1909. doi: 10.1074/mcp.M500112-MCP200. [DOI] [PubMed] [Google Scholar]
- Minn A.J., Velez P., Schendel S.L., Liang H., Muchmore S.W., Fesik S.W., Fill M., Thompson C.B. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Moghtaderi H., Sepehri H., Attari F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed. Pharmacother. 2017;88:582–594. doi: 10.1016/j.biopha.2017.01.072. [DOI] [PubMed] [Google Scholar]
- Mollereau B., Ma D. The p53 control of apoptosis and proliferation: lessons from Drosophila. Apoptosis. 2014;19:1421–1429. doi: 10.1007/s10495-014-1035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal A., Sawant D., Dent A.L. Transcriptional repressor BCL6 controls Th17 responses by controlling gene expression in both T cells and macrophages. J. Immunol. 2010;184:4123–4132. doi: 10.4049/jimmunol.0901242. [DOI] [PubMed] [Google Scholar]
- Moroni M.C., Hickman E.S., Denchi E.L., Caprara G., Colli E., Cecconi F., Muller H., Helin K. Apaf-1 is a transcriptional target for E2F and p53. Nat. Cell Biol. 2001;3:553–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- Muchmore S.W., Sattler M., Liang H., Meadows R.P., Harlan J.E., Yoon H.S., Nettesheim D., Chang B.S., Thompson C.B., Wong S.L., Ng S.L., Fesik S.W. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Nakano K., Vousden K.H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nechushtan A., Smith C., Hsu Y.T., Youle R. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A., Zou L., Chaudhury M., Signoretti S., Richardson A., Frank D. Reciprocal effects of STAT5 and STAT3 in breast cancer. Mol. Cancer Res. 2009;7:966–976. doi: 10.1158/1541-7786.MCR-08-0238. [DOI] [PubMed] [Google Scholar]
- Nevado J., Sanz R., Casqueiro J.C., Ayala A., Garcia-Berrocal J.R., Ramirez-Camacho R. Ageing evokes an intrinsic pro-apoptotic signaling pathway in rat cochlea. Acta Otolaryngol. 2006;126:1134–1139. doi: 10.1080/00016480600672592. [DOI] [PubMed] [Google Scholar]
- Nicotera T.M., Hu B.H., Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J. Assoc. Res. Otolaryngol. 2003;4:466–477. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H., Ye B.H., Dalla-Favera R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 1998;12:1953–1961. doi: 10.1101/gad.12.13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T., Tokino T., Taniguchi T., Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Spolski R., Ettinger R., Kim H.P., Wang G., Qi C.F., Hwu P., Shaffer D.J., Akilesh S., Roopenian D.C., Morse H.C., 3rd, Lipsky P.E., Leonard W.J. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- Perry M.E. The regulation of the p53 mediated stress response by MDM2 and MDM4. Cold Spring Harb. Perspect. Biol. 2010;2:a000968. doi: 10.1101/cshperspect.a000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflaum J., Schlosser S., Müller M. p53 family and cellular stress responses in cancer. Front. Oncol. 2014;4:285. doi: 10.3389/fonc.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan R.T., Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- Phan R.T., Saito M., Basso K., Niu H., Dalla-Favera R. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat. Immunol. 2005;6:1054–1060. doi: 10.1038/ni1245. [DOI] [PubMed] [Google Scholar]
- Pistritto G., Trisciuoglio D., Ceci C., Garufi A., D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8:603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranuncolo S.M., Polo J.M., Dieroy J., Singer M., Kuo T., Greally J., Green R., Carroll M., Melnick A. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat. Immunol. 2007;8:705–714. doi: 10.1038/ni1478. [DOI] [PubMed] [Google Scholar]
- Ranuncolo S.M., Wang L., Polo J.M., Dell'Oso T., Dieroy J., Gaymes T.J., Rassool F., Carroll M., Melnick A. BCL6-mediated attenuation of DNA damage sensing triggers growth arrest and senescence through a p53-dependent pathway in a cell context-dependent manner. J. Biol. Chem. 2008;283:22565–22572. doi: 10.1074/jbc.M803490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y., Altschuler R.A. Structure and innervation of the cochlea. Brain Res. Bull. 2003;60(5–6):397–422. doi: 10.1016/s0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Reed J.C. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Diff. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- Reed J.C., Doctor K., Rojas A., Zapata J.M., Stehlik C., Fiorentino L., Damiano J., Roth W., Matsuzawa S., Newman R., Takayama S., Marusawa H., Xu F., Salvesen G., Godzik A., RIKEN GER Group. GSL Members Comparative analysis of apoptosis and inflammation genes of mice and humans. Genome Res. 2003;13:1376–1388. doi: 10.1101/gr.1053803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid F.M., Vernham G.A., Jacobs H.T. A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Hum. Mutat. 1994;3:243–247. doi: 10.1002/humu.1380030311. [DOI] [PubMed] [Google Scholar]
- Riedl S.J., Salvesen G.S. The apoptosome: signaling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Riley M.F., You M.J., Multani A.S., Lozano G. MDM2 overexpression and p73 loss exacerbate genomic instability and dampen apoptosis, resulting in B-cell lymphoma. Oncogene. 2016;35:358–365. doi: 10.1038/onc.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz O., Rommel K., Dorsch K., Kelsch E., Melzner J., Buck M., Leroy K., Papadopoulou V., Wagner S., Marienfeld R., Bruderlein S., Lennerz J.K., Moller P. STAT6 mediated BCL6 repression in primary mediastinal B-cell lymphoma (PMBL) Oncotarget. 2013;4:1093–1102. doi: 10.18632/oncotarget.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak L.P., Whitworth C.A., Mukherjea D., Ramkumar V. Mechanism of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Sahara A., Aoto M., Eguchi Y., Imamoto N., Yoneda Y., Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature. 1999;401:168–173. doi: 10.1038/43678. [DOI] [PubMed] [Google Scholar]
- Saito M., Gao J., Basso K., Kitagawa Y., Smith P.M., Bhagat G., Pernis A., Pasqualucci L., Dalla-Favera R. A signaling pathway mediating down regulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Saito M., Novak U., Piovan E., Basso K., Sumazin P., Schneider C., Crespo M., Shen Q., Bhagat G., Califano A., Chadburn A., Pasqualucci L., Dalla-Favera R. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11294–11299. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano D., Kato A., Parikh N., McKnight K., Terry D., Stefanovic B., Kato Y. BCL6 canalizes Notch-dependent transcription, excluding Mastermind-like 1 from selected target genes during left-right patterning. Dev. Cell. 2010;18:450–462. doi: 10.1016/j.devcel.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax J.K., El-Deiry W.S. p53 downstream targets and chemosensitivity. Cell Death Diff. 2003;10:413–417. doi: 10.1038/sj.cdd.4401227. [DOI] [PubMed] [Google Scholar]
- Sax J.K., Fei P., Murphy M.E., Bernhard E., Korsmeyer S.J., El-Deiry W.S. Bid regulation by p53 contributes to chemo sensitivity. Nat. Cell Biol. 2002;4:842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- Scheeren F.A., Naspetti M., Diehl S., Schotte R., Nagasawa M., Wijnands E., Gimeno R., Vyth-Dreese F.A., Blom B., Spits H. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat. Immunol. 2005;6:303–313. doi: 10.1038/ni1172. [DOI] [PubMed] [Google Scholar]
- Schendel S.L., Xie Z., Montal M.O., Matsuyama S., Monal M., Reed J.C. Channel formation by antiapoptotic protein Bcl-2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfert V.L., Allman D., He Y., Staudt L.M. Transcriptional repression by the proto-oncogene BCL-6. Oncogene. 1996;12:2331–2342. [PubMed] [Google Scholar]
- Sha S.H., Taylor R., Forge A., Schacht J. Differential vulnerability of basal and apical hair ells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Sha S.H., Kanicki A., Dootz G., Talaska A.E., Halsey K., Dolan D., Altschuler R., Schacht J. Age-related auditory pathology in the CBA/J mouse. Hear Res. 2008;243:87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer A.L., Yu X., He Y., Boldrick J., Chan E.P., Staudt L.M. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Shvarts A., Brummelkamp T.R., Scheeren F., Koh E., Daley G.Q., Spits H., Bernards R. A senescence rescue screen identifies BCL6 as an inhibitor of anti-proliferative p19(ARF)-p53 signaling. Genes Dev. 2002;16:681–686. doi: 10.1101/gad.929302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky N.B. Structure of the mammalian cochlea. In: Dallos P., Papper A.N., Fay R.R., editors. The Cochlea. Springer-Verlag; New York: 1996. pp. 44–129. [Google Scholar]
- Smith R.J.H., Ranum P.T. GeneReviews®; Washington: 1998. Nonsyndromic Hearing Loss and Deafness, DFNA3. [PubMed] [Google Scholar]
- Someya S., Xu J., Kondo K., Ding D., Salvi R.J., Yamasoba T., Rabinovitch P.S., Weindruch R., Leeuwenburgh C., Tanokura M., Prolla T.A. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Proceedings of the International Symposium on “Frequency Analysis and Periodicity Detection in Hearing” Conference, 1969 June 23–27. Sijthoff; Sijthoff, The Netherlands: 1970. Structural basis of peripheral frequency analysis. [Google Scholar]
- Spolski R., Leonard W.J. IL-21 and T follicular helper cells. Int. Immunol. 2010;22:7–12. doi: 10.1093/intimm/dxp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S., Huang C., Geng H., Chen Z., Harvey R., Kang H., Ng C., Titz B., Hurtz C., Sadiyah M.F., Nowak D., Thoennissen G.B., Rand V., Graeber T.G., Koeffler H.P., Carroll W.L., Willman C.L., Hall A.G., Igarashi K., Melnick A., Muschen M. BACH2 mediates negative selection and p53-dependent tumor suppression at the pre-B cell receptor checkpoint. Nat. Med. 2013;19:1014–1022. doi: 10.1038/nm.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S., Duy C., Müschen M. BACH2-BCL6 balance regulates selection at the pre-B cell receptor checkpoint. Trend Immunol. 2014;35:131–137. doi: 10.1016/j.it.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.R., Nevill G., Forge A. Rapid hair cell loss: a mouse model for cochlear lesions. J. Assoc. Res. Otolaryngol. 2008;9:44–64. doi: 10.1007/s10162-007-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen E.A., Bosma S.C., Zuur C.L., Spijker R., van der Baan S., Dreschler W.A., de Boer J.P., Balm A.J., Rasch C.R. Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy: a systematic review of the literature. Head Neck. 2015;37:281–292. doi: 10.1002/hed.23551. [DOI] [PubMed] [Google Scholar]
- Thornborrow E.C., Patel S., Mastropieto A.E., Schwartzfarb E.M., Manfredi J.J. A conserved intronic response element mediates direct p53-dependent transcriptional activation of both the human and murine bax gene. Oncogene. 2002;21:990–999. doi: 10.1038/sj.onc.1205069. [DOI] [PubMed] [Google Scholar]
- Timmer J.C., Salvesen G.S. Caspase substrates. Cell Death Diff. 2007;14:66–72. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Marchenko N., Erster S., Nemajerova A., Dehner A., Klein C., Pan H., Kessler H., Pancoska P., Moll U.M. WT p53, but not tumor derived mutants, bind to Bcl2 via the DNA binding domain and induce mitochondrial permeabilization. JBC. 2006;281:8600–8606. doi: 10.1074/jbc.M507611200. [DOI] [PubMed] [Google Scholar]
- Toney L.M., Cattoretti G., Graf J.A., Merghoub T., Pandolfi P.P., Dalla-Favera R., Ye B.H., Dent A.L. BCL-6 regulates chemokine gene transcription in macrophages. Nat. Immunol. 2000;1:214–220. doi: 10.1038/79749. [DOI] [PubMed] [Google Scholar]
- Tran T.H., Utama F.E., Lin J., Yang N., Sjolund A.B., Ryder A., Johnson K.J., Neilson L.M., Liu C., Brill K.L., Rosenberg A.L., Witkiewicz A.K., Rui H. Prolactin inhibits BCL6 expression in breast cancer through a STAT5A dependent mechanism. Cancer Res. 2010;70:1711–1721. doi: 10.1158/0008-5472.CAN-09-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Ikegaki N., Croce C.M. Characterization of the protein product of bcl-2, the gene involved in human follicular lymphoma. Oncogene. 1987;2:3–7. [PubMed] [Google Scholar]
- Valls E., Lobry C., Geng H., Wang L., Cardenas M., Rivas M., Cerchetti L., Oh P., Yang S.N., Oswald E., Graham C.W., Jiang Y., Hatzi K., Agirre X., Perkey E., Li Z., Tam W., Bhatt K., Leonard J.P., Zweidler-McKay P.A., Maillard I., Elemento O., Ci W., Aifantis I., Melnick A. BCL6 antagonizes NOTCH2 to maintain survival of human follicular lymphoma cells. Cancer Discov. 2017;7:506–521. doi: 10.1158/2159-8290.CD-16-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van As J.W., van den Berg H., van Dalen E.C. Medical interventions for the prevention of platinum-induced hearing loss in children with cancer. Cochrane Database Syst. Rev. 2016;9:CD009219. doi: 10.1002/14651858.CD009219.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van As J.W., van den Berg H., van Dalen E.C. Platinum-induced hearing loss after treatment for childhood cancer. Cochrane Database Syst. Rev. 2016;8:CD010181. doi: 10.1002/14651858.CD010181.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S.D., Ahearne M., Ko Ferrigno P. The role of BCL6 in lymphomas and routes to therapy. Br. J. Haemotol. 2011;152:3–12. doi: 10.1111/j.1365-2141.2010.08420.x. [DOI] [PubMed] [Google Scholar]
- Walker S.R., Frank D.A. Targeting Bcl6 and STAT3 in triple negative breast cancer: the one-two punch? Oncoscience. 2015;2:912. doi: 10.18632/oncoscience.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.D., Fukuda T., Suzuki T., Hashimoto K., Liou S.Y., Momoi T., Kosaka T., Yamamoto K., Nakanishi H. Differential effects of Bcl-2 overexpression on hippocampal CA1 neurons and dentate granule cells following hypoxic ischemia in adult mice. J. Neurosci. Res. 1999;57:1–12. doi: 10.1002/(SICI)1097-4547(19990701)57:1<1::AID-JNR1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Wang X.T., Pei D.S., Xu J., Guan Q.H., Sun Y.F., Liu X.M., Zhang G.Y. Opposing effects of Bad phosphorylation at two distinct sites by Akt1 and JNK1/2 on ischemic brain injury. Cell Signal. 2007;19:1844–1856. doi: 10.1016/j.cellsig.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Wang J., Ruel J., Ladrech S., Bonny C., Van de Water T.R., Puel J.L. Inhibition of the c-Jun N-terminal kinase mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol. Pharmacol. 2007;71:654–666. doi: 10.1124/mol.106.028936. [DOI] [PubMed] [Google Scholar]
- Wei M.C., Lindsten T., Mootha V.K., Weiler S., Gross A., Ashiya, Thompson C.B., Korsmeyer S.J. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei M.C., Zong W.X., Cheng E.H., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter K.G., Hsu Y.T., Smith C.L., Nechushtan A., Xi X.G., Youle R.J. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worldwide Hearing . 2014. Hearing Loss Facts.http://www.wwhearing.org/ [Google Scholar]
- Wu C.C., Bratton S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013;19:546–558. doi: 10.1089/ars.2012.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Jelinek D.F. A-Miz-ing BCL6. Nat. Immunol. 2005;6:964–966. doi: 10.1038/ni1005-964. [DOI] [PubMed] [Google Scholar]
- Wu J., Li L., Jiang G., Zhan H., Wang N. B-cell CLL/Lymphoma 3 promotes glioma cell proliferation and inhibits apoptosis through oncogenic STAT3 pathway. Int. J. Oncol. 2016;49(6):2471–2479. doi: 10.3892/ijo.2016.3729. [DOI] [PubMed] [Google Scholar]
- Wu X., Li X., Song Y., Li H., Bai X., Liu W., Han Y., Xu L., Li J., Zhang D., Wang H., Fan Z. Allicin protects auditory hair cells and spiral ganglion neurons from cisplatin – induced apoptosis. Neuropharmacology. 2017;116:429–440. doi: 10.1016/j.neuropharm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Yamashita D., Miller J.M., Jiang H.Y., Minami S.B., Schacht J. AIF and EndoG in noise-induced hearing loss. Neuroreport. 2004;15:2719–2722. [PubMed] [Google Scholar]
- Yamasoba T., Lin F.R., Someya S., Kashio A., Sakamoto T., Kondo K. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res. 2013;303:30–38. doi: 10.1016/j.heares.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., Peng T.I., Jones D.P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Ye H., Wu Q., Guo M., Wu K., Lv Y., Yu F., Liu Y. Growth inhibition effects of ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid on colorectal carcinoma cells and colon carcinoma bearing mice. Mol. Med. Rep. 2016;13(4):3525–3532. doi: 10.3892/mmr.2016.4950. [DOI] [PubMed] [Google Scholar]
- Yu J., Wang Z., Kinzler K.W., Vogelstein B., Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Rao S., Tsai L.M., Lee S.K., He Y., Sutcliffe E.L., Srivastava M., Linterman M., Zheng L., Simpson N., Ellyard J.I., Parish I.A., Ma C.S., Li Q.J., Parish C.R., Mackay C.R., Vinuesa C.G. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Yu J.M., Sun W., Hua F., Xie J., Lin H., Zhou D.D., Hu Z.W. BCL6 induces EMT by promoting ZEB1-mediated transcription repression of E-cadherin in breast cancer cells. Cancer Lett. 2015;365:190–200. doi: 10.1016/j.canlet.2015.05.029. [DOI] [PubMed] [Google Scholar]
- Zaanan A., Okamoto K., Kawakami H., Khazaie K., Huang S., Sinicrope F.A. The mutant KRAS gene up-regulates Bcl-xL protein via STAT3 to confer apoptosis resistance that is reversed by BIM protein induction and Bcl-xL antagonism. J. Biol. Chem. 2015;290(39):23838–23849. doi: 10.1074/jbc.M115.657833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Liu H., McGee J., Walsh E.J., Soukup G.A., He D.Z. Identifying microRNAs involved in degeneration of the Organ of Corti during age-related hearing loss. PLoS One. 2013;8:e62786. doi: 10.1371/journal.pone.0062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Shen W., He D.Z.Z., Long K.B., Madison L.D., Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- Zheng H.W., Chen J., Sha S.H. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Diff. 2014;5:e1262. doi: 10.1038/cddis.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D., Coquet J.M., Zhang Y., Light A., D'Costa K., Kallies A., Corcoran L.M., Godfrey D.I., Toellner K.M., Smyth M.J., Nutt S.L., Tarlinton D.M. IL-21 regulates germinal center B cell differentiation and proliferation through a B-cell intrinsic mechanism. J. Exp. Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]