Abstract:
Despite the ubiquitous use of cardioplegia in cardiac surgery, there is a lack of agreement on various aspects of cardioplegia practice. To discover current cardioplegia practices throughout the world, we undertook a global survey to document contemporary cardiopulmonary bypass practices. A 16-question, Internet-based survey was distributed by regional specialist societies, targeting adult cardiac anesthesiologists. Ten questions concerned caseload and cardioplegia practices, the remaining questions examined anticoagulation and pump-priming practices. The survey was available in English, Spanish, and Portuguese. The survey was launched in June 2015 and remained open until May 2016. A total of 923 responses were analyzed, summarizing practice in Europe (269), North America (334), South America (215), and Australia/New Zealand (105). Inter-regional responses differed for all questions asked (p < .001). In all regions other than South America, blood cardioplegia was the common arrest technique used. The most commonly used cardioplegia solutions were: St. Thomas, Bretschneider, and University of Wisconsin with significant regional variation. The use of additives (most commonly glucose, glutamate, tris-hydroxymethyl aminomethane, and aspartate) varied significantly. This survey has revealed significant variation in international practice with regards to myocardial protection, and is a reminder that there is no clear consensus on the use of cardioplegia. It is unclear why regional practice groups made the choices they have and the clinical impact remains unclear.
Keywords: cardioplegia, blood, crystalloid, cardiopulmonary bypass
Cardiopulmonary bypass (CPB) was first used in 1951, enabling open heart surgery to become a reality (1). The practice has undergone significant evolution since its initiation, and is now safely used throughout the world. An essential adjunct to CPB is the ability to arrest the heart providing a motionless and bloodless operative site. This is achieved with cardioplegia solutions whose role is not only to arrest the heart, but also additionally protect the myocardium from ischemia. The first cardioplegia solution was developed in 1955 by Melrose, who observed that high concentration potassium citrate could be used to achieve “elective reversible cardiac arrest.” (2) Hyperkalemia results in membrane depolarization preventing conduction of action potentials resulting in diastolic cardiac arrest. Although Melrose’s solution was in fact found to result in myocardial necrosis and increased postoperative mortality, further study and modification of constituents has resulted in the use of cold, hypokalemic cardioplegia solutions (3).
Despite the ubiquitous use of cardioplegia in cardiac surgery today, there is lack of agreement on various aspects of cardioplegia practice. There remain many debates, for example the use of crystalloid versus cold blood cardioplegia (4–6) and the question of whole blood (microplegia) versus diluted blood cardioplegia (7) both of which have been the focus of recent meta-analysis, which do not demonstrate superiority of either approach.
In an attempt to document current cardioplegia practices and identify worldwide variations we administered a global survey of cardiac anesthetists to explore contemporary CPB practice.
METHODS
The Global CPB Survey was devised to understand current CPB practices worldwide (Appendix) (8). The survey contained 16 questions: four dealt with institution location and caseload, six concerned cardioplegia choices and additives, one concerned anticoagulation strategy and the remaining five concerned preferences for pump priming and associated additives. Institutional ethics approval was received from the Human Ethics Research Committee at Austin Health (Melbourne, Australia) (8).
The questionnaire was uploaded onto an online survey tool (SurveyMonkey, Palo Alto, CA) for distribution, facilitated by specialist cardiothoracic anesthesiology societies worldwide via their mailing lists, in some cases after individual societal ethics approval. Distribution was staggered due to differences in the time taken for societies to agree to participate. A reminder notice was sent approximately 2 weeks after the initial contact. The survey was translated into Spanish and Portuguese for the South American regions. The survey was open between June 2015 and May 2016 due to the staggered distribution and to leave sufficient time to maximize participation. It was open for a further 2 months after the last response was received. It became apparent that as some participants may have been members of more than one specialist society, there was the opportunity to inadvertently be invited and therefore respond more than once. After this possibility was realized, an additional question was added to the survey to identify and exclude duplicate responses.
After the survey was closed, the results were collated and analyzed. Those regions for which there was a poor response (defined as <100 individually identifiable participants) were eliminated from the final analysis as were duplicate responses. All data were obtained as categorical variables. Data were first analyzed descriptively using graphic methods. Statistical differences between region responses were evaluated using the Pearson χ2 test. The null hypothesis was rejected if the p < .05. An a priori sample size was derived using a χ2 sample size calculation. This used an effect size (w) value of .3 (estimating a moderate effect size), a significance level of .05, degrees of freedom set to 16, and a power of .95. This indicated that a total sample size of 317 individual responses across all the groups was required to achieve 95% power. Assuming a 15% response rate, this required the survey to be sent to at least 2,114 individual participants. A post hoc power calculation using the final number of total responses included in the analysis (n = 923) suggested that the study had a 99.999% power to detect a difference between the four regional groups. All statistical analyses was performed using Stata version 12 (StataCorp, College Station, TX).
This manuscript focuses on the cardioplegia practices. Data concerning pump priming and anticoagulation strategy have been previously analyzed and published (8).
RESULTS
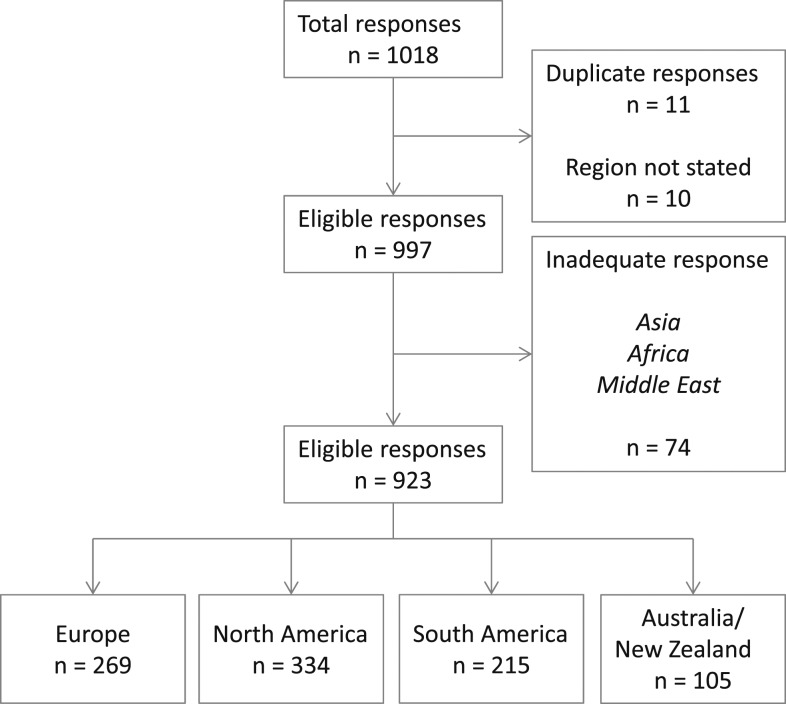
A total of 1,018 responses were received; of these, 11 (1.08%) were identified to be duplicates and were eliminated; a further 10 did not state their region. A further 74 (7.55%) were from regions where there were <100 responses (sub-Saharan Africa, Middle East, and Asia). Because of the low number of responses from these regions they were not analyzed further. The final cohort comprised 923 responses (Table 1, Figure 1), summarizing practice in Europe (269, 28.8%), North America (334, 35.8%), South America (215, 23.9%), and Australia/New Zealand (ANZ) (105, 11.5%). Inter-regional responses differed for all questions (p < .001).
Table 1.
Regional breakdown of responses by distributing society, number of potential participants, and number of responses.
| Regional Responses | Distributing Societies | Number of Potential Participants | Number of Responses | Percentage Response | Time Survey Active (months) |
|---|---|---|---|---|---|
| Europe | European Association of Cardiothoracic Anesthesiology | 810 | 269 | 19.8% | 12 |
| Association of Cardiac Anesthetists (UK) | 550 | ||||
| North America | Society of Cardiovascular Anesthesiologists | 4,145 | 334 | 8.1% | 10 |
| ANZ | Cardiovascular, Thoracic and Perfusion Special Interest Group - Australian and New Zealand College of Anaesthetists | 644 | 105 | 16.3% | 10 |
| South America | Confederacion Latinoamericana de Sociedades de Anestesiología | 2,350 | 215 | 1.7% | 8 |
| Brazilian Society of Anesthesiology | 10,443 | ||||
| Africa | Cardiothoracic Anaesthesia Society of South Africa | 50 | 22 | 46.0% | 10 |
| Asia | Asian Society of Cardiothoracic Anesthesia | Unknown | 33 | Unknown | 10 |
| Middle East | Various | Unknown | 19 | Unknown | 12 |
As the survey did not specifically ask which distributing society was involved, it is not possible to attribute an individual response to a specific organization, only to the region the participant was from. It is important to note that the percentage response is difficult to interpret as it is difficult to obtain the true denominator. For example, in South America, distribution was through non-specialist anesthesia societies hence the large number of potential participants—many of whom will not have cardiothoracic practice.
Figure 1.
CONSORT-style diagram describing the distribution of received responses and the reasons for not considering certain responses in the final analysis.
Caseload
In Europe, centers have higher case volumes than reported by respondents in other countries (Table 2). Of those responding, 73.0% worked in centers performing >750 cases per annum. By contrast, centers in North and South America tended to have much smaller volume, with 50.6% and 68.4% working in centers undertaking <500 cases per annum, respectively—in comparison with 12.3% in European centers. Centers in ANZ tended to be mid-range with 79.1% working in centers performing 250–750 cases per annum. There was not an assessment of whether the practices reported were from adult and/or pediatric centers.
Table 2.
Number of cardiopulmonary bypass cases undertaken per annum in centers in which individual respondents worked.
| CPB Cases per Annum | <250 Cases | 251–500 Cases | 501–750 Cases | 751–1,000 Cases | More Than 1,000 Cases | No Response |
|---|---|---|---|---|---|---|
| Europe | 10 (4.7%) | 16 (7.6%) | 31 (14.7%) | 70 (33.2%) | 84 (39.8%) | 58 (21.6%) |
| North America | 70 (24.6%) | 74 (26.0%) | 52 (18.2%) | 42 (14.7%) | 47 (16.5%) | 49 (14.7%) |
| ANZ | 3 (3.3%) | 32 (35.2%) | 40 (43.9%) | 9 (9.9%) | 7 (7.7%) | 14 (13.3%) |
| South America | 59 (43.4%) | 34 (25.0%) | 14 (10.3%) | 16 (11.8%) | 13 (9.5%) | 79 (36.7%) |
| Total | 142 (19.7%) | 156 (21.6%) | 137 (18.9%) | 137 (18.9%) | 151 (20.9%) | 200 (21.7%) |
Percentage values relate to proportion providing a response to the question. The far right column indicates the overall proportion not answering the question. p-value < .001, chi squared test. All tablulated values: number (percentage).
Arrest Technique
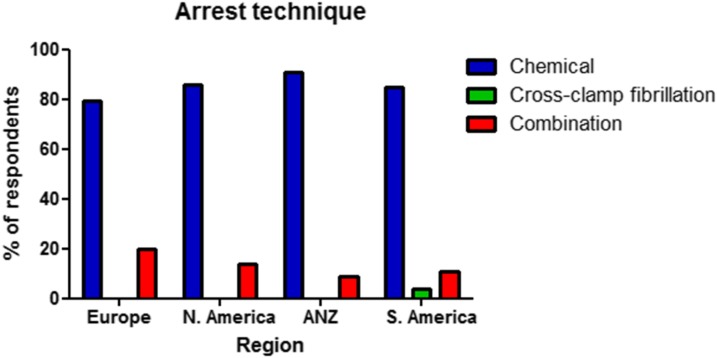
Respondents were asked whether chemical cardioplegia or cross-clamp fibrillation was the predominant arrest technique at their institution (Table 3, Figure 2). Chemical cardioplegia was the most frequent method, ranging from 79.5% in Europe to 90.6% in ANZ. Cross-clamp fibrillation is very rarely utilized as the only method of arresting the heart, with only four respondents (3.6%) from South America and one respondent each from Europe (.5%) and ANZ (.4%). There was some variability between the regions in the use of a combination of both arrest techniques, ranging from 20% of respondents in Europe to 9.4% in ANZ.
Table 3.
Arrest technique predominantly used in the respondents center.
| Arrest Technique | Chemical Cardioplegia | Cross Clamp Fibrillation | Combination | No Response |
|---|---|---|---|---|
| Europe | 155 (79.5%) | 1 (.5%) | 39 (20.0%) | 74 (27.5%) |
| North America | 202 (85.6%) | 1 (.4%) | 33 (14.0%) | 98 (29.3%) |
| ANZ | 77 (90.6%) | 0 (0%) | 8 (9.4%) | 20 (19.0%) |
| South America | 94 (84.7%) | 4 (3.6%) | 13 (11.7%) | 104 (48.4%) |
| Total | 528 (84.2%) | 6 (1.0%) | 93 (14.8%) | 296 (32.1%) |
Percentage values relate to proportion providing a response to the question. The far right column indicates the overall proportion not answering the question. p-value < .001, chi squared test. All tablulated values: number (percentage).
Figure 2.
Distribution of arrest technique as used by individual respondents by region. ANZ, Australia/New Zealand.
Chemical Cardioplegia
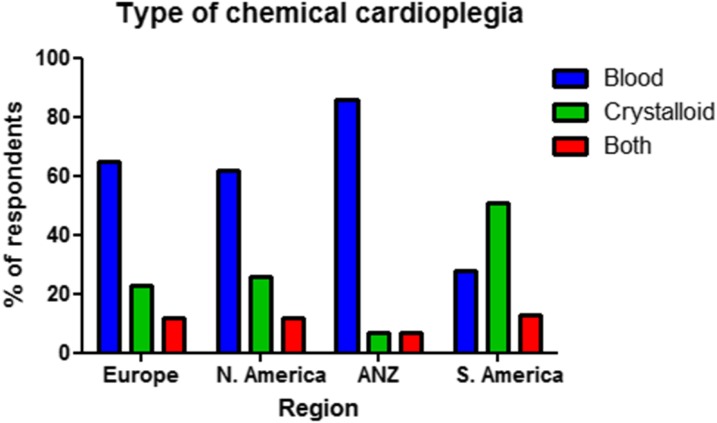
Respondents were asked to report on the type of chemical cardioplegia used at their center: blood, crystalloid, or a combination of both (Table 4, Figure 3). Significant regional variation was observed (p < .001). Apart from South America, blood cardioplegia was the most frequently reported type of chemical cardioplegia. In South America 50.9% reported crystalloid cardioplegia as the method of choice with only 27.8% using primarily blood cardioplegia, with the remainder using a combination of both. European and North American respondents described a similar pattern of chemical cardioplegia use with approximately two-thirds of respondents using blood cardioplegia, one-quarter of respondents using crystalloid cardioplegia and the remainder reporting both techniques being used. In ANZ, crystalloid cardioplegia was much less frequently utilized with only 14.2% reporting its use either alone or in combination.
Table 4.
Type of chemical cardioplegia predominantly used in the respondents center.
| Type of Chemical Cardioplegia | Blood Cardioplegia | Crystalloid Cardioplegia | Combination | No Response |
|---|---|---|---|---|
| Europe | 126 (65.3%) | 44 (22.8%) | 23 (11.9%) | 75 (27.8%) |
| North America | 146 (61.9%) | 62 (26.3%) | 28 (11.8%) | 97 (29.0%) |
| ANZ | 73 (85.8%) | 6 (7.1%) | 6 (7.1%) | 20 (19.0%) |
| South America | 30 (27.8%) | 55 (50.9%) | 23 (21.3%) | 106 (49.3%) |
| Total | 375 (60.3%) | 167 (26.8%) | 80 (12.9%) | 298 (32.3%) |
Percentage values relate to proportion providing a response to the question. The far right column indicates the overall proportion not answering the question. p-value < .001, chi squared test. All tablulated values: number (percentage).
Figure 3.
Distribution of type of chemical cardioplegia as used by individual respondents by region. ANZ, Australia/New Zealand.
Blood Cardioplegia Ratio?
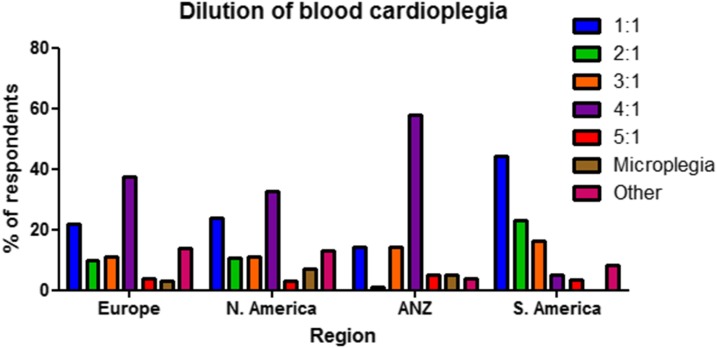
Respondents were asked to report on the dilution of blood cardioplegia used at their center (Table 5, Figure 4). There was varied practice both within and between regions. European and North American practice appeared broadly similar. Most frequent practice was a 4:1 (blood to cardioplegia) dilution with approximately one-third reporting this. Next most frequently used practice was a 1:1 dilution with a little more than 20% of responses, followed by 2:1 and 3:1 with approximately 10% of responses each in both regions.
Table 5.
Dilution of blood cardioplegia at centers where individual respondents worked.
| Blood Cardioplegia Dilution | 1:1 | 2:1 | 3:1 | 4:1 | 5:1 | Microplegia | Other | No Response |
|---|---|---|---|---|---|---|---|---|
| Europe | 36 (22.2%) | 16 (9.9%) | 18 (11.1%) | 61 (37.7%) | 6 (3.7%) | 5 (3.1%) | 20 (12.3%) | 88 (32.7%) |
| North America | 44 (24.3%) | 20 (11.0%) | 20 (11.0%) | 59 (32.6%) | 5 (2.8%) | 13 (7.3%) | 20 (11.0%) | 115 (34.4%) |
| ANZ | 12 (14.1%) | 1 (1.2%) | 12 (14.1%) | 49 (57.7%) | 4 (4.7%) | 4 (4.7%) | 3 (3.5%) | 22 (21.0%) |
| South America | 27 (44.3%) | 14 (23.0%) | 10 (16.4%) | 3 (4.9%) | 2 (3.2%) | 0 (0%) | 5 (8.2%) | 115 (53.5%) |
| Total | 119 (24.3%) | 51 (10.4%) | 60 (12.3%) | 172 (35.2%) | 17 (3.5%) | 22 (4.5%) | 48 (9.8%) | 340 (36.8%) |
Ratios are blood:crystalloid solution. Percentage values relate to proportion providing a response to the question. The far right column indicates the overall proportion not answering the question. p-value < .001, chi squared test. All tablulated values: number (percentage).
Figure 4.
Distribution of dilution of blood cardioplegia as used by individual respondents by region. ANZ, Australia/New Zealand.
Within the ANZ region there was more frequent use of less concentrated blood cardioplegia with 57.7% using 4:1 and 14.1% a 3:1 dilution. By contrast, in South America there was more frequent use of more concentrated blood cardioplegia: 44.3% using 1:1 and 23.0% a 2:1 dilution. A small proportion of respondents from Europe, North America, and ANZ describe the use of minimally diluted blood cardioplegia, (sometimes termed microplegia), with 3.1%, 7.3%, and 4.7% reporting this, respectively.
Type of Cardioplegia
Respondents were asked to report the type of cardioplegia solution used (Table 6). It is worth highlighting that the overall non-response rate to this question was significant −49.8%. There was significant variation between Europe and North America. In Europe, St. Thomas’ solution (Plegisol; Hospira Inc, Lake Forest, IL) is by far the most commonly used type (63.6%). By contrast, in North America, the University of Wisconsin solution is the most popular (34.9%) while St. Thomas’ was the second most frequent type (17.2%). Similar to Europe, in ANZ and South America, St. Thomas’ solution was the most frequently reported solution (67.1% and 56.6%, respectively). In both Europe and South America, Bretschneider solution (Custodiol; Essential Pharmaceuticals, LLC, Newton, PA) was the second most commonly reported solution (15.2% and 26.4%, respectively). In North America the use of del Nido cardioplegia (Compass; Baxter Healthcare Inc, Edison, NJ) was reported by 10.7% of respondents. This is typically used in pediatric cardiac surgery (9).
Table 6.
Type of cardioplegia solution used at centers where individual respondents worked.
| Cardioplegia Type | St. Thomas Solution | Bretschneider Solution | University of Wisconsin | Del Nido Solution | Unknown | Other | No Response |
|---|---|---|---|---|---|---|---|
| Europe | 117 (63.6%) | 28 (15.2%) | 2 (1.1%) | 0 (0%) | 2 (1.1%) | 35 (19.0%) | 116 (43.1%) |
| North America | 37 (17.2%) | 20 (9.3%) | 75 (34.9%) | 23 (10.7%) | 25 (11.6%) | 35 (16.3%) | 196 (58.7%) |
| ANZ | 55 (67.1%) | 6 (7.3%) | 3 (3.7%) | 1 (1.2%) | 2 (2.4%) | 15 (18.3%) | 40 (38.1%) |
| South America | 60 (56.6%) | 28 (26.4%) | 7 (6.6%) | 0 (0%) | 2 (1.9%) | 9 (8.5%) | 108 (50.2%) |
| Total | 269 (45.8%) | 82 (14.0%) | 87 (14.8%) | 24 (4.1%) | 31 (5.3%) | 94 (16.0%) | 460 (49.8%) |
Percentage values relate to proportion providing a response to the question. The far right column indicates the overall proportion not answering the question. p-value < .001, chi squared test. All tablulated values: number (percentage).
Additives to Cardioplegia
Respondents were asked whether any additives were routinely added to the cardioplegia used at their center (Table 7). In Europe this was rare, with only 9.2% reporting additive use. By contrast, in North America and ANZ the use of additives was reported by more than 40% of respondents. In South America, additive use was reported by 24%. Those who reported the use of additives were asked to identify the additives used (Table 8). A range of additives were reported. In North America the most frequently reported were glucose (53.3%), glutamate (25.7%), THAM (tris-hydroxymethyl aminomethane; 21.0%), and aspartate (19.1%). These were the commonest additives reported by all regions, although the proportions varied. For example, while in South America—similarly to North America—glucose was the most frequent additive, in Europe and ANZ aspartate was the most popular. A small proportion of respondents reported other additives including: esmolol, adenosine, lidocaine, magnesium, and bicarbonate.
Table 7.
Are additives added to the chemical cardioplegia solution used at the respondents center.
| Additives to Cardioplegia Solution | Additives | No Additives | No Response |
|---|---|---|---|
| Europe | 17 (9.2%) | 167 (90.8%) | 85 (31.6%) |
| North America | 89 (40.1%) | 133 (59.9%) | 112 (33.5%) |
| ANZ | 33 (40.7%) | 48 (59.3%) | 24 (22.9%) |
| South America | 25 (24.0%) | 79 (76.0%) | 111 (51.6%) |
| Total | 164 (27.8%) | 427 (72.2%) | 332 (36.0%) |
Percentage values relate to proportion providing a response to the question. The far right column indicates the overall proportion not answering the question. p-value < .001, chi squared test. All tablulated values: number (percentage).
Table 8.
Summary of additives added to cardioplegia solutions at centers where individual respondents worked.
| Additive Used | Glucose | Calcium Channel Blocker | THAM | Aspartate | Glutamate | Other | No Response |
|---|---|---|---|---|---|---|---|
| Europe | 7 (17.9%) | 3 (7.7%) | 8 (20.5%) | 9 (23.1%) | 7 (18.0%) | 6 (15.4%) | 230 (85.5%) |
| North America | 56 (53.3%) | 8 (7.6%) | 22 (21.0%) | 20 (19.1%) | 27 (25.7%) | 28 (26.7%) | 229 (68.6%) |
| ANZ | 7 (16.7%) | 1 (2.4%) | 1 (2.4%) | 23 (54.8%) | 8 (19.1%) | 9 (21.4%) | 61 (58.1%) |
| South America | 19 (40.4%) | 9 (19.1%) | 2 (4.3%) | 6 (12.8%) | 7 (14.9%) | 11 (23.4%) | 168 (78.1%) |
| Total | 89 (38.2%) | 21 (9.0%) | 33 (14.2%) | 58 (24.9%) | 49 (21.0%) | 54 (23.2%) | 688 (74.5%) |
Percentage values relate to proportion providing a response to the question. Respondents could select more than one answer. The far right column indicates the overall proportion not answering the question. p-value < .001, chi squared test. THAM, tris-hydroxymethyl aminomethane. All tablulated values: number (percentage).
DISCUSSION
We report the results of a global survey of cardiac anesthetists concerning CPB practice with representative cohorts from Europe, North America, ANZ, and South America. Questions were asked on cardioplegia practice and on anticoagulation and pump-priming practices with the aim of documenting contemporary practice. Data concerning the latter have previously been reported (8).
CPB is now well established within the practice of cardiac surgery. Myocardial protection is an essential requirement to counteract the global ischemia during aortic cross-clamp and necessary to achieve a bloodless operative field. This survey has revealed significant variation in international practice with regards to myocardial protection, likely due to a lack of clear consensus on best practice.
Chemical cardioplegia is the most frequent method of myocardial protection used in all regions. Between 9.4% and 20% of respondents in each region though reported the use of cross-clamp fibrillation on occasion. This technique of myocardial protection is usually reserved for coronary artery bypass grafting and involves repeated application of the cross-clamp and induction of ventricular fibrillation. It is thought that endogenous ischemic preconditioning occurs, protecting the myocardium (10). Several randomized controlled trials have reported equivalent outcomes with the use of cross-clamp fibrillation in terms of postoperative cardiac enzyme release and left ventricular function (11). However, aortic trauma and potential thromboemobolism from repeated applications of the aortic cross-clamp are the main concerns regarding this technique and as such it is infrequently used in the current era, as supported by this survey (10). Interestingly a small minority of respondents reported cross-clamp fibrillation as the only method of myocardial protection at their center, with four of the six such respondents reporting from South America.
Although most respondents reported the use of chemical cardioplegia, there was significant regional variation in the use of crystalloid versus blood cardioplegia. A significant body of literature has developed around answering the question of which is best: crystalloid or blood cardioplegia with many randomized controlled trials performed. Crystalloid cardioplegia has been associated with causing myocardial edema, particularly during repeated administration in long operations, which may contribute to a low cardiac output syndrome postoperatively (12). It has been shown that myocardial edema leads to ventricular wall stiffness reducing diastolic compliance and systolic elastance leading to impaired function (13). Cold blood cardioplegia was first introduced in 1978 (14) and several advantages over crystalloid cardioplegia have been proposed including: oxygen carrying capacity, inherent buffering capacity, endogenous antioxidants and oncotic properties minimising myocardial edema (15). However, it may not be this simple: for example, hypothermia will cause the oxygen-haemoglobin dissociation curve shifts to the left, and may in fact lead to less oxygen delivery with cold blood cardioplegia than oxygenated crystalloid cardioplegia (16). Several meta-analyses have been performed to summarise the literature comparing these two products, but fail to demonstrate clear superiority of either in terms of mortality or other early postoperative outcomes such as myocardial infarction and arrhythmia (4–6). In some of the primary studies, though there is benefit in favour of blood cardioplegia, particularly with regard to reducing the incidence of low cardiac output syndrome (17). Interestingly, one study followed up their patients 6 months later and found no difference in left ventricular function suggesting the impact is transient (18). The difficulty with comparing these studies is that varied cardioplegia preparations are used at different dilutions and in different patient groups. There certainly may be groups of patients for whom there are significant advantages of using blood cardioplegia, but these have not been clearly defined. As such, there is no consensus on which of these approaches are best, and this is reflected in the survey results. Nevertheless, it was interesting to see that such significant regional variation exists. In particular that bycontrast to all the other regions, in South America crystalloid cardioplegia appears to be the favored type. In the other regions, crystalloid cardioplegia use alone was only reported by between 7% and 26% of respondents, suggesting that the perceived benefits of cold blood cardioplegia have resulted in this being the favored approach. Why this should differ so much in South America is unclear but could be the subject of future investigation.
The next question concerned dilution factor of the blood cardioplegia. In addition to significant regional variation, there was also quite varied practice within each region. To generate blood cardioplegia, blood is added to the crystalloid cardioplegia solution. Proportionately, how much blood is added to the crystalloid solution determines how dilute the solution is. It is reported that there is increased risk of myocardial edema with crystalloid cardioplegia, and therefore dilute blood cardioplegia. Subsequently, there was a move by some proponents to using “microplegia,” minimally diluted whole blood cardioplegia which aims to avoid the potential disadvantages of using standard, diluted, blood cardioplegia (12,19). There have been studies, (such as that by Algarni et al.) that have shown improved cardiac function postoperatively using microplegia (20). However, a recent meta-analysis summarising the literature comparing the use of standard blood cardioplegia versus microplegia failed to demonstrate any difference in postoperative low cardiac output syndrome, perioperative myocardial infarction or postoperative rate of arrhythmia (7). Microplegia use was reported infrequently by respondents to our survey. Use was most popular in North America, but only 7.3% respondents reported using it. The proportion of crystalloid in blood cardioplegia did vary quite significantly. The most common ratio of blood:cardioplegia solution was 4:1 in Europe, North America and ANZ. However, there was more variability in Europe and North America with more dilute ratios also being frequently reported. In ANZ the majority reported using 3:1 or more concentrated. Bycontrast South American respondents described use of more concentrated cardioplegia with the majority reporting 1:1 or 2:1. It is worth noting with this observation that South American respondents also report the highest use of crystalloid cardioplegia. In view of the previous literature suggesting the risk of myocardial edema with more dilute solutions, there may be some benefit in future studies examining if there are selected patient groups who will benefit from minimising the amount of crystalloid in cardioplegia.
The final questions of the survey concerned type of cardioplegia solution and additives. A number of different preparations exist, and they are generally classified as either “extracellular” or “intracellular” based on their electrolyte constituents, primarily sodium and calcium, which are low concentration in “intracellular” type solutions (reflecting the intracellular fluid milieu). Generally “extracellular” type solutions are dosed every 20 minutes or so, whereas “intracellular” type solutions can provide satisfactory myocardial protection for up to 3 hours, although large volumes are administered (21). The commonest type of cardioplegia solution in all regions other than North America was St. Thomas’ solution, one of the oldest, which is an “extracellular” type. There much data from both animal models and clinical studies supporting St. Thomas’ solution as an effective cardioplegia solution, and it is clearly widely used throughout the world (22) In North America, there are several solutions used with high frequency, including St. Thomas’. The most commonly reported solution was University of Wisconsin solution. This is widely used as a preservation fluid for organ transplantation, and modified versions have been utilized as an effective cardioplegia solution (23). The other frequently reported solution in all regions was Bretschneider solution or Custodiol which is an “intracellular” type developed in Germany (24). There have been studies comparing St. Thomas’ solution with Bretschneider solution in both animals and humans without significant differences being demonstrated, which likely reflects the varied practice observed (21,25). Other components are sometimes added to these cardioplegia solutions as additives but the practice is variable. The commonest additives reported in this study included: glucose which is added as an additional energy source as concentration can be low particularly in “extracellular” solutions; glutamate and aspartate which have been shown to reduce hypoxic injury and act as preferential substrates by hypoxic myocardium on CPB (26); THAM which acts as an additional buffer supporting the endogenous buffering capacity of blood; and calcium channel blockers which have been found to prevent the myocardial damage associated with low calcium cardioplegia solutions after reperfusion (27).
A small number of surveys containing questions on myocardial preservation practice have been previously reported. Unlike this report, simultaneously documenting practice around the world, previous studies have described practice in a single country. The earliest to be published included a study of 984 surgeons in the United States which was published in 1995 (28). The authors reported a preference for blood cardioplegia among respondents (72.2%) over crystalloid cardioplegia (27.8%) with similar proportions identified in this study suggesting that practice has not changed over the last two decades. In this study 45.2% reported using St. Thomas solution as the crystalloid cardioplegia solution of choice, while Buckberg solution was the favored blood cardioplegia. Buckberg blood cardioplegia is based on St. Thomas solution and has similar constituents. This seems to have fallen out of favor with only 3.26% of North American respondents reporting its use in the current study. Another surveyed 118 surgeons from the UK in 2004, reporting on their universal practices (29). The authors identified that 11% of surgeons used cross-clamp fibrillation as their preferred myocardial protection strategy, which is higher than that identified for Europe in the current study, perhaps reflecting a change in practice over the last decade. For those using chemical cardioplegia the majority (83.5%) favored blood cardioplegia as is still the case in Europe. In 2009 a study from ANZ surveyed 34 perfusion leads (30). They reported a majority of centers using blood cardioplegia (77%), with 12% using cross-clamp fibrillation on occasion. Regarding dilution, just under half of respondents reported 4:1 ratio as the favored. Both of these results are very similar to the findings in the current study, validating the findings. Finally, a survey of 55 pediatric surgeons in the US was reported in 2013 (31). This study revealed a majority preference for blood cardioplegia (86%). The most common type of cardioplegia used was del Nido, which was developed for use in congenital surgery as a low volume product that can be given as a single dose. These results are not directly comparable with the current study because no distinction between adult and pediatric practice was made.
There are several limitations of this study. Some continents remain unrepresented in this survey, notably Asia and Africa. Despite contacting a number of specialist societies in Asia and distributing the survey through them, we had difficulty in achieving a reasonable response rate from this region. Specialist societies were judged as the best means of reaching a large number of cardiac anesthesiologists, rather than trying to identify and contact major cardiac centers on every continent. As larger centers employ a larger number of anesthesiologists, smaller centers may be relatively unrepresented in this survey.
The estimated response rates to the survey were relatively low, particularly for South America. However, it is important to highlight that the denominator is not accurately known for any of the regions as this is based on society membership, and in the case of South America the survey was distributed via general anesthesia societies rather than a specialist cardiothoracic society. Therefore, the true response rates are likely significantly higher.
Some questions had a high rate of failure to respond, despite respondents having elected to complete the survey. While this may reflect that the institution of the respondent does not use the practice in question, it may also reflect that the respondent may have a limited knowledge of cardioplegia practice at their center. It is noteworthy that non-response rate was particularly high in the South American respondents. Potentially related to this is the fact that the survey was administered to anesthetists rather than perfusionists. Access to international cardiothoracic anesthesia societies was the rationale, although it is acknowledged that reliability and completeness of data may have been increased if perfusionists were instead surveyed.
The low response rates, particularly to some questions, make it difficult to draw firm conclusions from the data. We have taken care to comment only on overall global results and have presented notable differences between regions, but have been cautious not to make unsupported conclusions. Statistically significant differences do not necessarily equate to clinically significant differences between regions bearing in mind the limitations outlined previously.
There are some aspects of cardioplegia practice that were not considered by the survey due to concerns regarding participant fatigue, and consequent effects on the response rate. Aspects such as the temperature of cardioplegia and whether centers perform leukocyte filtration of blood for cardioplegia for example would have provided interesting additional insights but were not examined. Additionally, details of administration such as routes used for cardioplegia delivery (antegrade, retrograde or both) and the pressure of administration would add further insight.
CONCLUSION
This work represents the first global survey of cardioplegia practice, presenting current practice in North America, Europe, ANZ and South America. The results of this survey have revealed significant regional variation in cardioplegia practices, in terms of the use of blood versus crystalloid cardioplegia, as well as in the type and dilution of cardioplegia solution and the use of additives.
The reasons why regional practice groups made the choices they have are not known and the clinical implications are uncertain. Although it is unlikely that these differences in practice will result in clinically significant mortality differences in all patients, it is possible that high-risk patients may benefit from alternative management. This lack of consensus opens the way for well-conducted, targeted, randomized studies to study the consequences of cardioplegia choices on clinical outcomes after cardiac surgery.
ACKNOWLEDGMENTS
The authors would like to extend their gratitude to Dr. Gudrun Kunst from the European Association of Cardiothoracic Anesthesiology, Dr. Paul Heerdt from the Society of Cardiovascular Anesthesiologists, Dr. Michael Fanshawe from the Australian and New Zealand College of Anesthetists, Tarryn Oschadleus and Professor Justiaan Swanevelder from the Groote Schuur Hospital in Cape Town, and Dr. Suhaini Kadiman and Professor Ruban Poopalalingam from the Asian Society of Cardiac Anesthesia for assisting with survey distribution. We would also like to thank Dr. Guillermo Martinez and Mr. Pedro Catarino for translating the survey.
APPENDIX
REFERENCES
- 1.Dennis C, Spreng DS Jr., Nelson GE, et al. Development of a pump-oxygenator to replace the heart and lungs; an apparatus applicable to human patients, and application to one case. Ann Surg. 1951;134:709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melrose DG, Dreyer B, Bentall HH, et al. Elective cardiac arrest. Lancet. 1955;269:21–2. [DOI] [PubMed] [Google Scholar]
- 3.Helmsworth JA, Kaplan S, Clark LC Jr., et al. Myocardial injury associated with asystole induced with potassium citrate. Ann Surg. 1959;149:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guru V, Omura J, Alghamdi AA, et al. Is blood superior to crystalloid cardioplegia? A meta-analysis of randomized clinical trials. Circulation. 2006;114:I331–8. [DOI] [PubMed] [Google Scholar]
- 5.Sa MP, Rueda FG, Ferraz PE, et al. Is there any difference between blood and crystalloid cardioplegia for myocardial protection during cardiac surgery? A meta-analysis of 5576 patients from 36 randomized trials. Perfusion. 2012;27:535–46. [DOI] [PubMed] [Google Scholar]
- 6.Zeng J, He W, Qu Z, et al. Cold blood versus crystalloid cardioplegia for myocardial protection in adult cardiac surgery: A meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth. 2014;28:674–81. [DOI] [PubMed] [Google Scholar]
- 7.Gong B, Ji B, Sun Y, et al. Is microplegia really superior to standard blood cardioplegia? The results from a meta-analysis. Perfusion. 2015;30:375–82. [DOI] [PubMed] [Google Scholar]
- 8.Miles LF, Coulson TG, Galhardo C, et al. Pump priming practices and anticoagulation in cardiac surgery: Results from the global cardiopulmonary bypass survey. Anesth Analg. 2017;125:1871–7. [DOI] [PubMed] [Google Scholar]
- 9.Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children’s Hospital. J Extra Corpor Technol. 2012;44:98–103. [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii M, Chambers DJ. Myocardial protection with intermittent cross-clamp fibrillation: Does preconditioning play a role? Eur J Cardiothorac Surg. 2005;28:821–31. [DOI] [PubMed] [Google Scholar]
- 11.Scarci M, Fallouh HB, Young CP, et al. Does intermittent cross-clamp fibrillation provide equivalent myocardial protection compared to cardioplegia in patients undergoing bypass graft revascularisation? Interact Cardiovasc Thorac Surg. 2009;9:872–8. [DOI] [PubMed] [Google Scholar]
- 12.Menasche P. Blood cardioplegia: Do we still need to dilute? Ann Thorac Surg. 1996;62:957–60. [DOI] [PubMed] [Google Scholar]
- 13.Hsu DT, Weng ZC, Nicolosi AC, et al. Quantitative effects of myocardial edema on the left ventricular pressure-volume relation. Influence of cardioplegia osmolarity over two hours of ischemic arrest. J Thorac Cardiovasc Surg. 1993;106:651–7. [PubMed] [Google Scholar]
- 14.Rosenkranz ER, Buckberg GD. Myocardial protection during surgical coronary reperfusion. J Am Coll Cardiol. 1983;1:1235–46. [DOI] [PubMed] [Google Scholar]
- 15.Rosenkranz ER, Buckberg GD. Advantages of blood cardioplegic solutions. Ann Chir Gynaecol. 1987;76:30–8. [PubMed] [Google Scholar]
- 16.Baer RW. Myocardial oxygen transport during leftward shifts of the oxygen dissociation curve by carbamylation or hypothermia. Adv Exp Med Biol. 1989;248:325–33. [DOI] [PubMed] [Google Scholar]
- 17.Sirlak M, Eryilmaz S, Yazicioğlu L, et al. Conduction disturbances in coronary artery bypass surgery. Int J Cardiol. 2003;92:43–8. [DOI] [PubMed] [Google Scholar]
- 18.Mullen JC, Christakis GT, Weisel RD, et al. Late postoperative ventricular function after blood and crystalloid cardioplegia. Circulation. 1986;74:III89–98. [PubMed] [Google Scholar]
- 19.Vinten-Johansen J. Whole blood cardioplegia: Do we still need to dilute? J Extra Corpor Technol. 2016;48:P9–14. [PMC free article] [PubMed] [Google Scholar]
- 20.Algarni KD, Weisel RD, Caldarone CA, et al. Microplegia during coronary artery bypass grafting was associated with less low cardiac output syndrome: A propensity-matched comparison. Ann Thorac Surg. 2013;95:1532–8. [DOI] [PubMed] [Google Scholar]
- 21.Korun O, Ozkan M, Terzi A, et al. The comparison of the effects of Bretschneider’s histidine-tryptophan-ketoglutarate and conventional crystalloid cardioplegia on pediatric myocardium at tissue level. Artif Organs. 2013;37:76–81. [DOI] [PubMed] [Google Scholar]
- 22.Robinson LA, Braimbridge MV, Hearse DJ. Comparison of the protective properties of four clinical crystalloid cardioplegic solutions in the rat heart. Ann Thorac Surg. 1984;38:268–74. [DOI] [PubMed] [Google Scholar]
- 23.Uesaka T, Chiba Y, Ihaya A, et al. Low-potassium University of Wisconsin solution for cardioplegia: Improved protection of the isolated ischemic neonatal rabbit heart. Cardiovasc Surg. 1999;7:723–9. [DOI] [PubMed] [Google Scholar]
- 24.Bretschneider HJ, Hubner G, Knoll D, et al. Myocardial resistance and tolerance to ischemia: Physiological and biochemical basis. J Cardiovasc Surg (Torino). 1975;16:241–60. [PubMed] [Google Scholar]
- 25.Aarsaether E, Stenberg TA, Jakobsen O, et al. Mechanoenergetic function and troponin T release following cardioplegic arrest induced by St Thomas’ and histidine-tryptophan-ketoglutarate cardioplegia–an experimental comparative study in pigs. Interact Cardiovasc Thorac Surg. 2009;9:635–9. [DOI] [PubMed] [Google Scholar]
- 26.Arsenian M. Potential cardiovascular applications of glutamate, aspartate, and other amino acids. Clin Cardiol. 1998;21:620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira MA, Brandi AC, Dos Santos CA, et al. The calcium paradox—What should we have to fear? Rev Bras Cir Cardiovasc. 2014;29:249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson LA, Schwarz GD, Goddard DB, et al. Myocardial protection for acquired heart disease surgery: Results of a national survey. Ann Thorac Surg. 1995;59:361–72. [DOI] [PubMed] [Google Scholar]
- 29.Karthik S, Grayson AD, Oo AY, et al. A survey of current myocardial protection practices during coronary artery bypass grafting. Ann R Coll Surg Engl. 2004;86:413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuble SC, Willcox TW, Baker RA, et al. Australian and New Zealand perfusion survey: Management and procedure. J Extra Corpor Technol. 2009;41:64–72. [PMC free article] [PubMed] [Google Scholar]
- 31.Kotani Y, Tweddell J, Gruber P, et al. Current cardioplegia practice in pediatric cardiac surgery: A North American multiinstitutional survey. Ann Thorac Surg. 2013;96:923–9. [DOI] [PubMed] [Google Scholar]