Abstract:
Acquired von Willebrand syndrome (VWS) due to loss of high-molecular-weight multimers (HMWMs) has been reported with longer term mechanical devices and is associated with mucosal bleeding, a primary hemostasis type of bleeding. However, little is known whether a similar defect occurs in patients with short-term mechanical circulatory support (STMCS) devices. We reviewed von Willebrand factor (VWF) profiles in patients with STMCS devices who underwent VWS workup from December 2015 to March 2017 at an academic quaternary care hospital. There were a total of 18 patients (57.0 ± 12.7 years old; 83.3% male) including nine with mucosal bleeding and nine with decreasing hemoglobin. The STMCS devices included Impella (n = 11), Impella and right ventricular assist device (n = 2), and an extracorporeal membrane oxygenator (n = 5). The mean HMWM by quantitative VWF multimer analysis was 3.6% ± 1.3% (normal cutoff: 18–34%). In all 10 cases in which VWF activity, fibrinogen, factor VIII, or VWF antigen level were obtained, they were either normal or elevated. All cases demonstrated high normal or elevated levels of low molecular weight multimers (LMWMs). These findings are consistent with type 2 VWS (qualitative defect). This is the first study that quantitatively describes STMCS device–associated HMWM loss, which may contribute to mucosal bleeding. This finding may have implications for intraoperative management during implantation of longer term devices or heart transplantation or other surgery while on STMCS.
Keywords: von Willebrand factor, bleeding, Impella, ECMO
Short-term mechanical circulatory support (STMCS) devices are used to support the circulatory and/or respiratory system for hours to days, or less commonly, for weeks. These devices may be placed in patients with cardiogenic shock as a bridge to decision, recovery, more durable device implantation, or heart transplantation, as well as for support in high-risk percutaneous interventions.
However, mechanical circulatory support (MCS) devices are associated with potential life-threatening complications, such as the competing risks of thrombosis and bleeding. Bleeding is the most common complication following MCS device implantation, occurring in about 20–25% of patients and is associated with increased morbidity and mortality (1,2).
One potential cause of severe bleeding is acquired von Willebrand syndrome (VWS), which is caused by the loss of high molecular weight multimers (HMWMs). Acquired VWS results is a primary hemostasis defect that places patients at risk for mucosal bleeding, an important complication, particularly in the perioperative durable device implantation or heart transplantation period.
Acquired VWS has been reported in patients receiving longer term MCS (LTMCS) devices, such as left ventricular assist devices (LVADs), but there are minimal data on whether a similar defect occurs in patients with STMCS devices (3–5). Thus, we sought to characterize the association between STMCS devices and von Willebrand factor (VWF) profiles in patients who presented with mucosal bleeding (i.e. epistaxis and gastrointestinal bleed) or decreasing hemoglobin concentration of uncertain etiology over a 16-month period and underwent VWS workup at an academic quaternary care hospital.
METHODS
We retrospectively reviewed the medical record of patients implanted with STMCS devices who underwent VWS workup from December 2015 to March 2017 at the Cedars-Sinai Medical Center, an academic quaternary care hospital. The STMCS devices used at the institution during this time period included an intra-aortic balloon pump, percutaneous mechanical circulatory assist devices (such as the Impella 2.5, CP, 5.0, right-sided Tandem Heart), and extracorporeal membrane oxygenator (ECMO) pumps.
We extracted the following data for each case: demographic, type of STMCS device, indication for VWS testing, device outcome, transfusion requirements, and laboratory data.
Quantitative VWF multimer analysis was performed at a specialized reference laboratory (Blood Center of Wisconsin, Milwaukee, WI), where citrated plasma samples underwent LiDS-horizontal gel agarose (.65%) electrophoresis and quantification using immunohistochemistry and densitometry analysis. Per reference laboratory, the cutoff result of 18–34% for HMWMs was considered normal and a HMWM result of <11% represented a loss of HMWMs.
All laboratory testing was performed at the Cedars-Sinai Medical Center except for the quantitative VWF multimer analysis. The VWF activity assay was performed by using the IL VWF activity kit (Instrumentation Laboratory, Bedford, MA) and Diagnostica Stago’s instrumentation. The activity of VWF is determined by measuring the increased turbidity produced by the agglutination of the latex reagent. A specific anti-VWF monoclonal antibody adsorbed onto the latex reagent, directed against the platelet-binding site of VWF (glycoprotein Ib receptor), reacts with the VWF of patient plasma. The degree of agglutination is directly proportional to the VWF activity in the sample and is determined by measuring the decrease in transmitted light caused by the aggregates.
VWF antigen (VWF:Ag) was determined by using Diagnostica Stago’s Liatest that uses the turbidimetric method. A suspension of latex microparticles coated by covalent bonding with antibodies specific for VWF was mixed with the patient’s platelet-poor plasma. Change in turbidity was measured photometrically at 540 nm and converted to percent of activity by the instrument (Diagnostica Stago’s STA-R Evolution, Parsippany, NJ).
Factor VIII activity was measured by determining the extent to which the patient’s plasma corrects the clotting time of Stago’s factor–deficient substrate plasma using Diagnostica Stago’s instrumentation and reagents. The percent activity was determined from a standard curve prepared using serial dilutions of a commercial assayed reference plasma.
The interpretation of each laboratory result was reviewed by a coagulation pathologist at the Cedars-Sinai Medical Center.
Our main outcome of interest was the overall mean percentage of HMWMs. Other outcomes of interest include percentage of cases with HMWMs below the normal reference range (<18%), overall mean percentage of low molecular weight multimers (LMWMs; cutoff: 16–24%), and recovery of HMWMs post-STMCS device removal.
Continuous data are presented as mean ± SD. Categorical data are presented as frequencies and percentages. The study was approved by the hospital’s institutional review board (Pro00020123).
RESULTS
There were 18 patients included in our analysis: 10 with Impella 5.0, one with Impella CP, two with Impella 5.0 and right ventricular assist device, and five with ECMO (Table 1). The mean age of the cohort was 57.0 ± 12.7 years and 83.3% were male. VWS workup was initiated because of mucosal bleeding and decreasing hemoglobin in nine cases and only decreasing hemoglobin concentration in nine cases. All cases (n = 18) had HMWMs below the reference range and the overall HMWM mean was 3.6% ± 1.3%. All cases (n = 18) had high normal or elevated level of LMWMs with a mean overall LMWM percentage of 38.6% ± 5.6%. All cases (n = 10) that had VWF:Ag, VWF activity, fibrinogen, and factor VIII levels available had normal or elevated values.
Table 1.
Cases of VWD workup during STMCS.
| Case | Age/Gender | STMCS Device | Indication for VWD Workup | Blood Products Used During STMCS | HMWMs (%) | IMWMs (%) | LMWMs (%) | VWF Ag | VWF Activity | F8 | Device Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59/M | Impella 5.0 | Hemoptysis | None | 3 | 46 | 51 | Removed; OHT | |||
| 2 | 40/F | Impella 5.0 | Epistaxis | 10 PRBCs | 4 | 59 | 37 | Removed; OHT | |||
| 3 | 69/M | ECMO | Hemoptysis | 16 PRBCs, 1 FFP, 5 platelets | 4 | 61 | 34 | 229 | 157 | 346 | Removed; LVAD |
| 4 | 73/M | Impella 5.0 | Hemoptysis | None | 3 | 60 | 37 | 222 | 210 | 318 | Removed; OHT |
| 5 | 56/M | ECMO | ↓ Hgb | None | 6 | 60 | 34 | Expired | |||
| 6 | 72/M | ECMO | ↓ Hgb | 2 PRBCs | 3 | 59 | 38 | 400 | 252 | 366 | Removed; RVAD |
| 7 | 60M | ECMO | GI bleed | 5 PRBCs, 4 FFPs, 3 Platelets, 1 cryo | 5 | 62 | 33 | 118 | 96 | 108 | Removed |
| 8 | 37/M | Impella 5.0 | Epistaxis | None | 4 | 49 | 47 | 291 | 237 | 434 | Removed; OHT |
| 9 | 57/M | Impella 5.0, RVAD | GI bleed | 7 PRBCs, 2 platelets | 2 | 52 | 46 | Expired | |||
| 10 | 40/M | Impella 5.0 | ↓ Hgb | 3 PRBCs | 2 | 52 | 46 | Expired | |||
| 11 | 74/M | Impella 5.0, RVAD | ↓ Hgb | 10 PRBCs, 1 platelets | 5 | 60 | 35 | Expired | |||
| 12 | 57/F | Impella 5.0 | GI bleed | 10 PRBCs | 2 | 59 | 38 | Removed | |||
| 13 | 57/F | Impella CP | ↓ Hgb | 2 PRBCs, 3 FFPs | 3 | 64 | 33 | 400 | 261 | 372 | Expired |
| 14 | 40/F | Impella 5.0 | ↓ Hgb | None | 5 | 59 | 37 | Removed; LVAD | |||
| 15 | 66/M | ECMO | ↓ Hgb | 12 PRBCs | 2 | 60 | 38 | 201 | 141 | 210 | Removed |
| 16 | 30/M | Impella 5.0 | GI bleed | 7 PRBCs, 5 FFPs, 1 platelets | 5 | 61 | 34 | 268 | 67 | 210 | Removed |
| 17 | 67/M | Impella 5.0 | ↓ Hgb | 11 PRBCs, 6 FFPs, 1 platelet | 4 | 62 | 34 | 400 | 330 | 714 | Expired |
| 18 | 50/M | Impella 5.0 | ↓ Hgb | 2 PRBCs, 1 FFP | 2 | 56 | 43 | 400 | 460 | 410 | Removed |
Ag, antigen; Cryo, cryoprecipitate; ECMO, extracorporeal membrane oxygenation; F, female; FFPs, fresh frozen plasma; GI, gastrointestinal; Hgb, hemoglobin; HMWMs, high molecular weight multimers; IMWM, intermediate molecular weight multimers; LMWMs, low molecular weight multimers; LVAD, left ventricular assist device; M, male; OHT, orthotopic heart transplantation; PRBCs, packed red blood cells; RVAD, right ventricular assist device; STMCS, short-term mechanical support; VWD, von Willebrand disease; VWF, von Willebrand factor.
Mucosal bleeding was due to epistaxis (n = 2), gastrointestinal bleeding (n = 4), and hemoptysis (n = 3). Blood product requirements during STMCS in patients with mucosal bleeding were 6.1 ± 5.2 units of packed red blood cells, 1.2 ± 3.5 units of platelets, and 1.1 ± 3.1 units of fresh frozen plasma. Only one patient received cryoprecipitate during STMCS. For patients who had the STMCS device removed and concurrently underwent orthotopic heart transplantation (n = 4) or LTMCS implantation (n = 3), perioperative blood product requirements were 3.6 ± 2.4 units of packed red blood cells, 1.0 ± .9 units of platelets, and 2.3 ± 2.6 units of fresh frozen plasma. Two patients (cases 1 and 2) received cryoprecipitate (2 units and 1 unit, respectively) in the perioperative period and two patients (cases 1 and 14) received a dose of tranexamic acid intraoperatively.
In patients who underwent VWS workup for only decreased hemoglobin levels (n = 9), the mean decrease in hemoglobin from the date of device implant to the date of VWD workup was 3.7 g/L (from 11.2 ± .8 to 7.5 ± .3, respectively). Gastroscopy was performed in two cases (17 and 18) and clinical judgment excluded other potential causes of decreasing hemoglobin levels in the other cases. Red blood cell transfusions were given in six of the nine cases. Cryoprecipitate was only given in one case (case 14) during implantation of a LTMCS device.
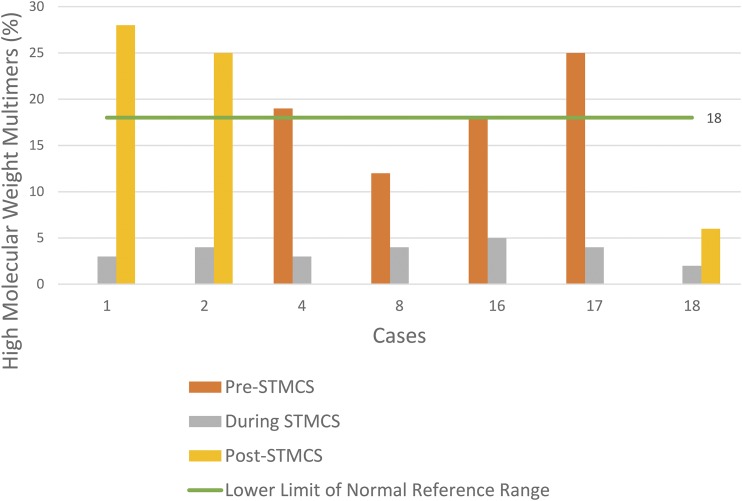
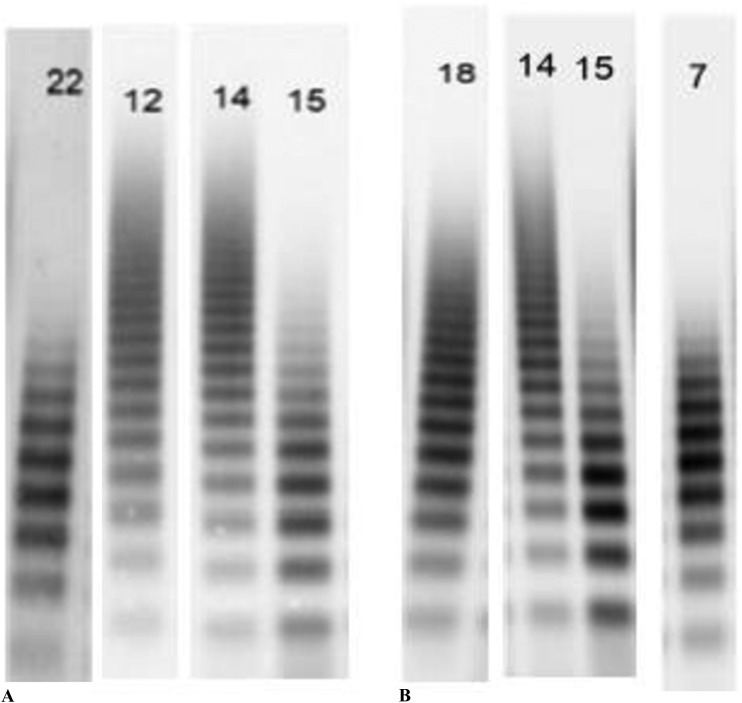
There were seven patients who had VWF multimer analysis during STMCS and had a second value obtained before device implantation or following device removal (Figure 1). For patients with VWF multimer analysis obtained during STMCS and post-explant (n = 3), the mean time to VWF testing post-device explant was 9.7 ± 7 days. A qualitative comparison of VWF multimers for cases 1 and 17 is presented in Figure 2. In patients who had a VWF analysis before STMCS implant (n = 4), there was a mean decrease in HMWMs by 14.5 absolute percentage points from prior to during STMCS (18.5% ± 4.6% to 4.0% ± .7%). In patients with VWF testing following STMCS device removal (n = 3), there was a mean increase in HMWMs by 16.7 absolute percentage points (3.0% ± .8% to 19.7% ± 9.7%).
Figure 1.
HMWMs in patients also tested before STMCS device implantation following device removal.
Figure 2.
Panel A shows a gel electrophoresis from the patient in case 1 while Impella 5.0 was in place (lane 22), from the same patient following device removal (lane 12), from a healthy control (lane 14), and from a patient with type 2B VWS (lane 15). Panel B shows a gel electrophoresis from the patient in case 17 before Impella 5.0 implant (lane 18), from a healthy control (lane 14), from a patient with type 2B VWS (lane 15), and from the patient in case 17 with the Impella in place (lane 7). In both cases, HMWMs disappear during MCS and resemble the distribution of the patient with type 2B VWS.
DISCUSSION
Our study represents the first case series quantitatively reporting the loss of HMWMs in patients with STMCS devices. All patients in our cohort had very low levels of HMWMs and normal or elevated levels of LMWMs during STMCS. We found that HMWMs decreased considerably during STMCS and improved following device removal. These findings are consistent with an acquired type 2 VWS (qualitative defect). We also found that these patients had considerable transfusion needs during STMCS use and during subsequent operations.
HMWM loss has been shown to occur almost universally in patients with LTMCS devices (6–8). In patients with STMCS devices, however, there has been only limited evidence for the loss of HMWMs. Davis et al. reported a case of decreased levels of HMWMs in a patient with an Impella 5.0 (3). Heilman et al. reported a case–control series in which 31 of the 32 patients on ECMO had absent HMWMs, compared with none of the 19 control subjects (9). However, prior studies in STMCS patients reported VWF multimer levels qualitatively by gel electrophoresis and did not perform quantitative VWF multimer analysis (3,9–11). We used quantitative VWF multimer analysis in our study, which allowed us to detect the degree of abnormality. In addition, using standard recommended first-tier assays alone for VWS diagnosis (VWF activity and antigen), we would have missed the diagnosis of VWS in many cases. Global assays of hemostasis, such as thromboelastogram and rotational thromboelastometry, are also not sensitive for VWS diagnosis.
VWF activity is currently measured by several assays and by different methods. Ristocetin cofactor activity (VWF:RCo) assay, the original assay, is still referred to as the gold standard for the measurement of VWF activity, despite being very labor intensive and having a high coefficient of variation. Our recent study evaluated VWF profiles after implantation of LTMCS devices using VWF activity assay and found that all patients had either normal (47.8%) or elevated (52.2%) VWF activity assay and normal (26.1%) or elevated (73.9%) VWF:Ag (12). The mean VWF activity assay/VWF:Ag ratio was .8 ± .3 and one-half of patients had a disproportionate ratio. Without quantitative multimer analysis and expertise in result interpretation, we would have missed the diagnosis of VWS in all patients included in this study.
The overall mean percentage of HMWMs in patients with STMCS devices in our study (3.6%) was much lower than that found in our prior study for patients with LVADs and total artificial hearts (12.4%) (12). Other studies in LTMCS devices have shown decreases in HMWMs to 30–34% (6,8). There is emerging data that each type of MCS device has its own unique hemostatic effect on blood flow, which may be related to variations in pump design, flow profiles, and pump speed (13,14). For example, the HeartMate 3 LVAD was designed to reduce circulatory shear stress and one study demonstrated a significantly greater preservation of HMWMs compared with the HeartMate II group (15). Compared with the HeartMate devices, percutaneous continuous flow pumps like the Impella 5.0 are at risk of even higher shear stress and more significant HMWM loss because of the micro axial design and high pump speed.
Type 2A VWS, the most commonly seen acquired form in MCS devices, refers to qualitative variants in which VWF-dependent platelet adhesion is decreased because the proportion of large VWF multimers is decreased. This deficiency of VWF HMWMs may be due to impaired large VWF multimers biosynthesis, increased sensitivity of plasma VWF multimers to ADAMTS-13 cleavage, or defective posttranslational processing (16–19). Type 2A acquired VWS is not unique to MCS devices. It has also been described in several other heart conditions, such as severe aortic stenosis (Heyde’s syndrome), mitral regurgitation, and hypertrophic cardiomyopathy (20–22). All these conditions have in common a significant disturbance in the cardiac blood flow (especially with continuous flow devices), shear stress, and turbulence that can induce conformational change in multimeric VWF and expose it to cleavage by the metalloproteinase ADAMTS-13. Factor VIII and platelet receptor GP1b alpha have synergistic effects that enhance VWF proteolysis by ADAMTS-13 under conditions of fluid shear stress (23). Changes in VWF structure and platelet-binding activity associated with LVAD implantation have been well-described (24,25). An increasing number of studies suggest that ADAMTS-13 plays an essential role in the decrease of HMWMs in patients with continuous flow devices (26,27). However, a recent study by Bartoli et al. (14) postulated that the major mechanism of VWF degradation was mechanical destruction of VWF.
There are a number of management considerations resulting from the STMCS-associated acquired type 2 VWS. Patients with Impella or ECMO support commonly receive systemic anticoagulation, placing them at further risk for bleeding events. There may also be an increased bleeding risk in the perioperative period of STMCS device removal and LTMCS device implant or orthotopic heart transplantation, as was seen in several cases in our study. Pharmacological and transfusion options that can be considered in the perioperative period for patients with overt bleeding include factor VIII concentrate or cryoprecipitate (which both contain VWF), factor VIIa, desmopressin, pentoxifylline, and tranexamic acid. However, desmopressin and cryoprecipitate may only provide VWF for the short term because VWF will be degraded and prothrombin complex concentrate could lead to a hypercoagulable state. There may be a need for a different anticoagulation strategy in patients at risk for VWF multimer loss. Doxycycline to reduce ADAMTS-13 activity and reducing the STMCS device speed may also be considered to decrease HMWM degradation (28). In addition, future MCS design may aim to decrease the high shear stress.
There are several limitations to our study. First, the decision to initiate VWS workup was based on clinician preference. It is probable that not all patients who met the inclusion criteria had quantitative VWF multimer analysis done. Given the consistency of our findings, it is highly likely that these patients would have low levels of HMWMs during STMCS as well. In addition, as we do not have prior and follow-up VWF testing for all patients, we cannot definitively conclude that STMCS causes HMWM degradation. However, our finding that all STMCS patients with mucosal bleeding had very low levels of HMWMs and the recovery of HMWMs in those with follow-up testing post-STMCS removal strongly suggest that STMCS is associated with HMWM degradation. There were four patients who had VWF testing before STMCS implantation, although the clinical indication for this testing was not clear. Finally, the overall percentage of patients on STMCS who developed VWS and the overall bleeding incidence for patients with STMCS during the study period are unknown.
CONCLUSION
This is the first study that quantitatively describes STMCS device–associated HMWM loss, which may contribute to bleeding. Using standard assays for VWS diagnosis such as VWF activity, VWF:Ag, and factor VIII levels may miss cases of VWS in patients with MCS devices. This may have further implications for intraoperative management during longer term device implantation or heart transplantation or other surgery while on STMCS.
ACKNOWLEDGMENT
The authors would like to acknowledge Sandra Haberichter, PhD, Blood Center of Wisconsin, who provided us with multimers analysis tracings.
REFERENCES
- 1.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–504. [DOI] [PubMed] [Google Scholar]
- 2.Harvey L, Holley CT, John R. Gastrointestinal bleed after left ventricular assist device implantation: Incidence, management, and prevention. Ann Cardiothorac Surg. 2014;3:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis ME, Haglund NA, Tricarico NM, et al. Development of acquired von Willebrand syndrome during short-term micro axial pump support: Implications for bleeding in a patient bridged to a long-term continuous-flow left ventricular assist device. ASAIO J. 2014;60:355–7. [DOI] [PubMed] [Google Scholar]
- 4.Klovaite J, Gustafsson F, Mortensen SA, et al. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53:2162–7. [DOI] [PubMed] [Google Scholar]
- 5.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–13. [DOI] [PubMed] [Google Scholar]
- 6.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–9. [DOI] [PubMed] [Google Scholar]
- 7.Geisen U, Heilmann C, Beyersdorf F, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–84. [DOI] [PubMed] [Google Scholar]
- 8.Meyer AL, Malehsa D, Budde U, et al. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC Heart Fail. 2014;2:141–5. [DOI] [PubMed] [Google Scholar]
- 9.Heilmann C, Geisen U, Beyersdorf F, et al. Acquired von Willebrand syndrome in patients with ventricular assist device or total artificial heart. J Thromb Haemost. 2010;103:962–7. [DOI] [PubMed] [Google Scholar]
- 10.Flierl U, Tongers J, Berliner D, et al. Acquired von Willebrand syndrome in cardiogenic shock patients on mechanical circulatory microaxial pump support. PLoS One. 2017;12:e0183193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalbhenn J, Schmidt R, Nakamura L, et al. Early diagnosis of acquired von Willebrand Syndrome (AVWS) is elementary for clinical practice in patients treated with ECMO therapy. J Atheroscler Thromb. 2015;22:265–71. [DOI] [PubMed] [Google Scholar]
- 12.Reich HJ, Morgan J, Arabia F, et al. Comparative analysis of von Willebrand factor profiles after implantation of left ventricular assist device and total artificial heart. J Thromb Haemost. 2017;15:1620–4. [DOI] [PubMed] [Google Scholar]
- 13.Birschmann I, Dittrich M, Eller T, et al. Ambient hemolysis and activation of coagulation is different between HeartMate II and HeartWare left ventricular assist devices. J Heart Lung Transplant. 2014;33:80–7. [DOI] [PubMed] [Google Scholar]
- 14.Bartoli CR, Kang J, Zhang D, et al. Left ventricular assist device design reduces von Willebrand factor degradation: A comparative study between the HeartMate II and the EVAHEART left ventricular assist system. Ann Thorac Surg. 2017;103:1239–44. [DOI] [PubMed] [Google Scholar]
- 15.Netuka I, Kvasnicka T, Kvasnicka J, et al. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous-flow left ventricular assist device in advanced heart failure. J Heart Lung Transplant. 2016;35:860–7. [DOI] [PubMed] [Google Scholar]
- 16.Rodeghiero F, Castaman G and Dini E. Epidemiological investigation of the prevalence of von Willebrand’s disease. Blood. 1987;69:454–9. [PubMed] [Google Scholar]
- 17.Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: A report of the subcommittee on von Willebrand factor. J Thromb Haemost. 2006;4:2103–14. [DOI] [PubMed] [Google Scholar]
- 18.Shida Y BC, Mewburn J, Sponagle K, et al. Impact of ADAMTS13-mediated regulation of von Willebrand factor multimer profile on hemostasis and VWF clearance. J Thromb Haemost. 2013;11(Suppl 2):288. [Google Scholar]
- 19.Werner EJ, Broxson EH, Tucker EL, et al. Prevalence of von Willebrand disease in children: A multiethnic study. J Pediatr. 1993;123:893–8. [DOI] [PubMed] [Google Scholar]
- 20.Pate GE, Chandavimol M, Naiman SC, et al. Heyde's syndrome: A review. J Heart Valve Dis. 2004;13:701–12. [PubMed] [Google Scholar]
- 21.Blackshear JL, Wysokinska EM, Safford RE, et al. Shear stress-associated acquired von Willebrand syndrome in patients with mitral regurgitation. J Thromb Haemost. 2014;12:1966–74. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu M, Masai H and Miwa Y. Occult gastrointestinal bleeding due to acquired von Willebrand syndrome in a patient with hypertrophic obstructive cardiomyopathy. Intern Med. 2007;46:481–5. [DOI] [PubMed] [Google Scholar]
- 23.Zheng XL. Structure-function and regulation of ADAMTS-13 protease. J Thromb Haemost. 2013;11(Suppl 1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nascimbene A, Neelamegham S, Frazier OH, et al. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 2016;127:3133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Susen S, Rauch A, Van Belle E, et al. Circulatory support devices: Fundamental aspects and clinical management of bleeding and thrombosis. J Thromb Haemost. 2015;13:1757–67. [DOI] [PubMed] [Google Scholar]
- 26.Jilma-Stohlawetz P, Quehenberger P, Schima H, et al. Acquired von Willebrand factor deficiency caused by LVAD is ADAMTS-13 and platelet dependent. Thromb Res. 2016;137:196–201. [DOI] [PubMed] [Google Scholar]
- 27.Tsai HM. von Willebrand factor, shear stress, and ADAMTS13 in hemostasis and thrombosis. ASAIO J. 2012;58:163–9. [DOI] [PubMed] [Google Scholar]
- 28.Bartoli CR, Kang J, Restle DJ, et al. Inhibition of ADAMTS-13 by doxycycline reduces von Willebrand factor degradation during supraphysiological shear stress: Therapeutic implications for left ventricular assist device-associated bleeding. JACC Heart Fail. 2015;3:860–9. [DOI] [PubMed] [Google Scholar]