Abstract
Respiratory syncytial virus (RSV) is associated with enhanced progression of chronic obstructive pulmonary disease (COPD) and COPD exacerbations. However, little is known about the role of IL-17 in RSV-induced lung injury. We first investigated the role of RSV infection in enhancing mucous cell hyperplasia (MCH) and airspace enlargement in the lungs of mice injured with elastase and LPS (E/LPS). Mice injured with E/LPS had an enhanced and prolonged neutrophilic response to RSV that was associated with decreased levels of type I IFN and increased levels of IL-17, IL-23, CXCL-1, granulocyte colony stimulating factor (GCSF), CXCL-5, and matrix metalloproteinase (MMP)-9. In addition, extent of MCH and mean weighted alveolar space were increased significantly in the lungs of E/LPS-injured mice infected with RSV compared with E/LPS-only or RSV-only controls. Interestingly, immunodepletion of IL-17 before viral infection diminished the RSV-driven MCH and airspace enlargement in the E/LPS-injured animals, suggesting that IL-17 may be a therapeutic target for MCH and airspace enlargement when enhanced by RSV infection.
Keywords: respiratory syncytial virus, LPS, elastase, IL-17, emphysema
Clinical Relevance
IL-17 has previously been linked to pulmonary inflammation, but little is known about its role in respiratory syncytial virus (RSV)-induced lung injury and the development of emphysema. IL-17 plays a key role in RSV-enhanced airway inflammation and emphysematous changes in the lungs of elastase and LPS-injured mice.
Chronic obstructive pulmonary disease (COPD) that is characterized by emphysema and chronic bronchitis is the third leading cause of death in the United States (1), but the risk factors for causing COPD are not fully understood. Exposure to cigarette smoke (CS) accounts for roughly 80% of all COPD cases (2), and other factors when combined with CS play a role in exacerbating CS-induced COPD (3). Environmental pollutants that may injure the lung, including wood smoke (4) and diesel exhaust (5), play a role in enhancing CS-induced inflammation. In addition, bacterial and viral infections are major causes for COPD exacerbations (6) that lead to rapid decline in lung function and concomitant deterioration of respiratory health (7).
Respiratory viral infections can induce sudden decline in clinical well-being of individuals with COPD that are otherwise relatively stable. As many as 40–60% of all exacerbations are attributed to respiratory viral infections alone (8), with rhinoviruses and respiratory syncytial virus (RSV) being the main responsible viruses (6). Although RSV accounts for a quarter of all virally induced COPD exacerbations (6), the mechanisms by which it causes worsening of this disease are not completely understood.
T helper (Th) 17 cells produce IL-17 and IL-22, and boost inflammation (9) by upregulating proinflammatory cytokines and chemokines (10). Expression of IL-17 is increased in human bronchial submucosa in patients with COPD (11), and genetic deletion of IL-17 attenuates CS-induced inflammation and alveolar type 2 apoptosis in mice (12). Furthermore, IL-17 increases mucus production by inducing mucin gene expression in the airway epithelium (13). Current antiinflammatory therapies are weakly effective in maintaining lung function and controlling symptoms of COPD (14). We recently found that, when mice are exposed to CS and infected with RSV, enhanced inflammation and extent of emphysema are accompanied by increased IL-17 (15). Therefore, in the present study, we investigated whether inhibition of IL-17 may dampen RSV-induced COPD exacerbation.
Viral infection is associated with acute exacerbations of COPD, which, in turn, accelerate disease progression. LPS is a major proinflammatory component of gram-negative bacteria that is present in CS in appreciable amounts (16), and mice exposed to elastase and LPS (E/LPS) once a week for 4 weeks show features of COPD, such as airway inflammation and obstruction, mucous cell hyperplasia (MCH), increased total lung volume, and increased alveolar space (17). In mice, at least 4 months of exposure to CS is required to observe the initial pathological features of COPD, and MCH is not evident even after prolonged exposure (18). Therefore, we used the E/LPS mouse model in combination with infection with RSV to induce a rapid onset of inflammation with pronounced MCH and emphysema (19). We show that RSV infection of E/LPS-injured mice display exaggerated cytokine/cellular inflammatory responses and increased MCH, and emphysematous-like changes in the lung tissues. Furthermore, we report that depletion of IL-17 diminishes RSV-driven MCH and airspace enlargement.
Methods
Animals
Female C57BL/6 mice (6–8 wk old) were purchased from Jackson Laboratories and housed in pathogen-free facility. All animal experimental procedures were approved by the Lovelace Respiratory Research Institute Animal Care and Use Committee.
RSV Propagation
Clinical isolate of RSV, RSV NM232, was obtained from the New Mexico Department of Health. The virus was plaque purified under agarose, inoculated into a subconfluent HEp-2 cell monolayer, and propagated as described in the data supplement.
Elastase and LPS Exposure, and Infection of Mice with RSV
Exposure of mice to E/LPS and infection with RSV were conducted as described in the data supplement and Figure 1.
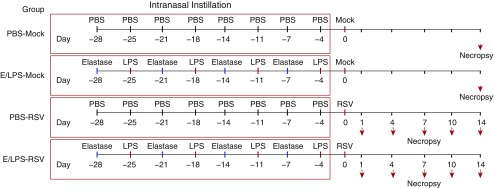
Figure 1.
Schematic diagram of the study design for elastase and LPS (E/LPS) exposure and infection with respiratory syncytial virus (RSV). Mice were intranasally instilled with 1.2 U of elastase on Day 1 followed by 7 μg of LPS on Day 4; this treatment regimen was performed for 4 consecutive weeks. Mice repeatedly treated with PBS instead of E/LPS were used as controls. At 3 days after the last LPS or PBS instillation, mice were inoculated with RSV (106 pfu) or vehicle (Mock) by intranasal instillation, and lung tissues were analyzed on Days 1, 4, 7, 10, and 14 after infection. Lung tissues for the mock-infected groups were analyzed on Day 14 postinfection. Arrows denote necropsy of mice.
Lung Inflammatory Cells
Mice were killed by lethal injection of pentobarbital sodium followed by exsanguination. Lung immune cells were recovered by BAL. Total cells and immune cell types in the BAL fluid (BALF) were quantified after staining cytospins using Diff-quik kit (Siemens Healthcare Diagnostics), per the manufacturer’s directions.
Cytokine and Chemokine Analysis
After removing cells, BALF samples were assayed for IL-17, CCL-5 (RANTES), CXCL-1 keratinocyte chemoattractant (KC), CXCL-2, granulocyte colony stimulating factor (GCSF), eotaxin, IL-1α, IL-1β, and IL-6 using the Millplex Bead ELISA platform (Millipore), per the manufacturer’s instructions. BALF levels of IFN-α, and IFN-β were determined by standard ELISA kit specific to mouse, but not to individual IFN-α levels, per the manufacturer’s recommendations (R&D Systems).
qRT-PCR
qRT-PCR was performed following the manufacturer’s instructions (Applied Biosystems) to measure mRNA levels of IL-23, CCL-2, CXCL-5, CCL-20, MMP-9, and GAPDH in lung tissue samples from mock- and RSV-infected, PBS- and E/LPS-treated mice at 1, 4, 7, 10, and 14 days postinfection. Details of the qRT-PCR analyses with primers and probes used to evaluate viral NS1 RNA are provided in the data supplement.
Lung Histology and Morphometry
To analyze inflammation-associated lung damage and measure the alveolar volume, hematoxylin and eosin–stained lung tissues were evaluated in a blinded manner using Visiomorph analysis software (Visiopharm) by a person unaware of slide identity. Details are provided in the data supplement.
Immunofluorescent Staining
Mouse lung tissues were analyzed for RSV positivity as described in the data supplement.
Immunodepletion of IL-17
Depletion of IL-17 was accomplished by a single intraperitoneal injection of an anti-mouse IL-17 antibody (50 μg/mouse, clone 50104; R&D Systems) immediately before infection with RSV. Control mice were injected with a nonspecific antibody (IgG2A, clone 54447; R&D Systems). The IL-17–neutralizing antibody is specific to IL-17A. The neutralizing activity of the anti–IL-17 antibody in vitro was determined by measuring its ability to neutralize IL-17–induced IL-6 secretion (20).
Statistical Analysis
Data were analyzed and graphed using GraphPad Prism version 5.04. When significance was detected, Bonferroni’s multiple comparison tests were used to determine differences between groups. Unpaired Student’s two-tailed t test was used to compare two groups. Data are presented as mean (±SEM) for at least six mice per group. Probability values less than 0.05 were considered significant.
Results
RSV Infection Increases Inflammatory Cell Recruitment in E/LPS-injured Lung
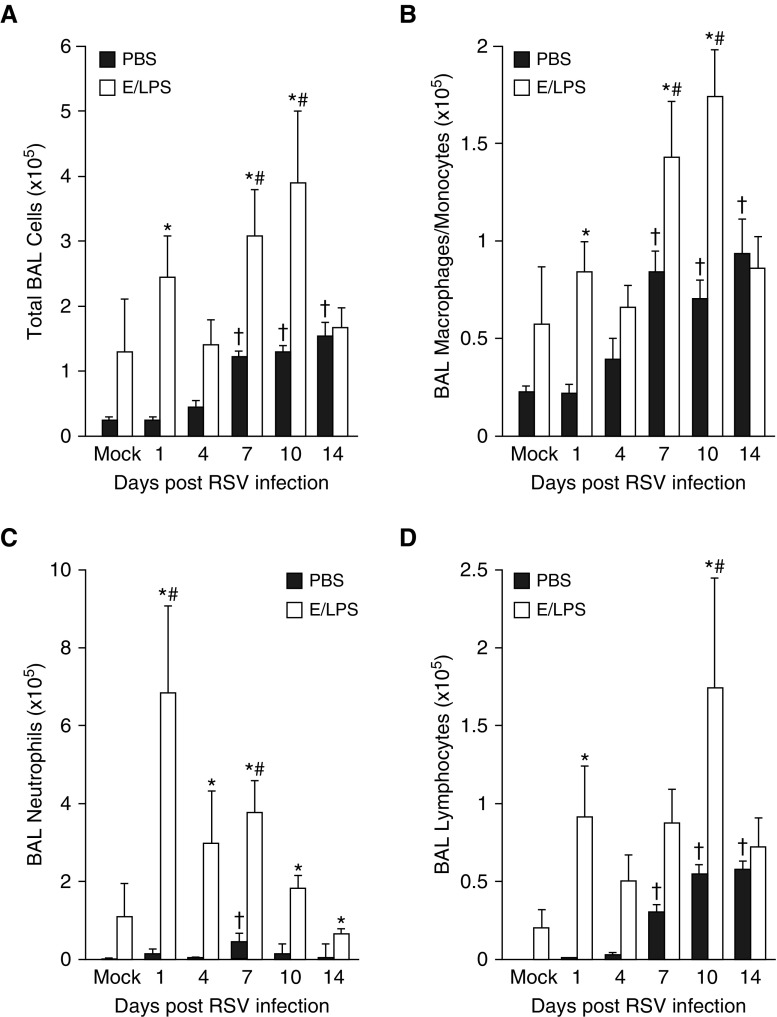
To investigate whether RSV causes significant increases in inflammatory cells in E/LPS-injured mouse lung, we analyzed the number of inflammatory cells in the BALF of mice infected with RSV in the presence or absence of E/LPS injury. RSV infection increased the total BALF cell number in both the E/LPS-treated and PBS-treated mice; however, on Days 1, 7, and 10 postinfection, total BAL cells were increased in the E/LPS-treated mice compared with the PBS-treated mice (Figure 2A). BALF macrophages were significantly increased on Days 1, 7, and 10 after RSV infection in the E/LPS-injured lung compared with the PBS control lung (Figure 2B). However, the number of neutrophils was significantly increased by RSV throughout all time points (Figure 2C). The number of lymphocytes was increased on Days 1 and 10 postinfection (Figure 2D). These data show that RSV infection enhanced cellular inflammation, characterized by macrophages, neutrophils, and lymphocytes in E/LPS-injured lungs.
Figure 2.
E/LPS lung injury alters inflammatory cell recruitment in response to RSV. BAL was performed on PBS + Mock–, E/LPS + Mock–, PBS + RSV–, and E/LPS + RSV–treated mice at Days 0, 1, 4, 7, 10, and 14 after RSV infection. (A) Total cell counts and differential analysis for (B) macrophages, (C) neutrophils, and (D) lymphocytes were performed on isolated BAL cells. E/LPS-treated mice show increased total BAL cells, macrophages, neutrophils, and lymphocytes in response to RSV when compared with PBS-treated mice; n = 6 mice per group; data reported as mean (±SEM); †P < 0.05, different from PBS + mock–infected group; #P < 0.05, different from E/LPS + mock–infected group; *P < 0.05, different from PBS group.
RSV Infection Enhances Cytokine Levels in E/LPS-injured Lungs
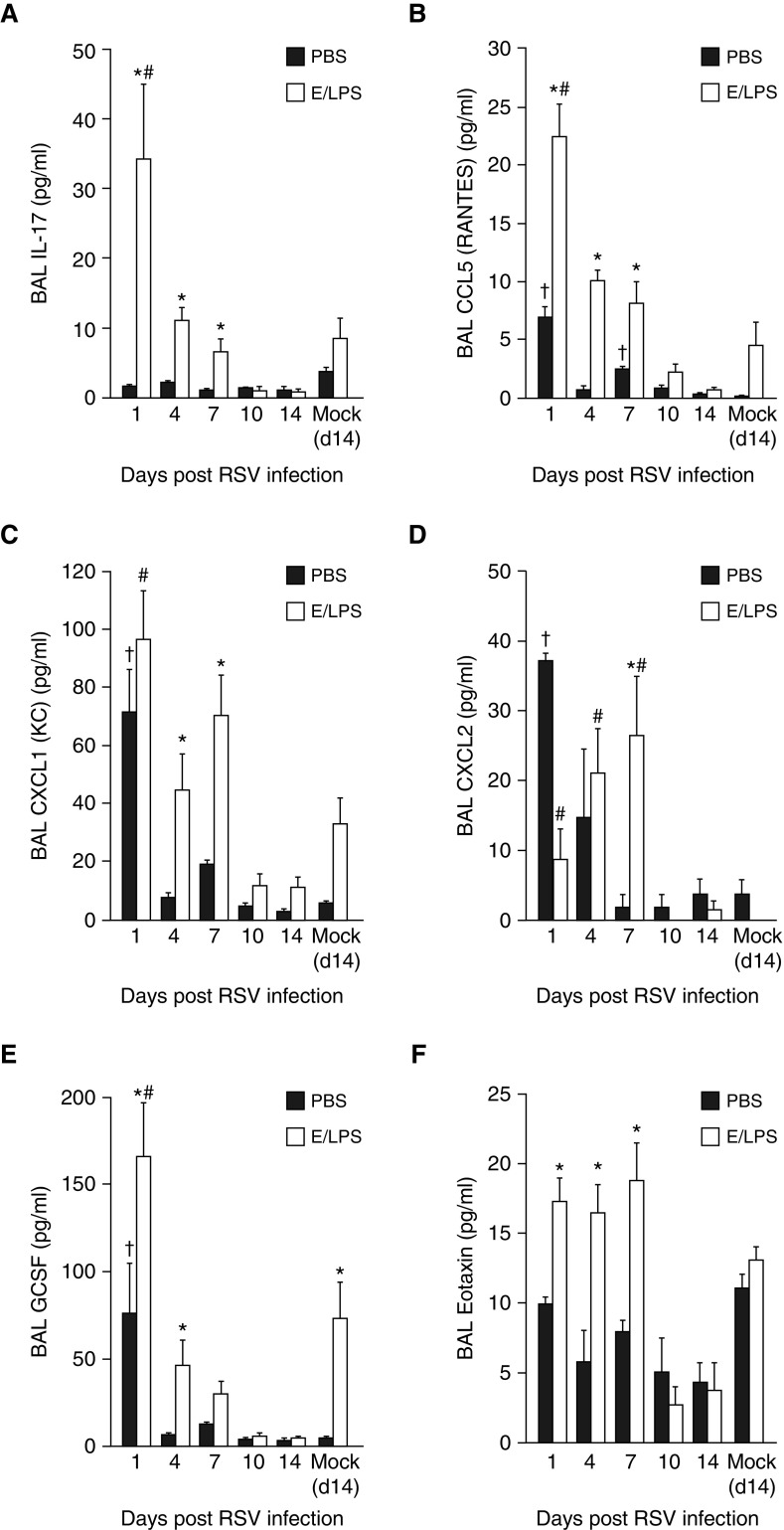
Analysis for inflammatory cytokines showed that RSV infection alone did not induce an IL-17 response in the PBS-treated mice, but significantly increased IL-17 levels in E/LPS-injured mice that peaked on Day 1 postinfection, and gradually decreased over 7 days after RSV infection (Figure 3A). In addition, compared with the PBS-treated mice, the level of CCL-5 (Figure 3B) was elevated by RSV infection of E/LPS-treated mice. Although when compared with PBS-treated groups, CXCL-1 was increased by E/LPS both in the absence and presence of RSV infection (Figure 3C), the level CXCL-2 was reduced in E/LPS groups, both in the absence RSV and on Day 1 of RSV infection. However, on Days 4 and 7 after RSV infection, CXCL-2 levels increased significantly by E/LPS compared with PBS instillation (Figure 3D). In addition, RSV also increased the levels of GCSF (Figure 3E) and eotaxin (Figure 3F) starting on Day 1 through Day 7 after RSV infection.
Figure 3.
E/LPS lung injury alters the BAL inflammatory mediator response to RSV. Luminex-based bead ELISA analysis for inflammatory cytokines and chemokines was performed on BAL fluid from PBS + Mock–, E/LPS + Mock–, PBS + RSV–, and E/LPS + RSV–infected mice at Days 0, 1, 4, 7, 10, and 14 postinfection. RSV infection in the E/LPS-injured mice increased the BAL levels of (A) IL-17, (B) CCL-5, (C) CXCL1, (D) CXCL2, (E) granulocyte colony stimulating factor (GCSF), and (F) eotaxin when compared with PBS-treated control mice; n = 6 mice per group; data reported as mean (±SEM); †P < 0.05, different from PBS + mock–infected group; #P < 0.05, different from E/LPS + mock–infected group; *P < 0.05, different from PBS group.
RSV Infection of E/LPS-injured Lungs Increases the IL-17 Signaling Cascade
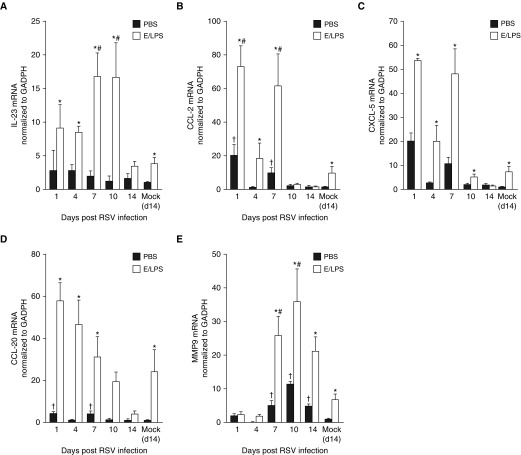
IL-23 is produced by macrophages and dendritic cells (DCs), and is a potent inducer of IL-17 expression in CD4+ T cells, CD8+ T cells, γδ T cells, natural killer (NK) cells, and neutrophils (21). Consistent with findings for IL-17 in the BALF, lung Il-23 mRNA levels remained unchanged in PBS-treated mice. but were elevated two- to fourfold on Days 1, 4, 7, and 10 after RSV infection of E/LPS-treated mice (Figure 4A). However, no significant changes were detected for IL-1α and IL-6 (Figure E1).
Figure 4.
E/LPS injury alters expression of upstream and downstream components of IL-17 pathway. TaqMan qPCR analysis for (A) IL-23, (B) CCL-2, (C) CXCL-5, (D) CCL-20, and (E) matrix metalloproteinase (MMP)-9 mRNA was performed on whole-lung RNA isolated from PBS + Mock–, E/LPS + Mock–, PBS + respiratory syncytial virus (RSV)–, and E/LPS + RSV–infected mice at Days 1, 4, 7, 10, and 14 postinfection. RSV infection in the E/LPS-injured mice significantly increased the levels on IL-23, CCL-2, CCL-20, and MMP-9 when compared with PBS-treated control mice; n = 6 mice per group; data reported as mean (±SEM); †P < 0.05, different from PBS + mock–infected group; #P < 0.05, different from E/LPS + mock–infected group; *P < 0.05, different from PBS group.
IL-17 induces expression of inflammatory cytokines and matrix-degrading proteinases, including CCL-2, CCL-20, and matrix metalloproteinase (MMP)-9 (22). We found that lung CCL-2 (Figure 4B), CXCL-5 (Figure 4C), and CCL-20 (Figure 4D) mRNA levels were elevated rapidly in response to RSV infection that persisted over 14 days after infection. MMP-9 mRNA expression was significantly elevated on Days 7, 10, and 14 after RSV infection in E/LPS-treated mice (Figure 4E).
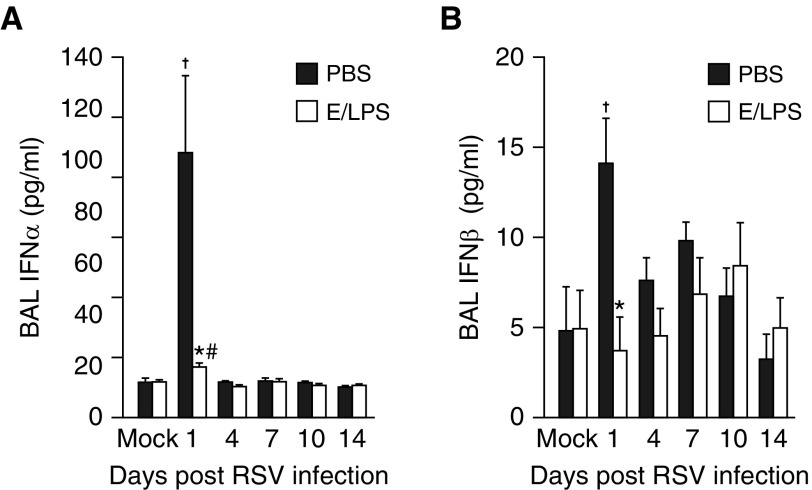
E/LPS Injury Impairs RSV-Dependent Induction of Type I IFN, but Not Viral Clearance
Analysis of BALF using ELISA showed that the level of IFN-α (Figure 5A) and IFN-β (Figure 5B) were significantly increased by RSV infection in PBS-treated mice only on Day 1 postinfection. In contrast, in the E/LPS-injured lungs IFN-α and IFN-β remained low throughout the 14 days after RSV infection. However, the viral titer remained high over 7 days postinfection and was cleared by 10 days postinfection in both PBS- and E/LPS-treated mice (Figure E2). No virus was detected in noninfected mice.
Figure 5.
E/LPS injury impairs RSV–dependent induction of type I IFN. ELISA analysis for IFN-α and IFN-β was performed on BAL fluid from PBS + Mock–, E/LPS + Mock–, PBS + RSV–, and E/LPS + RSV–infected mice at Days 0, 1, 4, 7, 10, and 14 postinfection. RSV infection in the E/LPS-injured mice significantly attenuated the BAL levels of (A) IFN-α and (B) IFN-β when compared with PBS-treated control mice; n = 6 mice per group; data reported as mean (±SEM); †P < 0.05, different from PBS + mock–infected group; #P < 0.05, different from E/LPS + mock–infected group; *P < 0.05, different from PBS group.
RSV increases the number of mucus-positive cells in the airways of E/LPS-injured mice
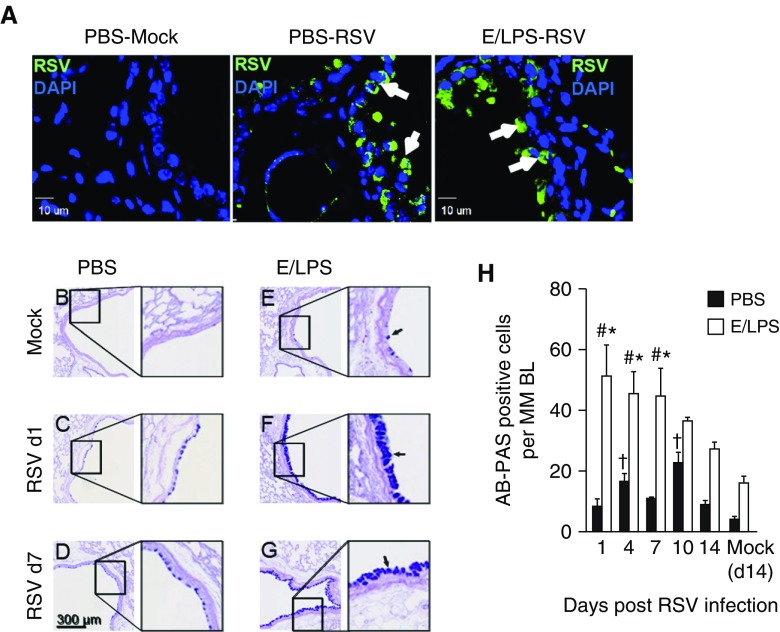
MCH in the bronchiolar airway epithelium is a common feature in patients with COPD with exacerbation caused by pathogen infection (23). Consistent with previous reports (24), immunostaining of lung tissues showed that RSV was detected mainly in the airways of infected mice (Figure 6A). In PBS-treated, noninjured lungs, RSV induced a significant increase in Alcian blue and periodic acid–Schiff–positive mucus-producing epithelial cells in the large airways (Figure 6B vs. Figure 6C). E/LPS lung injury alone modestly increased the number of mucus-producing cells when compared with PBS-treated mice (Figure 6B vs. Figure 6E). RSV infection synergistically increased the number of Alcian blue and periodic acid–Schiff–positive epithelial cells in the large airways of E/LPS-injured lungs (Figures 6F and 6G vs. Figures 6C–6E). This increase peaked at Day 1 after RSV infection and persisted throughout Day 7, and declined by Days 10 and 14 postinfection (Figure 6H). RSV caused increases in perivascular edema in the lungs of infected mice. However, the RSV-induced perivascular edema does not appear to be increased further in E/LPS-injured lungs (data not shown).
Figure 6.
RSV infection increases the number of mucus-producing cells in the airways of E/LPS–injured mice. (A) Immunostaining of lung tissues from mock- or RSV-infected mice on Day 4 postinfection. White arrows show RSV-positive cells. Scale bars: 10 μm. (B–H) Lung sections were stained with Alcian blue and periodic acid–Schiff (AB-PAS) reagent to identify airway cells containing acidic and neutral mucins (B) PBS + Mock–treated mice had very few AB-PAS–positive airway cells. (C and D) RSV infection of the PBS-treated mice caused transient increase in AB-PAS–positive cells in large airways; scale bar: 300 μm. (E) E/LPS + Mock–treated mice had increased AB-PAS–positive cells in airways. (F and G) RSV infection induced a robust increase in large and small airways of E/LPS-treated mice. Images are representative of lungs from six different mice per group. Black arrows in E, F, and G represent AB-PAS positive cells. (H) The number of AB-PAS–positive airway cells per millimeter basement membrane was quantified from three measurements made on all primary and secondary airways from five lung lobes; n = 6 mice per group; data reported as mean (±SD); †P < 0.05, different from PBS + Mock–infected group; #P < 0.05, different from E/LPS + Mock–infected group; *P < 0.05, different from PBS group.
RSV Infection Enhances Airspace Enlargements Caused by E/LPS Injury
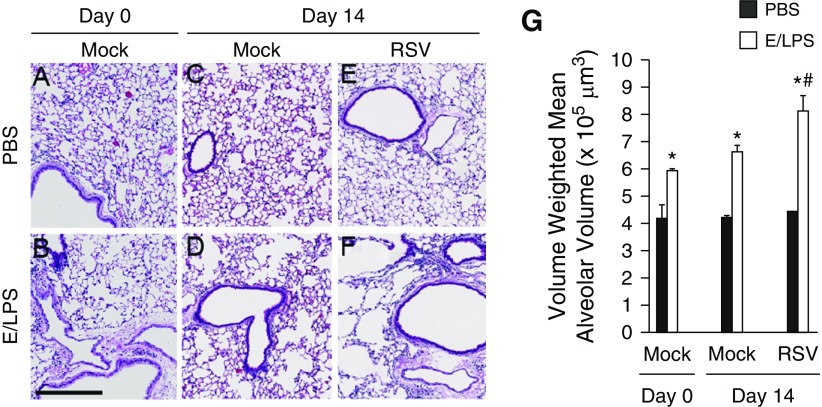
E/LPS alone caused persistent inflammation over a period of 28 days before RSV infection, resulting in increased alveolar space (Figures 7B and 7G). In the absence of RSV infection, at Day 14 after mock infection, E/LPS injury alone caused a 50% increase in volume-weighted mean alveolar volume compared with PBS-treated control mice (Figures 7C, 7D, and 7G). RSV infection alone did not cause a significant increase in alveolar destruction (Figures 7E and 7G). However, RSV combined with E/LPS caused significant increases in the volume-weighted mean alveolar volume on Day 14 after RSV infection (Figures 7F and 7G). These data suggest that, although RSV has little effect on the parenchymal structure of noninjured lungs, it significantly enhances the alveolar destruction after repeated exposure to E/LPS.
Figure 7.
RSV infection worsens emphysematous changes in E/LPS–injured mice. Lungs of (A) PBS + Mock and (B) E/LPS + Mock at Day 0, and (C) PBS + Mock–, (D) E/LPS + Mock–, (E) PBS + RSV–, and (F) E/LPS + RSV–treated mice at Day 14 were inflated to a constant pressure, paraffin embedded, sectioned, and stained with hematoxylin and eosin. Lungs from the PBS + Mock or PBS + RSV group (Day 14 postinfection) showed normal alveolar morphology. In contrast, lungs from E/LPS + Mock or E/LPS + RSV groups (Day 14 postinfection) had mild to moderate emphysema. Images are representative of lungs from eight different mice per group. (G) The volume-weighted mean alveolar volume was calculated from measurements made on at least 25 random fields per slide. Lungs from E/LPS + Mock–treated mice had increased volume-weighted mean alveolar volume compared with PBS + Mock–treated mice. RSV infection of the E/LPS-treated mice increased volume-weighted mean alveolar volume when compared with E/LPS + PBS–treated mice; n = 8 mice per group; data reported as mean (±SD); *P < 0.05, different from PBS group; #P < 0.05, different from E/LPS + PBS group; scale bar: 250 μm.
Immunodepletion of IL-17 Partially Inhibits RSV-induced MCH and Emphysema in E/LPS-Injured Mice
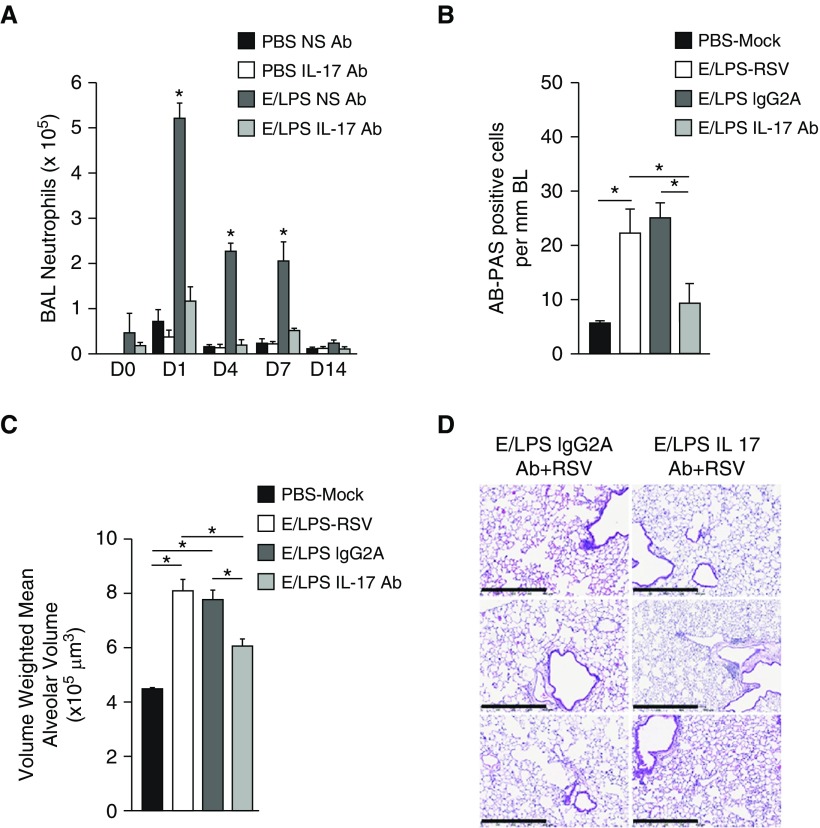
Patients with COPD have increased levels of IL-17 (11), and the IL-17 pathway is essential for the development of emphysema from long-term CS exposure in mice (22), and is increased in the lungs of RSV-infected mice exposed to CS (15). Therefore, we investigated the role of IL-17 in the enhanced response to RSV of the E/LPS-injured lung. Because RSV caused significant increases in both MCH and emphysema in E/LPS-injured lungs at Day 7 postinfection, we injected mice intraperitoneally with anti–IL-17 antibodies or IgG2A nonspecific antibody immediately before RSV infection, and analyzed lung tissues 7 days after RSV infection. Anti–IL-17 treatment significantly reduced BAL neutrophil levels (Figure 8A). Treatment with anti–IL-17 caused a significant reduction in MCH in E/LPS-injured mice infected with RSV compared with IgG2A-injected controls (Figure 8B). In E/LPS-treated mice, RSV caused maximum increases in volume-weighted mean alveolar volume at Day 14 (Figure 7E). Therefore, we tested whether treatment with anti–IL-17 reduces E/LPS–RSV–induced emphysema 14 days postinfection. We found that, compared with the control antibody, anti–IL-17 antibody reduced emphysema by 50% in the E/LPS-treated mice (Figures 8C and 8D). Together, these results suggest that IL-17 plays a role in the viral exacerbation of MCH and airspace enlargement in the E/LPS-injured lungs.
Figure 8.
Antibody (Ab)-mediated depletion of IL-17 protects against RSV-mediated injury in E/LPS-treated mice. (A) BAL neutrophil numbers in the lungs of PBS NS Ab–, PBS–IL-17 Ab–, E/LPS NS Ab–, and E/LPS IL-17 Ab–treated groups. Lungs of PBS + Mock, E/LPS + RSV, E/LPS + RSV + IgG2A Ab, or E/LPS + RSV + IL-17 Ab groups were inflated to a constant pressure at Day 14 postinfection. (B) AB-PAS– or (C) hematoxylin and eosin (H&E)–stained lung tissues were analyzed for mucous cell positivity and emphysematous changes. (B) The number of AB-PAS–positive airway cells per millimeter basement membrane was quantified from three measurements made on all primary and secondary airways from five lung lobes. (C) The volume-weighted mean alveolar volume was calculated from measurements made on at least 25 random fields per slide. (D) Representative (images from three independent animals per group) H&E-stained lung sections from mice injured with E/LPS and RSV and treated with NS Ab or IL-17 Ab; n = 8 mice per group; data reported as mean (±SD). *P < 0.05. Scale bars: 250 μm. NS = nonspecific.
Discussion
The present study shows that RSV infection in the E/LPS-injured lung enhances and prolongs cellular inflammatory responses, elevates levels of IL-17, IL-23, CXCL-1, GCSF, and CCL-5, and synergistically increases MCH and emphysema. Furthermore, depletion of IL-17 in vivo diminished RSV-driven MCH and airspace enlargement.
The mouse model we used in the current study recapitulated essential aspects of COPD exacerbations seen in humans infected with viruses. RSV infection of E/LPS-injured mice resulted in robust mucous cell metaplasia and emphysema. RSV was detected mainly in the airways of both E/LPS-treated and PBS-treated mice. These findings suggest that emphysema and MCM may not directly be caused by RSV itself, but by the ensuing increases in inflammatory responses. In contrast, mice exposed to CS for 6 months show emphysematous lesions without evidence of mucous cell metaplasia (3).
RSV infection caused significant increases in the total BALF cell numbers at Days 1, 7, and 10 postinfection in E/LPS-treated than PBS-treated mice. Furthermore, lung injury with E/LPS caused a robust neutrophil, lymphocytic, and macrophage recruitment to the lung in response to RSV. We found that RSV infection superimposed on E/LPS-injured lungs leads to a more vigorous cellular influx, with drastic increases in the proportion of neutrophils and lymphocytes as compared with noninjured lungs. Despite enhanced inflammation by RSV in E/LPS-injured lungs, the RSV-induced perivascular edema did not appear to increase further by RSV in the lungs of E/LPS-injured mice (data not shown). We have recently shown that RSV increases the level of neutrophils and lymphocytes in the lungs of CS-exposed mice (15). T lymphocytes, particularly CD8+ T cells, have the capacity to regulate macrophage activation and accumulation (25) and produce cytokines that may contribute to emphysema (26). The recruitment of neutrophils and lymphocytes is required for IL-17–mediated airway inflammation and modulation of lung and airway structural cells, which are associated with COPD progression in humans (22). Neutrophils and macrophages are the main sources of proteases, such as MMP-12 and -9 in lungs (27), which are involved in causing lung damage and development of emphysema (28). The recruitment of macrophages in the lung could also contribute to the alveolar epithelial cell apoptosis (29). Macrophages are also involved in the secretion of IL-23, which plays a major role in inflammation, including the induction of Th17 cells (30). Thus, the RSV-enhanced increases in neutrophil, lymphocytes, and macrophages in E/LPS-injured lungs, may be the main drivers of IL-17 and IL-23 levels and emphysematous changes. Interestingly, RSV alone also caused modest increases in the levels of neutrophilic inflammation and substantial increases in macrophages and lymphocytes in BALF without inducing IL-17 production. Inflammation characterized by these cells and molecules independent of IL-17 may account for the airspace enlargement that was not inhibited by IL-17 antibodies.
We found that increased RSV-related pathology in the E/LPS mice correlated with elevated level of inflammatory cells and IL-17–related cytokines and chemokines in the lungs of mice. RSV infection induced production of IL-17 only in the lungs of E/LPS-injured mice. In addition, our previous data using a CS exposure model showed similar increases in IL-17 by RSV infection (15). IL-17 acts on airway epithelial cells, smooth muscle cells, and airway fibroblasts to release neutrophil chemoattractant, including CXCL-8 (31). The higher lymphocyte counts and higher IL-17 levels in the BALF of injured animals may account for the rise in IL-23 mRNA levels, as IL-23 mRNA was induced more robustly in the lungs of E/LPS-injured mice compared with uninjured controls.
Depending on the balance of cytokines present during their differentiation, naive CD4 T cells can mature in three cell types of helper T cells—Th1, Th2, and Th17—distinguished by the profile of cytokines they produce. Transforming growth factor-β1, IL-1β, IL-6, and IL-23 have been implicated in the differentiation of Th17 cells, and IL-23 in particular has been shown to be a potent inducer of IL-17, not only in CD4 T cells, but in CD8 T cells, γδ T cells, natural killer (NK) cells, and neutrophils (32). We found that the IL-23 mRNA was induced much more strongly by RSV in the lungs of E/LPS-injured mice as compared with uninjured mice, consistent with the higher lymphocyte counts and higher IL-17 levels in the BALF of injured animals. The main source of IL-23 is antigen-presenting cells responding to an infectious agent, and production of IL-23 is inhibited by type I IFN in a signal transducer and activator of transcription factor-1 (STAT1)-dependent manner (33). We found that both IFN-α and IFN-β levels in BALF were not increased in response to RSV in E/LPS-injured mice, whereas both were robustly induced in PBS-treated controls; similar lack of type I IFN upregulation was reported for rhinovirus RV1b infection in E/LPS-injured lungs (19). Toll-like receptor (TLR) 7, a pattern-recognition receptor expressed by antigen-presenting cells, activates the expression of IFN-α/β in a myeloid differentiation primary response 88 (MyD88)- and interferon regulatory factor-7 (IRF7)-dependent manner (34). The TLR7 response is important in RSV infection, because infection of TLR7−/− mice induced an IL-23–dominated response that resulted in increased IL-17 and airway mucus production (35). Therefore, a failure of the E/LPS-injured lung epithelium and/or macrophages to respond to RSV infection by upregulating type I IFN production may lead to exaggerated expression of IL-23 by antigen-presenting cells with subsequent increases in IL-17 levels.
LPS causes enlarged alveolar spaces and aggravates emphysema in the lungs of mice treated with elastase (17) when given alone or concomitant with CS (36). In the current study, although RSV infection alone did not cause significant changes in the alveolar volume, E/LPS injury caused significant damage to the alveolar structures, which was enhanced by RSV infection. This finding suggests that the recruitment of inflammatory cells and induction of IL-17 and IL-23 in the lungs of E/LPS-injured mice compared with uninjured controls play significant roles in the alveolar destruction after repeated exposure to E/LPS. Treatment with anti–IL-17 antibodies only partially inhibited the RSV-dependent exacerbation of airspace injury in E/LPS-injured mice. Previous studies showed that deletion of IL-17 reduced elastase-induced emphysematous changes in mice, but was not completely attenuated (37). The fact that IL-17 Ab treatment reduced the volume-weighted mean alveolar volume to the levels seen in the E/LPS-mock group suggests that suppressing IL-17 reduces virus-enhanced mucus cell metaplasia and increases in lung alveolar tissue destructions.
The recruitment of neutrophils and lymphocytes is required for IL-17–mediated airway inflammation and modulation of lung and airway structural cells (22). As we stated above, RSV infection causes early spikes mainly in neutrophils in the lungs of E/LPS-injured mice. These early recruitment of inflammatory cells by RSV may be responsible for induction of IL-17–related cytokines and chemokines, leading to exacerbation of tissue damage and mucous production. Anti–IL-17 treatment significantly reduced BAL neutrophil levels consistent with previous reports showing IL-17 overproduction increased neutrophilic infiltration and exacerbation of tissue damage and mucus production (38). Therefore, this early spike in IL-17 is likely playing a significant role in causing MCH. This finding is supported by previous reports that IL-17 controls mucus production (39) and that deletion of IL-17 reduces elastase-induced emphysema in mice (37). Neutralization receptor knockout of IL-17 in RSV-infected IL-27 mice resulted in a significant decrease in the pulmonary mucus response and inhibition of the Th2-associated cytokines. Furthermore, IL-17 blockage leads to an increase in the expression of IL-27 in the lungs of RSV-infected mice (38), suggesting that IL-27 functions as a regulatory cytokine during RSV pathogenesis by suppressing the development of Th17 cells or that RSV-induced IL-17 regulates IL-27.
Together, these data suggest that IL-17 is essential, but additional factors may contribute to worsening of emphysema. In elastase-instilled mice, increases in IL-23 are followed by increases in IL-17, supporting the importance of IL-23/IL-17 pathway in elastase-induced lung pathology. Antagonizing IL-23 with anti–IL-23p40 antibody reduced airway inflammation and emphysematous changes in mice (40), suggesting that, in addition to IL-17, IL-23 may play a significant role in elastase-induced lung inflammation and emphysema. Overexpression of IL-18 induces emphysema via IFN-γ and IL-17A, and mucus metaplasia via IL-13 (41), suggesting that IL-18 and IL-13 may have important roles in the pathogenesis of emphysema (42). The key role of IL-17 in this animal model is supported by other reports that patients with COPD have increased levels of IL-17 (11). Two different clones of anti–IL-17 antibody, LY2439821 and AIN457, are currently undergoing clinical trials in patients with rheumatoid arthritis.
IL-17 and IL-23 play a role not only in elastase-induced emphysema (40), but also in enhancing mucus cell metaplasia. IL-17 increases MUC5AC mRNA and protein synthesis (43), leading to increased mucus production (44) in the airway epithelium. Consistent with our finding, injection of anti–IL-17 antibody intraperitoneally in tobacco smoke–exposed mice significantly reduced neutrophil levels in the BALF and the pathological score of small airway inflammation (45). In the current study, treatment with anti–IL-17 antibodies completely blocked RSV-induced mucus cell metaplasia in E/LPS-injured lungs, suggesting that increased expression of promucogenic IL-17 essentially accounted for increased mucus production, a hallmark of chronic bronchitis.
IFNs play important roles in limiting viral spread in the early stages of infection (46). However, in our model, both IFN-α and IFN-β levels were not increased in BALF of E/LPS-injured mice infected with RSV. Varying responses have been demonstrated in mouse models of COPD. For instance, mice exposed to four weekly doses of E/LPS followed by rhinovirus infection had an impaired IFN-α/β response with increased virus load compared with mice receiving rhinovirus alone (19). Type I IFNs and their major producers, plasmacytoid DCs (pDCs), are important in protection from RSV disease. RSV stimulates pDCs from human peripheral blood mononuclear cells or murine bone marrow–derived pDCs (47) to produce type I IFN (48). The reduced RSV-induced type I IFN responses in E/LPS-injured lungs in the current study may be explained by reduced recruitment of pDCs in E/LPS-injured lungs because of defective innate IFN responses, or BALF may not be a good indicator of type I IFN response. Further studies on IFN levels in the micromilieu of the lung in response to RSV infection in the E/LPS mouse COPD model are needed to characterize the IFN responses in detail.
In summary, the RSV-induced exacerbation of E/LPS-induced lung injury shares key features with respiratory virus–induced exacerbation of COPD in humans. Infection with RSV in E/LPS-injured mice is associated with enhanced increases in IL-23 and IL-17 expression that translates into increased mucus cell hyperplasia and emphysema. Depletion of IL-17 in vivo diminished RSV-driven MCH and airspace enlargement in the E/LPS-injured mice, suggesting that IL-17 may be a therapeutic target to reduce RSV-driven MCH and airspace enlargement.
Acknowledgments
Acknowledgment
The authors thank Ms. Elise Calvillo from technical communication department at Lovelace Respiratory Research Institute for helping with preparation of graphics and images.
Footnotes
This work was supported by National Institutes of Health grants 1R21AI115291-01A1 (Y.A.M.), and HL68111 and ES015482 (Y.T.).
Author Contributions: Design and conception of study—Y.A.M. and Y.T.; conducting the experiments—Y.A.M.; analysis and interpretation—Y.A.M. and Y.T.; drafting the manuscript for intellectual content—Y.A.M. and Y.T.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0265OC on January 9, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Miniño AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. Natl Vital Stat Rep. 2010;59:1–52. [PubMed] [Google Scholar]
- 2.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 3.Awji EG, Seagrave JC, Tesfaigzi Y. Correlation of cigarette smoke–induced pulmonary inflammation and emphysema in C3H and C57Bl/6 mice. Toxicol Sci. 2015;147:75–83. doi: 10.1093/toxsci/kfv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awji EG, Chand H, Bruse S, Smith KR, Colby JK, Mebratu Y, et al. Wood smoke enhances cigarette smoke–induced inflammation by inducing the aryl hydrocarbon receptor repressor in airway epithelial cells. Am J Respir Cell Mol Biol. 2015;52:377–386. doi: 10.1165/rcmb.2014-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, et al. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 2015;136:441–453. doi: 10.1016/j.jaci.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117(5 suppl 2):398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 8.Wright JL, Churg A. Animal models of cigarette smoke–induced COPD. Chest. 2002;122(6 suppl):301S–306S. doi: 10.1378/chest.122.6_suppl.301s. [DOI] [PubMed] [Google Scholar]
- 9.Criado G, Simelyte E, Inglis JJ, Essex D, Williams RO. Indoleamine 2,3 dioxygenase–mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009;60:1342–1351. doi: 10.1002/art.24446. [DOI] [PubMed] [Google Scholar]
- 10.McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev. 2014;260:129–144. doi: 10.1111/imr.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Y, Nadigel J, Boulais N, Bourbeau J, Maltais F, Eidelman DH, et al. CD8 positive T cells express IL-17 in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:43. doi: 10.1186/1465-9921-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang Y, Al-Alwan L, Audusseau S, Chouiali F, Carlevaro-Fita J, Iwakura Y, et al. Genetic deletion of IL-17A reduces cigarette smoke–induced inflammation and alveolar type II cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2014;306:L132–L143. doi: 10.1152/ajplung.00111.2013. [DOI] [PubMed] [Google Scholar]
- 13.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:478–485. doi: 10.1513/pats.200802-014ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mebratu YA, Smith KR, Agga GE, Tesfaigzi Y. Inflammation and emphysema in cigarette smoke–exposed mice when instilled with poly (I:C) or infected with influenza A or respiratory syncytial viruses. Respir Res. 2016;17:75. doi: 10.1186/s12931-016-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W. Bacterial endotoxin is an active component of cigarette smoke. Chest. 1999;115:829–835. doi: 10.1378/chest.115.3.829. [DOI] [PubMed] [Google Scholar]
- 17.Ishii T, Hosoki K, Nikura Y, Yamashita N, Nagase T, Yamashita N. IFN regulatory factor 3 potentiates emphysematous aggravation by lipopolysaccharide. J Immunol. 2017;198:3637–3649. doi: 10.4049/jimmunol.1601069. [DOI] [PubMed] [Google Scholar]
- 18.Beckett EL, Stevens RL, Jarnicki AG, Kim RY, Hanish I, Hansbro NG, et al. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J Allergy Clin Immunol. 2013;131:752–762. doi: 10.1016/j.jaci.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajjan U, Ganesan S, Comstock AT, Shim J, Wang Q, Nagarkar DR, et al. Elastase- and LPS-exposed mice display altered responses to rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2009;297:L931–L944. doi: 10.1152/ajplung.00150.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 21.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesfaigzi Y. Roles of apoptosis in airway epithelia. Am J Respir Cell Mol Biol. 2006;34:537–547. doi: 10.1165/rcmb.2006-0014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foronjy RF, Taggart CC, Dabo AJ, Weldon S, Cummins N, Geraghty P. Type-I interferons induce lung protease responses following respiratory syncytial virus infection via RIG-I–like receptors. Mucosal Immunol. 2015;8:161–175. doi: 10.1038/mi.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T cells are required for inflammation and destruction in cigarette smoke–induced emphysema in mice. J Immunol. 2007;178:8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- 26.Majo J, Ghezzo H, Cosio MG. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur Respir J. 2001;17:946–953. doi: 10.1183/09031936.01.17509460. [DOI] [PubMed] [Google Scholar]
- 27.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 28.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 29.Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 32.Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Jin J, Tang Y, Speer D, Sujkowska D, Markovic-Plese S. IFN-β1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182:3928–3936. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]
- 34.Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, et al. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci USA. 2004;101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virus–induced TLR7 activation controls IL-17–associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1–L15. doi: 10.1152/ajplung.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurimoto E, Miyahara N, Kanehiro A, Waseda K, Taniguchi A, Ikeda G, et al. IL-17A is essential to the development of elastase-induced pulmonary inflammation and emphysema in mice. Respir Res. 2013;14:5. doi: 10.1186/1465-9921-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Almeida Nagata DE, Demoor T, Ptaschinski C, Ting HA, Jang S, Reed M, et al. IL-27R–mediated regulation of IL-17 controls the development of respiratory syncytial virus–associated pathogenesis. Am J Pathol. 2014;184:1807–1818. doi: 10.1016/j.ajpath.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimoto K, Graham BS, Ho SB, Adler KB, Collins RD, Olson SJ, et al. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am J Respir Crit Care Med. 2004;170:306–312. doi: 10.1164/rccm.200301-030OC. [DOI] [PubMed] [Google Scholar]
- 40.Fujii U, Miyahara N, Taniguchi A, Waseda K, Morichika D, Kurimoto E, et al. IL-23 is essential for the development of elastase-induced pulmonary inflammation and emphysema. Am J Respir Cell Mol Biol. 2016;55:697–707. doi: 10.1165/rcmb.2016-0015OC. [DOI] [PubMed] [Google Scholar]
- 41.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, et al. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoshino T, Kato S, Oka N, Imaoka H, Kinoshita T, Takei S, et al. Pulmonary inflammation and emphysema: role of the cytokines IL-18 and IL-13. Am J Respir Crit Care Med. 2007;176:49–62. doi: 10.1164/rccm.200603-316OC. [DOI] [PubMed] [Google Scholar]
- 43.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1β and IL-17A; the NF-κB paradigm. J Immunol. 2009;183:6236–6243. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, et al. IL-17–induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011;179:248–258. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen N, Wang J, Zhao M, Pei F, He B. Anti–interleukin-17 antibodies attenuate airway inflammation in tobacco-smoke–exposed mice. Inhal Toxicol. 2011;23:212–218. doi: 10.3109/08958378.2011.559603. [DOI] [PubMed] [Google Scholar]
- 46.Isaacs A, Lindenmann J. Virus interference: I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 47.Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, et al. Replication-dependent potent IFN-α induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 48.Cormier SA, Shrestha B, Saravia J, Lee GI, Shen L, DeVincenzo JP, et al. Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J Virol. 2014;88:9350–9360. doi: 10.1128/JVI.00818-14. [DOI] [PMC free article] [PubMed] [Google Scholar]